Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Immunofluorescence description | Positive stains | Molecular / cytogenetics description | Electron microscopy description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Pelkey L, Parker DC, Wolner Z. Interface dermatitis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skinnontumorinterfacedermatitis.html. Accessed September 17th, 2025.
Definition / general
- Interface dermatitis is a tissue reaction pattern characterized by the destruction of basal keratinocytes, which appears in the form of cell death or vacuolar change
Essential features
- Interface dermatitis is an inflammatory tissue reaction pattern characterized by lymphocyte mediated damage to basal keratinocytes, which manifests as cell death or vacuolar change
- Dyskeratotic keratinocytes, apoptotic keratinocytes or intracytoplasmic vacuoles are present in the basal epithelium
- Key features to narrow the differential diagnosis for interface dermatoses
- Type of basal cell damage
- Vacuolar (cell poor): erythema multiforme (EM) (prototypical example), Stevens-Johnson syndrome / toxic epidermal necrolysis (SJS / TEN), pityriasis lichenoides, acute graft versus host reaction, fixed drug eruption
- Connective tissue diseases: cutaneous lupus and dermatomyositis are also important examples of vacuolar interface change but are topics more thoroughly discussed elsewhere
- Lichenoid (cell rich): lichen planus (prototypical example), lichenoid drug eruption, lichen planus-like keratosis, lichen striatus, lichen nitidus
- Vacuolar (cell poor): erythema multiforme (EM) (prototypical example), Stevens-Johnson syndrome / toxic epidermal necrolysis (SJS / TEN), pityriasis lichenoides, acute graft versus host reaction, fixed drug eruption
- Distribution of the dermal inflammatory infiltrate
- Superficial only: erythema multiforme, SJS / TEN, lichen planus, lichenoid keratosis, lichen nitidus
- Superficial to deep: drug associated eruptions (lichenoid drug eruption, fixed drug), pityriasis lichenoides, graft versus host reaction, lichen striatus
- Prominent pigment incontinence
- Lichen planus, lichenoid reactions in darker pigmented patients, some drug associated eruptions
- Satellite cell necrosis (lymphocyte assisted apoptosis)
- Erythema multiforme, acute graft versus host reaction, some drug associated eruptions
- Type of basal cell damage
Terminology
- Lichenoid reaction pattern
- Lichenoid tissue reaction
- Lichenoid interface dermatitis
- Vacuolar interface dermatitis
- Vacuolar change
- Epidermal interface alteration
ICD coding
Epidemiology
Vacuolar
Lichenoid
- Erythema multiforme
- Young adults with modest predilection for men; median age of 24 years (Arch Dermatol 2002;138:1019)
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- M:F = 1:1.5 (Medicina (Kaunas) 2021;57:895)
- Predilection for older individuals; median age of 45 years (Arch Dermatol 2002;138:1019)
- Pityriasis lichenoides
- Average age of 29 years, no significant sex predisposition (J Am Acad Dermatol 2006;55:557)
- Graft versus host disease (GVHD)
- Patients who have received allogenic hematopoietic stem cell transplantation (HSCT) or rarely solid organ transplantation or blood transfusion (Transfus Apher Sci 2022;61:103402)
- Acute cutaneous GVHD occurs in 30 - 50% of patients within 100 days after allogenic HSCT (Bone Marrow Transplant 1998;22:755)
- Chronic GVHD develops in 30 - 40% of all long lived HSCT survivors (Blood 2015;125:606)
- Most (66%) cases of chronic GVHD have a history of acute GVHD, with an increasing likelihood with an increasing histologic grade of acute GVHD (Leukemia 2013;27:1196)
- Risk of GVHD is increased in human leukocyte antigen (HLA) disparity, peripheral blood and bone marrow grafts, donor - recipient sex mismatch and donors who have been previously pregnant or transfused as well as underlying disease and older age of recipient (Br J Haematol 1987;67:397, J Clin Oncol 2008;26:5728)
- Fixed drug eruption (FDE)
- M = F
- Patients taking antibiotics or other drugs (see Diagrams / tables)
- Predilection for ages 20 - 40 years old
- Generalized and bullous FDE occurs
Lichenoid
- Lichen planus (LP)
- M = F, though oral LP and lichen planopilaris are more common in female patients (Cutis 2003;72:63)
- Age of onset is typically 30 - 50 years old (J Am Acad Dermatol 2019;81:1397)
- Lichenoid drug eruption
- Adults, ~50 years old (Indian J Dermatol 2024;69:137)
- Patients taking antihypertensives or other photosensitizing drugs (see Diagrams / tables)
- Lichen striatus
- M < F
- Children, ~5 months - 15 years old (Pediatr Dermatol 2004;21:197)
- Lichen planus-like keratosis
- M:F = 1:2
- Adults, wide age range of 30 - 80 years old
- More common in White patients
- Lichenoid drug eruption
- Adults, ~50 years old (Indian J Dermatol 2024;69:137)
- Patients taking antihypertensives or other photosensitizing drugs (see Diagrams / tables)
- Lichen nitidus
- M > F
- Childhood, mean age of 9 years (Pediatr Dermatol 2019;36:189)
Sites
Vacuolar
Lichenoid
- Erythema multiforme
- Mucous membranes involved in 2 - 10% of individuals (StatPearls: Erythema Multiforme [Accessed 3 February 2025])
- Skin, face and symmetrically distributed lesions on the hands and feet
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- Systemic symptoms are typically present in TEN and SJS / TEN overlap and usually present in SJS
- Prodrome of fever, malaise, sore throat and cough (Adv Wound Care (New Rochelle) 2020;9:426)
- Multiple mucous membranes often involved (oral, ocular, genital)
- Skin of the face and trunk most common
- Body surface area involvement determines classification (Arch Dermatol 1993;129:92)
- SJS: < 10%
- SJS / TEN: 10 - 30%
- TEN: > 30%
- Body surface area involvement determines classification (Arch Dermatol 1993;129:92)
- Systemic symptoms are typically present in TEN and SJS / TEN overlap and usually present in SJS
- Pityriasis lichenoides
- Skin of the anterior trunk, flexor surfaces of the proximal extremities (J Am Acad Dermatol 2006;55:557)
- Graft versus host disease
- Skin (epidermis to deep fascia), gastrointestinal tract, liver, oral mucosa, lung, kidney
- Acute: skin of face, trunk, extremities, acral
- Chronic: skin of oral mucosa, trunk, anogenital, acral, eyes (keratitis sicca)
- Fixed drug eruption
- Recurs at the same site of mucous membranes or skin with repeated drug intake
- Can occur anywhere but sites of predilection include the lip, sacral region and genitalia (Arch Dermatol 1984;120:520)
- Palmar and plantar skin can also be involved (Arch Dermatol 1984;120:520)
- Connective tissue disease
Lichenoid
- Lichen planus
- Mucous membranes, oral and genital
- Skin, nails, flexor surfaces of the wrist, trunk, thighs, ankles
- Hypertrophic LP is usually confined to the shins
- Terminal hairs of the scalp are the main site of involvement in lichen planopilaris (Int J Womens Dermatol 2018;4:180)
- Palms and soles (palmoplantar LP) (Br J Dermatol 2000;142:310)
- Esophageal involvement may occur (World J Gastroenterol 2022;28:5893)
- Lichenoid drug eruption
- Mucous membranes, oral and genital
- Skin, photo distributed sites (face, forearms) or generalized
- Lichen striatus
- Skin, along the lines of Blaschko
- Usually involves the length of 1 extremity (J Cutan Pathol 1995;22:18)
- Nail changes may manifest as longitudinal fissures (Int J Dermatol 2015;54:1255)
- Lichen planus-like keratosis
- Skin, chest, back
- Lichen nitidus
- Skin, upper extremities, genitalia, abdomen, chest
- Generalized lichen nitidus involves the entire body (JAMA Dermatol 2018;154:367)
- Nail changes occur
Pathophysiology
- Basal cell damage in interface dermatitis is primarily mediated by apoptotic mechanisms, more so than epidermal necrosis (Am J Dermatopathol 1979;1:133, Adv Dermatol 1990;5:243)
- Apoptosis of the basal keratinocytes occurs by an active budding process that involves single cells condensing and fragmenting into small bodies (Civatte / colloid bodies)
- Colloid bodies in the papillary dermis appear to trap immunoglobulins nonspecifically, particularly IgM (Am J Dermatopathol 2002;24:130)
- Cytotoxic T lymphocyte attack on the basal keratinocytes is thought to be mediated by increased ICAM1 (intracellular adhesion molecule 1) expression on basal keratinocytes, secondary to cytokine (TNFα and interferon γ [IFNγ]) release by the mononuclear inflammatory infiltrate in interface dermatitis (subacute cutaneous lupus erythematosus [SCLE], erythema multiforme and lichen planus) (J Invest Dermatol 1995;105:71S)
- Increased IKKB (a subunit of the IKB kinase complex required for NFκB activation) activity resulted in interface dermatitis in transgenic mice, a finding dependent on inflammatory cytokines, macrophages and other CD45+ infiltrating cells in the skin and independent of T and B lymphocytes (J Invest Dermatol 2010;130:1598)
- Type 1 IFN system is involved in cutaneous autoimmune diseases characterized by interface dermatitis (cutaneous lupus erythematosus, lichen planus) (J Invest Dermatol 2008;128:2392)
- IFNγ and FasL / Fas exposure induces the histologic hallmarks of acute cutaneous GVHD in a reconstructed human epidermis model (Am J Dermatopathol 2011;33:244)
Etiology
Vacuolar
Lichenoid
- Erythema multiforme
- Associated with recent or recurrent infection, primarily herpes simplex infection elsewhere in the body (no viral infection of the EM lesions themselves)
- May be associated with Mycoplasma pneumoniae or Epstein-Barr virus (EBV) (Arch Dermatol 2002;138:1019)
- Infections are the most common trigger for EM (Am Fam Physician 2019;100:82)
- See Diagrams / tables for list of some associations / causative triggers for EM
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- Associated with medications; antiepileptic drugs, antibiotics and NSAIDs
- See Diagrams / tables for list of drugs causing SJS / TEN
- Those most at risk include those with hematologic malignancies, central nervous system cancer and infections with human immunodeficiency virus (HIV), tuberculosis and mycoplasma but no HSV association was found (J Invest Dermatol 2016;136:1387)
- Pityriasis lichenoides
- Is a spectrum of disease, with an acute form (pityriasis lichenoides et varioliformis acuta [PLEVA]) and a chronic form (pityriasis lichenoides chronica [PLC])
- Lesions of both PLEVA and PLC can coexist on the same patient
- PLEVA has been referred to as Mucha-Habermann disease but recently this terminology applies to the febrile ulceronecrotic subtype of PLEVA only (J Am Acad Dermatol 2006;55:557)
- Why some progress to the febrile ulceronecrotic Mucha-Habermann disease (FUMHD) variant is unknown
- PLEVA has been referred to as Mucha-Habermann disease but recently this terminology applies to the febrile ulceronecrotic subtype of PLEVA only (J Am Acad Dermatol 2006;55:557)
- Associated with HIV1, varicella zoster virus, as well as various medications and immunizations (Int J Dermatol 1997;36:104, Clin Exp Dermatol 2018;43:703)
- May progress to mycosis fungoides or another lymphoma (Br J Dermatol 2005;152:1327)
- Has clinicopathologic overlap with lymphomatoid papulosis (Br J Dermatol 2005;152:1327)
- Graft versus host disease
- Acute
- Interaction of donor T lymphocytes with host major histocompatibility complex (MHC) or host minor histocompatibility antigen (miH) (N Engl J Med 2017;377:2167)
- Mismatches in MHC class or minor histocompatibility antigens are associated with increased risk of severe acute GVHD
- Th2 predominant cytokines / chemokines and increase in IL22 producing CD4+ T cells, with an elevated thymic stromal lymphopoietin (a cytokine inducing Th2 differentiation) at day 20 - 30 post-HSCT in the skin of patients who later developed acute GVHD (Blood 2014;123:290)
- Late onset acute GVHD
- Mean levels of epidermal growth factor are 3 fold higher than in classic acute GVHD and not increased in chronic GVHD (Blood 2016;128:2350)
- Chronic
- Chronic lichenoid has mixed Th1 / Th17 with upregulated IFNγ and IL17 producing CD8+ T cells (Blood 2014;123:290)
- Acute
- Fixed drug eruption
- Occurs anytime from same day to 45 days after causative drug intake (Indian J Dermatol 2018;63:332)
- See Diagrams / tables for list of drugs causing fixed drug eruption
Lichenoid
- Lichen planus
- Prototypical lichenoid interface dermatitis reaction pattern
- Often unknown; associated with autoimmune disorders such as primary biliary cholangitis, autoimmune thyroiditis, hypothyroidism, ulcerative colitis and alopecia areata (J Am Acad Dermatol 1983;9:540, Int J Trichology 2015;7:87, J Am Acad Dermatol 2014;70:889, Arch Dermatol 1991;127:688)
- May be associated with hepatitis C infection and dyslipidemia (Br J Dermatol 1996;134:715, Int J Dermatol 2016;55:e295)
- Lichenoid drug eruption
- Occurs 15 days - 6 months after causative drug intake (Indian J Dermatol 2024;69:137)
- See Diagrams / tables for list of drugs producing a lichenoid reaction pattern
- Lichen striatus
- Unknown etiology
- Reported after viral infections, certain vaccinations
- Associated with atopic disease (Pediatr Dermatol 2004;21:197)
- Lichen planus-like keratosis
- Usually represents the attempted cell mediated immune rejection of a variety of epidermal lesions (solar lentigo, seborrheic keratosis) (Am J Surg Pathol 1993;17:259)
- Lichen nitidus
- Unknown etiology
- May be associated with Down syndrome and Russell-Silver syndrome (Indian J Dermatol Venereol Leprol 2009;75:627, Pediatr Dermatol 2013;30:150)
Diagrams / tables
Clinical features
Vacuolar
- Erythema multiforme
- Mucocutaneous eruption characterized by a few to numerous papules or plaques
- Erythematous, raised bullseye or targetoid lesions
- Lesions have a predilection for the extremities and the face
- Unlikely to involve multiple mucous membranes, such as oral, ocular or genital
- Demonstrates a high rate of recurrence (Arch Dermatol 2002;138:1019)
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- Ill defined erythematous macules with purpuric centers, with minimal (SJS) to widespread (TEN) coalescing of individual lesions
- Hemorrhagic erosions of the mucous membranes
- Positive Nikolsky sign with blistering and detachment of epidermis with light pressure (Adv Wound Care (New Rochelle) 2020;9:426)
- Classically mucocutaneous tenderness where the skin pain is out of proportion with the clinical appearance (Open Access Maced J Med Sci 2018;6:730)
- Pityriasis lichenoides
- Acute (PLEVA) presentation is a sudden onset of numerous erythematous 3 - 15 mm papules and small plaques with hemorrhagic and necrotic crusts that resolve to leave varioliform scars (Cureus 2020;12:e8725)
- Waxing and waning course over months to years
- Can progress to febrile ulceronecrotic Mucha-Habermann disease
- Systemic involvement along with cutaneous lesions
- Necrotic papules to large ulcers with necrotic crusts, hemorrhagic bullae and pustules
- Systemic symptoms include high fever, lymphadenopathy, arthritis or bacteremia, with mucosal, gastrointestinal and pulmonary involvement (Indian J Dermatol 2017;62:675)
- Graft versus host disease
- Cutaneous and systemic manifestations (hepatic dysfunction, gastrointestinal symptoms)
- Acute cutaneous
- Develops within 100 days posttransplant
- Erythematous maculopapular rash
- With progression, there can be bullae and epidermal necrosis
- Severe cases resemble toxic epidermal necrolysis
- Late acute GVHD may arise > 100 days posttransplant, after the cessation or tapering of immunosuppressive drugs (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Chronic cutaneous
- Develops > 100 days posttransplant
- Early lichenoid phase mimics lichen planus clinically and histologically, including Wickham striae of oral mucosa
- Late chronic change clinically and histologically mimics lichen sclerosus, morphea or systemic sclerosis (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Scaly, erythematous hyperkeratotic papules
- Fixed drug eruption
- Oral ingestion of certain drugs is associated with a recurrent reddish brown patch at the same location, normally presents as localized FDE
- Vesicles and bullae can rupture to leave superficial ulcers
- Blistering and erosions involving > 10% of body surface area and at least 3 of 6 different sites (head and neck, anterior trunk, back, upper extremities, lower extremities, genitalia) warrants a diagnosis as generalized fixed drug eruption (J Am Acad Dermatol 2014;70:539)
Lichenoid- Lichen planus
- Multiple purplish, pruritic, polygonal, planar, papules and plaques (6 Ps)
- Frequently bilateral and symmetric
- Wickham striae, a network of fine white lines seen on the surface of mucosal lesions
- May exhibit the Koebner phenomenon, where new skin lesions appear on previously unaffected skin, secondary to trauma
- Hypertrophic variant clinically may appear verrucous (Dermatol Reports 2014;6:5113)
- Many variants of LP: atrophic, hypertrophic, annual, linear, ulcerative (erosive), oral, lichen planus pigmentosus, actinicus, planopilaris, pemphigoides, bullous, keratosis lichenoides chronica and lupus erythematosus - lichen planus overlap syndrome
- Lichenoid drug eruption
- Eruption can mimic lichen planus, though it can have marked desquamation (Eur J Case Rep Intern Med 2020;7:002103)
- Pronounced postinflammatory hyperpigmentation
- After (ranges from 1 month to a year or more) oral intake of a wide range of possible drugs (see Diagrams / tables)
- Lichen striatus
- Discrete flesh colored small papules that eventually coalesce to form an erythematous band-like, linear papular eruption along Blaschko lines, usually unilateral
- Lichen planus-like keratosis
- Solitary, discrete, violaceous to pink thin papule or plaque of sudden onset and short duration, mildly pruritic
- 3 mm - 1.8 cm in diameter (J Cutan Pathol 2002;29:291)
- Clinically, may mimic superficial basal cell carcinoma or squamous cell carcinoma in situ
- Lichen nitidus
- Multiple, round, 1 - 3 mm, flesh colored papules
- Usually asymptomatic
Diagnosis
- Clinical history, exam and correlating skin biopsy
Laboratory
Vacuolar
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- Fever of > 38.5 °C; however, this also may be seen in EM (Arch Dermatol 2002;138:1019)
- Graft versus host disease
- Liver: elevated serum liver enzymes (can present with both acute hepatitis picture or cholestatic picture) (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Hepatitis: elevated aminotransferases
- Cholestatic: hyperbilirubinemia, elevated alkaline phosphatase and gamma glutamyl transpeptidase (GGT)
- GI tract: low serum albumin (protein losing enteropathy)
- Liver: elevated serum liver enzymes (can present with both acute hepatitis picture or cholestatic picture) (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
Prognostic factors
Vacuolar
Lichenoid
- Erythema multiforme
- Self limiting disease, spontaneous remission within a few weeks (Arch Dermatol 1996;132:711)
- Recurrent EM, in contrast, occurs with a mean frequency of 6 attacks/year (range: 2 - 24), with a mean duration of 9.5 years (range: 2 - 36) (Br J Dermatol 1993;128:542)
- Recalcitrant cases of recurrent EM were associated with severe oral mucosal involvement, the inability to identify a specific cause, a lack of improvement with continuous antiviral therapy, near continuous corticosteroid therapy for at least 1 year, hospitalization and immunosuppressive therapy with 2 or more agents (J Am Acad Dermatol 2010;62:45)
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- Life threatening disease
- Higher mortality in Medicaid and Medicare insurance patients as well as those > 60 years of age with non-Hodgkin lymphoma, leukemia, septicemia, tuberculosis, pneumonia and renal failure (J Invest Dermatol 2016;136:1387)
- SJS: 4.8% mortality
- SJS / TEN: 19.4% mortality
- TEN: 14.8% mortality (J Invest Dermatol 2016;136:1387)
- Pityriasis lichenoides
- Self limiting disease, duration between months and several years
- Resolution leaves varioliform or postinflammatory hyper or hypopigmented scars (J Eur Acad Dermatol Venereol 2019;33:2039)
- Life threatening in the febrile ulceronecrotic Mucha-Habermann disease variant
- 15% mortality rate (Indian J Dermatol 2017;62:675)
- Graft versus host disease
- Worse prognosis is associated with late acute GVHD, overlap syndrome (concurrent acute and chronic GVHD) and multiple organ systems involved (GI, liver, pulmonary, etc.) (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Numerous clinical staging systems to help in risk stratification (International Bone Marrow Transplant Registry [IBMTR]) and Glucksberg grade (Br J Haematol 1997;97:855)
- Fixed drug eruption
- Repeat ingestion of the same drug can cause an increase in number and size of lesions (Int J Dermatol 1998;37:833)
- Generalized bullous FDE may have clinicopathologic overlap with SJS / TEN; one study reported a mortality rate of 22% in these cases (Br J Dermatol 2013;168:726)
- Localized FDE has a spontaneous resolution with avoidance of offending agent, with residual postinflammatory pigmentary alteration (Am J Clin Dermatol 2020;21:393)
Lichenoid
- Lichen planus
- Cutaneous lichen planus is usually a self limiting disorder
- Palm and sole lesions (palmoplantar LP) are especially painful and therapy resistant
- Oral lichen planus
- Malignant transformation is more likely in smokers, alcoholics and hepatitis C virus (HCV) infected patients (Oral Oncol 2017;68:92)
- Hypertrophic lichen planus (Int J Dermatol 2016;55:e295)
- Squamous cell carcinoma may arise from these lesions (Dermatol Surg 2015;41:1411)
- Lichenoid drug eruption
- Usually, complete resolution weeks to months after discontinuing the offending drug
- Patients who require treatment are those who cannot discontinue the drug, those with intractable symptoms, extensive generalized skin involvement and persistence of the lesions several months after discontinuing the offending agent (J Eur Acad Dermatol Venereol 2023;37:965)
- Lichen striatus
- Spontaneous regression, mean duration of 6 months (Pediatr Dermatol 2004;21:197)
- Lichen planus-like keratosis
- Benign, spontaneous resolution or cryotherapy
- Lichen nitidus
- Spontaneous remission
- Palmar lichen nitidus can persist or be refractory to usual treatments (Indian J Dermatol 2015;60:308)
Case reports
Vacuolar
Lichenoid
- 38 year old man who returned from Ecuador presented with loose, watery diarrhea and targetoid hemorrhagic bullae on the right palm (J Cutan Pathol 2002;29:291)
- 52 year old man presented with SJS-like lesions and a diffuse erythematous and violaceous maculopapular exanthem 1 month after liver transplantation (Front Immunol 2022;13:917782)
Lichenoid
- 6 year old boy, recently diagnosed with lichen planus, presented with vesicles and bullae arising on both lichen planus lesions and clinically uninvolved skin (Pediatr Dermatol 2009;26:569)
- 75 year old woman with a 2 year history of intermittently pruritic pink scaly plaques on the bilateral shins and oromucosal reticulate white patches (JAAD Case Rep 2023;44:58)
Treatment
Vacuolar
Lichenoid
- Erythema multiforme
- Symptomatic treatment is important (Br J Dermatol 2018;179:1009)
- Topical corticosteroids
- Medicated mouthwash for oral involvement
- Extensive oral involvement may require systemic glucocorticoids but it is unknown if this changes the outcome (Br J Dermatol 2018;179:1009)
- Antivirals may be indicated
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- Multidisciplinary care in a specialized burn unit
- Withdrawal of the culprit drug
- Systemic treatments (systemic corticosteroids, intravenous immunoglobulin [IVIg], cyclosporine, biologics) may or may not be beneficial (Biomedicines 2022;10:2105)
- Pityriasis lichenoides
- Treatments include phototherapy, erythromycin, topical corticosteroids, low dose methotrexate (J Eur Acad Dermatol Venereol 2019;33:2039)
- Febrile ulceronecrotic variant (FUMHD)
- IV methotrexate use has been reported to be effective (J Am Acad Dermatol 1994;30:261)
- Graft versus host disease
- Prophylaxis
- Cyclosporine, tacrolimus, methotrexate
- Antithymocyte globulin (ATG) given before hematopoietic cell transplantation significantly reduces the risk of grade 3 or 4 acute GVHD
- Other immunosuppressants may be added (mycophenolate mofetil [MMF], sirolimus)
- Extracorporeal photopheresis (Transfus Med Hemother 2020;47:214)
- Acute
- Topical corticosteroids if only skin involvement (Nat Rev Dis Primers 2023;9:27)
- Systemic treatment for grade 2 - 4 GVHD, which includes calcineurin inhibitors plus methylprednisolone (Int J Hematol 2015;101:452)
- Secondary therapy includes Janus associated kinase inhibitor ruxolitinib (PLoS One 2022;17:e0271979)
- ATG, sirolimus, etc. (Bone Marrow Transplant 2017;52:438)
- Chronic
- Methylprednisolone / prednisolone and calcineurin inhibitor (Best Pract Res Clin Haematol 2008;21:271)
- Prophylaxis
- Fixed drug eruption
- Discontinuation of offending drug
- Medium to high potency topical corticosteroids, systemic antihistamines (Am J Clin Dermatol 2020;21:393)
- Generalized bullous FDE requires systemic treatments, similar to SJS / TEN, such as systemic corticosteroids (Br J Dermatol 2013;168:726)
Lichenoid
- Lichen planus
- First line therapy
- Topical corticosteroid (high or super high potency for extremities / trunk, low or mid potency for intertriginous / facial skin) (N Engl J Med 2012;366:723)
- Intralesional corticosteroid for thick plaques of hypertrophic lichen planus (Int J Dermatol 2009;48:682)
- Second line therapy
- Systemic corticosteroids, phototherapy (such as PUVA, narrowband ultraviolet B), oral acitretin, other systemic agents (J Am Acad Dermatol 2019;81:1397, J Am Acad Dermatol 1991;24:434)
- First line therapy
- Lichenoid drug eruption
- Discontinue offending drug
- Topical corticosteroid (Cancer Treat Res Commun 2022;30:100506)
- Systemic corticosteroids
- Systemic retinoids, others
- Phototherapy
- Lichen striatus
- Mild or medium strength topical steroids may be used but do not appear to shorten disease duration or prevent postinflammatory hypopigmentation (Pediatr Dermatol 2004;21:197)
- Lichen planus-like keratosis
- Lesions can be removed by cryotherapy (liquid nitrogen) (Cureus 2021;13:e13292)
- Lichen nitidus
- Systemic and topical corticosteroids
- Antihistamines, antituberculous drugs, antifungals (Int J Dermatol 2006;45:615)
- Narrowband ultraviolet B phototherapy has been reportedly successful in generalized lichen nitidus (J Am Acad Dermatol 2006;54:545)
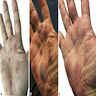
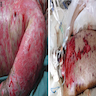
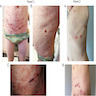
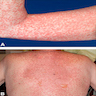
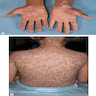
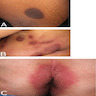
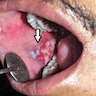
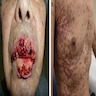
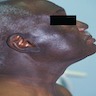
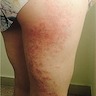
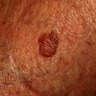
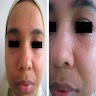
Clinical images
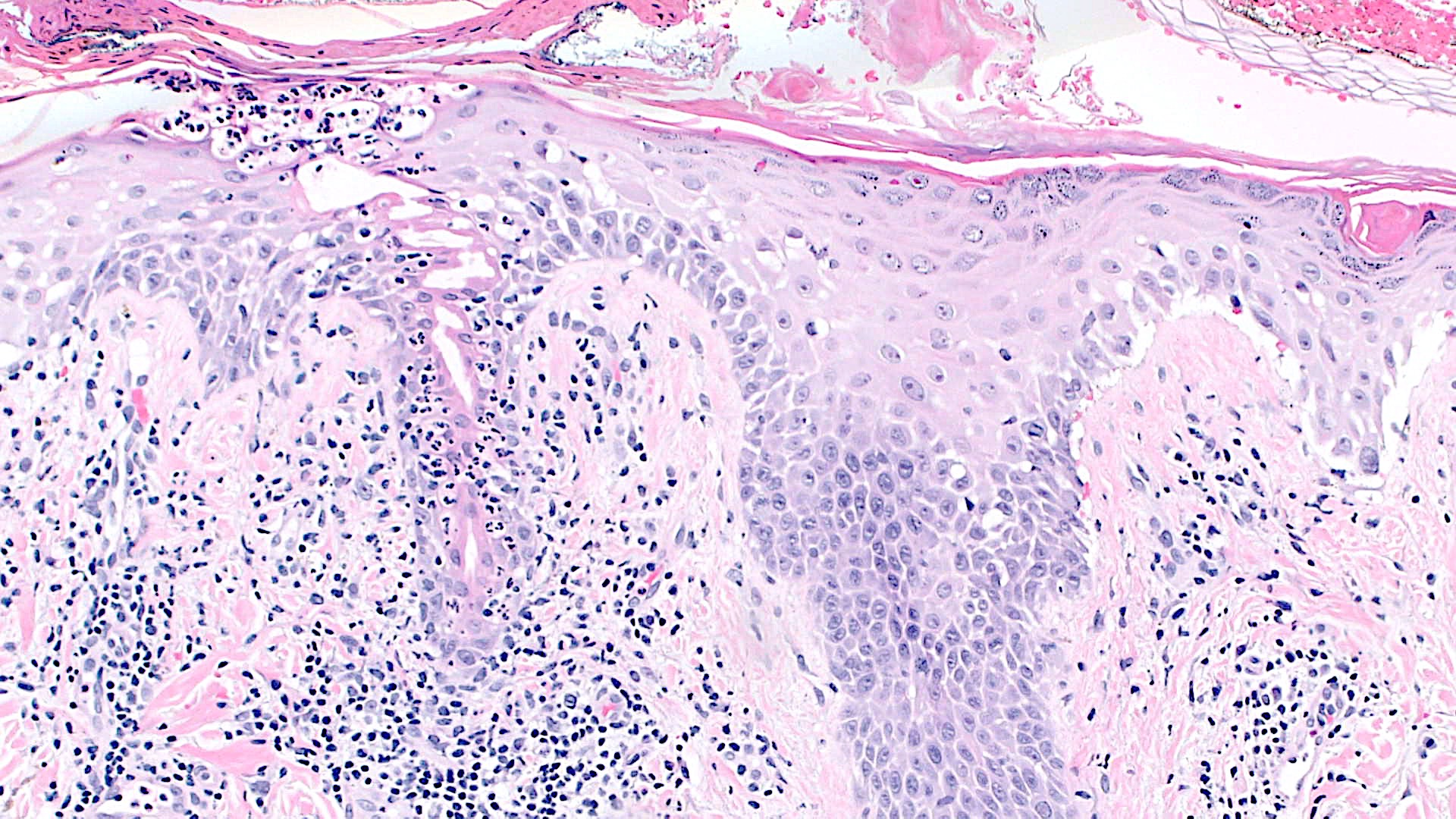
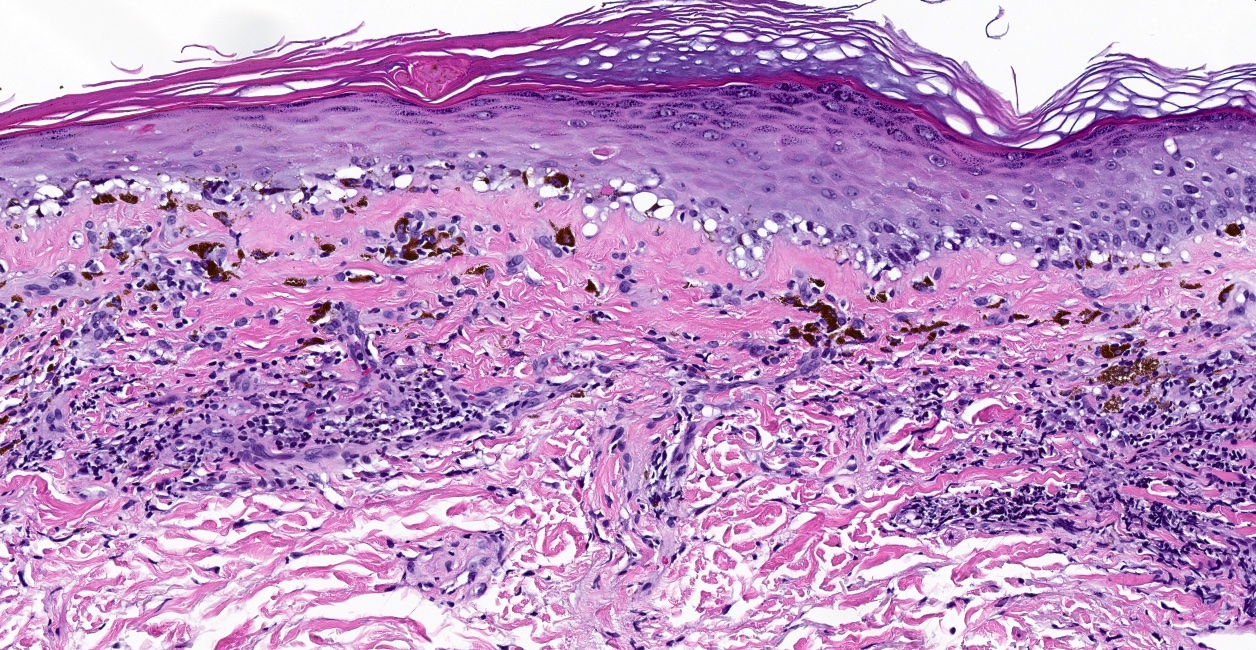
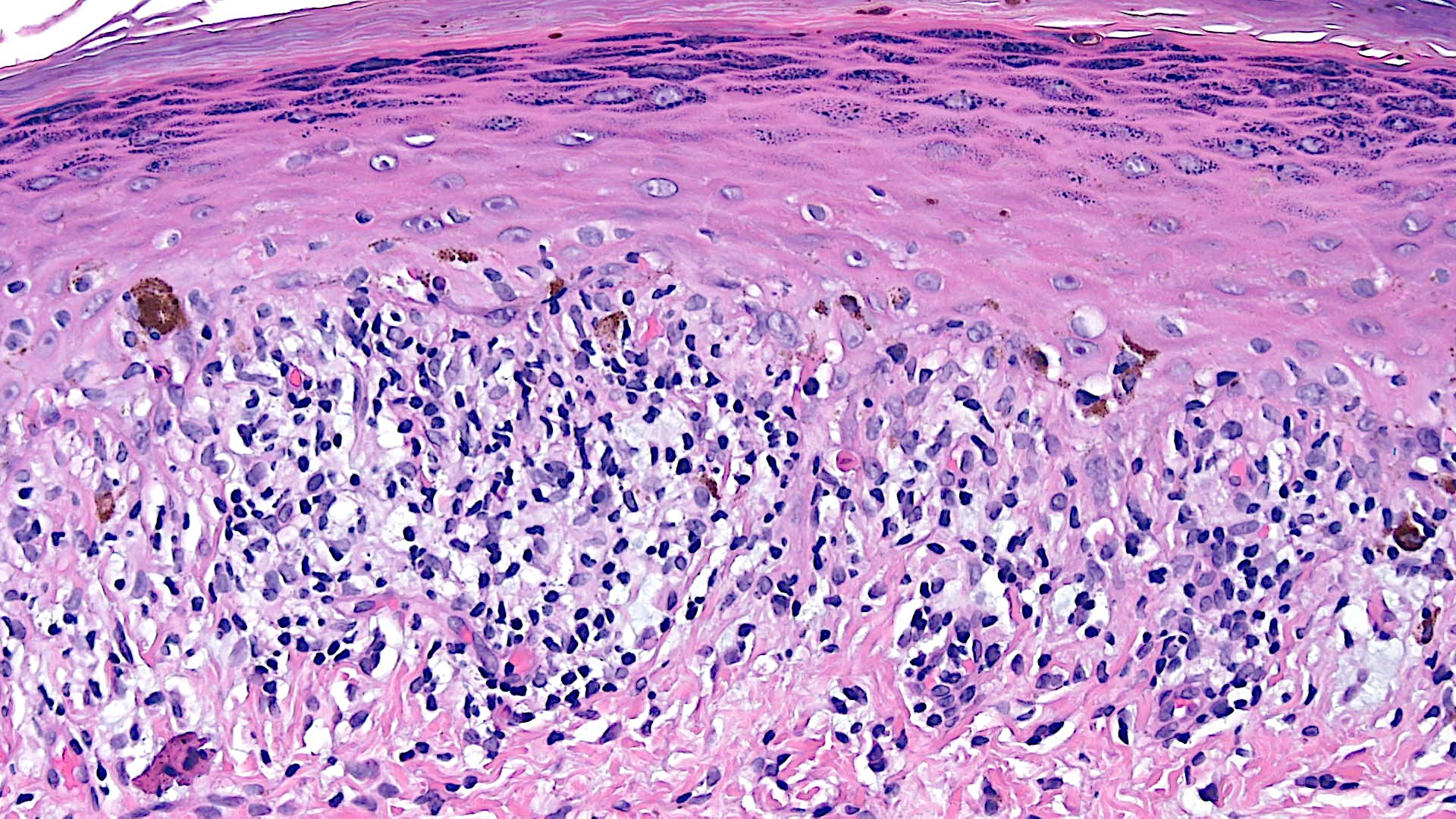
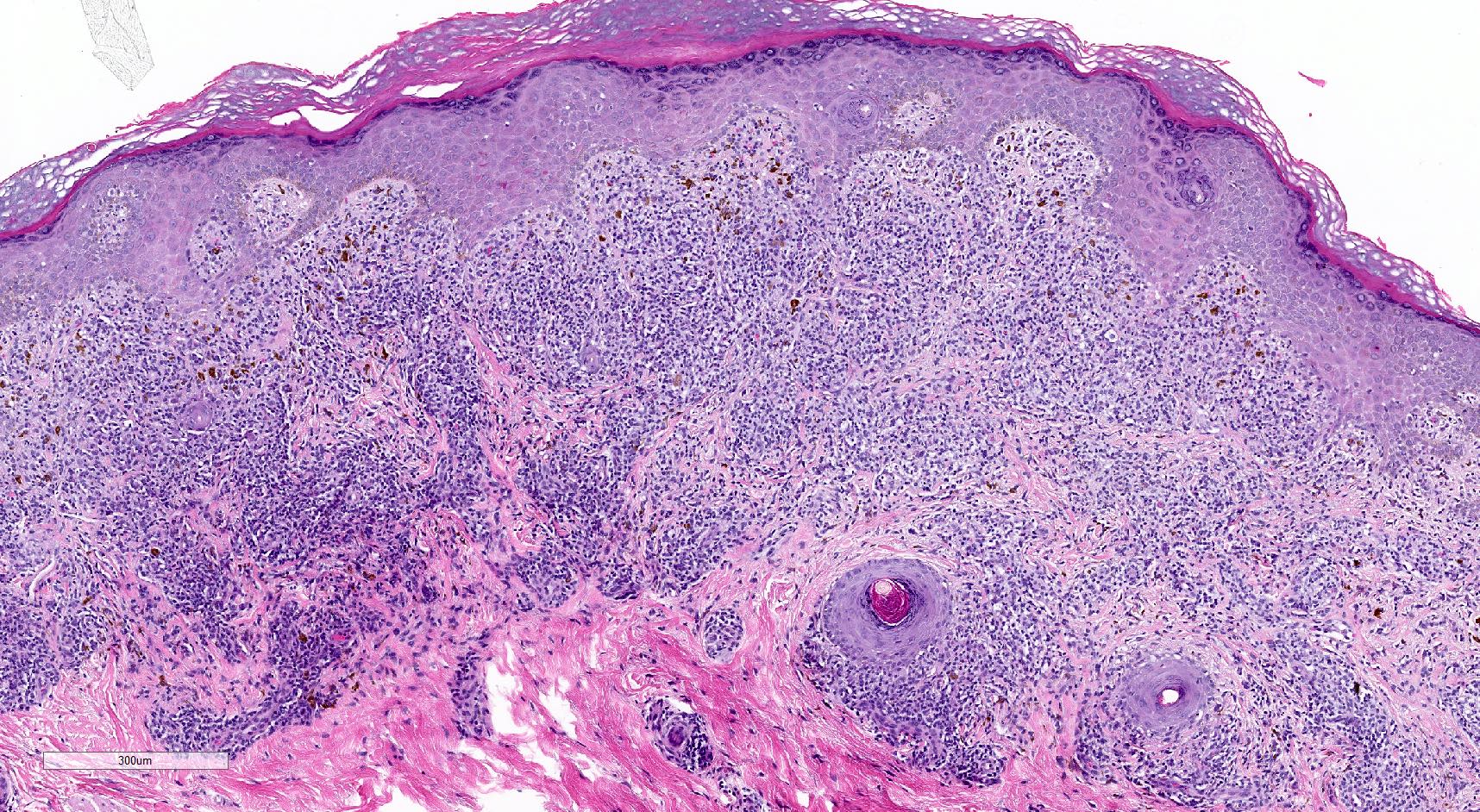
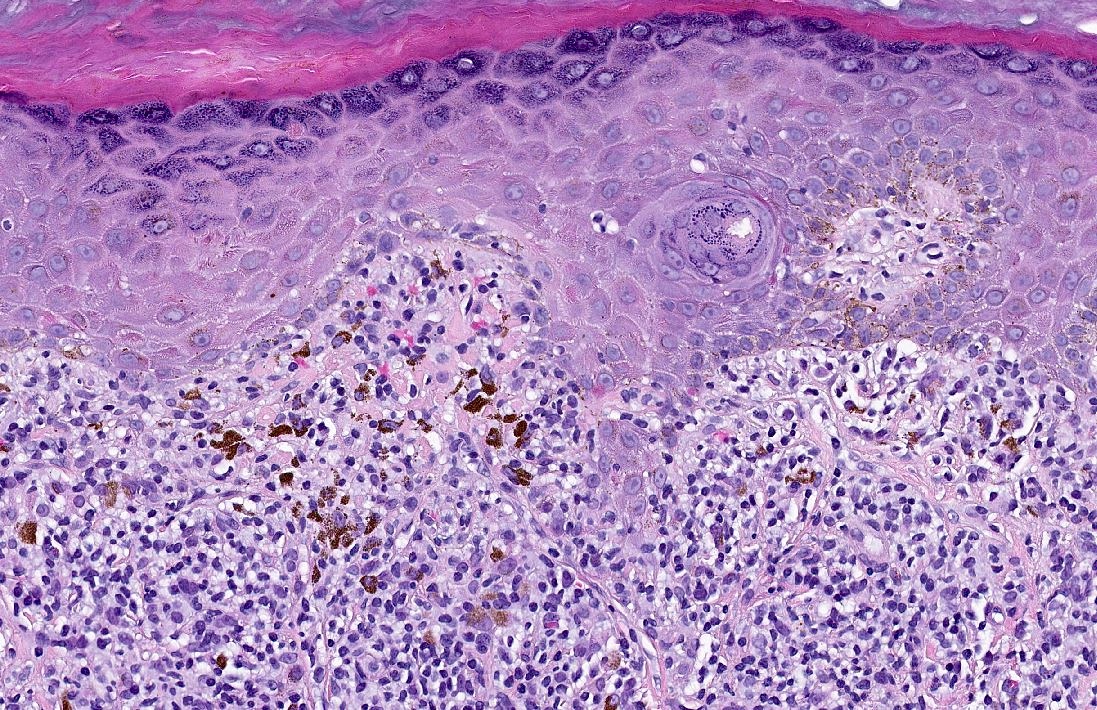
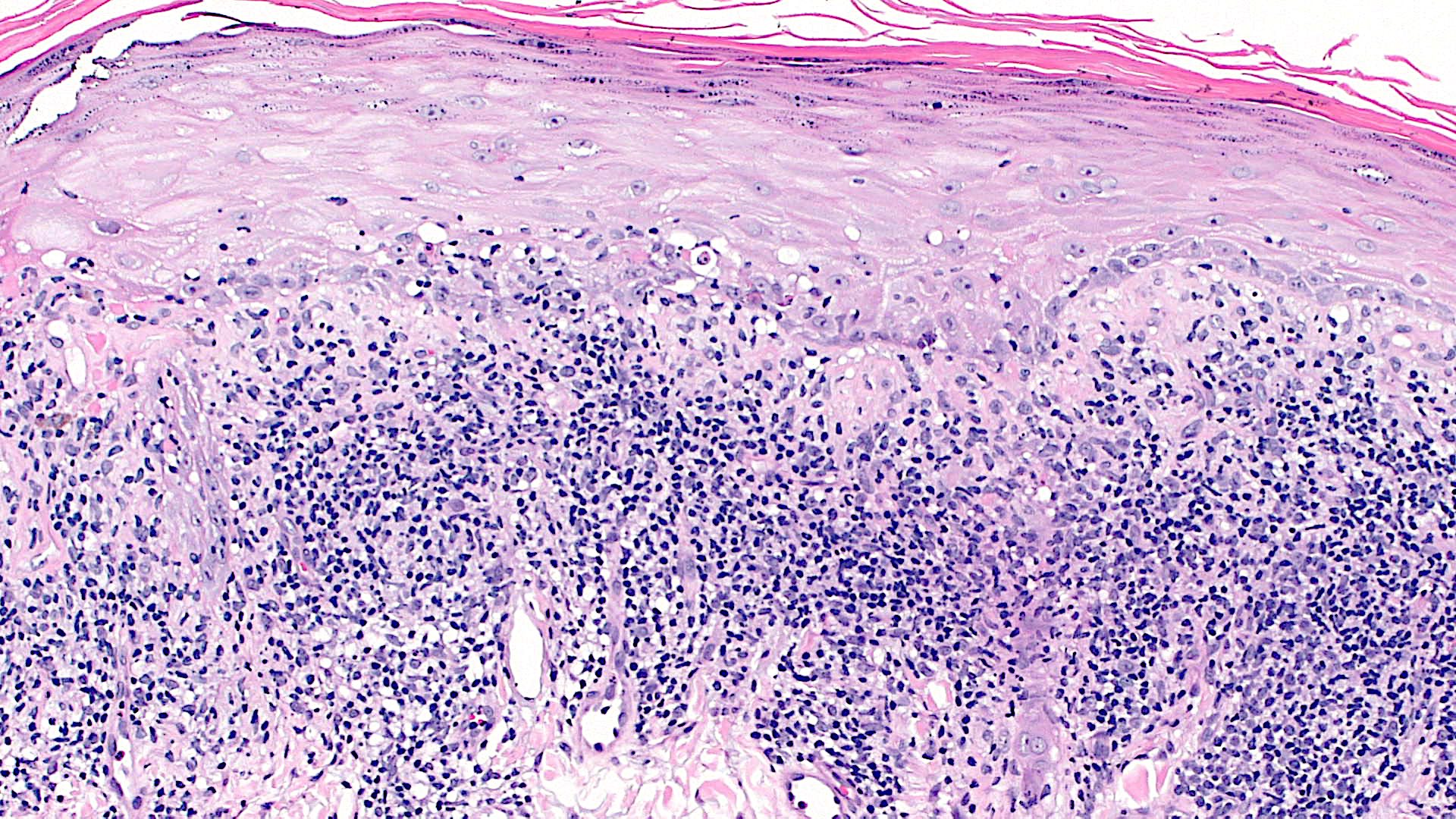
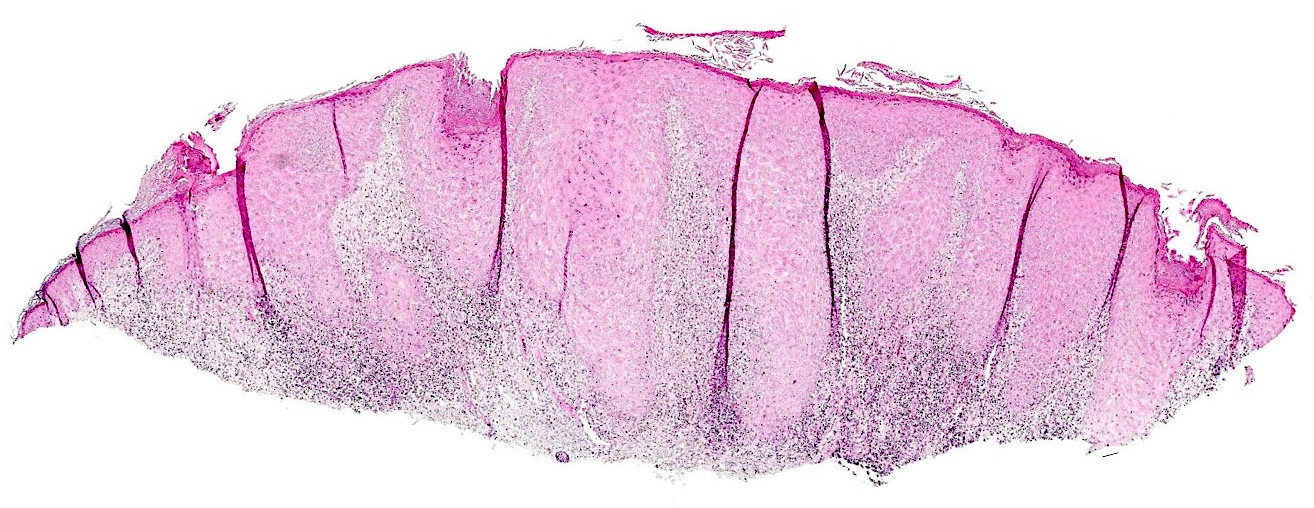
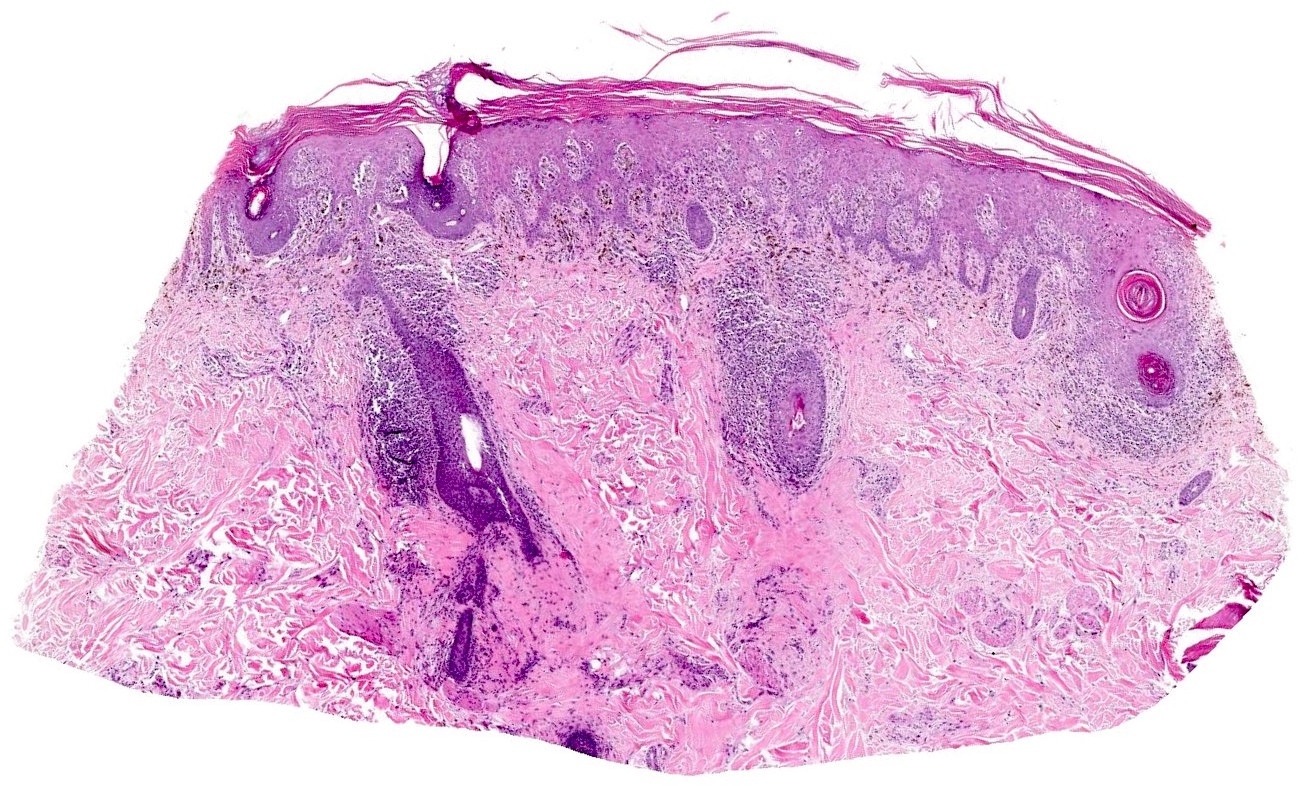
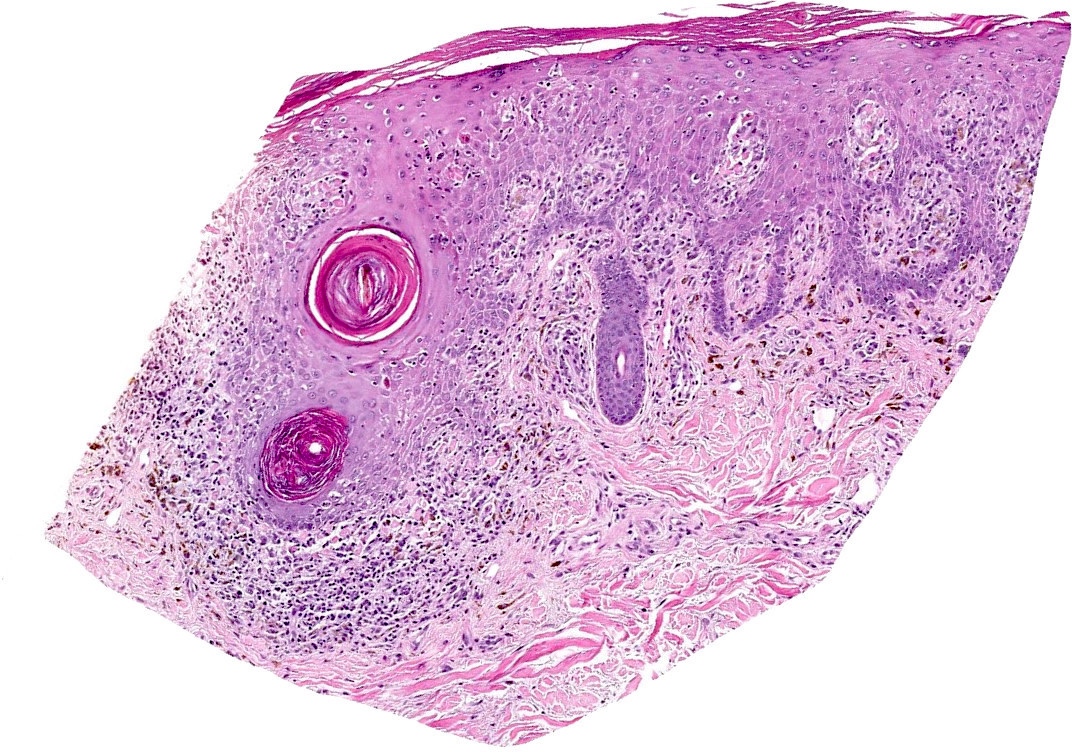
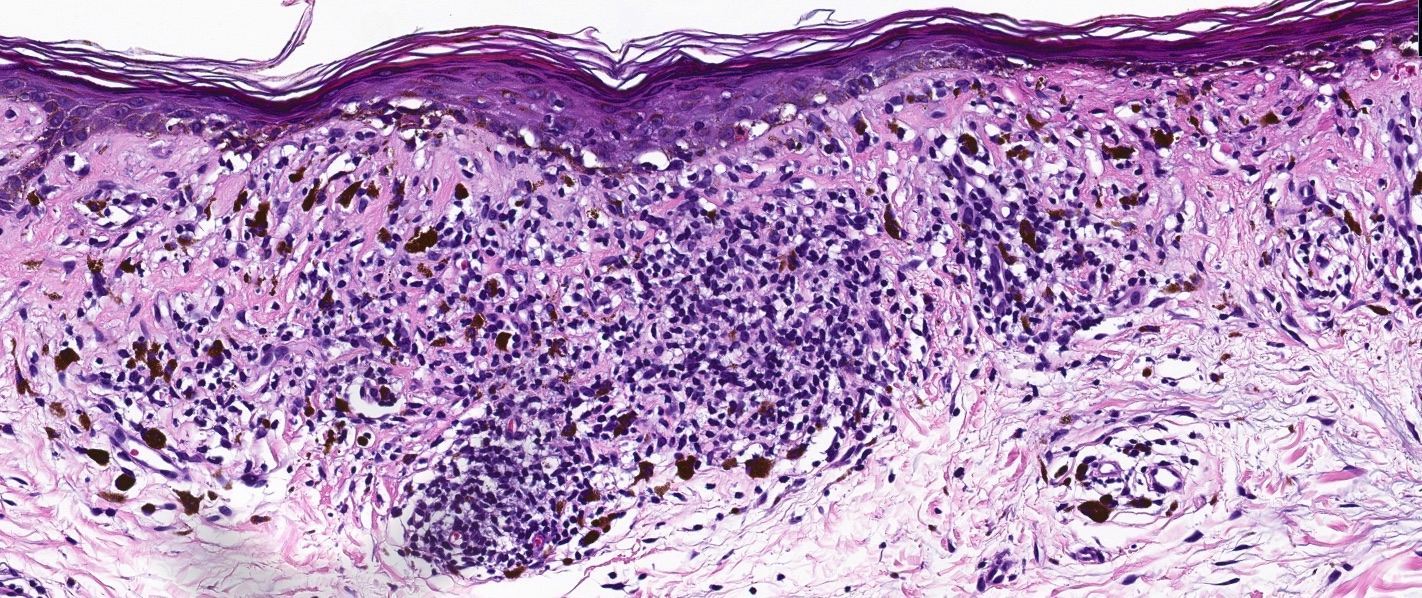
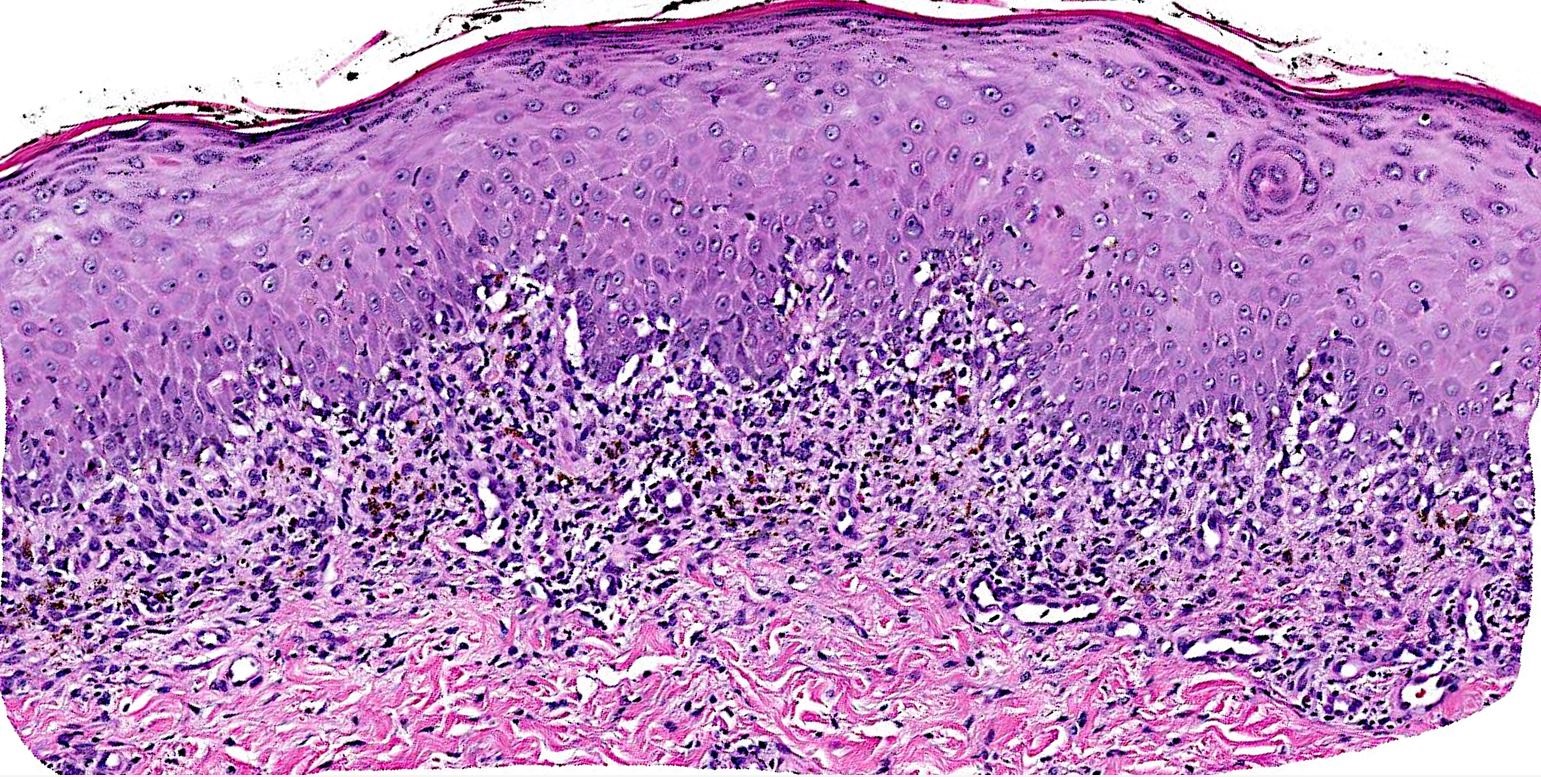
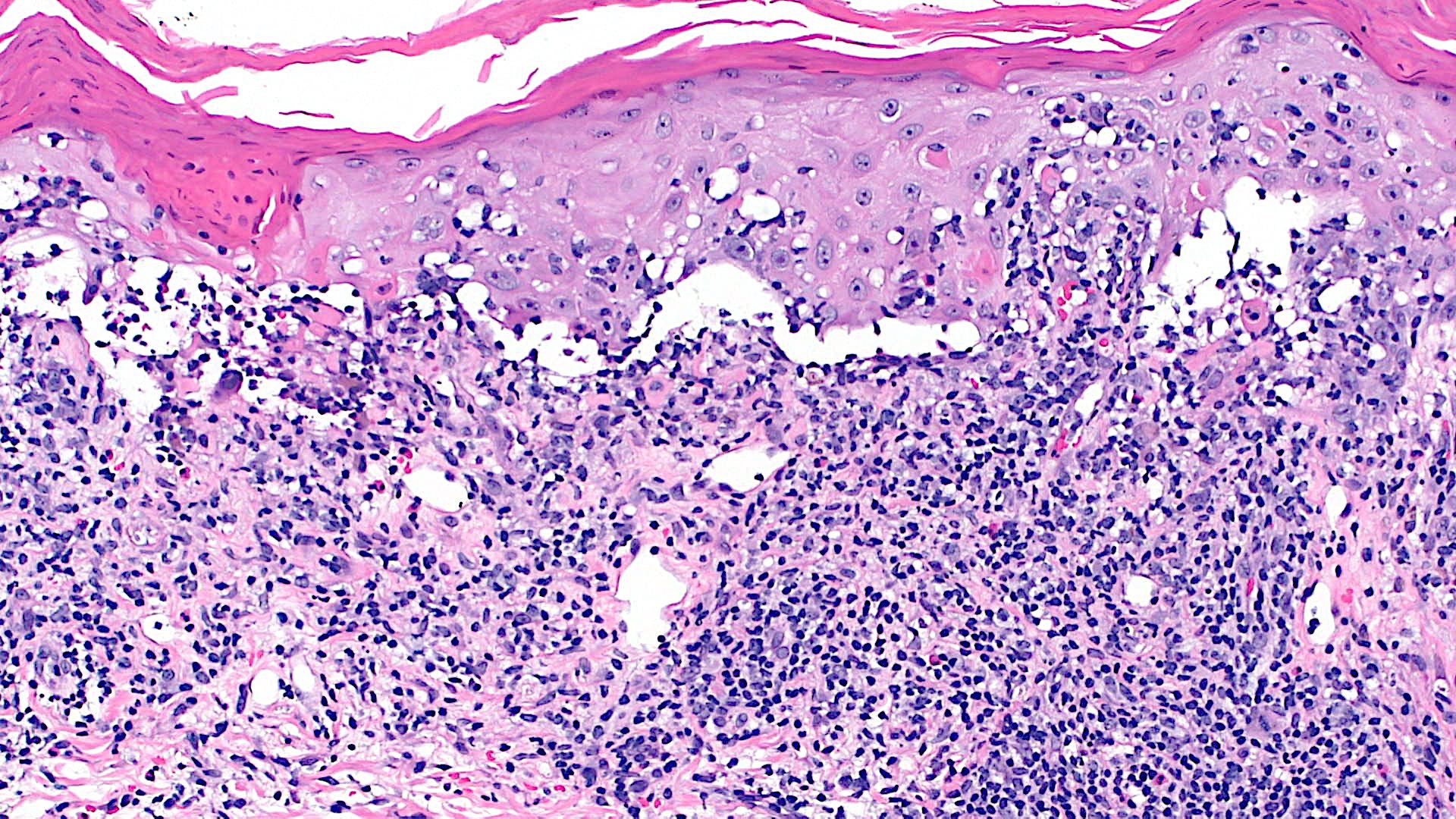
Microscopic (histologic) description
- Interface dermatitis is characterized by epithelial (epidermal or mucosal) basal cell damage, which manifests as basal keratinocyte vacuoles and dyskeratotic / apoptotic keratinocytes
- Vacuolar pattern has a sparse inflammatory infiltrate
- Lichenoid pattern has a moderate to dense, band-like lymphocytic inflammatory infiltrate closely approximating the basal epidermis and dyskeratotic / apoptotic keratinocytes
- Dyskeratotic / apoptotic keratinocytes appear as
- Shrunken, eosinophilic keratinocytes with pyknotic / absent nuclei scattered about the basal layer of the epidermis
- Colloid bodies, which are brightly eosinophilic apoptotic keratinocytes that have been extruded into the papillary dermis
Vacuolar
- Erythema multiforme
- Apoptotic keratinocytes within and above the basal layer, in the form of single cells or full thickness necrosis
- Ragged subepidermal split, where dyskeratotic keratinocytes form the roof of subepidermal vesicle
- Acrosyringial concentration of keratinocyte necrosis has been reported in drug induced EM (J Cutan Pathol 1997;24:235)
- Mild to moderate lymphocytic interface obscuring inflammatory infiltrate
- Usually, absent to occasional eosinophils (Arch Pathol Lab Med 1989;113:36)
- Cannot distinguish from SJS / TEN without clinical data
- Stevens-Johnson syndrome / toxic epidermal necrolysis
- Subepidermal bulla with overlying partial to full thickness epidermal necrosis
- Individual cell necrosis may take the form of lymphocyte associated apoptosis (i.e., satellite cell necrosis)
- Inflammatory infiltrate may be denser than in EM
- Pityriasis lichenoides
- Acute form (PLEVA) has confluent mounded parakeratosis containing lymphocytes / neutrophils or serum
- Epidermal necrosis can range from single dyskeratotic cells to full thickness (the latter representing the ulcerated papules seen clinically)
- Intravascular fibrin deposition may be present
- Wedge shaped or band-like lymphocytic infiltrate with lymphocyte exocytosis, red cell extravasation and spongiosis
- Superficial and deep perivascular infiltrates in PLEVA, only superficial in PLC
- Lymphocytes can have an atypical morphology, with reduced expression of pan-T cell markers and have a positive T cell receptor rearrangement
- There can be scattered large atypical CD30+ cells, which lends consideration to lymphomatoid papulosis (Int J Dermatol 2003;42:26)
- Clinical correlation is necessary for diagnosis of the febrile ulceronecrotic variant (FUMHD)
- Chronic form (PLC) is a milder version of acute, with a lack of ulceration
- Graft versus host disease
- Ballooning and individual necrotic keratocytes
- Acute
- Keratinocyte apoptosis in basilar and lower spinous layers, especially in the tips of epidermal rete, pilosebaceous units and eccrine coils (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Apoptosis can involve all levels of the epidermis
- Satellite cell necrosis (lymphocytes surrounding intraepidermal apoptotic keratinocytes)
- Diffuse vacuolization of the interface
- Melanophages and red blood cell (RBC) extravasation may be present
- Sparse, superficial lymphocytic infiltrate
- Occasional eosinophils may be present
- Grading for acute GVHD by Horn (J Cutan Pathol 1994;21:385)
- Grade 0: normal skin
- Grade 1: basal vacuolar change
- Grade 2: dyskeratotic cells in the epidermis or follicle, dermal lymphocytic infiltrate
- Grade 3: fusion of basilar vacuoles to form clefts and macrovesicles
- Grade 4: separation of epidermis from dermis
- Chronic
- Early lichen planus-like inflammatory phase
- Band-like lymphocytic infiltrate at the DEJ with orthohyperkeratosis, hypergranulosis and acanthosis
- Vacuolar interface change of eccrine coils (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Lichen sclerosus-like variant
- Homogenization or sclerosis of papillary dermal collagen with overlying vacuolar interface change, with an underlying sparse infiltrate and melanophages (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Morphea-like variant
- Localized thickening and homogenization of reticular dermal collagen with minimal apoptosis and preserved adnexal structures, often with overlying vacuolar change (Arch Dermatol 2008;144:1229)
- Sclerodermoid variant
- Pandermal sclerosis extending from the papillary to reticular dermis and into the subcutis, with destruction of adnexal structures and prominent vacuolar interface changes (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Fasciitis-like variant
- Thickening of fascial septa with adjacent inflammation with or without sclerosis in the subcutis, often compressing or destroying adnexal structures (Yeung: Pathology of Graft vs. Host Disease, 1st Edition, 2019)
- Early lichen planus-like inflammatory phase
- Fixed drug eruption
- Ballooning and individual necrotic keratinocytes
- Classic features are
- Interface alteration with overlapping lichenoid and vacuolar pattern
- Papillary dermal fibrosis with melanophages / pigment incontinence
- Dermal eosinophils
- Basketweave stratum corneum
- Neutrophil component may be prominent in acute lesions and take the form of subcorneal or intraepidermal microabscesses, a dermal neutrophilic infiltrate or a perivascular inflammatory infiltrate
Lichenoid
- Lichen planus
- Jagged epidermal hyperplasia (sawtooth appearance in lower epidermis), wedged hypergranulosis (histologic correlate to Wickham striae), compact orthokeratosis
- Interface obscuring band-like lymphocytic infiltrate at the dermoepidermal junction (DEJ)
- Eosinophils and plasma cells are rare
- Numerous colloid bodies at the DEJ
- Acanthotic or atrophic epidermis
- Clefting artifact between the epidermis and dermis, secondary to basal damage (Caspary-Joseph spaces) (Int J Dermatol 1977;16:842)
- Lichen planopilaris (subtype of LP)
- Peri-infundibular and peri-isthmic (bulge region of the hair follicle) band-like inflammatory infiltrate that abuts the basilar follicular epithelium (J Cutan Pathol 2005;32:675)
- Perifollicular mucinous fibrosis (J Am Acad Dermatol 2008;59:91)
- Late stage: there is a loss of hair follicles and sebaceous glands that are replaced by bands of fibrous tissue without elastic fibers (J Cutan Pathol 2000;27:147)
- Hypertrophic lichen planus (subtype of LP)
- Markedly acanthotic epidermis
- Dermal infiltrate is concentrated near the tips of the rete ridges
- Margins contain changes of lichen simplex chronicus (acanthotic epidermis, hypergranulosis, compact orthokeratosis, stratum lucidum)
- Can progress to squamous cell carcinoma, usually well differentiated (Int J Dermatol 2022;61:1527)
- Dermal eosinophils are usually present, in contrast to conventional LP
- Lichenoid drug eruption
- See lichen planus, except may have eosinophils, plasma cells
- Pigment incontinence
- Photolichenoid eruption (subtype of lichenoid drug eruption)
- Deep extension of the infiltrate
- Lichen striatus
- Distinguishing feature is deep perivascular and perieccrine lymphocytic inflammatory infiltrate
- Epidermis will show spongiosis, most likely the subacute type (which is characterized by parakeratosis and acanthosis)
- Intraepidermal vesicles filled with Langerhans cells may be present (J Cutan Pathol 1995;22:18)
- Lichen planus-like keratosis
- Necrotic keratinocytes, focal parakeratosis, residuum or adjacent solar lentigo or seborrheic keratosis, large cell acanthoma or a verruca (Int J Dermatol 2019;58:830)
- Epidermal atrophy
- Plasma cells, eosinophils and neutrophils may be present in the brisk inflammatory infiltrate
- Pigment incontinence may be prominent
- Papillary dermal fibrosis
- Lichen nitidus
- Discrete foci in papillary dermis
- Histiocyte predominant dermal inflammatory infiltrate
- Also, infiltrate contains lymphocytes, histiocytes, melanophages, with or without granulomas / multinucleated giant cells
- Inflammatory infiltrate is very focal - limited to 1 or 2 adjacent dermal papillae
- Ball and claw configuration where a well circumscribed inflammatory infiltrate (ball) expands the dermal papillae, pushing the finger-like epidermal rete to the periphery (claw) (Pediatr Dermatol 2019;36:189)
- Epidermis with mild hyperkeratosis
- No deep component
- Discrete foci in papillary dermis
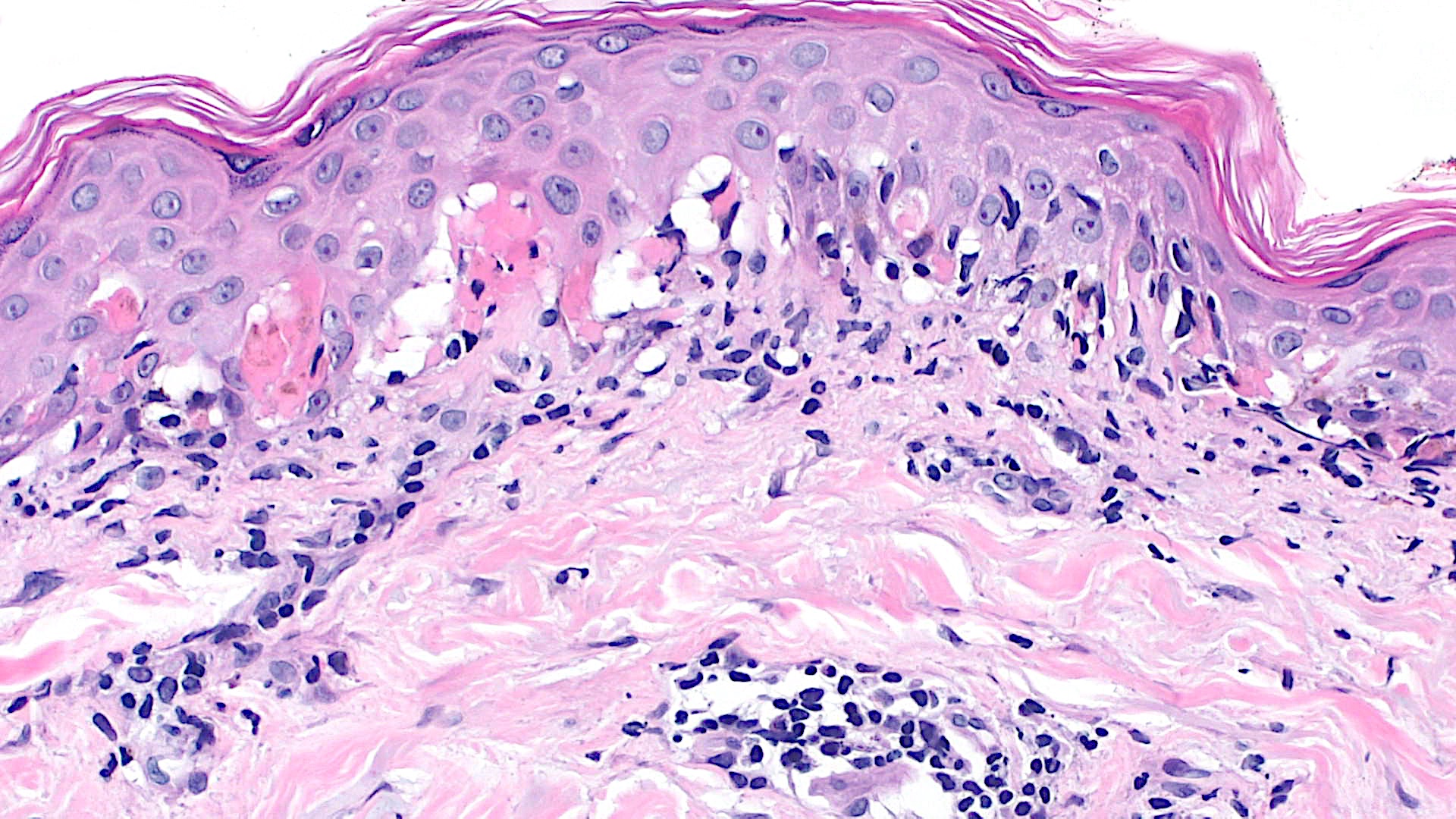
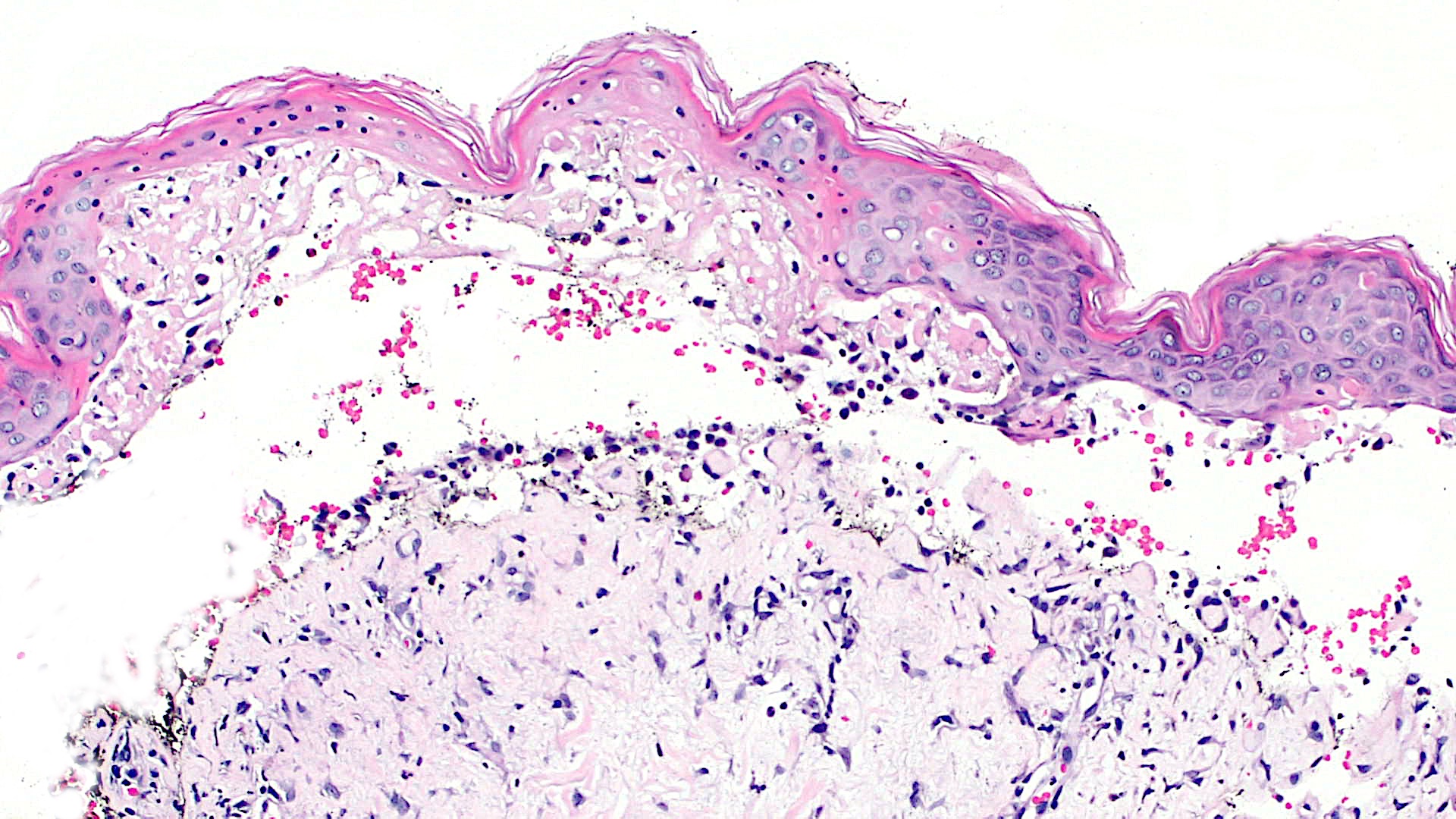
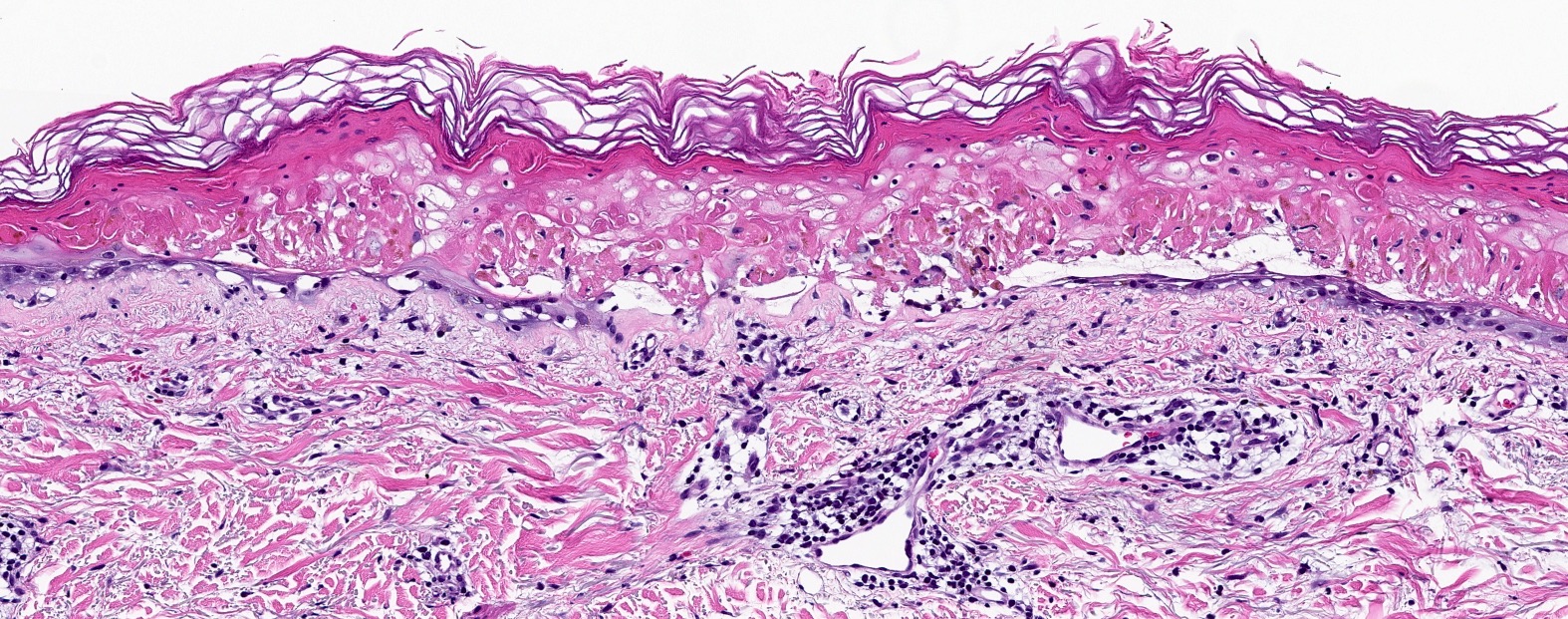
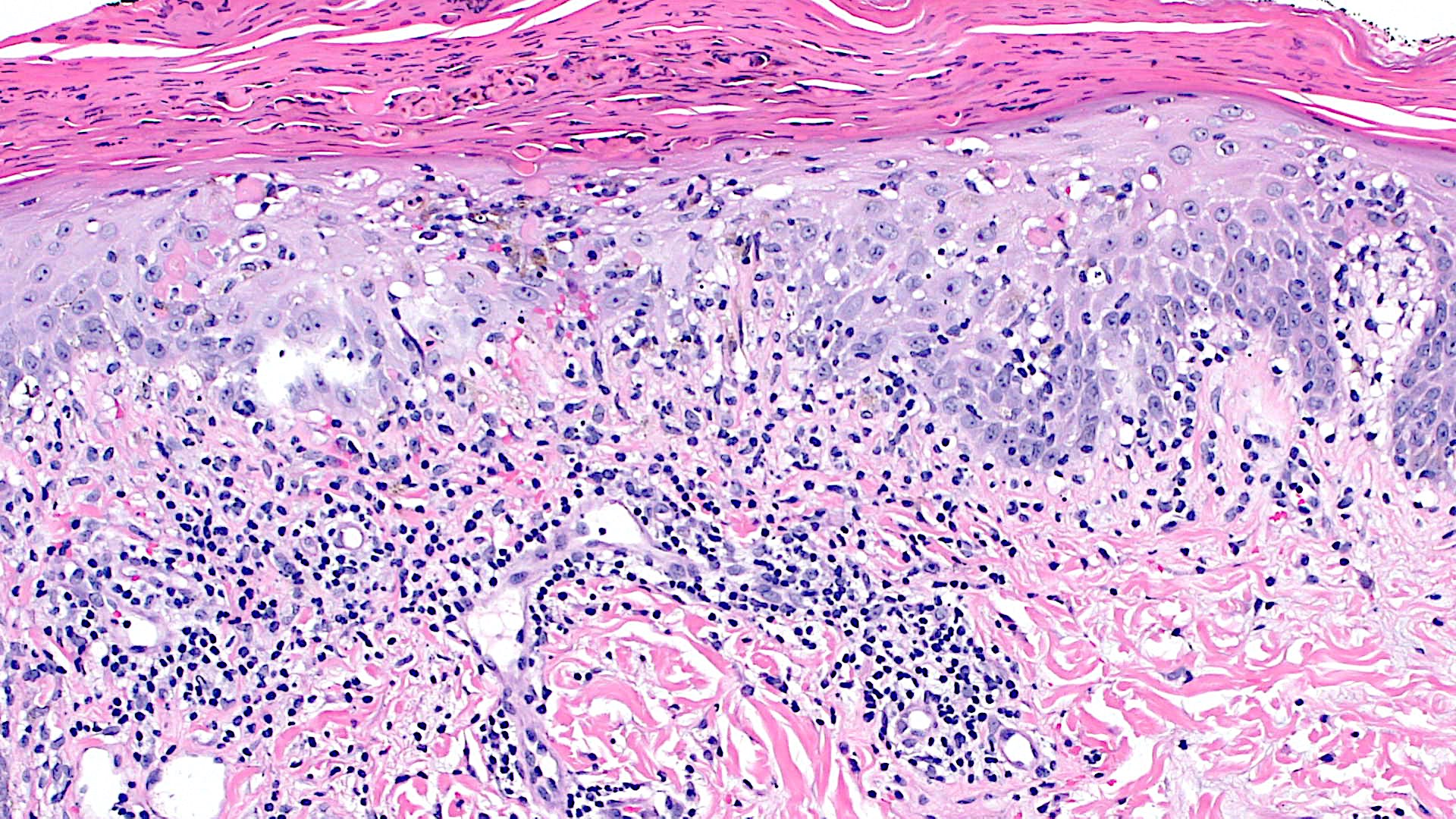
Microscopic (histologic) images
Contributed by Lauren Pelkey, D.O.
Vacuolar
Lichenoid
Immunofluorescence description
- Nonspecific findings that may be present in interface dermatitides include
- Colloid bodies may be positive for complement and immunoglobulins, especially IgM (Am J Dermatopathol 2002;24:130)
- Fibrin band at the basement membrane zone, with irregular extension into the underlying papillary dermis (Indian J Dermatol Venereol Leprol 2010;76:373)
Vacuolar
- Erythema multiforme
- Granular IgM and C3 along the DEJ and in papillary dermal vessels
- Pityriasis lichenoides et varioliformis acuta (PLEVA)
- Granular IgM and C3 along the DEJ and in papillary dermal vessels (Br J Dermatol 1978;99:491)
- Systemic lupus erythematosus
- General features (all cutaneous lupus erythematosus (CLE) subtypes)
- Immunoreactive for IgG, IgM, IgA and C3 (full house) (Indian Dermatol Online J 2015;6:172)
- Granular to continuous linear deposition at the DEJ
- Deposits typically present in lesional and sometimes nonlesional sun exposed skin
- Discoid lupus erythematosus (DLE)
- High frequency of positive DIF (up to 90% in lesional skin) (Int J Dermatol 1992;31:260)
- Intense, thick, continuous linear or granular immunoreactant deposition (Int J Dermatol 1992;31:260)
- Prominent C3 and IgG deposition
- Folliculocentric pattern common, reflecting perifollicular inflammation (J Cutan Pathol 2013;40:230)
- Subacute cutaneous lupus erythematosus (SCLE)
- Lower frequency of positive DIF (50 - 60%) (J Am Acad Dermatol 1981;4:471)
- Typically less intense than discoid lupus erythematosus
- Granular to continuous linear deposition, often at the DEJ (J Am Acad Dermatol 1981;4:471)
- May involve epidermal staining in active lesions
- Less follicular involvement compared to discoid lupus erythematosus
- Lupus band test
- Positive in both lesional and nonlesional sun exposed skin in cutaneous lupus erythematosus (Indian Dermatol Online J 2015;6:172)
- More commonly positive in discoid lupus erythematosus and chronic cutaneous lupus (Indian Dermatol Online J 2015;6:172)
- General features (all cutaneous lupus erythematosus (CLE) subtypes)
Lichenoid
- Lichen planus
- Shaggy fibrinogen at basement membrane zone (J Clin Diagn Res 2015;9:ZC34)
- IgM deposition on the colloid bodies
Positive stains
- PAS / PASD is positive in colloid / Civatte bodies
- None required
Vacuolar
- Pityriasis lichenoides
- Lichen planoplanus
- Verhoeff-van Gieson elastic stain shows loss of elastic fibers
- Lichen nitidus
- Inflammatory infiltrates contain CD68+ histiocytes and S100+, CD1a+ Langerhans cells (Clin Exp Dermatol 2011;36:811)
Molecular / cytogenetics description
Lichenoid
- Lichen planus
- Can be monoclonal via T cell receptor rearrangement studies (25% of cases) (Arch Dermatol Res 2000;292:568)
- Pityriasis lichenoides
- Can be monoclonal via T cell receptor rearrangement studies (65% of cases) (Arch Dermatol 2000;136:1483)
Electron microscopy description
- Basal keratinocyte damage
- Cytoplasmic vacuolization, nuclear pyknosis, chromatin condensation and mitochondrial swelling with disrupted cristae (J Pathol 2024;264:30)
- Basement membrane zone (BMZ) alterations
- Fragmentation and thinning of the basal lamina
- Reduced or absent hemidesmosomes
- Loss of anchoring fibrils, occasionally with immune complex deposition (J Pathol 2024;264:30)
- Interface changes
- Widening of the dermoepidermal junction
- Subepidermal edema and dense band formation with immune complex deposition (Br J Dermatol 1980;102:161)
- Inflammatory infiltrate
- Lymphocytic infiltration at the DEJ, satellite cell necrosis and apoptotic keratinocytes (J Pathol 2024;264:30)
Videos
Vacuolar versus lichenoid interface dermatitis pattern
Sample pathology report
Vacuolar
Lichenoid
- Skin, right arm, punch biopsy:
- Vacuolar interface dermatitis (see comment)
- Comment: Microscopic examination shows vacuolar alteration in the basal keratinocytes with associated dyskeratotic keratinocytes. There is an accompanying superficial, sparse to mild lymphocytic inflammatory infiltrate. Melanophages are scattered in the papillary dermis. The differential diagnosis includes erythema multiforme / SJS / TEN spectrum of disorders, acute graft versus host disease (grade 2), drug eruption and connective tissue diseases. Clinical correlation is recommended for further classification.
Lichenoid
- Skin, right inner wrist, punch biopsy:
- Lichenoid interface dermatitis (see comment)
- Comment: Microscopic examination shows a dense, superficial, band-like lymphocytic infiltrate, closely approximating the epidermis, with numerous dyskeratotic keratinocytes in the epidermal basal layer. The differential diagnosis includes lichen planus, lichenoid drug eruption and lichen planus-like keratosis. Clinical correlation is recommended for further classification.
Differential diagnosis
Interface
Lichenoid
- Erythema multiforme / SJS / TEN spectrum
- Bullous pemphigoid:
- Clean subepidermal split with no dyskeratotic keratinocytes forming the roof of the blister
- Eosinophilic infiltrate in superficial to mid dermis
- Linear deposition of IgG and C3 along the basement membrane zone on direct immunofluorescence (DIF)
- Fixed drug eruption:
- Patchy parakeratosis
- Classically associated with superficial and deep perivascular inflammation (Dermatol Pract Concept 2011;1:33)
- Predominantly neutrophilic or eosinophilic inflammatory infiltrate but this depends on the age of lesion
- Acanthosis is more pronounced than EM
- PLEVA:
- Parakeratosis, with or without neutrophils
- Red cell extravasation, lymphocyte exocytosis
- Bullous pemphigoid:
- Pityriasis lichenoides
- PLEVA:
- Erythema multiforme:
- May also have vacuolar interface alteration and an interface obscuring infiltrate
- Usually no parakeratosis
- Less prominent lymphocyte exocytosis
- No wedge shaped inflammatory infiltrate
- No lymphocytic vasculitis (endothelial swelling, erythrocyte exocytosis, fibrinoid necrosis)
- Erythema multiforme:
- PLC:
- Chronic superficial dermatitis:
- Spongiotic reaction pattern
- May also have parakeratosis and lymphocyte exocytosis
- No lymphocytic vasculitis
- Chronic superficial dermatitis:
- PLEVA:
- Graft versus host disease
- Subacute radiation dermatitis:
- Develops weeks to months after radiation
- Both entities may have interface dermatitis with satellite cell necrosis (J Am Acad Dermatol 1989;20:236)
- Cytologically atypical keratinocytes
- Effacement of dermal papillae and rete ridges
- Large, atypical fibroblast nuclei (i.e., radiation fibroblasts in altered dermal collagen) (J Am Acad Dermatol 1989;20:236)
- Eruption of lymphocyte recovery:
- Develops 2 - 3 weeks after chemotherapy
- Both entities may have dyskeratotic keratinocytes and satellite cell necrosis (Arch Dermatol 1993;129:855)
- Erythematous maculopapular rash during bone marrow aplasia induced by chemotherapy
- Epidermal spongiosis, keratinocyte atypia secondary to chemotherapy effect, vacuolar interface alteration, mild superficial perivascular lymphocytic inflammation
- May need clinical correlation to distinguish from grade 2 GVHD (Arch Dermatol 1989;125:1512, Arch Dermatol 1993;129:855)
- Engraftment syndrome:
- Usually occurs 3 - 10 days after HSCT, as this is the period of neutrophil recovery
- Risk factors include less intense chemotherapy regimens, use of peripheral blood stem cells, use of granulocyte colony stimulating factor (G-CSF) (Ann Transplant 2024;29:e944043, Med Oncol 2022;40:36)
- Noninfectious fever, diarrhea, skin rash, pulmonary edema, weight gain, deranged renal function tests and liver function tests (Med Oncol 2022;40:36)
- Lower numbers of epidermal CD8+ cells and CD1a+ cells in engraftment syndrome than in GVHD (Histol Histopathol 2012;27:235)
- Drug hypersensitivity reaction:
- Both may have vacuolar interface changes
- More spongiosis and dermal eosinophils than in GVHD (J Cutan Pathol 2015;42:39)
- Viral exanthem:
- In immunocompromised adults, diffuse morbilliform rashes can mimic GVHD (J Am Acad Dermatol 2017;76:538, Allergol Int 2006;55:1)
- Causative agents may include influenza virus, enteroviruses (coxsackie, echovirus), herpes simplex, HHV6 / 7, parvovirus B19, measles, cytomegalovirus (CMV), EBV, HIV and others (J Am Acad Dermatol 2017;76:538, Future Microbiol 2017;12:171)
- Clinically, vesicles and petechiae favor viral etiology (J Am Acad Dermatol 2012;67:1282)
- Serology, polymerase chain reaction (PCR) or viral antigen detection methods can be used
- Subacute radiation dermatitis:
- Fixed drug eruption
- Erythema multiforme:
- Superficial inflammatory infiltrate only
- Inflammatory infiltrate is more sparse and does not contain significant neutrophils and eosinophils
- Lack of pigment incontinence and melanophages
- Stevens-Johnson syndrome / toxic epidermal necrolysis:
- Has a longer latency period (order of weeks versus hours), more mucosal involvement, more severe constitutional symptoms
- Herpes simplex / zoster:
- Considered when the fixed drug has abundant intraepidermal vesiculation
- Features include multinucleation, molding and nuclear margination with intranuclear inclusions
- HSV immunohistochemical stain is positive
- Other intraepidermal pustular conditions:
- Acute generalized exanthematous pustulosis:
- Clinically, innumerable minute pustules on a background of intense erythema with desquamation, over the trunk and intertriginous areas, instead of a recurrent single round to oval lesion
- Subcorneal pustules
- Dermatitis herpetiformis:
- Clinically associated with celiac disease (gluten sensitivity) (Acta Derm Venereol 2020;100:adv00056)
- Subepidermal vesicle formation (Acta Derm Venereol 2020;100:adv00056)
- Neutrophilic microabscesses at the tips of dermal papillae (Acta Derm Venereol 2020;100:adv00056)
- Granular IgA in dermal papillae on perilesional skin is nearly pathognomonic for dermatitis herpetiformis (Acta Derm Venereol 2020;100:adv00056)
- Pemphigus group of conditions:
- Intraepidermal blister formation in response to ultraviolet exposure, heat, friction (Dermatol Pract Concept 2020;10:e2020050)
- Acantholytic keratinocytes (Dermatol Pract Concept 2020;10:e2020050)
- Intercellular IgG / C3, fishnet pattern (Am J Dermatopathol 2021;43:429)
- Desmoglein autoantibodies (Am J Dermatopathol 2021;43:429)
- Acute generalized exanthematous pustulosis:
- Erythema multiforme:
Lichenoid
- Lichen planus
- Secondary syphilis:
- Often acral involvement in an immunocompromised patient
- Plasma cells are often present
- Elongated rete
- Apoptotic keratinocytes are usually absent or far fewer than expected, given the density of the lymphocytic infiltrate
- Treponemal immunostain is positive for spirochetal organisms in the basal epidermis / superficial dermis
- Cutaneous lupus erythematous:
- Varying vacuolar interface pattern pending subtype
- Basement membrane zone thickening
- Interstitial dermal mucin
- Inflammation in discoid lupus erythematosus (DLE) is more widespread than in LP and can involve the entire hair follicle, the sweat glands, deep perivascular plexus and the subcutaneous fat
- Panniculitis may manifest as mild patchy lymphocytic infiltrates, mucin deposition or lipoatrophy
- Lichen planus-like keratosis (DermNet: Lichenoid Keratosis [Accessed 5 February 2025]):
- Parakeratosis
- Plasma cells present
- Adjacent solar lentigo or seborrheic keratosis
- Solitary lesion
- Early lichen sclerosus:
- Early homogenization of the papillary collagen
- Dermal edema
- Secondary syphilis:
- Lichenoid drug eruption
- Lichen planus:
- Orthokeratosis only
- No eosinophils
- More pronounced band-like inflammatory infiltrate
- No deep involvement (in contrast to photolichenoid eruptions)
- Can be indistinguishable
- Lichen planus:
- Lichen striatus
- Lichen planus:
- No inflammation surrounding eccrine coils
- No parakeratosis
- Lichen planus:
- Lichen planus-like keratosis
- Lichenoid actinic keratosis:
- Large atypical basal layer keratinocyte nuclei with a loss of polarity, loss of granular layer
- Atypical basal keratinocytes extend beyond the zone of most dense lymphocytic inflammation
- Focal parakeratosis
- Prominent solar elastosis
- Lichen planus:
- No plasma cells
- No parakeratosis
- Clinically more diffuse
- Lichenoid drug eruption:
- Clinically more diffuse
- No adjacent solar lentigo or seborrheic keratosis
- Lichenoid actinic keratosis:
- Lichen nitidus
- Arthropod reactions:
- Inflammation is more widely distributed (not limited to 1 or 2 adjacent dermal papillae)
- Eosinophils and neutrophils may be prominent
- Disseminated granuloma annulare:
- Granulomatous foci can form a dense ball in the superficial dermis in disseminated granuloma annulare
- However, there are no finger-like epidermal rete on the periphery of the inflammatory focus (no claw)
- Necrobiosis may be present, along with increased dermal mucin
- Arthropod reactions:
Additional references
Practice question #1
A 41 year old woman presented with alopecia. Which histopathologic feature, if present, would most favor a diagnosis of lichen planopilaris?
- Deep dermal mucin
- Follicular epithelial interface alteration
- Subepidermal blister formation
- Well circumscribed lymphohistiocytic infiltrate in the upper dermis
Practice answer #1
B. Follicular epithelial interface alteration. The primary lymphocytic inflammatory infiltrate in lichen planopilaris (LPP) targets the follicular epithelium, particularly the bulge region, leading to permanent follicular destruction (J Cutan Pathol 2005;32:675). LPP is a primary scarring alopecia characterized by perifollicular inflammation and fibrosis, which eventually leads to irreversible hair loss.
Answer A is incorrect because deep dermal mucin is more characteristic of lupus erythematosus, particularly discoid lupus erythematosus (DLE). Lupus specific alopecias typically involve the entire hair follicle and its appendages, with prominent dermal mucin deposition and perivascular inflammation that extends to the deep vascular plexus (Lupus Sci Med 2018;5:e000291).
Answer C is incorrect because this is a hallmark of subepidermal blistering diseases like bullous pemphigoid, which show subepidermal separation with prominent eosinophilic or neutrophilic infiltrates, not the follicular epithelial damage seen in LPP (Arch Pathol Lab Med 1989;113:36).
Answer D is incorrect because this finding is more typical of lichen nitidus, which presents as small, flesh colored papules and shows a ball and claw configuration, where a dense aggregate of lymphocytes and histiocytes is confined to the upper dermis, cupped by elongated rete ridges (Pediatr Dermatol 2019;36:189).
Comment Here
Reference: Interface dermatitis
Answer A is incorrect because deep dermal mucin is more characteristic of lupus erythematosus, particularly discoid lupus erythematosus (DLE). Lupus specific alopecias typically involve the entire hair follicle and its appendages, with prominent dermal mucin deposition and perivascular inflammation that extends to the deep vascular plexus (Lupus Sci Med 2018;5:e000291).
Answer C is incorrect because this is a hallmark of subepidermal blistering diseases like bullous pemphigoid, which show subepidermal separation with prominent eosinophilic or neutrophilic infiltrates, not the follicular epithelial damage seen in LPP (Arch Pathol Lab Med 1989;113:36).
Answer D is incorrect because this finding is more typical of lichen nitidus, which presents as small, flesh colored papules and shows a ball and claw configuration, where a dense aggregate of lymphocytes and histiocytes is confined to the upper dermis, cupped by elongated rete ridges (Pediatr Dermatol 2019;36:189).
Comment Here
Reference: Interface dermatitis
Practice question #2
A 70 year old man, 26 days postallogeneic hematopoietic stem cell transplant for acute myeloid leukemia, develops a tender, diffuse rash over his bilateral lower extremities, trunk and scalp. He has gastrointestinal upset and elevated liver enzymes. A skin biopsy is performed. What is the most likely diagnosis given the clinical findings?
- Acute graft versus host disease
- Drug hypersensitivity syndrome
- Engraftment syndrome
- Morphea
- Scabies
Practice answer #2
A. Acute graft versus host disease (GVHD). The timing (< 100 days posttransplant) and multiorgan involvement (skin, liver, gastrointestinal tract) are classic for acute GVHD. Histologically, acute GVHD typically shows keratinocyte apoptosis, basal vacuolization and a vacuolar lymphocytic infiltrate (Actas Dermosifiliogr 2016;107:183). The apoptosis can be focal or diffuse, involving the entire epidermis or limited to the basal and lower spinous layers. Interface change often extends to adnexal structures, including the hair follicles and acrosyringium.
Answer B is incorrect because drug hypersensitivity syndrome (DRESS) usually presents 2 - 8 weeks after starting the offending medication, often an anticonvulsant or other drug. It is characterized by fever, facial edema, maculopapular rash, eosinophilia and visceral organ involvement (i.e., liver, kidney). Histologically, DRESS can show vacuolar and lichenoid interface dermatitis, spongiosis, eosinophilic infiltrates and small granulomas, which are not the predominant findings in acute GVHD (Arch Intern Med 1995;155:2285). The absence of eosinophilia and the timing in this patient make DRESS unlikely.
Answer C is incorrect because engraftment syndrome typically occurs 3 - 10 days posttransplant during the early neutrophil recovery phase. It presents with a maculopapular rash, fever, noncardiogenic pulmonary edema and sometimes diarrhea but lacks the multiorgan involvement typical of acute GVHD (Histol Histopathol 2012;27:235). Histologically, it can resemble GVHD but the later timing in this patient (26 days posttransplant) makes this diagnosis less likely.
Answer D is incorrect because this is a manifestation of chronic GVHD, which generally occurs > 100 days posttransplant. It presents with sclerotic, indurated plaques due to collagen deposition and fibrosis in the deep dermis (Arch Dermatol 2002;138:924). Early acute GVHD is less likely to show these changes and the timing in this patient is more consistent with acute GVHD.
Answer E is incorrect because scabies typically presents with intensely pruritic papules and burrows in the finger web spaces, wrists and axillae. Histologically, it is diagnosed by identifying Sarcoptes scabiei mites, eggs or scybala within the stratum corneum (Parasitol Res 2024;123:149).
Comment Here
Reference: Interface dermatitis
Answer B is incorrect because drug hypersensitivity syndrome (DRESS) usually presents 2 - 8 weeks after starting the offending medication, often an anticonvulsant or other drug. It is characterized by fever, facial edema, maculopapular rash, eosinophilia and visceral organ involvement (i.e., liver, kidney). Histologically, DRESS can show vacuolar and lichenoid interface dermatitis, spongiosis, eosinophilic infiltrates and small granulomas, which are not the predominant findings in acute GVHD (Arch Intern Med 1995;155:2285). The absence of eosinophilia and the timing in this patient make DRESS unlikely.
Answer C is incorrect because engraftment syndrome typically occurs 3 - 10 days posttransplant during the early neutrophil recovery phase. It presents with a maculopapular rash, fever, noncardiogenic pulmonary edema and sometimes diarrhea but lacks the multiorgan involvement typical of acute GVHD (Histol Histopathol 2012;27:235). Histologically, it can resemble GVHD but the later timing in this patient (26 days posttransplant) makes this diagnosis less likely.
Answer D is incorrect because this is a manifestation of chronic GVHD, which generally occurs > 100 days posttransplant. It presents with sclerotic, indurated plaques due to collagen deposition and fibrosis in the deep dermis (Arch Dermatol 2002;138:924). Early acute GVHD is less likely to show these changes and the timing in this patient is more consistent with acute GVHD.
Answer E is incorrect because scabies typically presents with intensely pruritic papules and burrows in the finger web spaces, wrists and axillae. Histologically, it is diagnosed by identifying Sarcoptes scabiei mites, eggs or scybala within the stratum corneum (Parasitol Res 2024;123:149).
Comment Here
Reference: Interface dermatitis