Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Gagné A, Joubert P. Atypical carcinoid tumor / neuroendocrine tumor, grade 2. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumoratypicalcarcinoid.html. Accessed September 15th, 2025.
Definition / general
- Well differentiated neuroendocrine carcinoma with atypical features
- Defined as tumor displaying 2 - 10 mitoses per 2 mm² or foci of necrosis (Travis: WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th Edition, 2015)
Essential features
- Atypical carcinoids are defined as neuroendocrine tumors with 2 - 10 mitoses per 2 mm² or foci of necrosis
- Tumors with morphologic features of carcinoid and > 10 mitoses per 2 mm² have been reported and are the focus of active research
- Histologic features include neuroendocrine differentiation, with neuroendocrine growth patterns, salt and pepper chromatin with an inconspicuous nucleolus and moderate to abundant cytoplasm
- Differentiation from typical carcinoid is crucial as they have a poorer prognosis and are more likely to metastasize
Terminology
- Not recommended: moderately differentiated lung neuroendocrine carcinoma, grade 2 neuroendocrine carcinomas
- Well differentiated neuroendocrine tumors G1 to G3 nomenclature is not currently applied in pulmonary carcinoids (Mod Pathol 2018;31:1770)
ICD coding
- ICD-O: 8249/3 - atypical carcinoid tumor
- ICD-10: C7A.090 - malignant carcinoid tumor of the bronchus and lung
- ICD-11: 2C25.4 & XH51K1 - carcinoid or other malignant neuroendocrine neoplasms of bronchus or lung & neuroendocrine tumor, grade 2
Epidemiology
- Rare tumors (Arch Pathol Lab Med 2010;134:1628, Ann Oncol 2015;26:1604):
- All pulmonary carcinoids account for less than 2% of all primary lung tumors
- Atypical carcinoids represent only 10% of all lung carcinoids
- Exact incidence reported as around 0.05% (J Thorac Oncol 2015;10:479)
- More frequent (J Thorac Oncol 2015;10:479, Eur J Cardiothorac Surg 2011;39:565, Ann Oncol 2015;26:1604):
- Females (69%)
- Caucasian
- Mean age at diagnosis: 65, 5 - 10 years older than typical carcinoid
- Less likely to be associated to MEN1 syndrome than typical carcinoid (Ann Oncol 2015;26:1604)
- Unrelated to smoking status
Sites
- Can be found anywhere from the trachea to the distal bronchioles (Cancer 2008;113:5)
- More likely to be peripherally located than typical carcinoids (J Thorac Oncol 2017;12:425, Eur J Cardiothorac Surg 2011;39:565)
Etiology
- Unknown
- Can arise in the context of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia and tumorlets (Thorax 2007;62:248)
Clinical features
Diagnosis
- Even if a diagnosis of carcinoid tumor can be made with confidence on a biopsy or cytology sample, the definitive diagnosis of atypical carcinoid can only be made on a surgical resection, unless necrosis or increased mitotic activity is seen (Ann Oncol 2015;26:1604)
Laboratory
Radiology description
- Similar to Typical carcinoid
- Standardized uptake value (SUV) is generally higher in atypical carcinoid than in typical carcinoid (Ann Oncol 2015;26:1604)
Radiology images
Prognostic factors
- 5 year survival: 60% (Arch Pathol Lab Med 2010;134:1628)
- Lymph node metastasis: 50%
- Distant metastasis: 20% (late metastasis can occur up to 10 years following the initial diagnosis)
- Recurrence
- Prognosis related to (Lung Cancer 2020;139:94):
- TNM stage (J Thorac Oncol 2019;14:184)
- More likely to present at a higher stage disease than typical carcinoid (Eur J Cardiothorac Surg 2011;39:565)
- Complete surgical resection: associated with a better prognosis
- Spread through air spaces (STAS): identified as a factor of poor prognosis (J Thorac Oncol 2019;14:1583, Virchows Arch 2019;475:325)
- TNM stage (J Thorac Oncol 2019;14:184)
Case reports
- 25 year old woman with a postpneumonectomy-like syndrome due to a bronchial atypical carcinoid tumor (BMC Pulm Med 2019;19:44)
- 30 year old woman with a subcutaneous metastasis of a lung atypical carcinoid tumor (Medicine (Baltimore) 2018;97:e9415)
- 45 year old woman with an endobronchial atypical carcinoid tumor with postobstructive mycobacterial infection (BMC Pulm Med 2019;19:41)
- 77 year old woman with a bronchial typical carcinoid lung tumor and diffuse idiopathic neuroendocrine cell hyperplasia in the distal lung (J Thorac Dis 2017;9:E774)
Treatment
- Complete surgical resection is the most efficient treatment for localized disease and has a significant impact on prognosis (Lung Cancer 2020;139:94, Chest 2017;151:1141, NCCN: NCCN Guidelines - Neuroendocrine and Adrenal Tumors [Accessed 11 November 2020])
- Chemotherapy should be considered for stage IIIA resectable tumors
- Absence of consensus for nonresectable and metastatic tumors; options include combinations of chemotherapy and radiotherapy, octreotide or lanreotide or everolimus
- Recent clinical trial explored the role of a combination of anti-CTLA4 (ipilimumab) and anti-PD1 (nivolumab) for advanced stage atypical tumors with promising results (JAMA Oncol 2020;6:1405)
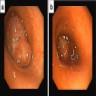
Clinical images
Gross description
- Can be similar to typical carcinoids, as they are well circumscribed and round / ovoid tumors but differ in certain points (Cancer 2008;113:5)
- On average, atypical carcinoids are larger
- Cut surface is white-gray or tan like typical carcinoids but can be less homogeneous with pink or yellow-brown or red areas
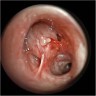
Gross images
Frozen section description
Frozen section images
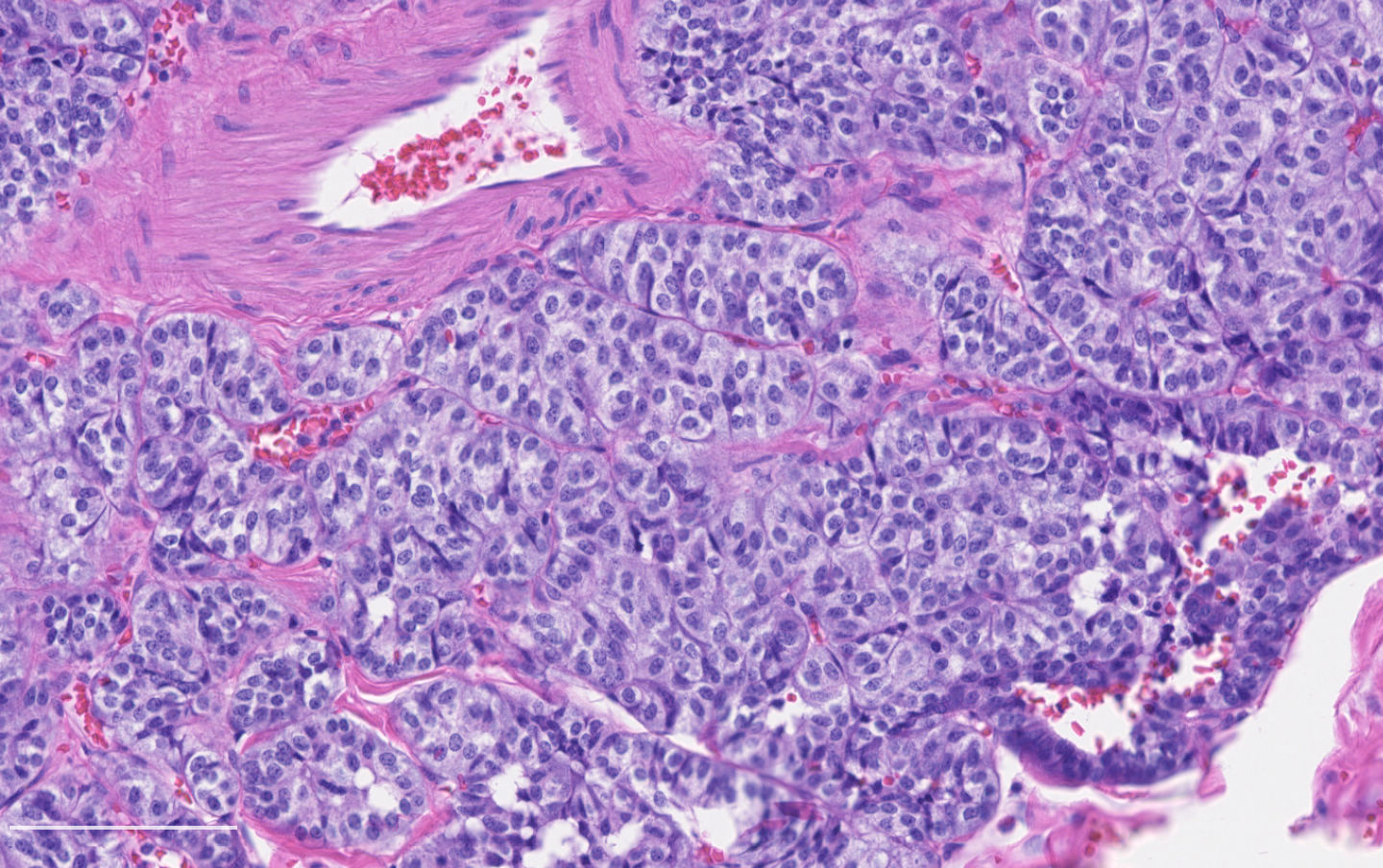
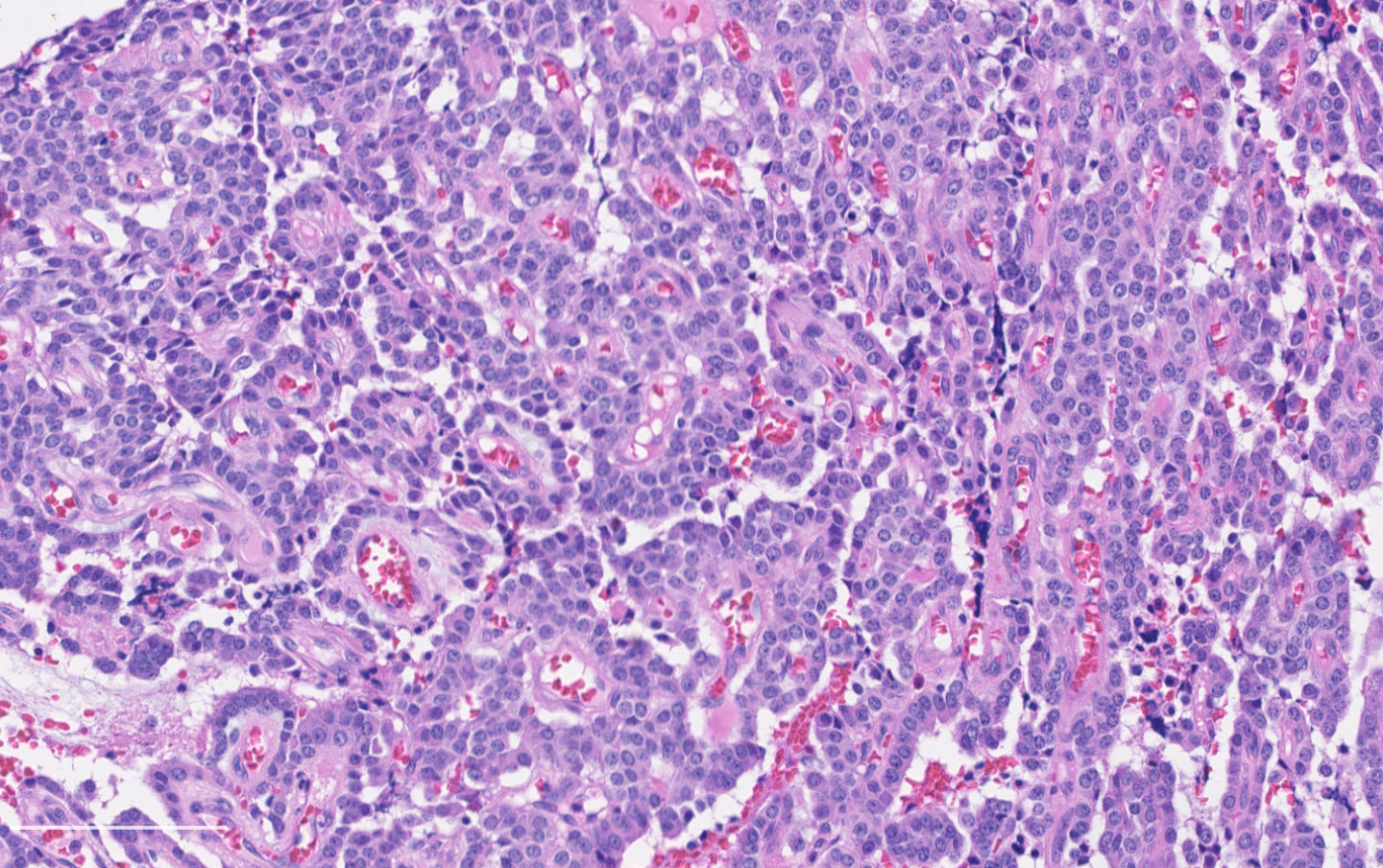
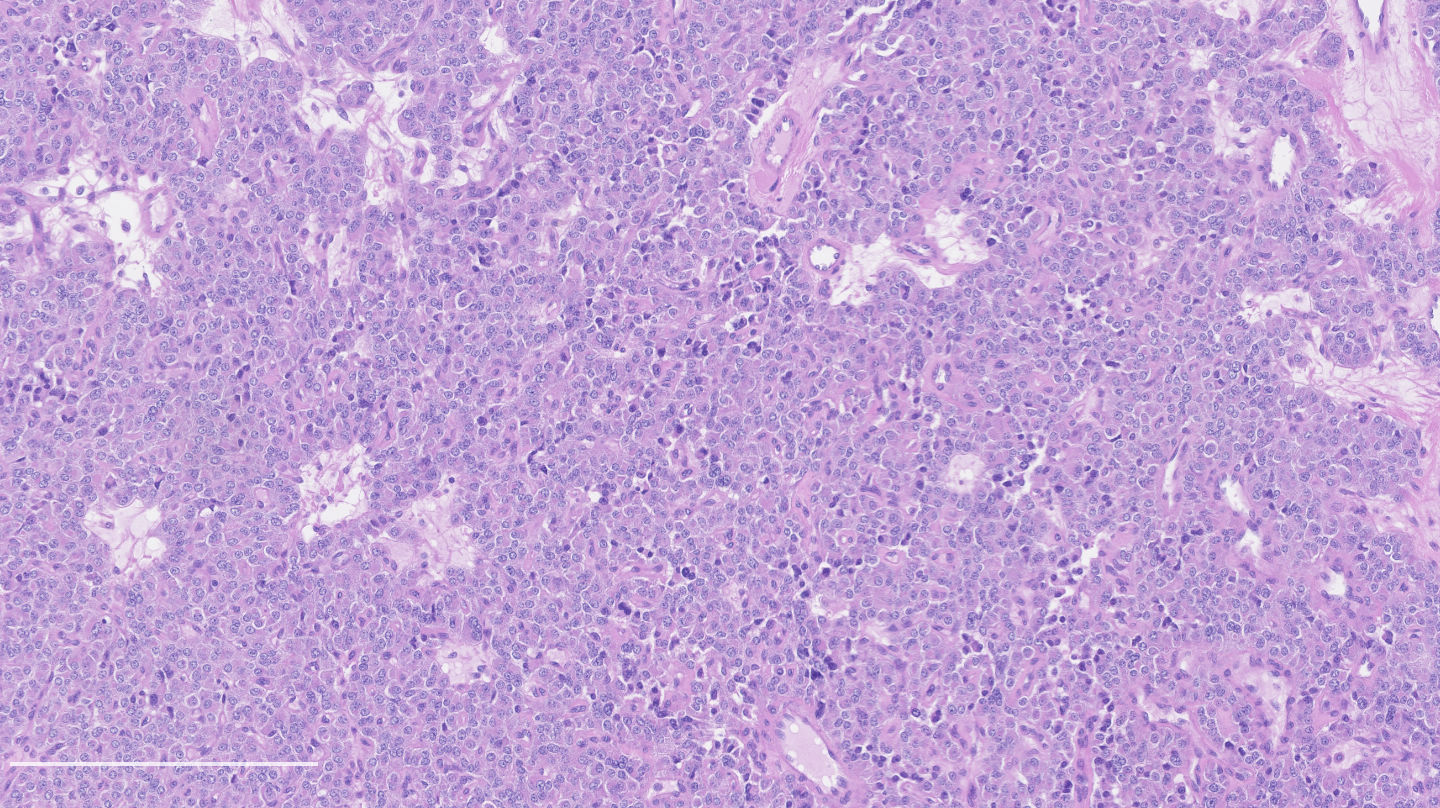
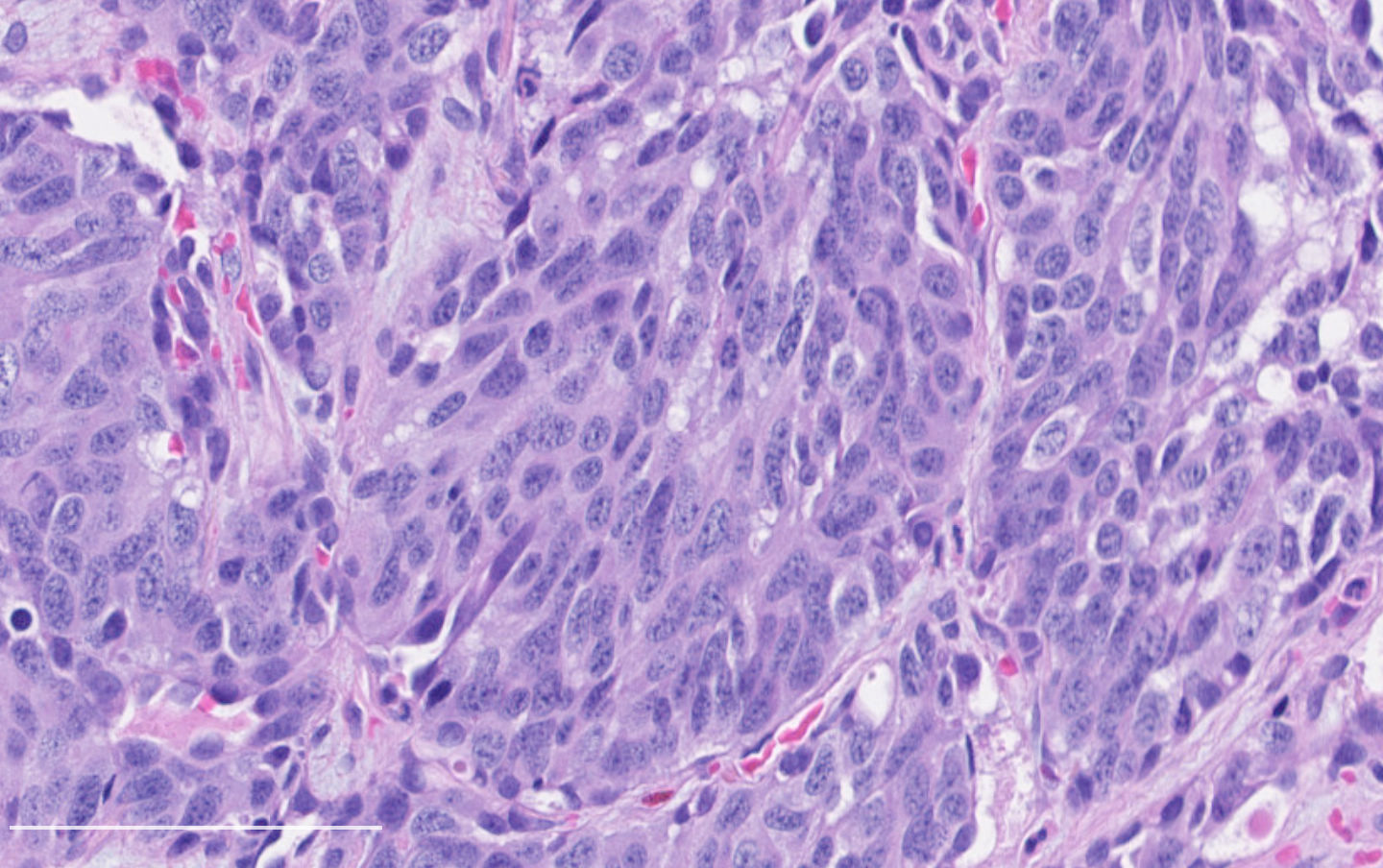
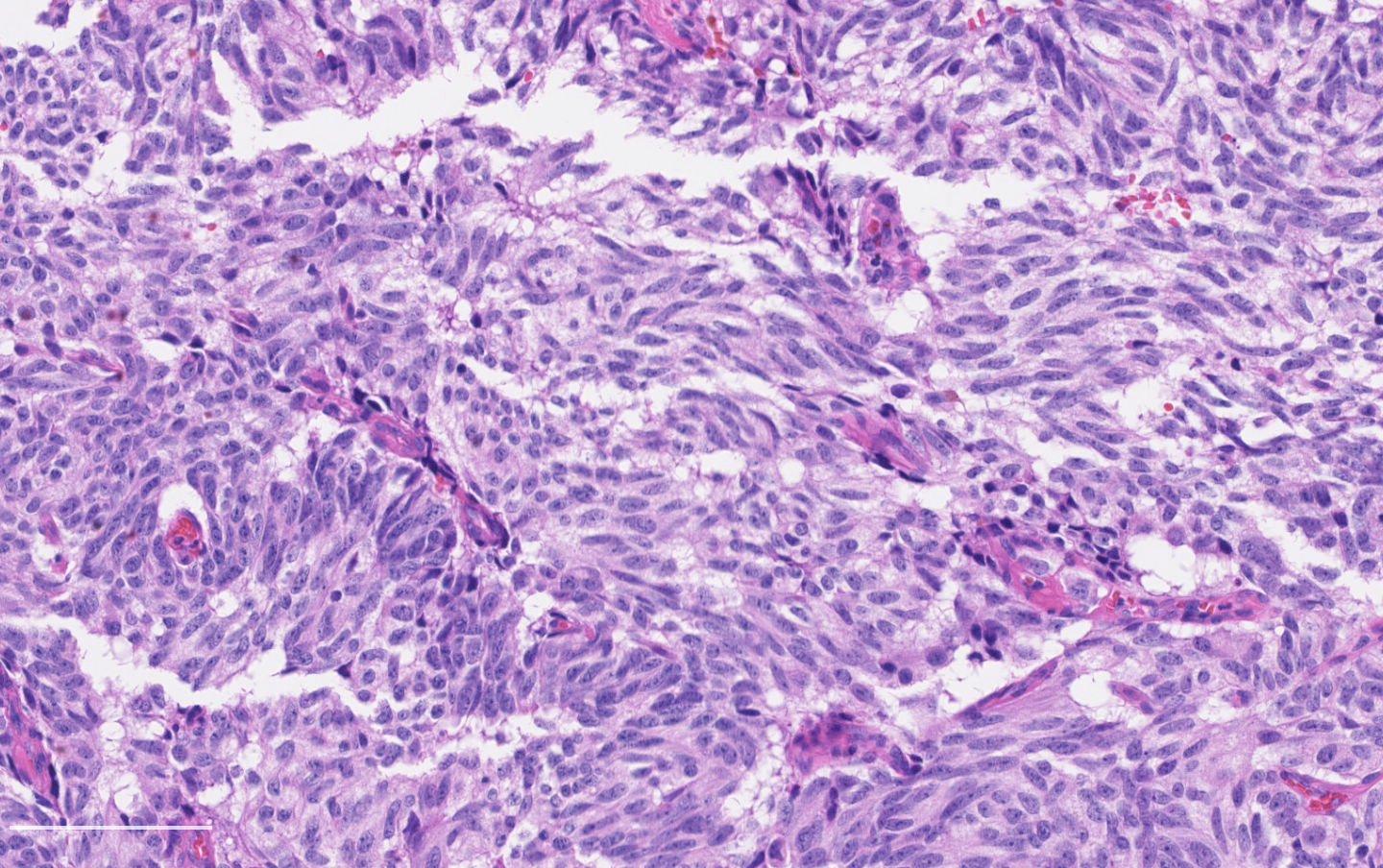
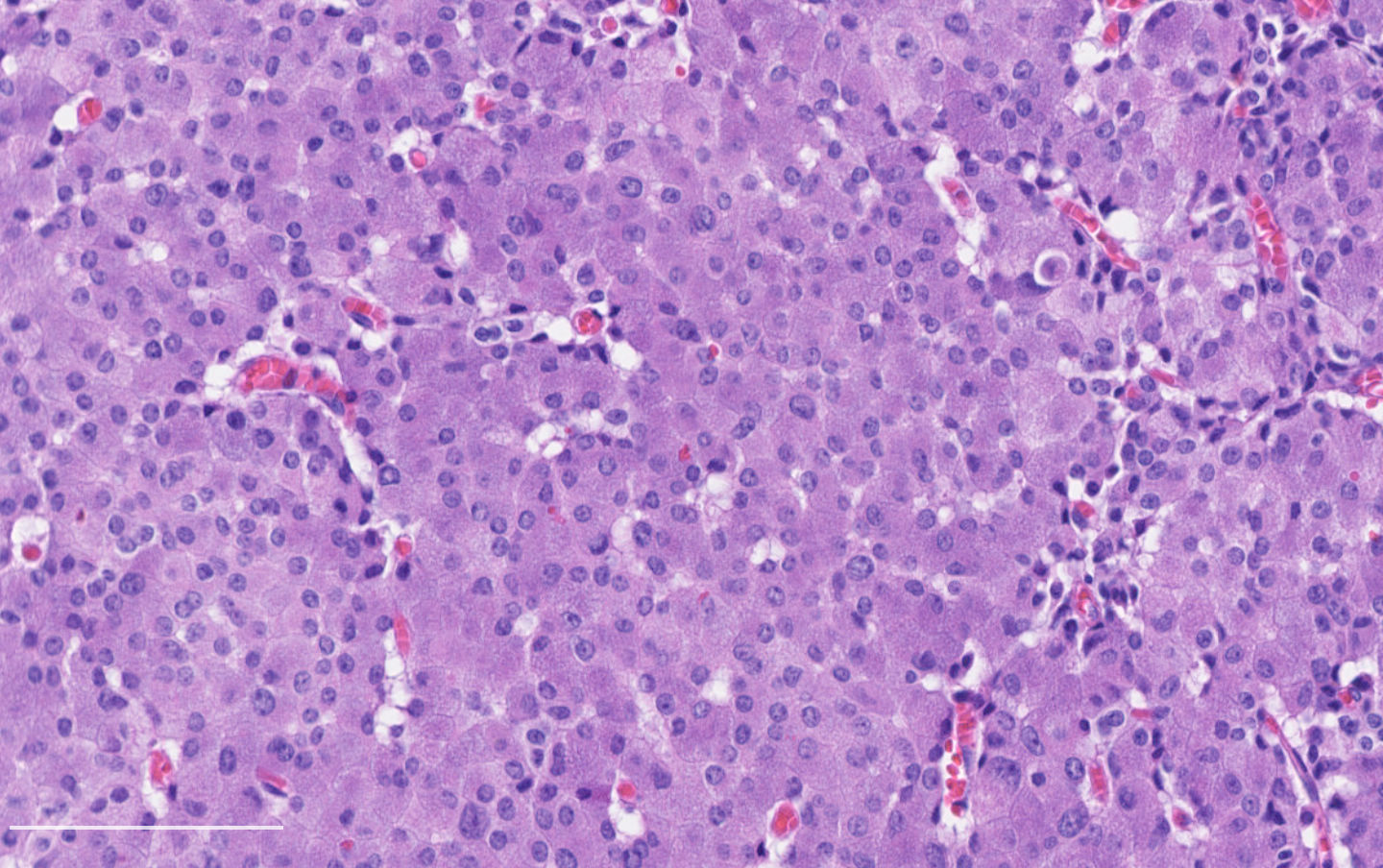
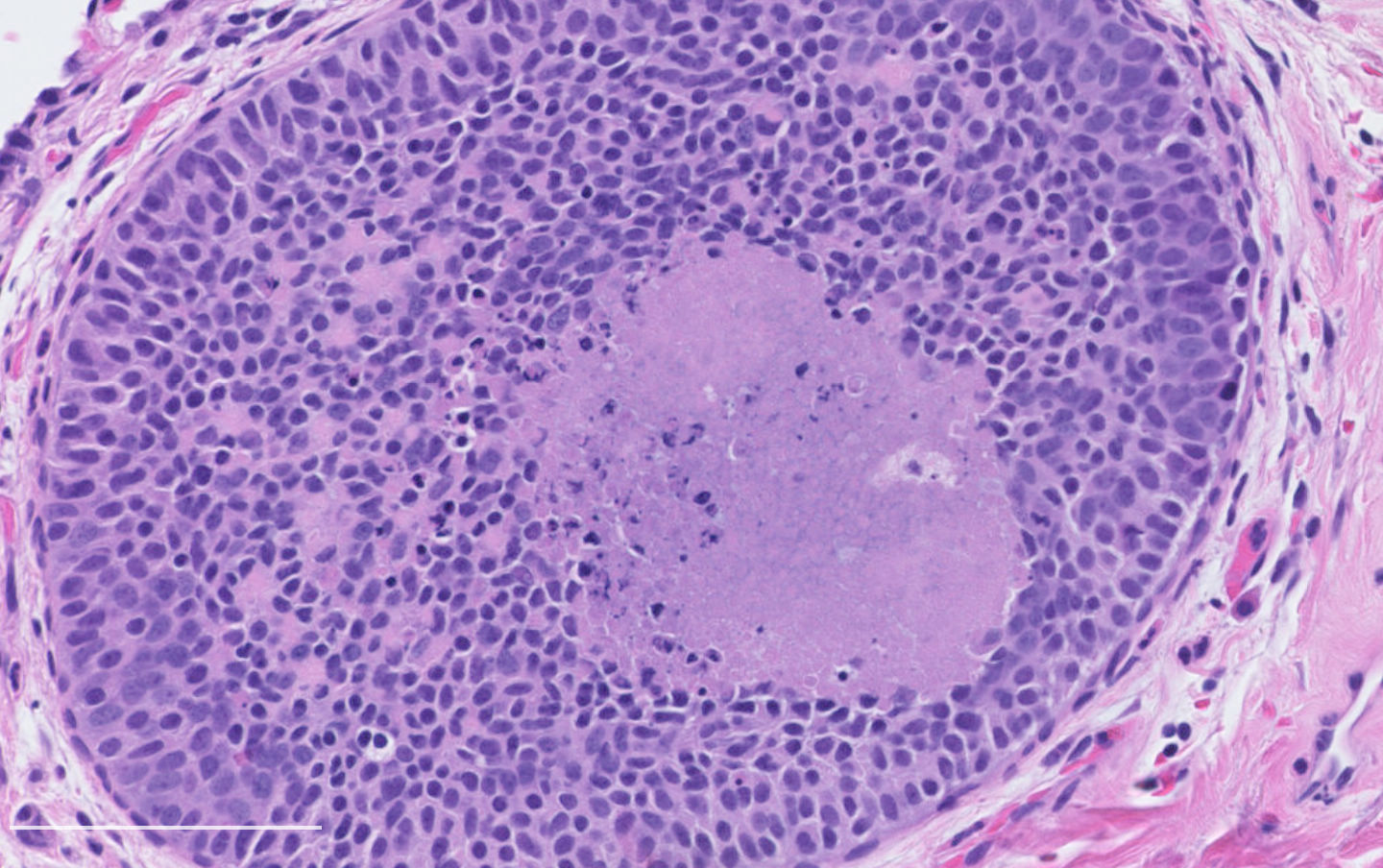
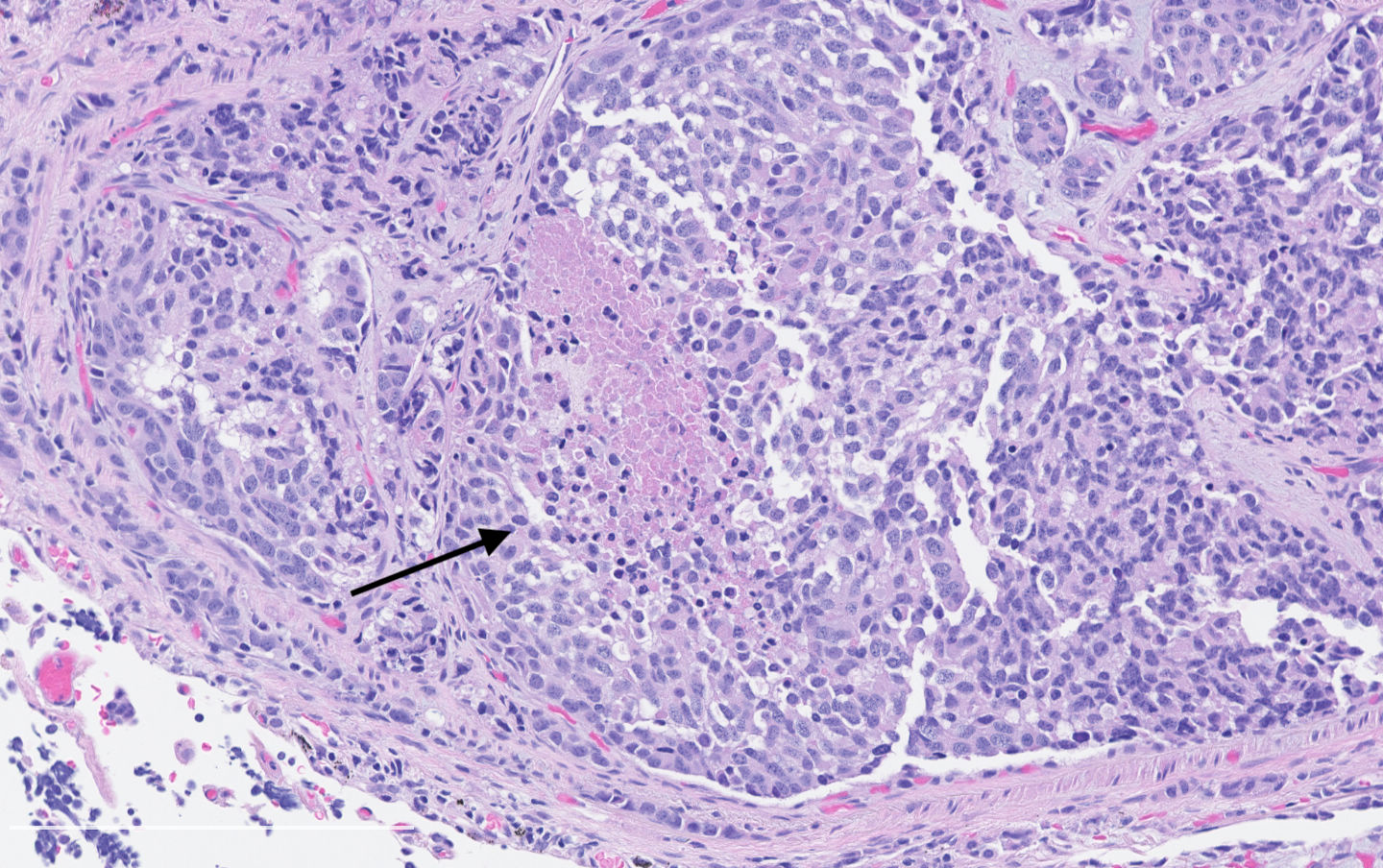
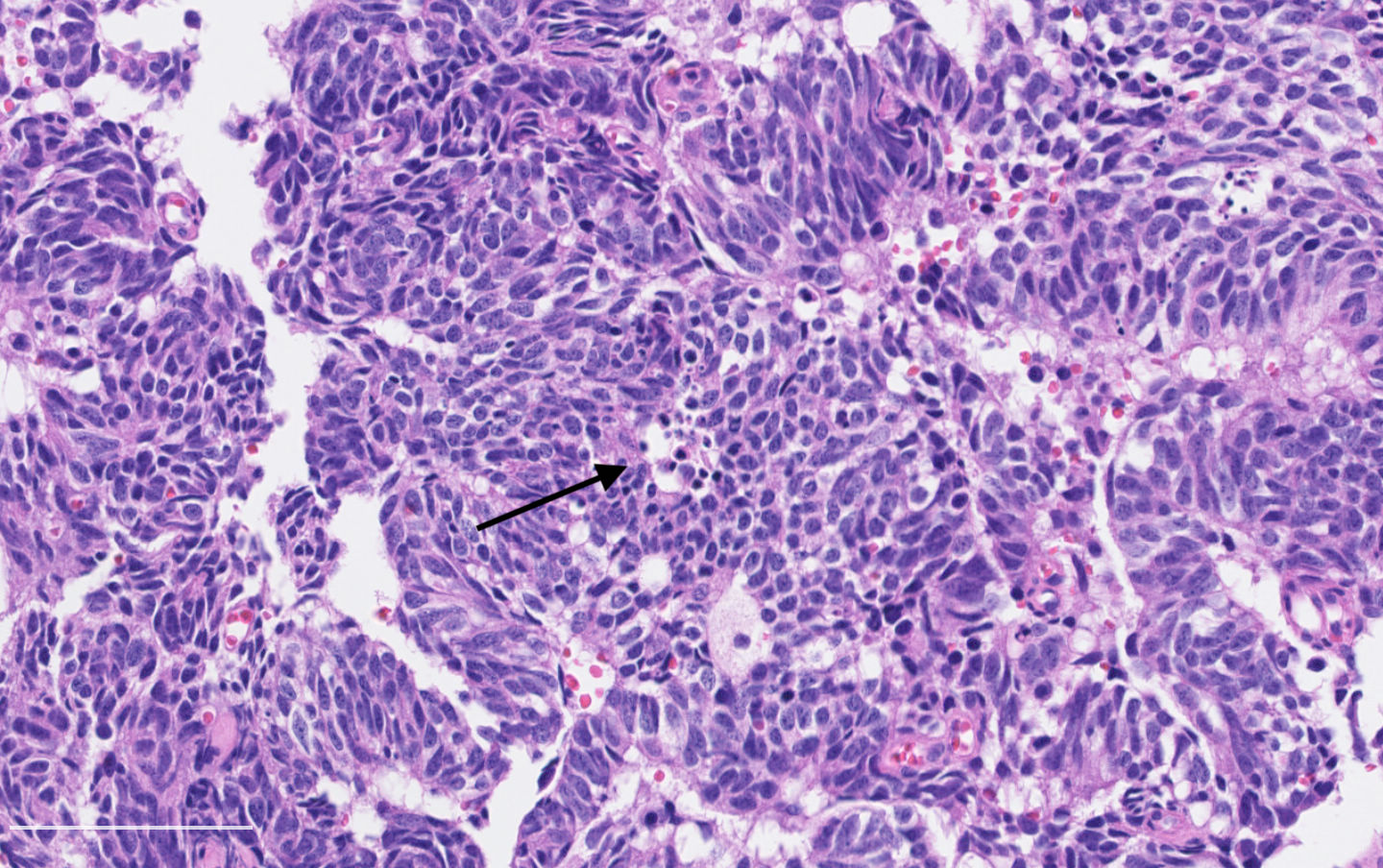
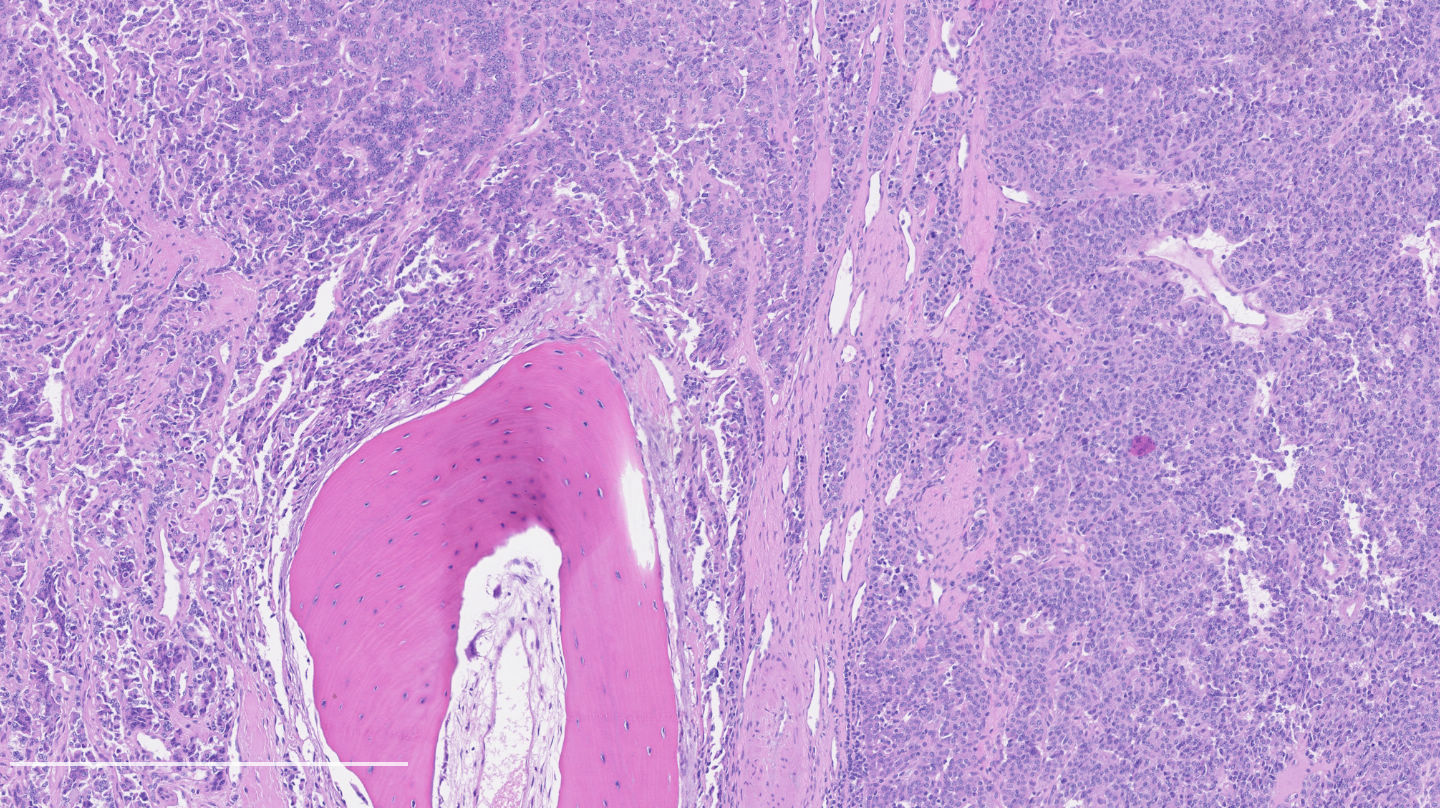
Microscopic (histologic) description
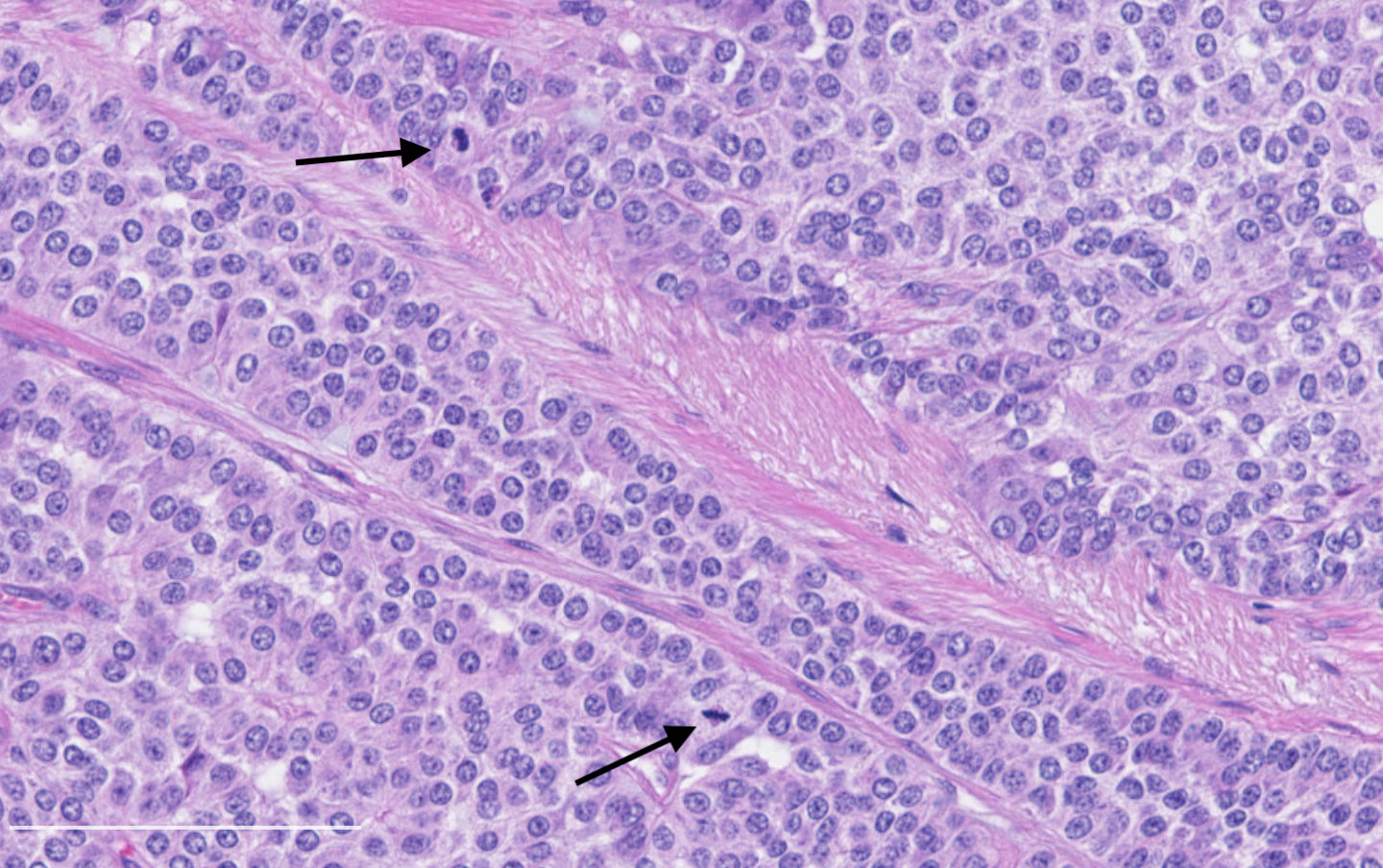
- Diagnostic criteria:
- Neuroendocrine morphology with 2 - 10 mitoses per 2 mm² or presence of necrosis
- Necrosis can be in large zones but is usually punctate
- Mitotic rate should be counted in the area with the highest proliferation rate (hot spot)
- If mitotic rate is near cutoffs, assessment should be made on three sets of 2 mm² and their mean should count as the final mitotic rate
- Rare tumors with morphologic features of carcinoid and > 10 mitoses per 2 mm² have been reported (Virchows Arch 2017;471:713, Am J Surg Pathol 2017;41:263, Diagn Pathol 2019;14:104)
- According to WHO classification, these tumors should be classified as large cell neuroendocrine carcinomas
- However, recent clinical and molecular data support a relationship with carcinoid for some of those tumors and they are the focus of active research (Clin Cancer Res 2016;22:3618, Mod Pathol 2019;32:1106, Mod Pathol 2020;33:1712, Nat Commun 2019;10:3407)
- Neuroendocrine morphology with 2 - 10 mitoses per 2 mm² or presence of necrosis
- Neuroendocrine histologic patterns similar to typical carcinoids: organoid, trabecular, rosette formation, papillary, pseudoglandular, follicular
- Tumor cells are as typical carcinoid: uniform with a polygonal shape, round to oval nuclei with salt and pepper chromatin and inconspicuous nucleoli, along with moderate to abundant eosinophilic cytoplasm
- Greater pleomorphism than for typical carcinoid is common (Arch Pathol Lab Med 2010;134:1628)
- Spindle cells and clear cell features can be seen
- Stroma is fine and highly vascularized; hyalinization, cartilage or bone formation are possible
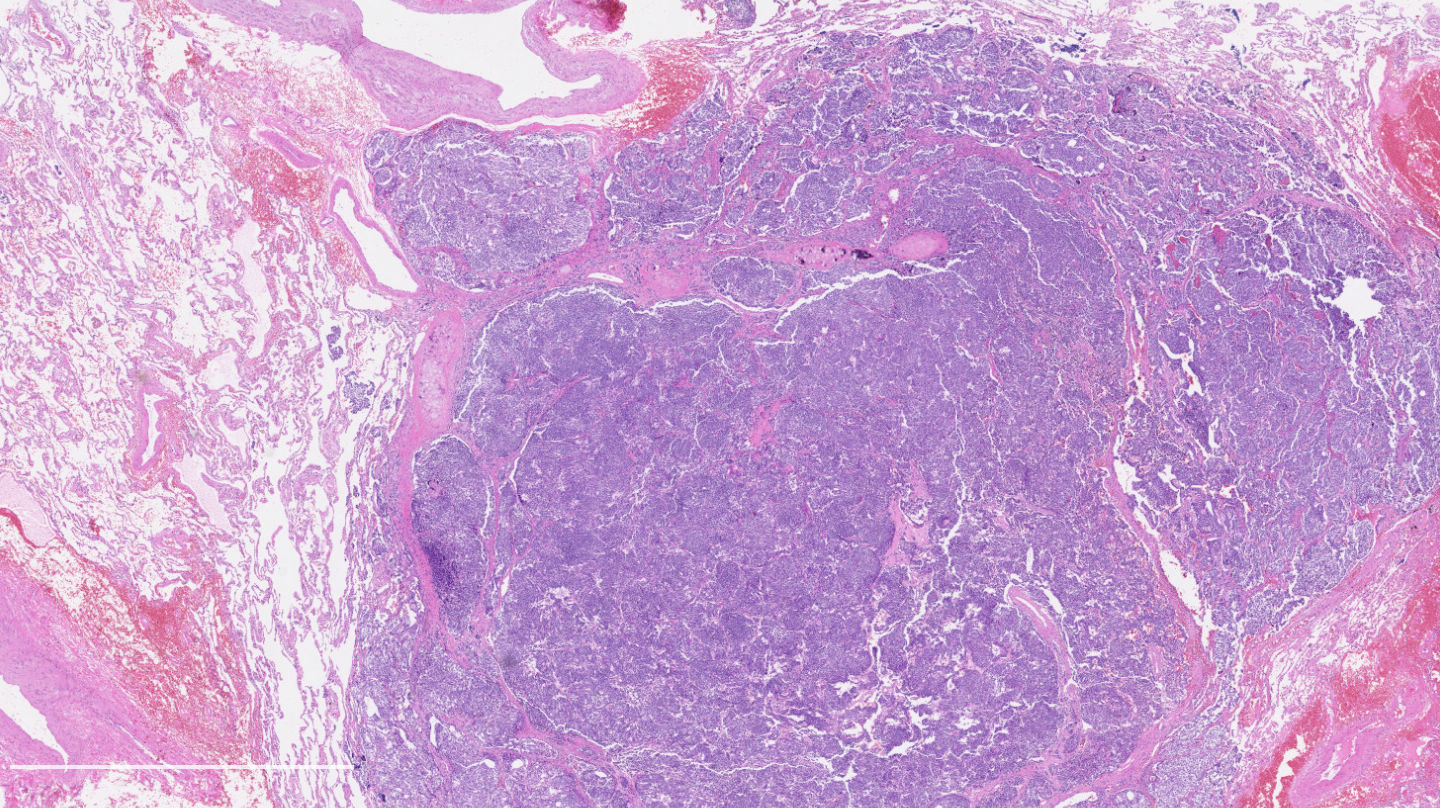
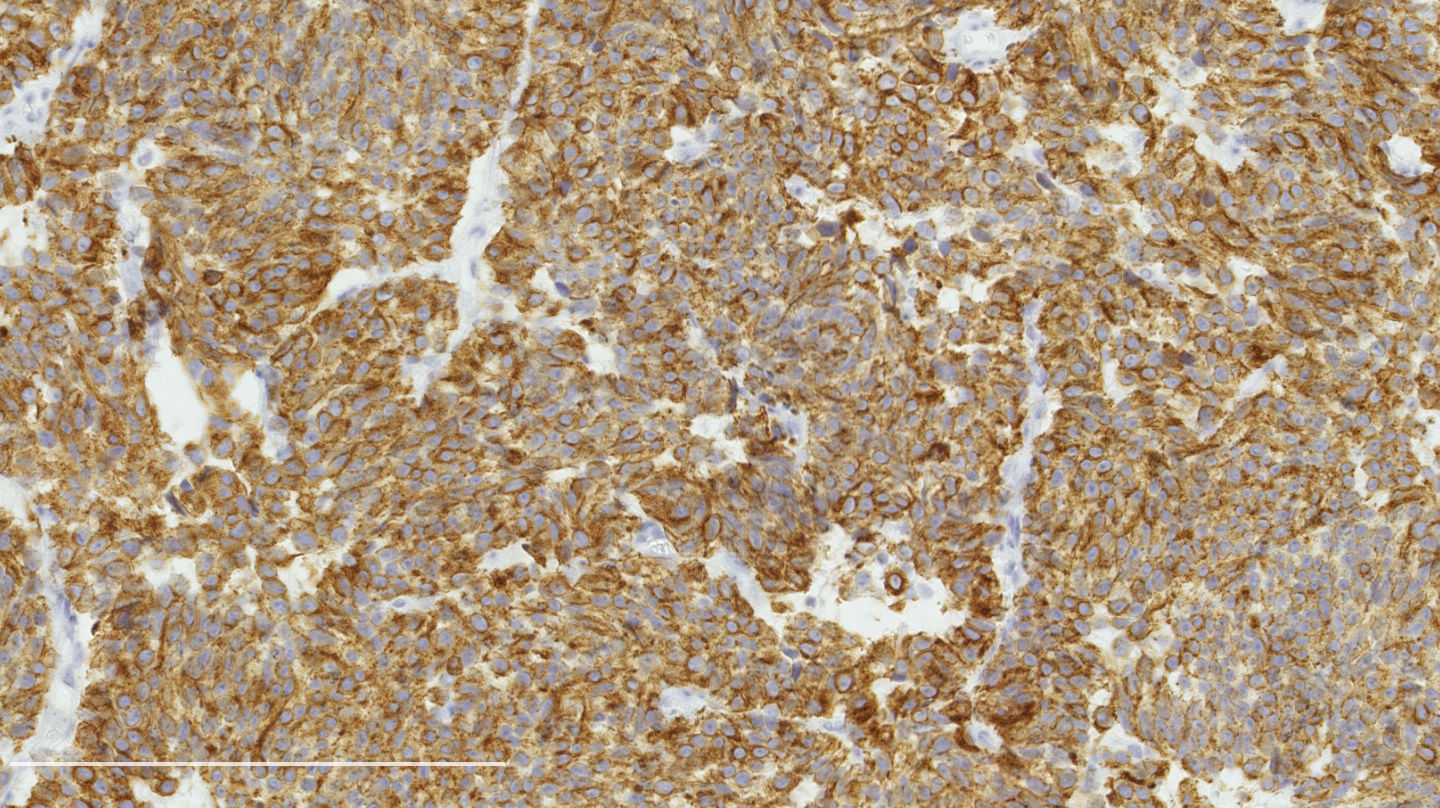
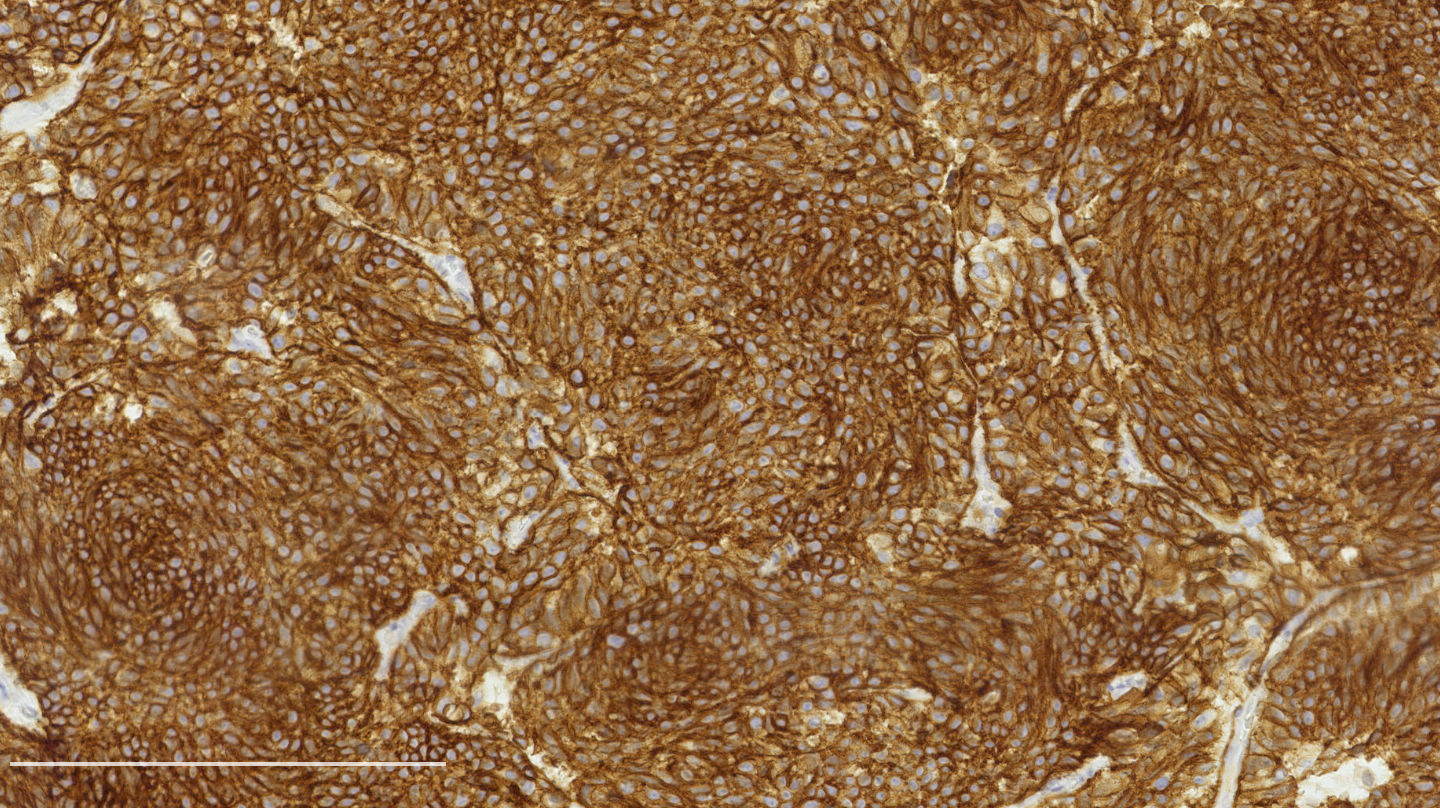
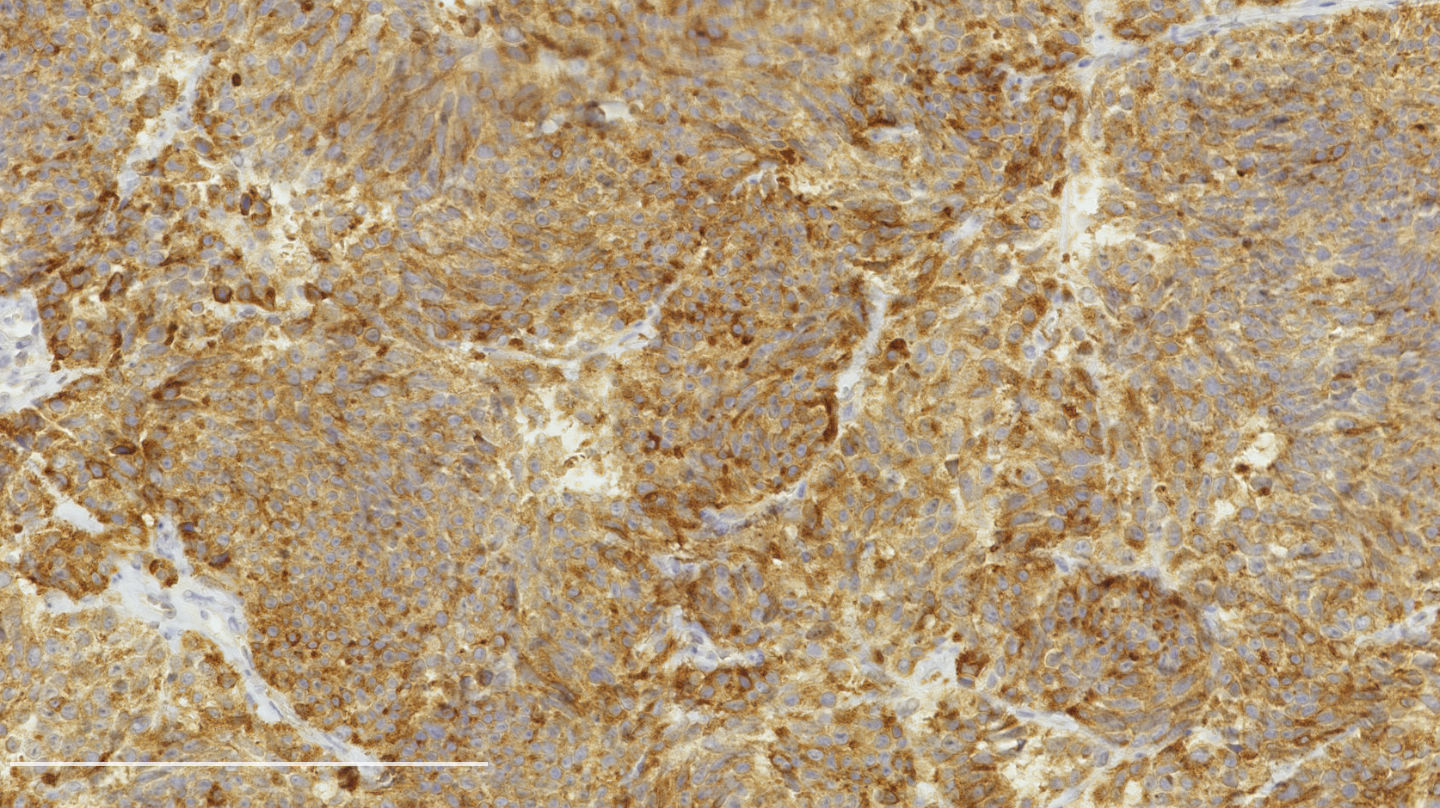
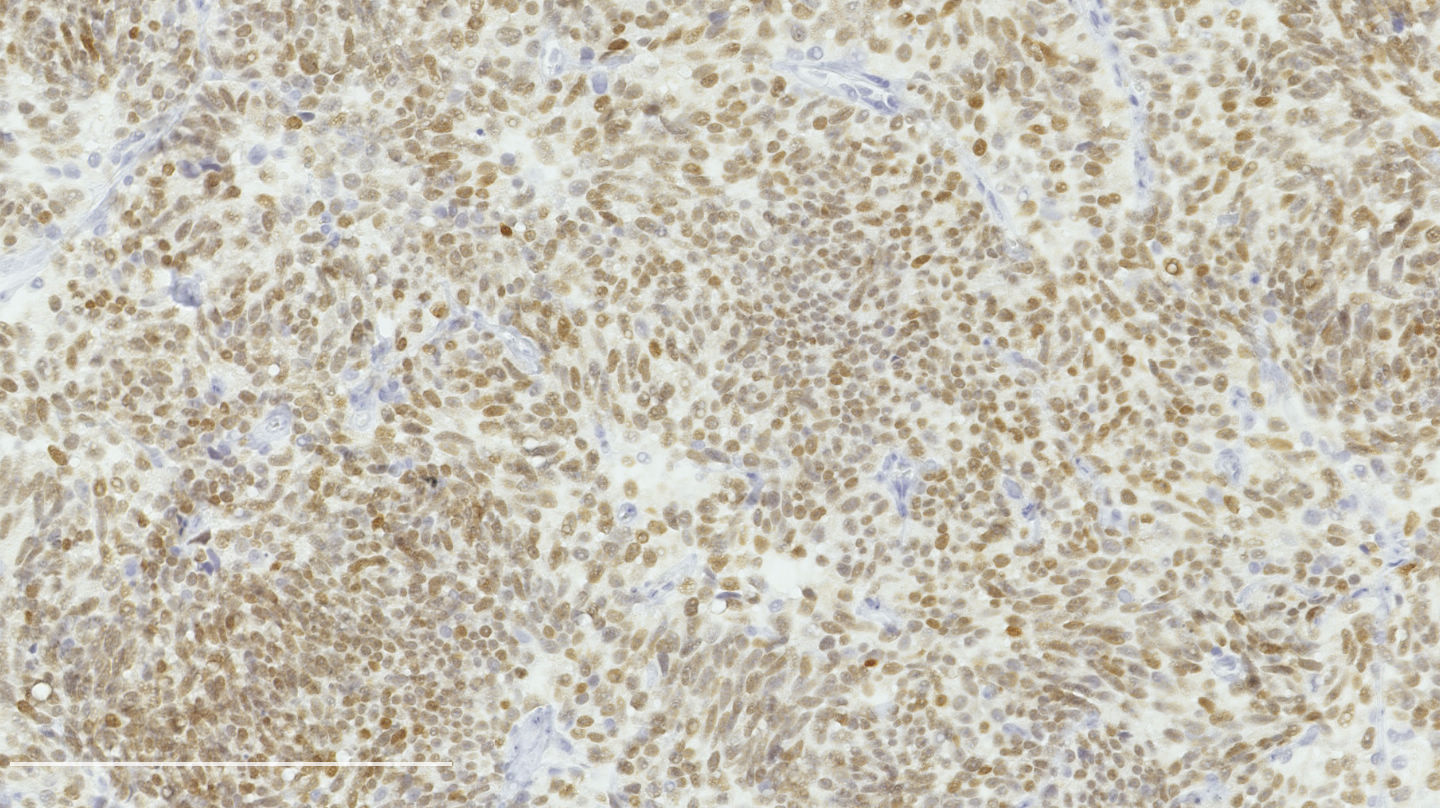
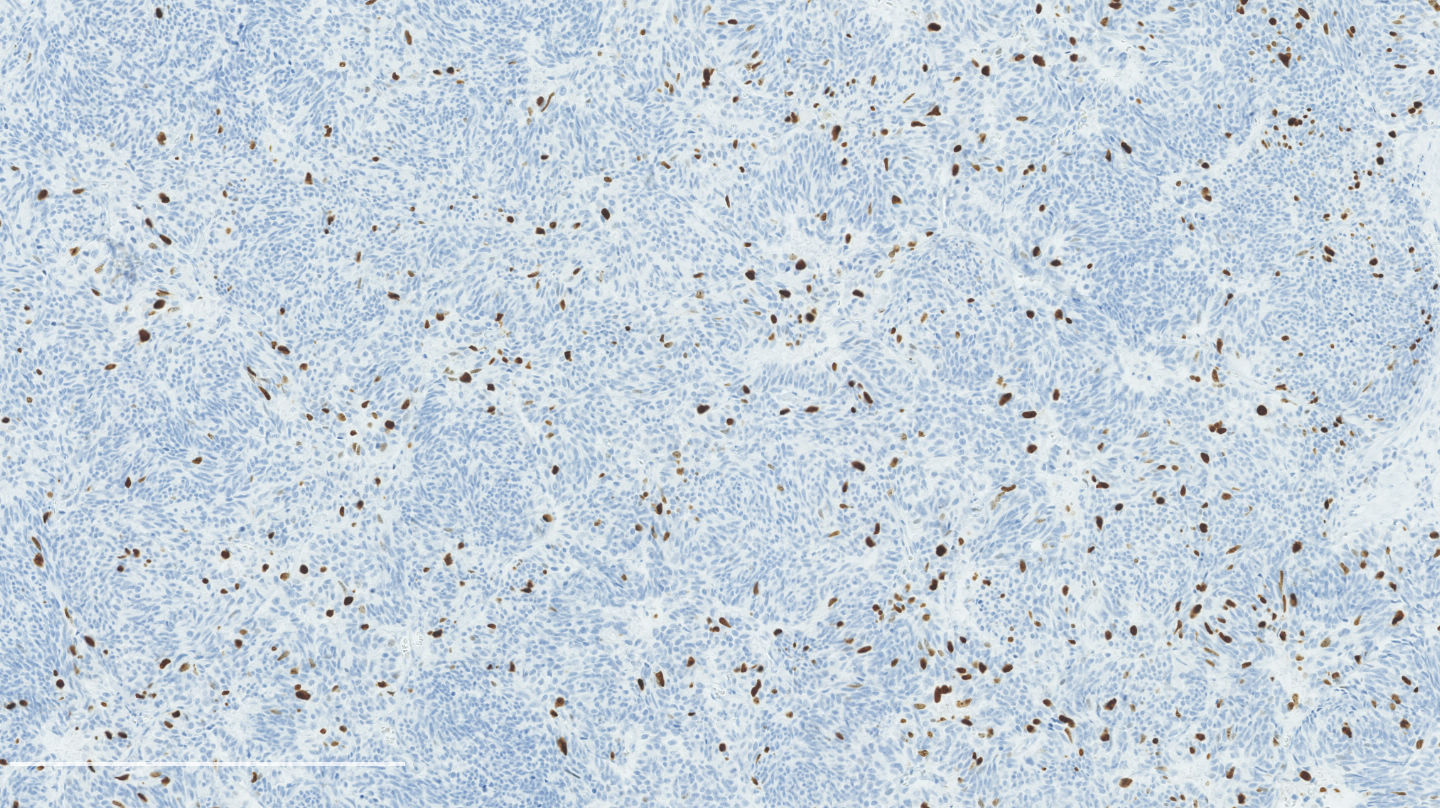
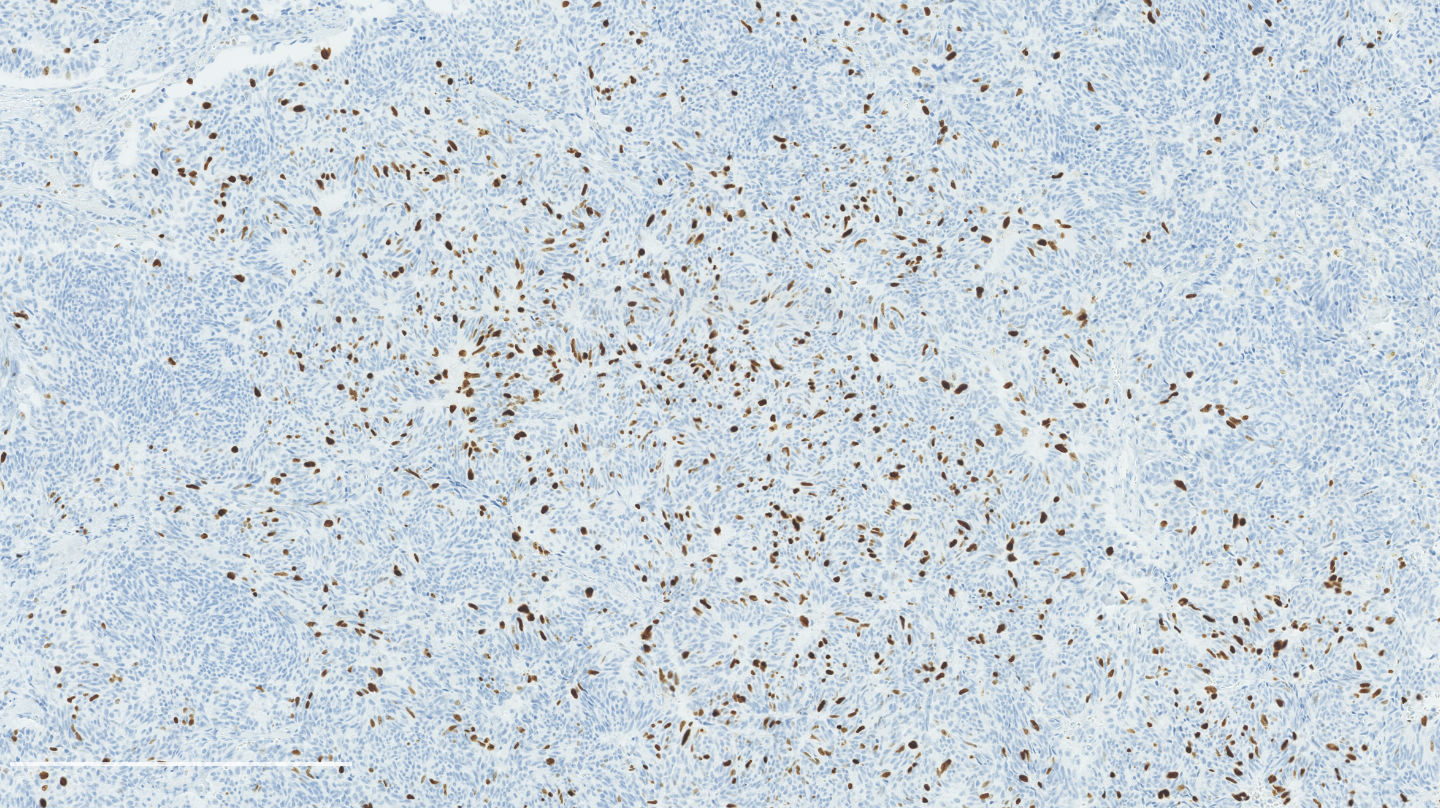
Microscopic (histologic) images
Contributed by Philippe Joubert, M.D., Ph.D.
Cytology description
- Cells and architecture similar to typical carcinoid tumors but can differ in several ways (Cibas: Cytology - Diagnostic Principles and Clinical Correlates, 4th edition, 2014)
- Groups of cells tend to be looser, with more isolated cells; rosette structures might be seen
- Population of tumor cells can be less uniform with slight pleomorphism
- Focal necrosis can be seen
- Mitoses can be seen but should be rare
- Even though the diagnosis can be suggested, a thorough examination of the surgical resection remains necessary to classify the tumor
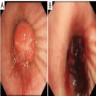
Cytology images
Positive stains
- Chromogranin, synaptophysin, CD56: diffusely and strongly positive; can be negative in a small number of atypical carcinoids (Hum Pathol 2000;31:1255)
- Pancytokeratins: positive but up to 20% can be negative
- TTF1: useful to establish a pulmonary lineage but positive in only 50% of tumors
- Ki67: should not be used as a diagnostic criterion (J Thorac Oncol 2019;14:377)
- Mostly useful to differentiate lung carcinoids from high grade neuroendocrine carcinomas, in particular in small biopsies or cytology samples (Arch Pathol Lab Med 2018;142:947, J Thorac Oncol 2014;9:273)
- Usually < 20% but a > 30% cutoff has been proposed (Virchows Arch 2017;470:153, J Thorac Oncol 2014;9:273)
- Reported to be higher than typical carcinoid (2 - 5% versus 9 - 18%) but not proven to be a reliable marker
- Rare tumors with > 10 mitoses per 2 mm² can have a > 30% proliferation rate (Neuroendocrinology 2019;108:109)
- Mostly useful to differentiate lung carcinoids from high grade neuroendocrine carcinomas, in particular in small biopsies or cytology samples (Arch Pathol Lab Med 2018;142:947, J Thorac Oncol 2014;9:273)
Negative stains
- CDX2: useful to differentiate from a metastatic gastrointestinal or pancreatic neuroendocrine tumor (Appl Immunohistochem Mol Morphol 2007;15:407)
Molecular / cytogenetics description
- Mutations in the chromatin remodeling genes, including MEN1 and SWI/SNF complex (Transl Lung Cancer Res 2017;6:513)
- RB1 and TP53 are uncommon
- Low number of chromosomal imbalances
Sample pathology report
- Right lung, superior lobe, transbronchial biopsy:
- Carcinoid tumor, not further classified (see comment)
- Comment: The presence of focal necrosis or increased mitotic activity on this biopsy combined with the classic morphology of a carcinoid tumor favors a diagnosis of atypical carcinoid. However, the definitive diagnosis will be made on the resection specimen.
Differential diagnosis
Practice question #1
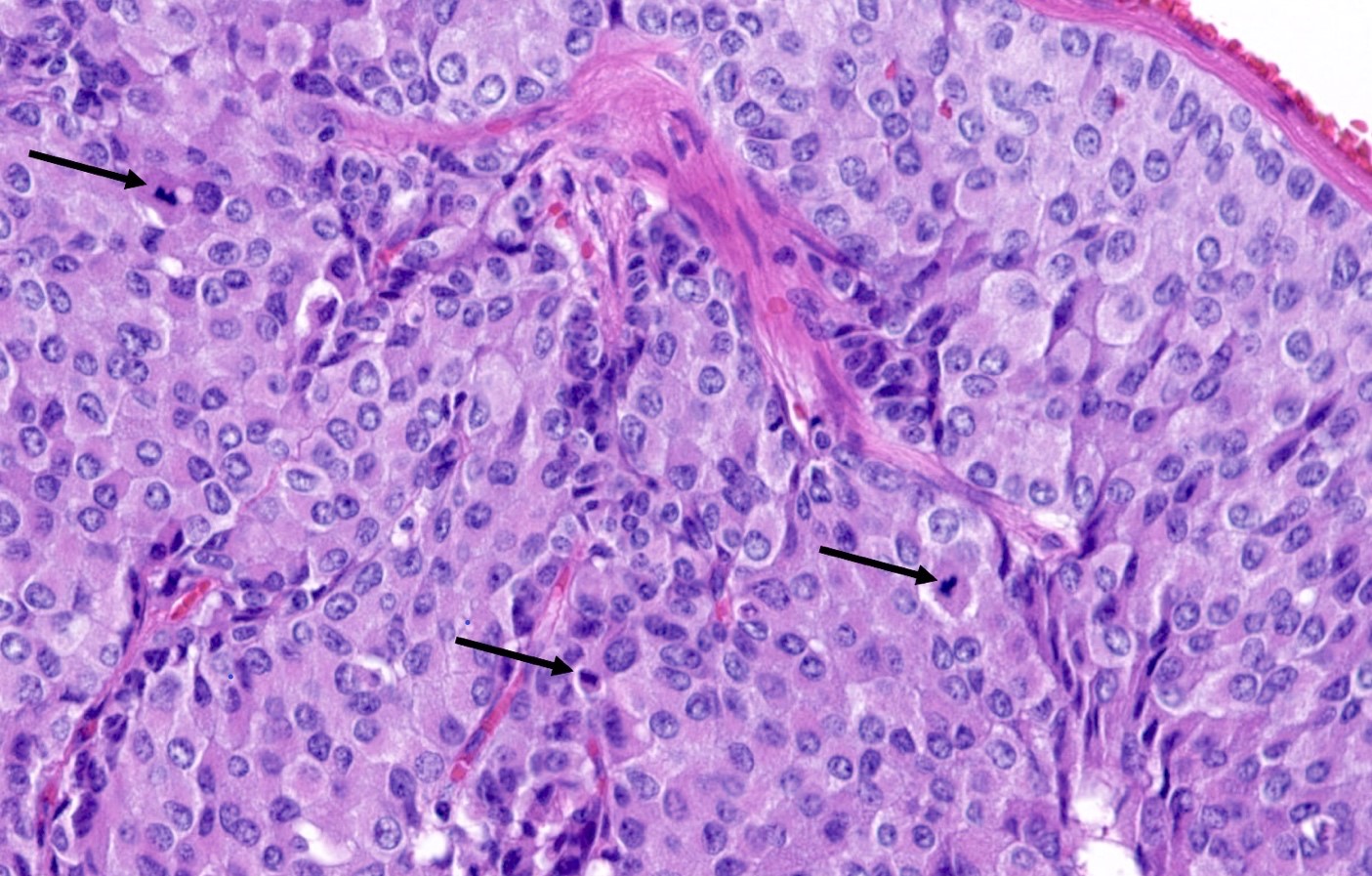
- A patient undergoes a lobectomy for a well circumscribed nodule. On H&E slide, the tumor exhibits a well differentiated neuroendocrine morphology and you observe the histologic features presented in the image. Which of the following statements is true?
- A Ki67 proliferation rate of > 10% is diagnostic
- It is defined as a well differentiated neuroendocrine tumor with 2 - 10 mitoses per 2 mm² or foci of necrosis
- It is defined as a well differentiated neuroendocrine tumor with 2 - 10 mitoses in 1 high power field or foci of necrosis
- This diagnosis can be made with certainty on small samples (biopsies and cytology)
Practice answer #1
B. It is defined as a well differentiated neuroendocrine tumor with 2 - 10 mitoses per 2 mm² or foci of necrosis. The picture shows a carcinoid lung tumor with a classical neuroendocrine morphology and 2 mitoses in 1 high power field. Even though the whole tumor is not presented here, the presence of 2 mitoses is sufficient for an atypical carcinoid diagnosis.
While Ki67 proliferation rate is frequently > 10% in atypical carcinoids, this feature is not part of the diagnosis (A). C is nearly exact but mitotic count is not made on 1 high power field. Finally, the diagnosis can be suggested on small samples but a thorough examination of a resection specimen is necessary to confirm an atypical carcinoid diagnosis (D).
Comment Here
Reference: Atypical carcinoid
While Ki67 proliferation rate is frequently > 10% in atypical carcinoids, this feature is not part of the diagnosis (A). C is nearly exact but mitotic count is not made on 1 high power field. Finally, the diagnosis can be suggested on small samples but a thorough examination of a resection specimen is necessary to confirm an atypical carcinoid diagnosis (D).
Comment Here
Reference: Atypical carcinoid
Practice question #2
- Regarding pulmonary atypical carcinoids, which of the following statements is true?
- Differentiating them from typical carcinoid is crucial as they have a poorer prognosis and are more likely to metastasize
- Neuroendocrine immunohistochemical markers (chromogranin, synaptophysin and CD56) are systematically positive
- They are less likely to be peripherally located than typical carcinoids
- They frequently harbor ALK-ELM4 fusions
Practice answer #2
A. Differentiating them from typical carcinoid is crucial as they have a poorer prognosis and are more likely to metastasize. Neuroendocrine immunohistochemical markers are not always positive and can be completely negative in a small subset of atypical carcinoids (B). Regarding their location, atypical carcinoids are more frequently peripherally located and typical carcinoids are more frequently central (C). ALK-EML4 fusions are not found in atypical carcinoids (D).
Comment Here
Reference: Atypical carcinoid
Comment Here
Reference: Atypical carcinoid