Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Ahmed A, Shalin SC. Cutaneous mixed tumor / chondroid syringoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticchondroidsyringoma.html. Accessed September 17th, 2025.
Definition / general
- Benign adnexal tumor of the skin composed of mesenchymal and sweat gland components
- Morphologically identical to pleomorphic adenoma / benign mixed tumor of salivary gland
- Most mixed tumors have both eccrine and apocrine components
- Most are currently classified as apocrine type
Essential features
- Slow growing, painless nodule on head and neck
- Benign tumor of sweat glands with a myxoid stroma and cartilaginous metaplasia (Stedman: Medical Dictionary, 28th Edition, 2006)
Terminology
- Other names include:
- Apocrine mixed tumor
- Eccrine mixed tumor
- Benign mixed tumor of skin
- Chondroid syringoma
ICD coding
- ICD-10: D23.9 - other benign neoplasm of skin, unspecified
Epidemiology
- Less than 0.1% of cutaneous tumors (Dermatol Surg 2003;29:179)
- Middle aged men most commonly affected (J Am Acad Dermatol 2007;57:467)
Sites
- Head and neck region involvement is common (nose most common site)
- Extremities are less common (axilla most common site)
- Rare cases of scrotum reported (J Int Med Res 1996;24:482, Int J Urol 2008;15:944)
Pathophysiology
- Benign adnexal proliferation with a wide range of differentiation and metaplastic changes in its epithelial, myoepithelial and stromal components
Etiology
- Unclear
Clinical features
- Benign; rarely recurs
- No distinctive clinical characteristics but may mimic pilomatricoma
- Most often seen in middle aged men; mean age at presentation is 48 years
- Most commonly in nose, followed by cheek and upper lip
- Face / head and neck > extremities, trunk or back
- Calcification / ossification may occur
- Typically small (0.5 - 3 cm) and painless
- No specific findings on dermoscopy
- Rapid, ulcerative growth can be seen in malignant mixed tumor
- Reference: Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Diagnosis
- Biopsy required for diagnosis
Radiology description
- Findings are not highly specific for chondroid syringoma over other dermal and soft tissue neoplasms (Australas Radiol 2001;45:240)
- MRI can be helpful to determine the extent and depth of the lesion and relation to anatomic structures in large tumors
- In the few cases studied, radiography of malignant chondroid syringoma tends to show higher signal intensity and enhancement (Australas Radiol 2001;45:240)
Prognostic factors
- Benign, with rare recurrence
- Malignant counterpart (malignant chondroid syringoma) rare with high rate of metastasis (60%) and death (25%) and should be ruled out on microscopic morphology (Pathology 1994;26:237)
Case reports
- 14 year old boy with eccrine spiradenoma with chondroid syringoma in Blaschkoid distribution (Indian J Dermatol Venereol Leprol 2009;75:600)
- 20 year old man with chondroid syringoma with extensive bone formation (J Clin Diagn Res 2014;8:FD15)
- 34 year old man with rapidly growing chondroid syringoma of external auditory canal (Case Rep Med 2011;2011:589680)
- 43 year old woman with chondroid syringoma of arm (Dermatol Online J 2006;12:14)
- 47 and 59 year old men whose tumors had prominent pilomatricomal component (J Cutan Pathol 2009;36:882, J Cutan Pathol 2012;39:718)
- 53 year old man with chondroid syringoma in the middle finger (Hand Surg Rehabil 2021;40:353)
- 55 year old man with 10 x 10 cm chondroid syringoma (Dermatol Surg 2003;29:977)
- 67 year old man with lipomatous mixed tumor with follicular differentiation (J Cutan Pathol 2006;33:389)
- 72 year old man with chondroid syringoma of the toe (Medicine (Baltimore) 2018;97:e9825)
- Apocrine mixed tumors of the skin with architectural or cytologic atypia (Am J Surg Pathol 2007;31:1094)
- Cutaneous apocrine mixed tumor with intravascular tumor deposits (Am J Dermatopathol 2011;33:775)
Treatment
- Surgical excision is curative
- Recurrence is rare
- Electrodissection and CO2 laser have been used with slightly higher recurrence rate
- Reference: Dermatology Advisor: Chondroid Syringoma (Mixed Tumor of the Skin) [Accessed 17 June 2021]
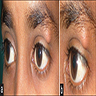
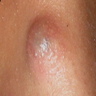
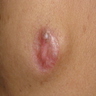
Clinical images
Gross description
- Nodular, circumscribed, nonulcerated, with marked variation in size
- Cut surfaces are tan-white, often with grossly apparent chondroid component
- Slow growing, painless, firm, deep dermal to subcutaneous nodule
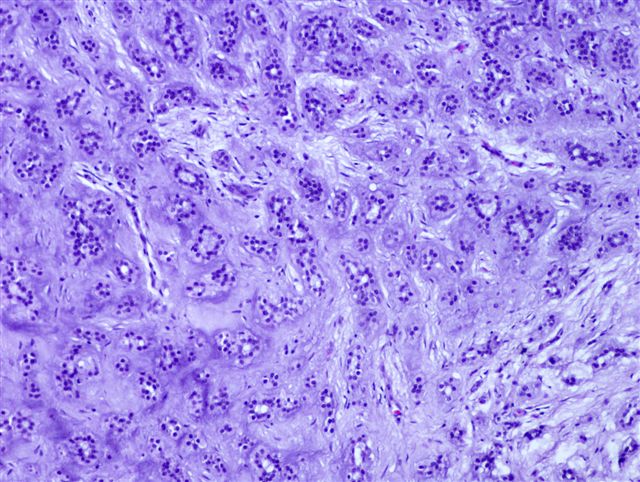
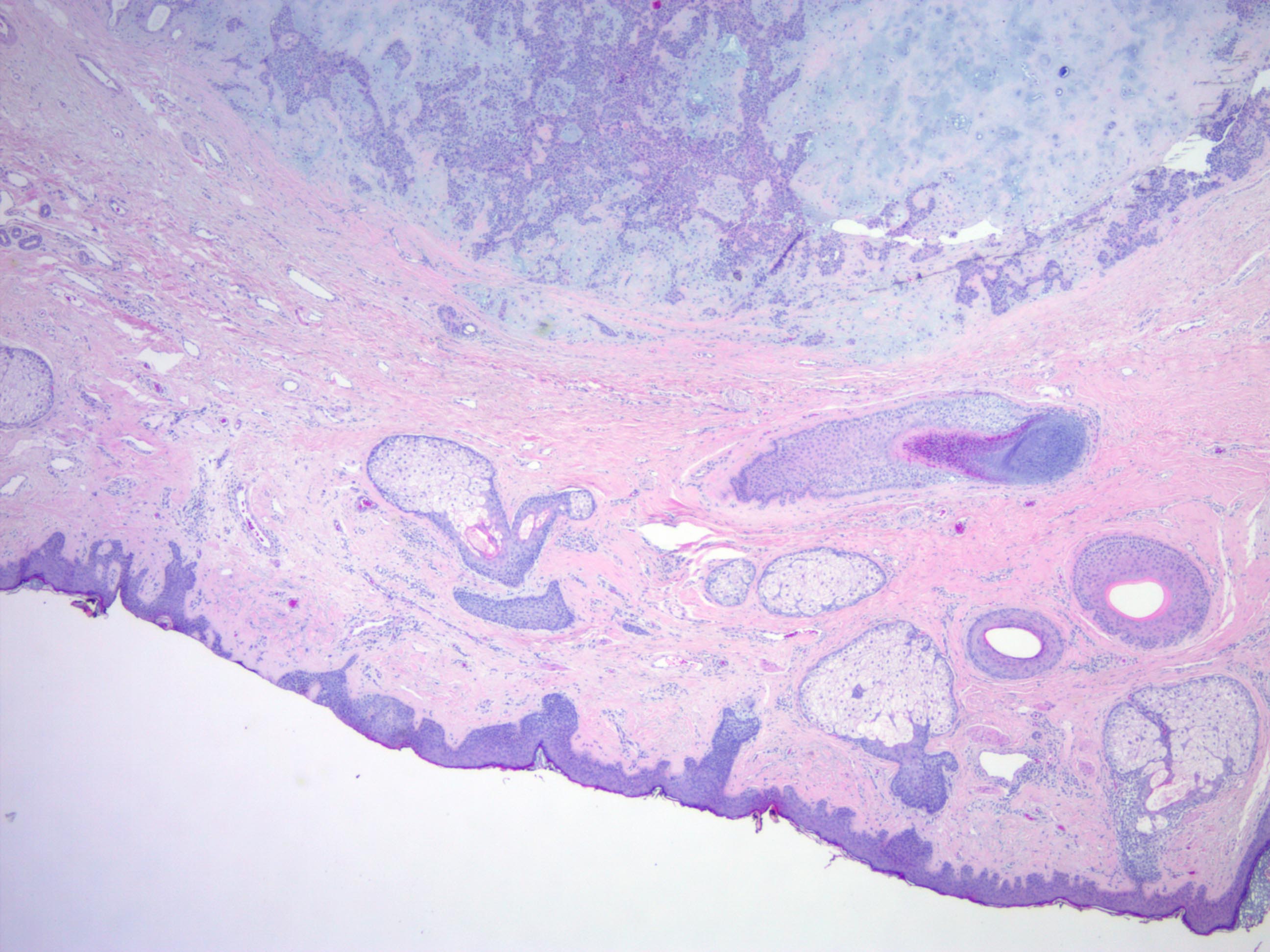
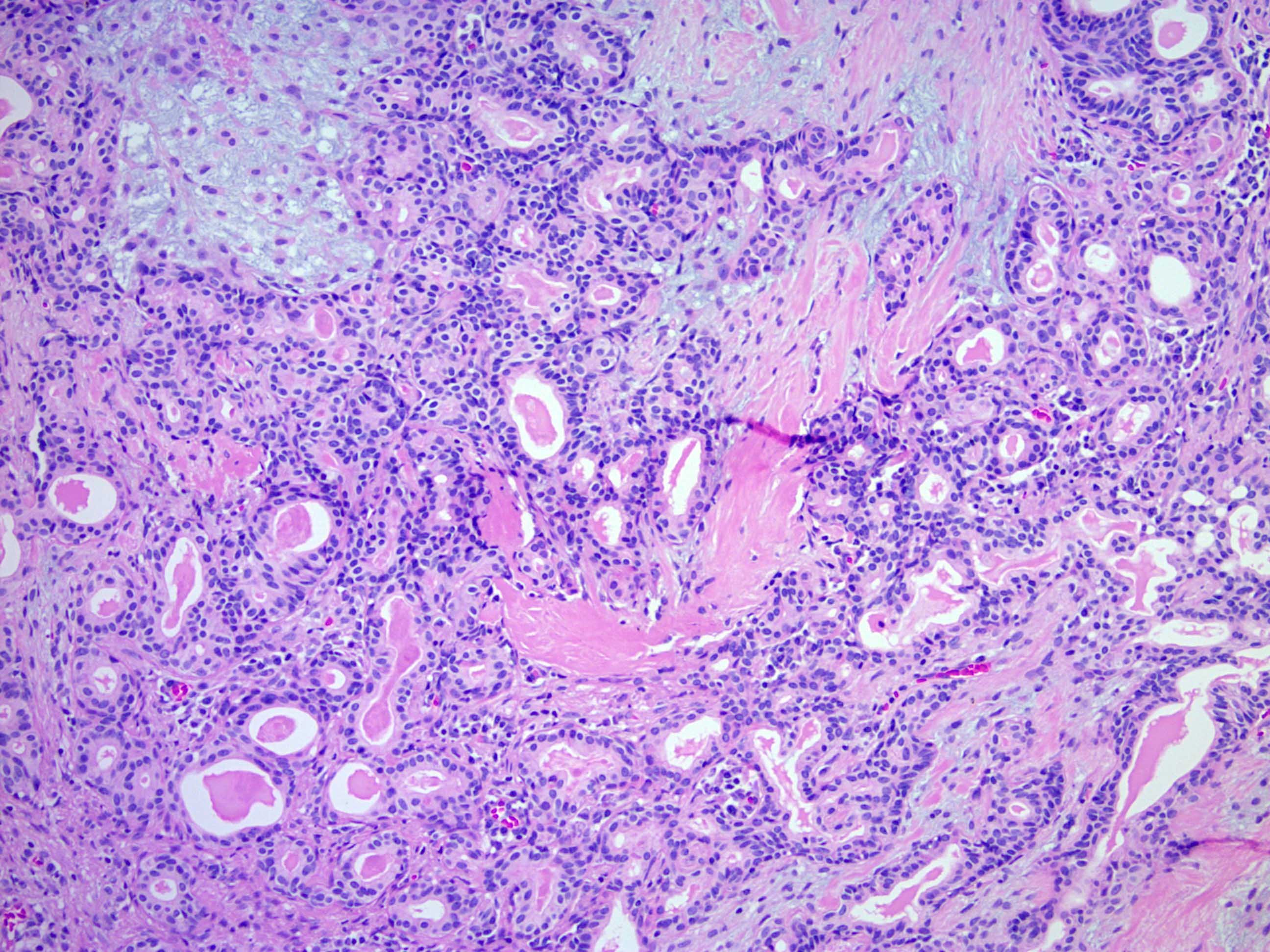
Microscopic (histologic) description
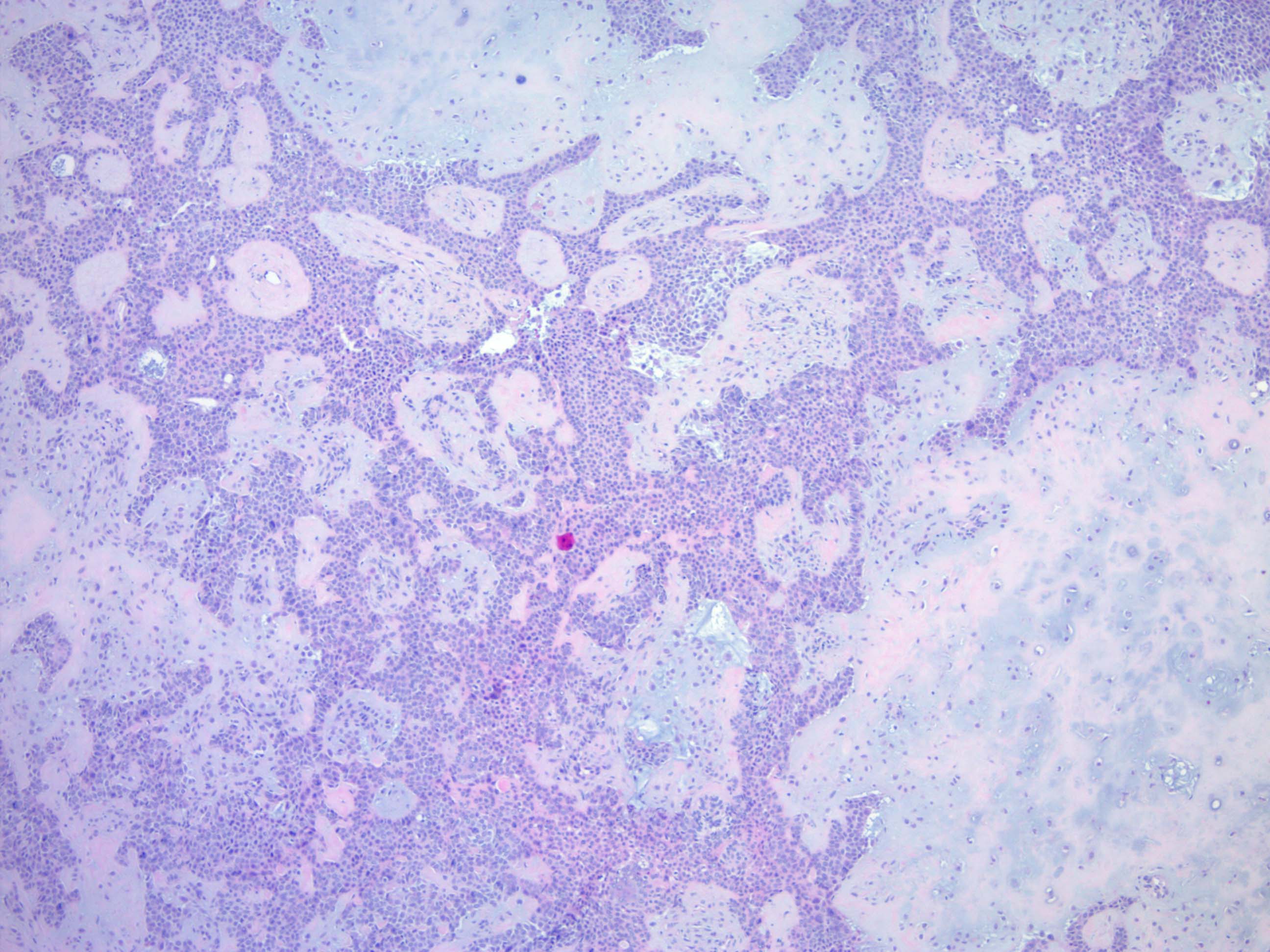
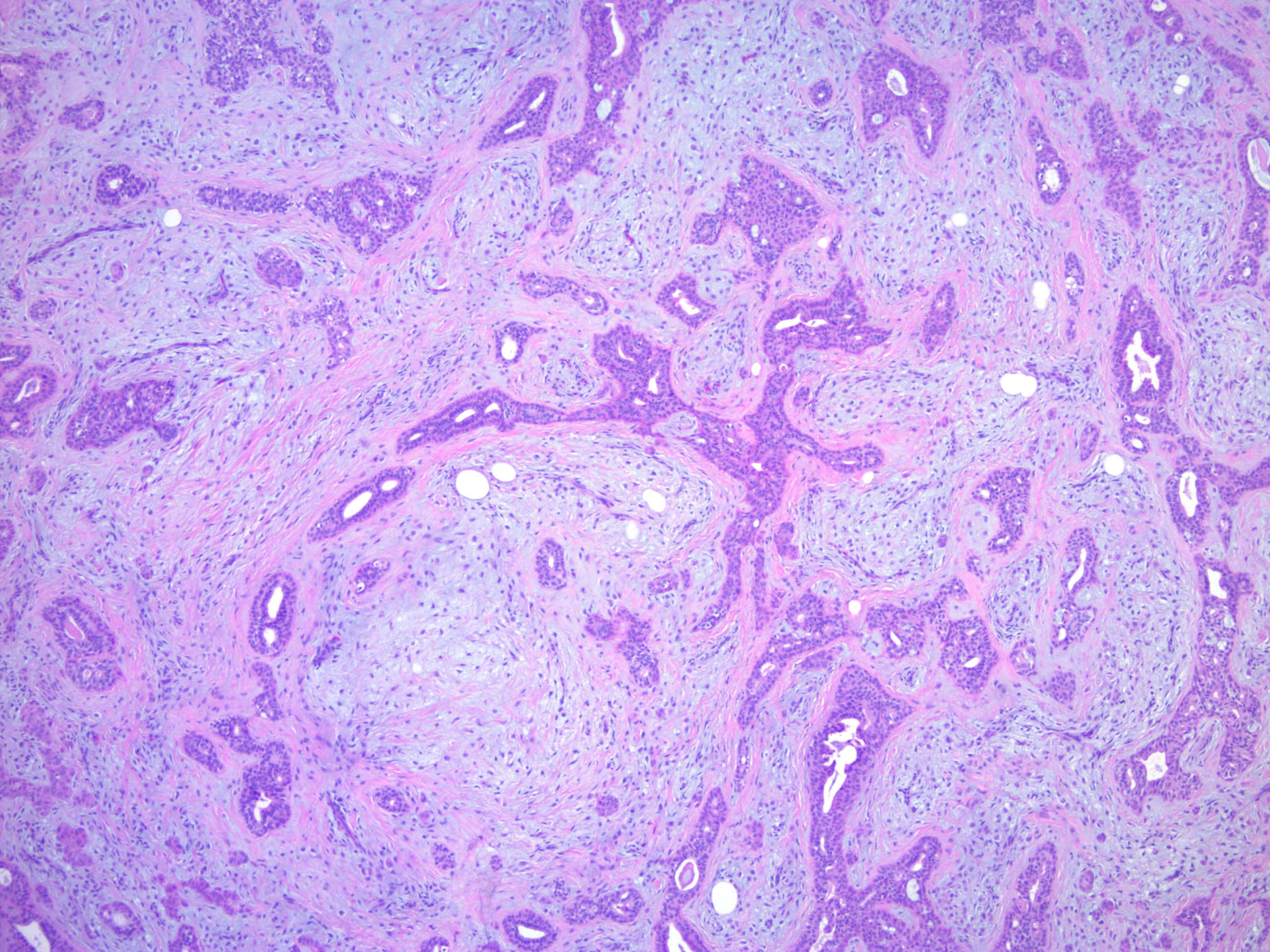
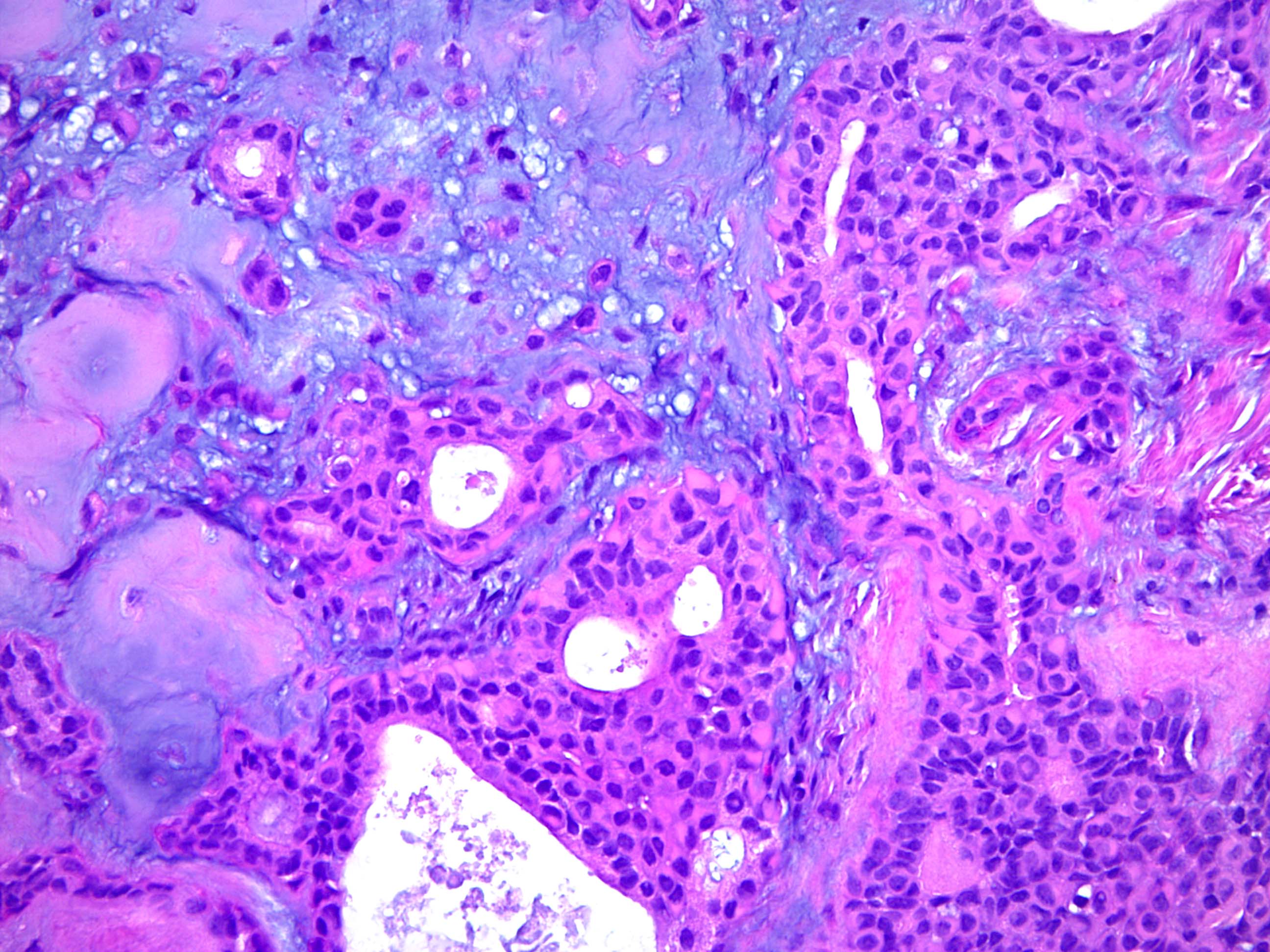
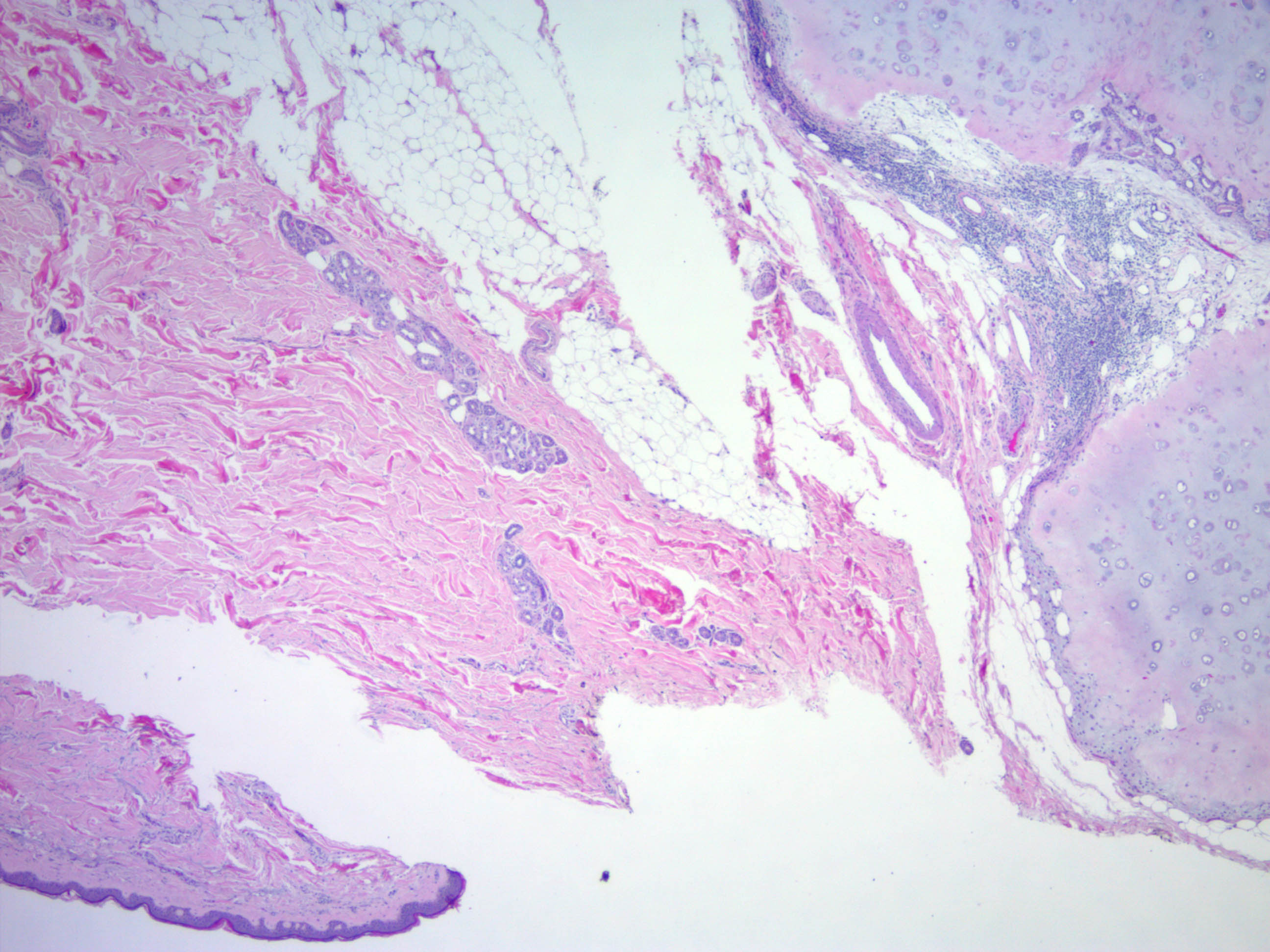
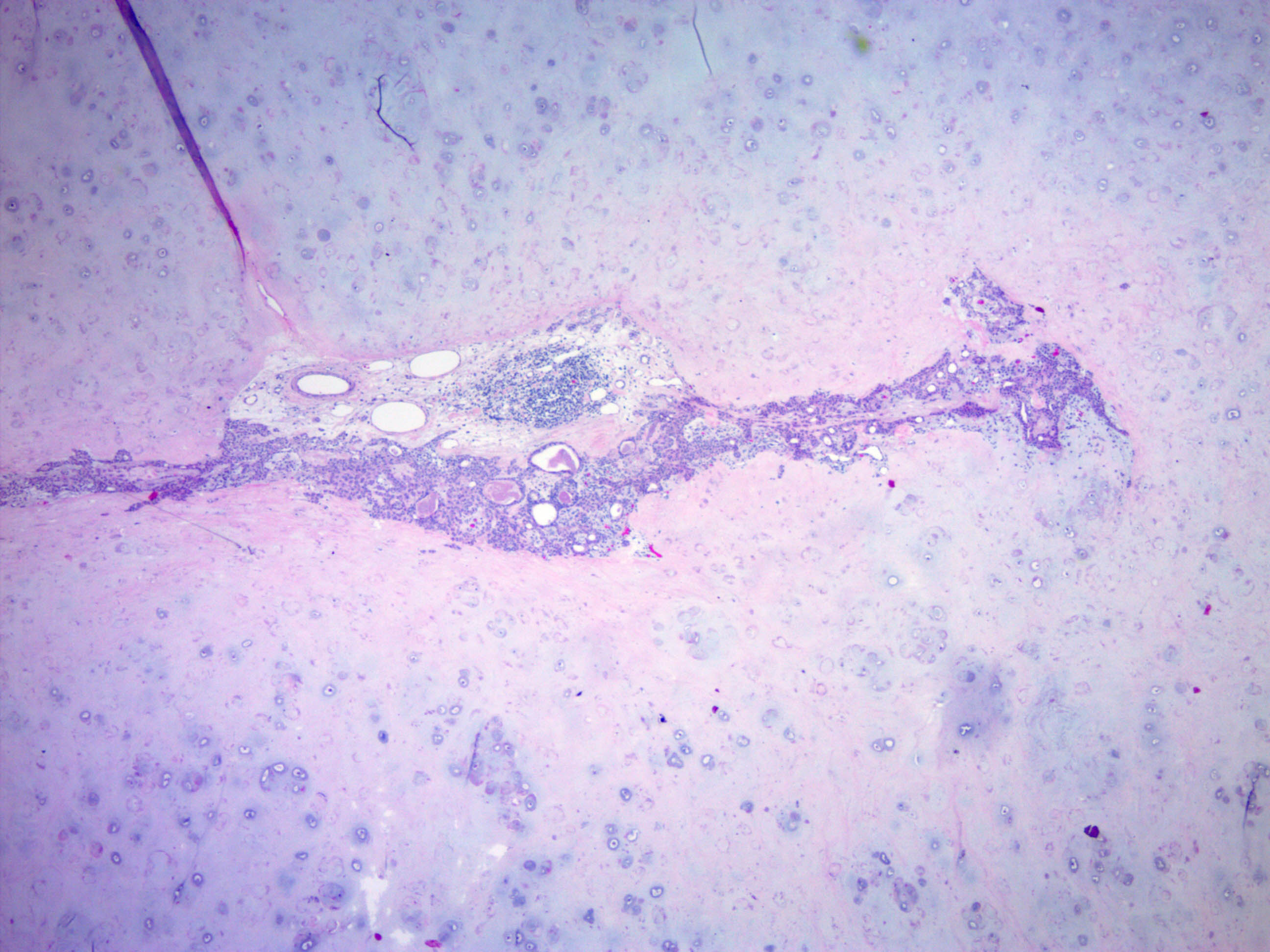
- Well circumscribed but unencapsulated, multilobulated mass (separated by fibrous septae) centered in deep dermis or subcutaneous fat, with a prominent chondroid or myxoid stroma enveloping benign bland appearing epithelial and myoepithelial cells that form secondary structures (glands, ducts, cysts, reticular lace-like networks, nests)
- Biphasic, with both epithelial and stromal components
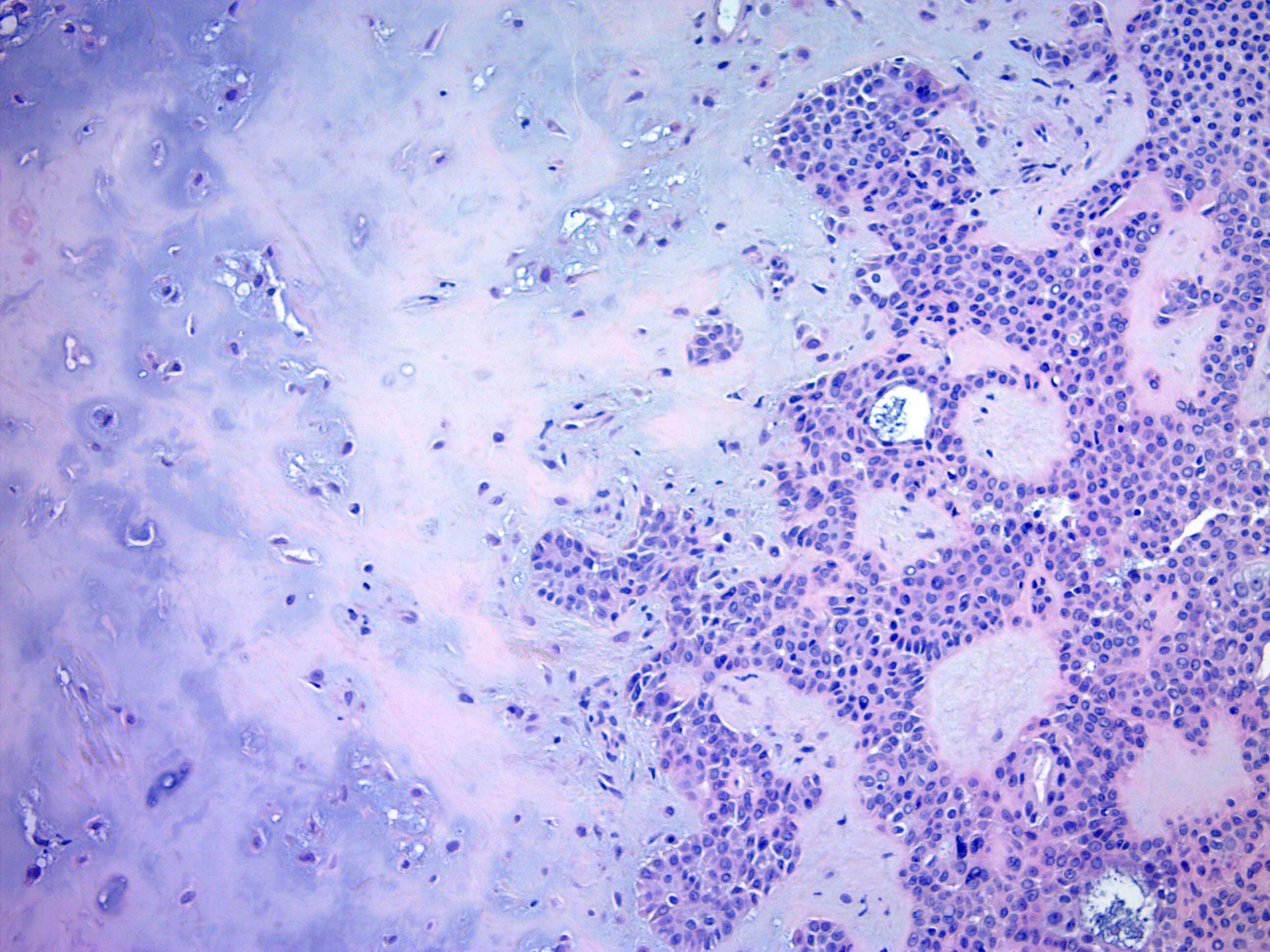
- Epithelial: elongated branching tubular structures with 2 cell layers
- Outer layer is cuboidal or columnar; inner layer is columnar with eosinophilic cytoplasm
- Decapitation type secretion, often squamous metaplasia and basal nuclei
- Often has areas of apocrine, follicular and sebaceous differentiation
- Presence of any follicular or sebaceous features rules out pure eccrine mixed tumor
- Wide array of morphologies can be seen with all types of differentiation along the folliculosebaceous unit
- Occasional cases have neoplastic tubules connecting directly to normal follicular infundibulum
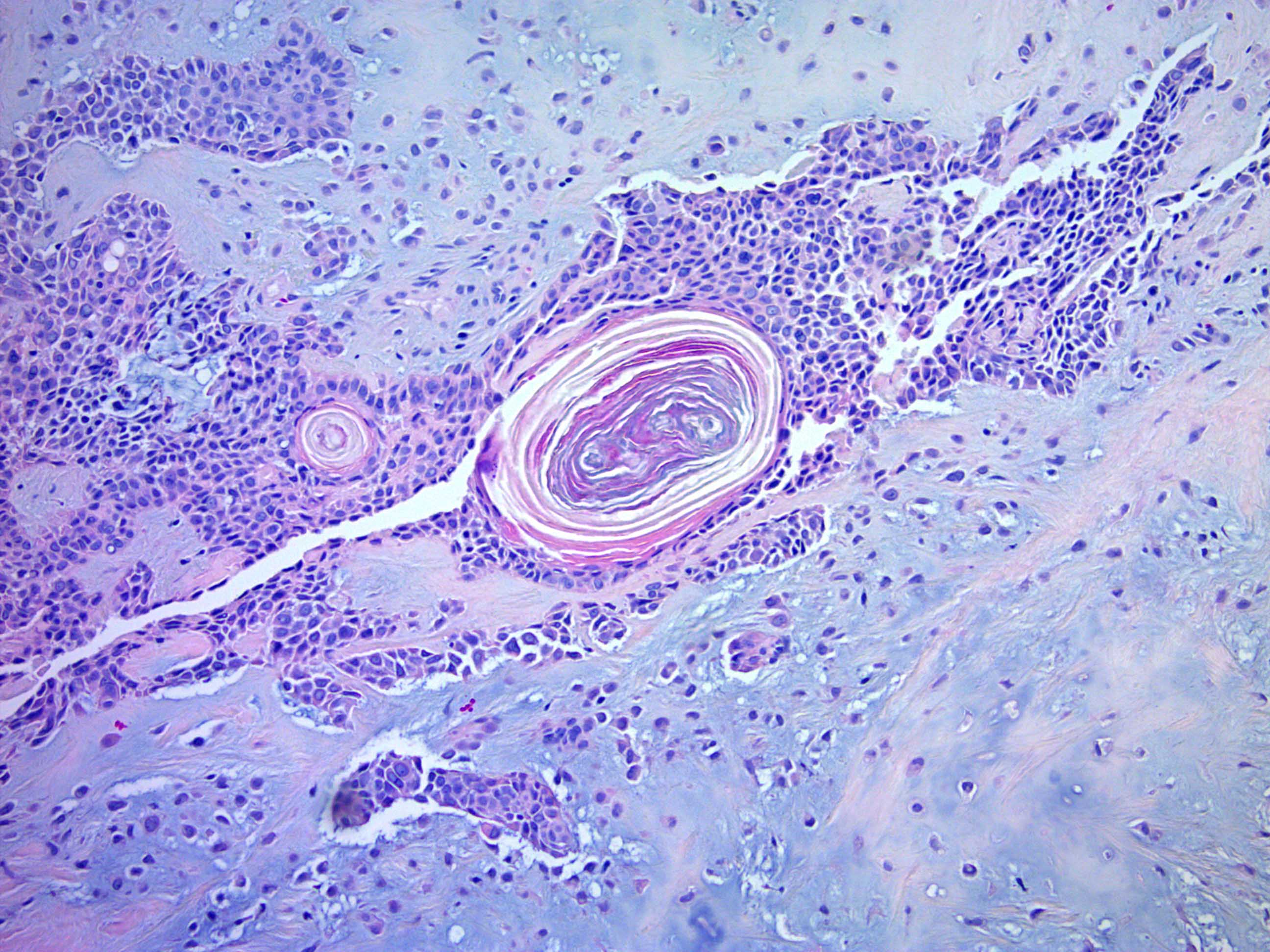
- Follicular differentiation is the most common type of change in the epithelium and may include:
- Keratinous cysts with infundibular keratinization
- Isthmic epithelium
- Hair bulbs with papillary mesenchyme
- Matrical differentiation with basaloid, transitional and ghost cells
- Trichohyaline granules
- Vellous hair shafts
- Anagen differentiation
- Sebaceous differentiation may include single cells or islands of mature sebocytes
- Metaplastic epithelial changes include clear cell change, columnar change, cytoplasmic vacuolization (may be abortive luminal differentiation), hobnailing, mucinous, oxyphilic, squamous
- Metaplastic myoepithelial changes include clear cell change, collagenous spherulosis, hyaline cells, plasmacytoid cells, spindled cells
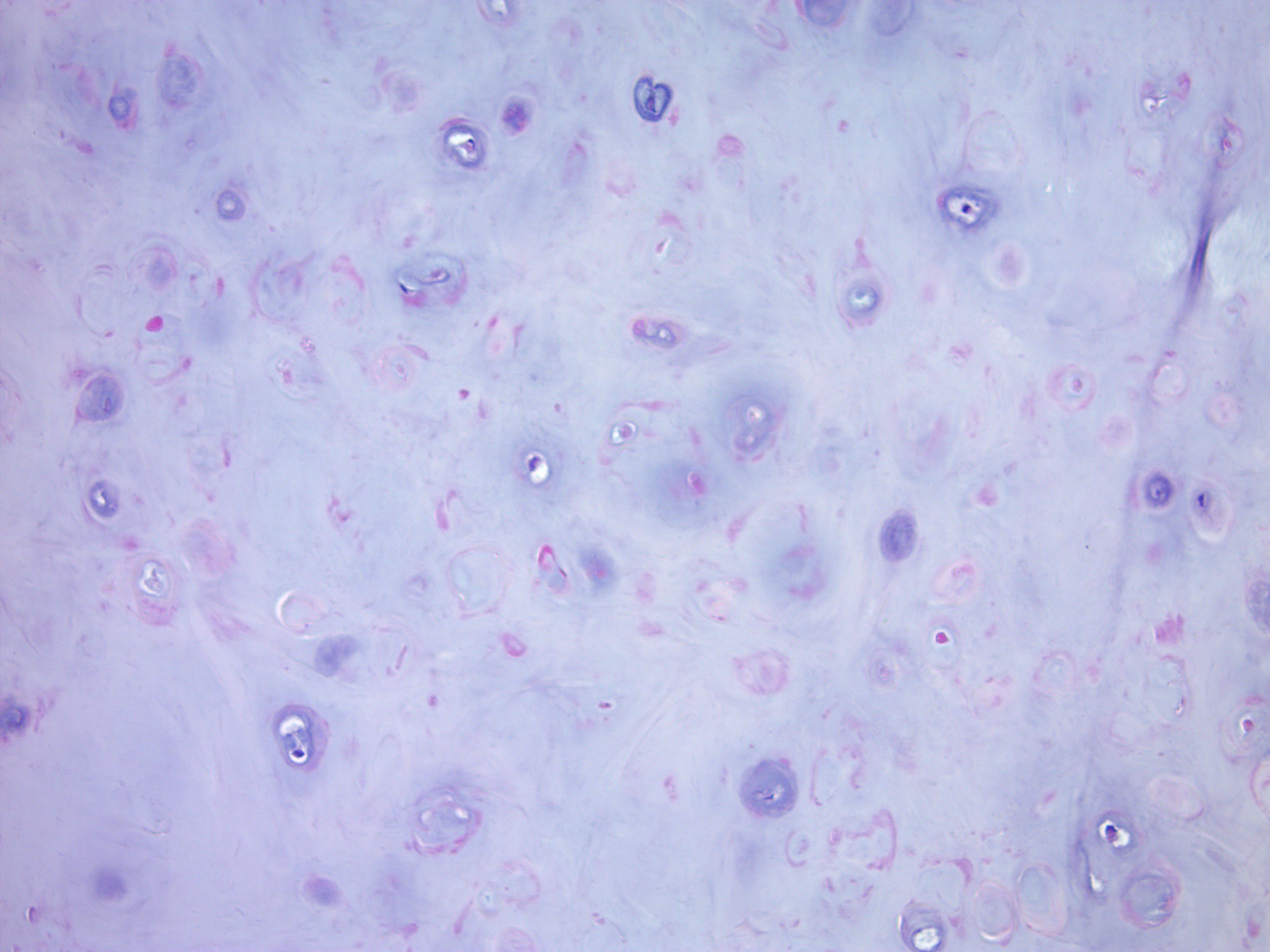
- Stroma: chondroid, mucoid or focally fibrous / hyaline
- Metaplastic stromal changes include adipocytic, chondroid and osseous metaplasia
- Adipocytic metaplasia may be extensive; some adipocytes may have Lochkern nuclei with a single small vacuole indenting the nucleus that resemble lipoblasts
- Epithelial: elongated branching tubular structures with 2 cell layers
- In contrast to eccrine mixed tumors, apocrine mixed tumors do not have clear cell change, cribriform areas, osseous metaplasia (prominent), physaliferous-like cells, pseudorosettes
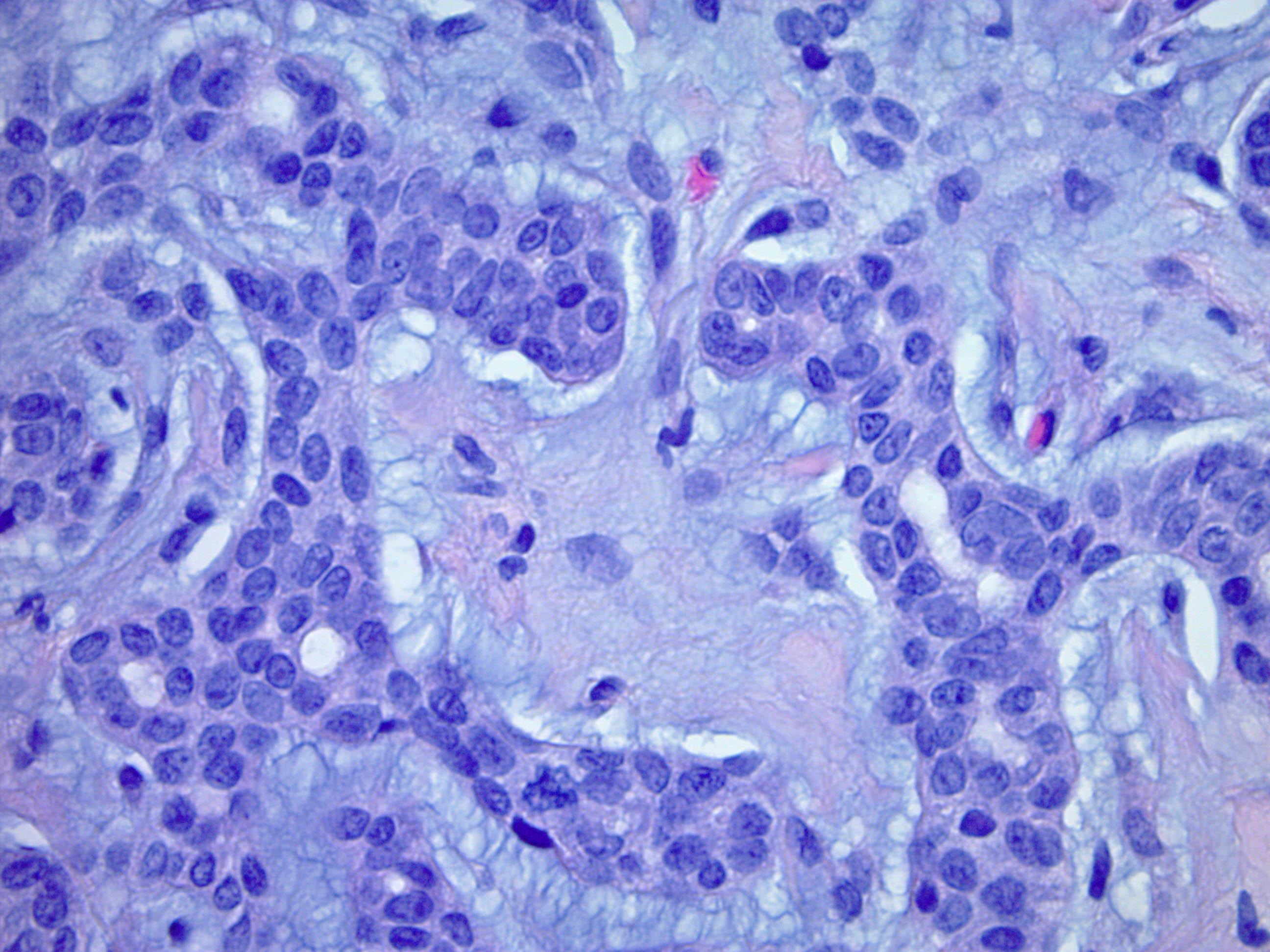
- No pleomorphism or necrosis, sparse mitoses
- Benign tumors may have:
- Asymmetry
- Mild nuclear pleomorphism within the ductal component
- Multinucleated, bizarre, hyperchromatic cells
- Infiltrative or pushing borders
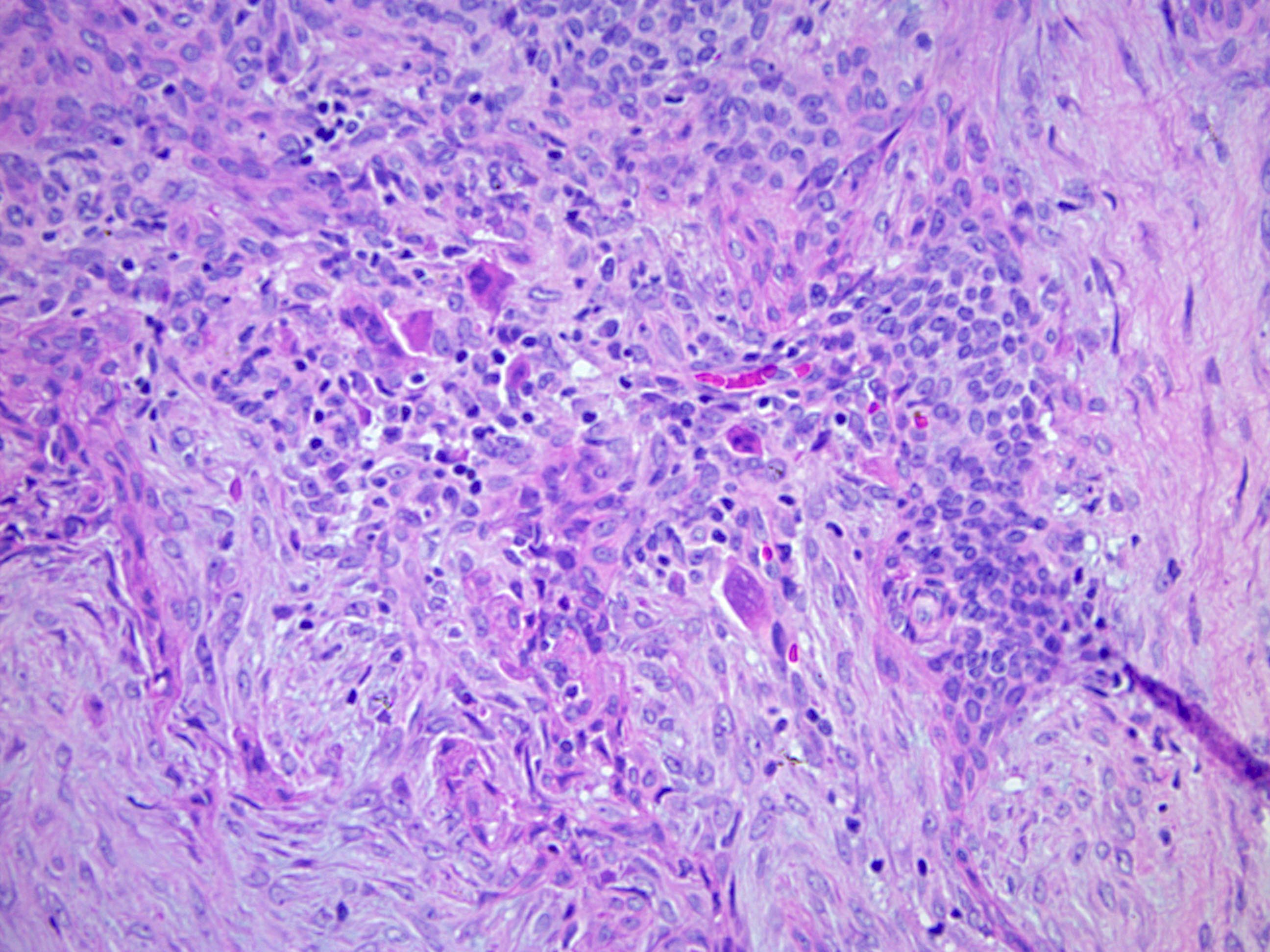
- Features suggestive of malignant transformation include:
- Severe atypia
- Markedly increased cellularity
- Increased mitotic rate
- Destruction of surrounding tissue
- Tumor necrosis
- Reference: Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Microscopic (histologic) images
Cytology description
- Many epithelial cells with bland nuclei in a chondromyxoid background
- Marked nuclear pleomorphism and prominent nucleoli may be used to identify malignant features on fine needle aspiration (Acta Cytol 1998;42:1155)
- Findings for diagnosis of chondroid syringoma on fine needle aspiration (Acta Cytol 2010;54:183)
- Fibrillary chondromyxoid background
- Prominent cellular component of epithelial and myoepithelial cells
- Absence of atypia and tumor diathesis (however, histology still required for definitive exclusion of malignant features)
Positive stains
- Immunohistochemistry generally not required for diagnosis
- Inner epithelial layer: keratin, EMA, CEA, GCDFP-15, actin, cytokeratin 15
- Outer myoepithelial layer / stromal component: S100, SOX10, NSE, GFAP, SMA, calponin, p63
- Variable SMA, GFAP
- p53 positivity of varying intensity
- PLAG1 nuclear expression in apocrine mixed tumors (Am J Dermatopathol 2020;42:251)
Negative stains
- Desmin (usually) (J Clin Pathol 2007;60:145)
- p16 negative, in contrast to strong nuclear positivity in malignant chondroid syringoma (CRCP 2015;2:28)
Molecular / cytogenetics description
- Positive for gene rearrangement of PLAG1 in largest study to date but lacks the characteristic translocations of PLAG1 seen in pleomorphic adenoma of salivary gland (Genes Chromosomes Cancer 2012;51:140, Virchows Arch 2011;459:539)
- Small subset with ESWR1 rearrangement (Mod Pathol 2011;24:1444)
Sample pathology report
- Skin, nose, biopsy:
- Chondroid syringoma (benign cutaneous mixed tumor)
- Microscopic description: Histologic sections demonstrate a well circumscribed nodular dermal (subcutaneous) neoplasm. The tumor consists of cords and islands of banal epithelial cells set in a variably fibrous and chondromyxoid stroma, creating a biphasic appearance. Ductal structures are present, with inner epithelial and outer myoepithelial layers evident. Cystic (or other metaplastic) change is seen. There is minimal nuclear atypia and mitoses are not abundant.
Differential diagnosis
- Cutaneous chondroma:
- Lacks the epithelial and myoepithelial elements
- Malignant mixed tumor / malignant chondroid syringoma:
- Necrosis, infiltrative growth, marked pleomorphism and greater cellularity
- Metastatic carcinoma:
- Can often be distinguished based on immunohistochemistry and cytologic features of malignancy
- Parachordoma:
- Rare, no true ducts, actin negative, presence of physaliferous cells, arises adjacent to tendon and synovium in extremities
- Now often considered a variant of myoepithelioma
- Collagenous spherulosis:
- More common in breast, lumina contain dense eosinophilic secretion, no biphasic appearance
- Hidradenoma papilliferum:
- No chondroid matrix, not biphasic
- Myopeithelioma / adenomyoepithelioma of the skin:
- More common on extremities, myoepithelial areas should predominate, lacks luminal epithelial cells
- Negative for cytokeratin and CEA
- Other adnexal tumors with apocrine differentiation
Practice question #1
Features that are most suggestive of malignant behavior in mixed tumor are
- Asymmetry, osseous metaplasia and scattered pleomorphic cells
- Cytoplasmic vacuolization and plasmacytoid cells
- Increased cellularity and necrosis
- Infiltrative borders and asymmetry
- Infiltrative borders and bizzare, multinucleated cells
Practice answer #1
C. Increased cellularity and necrosis. Benign tumors may exhibit asymmetry, infiltrative borders, mild atypia, metaplasia and multinucleated cells. Features that suggest malignant mixed tumor are prominent pleomorphism / atypia, necrosis, increased cellularity and mitoses with tissue destruction.
Comment Here
Reference: Chondroid syringoma
Comment Here
Reference: Chondroid syringoma
Practice question #2
Most common molecular abnormality seen in chondroid syringoma is
- EWSR1 amplification
- EWSR1 rearrangement
- HMGA2 fusion
- PLAG1 deletion
- PLAG1 rearrangement
Practice answer #2
E. PLAG1 rearrangement. Although the characteristic PLAG1 or HMGA2 fusions seen in pleomorphic adenoma of salivary glands are not seen in chondroid syringoma, PLAG1 rearrangement has been detected in a subset of cutaneous mixed tumors. Nuclear immunohistochemical expression of PLAG1 is also seen. A subset of tumors show EWSR1 rearrangement.
Comment Here
Reference: Chondroid syringoma
Comment Here
Reference: Chondroid syringoma