Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology and etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Statz E, Jorns JM. Atypical lobular hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastalh.html. Accessed October 3rd, 2025.
Definition / general
- Clonal proliferation of discohesive epithelial cells arising in terminal duct lobular units
- Similar histologic features to lobular carcinoma in situ (LCIS) but of insufficient quantity
Essential features
- Clonal proliferation of discohesive cells identical to lobular carcinoma in situ but in smaller quantity
- Typical dysfunction / loss of E-cadherin
- Generally considered incidental finding (clinically / radiologically occult)
- 4 - 5 times increased risk of (bilateral risk but higher risk ipsilateral) invasive breast carcinoma compared with general population
- Does not necessarily require excision if isolated finding
Terminology
- Lobular intraepithelial neoplasia 1 (LIN1)
ICD coding
Epidemiology
- F > M (Ann Plast Surg 2015;74:163)
- Typically adults with wide age range, most commonly diagnosed in 50s (Arch Pathol Lab Med 2018;142:391)
- Found in less than 1% of core needle biopsies performed for BI-RADS 4 assessment (Am J Surg 2014;207:24)
Sites
- Bilateral breasts with no specific location
Pathophysiology and etiology
- 16q loss / CDH1 deletion and loss of function of E-cadherin, a transmembrane cell adhesion molecule (Breast Cancer Res 2019;21:76)
- Change in function of E-cadherin rather than loss in some cases and rarely other adhesion proteins implicated with E-cadherin stable
- Low grade neoplasia pathway / family (Breast Cancer Res 2019;21:76)
- Increased expression ESR1, decreased expression SFRP1 (Breast Cancer Res 2019;21:76)
Clinical features
- Frequently associated with columnar cell lesions and flat epithelial atypia; less commonly associated with low grade invasive carcinomas which may have mammographically detectable calcifications, density or mass targeted on biopsy (Am J Surg Pathol 1998;22:1521, Am J Surg Pathol 2007;31:417)
- Does not form a palpable mass
- 19% develop invasive cancer at mean 15 years after diagnosis (4 - 5 times usual risk), 42% are special subtypes with good prognosis (Cancer 2006;107:1227)
Diagnosis
- Core biopsy
- Incidental after mammography, ultrasound or MRI
Radiology description
- Classically considered to be an incidental finding with no radiologic correlates
- Calcifications, if associated with atypical lobular hyperplasia in biopsy or non-mass enhancement on MRI, may be considered concordant after biopsy is read (Breast Dis 2016;36:5)
Radiology images
Prognostic factors
- Lower risk of upgrade than lobular carcinoma in situ
- 4 - 5 times usual risk of carcinoma, higher in ipsilateral breast, higher if age < 50 years; risk increased further if ductal involvement (Am J Surg Pathol 2002;26:421, Cancer 2007;109:180, Hum Pathol 1988;19:201)
- If present at core biopsy, is surgical excision appropriate (controversial)?
- Many studies support excision due to risk of subsequent in situ or invasive carcinoma
- Yes: subsequent DCIS / invasive carcinoma occurs in 6 - 8%, invasive ductal / lobular carcinoma occurs in 25% (Arch Pathol Lab Med 2008;132:979, Am J Surg Pathol 2007;31:717, Am J Surg Pathol 2005;29:534, AJR Am J Roentgenol 2008;190:637)
- No, unless:
- Radiologic pathologic discordance suggests that the targeted lesion was not excised
- Other high risk lesions such as atypical ductal hyperplasia are present that would warrant further surgery
- Atypical lobular hyperplasia has features resembling ductal carcinoma in situ (Am J Surg Pathol 2002;26:1095)
- Atypical lobular hyperplasia has pleomorphic features (Mod Pathol 2008;21:1208, Cancer 2008;112:2152)
- There is diffuse lobular neoplasia at core biopsy (Breast J 2007;13:55)
- No: because there is no significantly increased risk and any subsequent cancer is diffuse, in either breast and not at location of biopsy (Am J Surg 2009;198:792)
Case reports
- 42 year old woman with microcalfications on routine screening mammogram (Arch Pathol Lab Med 2000;124:463)
- 44 year old woman with 1.5 cm palpable smooth lump in the right breast with popcorn-like calcifications (BMC Res Notes 2013;6:267)
- 50 year old woman with abnormal calcifications on screening (Arch Pathol Lab Med 2014;138:1344)
- 53 year old woman with asymmetry on annual screening mammogram (Ann Diagn Pathol 2015;19:20)
- 59 year old woman with 5 cm opacity in upper inner quadrant of left breast detected by mammogram (J Clin Pathol 1992;45:936)
- Premenopausal woman with family history of breast cancer was found to have a subcentimeter mass with calcifications on mammogram (Case of the month #533)
Treatment
- Controversial; some surgeons excise, others get lifelong screening / follow up screening intervals
- Growing evidence to avoid excision for well sampled lesions (Ann Surg Oncol 2017;24:2848)
- Selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) can lower risk of subsequent invasive carcinoma in patients with atypical hyperplasias (atypical lobular hyperplasia or atypical ductal hyperplasia not separated) (J Natl Compr Canc Netw 2018;16:1391)
Gross description
- No specific gross findings
- May be associated with calcifications but typically due to other coexisting lesions
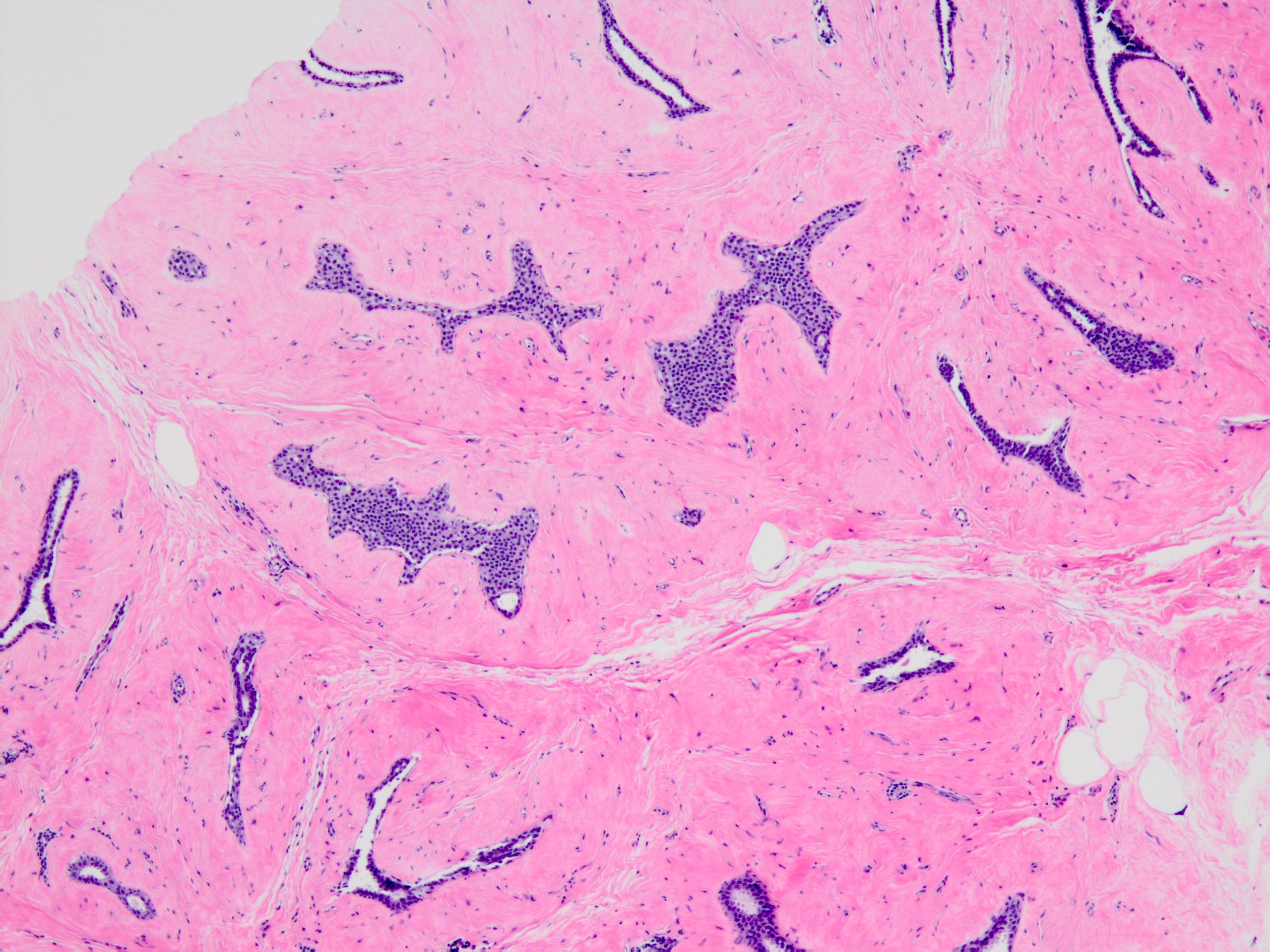
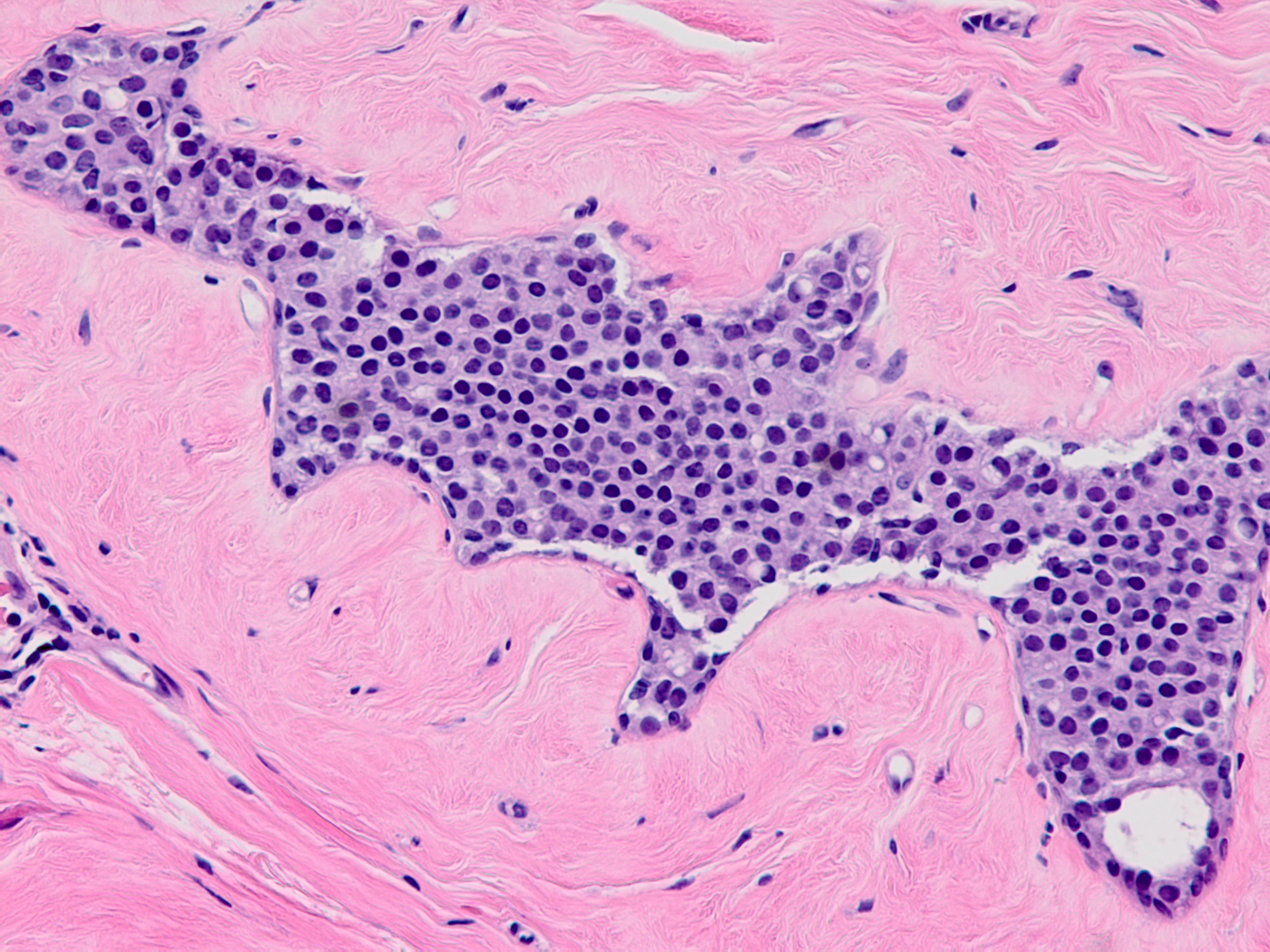
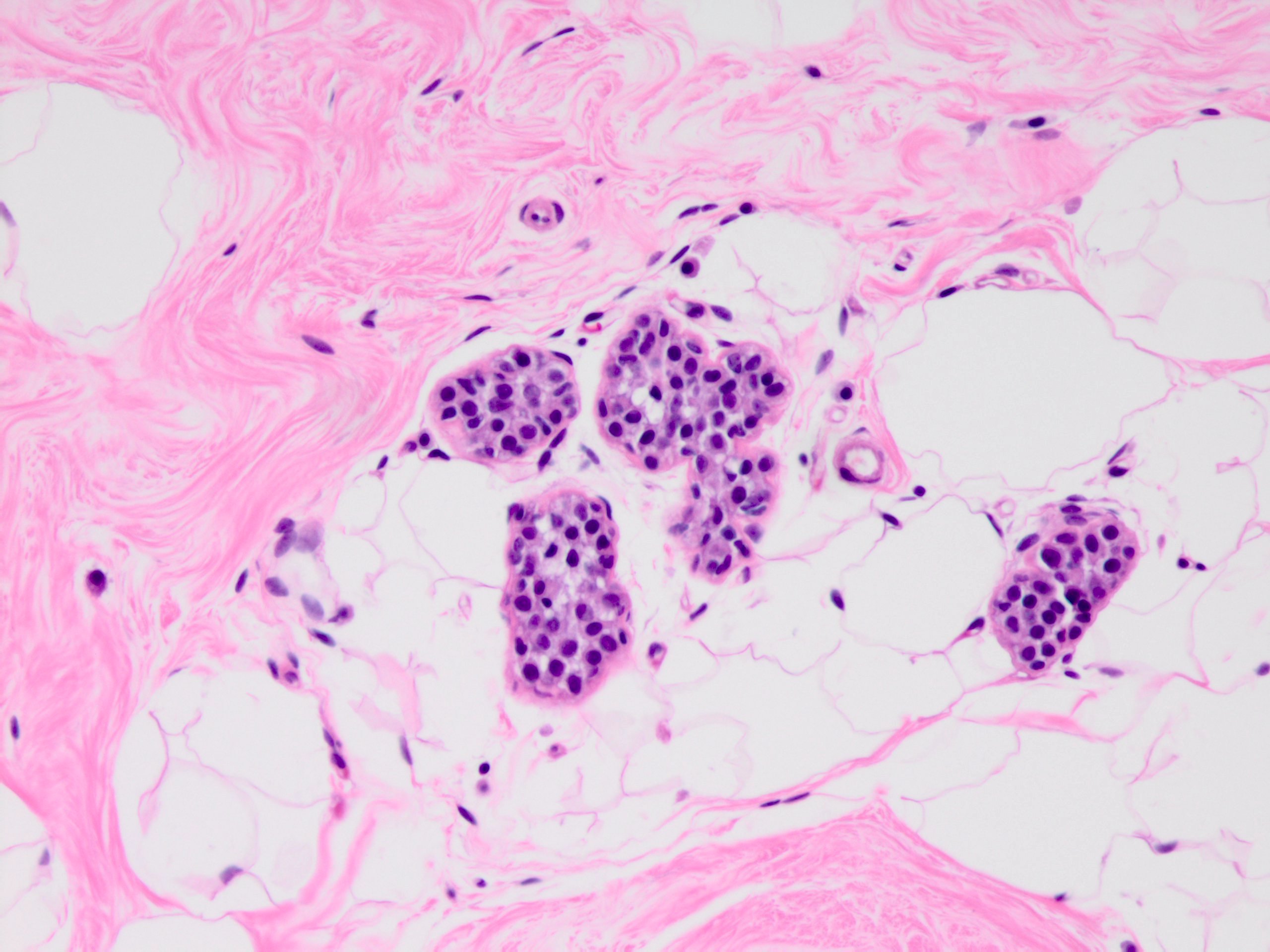
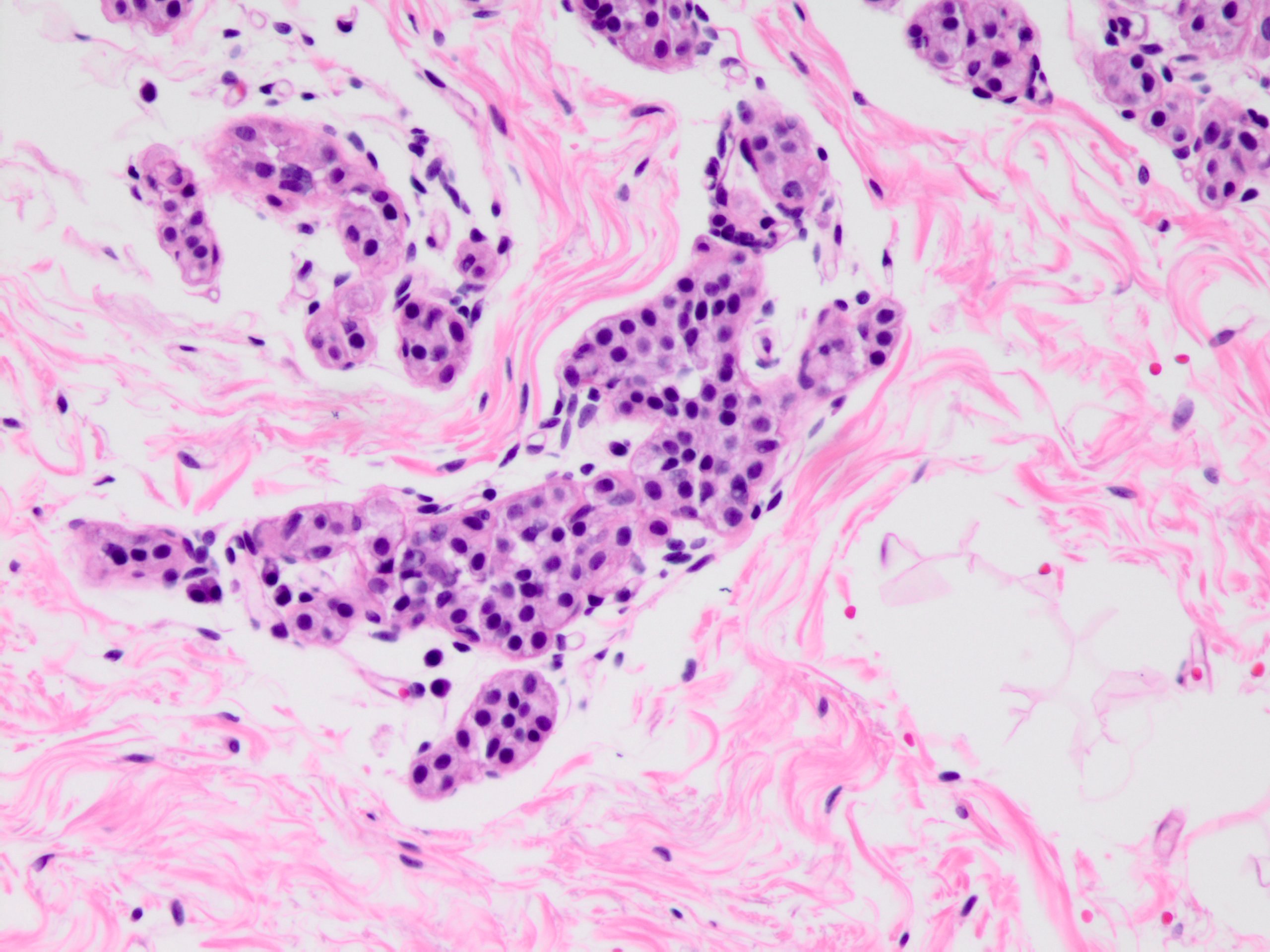
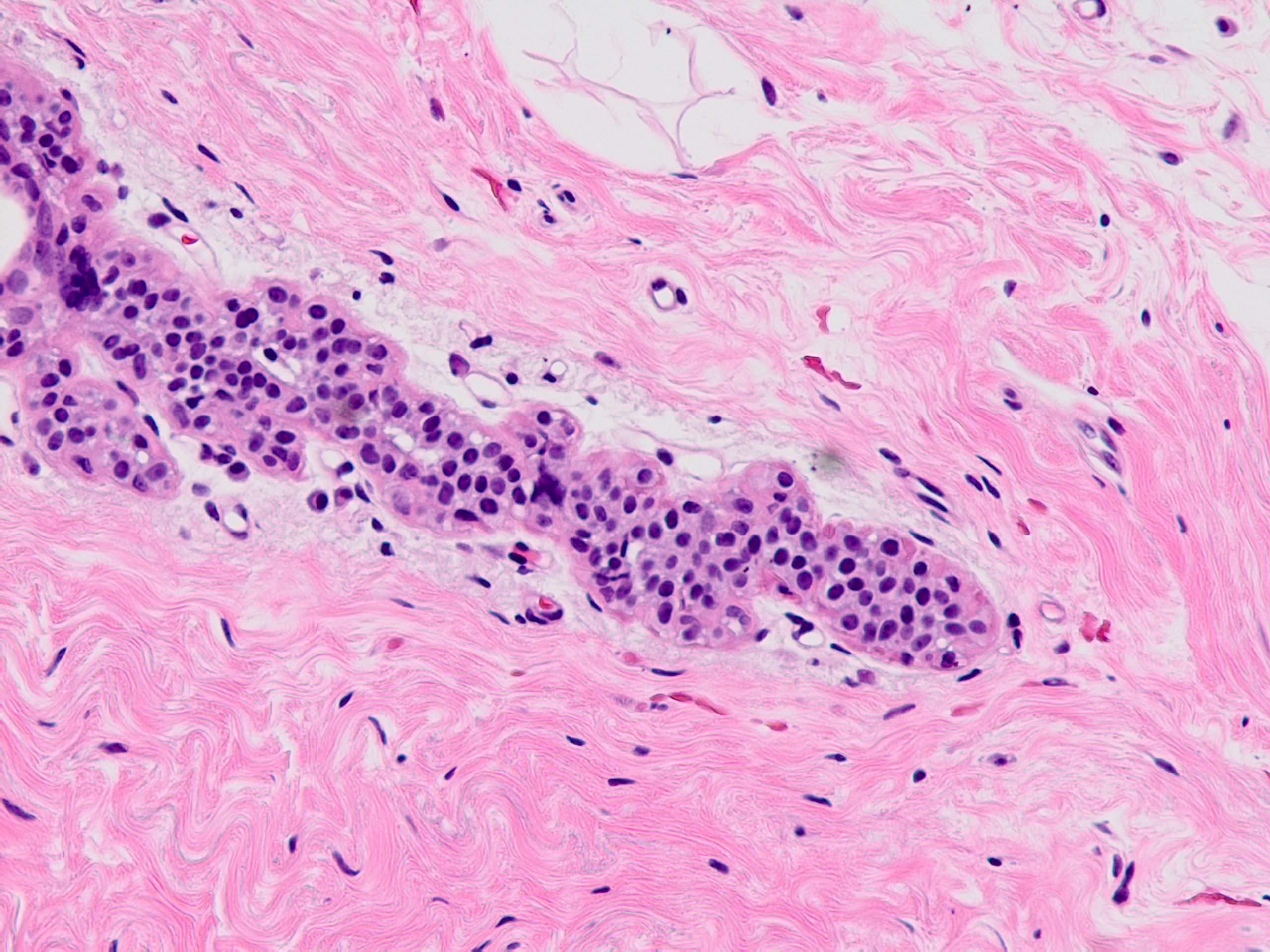
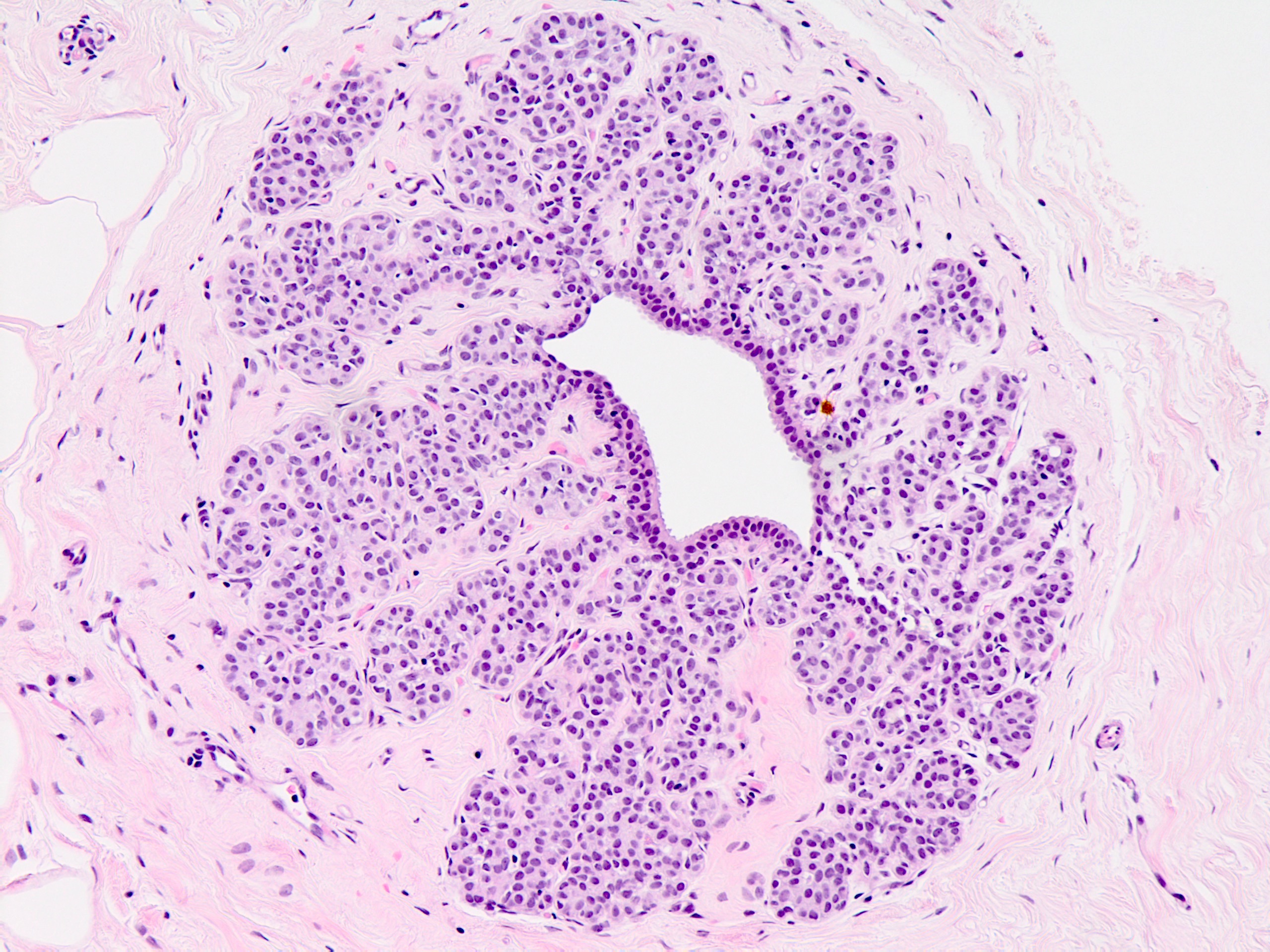
Microscopic (histologic) description
- Solid, discohesive proliferation of cells that are monomorphic, small, have pale pink cytoplasm, uniform oval nuclei and indistinct nuclei
- Some cells have plasmacytoid or signet ring appearance (intracytoplasmic vacuoles)
- Do not form arcades, lumens or papillary projections
- Criteria of Page et al. (Schnitt: Biopsy Interpretation of the Breast, 3rd Edition, 2017):
- Distends 50% or more acini within a lobule (so resembles lobular carcinoma in situ) but not uniformly present throughout entire lobule OR
- Involves all acini in a terminal duct lobular unit (TDLU) and so resembles lobular carcinoma in situ but does not distend the acini (i.e. caliber of the involved acini is similar to that of uninvolved acini)
- Can involve ducts; alteration occurs around the duct as outpouchings producing a cloverleaf pattern
- Lacks intracytoplasmic mucin
- May be no / minimal inflammatory response
Microscopic (histologic) images
Cytology description
- Loosely cohesive cell clusters composed of uniform cells with occasional intracytoplasmic lumina, minimal nuclear atypia but frequent eccentric nuclei
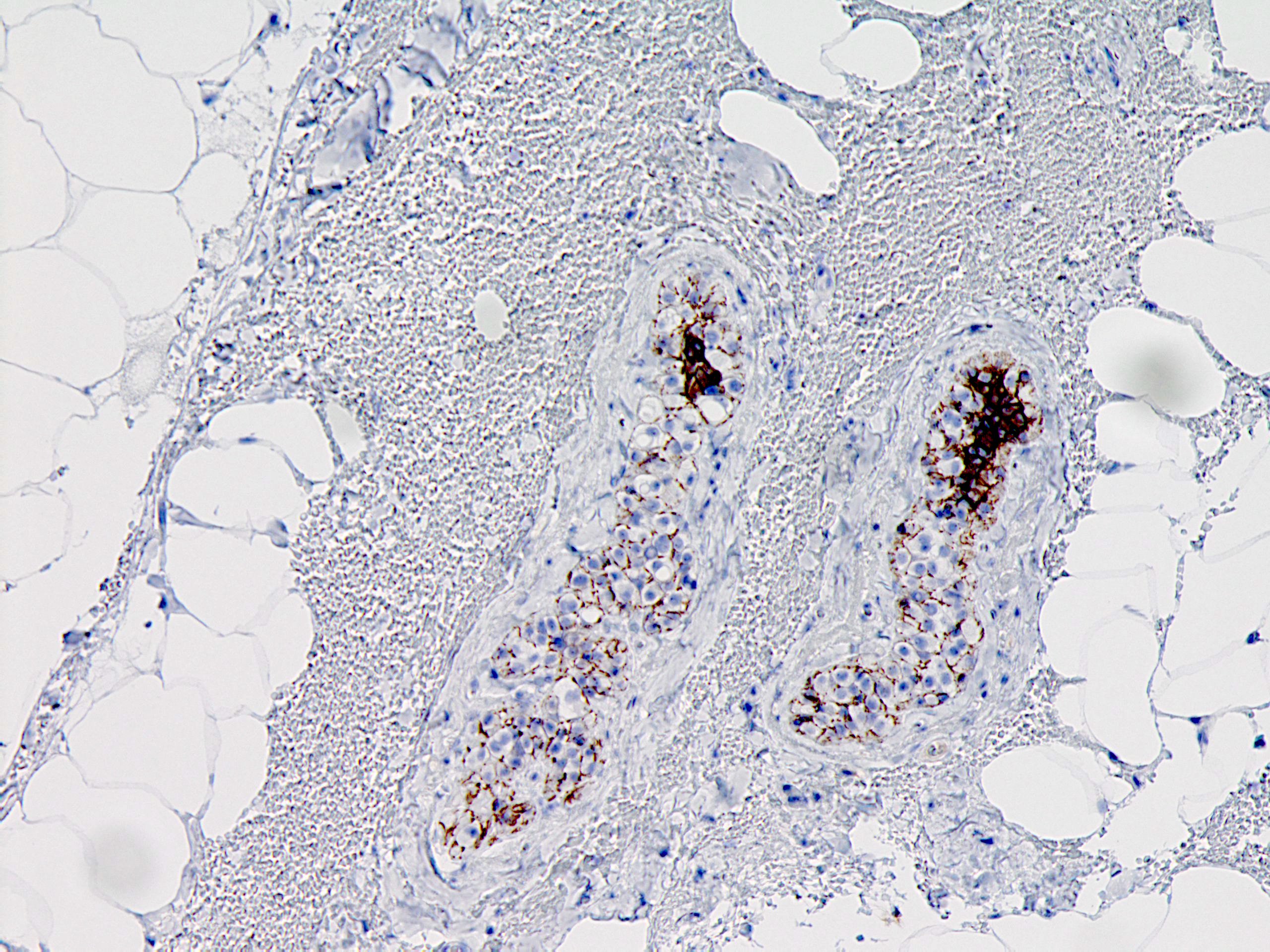
Positive stains
- ER: ER alpha stronger than ER beta (Virchows Arch 2007;451:893)
- PR
- 34betaE12: polarized
- p120 catenin: cytoplasmic, not membranous (Am J Surg Pathol 2007;31:427)
Negative stains
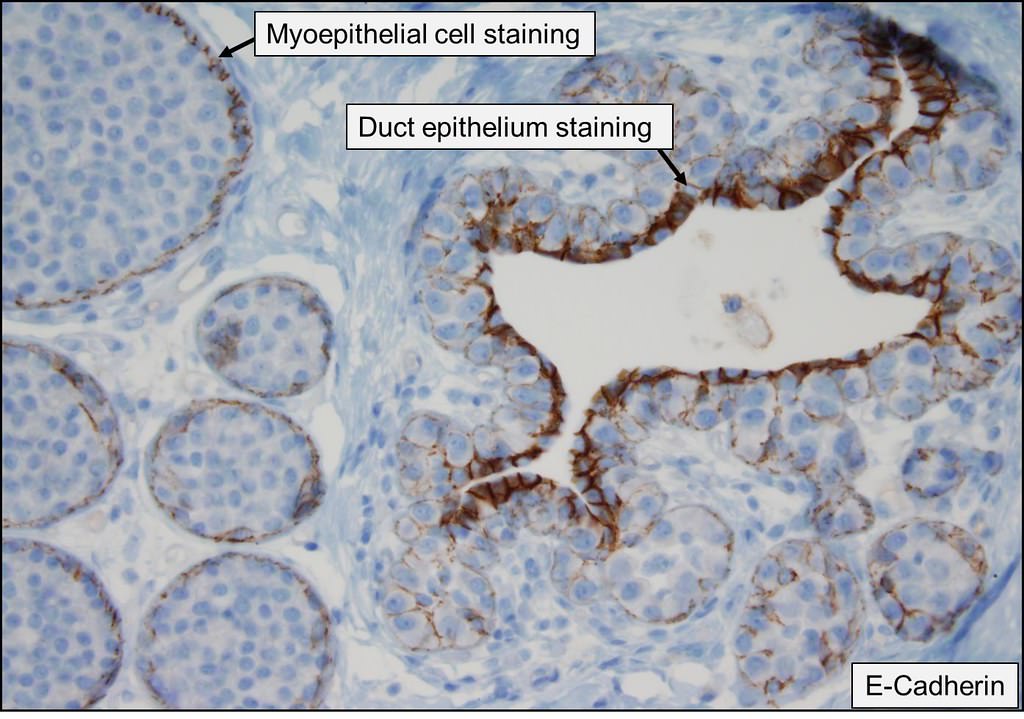
- E-cadherin (Mod Pathol 2005;18:741): reduced or absent
- HER2
- CK5/6 (Histopathology 2000;37:232)
- Alpha and beta catenin
Electron microscopy description
- Intracytoplasmic lumina, microvilli with secretory droplets; basement membrane and myoepithelial cells are present
Molecular / cytogenetics description
- Diploid
- LOH 16q (Mod Pathol 2005;18:741)
- CDH1 methylation in subset (Breast Cancer Res Treat 2018;167:9)
- Multiple others seen including 22q loss, 2p11.2 gain, 5q32-33.1 (CSF1R) gain, 6q gain, 11q13 gain, 14q32.33 gain, 16p loss (Breast Cancer Res Treat 2018;167:9)
Sample pathology report
- Left breast, 12 o'clock, core biopsy:
- Atypical lobular neoplasia (ALH) (see comment)
- Comment: An immunohistochemical stain for E-cadherin is negative in the atypical focus, supporting the diagnosis of ALH. Controls are appropriate.
Differential diagnosis
- Atypical ductal hyperplasia (ADH):
- Cells should be cohesive with rigid architecture
- E-cadherin positive
- p120 catenin membranous positive
- Lobular carcinoma in situ (LCIS):
- Greater than 50% distension
- Greater than 50% involvement of a lobule
- Benign lobule with clear cell change
Practice question #1
Practice answer #1
C. Atypical lobular hyperplasia (ALH)
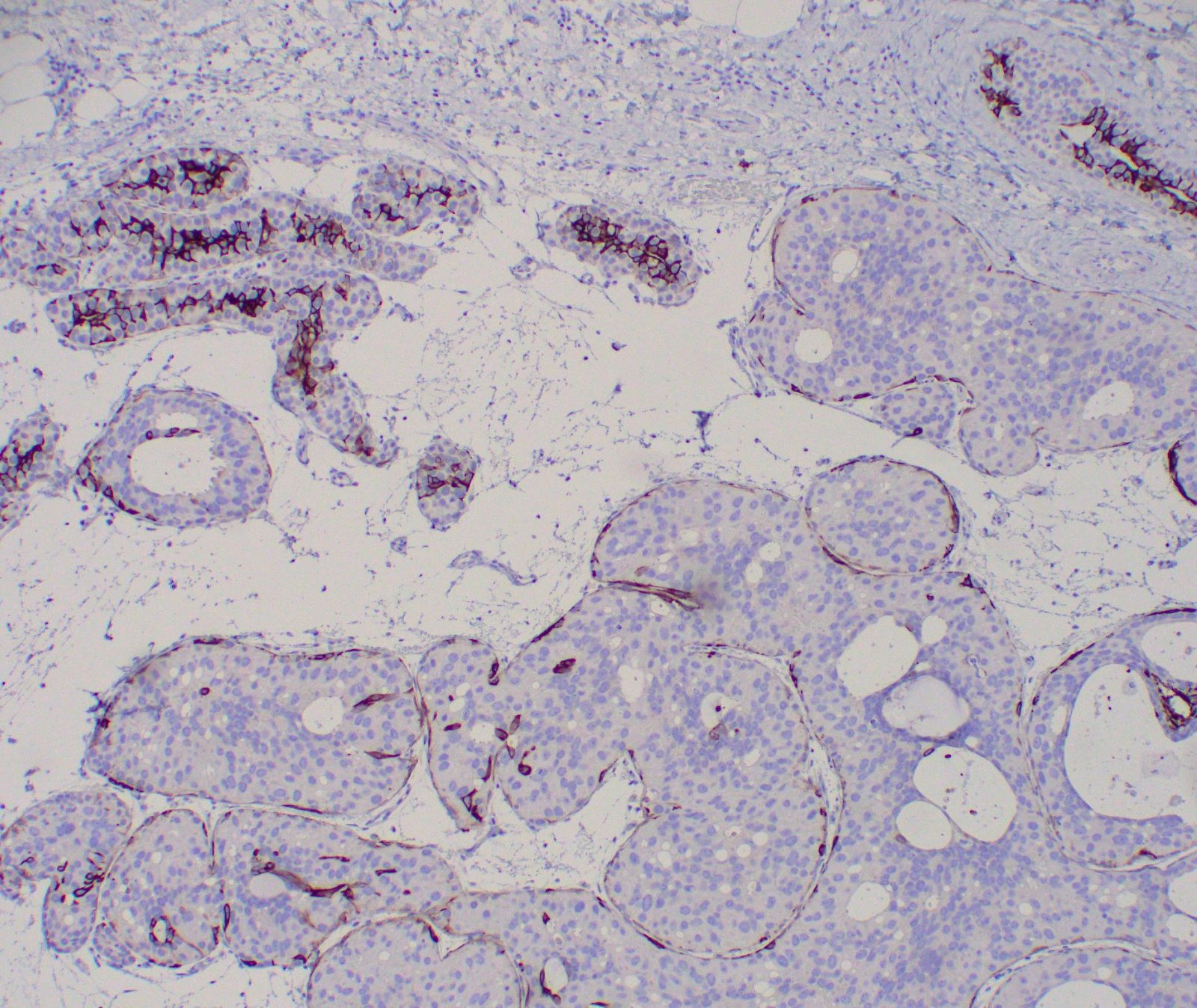
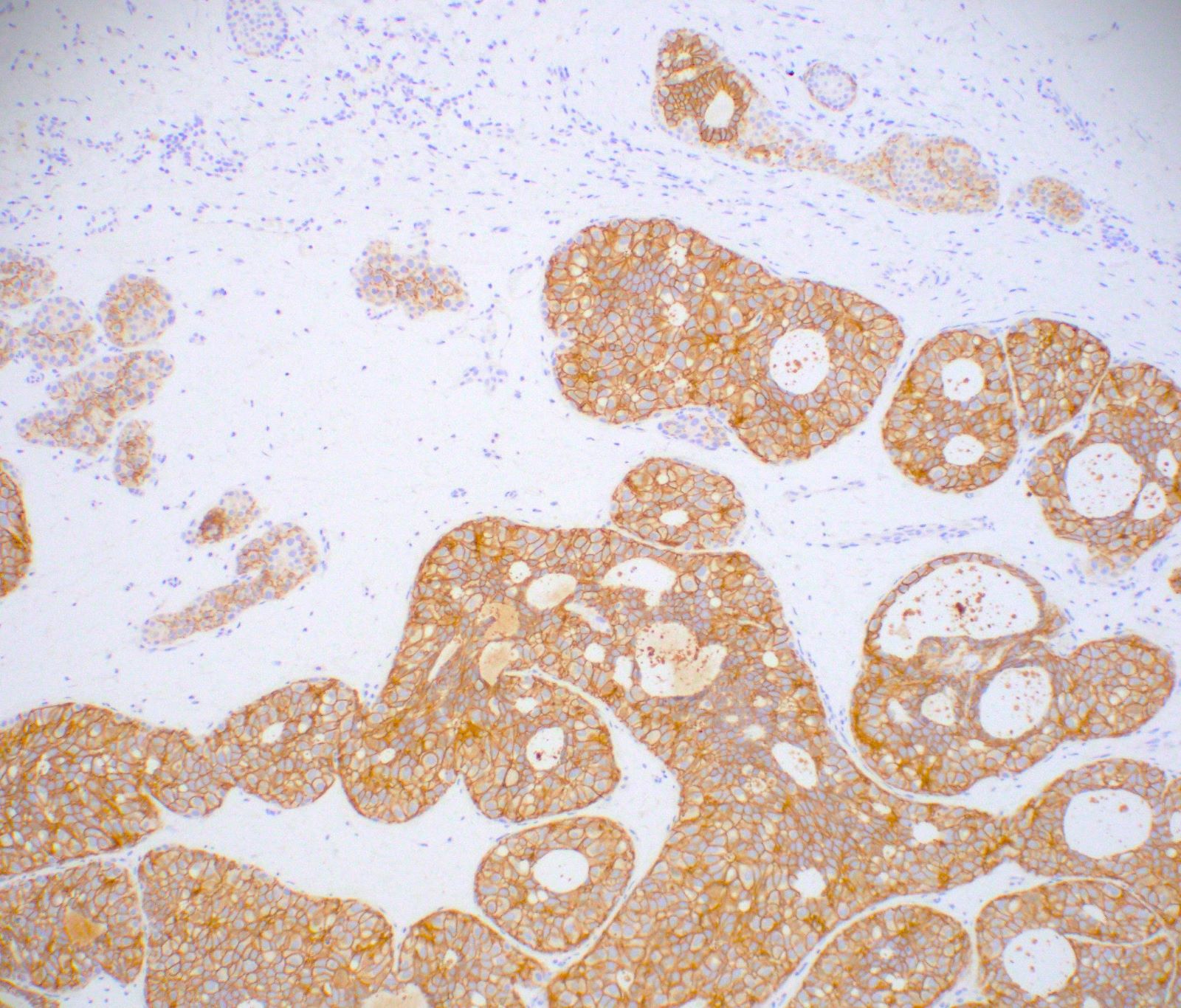
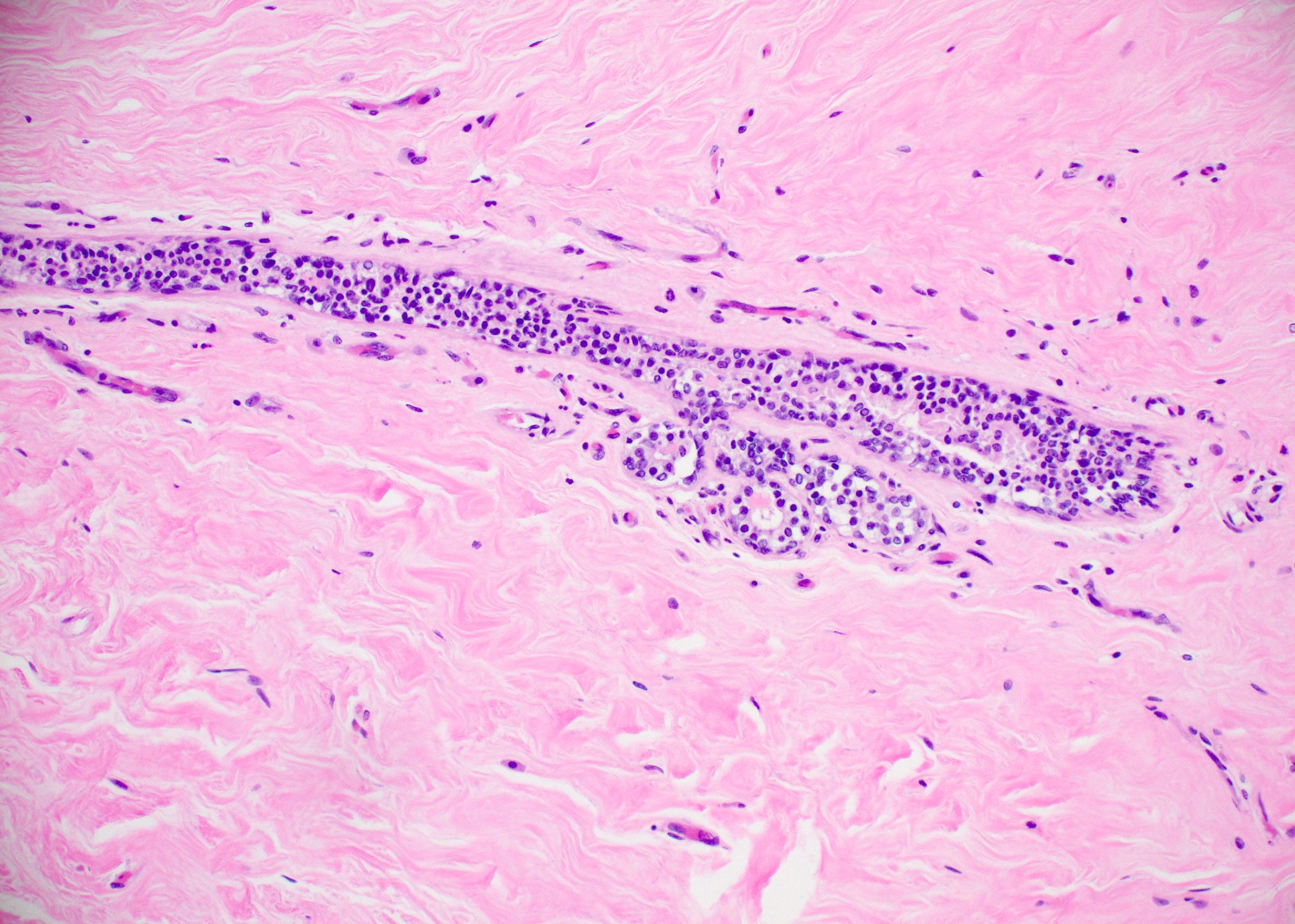
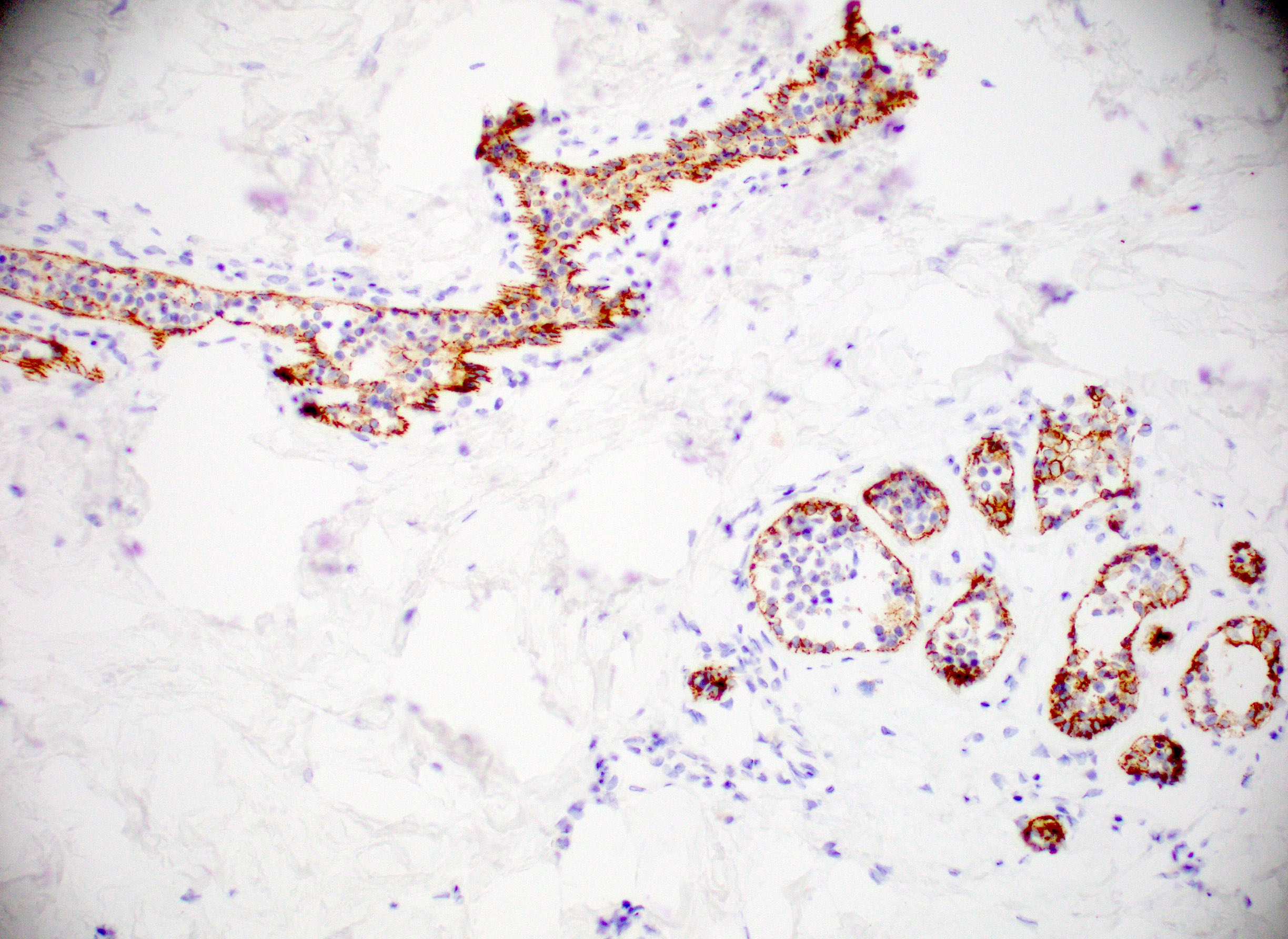
Explanation: The above images show an in situ proliferation of small to moderate sized, monotonous epithelial cells with mild expansion of involved glands. There are a few cells with intracytoplasmic vacuoles as well as variably prominent cell borders. Thus, the differential diagnosis includes atypical ductal hyperplasia (ADH) with a solid growth pattern and atypical lobular hyperplasia (ALH). Atypical apocrine adenosis (AAA) has larger cells with apocrine cytology including large, round nuclei with prominent nucleoli and abundant eosinophilic cytoplasmic. Flat epithelial atypia (FEA) has dilated glands with atypical low columnar to cuboidal epithelium with high nuclear cytoplasmic ratio and lack of orientation toward lumina. Characteristic features of AAA and FEA are lacking in the pictured lesion. In the differential of ALH and solid ADH immunohistochemistry can be very helpful, with ALH showing lack or attenuation of E-cadherin and cytoplasmic staining via p120 as shown above. ADH in contrast has strong, diffuse E-cadherin staining and membranous staining via p120.
Comment Here
Reference: Atypical lobular hyperplasia (ALH)
Explanation: The above images show an in situ proliferation of small to moderate sized, monotonous epithelial cells with mild expansion of involved glands. There are a few cells with intracytoplasmic vacuoles as well as variably prominent cell borders. Thus, the differential diagnosis includes atypical ductal hyperplasia (ADH) with a solid growth pattern and atypical lobular hyperplasia (ALH). Atypical apocrine adenosis (AAA) has larger cells with apocrine cytology including large, round nuclei with prominent nucleoli and abundant eosinophilic cytoplasmic. Flat epithelial atypia (FEA) has dilated glands with atypical low columnar to cuboidal epithelium with high nuclear cytoplasmic ratio and lack of orientation toward lumina. Characteristic features of AAA and FEA are lacking in the pictured lesion. In the differential of ALH and solid ADH immunohistochemistry can be very helpful, with ALH showing lack or attenuation of E-cadherin and cytoplasmic staining via p120 as shown above. ADH in contrast has strong, diffuse E-cadherin staining and membranous staining via p120.
Comment Here
Reference: Atypical lobular hyperplasia (ALH)
Practice question #2
- Atypical lobular hyperplasia (ALH) seen on core biopsy most frequently correlates to the following feature via breast imaging
- Asymmetry
- Calcifications
- Mass
- No specific finding
Practice answer #2
D. No specific finding
Explanation: Atypical lobular hyperplasia (ALH) is typically not identified via standard imaging modalities. Occasionally ALH has calcifications, although if a core biopsy that has ALH has an indication of calcifications, these are more frequently identified in other lesions in the biopsy such as fibrocystic change or other atypias (e.g. flat epithelial atypia or atypical ductal hyperplasia). ALH is not typically identified as a mass or asymmetry on breast imaging.
Comment Here
Reference: Atypical lobular hyperplasia (ALH)
Explanation: Atypical lobular hyperplasia (ALH) is typically not identified via standard imaging modalities. Occasionally ALH has calcifications, although if a core biopsy that has ALH has an indication of calcifications, these are more frequently identified in other lesions in the biopsy such as fibrocystic change or other atypias (e.g. flat epithelial atypia or atypical ductal hyperplasia). ALH is not typically identified as a mass or asymmetry on breast imaging.
Comment Here
Reference: Atypical lobular hyperplasia (ALH)