Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Afifi A, Gonzalez RS. Carcinoma of extrahepatic bile ducts. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/gallbladdercarcinomaextrahepatic.html. Accessed May 13th, 2024.
Definition / general
- Rare malignant adenocarcinoma arising from extrahepatic bile ducts
- Biliary adenocarcinomas (cholangiocarcinomas) typically divided into intrahepatic bile duct origin, gallbladder origin, perihilar bile origin (this topic) and distal extrahepatic bile duct origin (this topic), including right and left hepatic ducts, common hepatic duct and common bile duct
Essential features
- Adenocarcinoma arising from the biliary system outside the liver, with several risk factors
- Histology is similar to intrahepatic cholangiocarcinoma, particularly large duct type
- Poor prognosis
Terminology
- Some prefer to restrict the term cholangiocarcinoma to intrahepatic adenocarcinomas only but others use the term for any adenocarcinoma of the biliary system
- Klatskin tumor refers to a hilar cholangiocarcinoma arising at the bifurcation of the right and left hepatic ducts
ICD coding
Epidemiology
- 0.53 - 2 per 100,000 in population
- Incidence is decreasing in recent years (StatPearls: Biliary Tract Cholangiocarcinoma [Accessed 17 November 2023])
- Slight male predominance due to primary sclerosing cholangitis being more common in men (StatPearls: Biliary Tract Cholangiocarcinoma [Accessed 17 November 2023], Ann Hepatobiliary Pancreat Surg 2023;27:151)
- Occurs primarily in sixth and seventh decades of life (Ann Hepatobiliary Pancreat Surg 2023;27:151)
Sites
- Can develop at any level of the extrahepatic biliary tree, including within the pancreas
- Bismuth-Corlette classification relates to extent of infiltration of ducts
Pathophysiology
- Risk factors such as infections, genetics and primary sclerosing cholangitis can play a role in disease pathogenesis (StatPearls: Biliary Tract Cholangiocarcinoma [Accessed 17 November 2023])
- Often arises from precursor lesions, namely biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile ducts
- Various mutations in oncogenes and tumor suppressor genes transform normal epithelium into premalignant lesions
Etiology
- Several risk factors exist for development of extrahepatic cholangiocarcinoma (Liver Int 2019;39:19)
- Choledochal cyst
- Primary sclerosing cholangitis
- Parasites (Opisthorchis viverrini and Clonorchis sinensis)
- Caroli disease
- Cholelithiasis
- Chronic pancreatitis
- May arise de novo, without risk factors being present
Clinical features
- Signs of biliary obstruction may occur, including jaundice (most characteristic and common symptom), pruritis, acholic stools and dark urine (Liver Int 2019;39:98)
- Patients can present with nonspecific symptoms, including abdominal pain, nausea / vomiting, anorexia, weight loss and malaise (Liver Int 2019;39:98)
- Many patients present with unresectable disease
Diagnosis
- Early diagnosis is challenging and is often delayed due to asymptomatic early stage disease (World J Hepatol 2021;13:166)
- Lack of a standardized screening protocol
- MRI and magnetic resonance cholangiopancreatography (MRCP) can assess size and extent of tumor
- Endoscopic ultrasound (EUS) and EUS guided FNA can be utilized in the diagnosis and staging of cholangiocarcinoma
- Intraductal ultrasound is suggested for patients with obstructive jaundice for local tumor staging and bile duct stricture assessment
Laboratory
- Elevated serum CA 19-9, CEA-M, CA125 (BJS Open 2021;5:zrab127)
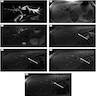
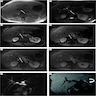
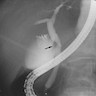
Radiology description
- May form a mass or cause thickening of bile duct walls
- CT and MRI (Liver Int 2019;39:98)
- Duct wall thickening and lumen obliteration, indicating infiltration
- Mass lesion
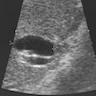
- Ultrasound (Liver Int 2019;39:98)
- Duct dilation, indicating obstruction
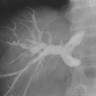
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Stricture of the bile duct
Radiology images
Prognostic factors
- Favorable prognostic factors
- Absence of comorbidities and presence of dysplasia are favorable prognostic factors (Hepatobiliary Pancreat Dis Int 2020;19:153)
- Increase in albumin and a decrease in total bilirubin improve the prognosis of patients with hilar cholangiocarcinoma (Hepatobiliary Pancreat Dis Int 2020;19:153)
- Intrapancreatic cases have a better prognosis than pancreatic ductal adenocarcinoma but a worse prognosis than ampullary adenocarcinoma (Mod Pathol 2016;29:1358)
- Prognostic biomarkers
- Albumin, ALT, CA 19-9 and CEA are independent prognostic biomarkers for overall survival (Cancers (Basel) 2022;14:1026, Medicine (Baltimore) 2019;98:e14556)
- Preoperative C reactive protein to albumin ratio (CAR) is an accurate prognostic biomarker, predicting overall survival and disease free survival (J Surg Oncol 2020;122:1516)
- CAR is the most valuable prognostic biomarker in patients with resected extrahepatic cholangiocarcinoma (Dig Surg 2022;39:65)
- Lymph node metastasis is one of the most important prognostic factors (J Surg Oncol 2020;122:1516)
- Presence of venous, lymphatic and perineural invasion indicate a worse prognosis (J Surg Oncol 2020;122:1516)
- pT category staging for extrahepatic cases is based on depth of invasion into bile duct wall (Am J Surg Pathol 2007;31:199)
Case reports
- 17 year old boy with perihilar mass in an explanted liver (Pathol Int 2020;70:888)
- 57 year old man with obstructive jaundice (Front Oncol 2022;12:948799)
- 71 year old woman with extrahepatic cholangiocarcinoma and thigh mass (Front Surg 2022;9:922834)
Treatment
- Surgical resection is the only hope for long term survival (Rozhl Chir 2022;101:416)
- Chemotherapy can be used as adjuvant treatment or for palliation in unresectable cases (J Clin Oncol 2019;37:1015)
Gross description
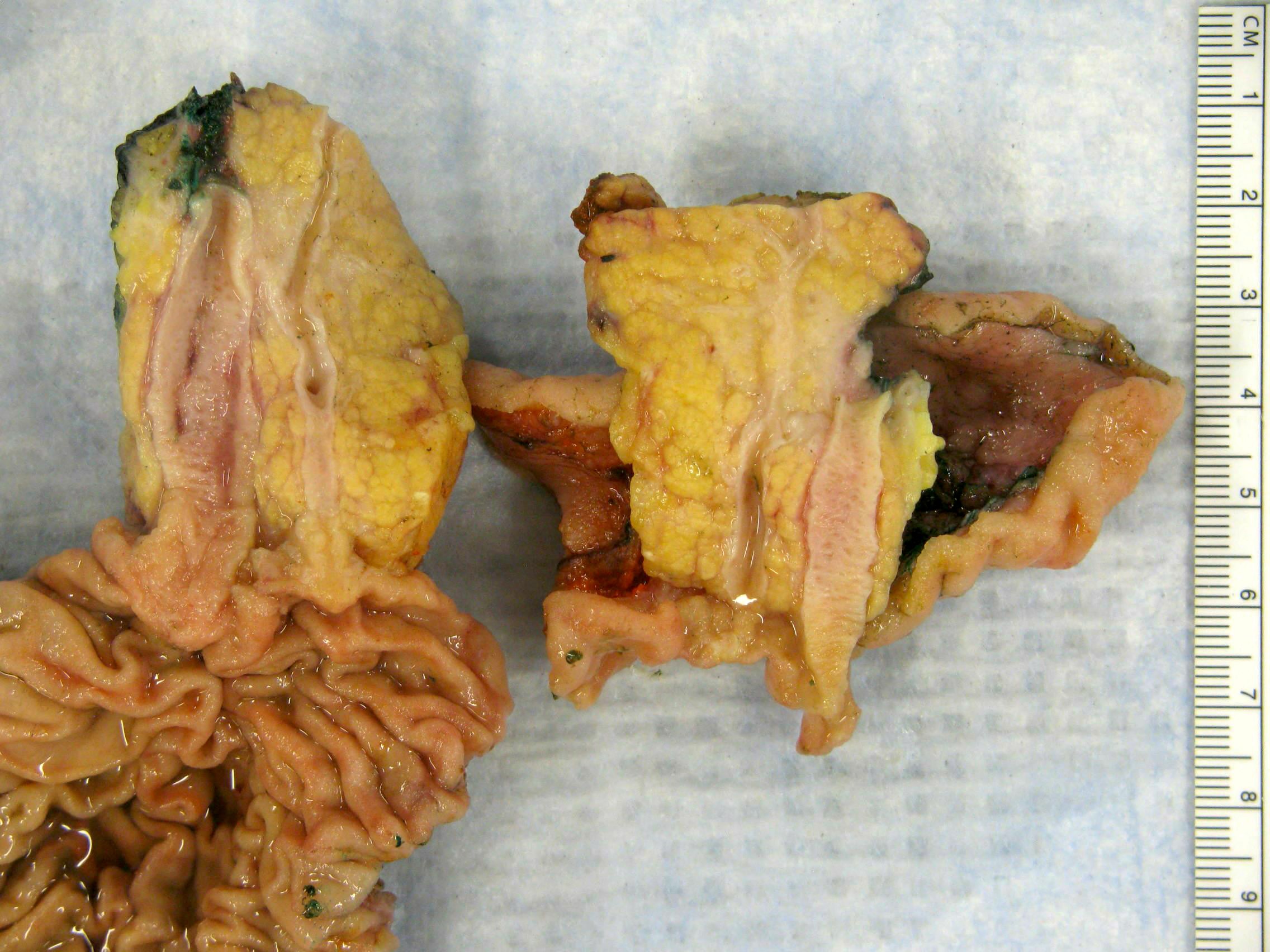
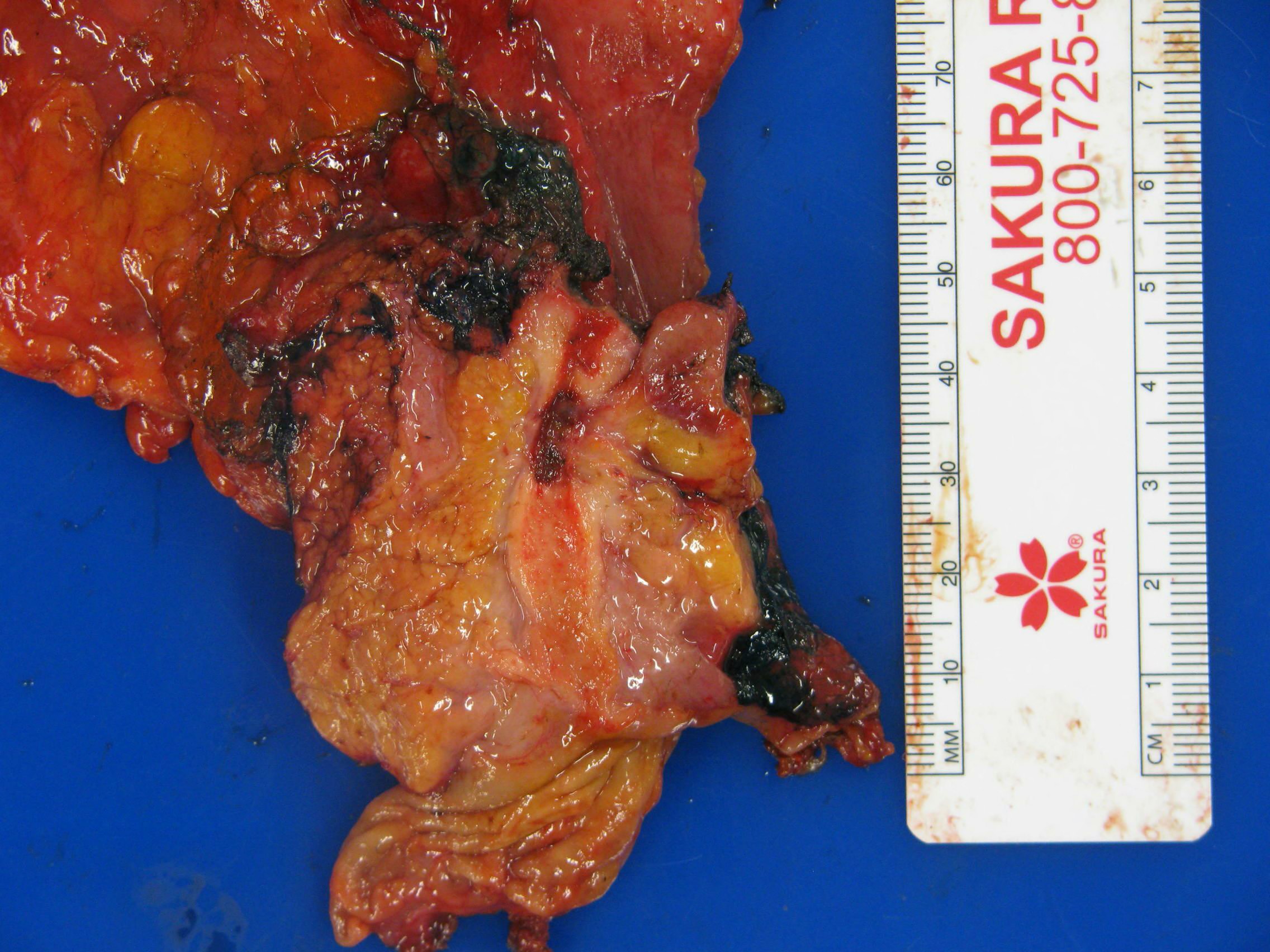
- Various configurations possible, including stenotic, nodular, polypoid, sclerosing and diffusely infiltrating
- Mass forming cases have a firm, white, gritty cut surface
Gross images
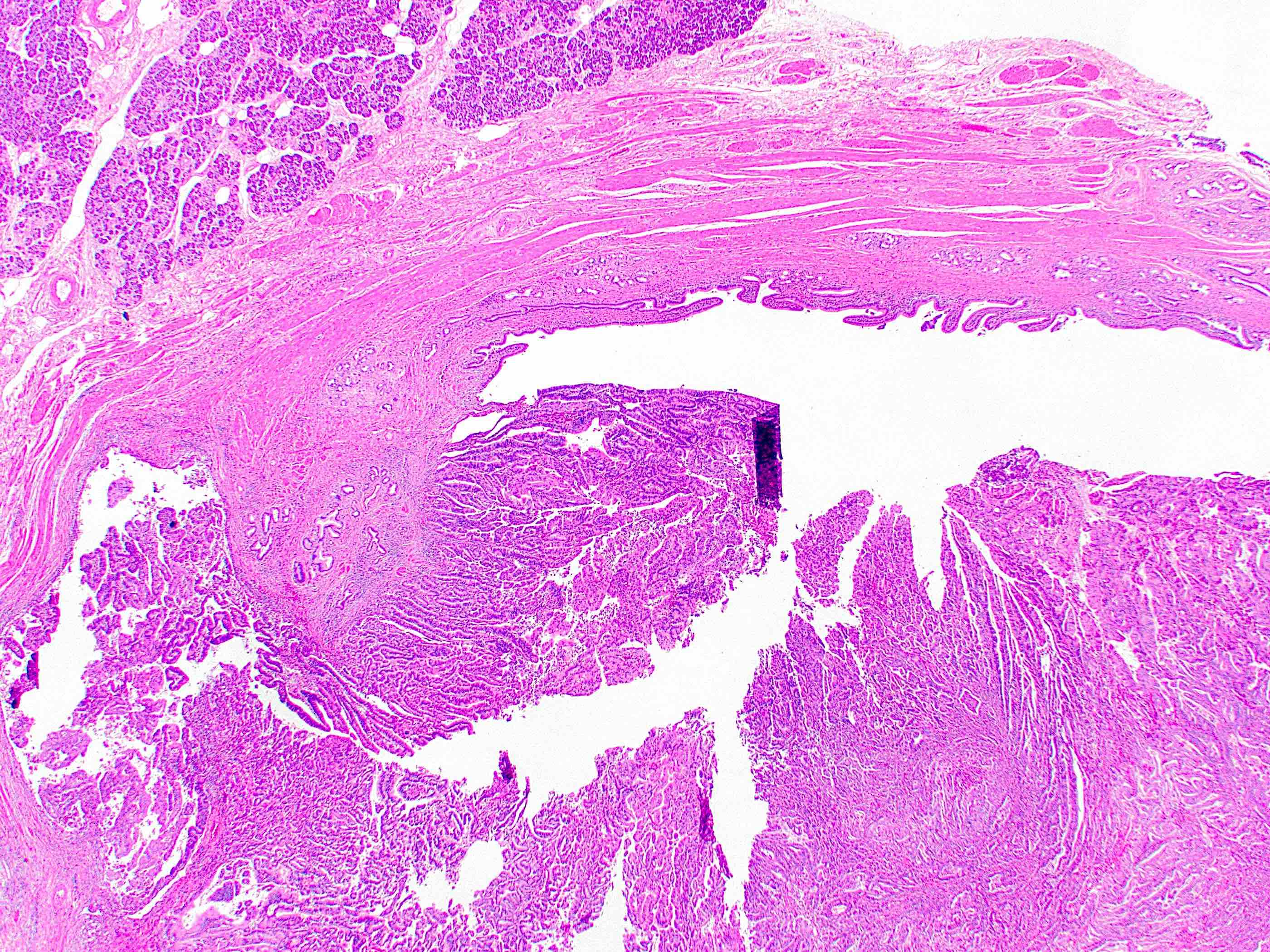
Microscopic (histologic) description
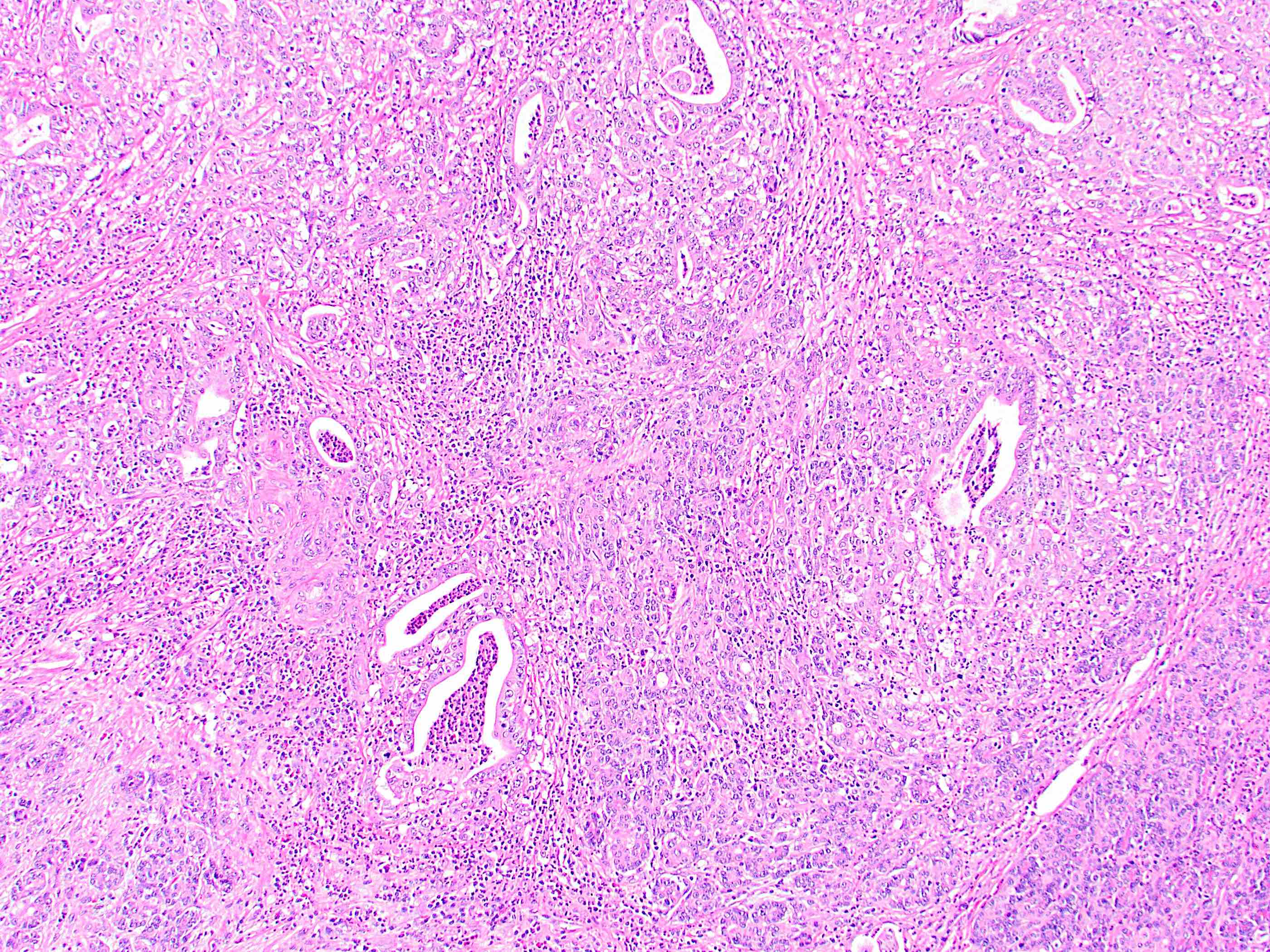
- Generally resembles the large duct type of intrahepatic cholangiocarcinoma (Mod Pathol 2014;27:1163)
- Invasive malignant adenocarcinoma with variable nuclear and cytologic pleomorphism (World J Gastrointest Oncol 2022;14:607)
- Widely spaced glands haphazardly infiltrate duct wall
- Glands may be well formed, irregular, solid, cord-like, cribriform, abortive or forming papillary structures
- Individual infiltrating cells
- Variable necrosis can be present
- Often a prominent associated neutrophilic infiltrate
- May show intraductal growth with papillary, tubular or superficial spreading patterns (World J Gastrointest Oncol 2022;14:607)
- Precursor lesion may be visible (biliary intraepithelial neoplasia, intraductal papillary neoplasm of the bile ducts)
Microscopic (histologic) images
Cytology description
- Cytologic features include (Cytopathology 2022;33:257)
- Irregular, variably sized sheets of atypical to malignant glandular cells
- Peripheral palisading nuclei
- Branching tapered columns (resembling bile duct epithelium)
- Disorderly growth with crowding and piling up
- Loss of nuclear polarity (drunken honeycomb appearance)
Negative stains
- CK20 (Surg Case Rep 2020;6:264)
- Generally negative for markers indicating origin in other organ systems (e.g., TTF1, GATA3, PAX8)
Molecular / cytogenetics description
- KRAS, TP53, ARID1A and SMAD4 mutations most common (J Hepatol 2020;73:315)
- 4 molecular classes have been proposed: metabolic class, proliferation class, mesenchymal class and immune class
Sample pathology report
- Extrahepatic bile duct, excision:
- Segment of bile duct with extrahepatic cholangiocarcinoma (2.5 cm), extending 0.7 cm into bile duct wall (pT2) (see synoptic report)
- Margins of resection unremarkable
- Lymphovascular and perineural invasion present
- One lymph node positive for carcinoma (1/1)
- Background bile duct with acute and chronic inflammation
Differential diagnosis
- Secondary involvement of pancreatic ductal adenocarcinoma or ampullary carcinoma (Cancers (Basel) 2023;15:1454):
- Most relevant in pancreatoduodenectomy specimens
- Requires careful gross examination and correlation with clinical and imaging findings
- May be impossible to definitively determine for advanced lesions
- Reactive change:
- Can involve biliary surface epithelium or peribiliary glands
- On cytology specimens, features of malignancy include 3 dimensional clusters, pleomorphism, 2 cell population and chromatin pattern changes (Mod Pathol 2017;30:1273)
Board review style question #1
This image shows a cholangiocarcinoma arising within the pancreas. Which of the following is true regarding extrahepatic cholangiocarcinoma?
- It has a good prognosis
- It often shows a prominent neutrophilic infiltrate
- It resembles the small duct type of intrahepatic cholangiocarcinoma
- Peak onset is in the third decade of life
- Primary biliary cholangitis is a risk factor
Board review style answer #1
B. It often shows a prominent neutrophilic infiltrate. This finding is often seen in pancreatobiliary adenocarcinomas in general, including extrahepatic cholangiocarcinoma. Answer A is incorrect because this disease has a poor prognosis. Answer D is incorrect because peak onset is in the sixth and seventh decades of life. Answer E is incorrect because primary sclerosing cholangitis is a risk factor, not primary biliary cholangitis. Answer C is incorrect because histologically, it most resembles the large duct type of intrahepatic cholangiocarcinoma.
Comment Here
Reference: Carcinoma of extrahepatic bile ducts
Comment Here
Reference: Carcinoma of extrahepatic bile ducts
Board review style question #2
Which of the following immunohistochemical stains is typically positive in extrahepatic cholangiocarcinoma?
- CDX2
- CK7
- CK20
- PAX8
- TTF1
Board review style answer #2
B. CK7. As with most pancreatobiliary adenocarcinomas, extrahepatic cholangiocarcinoma is positive for CK7. Answers A and C are incorrect because CDX2 and CK20 are variable but more often negative than positive. Answer E is incorrect because TTF1 positivity would suggest lung origin. Answer D is incorrect because PAX8 positivity would suggest renal or gynecologic origin.
Comment Here
Reference: Carcinoma of extrahepatic bile ducts
Comment Here
Reference: Carcinoma of extrahepatic bile ducts