Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Lérias S, Lerwill M. Solid papillary carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantsolidpapillarycarcinoma.html. Accessed September 17th, 2025.
Definition / general
- Subtype of ductal carcinoma characterized by solid cellular nodules punctuated by thin fibrovascular cores
- Frequently demonstrates neuroendocrine differentiation
- May be either in situ or invasive
Essential features
- Circumscribed solid growth pattern with interspersed delicate fibrovascular cores
- Low or intermediate grade nuclear atypia
- Neuroendocrine differentiation is common
- In situ carcinoma is diagnosed when the tumor is composed of well circumscribed solid nests in a distribution pattern consistent with an intraductal process; myoepithelial cells may or may not be demonstrable at the periphery
- Invasive carcinoma is diagnosed when there is a lack of myoepithelium and a convincing pattern of infiltrative growth
- ER+ / PR variable / HER2-
- Associated with an excellent prognosis (Breast 2016;26:67)
Terminology
- In situ or invasive nature of the tumor should be specified
- Also called neuroendocrine breast carcinoma in situ, spindle cell ductal carcinoma in situ, neuroendocrine ductal carcinoma in situ, endocrine ductal carcinoma in situ
ICD coding
- ICD-O: 8509/2 - solid papillary carcinoma in situ
- ICD-O: 8509/3 - solid papillary carcinoma with invasion
- ICD-10: C50 - malignant neoplasm of breast
- ICD-11: 2E65.Y & XH0134 - other specified carcinoma in situ of breast & solid papillary carcinoma in situ
- ICD-11: 2C64 - solid papillary carcinoma of breast with evidence of invasion
Epidemiology
- Postmenopausal women (median age = 73 years) (Am J Surg Pathol 1995;19:1237)
Sites
- Any part of the breast but the central / subareolar region is most common
Pathophysiology
- Unknown
Etiology
- No specific etiologic factors
Clinical features
- May present as a mammographic abnormality, palpable mass or bloody nipple discharge (Am J Surg Pathol 1995;19:1237)
Diagnosis
- Diagnosed by histologic examination of tissue removed by biopsy or surgical excision
Radiology description
- On mammography, solid papillary carcinoma can appear as a lobulated, circumscribed mass, with or without calcifications
- Margins may be indistinct in invasive cases (AJR Am J Roentgenol 2012:198;264)
- Ultrasonography may demonstrate a hypoechoic or heterogeneous solid mass (AJR Am J Roentgenol 2012:198;264)
- Magnetic resonance imaging findings include mass enhancement with circumscribed margins, oval or irregular shapes, heterogeneous signal intensity and rapid enhancement in initial contrast phases (BMC Cancer 2017;17:525)
Prognostic factors
- Excellent prognosis
- Axillary lymph node metastasis (6%) and distant metastasis are rare and appear limited to cases with an overt invasive component (Breast 2016;26:67)
Case reports
- 46 year old woman with a left breast mass (Surg Case Rep 2020;6:143)
- 73 year old woman with synchronous bilateral solid papillary carcinomas (Case Rep Surg 2013;2013:812129)
- 86 year old woman with a solid papillary carcinoma diagnosed by fine needle aspiration (Diagn Cytopathol 2020;48:53)
Treatment
- Solid papillary carcinoma in situ is a form of ductal carcinoma in situ and is managed as such
- Surgical excision
- Radiation therapy in the setting of breast conserving surgery
- Consideration of endocrine therapy for risk reduction
- Invasive solid papillary carcinoma is managed as an invasive breast carcinoma
- Surgical excision and sentinel node evaluation
- Radiation therapy in the setting of breast conserving surgery
- Endocrine therapy is typically recommended due to the strong ER positivity of these tumors
- Systemic therapy decisions should factor in the favorable prognosis of this histologic subtype
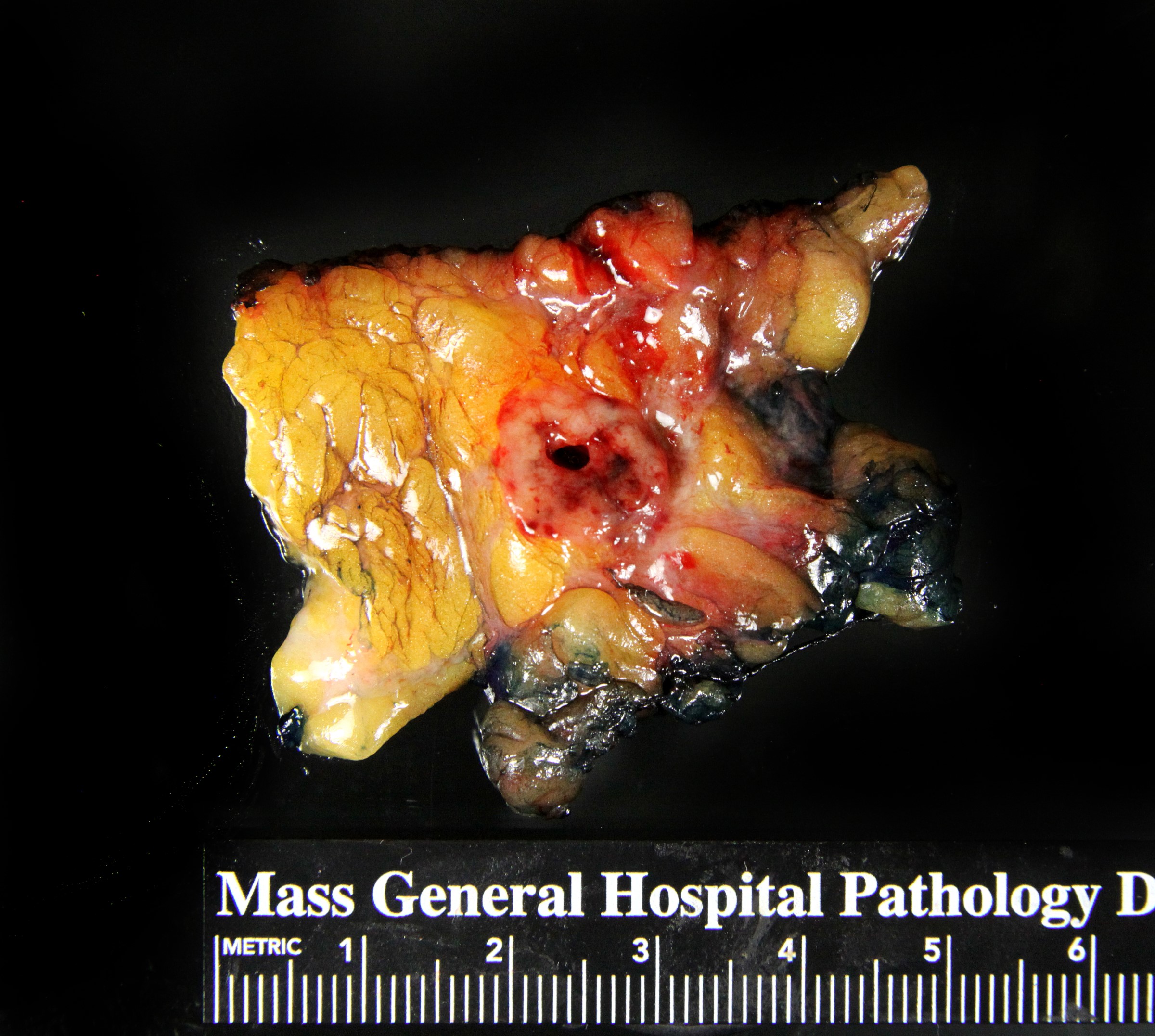
Gross description
- Mass forming examples demonstrate a well circumscribed, firm to soft tumor with tan-white to tan-pink cut surfaces (Am J Surg Pathol 1995;19:1237)
- In situ examples with less confluent growth may demonstrate scattered firm nodularity
Frozen section description
Microscopic (histologic) description
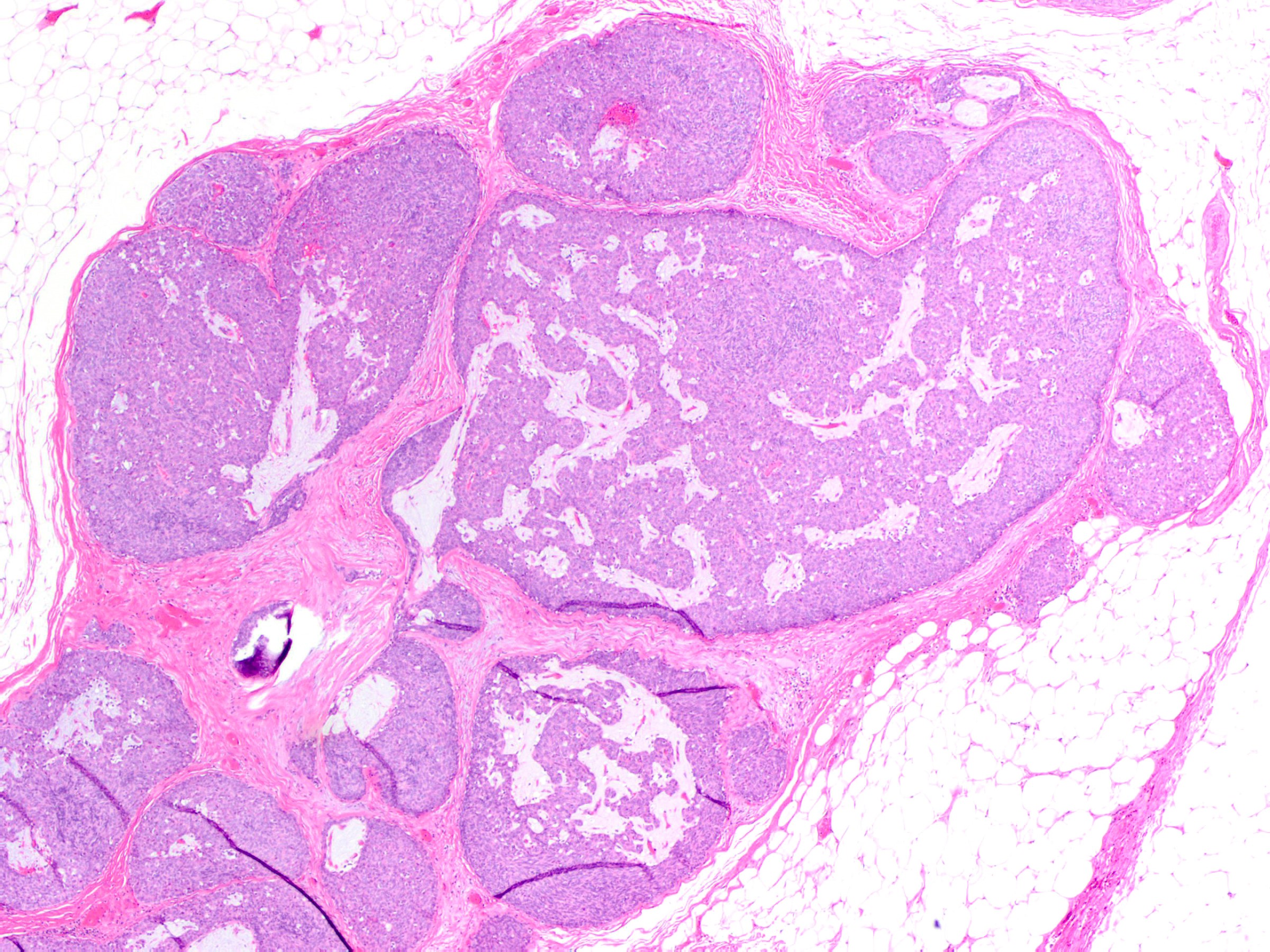
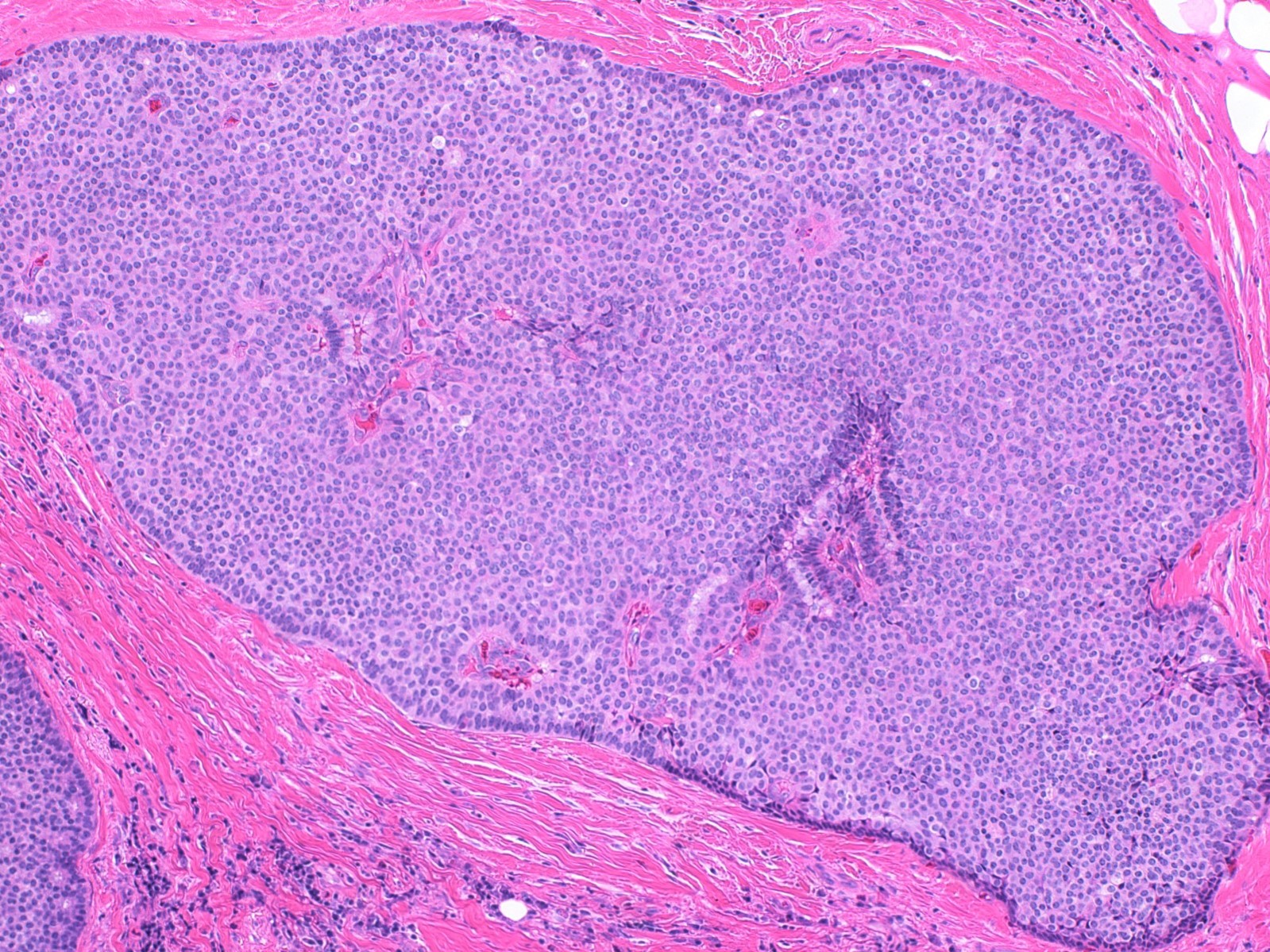
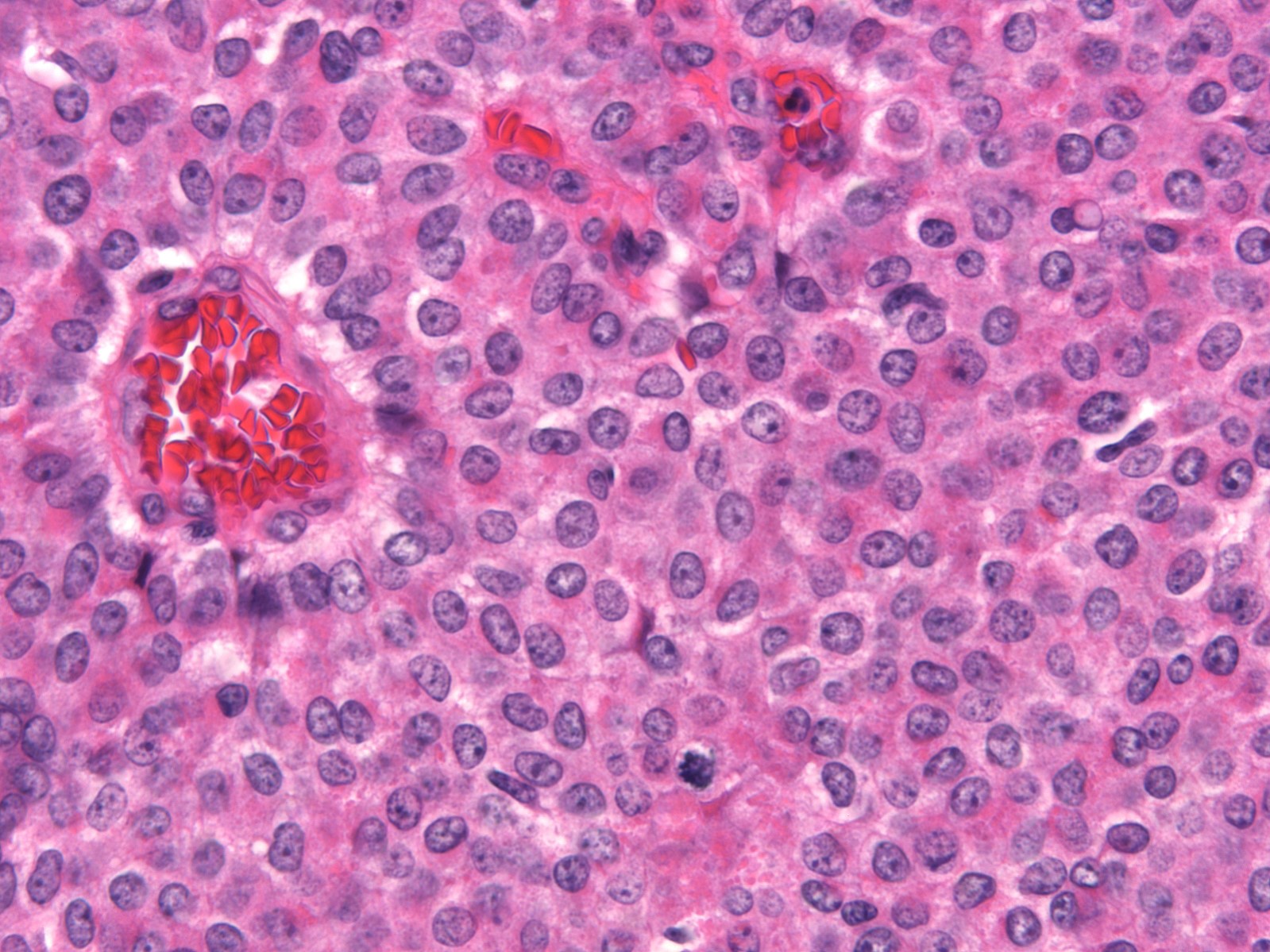
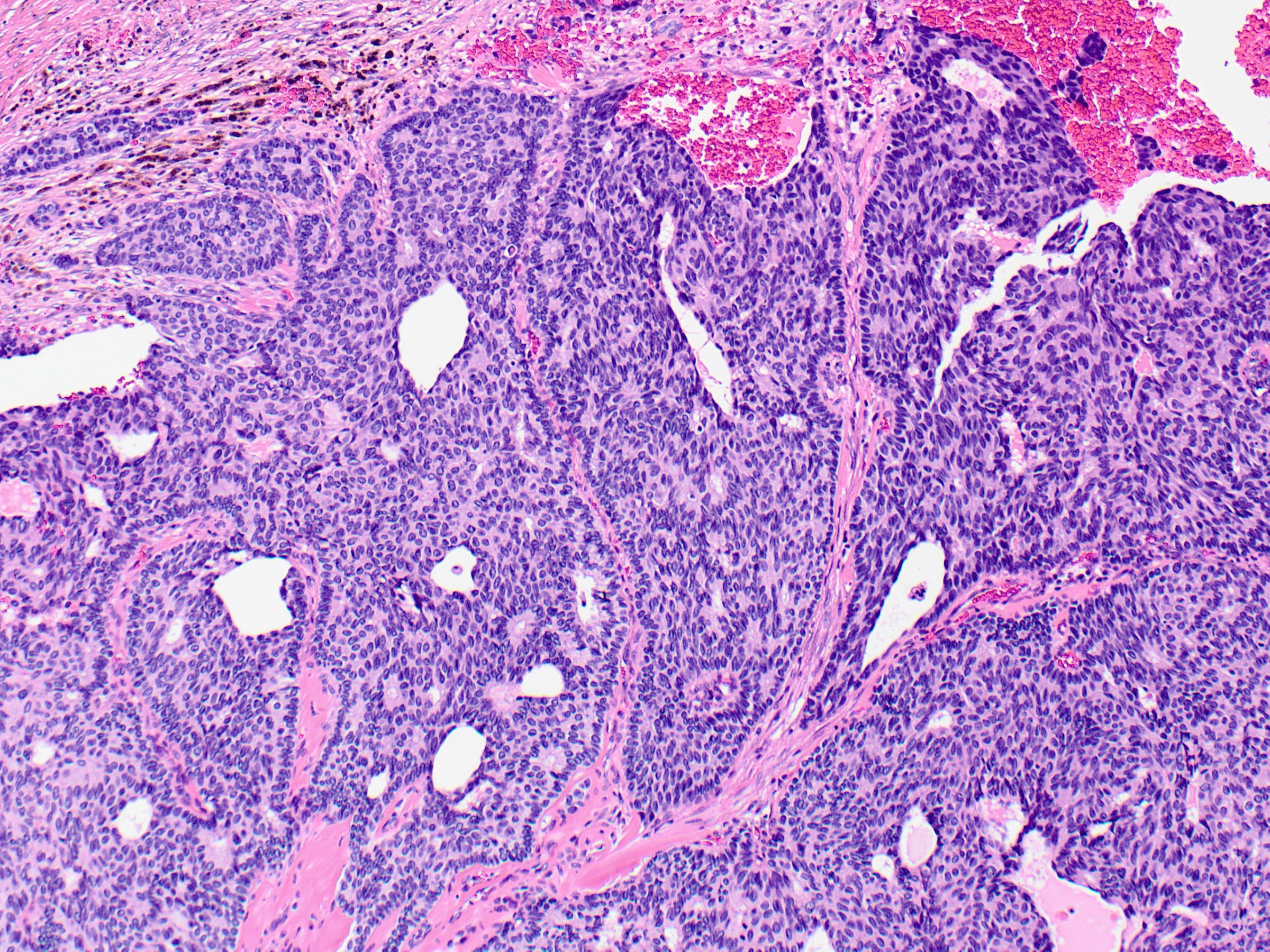
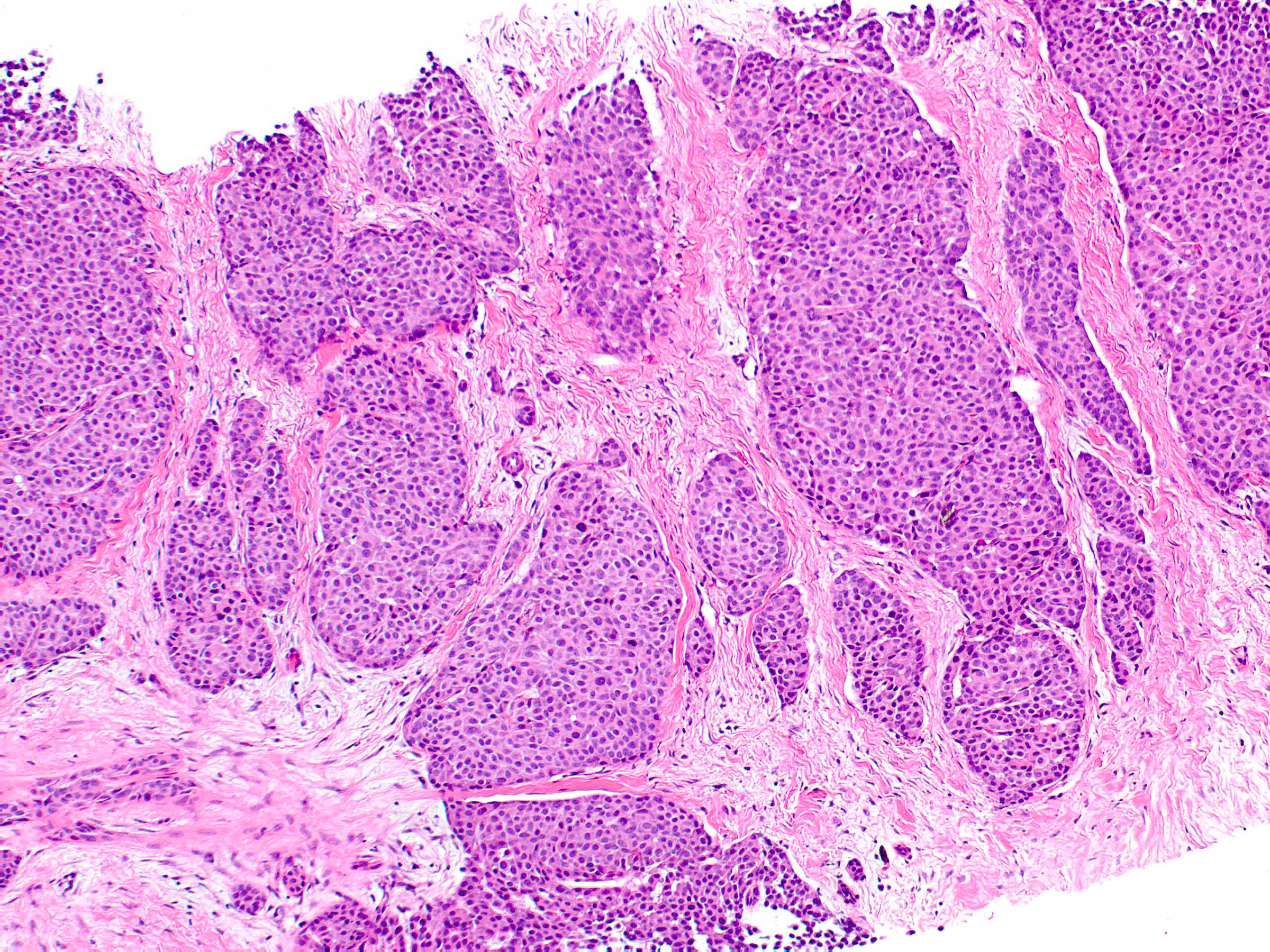
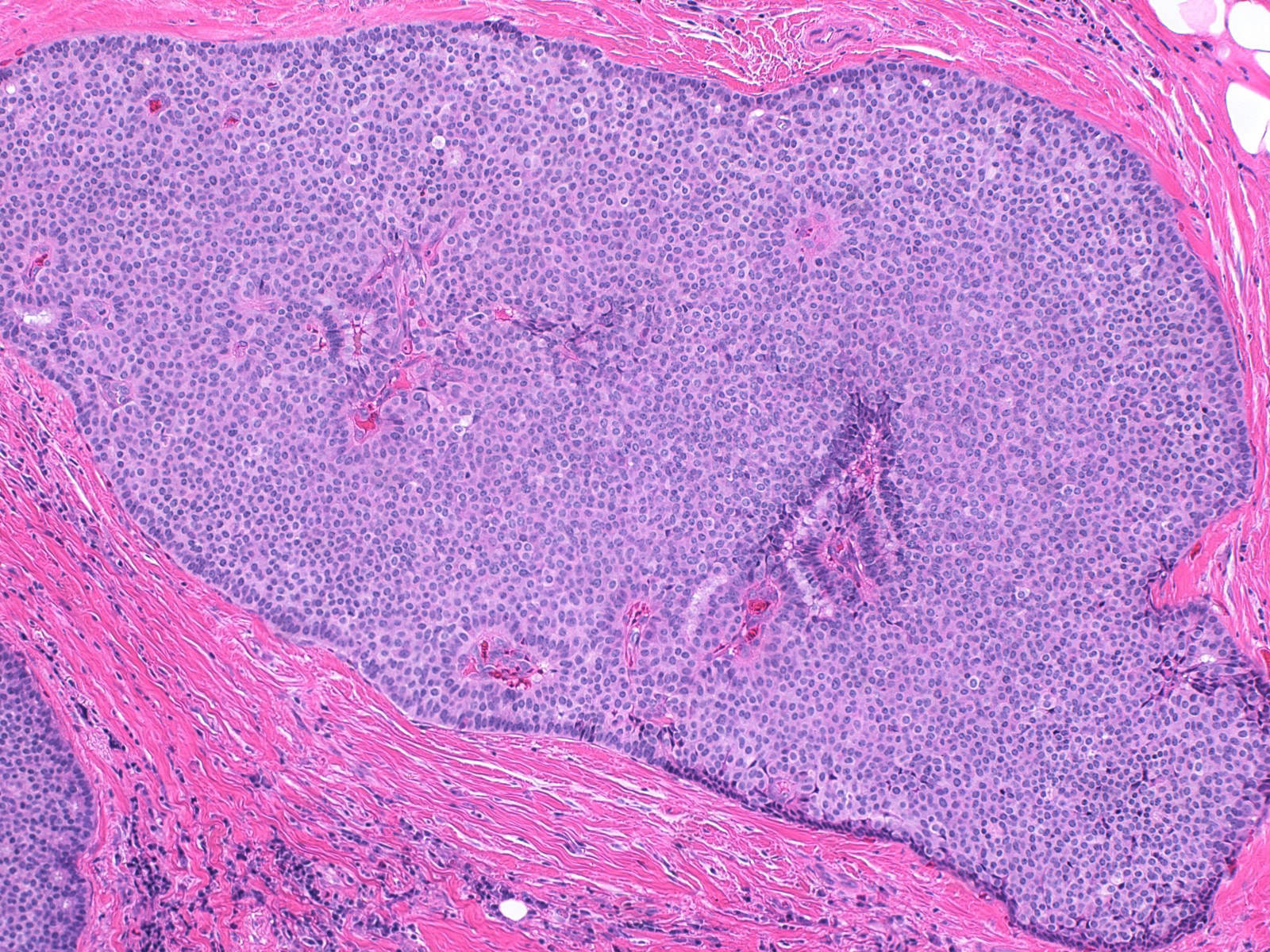
- Expansile nodules composed of a solid epithelial proliferation punctuated by delicate fibrovascular cores
- Cytologic features (Am J Surg Pathol 1995;19:1237)
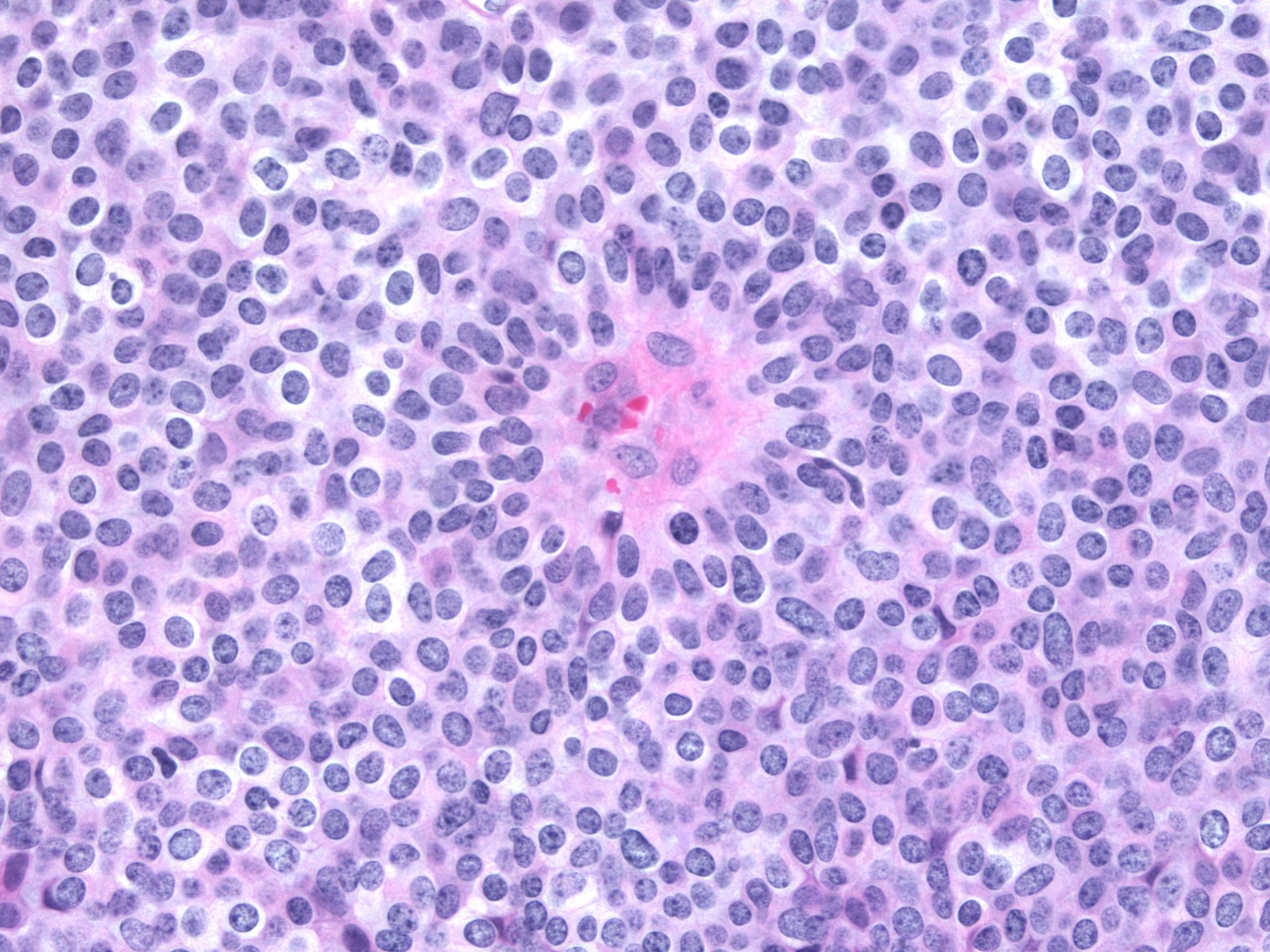
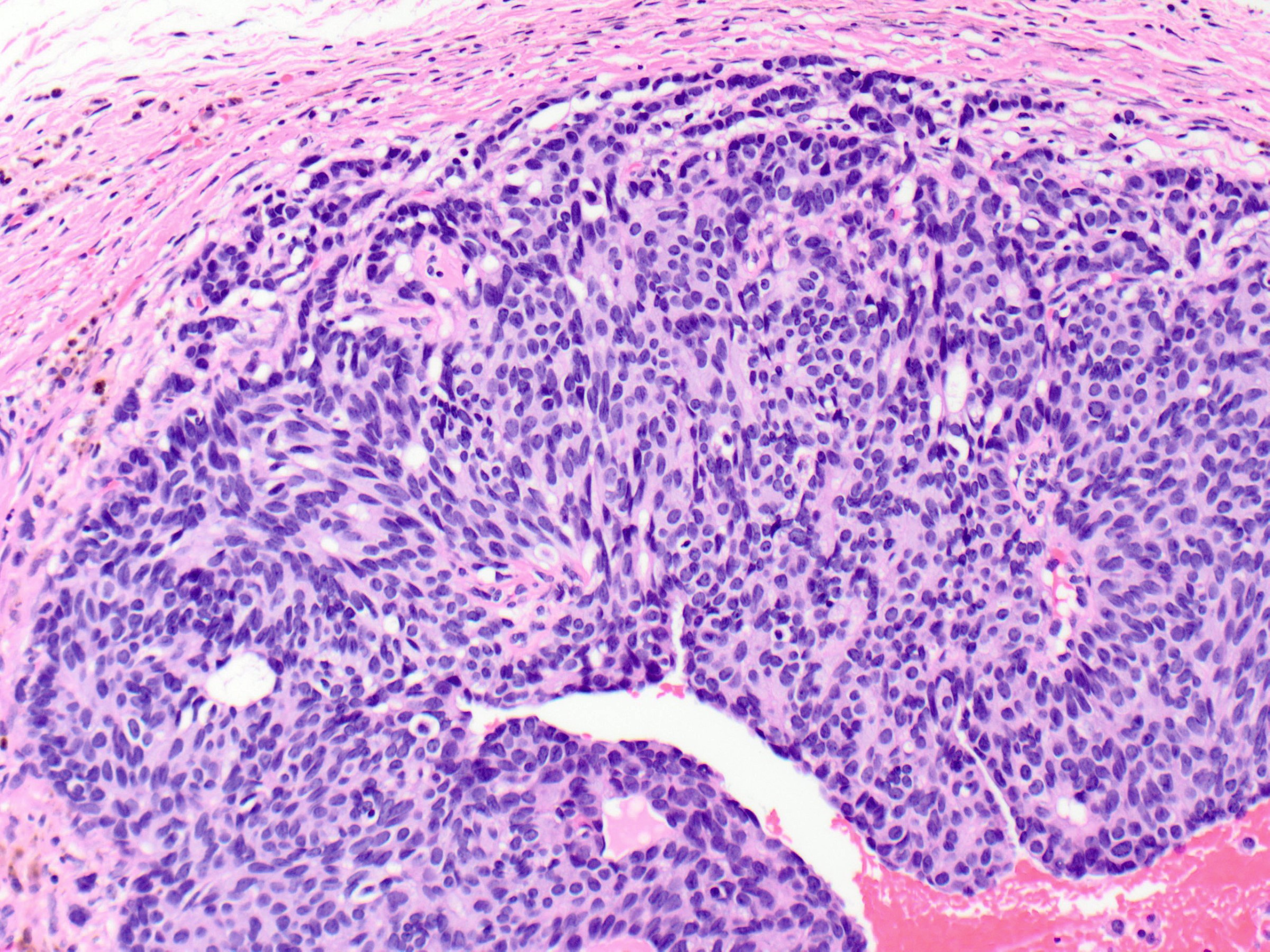
- Monomorphic cytologic appearance
- Round, oval, plasmacytoid or spindle shaped cells
- Low to intermediate grade nuclear atypia
- Mildly enlarged, round to oval nuclei with fine chromatin and indistinct nucleoli
- Some examples have slightly more open, granular chromatin and small nucleoli
- Nuclear grooves are occasionally conspicuous
- Variable mitotic activity
- Cytoplasm ranges from pale and amphophilic to eosinophilic and granular
- Intracellular and extracellular mucin are common and signet ring cells are occasionally observed
- Architectural features (Am J Surg Pathol 1995;19:1237)
- Solid epithelial growth pattern with interspersed thin fibrovascular cores
- Cellular palisading around fibrovascular cores
- Streaming growth pattern is common in cases with spindle cells
- Microcystic spaces are occasionally present
- Fibrovascular cores can demonstrate hyalinization
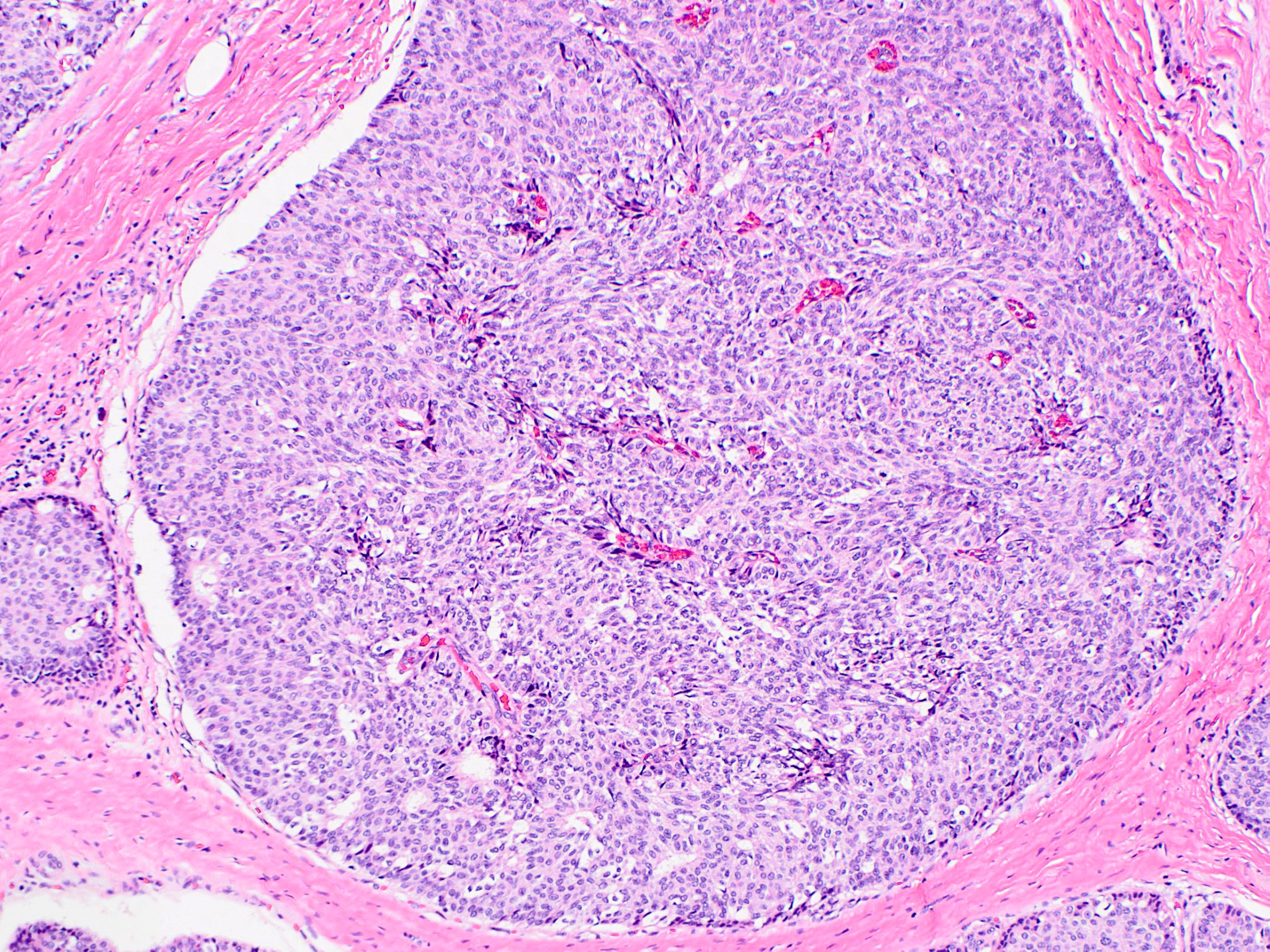
- Solid papillary carcinoma in situ
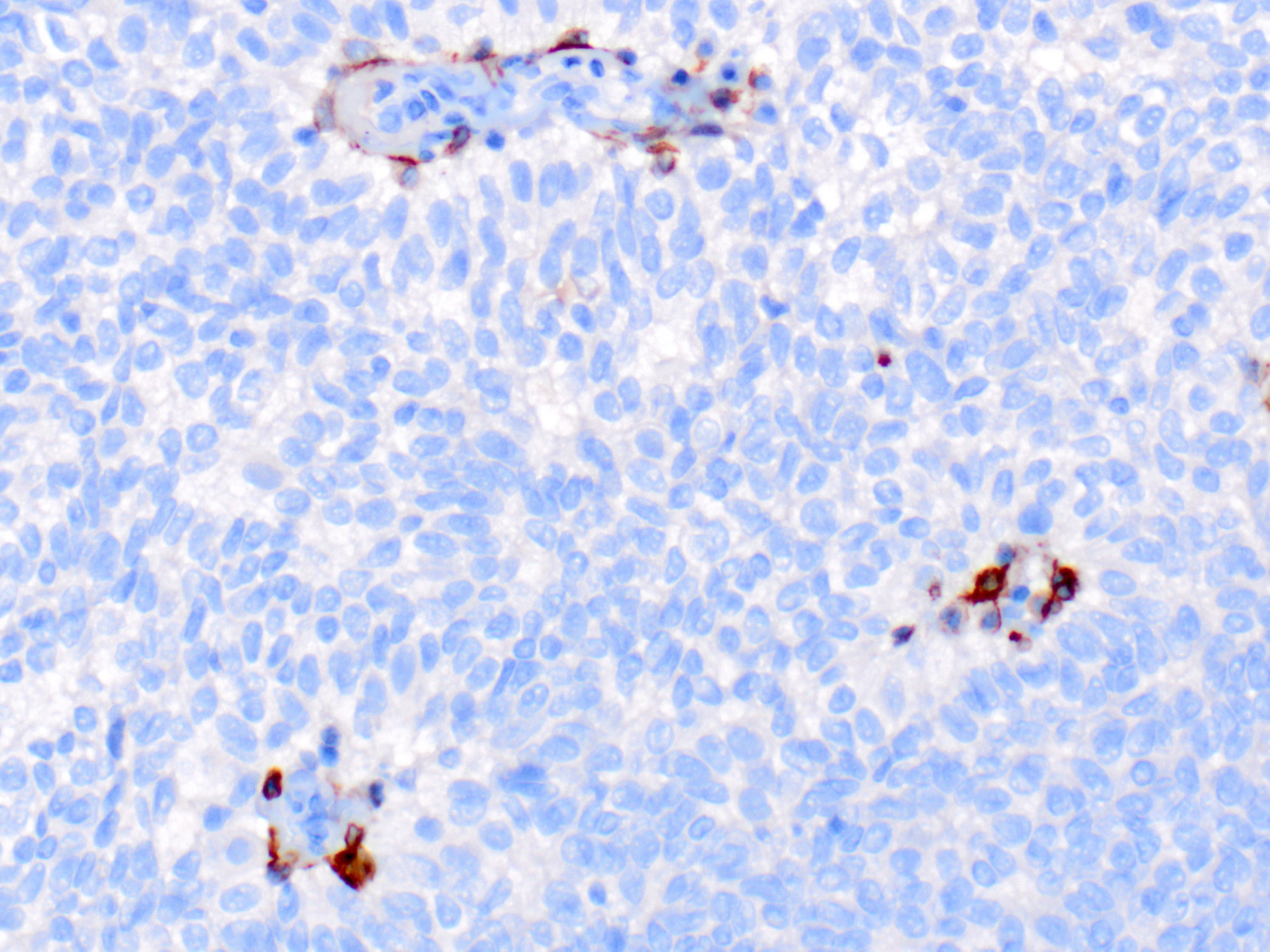
- In situ carcinoma is diagnosed when the nodules have smooth rounded contours and a distribution pattern consistent with involvement of the underlying glandular tree
- Myoepithelial cells may be retained, attenuated or absent around the perimeter of the nodules
- If the morphology is consistent with an intraductal process, in situ carcinoma (pTis) is diagnosed even if no myoepithelial cell layer is seen
- Careful histologic assessment of tumor distribution and borders is required in cases without an identifiable myoepithelial cell layer
- Myoepithelial cells are often attenuated or absent along the internal fibrovascular cores
- Graded according to nuclear features
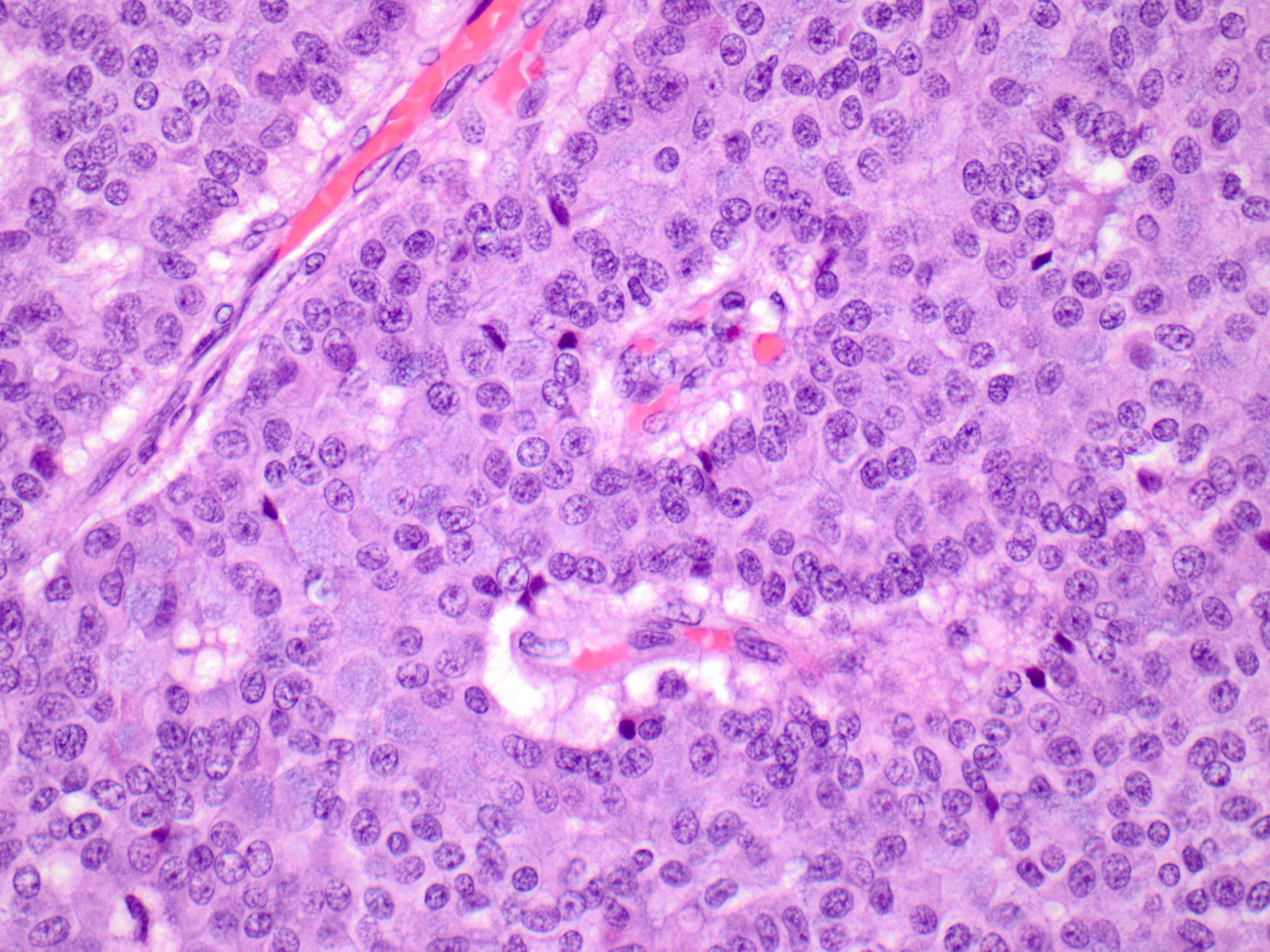
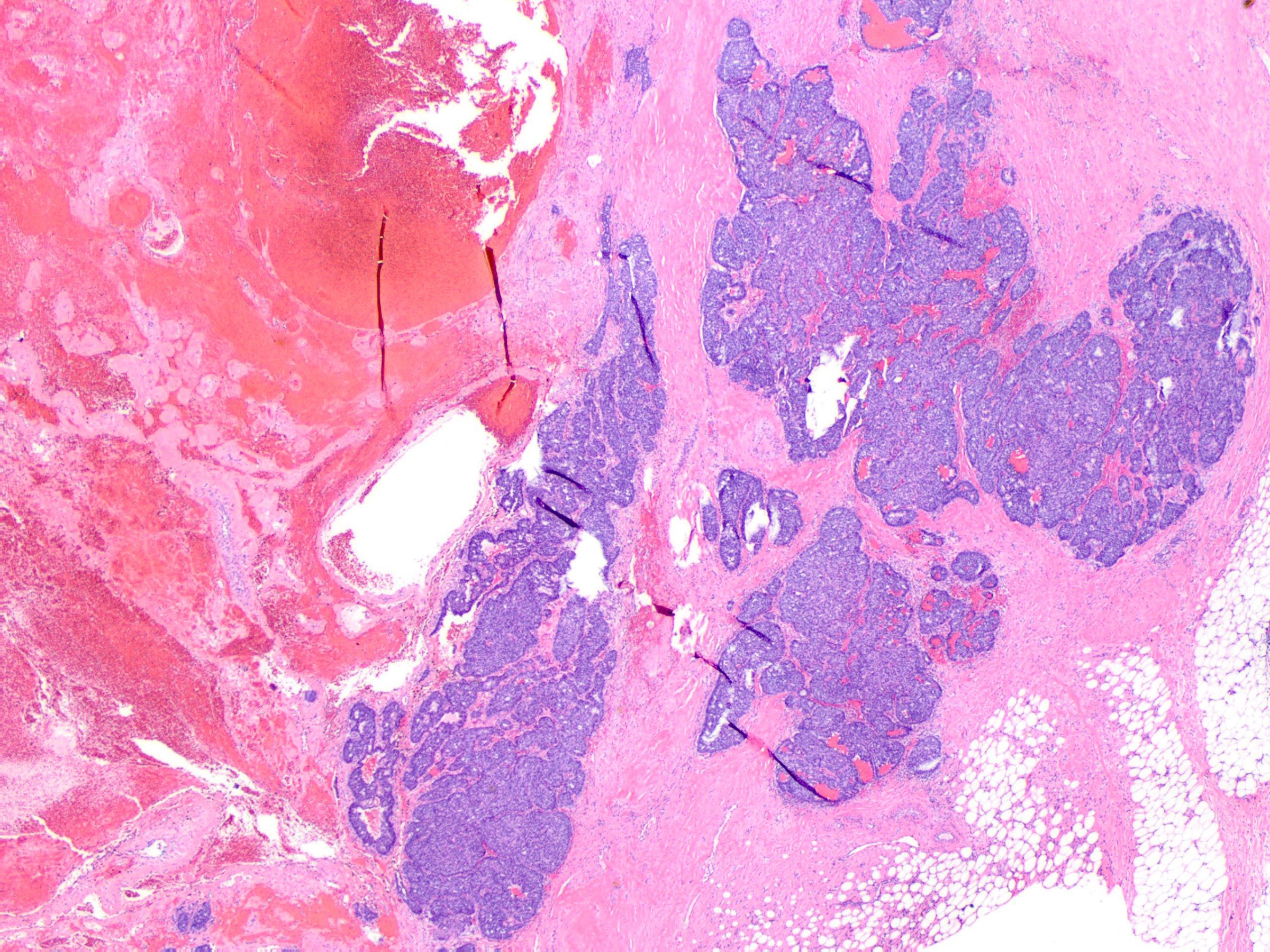
- Solid papillary carcinoma with invasion
- Invasive carcinoma arising in association with solid papillary carcinoma in situ may take one of several forms:
- Invasive solid papillary carcinoma
- Mucinous carcinoma
- Type B / cellular mucinous carcinoma is the most common pattern in this setting (Am J Surg Pathol 1995;19:1237)
- Conventional pattern of invasive carcinoma (most often invasive ductal carcinoma, NOS)
- Admixture of the above patterns
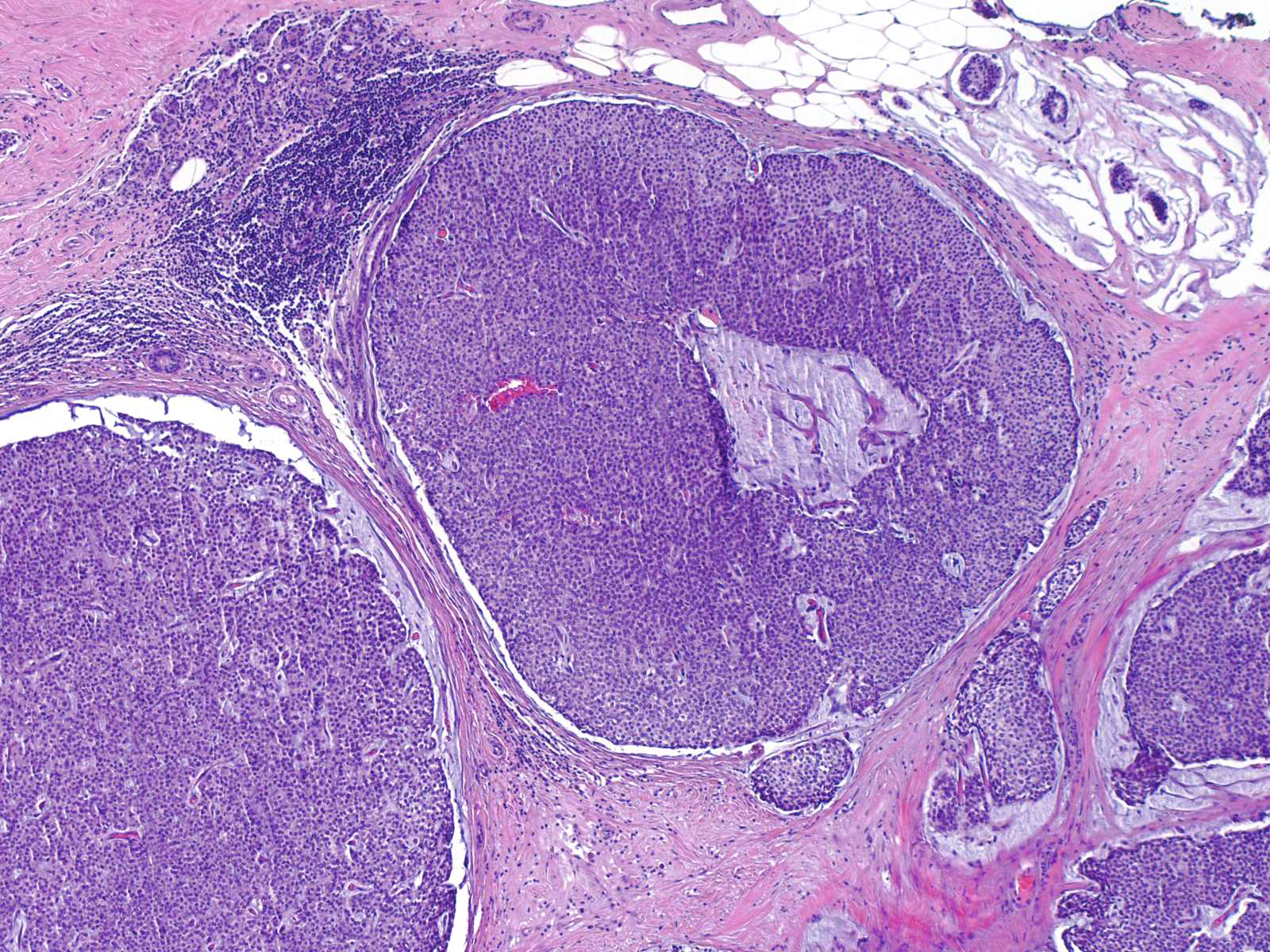
- Invasive solid papillary carcinoma is diagnosed when a solid papillary carcinoma shows evidence of destructive growth within extralobular stroma
- Tumor nests have angulated or ragged contours or form irregularly anastomosing islands
- Jigsaw-like appearance
- Distribution is too confluent and haphazard to represent involvement of the underlying glandular tree
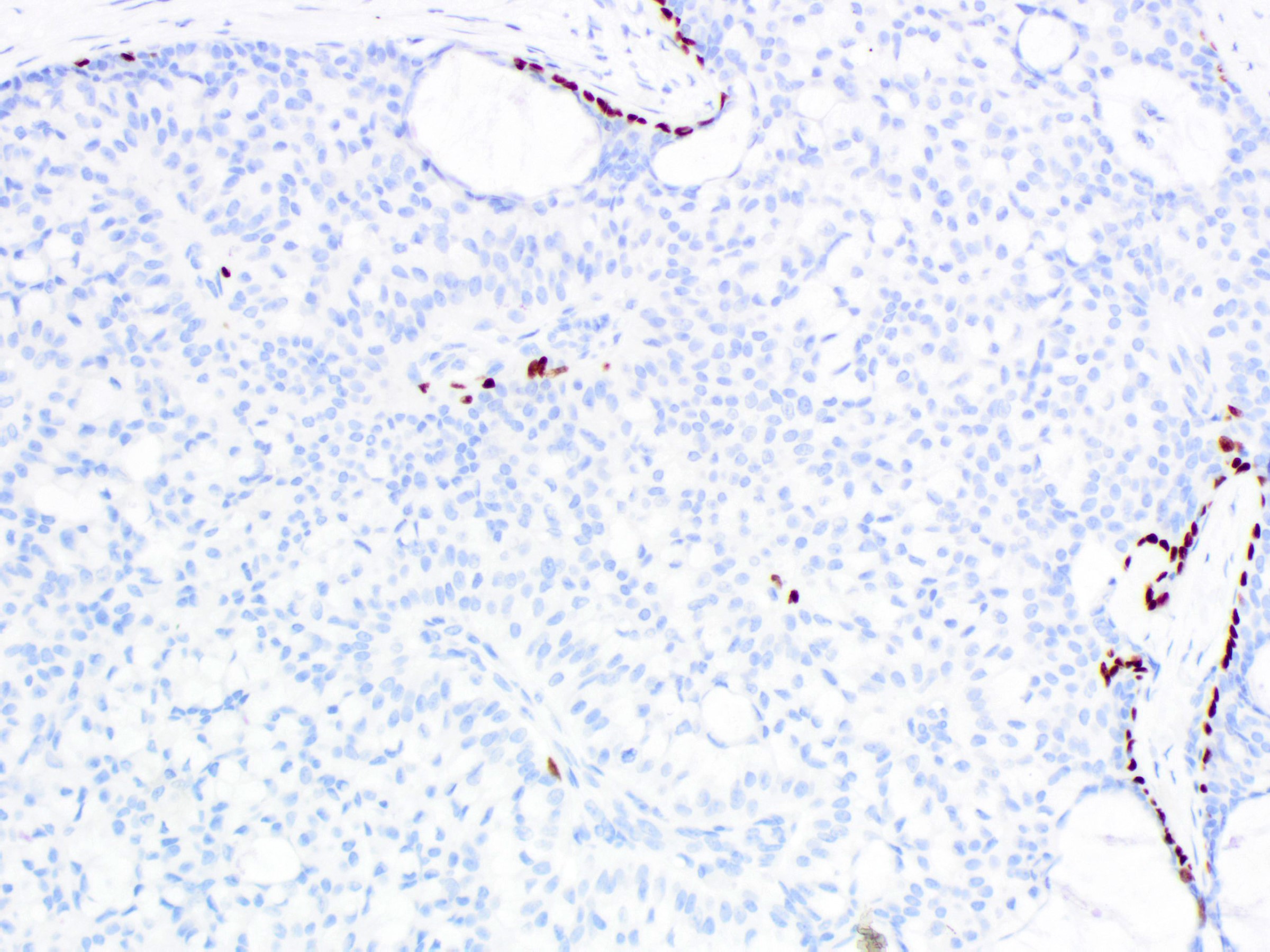
- Myoepithelial cells are absent
- Additional clues to infiltrative growth:
- Desmoplastic stroma
- Engulfment of normal glands or fat
- Vascular invasion
- In rare examples, the invasive nests may have more rounded shapes but their geographic confluence, expansile mass forming growth, random distribution and association with reactive stroma are indicative of growth beyond the confines of the ductal tree
- Nottingham grading and ER, PR and HER2 studies are applied to the invasive component only
- Tumor is staged according to the size of the invasive component only
- Invasive carcinoma arising in association with solid papillary carcinoma in situ may take one of several forms:
Microscopic (histologic) images
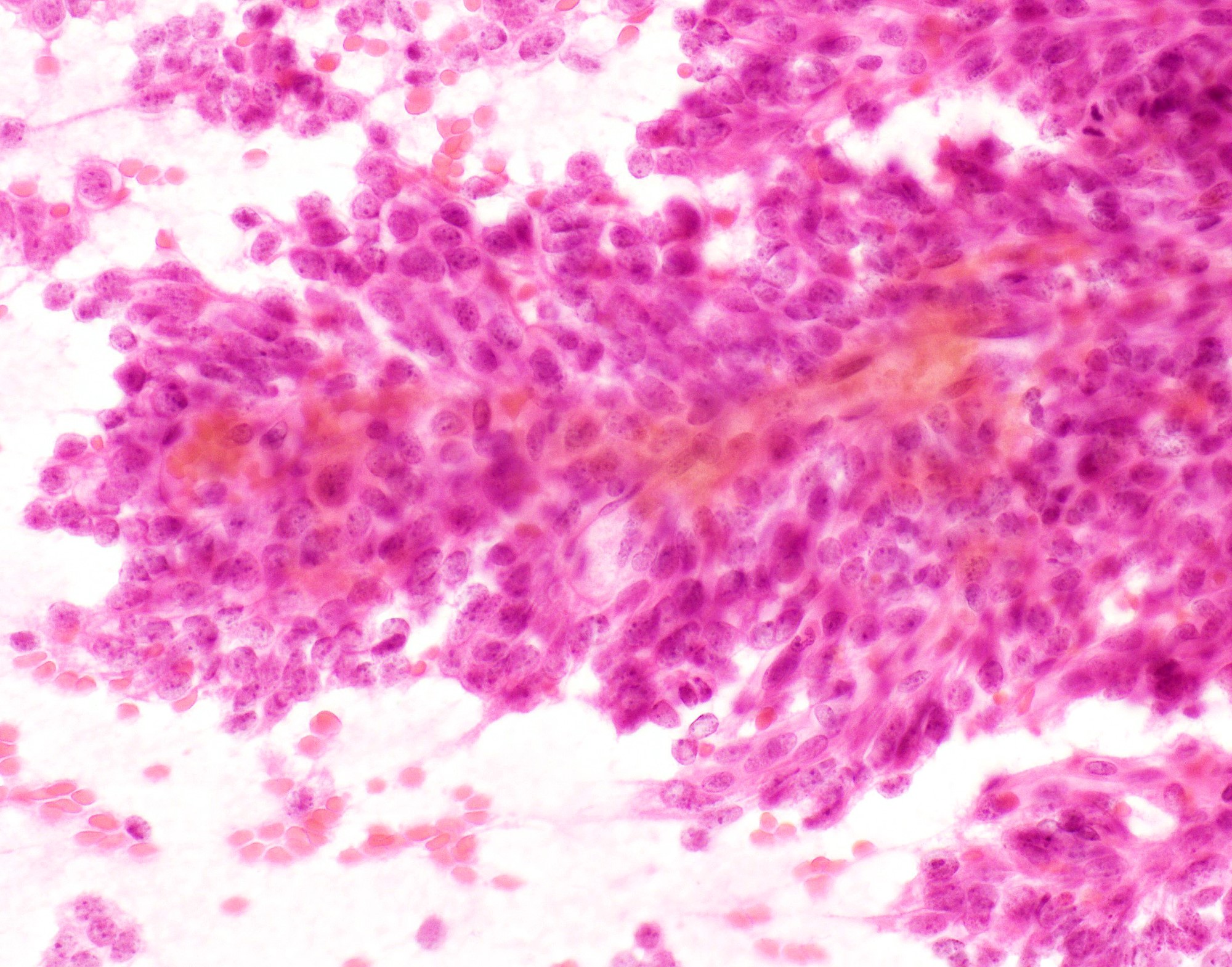
Cytology description
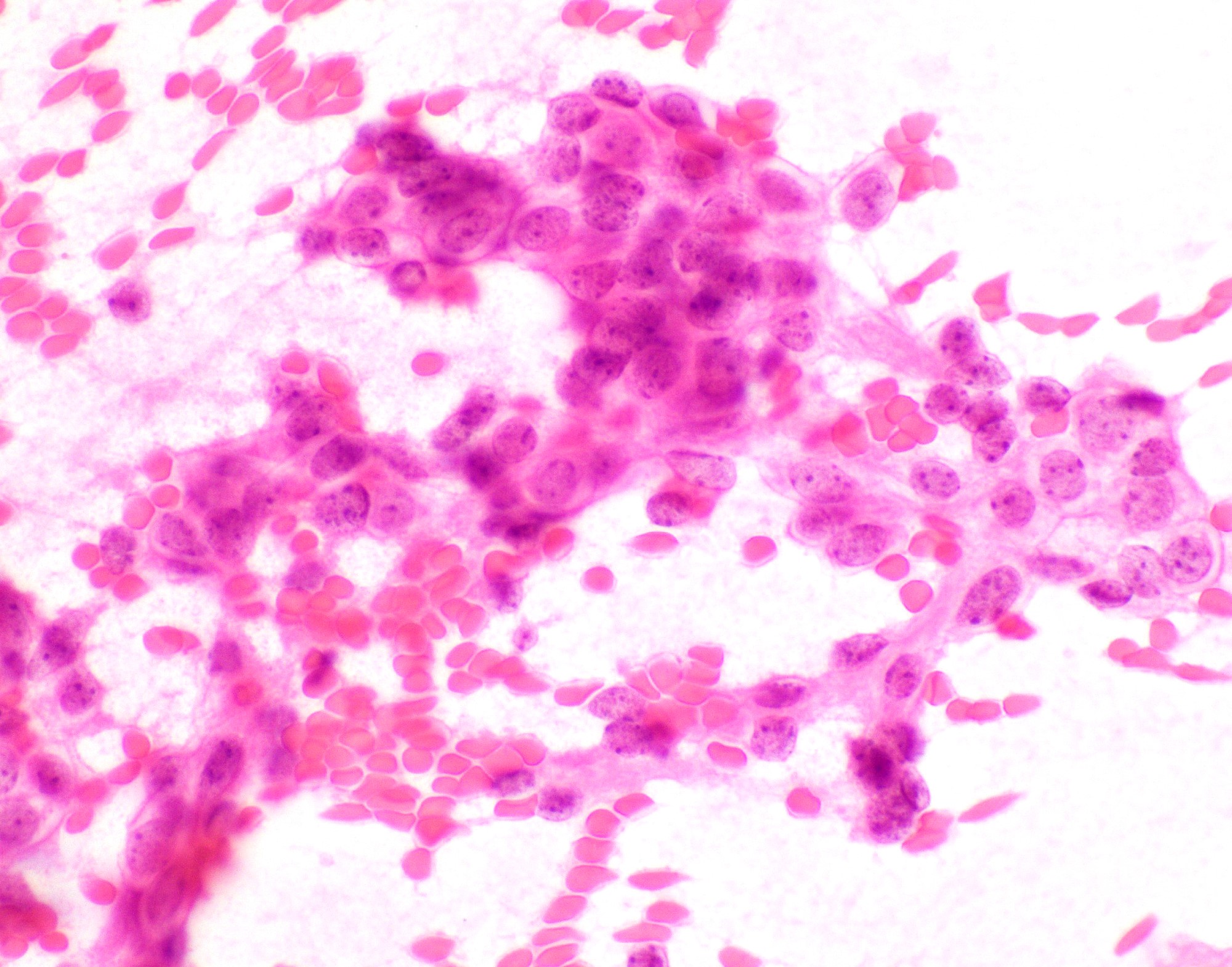
- Hypercelluar specimen
- Small to large discohesive epithelial clusters and many single isolated cells with a low nuclear cytoplasmic ratio (Diagn Cytopathol 2007;35:417)
- Small round to oval, bland nuclei with finely granular chromatin, eccentrically placed nuclei and inconspicuous nucleoli
- Lack of naked myoepithelial cell nuclei in the background
- Mucin and capillary vascular structures are infrequently noted (Diagn Cytopathol 2007;35:417)
Positive stains
- Estrogen receptor: strong and diffuse
- Progesterone receptor: variable but often strong and diffuse
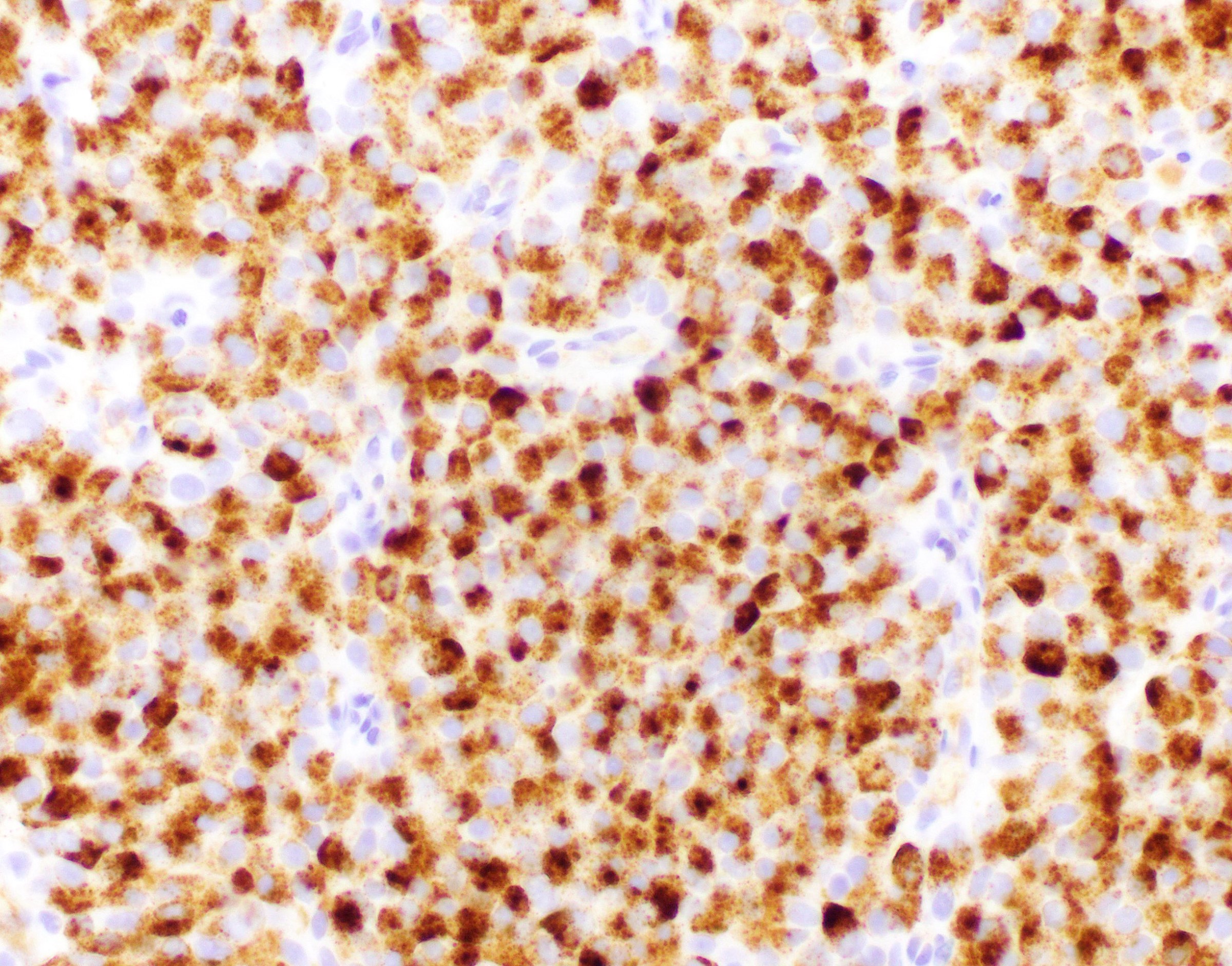
- Chromogranin and synaptophysin are positive in approximately half of cases (Am J Surg Pathol 2016;40:1334)
- INSM1 is positive in half of cases and it can be positive in cases that are negative for synaptophysin and chromogranin (Pathol Int 2021;71:51)
- Ki67 is usually low or intermediate
Negative stains
- High molecular weight cytokeratins (e.g. CK5/6, CK14, 34 beta E12) (Hum Pathol 2006;37:787)
- ERBB2 (HER2)
Electron microscopy description
- Variety of granule types, including mucinous, small dense core and large serous-like granules, are observed (Ultrastruct Pathol 1997;21:153)
Molecular / cytogenetics description
- Luminal phenotype with relatively simple genomes and few copy number aberrations (J Pathol 2012;226:427)
- 16q loss, 16p gains and 1q gains (J Pathol 2012;226:427)
- Genes related to neuroendocrine differentiation, including RET, ASCL1 and DOK7, are expressed at higher levels in solid papillary carcinomas than in encapsulated papillary carcinomas (Mol Oncol 2014;8:1588)
Sample pathology report
- Left breast, excision:
- Solid papillary carcinoma in situ, grade 1, spanning at least 1.0 cm
- Alternative terminology: ductal carcinoma in situ, solid papillary type
- Right breast, excision:
- Invasive solid papillary carcinoma (1.2 x 1.0 cm), grade 2
- Alternative terminology: invasive ductal carcinoma, solid papillary type
Differential diagnosis
- Usual ductal hyperplasia involving an intraductal papilloma:
- Polymorphic rather than monomorphic cell population
- Cellular maturation often present
- Broad fibrovascular cores
- Positivity for high molecular weight keratins
- Heterogeneous positivity for estrogen receptor
- Negative for neuroendocrine markers (Virchows Arch 2007;450:539)
- Typically shows a robust myoepithelial cell layer around the periphery and along fibrovascular cores
- Conventional ductal carcinoma in situ with solid growth pattern:
- Lacks interspersed thin fibrovascular cores
- Spindle cell morphology and neuroendocrine differentiation uncommon
- Encapsulated papillary carcinoma:
- Complex expansile mass formed by filiform, branching papillae that are lined by columnar cells
- Often partially cystic
- Examples with epithelial bridging between papillae often show prominent cribriforming rather than solid growth
- Lacks neuroendocrine differentiation
- Tall cell carcinoma with reverse polarity (Cancer Res 2016;76:7118):
- Apical nuclei
- Triple negative phenotype
- Positive for high molecular weight keratins and calretinin (Mod Pathol 2018;31:1367)
- IDH2 mutations
- Neuroendocrine tumor / carcinoma:
- Despite its frequent neuroendocrine differentiation, solid papillary carcinoma is a distinct breast tumor and should not be classified as a neuroendocrine tumor or neuroendocrine carcinoma (WHO Classification of Tumours Editorial Board: Breast Tumours, 5th Edition, 2019)
- Neuroendocrine neoplasms of the breast (neuroendocrine tumor, neuroendocrine carcinoma) demonstrate morphological features similar to those originating in the gastrointestinal tract or lung (Mod Pathol 2021:34:1062)
- Terminology excludes solid papillary carcinoma and the cellular subtype of mucinous carcinoma
- Unique morphologic features of solid papillary carcinoma (expansile cellular nodules punctuated by thin fibrovascular cores, frequent presence of mucin) enable its differentiation from neuroendocrine neoplasms of the breast
Practice question #1
Which of the following statements most accurately characterizes the illustrated breast lesion?
- Solid papillary carcinoma in situ frequently demonstrates neuroendocrine differentiation
- Solid papillary carcinoma in situ is associated with a poor prognosis
- Solid papillary carcinoma in situ is positive for high molecular weight cytokeratins
- Usual ductal hyperplasia involving a papilloma is associated with a 7 fold increase in risk for subsequent breast cancer
- Usual ductal hyperplasia involving a papilloma is most commonly observed in women in the eighth decade of life
Practice answer #1
A. Solid papillary carcinoma in situ frequently demonstrates neuroendocrine differentiation. The illustrated lesion is an example of solid papillary carcinoma in situ. Solid papillary carcinoma is characterized by a solid cellular proliferation of monomorphic, atypical ductal cells with interspersed delicate fibrovascular cores. It demonstrates neuroendocrine differentiation in approximately half of cases and is associated with an excellent prognosis. Lack of expression of high molecular weight cytokeratins can aid in distinguishing it from usual ductal hyperplasia.
Comment Here
Reference: Solid papillary carcinoma
Comment Here
Reference: Solid papillary carcinoma
Practice question #2
Invasive solid papillary carcinoma of the breast demonstrates
- Branching filiform papillae within a cystic space
- ER-, PR- , HER2- immunophenotype
- Frequent lymph node metastases
- Irregular tumor nests in a jigsaw-like pattern
- Myoepithelial cells along fibrovascular cores but not along the periphery of the tumor
Practice answer #2
D. Irregular tumor nests in a jigsaw-like pattern. Invasive solid papillary carcinoma is diagnosed when there is destructive growth of the tumor within extralobular stroma, most readily recognized by the presence of irregularly shaped nests in a confluent, jigsaw-like pattern. Myoepithelial cells are absent. Invasive papillary carcinoma is rarely associated with lymph node metastases and is typically ER / PR+ and HER2-.
Comment Here
Reference: Solid papillary carcinoma
Comment Here
Reference: Solid papillary carcinoma