Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Punsoni M, Asa SL. Pituitary neuroendocrine tumor (PitNET). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/CNSpituitaryendocrine.html. Accessed May 10th, 2024.
Definition / general
- Neuroendocrine tumors of the anterior pituitary gland that are composed of secretory cells with pituitary hormone production
- Main tumor types include somatotroph, lactotroph, mammosomatotroph, thyrotroph, corticotroph, gonadotroph, null cell and plurihormonal tumors
Essential features
- Routine assessment of histology includes determination of:
- Architecture: solid growth, nests, trabeculae or perivascular rosettes
- Cytoplasmic features: acidophilia, basophilia, inclusions indicative of fibrous bodies
- Nuclear features: mitoses, pleomorphism, giant cells
- Other features: inflammatory changes, stroma, hemorrhage, vascular features
- Determination of cell lineage and hormonal activity using stains for transcription factors, hormones and cytokeratins (Endocr Pathol 2022;33:6)
- Evaluation of tumor proliferation potential by mitotic count and Ki67 labeling index
- Evaluation of tumor invasion if possible to identify clinically aggressive tumors
- Ultrastructural features of tumor cells may be important for classification of unusual tumors
Terminology
- Pituitary neuroendocrine tumor (preferred nomenclature)
- Pituitary adenoma (still acceptable but not preferred terminology)
- Metastatic pituitary neuroendocrine tumor (formerly known as pituitary carcinoma)
ICD coding
- ICD-O: 8272/3 - pituitary carcinoma, NOS
- ICD-11: 2F9A & XH94U0 - neoplasms of unknown behavior of endocrine glands & pituitary adenoma, NOS
Epidemiology
- 10 - 15% of intracranial neoplasms
- Mainly occurs in fourth to seventh decade
- F > M
- Incidental tumors are seen in ~14.4% of autopsies and 22.5% of radiologic studies (Cancer 2004;101:613)
Sites
- Majority occur in the sella turcica, originating within the adenohypophysis / anterior pituitary lobe, with variable extension upwards into suprasellar region and into adjacent structures, such as cavernous sinuses, sphenoid sinus and sinonasal mucosa
- Ectopic sites include primary location in the sphenoid sinus; rare examples occur in the suprasellar pituitary stalk and clivus (Neurol Med Chir (Tokyo) 2004;44:380, Arq Neuropsiquiatr 2012;70:744, BMC Res Notes 2013;6:411, Pituitary 2020;23:457)
- Rare tumors are seen as a part of ovarian teratoma (Endocr Pathol 2014;25:321, Eur J Intern Med 2005;16:359, Case Rep Womens Health 2020;29:e00279, Endocr Pathol 2020;31:315)
Pathophysiology
- Cell differentiation is driven by transcription factors leading to 3 main cell lineages
- Transcription factors are (Endocr Pathol 2022;33:6):
- Pit1 (pituitary specific POU class homeodomain transcription factor), which leads to differentiation of somatotrophs, mammosomatotrophs, lactotrophs and thyrotrophs
- SF1 (steroidogenic factor 1), which regulates gonadotroph cell differentiation
- Tpit (T box family member TBX19), which has a transcription factor driving proopiomelanocortin (POMC) lineage with differentiation of corticotrophs
Etiology
- Most tumors are of unknown etiology and lack mutations that are considered causative; epigenetic alterations are common (Endocr Pathol 2021;32:3)
- Inherited syndromes:
- MEN1 with involvement of the MEN1 gene and loss of menin
- MEN4 with mutation of CDKN1B and loss of p27
- MEN5 with mutation of MAX
- Carney complex with mutation of PRKR1ɑ
- Familial acromegaly with mutation of AIP
- SDHx related disease due to mutation in any of the genes encoding the SDH complex
- Hyperparathyroidism jaw tumor syndrome due to mutation of CDC73 and loss of parafibromin
- Lynch syndrome due to mutation in MLH1 / MSH2 / MSH6 / PMS2
- Noninherited germline predisposition syndromes include:
- X linked acrogigantism due to Xq26 microduplication and GPR101 alterations
- McCune-Albright syndrome with GNAS activating mutations
Diagrams / tables
| Tumor type | Transcription factor(s) | Hormone(s) | Other biomarkers | Clinical features |
| Tpit family | ||||
| Densely granulated corticotroph tumor | Tpit | ACTH | PAS; intense keratins | Florid Cushing disease or silent |
| Sparsely granulated corticotroph tumor | Tpit | ACTH (weak) | PAS (weak); strong keratins | Cushing disease or silent |
| Crooke cell tumor | Tpit | ACTH | PAS; ring-like keratins | Cushing disease or silent |
| Pit1 family | ||||
| Densely granulated somatotroph tumor | Pit1 | GH | Perinuclear keratins | Acromegaly |
| Sparsely granulated somatotroph tumor | Pit1 | GH (weak) | Keratin fibrous bodies (> 70%) | Acromegaly or silent |
| Mammosomatotroph tumor | Pit1, ER | GH, PRL | Perinuclear keratins | Acromegaly and hyperprolactinemia |
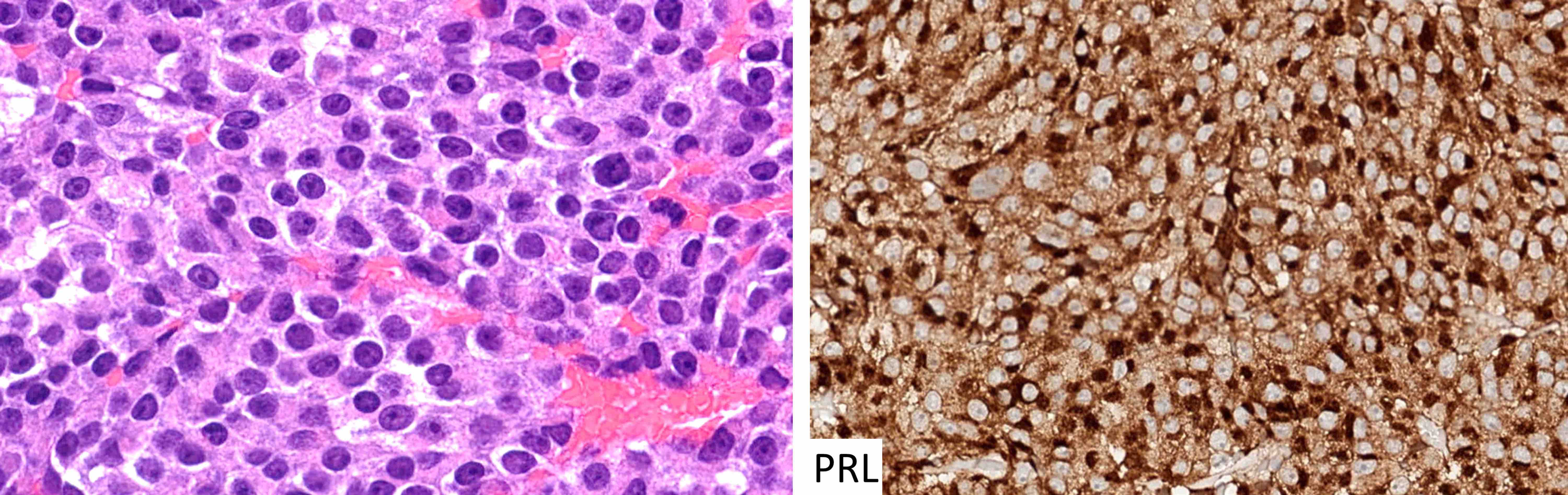
| Sparsely granulated lactotroph tumor | Pit1, ER | PRL (juxtanuclear) | Variable keratins | Hyperprolactinemia (tumor size correlates with PRL level) |
| Densely granulated lactotroph tumor | Pit1, ER | PRL | Variable keratins | Hyperprolactinemia |
| Acidophil stem cell tumor | Pit1, ER | PRL with or without GH (weak, focal) | Variable keratins with or without fibrous bodies | Hyperprolactinemia |
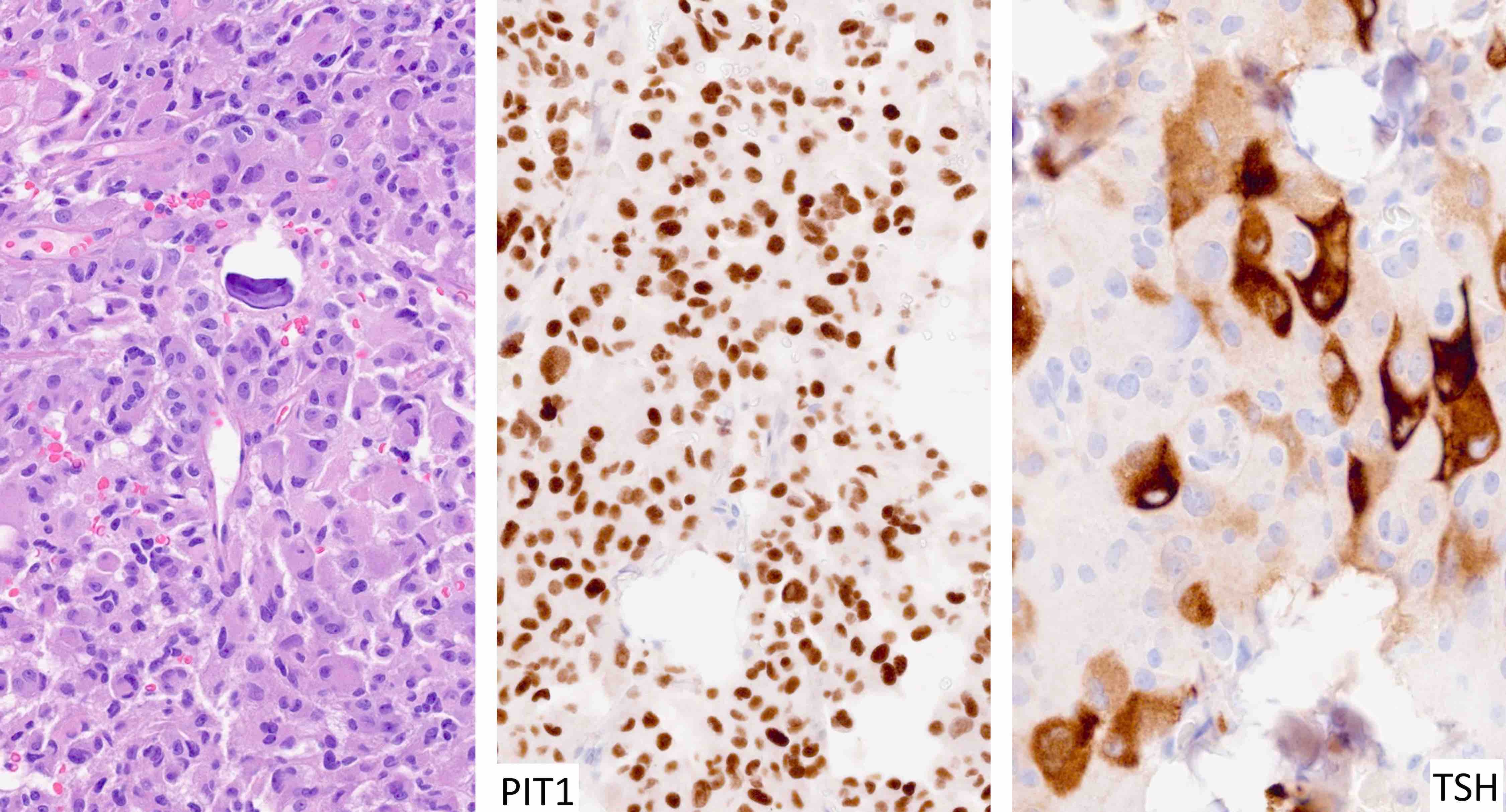
| Thyrotroph tumor | Pit1, GATA3 | TSH | Variable keratins | Elevated TSH with or without hyperthyroidism |
| Mature plurihormonal Pit1 lineage tumor | Pit1, GATA3, ER | Variable, often multiple | Perinuclear keratins | Acromegaly, hyperprolactinemia and hyperthyroidism |
| Immature Pit1 lineage tumor | Pit1 with or without GATA3 with or without ER | Variable, often limited / focal | Variable keratins with or without fibrous bodies | Silent or acromegaly or hyperprolactinemia or hyperthyroidism |
| SF1 family | ||||
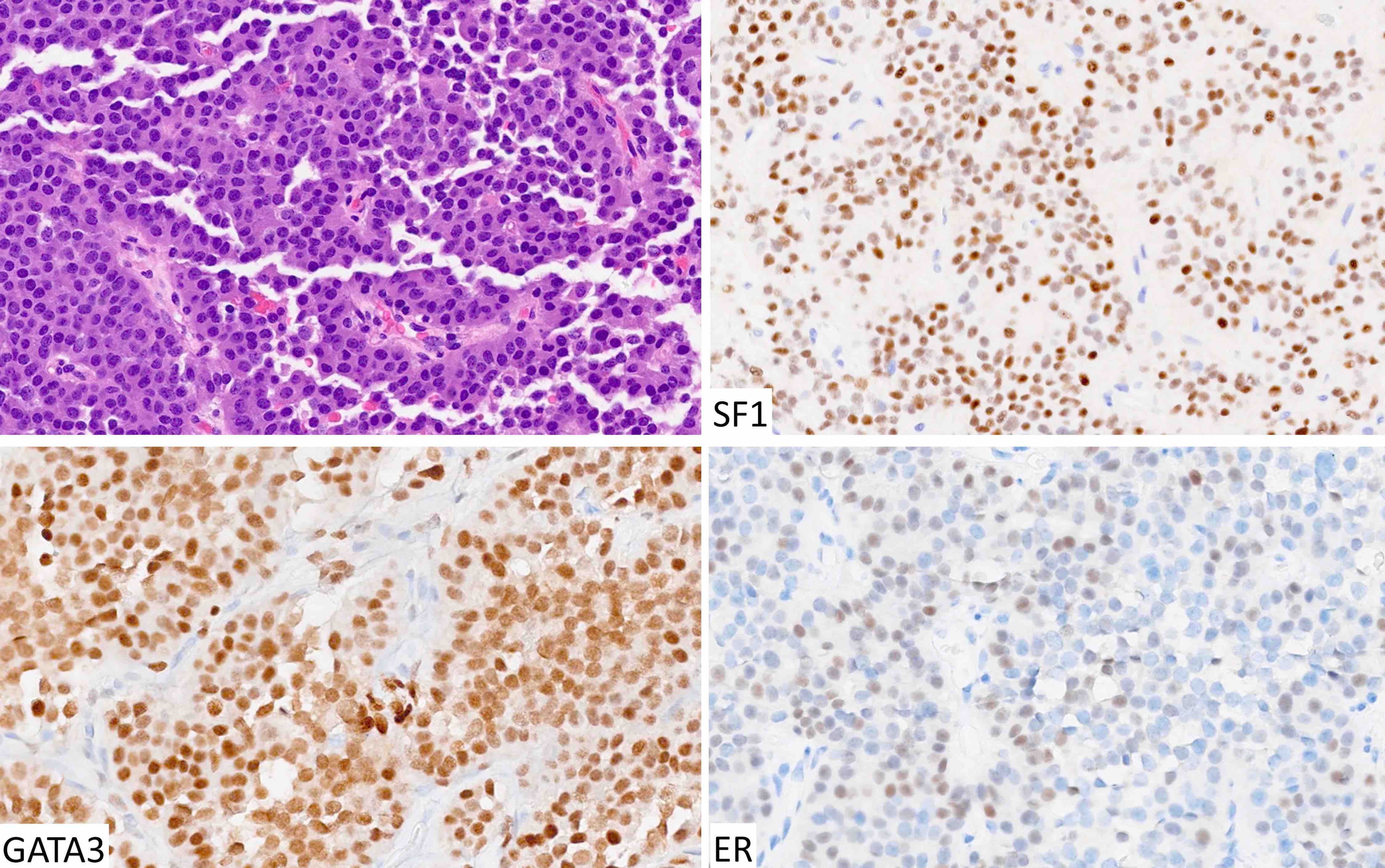
| Gonadotroph tumor | SF1, GATA3, ER | FSH, LH | Variable keratins, follicular cells | Silent with or without elevated gonadotropins |
| No lineage fidelity | ||||
| Null cell tumor | None | None | Variable | Silent |
| Unclassified plurihormonal tumor | Multiple combinations | Multiple combinations | Variable | Variable |
Clinical features
- Clinical features of hormone excess: acromegaly, gigantism, Cushing disease, sequelae of hyperprolactinemia, hyperthyroidism, rarely gonadotropin excess (Endocr Pathol 2022;33:6)
- Larger tumors (> 1 cm) can be associated with mass effects such as headache, visual disturbance and hypopituitarism
- Hemorrhagic necrosis of large tumors (pituitary apoplexy) may be a surgical emergency
Diagnosis
- Current consensus classifies tumors according to pituitary cell lineage and cell differentiation (see Diagrams / tables)
- Classification requires immunohistochemical staining for pituitary transcription factors including the 3 main ones (Pit1, Tpit and SF1) as well as ER and GATA3
- Main pituitary hormones (growth hormone, prolactin, adrenocorticotropic hormone [ACTH], beta thyroid stimulating hormone [TSH], beta luteinizing hormone [LH], beta follicle stimulating hormone [FSH] and alpha subunit of glycoproteins) and keratins (usually CAM 5.2 but also AE1 / AE3 or CK18) (Hum Pathol 2021;107:87)
Laboratory
- Biochemical assessment should be performed in all patients with pituitary tumors (Endocr Pathol 2022;33:6)
- Serum prolactin can be elevated in any patient with a sellar lesion since it is under tonic inhibition and interruption of hypothalamic signaling can result in hyperprolactinemia
- However, this so called stalk effect does not usually result in a level > 200 mcg/L; levels above this usually indicate a tumor producing prolactin
- In patients with true lactotroph tumors, prolactin levels are usually proportional to tumor size
- Patients with large tumors and prolactin levels of 500 mcg/L or less usually do not have a lactotroph tumor
- Growth hormone and IGF1 are biomarkers of acromegaly or gigantism; since growth hormone secretion is pulsatile, IGF1 provides an integrated measure that is more reliable
- Oral glucose tolerance test is a definitive confirmatory test for the diagnosis of acromegaly
- Cortisol and ACTH are elevated in Cushing disease
- The relationship between these is important; a high normal ACTH in a patient with high normal or elevated cortisol should be assessed for Cushing disease
- High dose dexamethasone suppression testing can be useful
- TSH secreting tumors cause increased TSH levels and are usually associated with elevated T3 and T4 levels, hyperthyroidism and goiter
- Gonadotropin excess can be physiological in postmenopausal women; serum levels of FSH and LH as well as testosterone levels in men should be screened
- Cerebrospinal fluid may be xanthochromic with crenated red blood cells and high protein levels in pituitary apoplexy
Radiology description
- Method of classification is based on tumor size and degree of invasion (Endocr Pathol 2022;33:6)
- Important for planning of surgical resection
- Microtumors measure < 1 cm in diameter; macrotumors are > 1 cm
- Identification of tumor extension is important: suprasellar, lateral (cavernous sinus), inferior (sphenoid sinus), posterior fossa
- Density on T2 weighted imaging distinguishes sparsely from densely granulated tumors
Prognostic factors
- Tumor subtypes are predictive of responsiveness to medical therapies
- For example, densely granulated somatotroph tumors are usually responsive to long acting somatostatin analogues whereas sparsely granulated somatotroph tumors are not; densely granulated corticotroph tumors tend to respond to pasireotide therapy
- Invasive, aggressive tumors adenomas often recur over several years; presurgical growth rates can predict temporal pattern of regrowth
- Immature Pit1 lineage tumors tend to be aggressive (Mod Pathol 2016;29:131)
- Crooke cell tumors are aggressive (Endocr Pathol 2022;33:6)
- ATRX loss has been described in some aggressive corticotroph tumors (J Clin Endocrinol Metab 2021;106:1183, Endocrine 2022;76:228)
Case reports
- 11 year old boy with a silent corticotroph tumor with adrenocortical choristoma (J Clin Res Pediatr Endocrinol 2022;14:126)
- 17 year old boy with an aggressive prolactinoma treated with temozolomide (Medicine (Baltimore) 2017;96:e8733)
- 19 year old woman with an ACTH producing tumor with signet ring cells (Appl Immunohistochem Mol Morphol 2020;28:e13)
- 33 year old man with Cushing disease and pituitary corticotroph tumor with adrenocortical cells (Neuropathol Appl Neurobiol 2022;48:e12754)
- 37 year old man with Cushing disease and co-occurring infundibular pituicytoma and pituitary adenoma (Endocr Regul 2019;53:263)
- 41 year old woman with plurihormonal tumor of Pit1 and SF1 lineages and collision with corticotroph tumor (Endocr Pathol 2019;30:74)
- 45 year old man with a multihormonal tumor with Pit1 and Tpit lineage cells (BMC Endocr Disord 2017;17:54)
- 47 year old man with a lactotroph PitNET / adenoma associated to granulomatous hypophysitis (Neuropathology 2022 Aug 10 [Epub ahead of print])
- 56 year old woman with a xanthogranulomatous tumor (Mol Clin Oncol 2018;8:445)
- 58 year old women with meningioma pituitary adenoma collision tumors (Medicine (Baltimore) 2017;96:e9139)
- 60 year old woman with a plurihormonal ACTH growth hormone tumor (World Neurosurg 2018;114:e158)
Treatment
- Surgical approaches
- Commonly via transsphenoidal route (minimally invasive)
- Transfrontal approach for larger invasive tumors
- Partial versus gross total resection
- Dopamine agonists can reduce prolactin levels and the size of sparsely granulated lactotroph tumors
- Somatostatin analogs can reduce hormone (growth hormone and TSH) secretion but may cause little reduction in tumor size
- Clinically nonfunctioning tumors may show little response to medical therapy
- Radiation therapy may be used for tumors resistant to medical therapy or for surgical remnants from subtotal resections
- Therapy may include linear accelerator radiotherapy, stereotactic radiotherapy or radiosurgery
- Single fraction versus fractionated radiosurgery are options (Pituitary 2017;20:489)
- Several studies have evaluated tumor response to temozolomide therapy, particularly in aggressive pituitary tumors (Front Neurol 2021;12:700007, Rev Endocr Metab Disord 2020;21:263)
- Peptide receptor radionuclide therapy with lutetium oxodotreotide has been reported (Endocr Pathol 2019;30:118, Endocr Connect 2019;8:528, J Endocr Soc 2021;5:bvab133)
Gross description
- These tumors are usually resected as small fragments; however, occasionally they can be removed in a single piece that allows assessment of margins
Frozen section description
- Frozen sections are discouraged
- Most cases do not require intraoperative consultation but in unusual situations (e.g., where the differential diagnosis is inflammation), touch preps can be used for cytologic assessment without compromising residual tissue that is retained for proper immunohistochemical analysis
Microscopic (histologic) description
- Architecture varies from solid sheets to nests within a fibrovascular stroma (Endocr Pathol 2022;33:6)
- Some tumors, usually gonadotroph tumors, form pseudorosettes around vascular channels
- Dyscohesive growth is characteristic of sparsely granulated somatotroph tumors (it is associated with loss of E-cadherin expression)
- Most tumor cells are epithelioid but some can be spindle shaped; this is a feature of some immature Pit1 lineage tumors
- Most tumors have a uniform nuclear morphology with stippled chromatin, inconspicuous nucleoli and moderately abundant cytoplasm
- Cells may be classified as acidophilic, basophilic or chromophobic based on tinctorial differences; this usually correlates with content of hormone containing secretory cells (i.e., densely versus sparsely granulated)
- Crooke hyaline change is characterized by large chromophobic or eosinophilic cells with a glassy hyaline appearance (due to accumulation of keratin filaments); it can be seen in neoplastic and nonneoplastic corticotrophs
- Oncocytic change is a feature of some gonadotroph tumors where it tends to be variable; diffuse oncocytosis is a feature of acidophil stem cell tumors
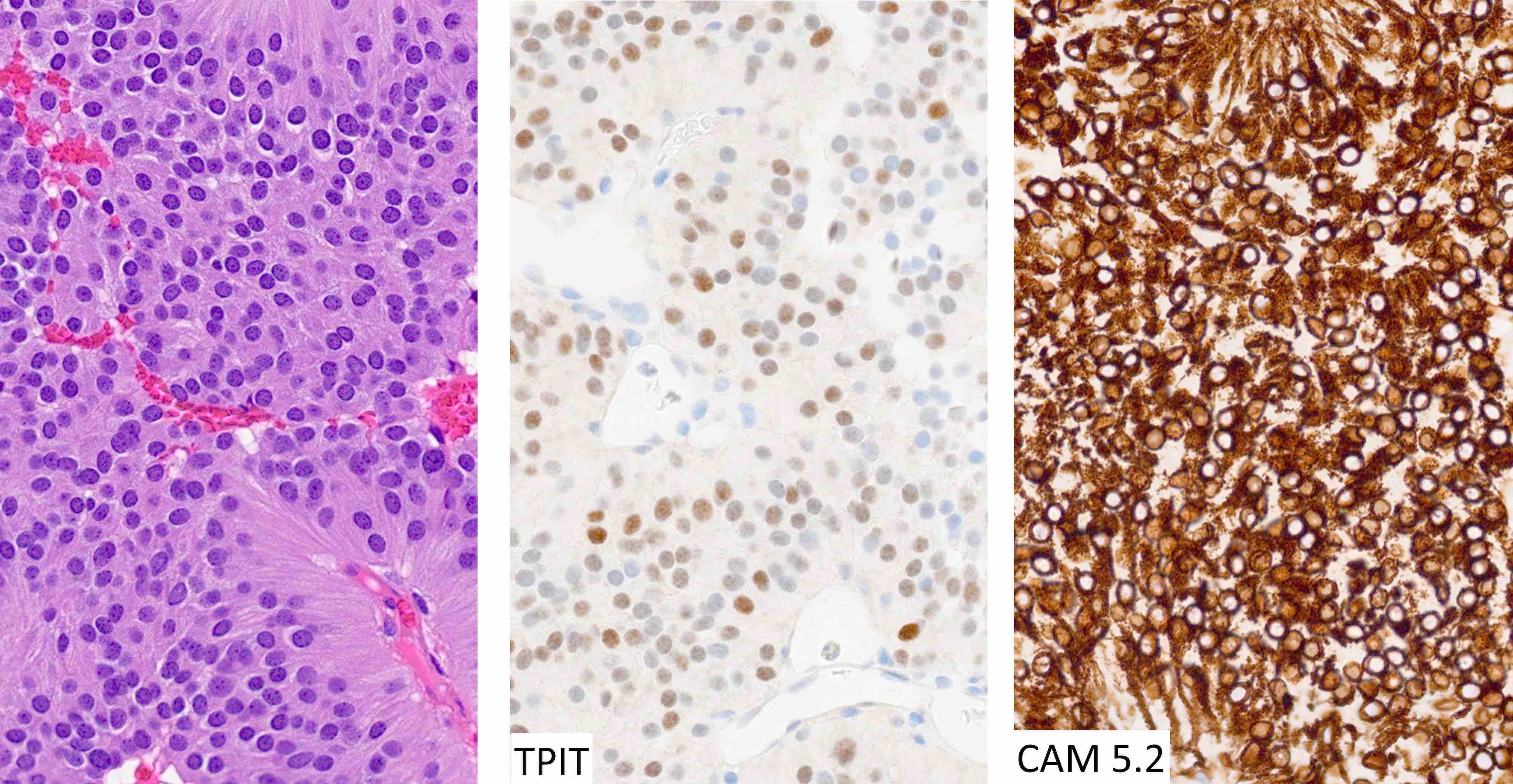
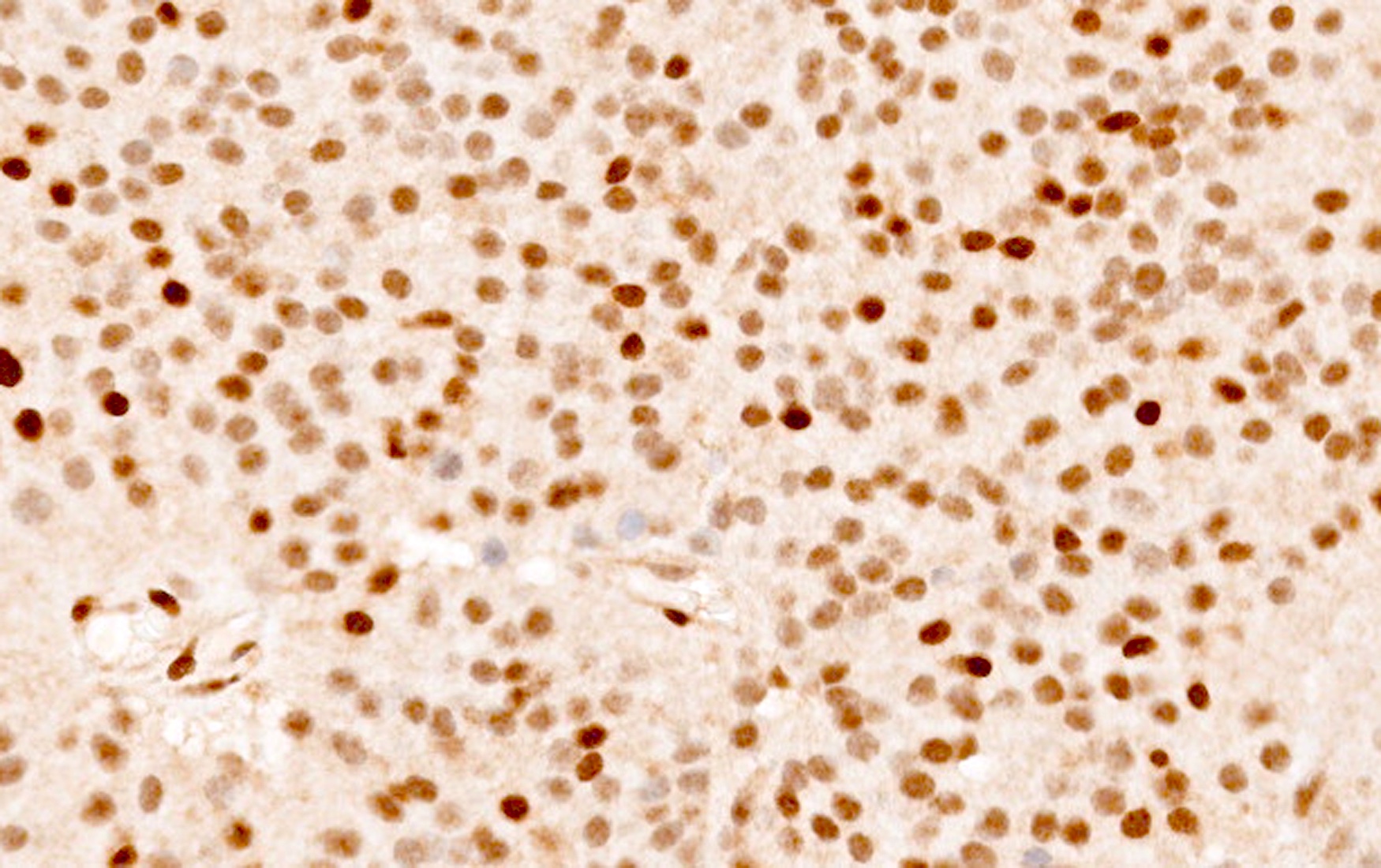
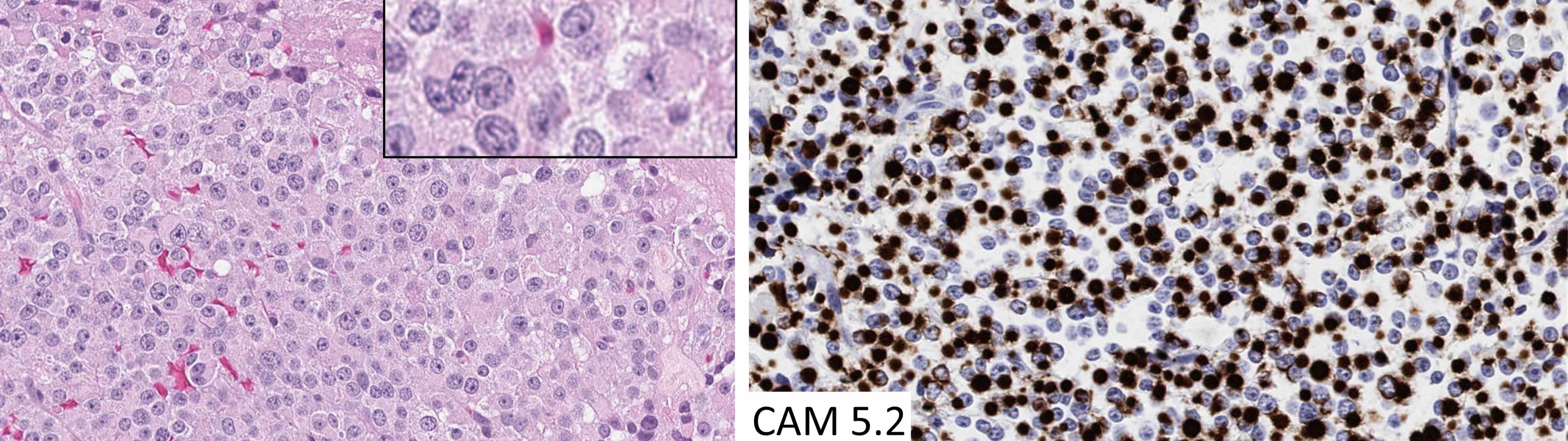
Microscopic (histologic) images
Contributed by Sylvia L. Asa, M.D., Ph.D.
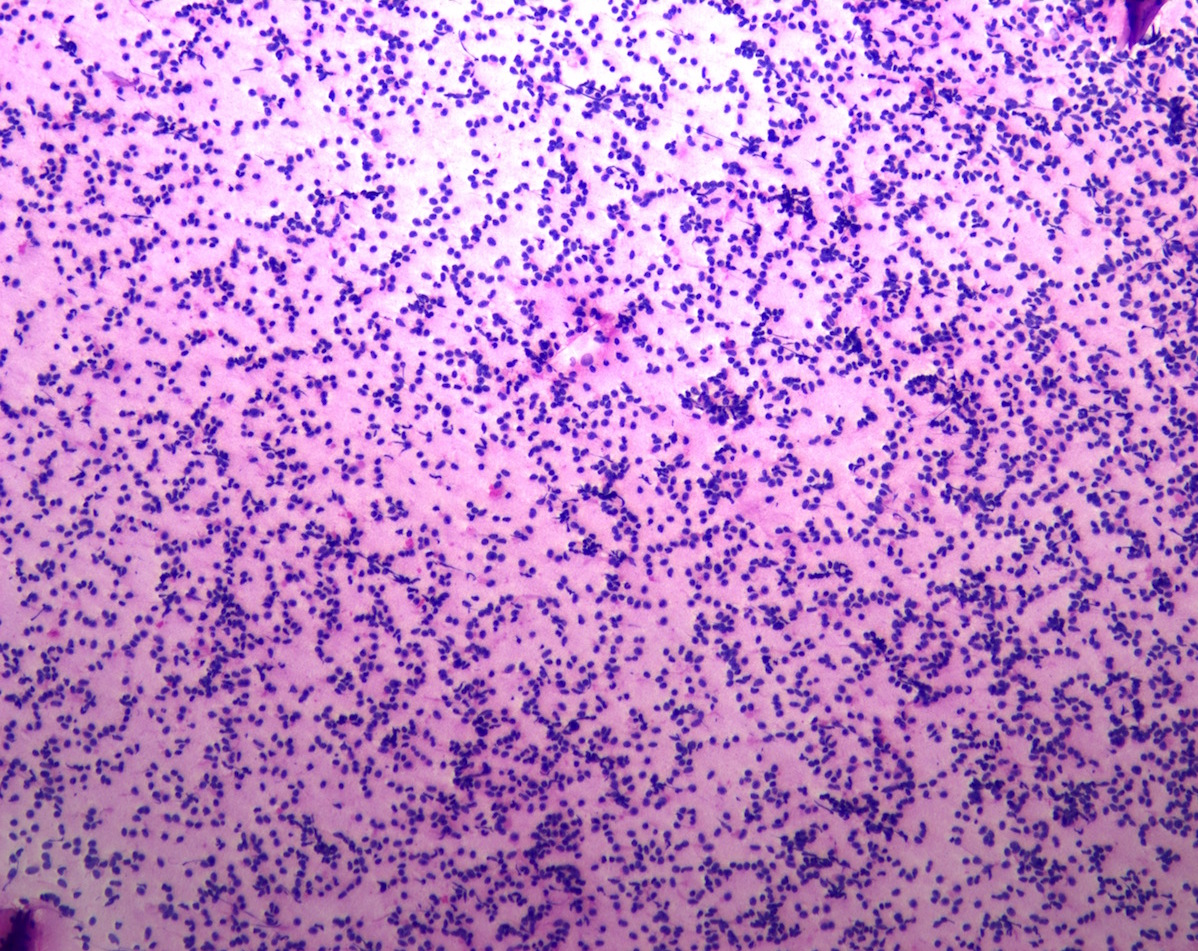
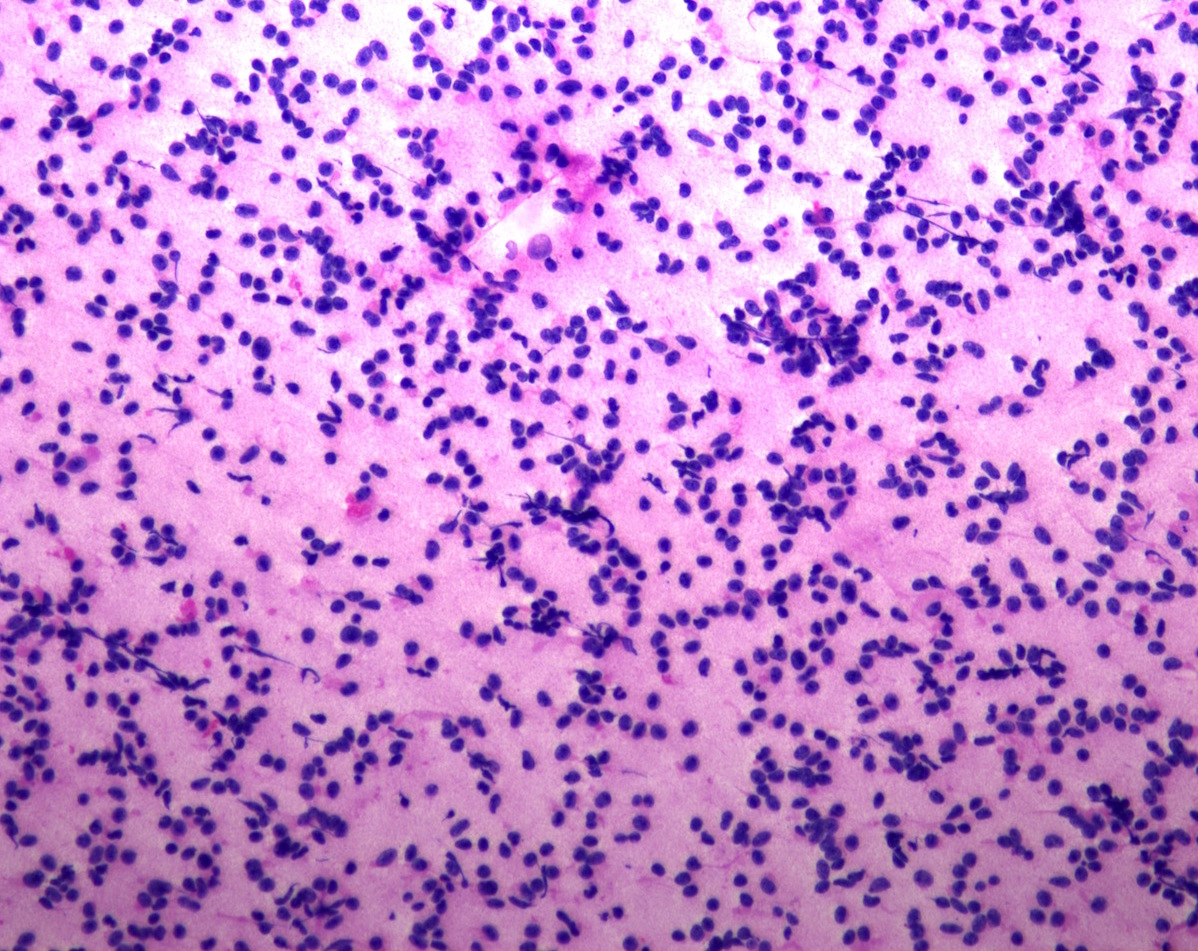
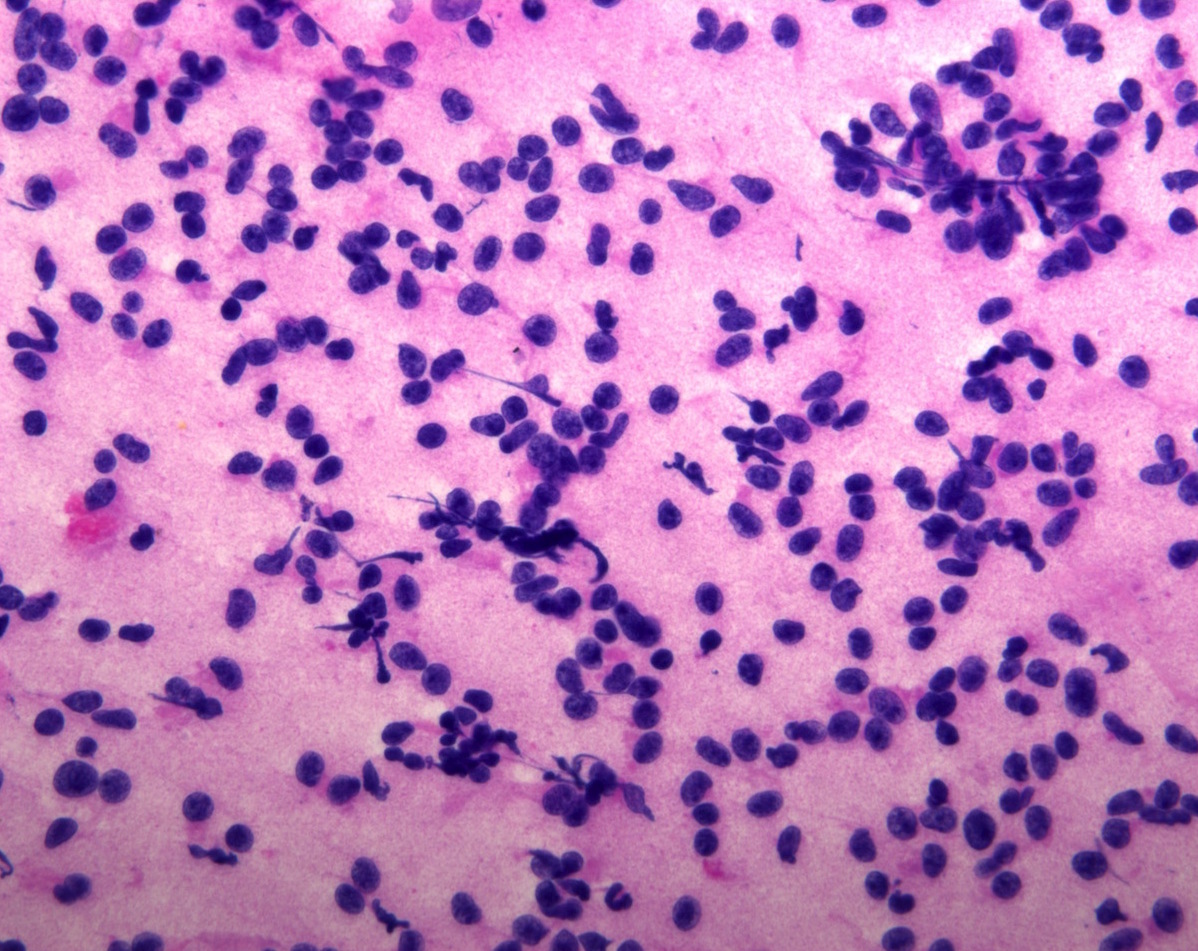
Cytology description
- Normal pituitary has mixed cell types on smear preparation whereas tumors show uniform morphology and cell type
- Tumors produce cellular smear with discohesive small round blue cells
- Some tumors have specific cytologic atypia (e.g., fibrous bodies of sparsely granulated somatotroph tumors, Crooke hyaline of Crooke cell tumors)
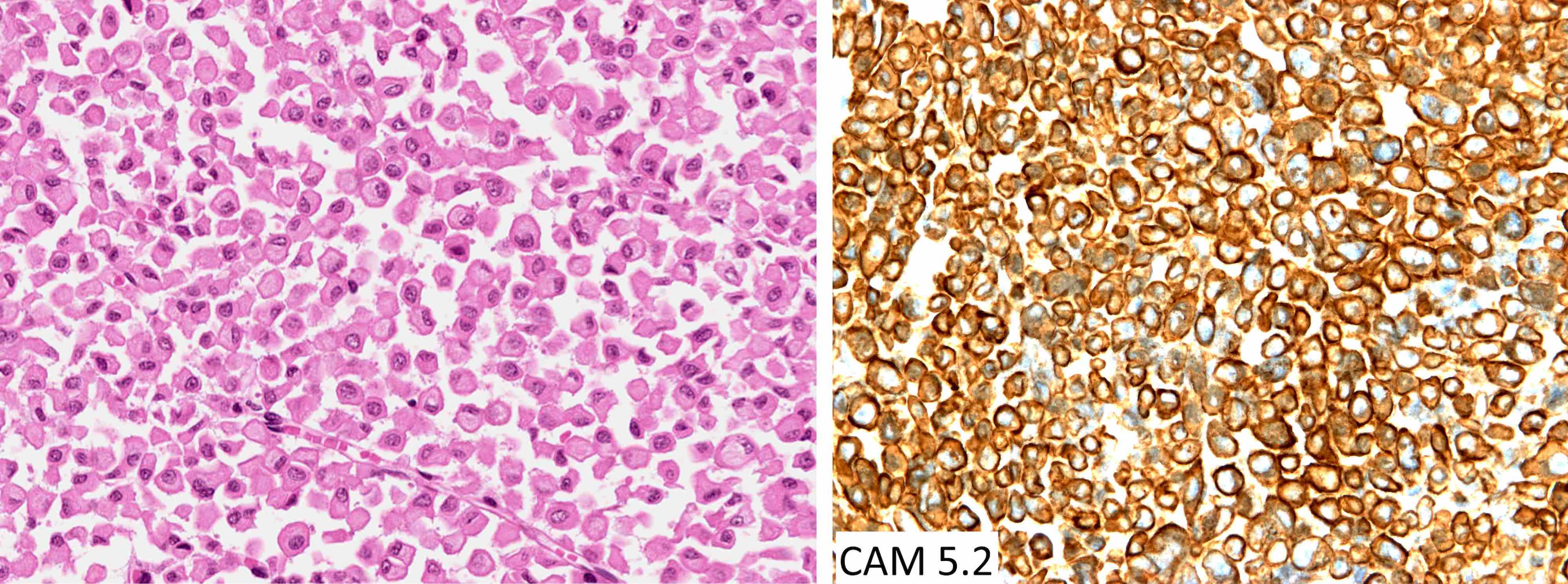
Cytology images
Positive stains
- All pituitary neuroendocrine tumors express INSM1, synaptophysin and chromogranin; positivity for chromogranin A is variable
- Stains for pituitary transcription factors (Pit1, Tpit, SF1, ER and GATA3) and hormones provide the basis for tumor classification (see Diagrams / tables)
- Reticulin staining is useful to distinguish normal acinar architecture, enlarged acini of hyperplasia versus disrupted staining pattern in neoplasms
- Perinuclear CAM 5.2 staining pattern is a feature of densely granulated somatotroph tumors, mammosomatotroph tumors and mature plurihormonal Pit1 lineage tumors
- Paranuclear CAM 5.2 positive fibrous bodies are a conspicuous feature present in > 70% of tumor cells of sparsely granulated somatotroph tumors
- Occasional fibrous bodies can be seen in poorly differentiated Pit1 lineage tumors and acidophil stem cell tumors
- Diffuse and strong cytoplasmic CAM 5.2 staining is characteristic of corticotroph tumors
- Ring-like CAM 5.2 positivity is characteristic of Crooke cell tumors (Brain Pathol 2012;22:443)
- Ki67 immunolabeling often used to characterize tumors with elevated proliferative indices
- Antimitochondrial antibody staining is a feature of oncocytic tumors, including some gonadotroph tumors and the characteristically oncocytic acidophil stem cell tumor
Negative stains
- GFAP: positive in ependymomas
- CK7: positive in choroid plexus tumors and metastatic lesions
- CK20: positive in metastatic lesions
- TTF1: positive in tumors of posterior lobe pituicytes including conventional pituicytoma, granular cell pituicytoma, oncocytic pituicytoma (formerly known as spindle cell oncocytoma) and ependymal pituicytoma (formerly known as sellar ependymoma) (Am J Surg Pathol 2013;37:1694, Endocr Pathol 2014;25:436)
Electron microscopy description
- Mostly supplanted by immunohistochemistry; however, electron microscopy may still play a role in characterization of unusual pituitary tumors and identification of giant mitochondria in acidophil stem cell tumor and spheridia in immature Pit1 lineage tumors (formerly known as silent subtype III adenomas) (Brain Pathol 2012;22:443)
Electron microscopy images
Molecular / cytogenetics description
- No specific molecular characteristics in routine clinical diagnostic workup
- Most pituitary tumors are sporadic; minority are part of hereditary or familial syndromes
- Somatic mutations in GNAS and USP8 genes have been found in ~40% of sporadic somatotroph tumors and 30 - 60% of sporadic corticotroph tumors, respectively (Endocr Pathol 2021;32:3)
Sample pathology report
- Pituitary, transsphenoidal resection specimen:
- Mature plurihormonal Pit1 lineage tumor (see synoptic report)
- Synoptic report:
- Clinical features: functional
- Hormone excess (specify): acromegaly, hyperthyroidism
- Tumor size (from imaging): greatest dimension was 3.8 cm
- Additional dimensions: 3.5 x 2.4 cm
- Received: in formalin
- Specimen integrity: fragmented
- Specimen size: 2.4 x 2.2 x 0.4 cm
- Histologic features:
- Reticulin: disrupted
- PAS: negative
- Infiltrating tumor: cannot be determined
- Immunohistochemistry:
- Tpit is negative
- Pit1 is positive
- ER is focally positive
- GATA3 is focally positive
- SF1 is negative
- ACTH is negative
- Growth hormone is positive
- Prolactin is focally positive
- Beta TSH is focally positive
- Alpha subunit is diffuse positive
- Beta FSH is negative
- Beta LH is negative
- Keratin (CAM 5.2) is diffuse
- Ki67 labeling index is 5%
- Tumor type: pituitary neuroendocrine tumor
- Subtype: mature plurihormonal Pit1 lineage tumor
- Additional pathologic findings
- Nontumorous adenohypophysis: present
- Neurohypophysis: not identified
- Gross description: Labeled with the patient's name and hospital number and received in formalin as multiple fragments of tan-pink friable tissue that measure 2.4 x 2.2 x 0.4 cm in aggregate. The specimen is submitted in toto in 1 cassette.
Differential diagnosis
- Normal adenohypophysis:
- Normal acini by reticulin staining
- Pituitary nodular hyperplasia:
- Enlarged acini by reticulin staining
- Metastatic neuroendocrine tumor:
- Pituicytoma:
- Ependymoma:
- Positive for GFAP
- Negative for synaptophysin
- Pituitary blastoma:
- Early childhood tumor, small blastema-like cells, true rosettes, large glandular structures, DICER1 mutation
Board review style question #1
Which of the following is true of pituitary neuroendocrine tumors?
- Diabetes insipidus is often associated with PitNETs
- GNAS mutations have been described in pituitary tumors
- Most PitNETs are negative for pituitary hormones
- Most PitNETs are part of hereditary or familial syndromes
- The diagnosis of metastatic behavior can be predicted by morphological or immunohistochemical features
Board review style answer #1
B. GNAS mutations have been described in pituitary tumors. GNAS mutations have been described in a subset of pituitary somatotroph tumors.
Comment Here
Reference: Pituitary neuroendocrine tumor (PitNET)
Comment Here
Reference: Pituitary neuroendocrine tumor (PitNET)
Board review style question #2
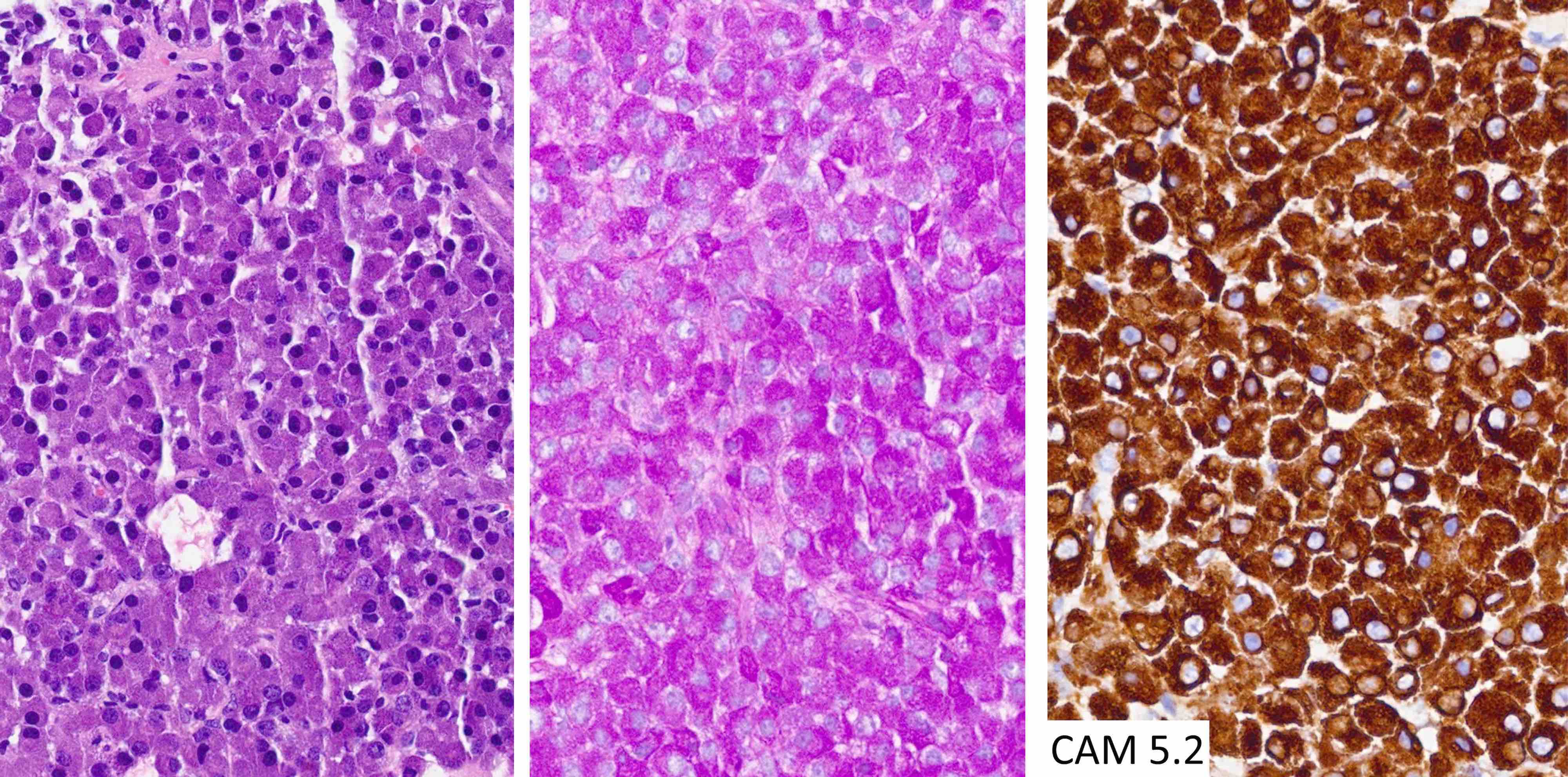
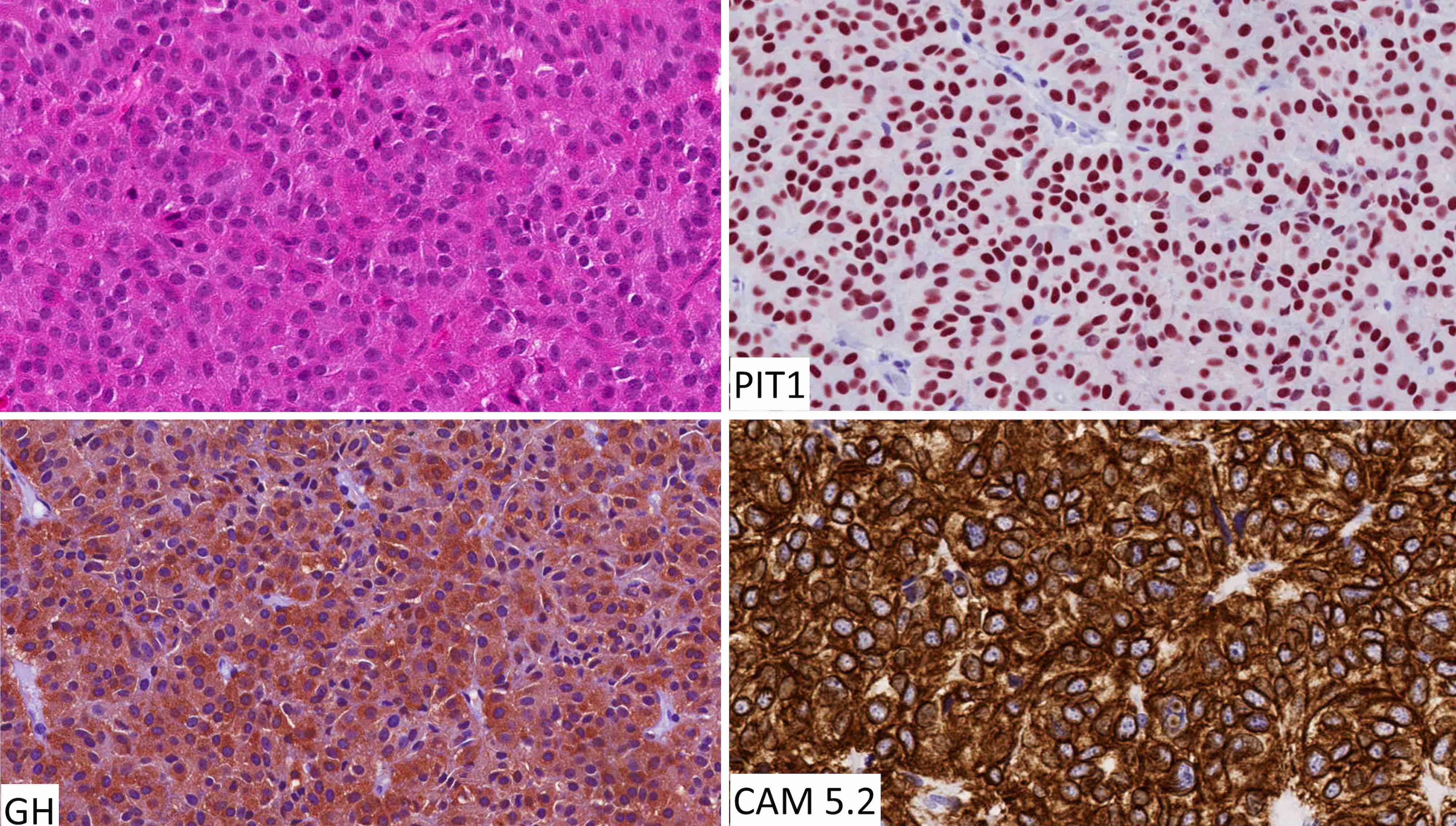
The chromophobic tumor in the image above has the keratin pattern shown on the right. Which of the following is correct?
- This patient had acromegaly
- This patient had Cushing disease
- This patient would not have had hyperprolactinemia
- This tumor is likely to respond to first generation somatostatin analogues
- This tumor stains for Tpit
Board review style answer #2
A. This patient had acromegaly. This sparsely granulated somatotroph tumor stains for Pit1 (not Tpit) and causes acromegaly, which may be associated with hyperprolactinemia due to the stalk section effect since sparsely granulated tumors are often large at the time of diagnosis. These tumors generally do not respond to first generation somatostatin analogues. Somatotroph tumors do not cause Cushing disease.
Comment Here
Reference: Pituitary neuroendocrine tumor (PitNET)
Comment Here
Reference: Pituitary neuroendocrine tumor (PitNET)