Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Bennett J. Perivascular epithelioid cell tumor (PEComa). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uteruspecoma.html. Accessed September 18th, 2025.
Definition / general
- Rare mesenchymal neoplasm with myomelanocytic differentiation that is favored to arise from perivascular epithelioid cells
Essential features
- Best classified as a tumor of uncertain malignant potential since recurrences may arise years after initial diagnosis
- Characterized by sheets or nests of epithelioid cells with clear to eosinophilic and granular cytoplasm, surrounded by delicate vasculature
- Coexpresses melanoma and smooth muscle markers, with variable staining intensity and distribution
- 2 main molecular subgroups: TSC1 / TSC2 alterations, TFE3 fusions
ICD coding
Epidemiology
- Most commonly presents at 40 - 60 years (range: 6 - 79) (Genes Chromosomes Cancer 2021;60:168)
Sites
- Uterine corpus is most common in gynecologic tract
- Rare in cervix, vagina, ovary, broad ligament and vulva
Pathophysiology
- Somatic or germline mutations in TSC1 / TSC2 inactivate the tuberous sclerosis complex protein, leading to activation of the mTOR kinase (Nat Cell Biol 2019;21:63, Genes Chromosomes Cancer 2021;60:168)
Etiology
- ~10% associated with tuberous sclerosis complex (Am J Surg Pathol 2018;42:1370)
Clinical features
- Generally present with nonspecific gynecologic symptoms (abnormal uterine bleeding, abdominal / pelvic pain) (Am J Surg Pathol 2018;42:1370)
- May be discovered incidentally during evaluation for adnexal mass, leiomyomas, infertility or at cesarean section
- Rarely present with uterine rupture or hemoperitoneum, especially in pregnancy (J Obstet Gynaecol Res 2019;45:709, Int J Gynecol Cancer 2020;30:2008)
Diagnosis
- Rarely made on endometrial sampling
- Myomectomy or hysterectomy
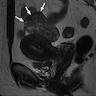
Radiology description
- No defining features to distinguish from other uterine mesenchymal neoplasms on imaging
Radiology images
Prognostic factors
- 3 main algorithms have been proposed for predicting behavior
- Nongynecologic specific criteria (Am J Surg Pathol 2005;29:1558):
- Benign: < 5 cm, noninfiltrative, no high grade atypia, mitoses ≤ 1/50 high power fields (HPFs), no necrosis, no lymphovascular invasion (LVI)
- Uncertain malignant potential: nuclear pleomorphism / multinucleated giant cells or > 5 cm
- Malignant: 2+ features (> 5 cm, noninfiltrative, high grade atypia, mitoses > 1/50 HPFs, necrosis, LVI)
- Gynecologic specific criteria (Am J Surg Pathol 2014;38:176):
- Benign / uncertain malignant potential: < 4 features (≥ 5 cm, high grade atypia, mitoses > 1/50 HPFs, necrosis, LVI)
- Malignant: ≥ 4 features
- Modified gynecologic specific criteria (adopted by the WHO 2020) (Am J Surg Pathol 2018;42:1370)
- Uncertain malignant potential: < 3 features (> 5 cm, high grade atypia, mitoses > 1/50 HPFs, necrosis, LVI)
- Malignant: ≥ 3 features
- Nongynecologic specific criteria (Am J Surg Pathol 2005;29:1558):
- Recurrences can occur years after original diagnosis
Case reports
- 21 year old woman with a subserosal uterine nodule identified at cesarean section (Gynecol Oncol Rep 2022;40:100962)
- 38 year old woman with adnexal mass and 62 year old woman with abnormal uterine bleeding (Eur J Med Res 2017;22:7)
- 65 year old woman with postmenopausal bleeding and abdominal pain (Int J Clin Exp Pathol 2021;14:993)
Treatment
- Surgery (hysterectomy, myomectomy) is first line therapy as many are presumed leiomyomas
- Chemotherapy and radiation may be administered in patients with metastatic or recurrent disease
- mTOR inhibitors have shown variable results (Genes Chromosomes Cancer 2021;60:168, J Clin Oncol 2021;39:3660)
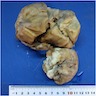
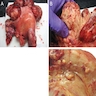
Gross description
- Mean: 6.5 cm; range: 0.2 - 25 cm (Genes Chromosomes Cancer 2021;60:168)
- Typically intramural but may be polypoid
- Tan, white, pink or gray solid mass
- Subset show hemorrhage or necrosis
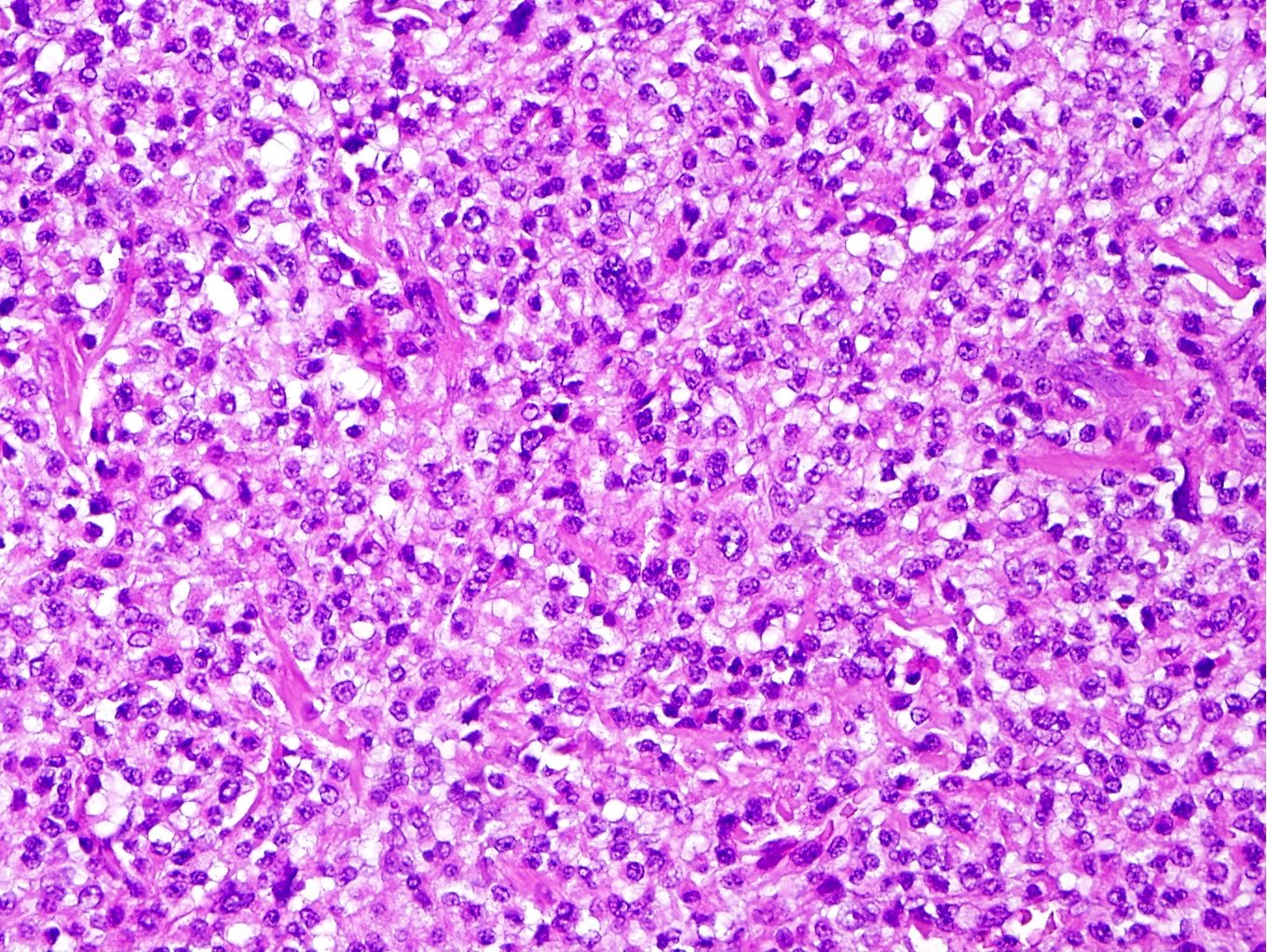
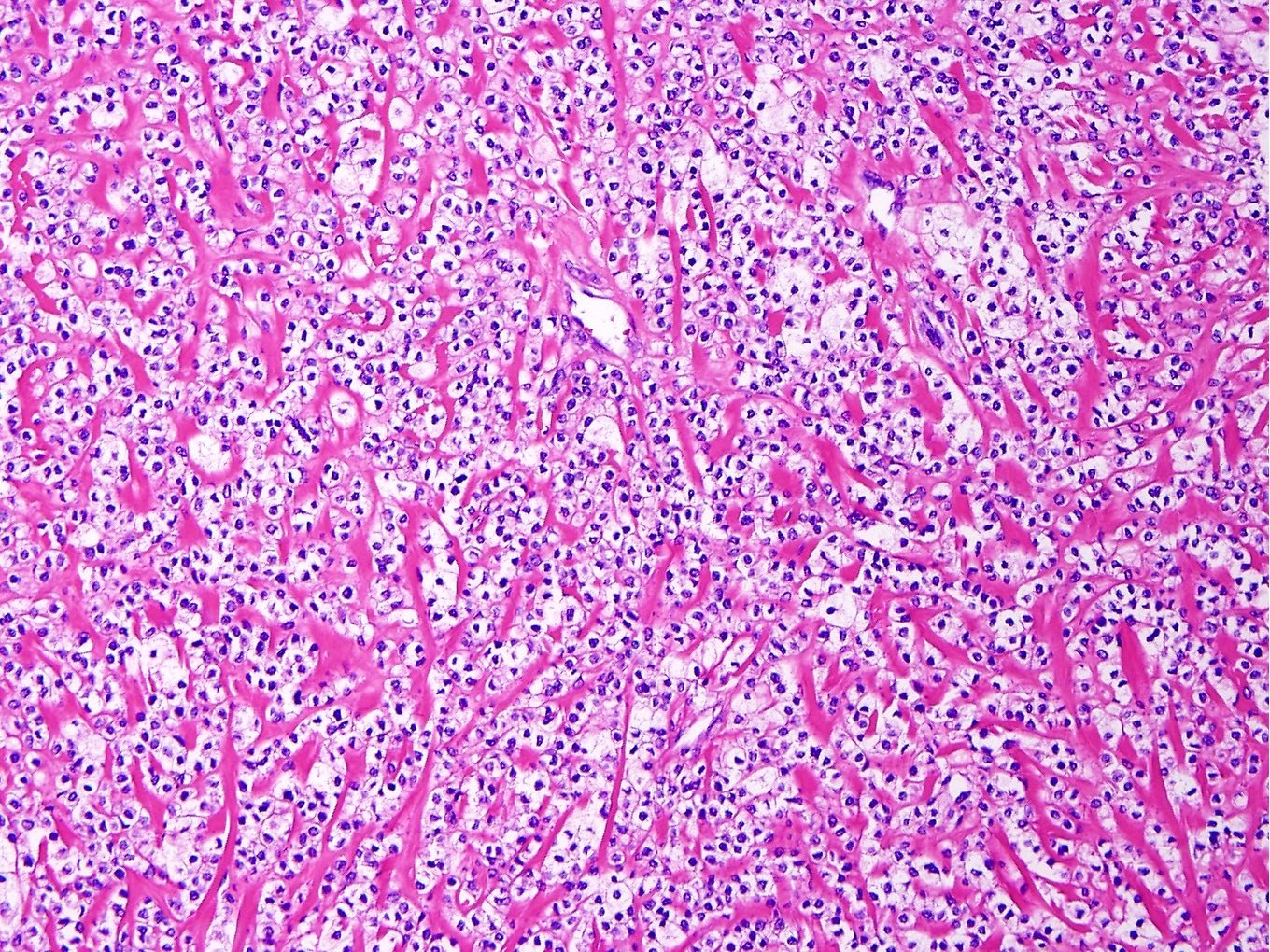
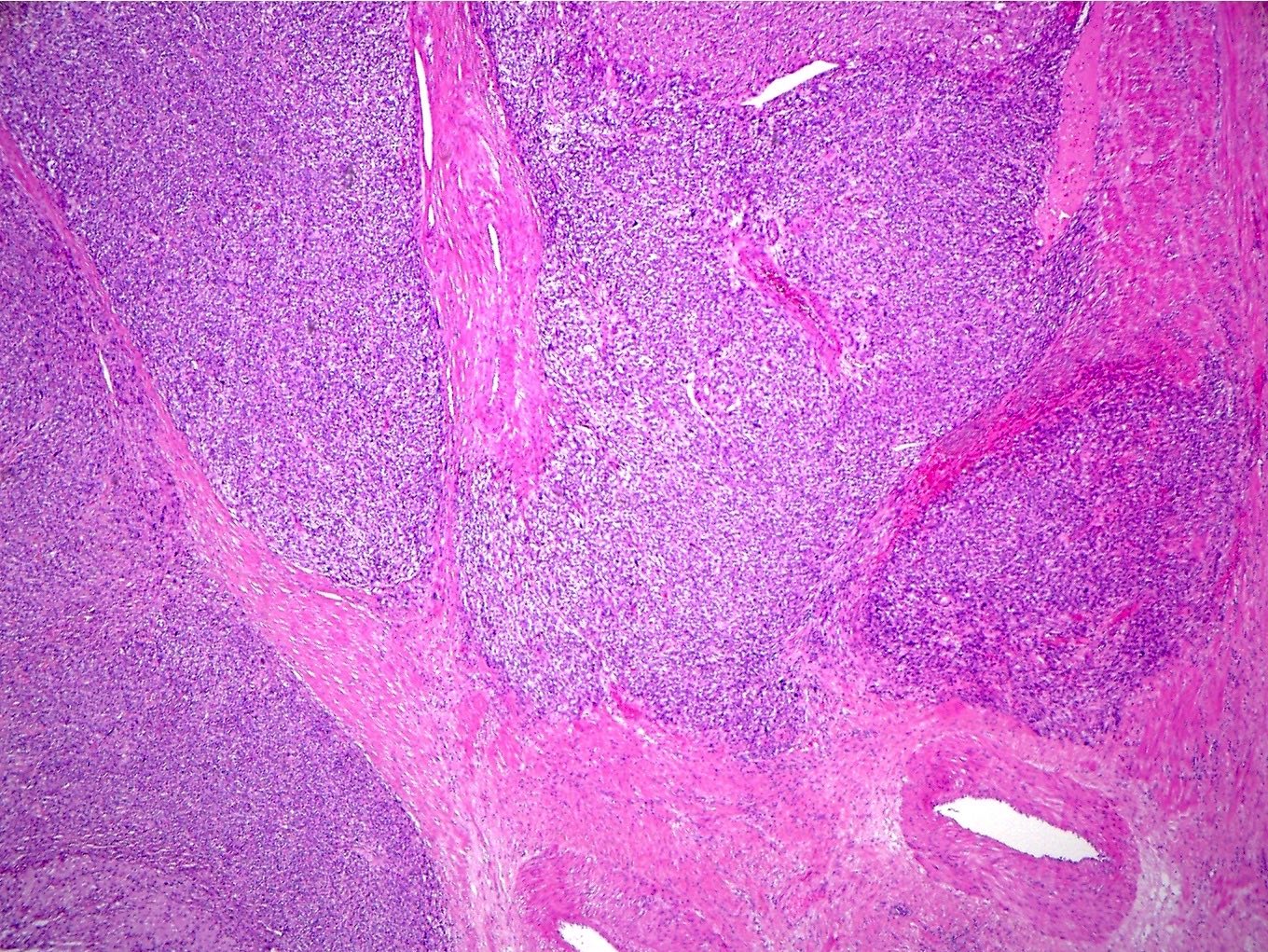
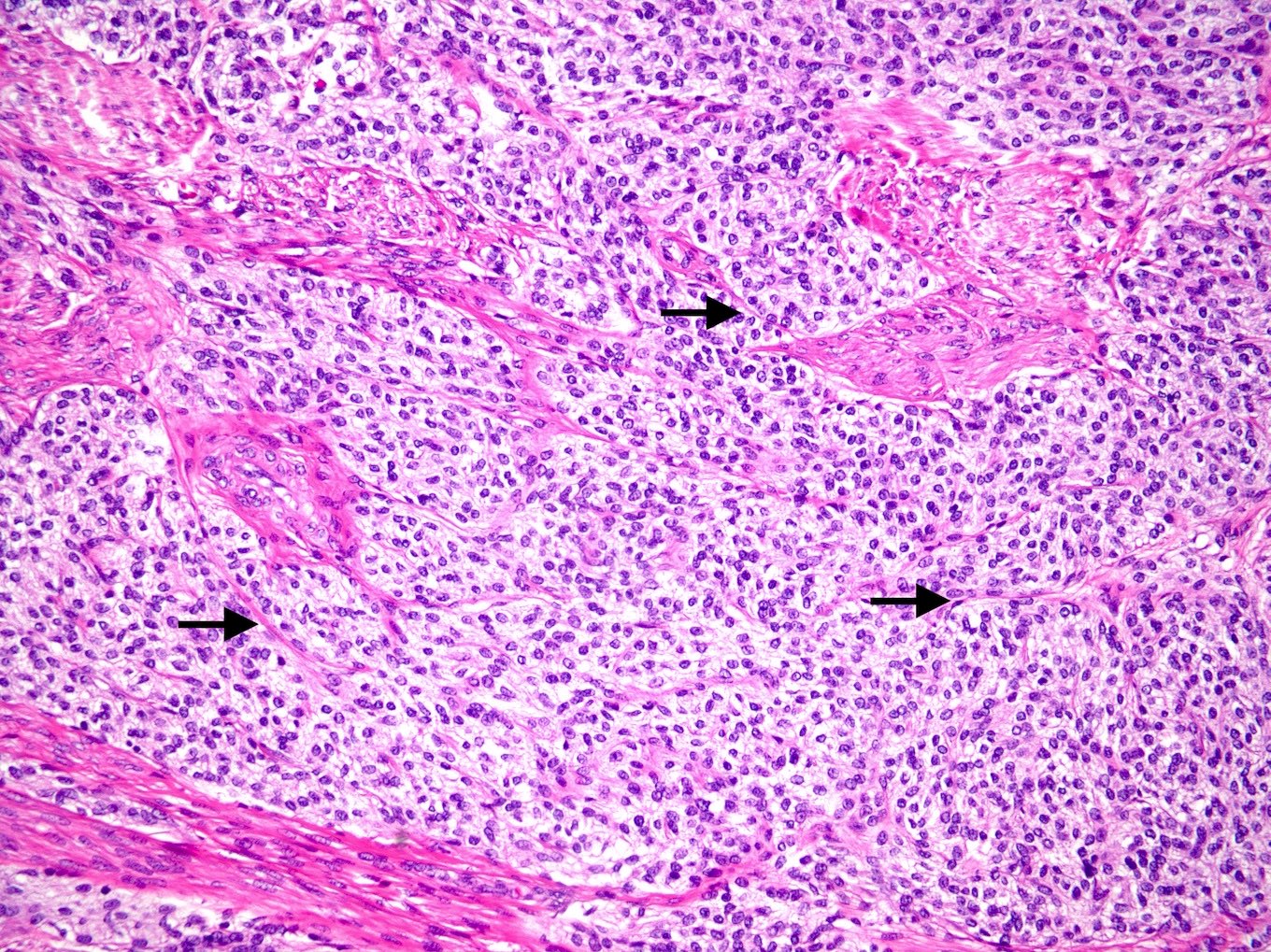
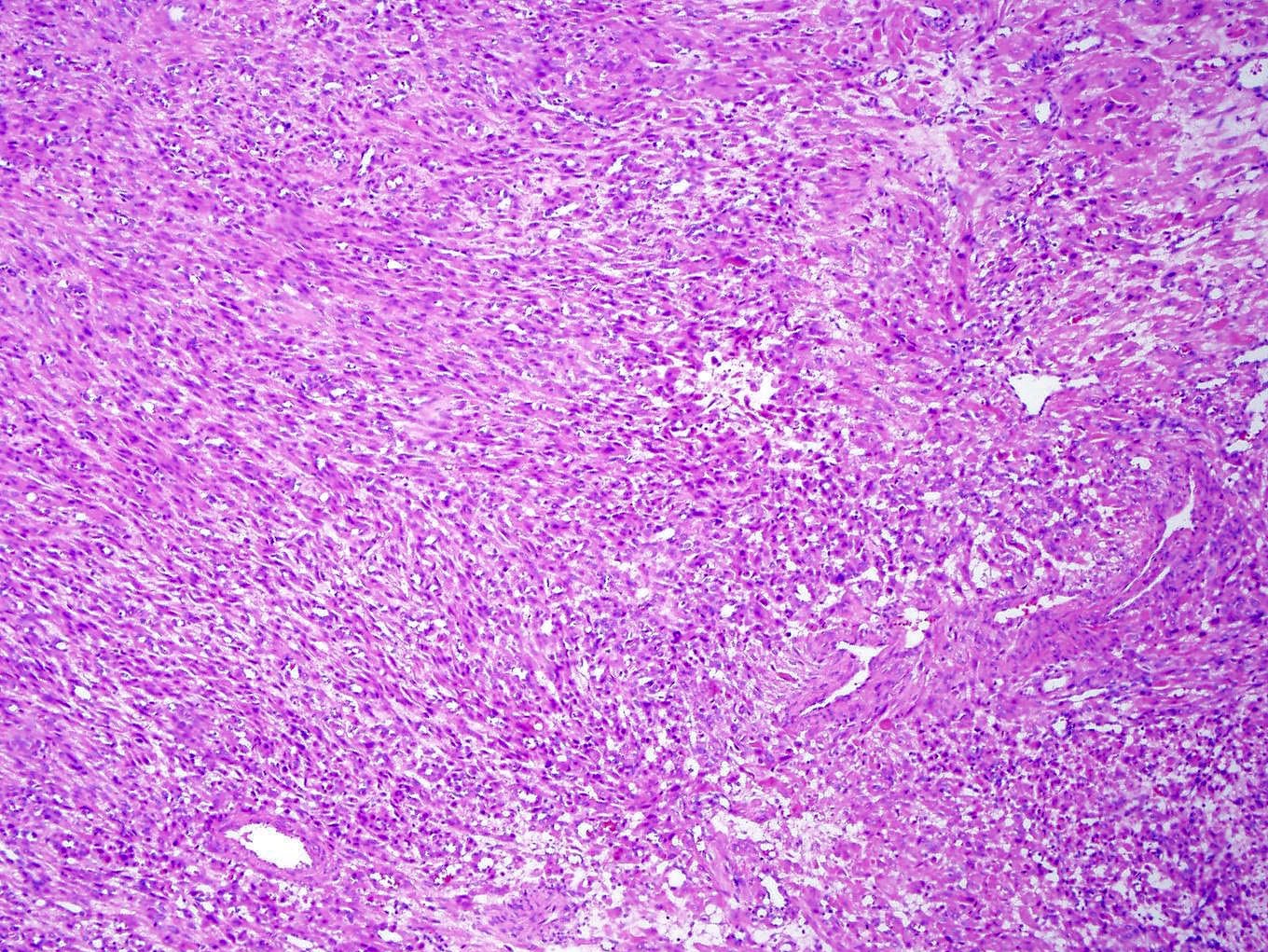
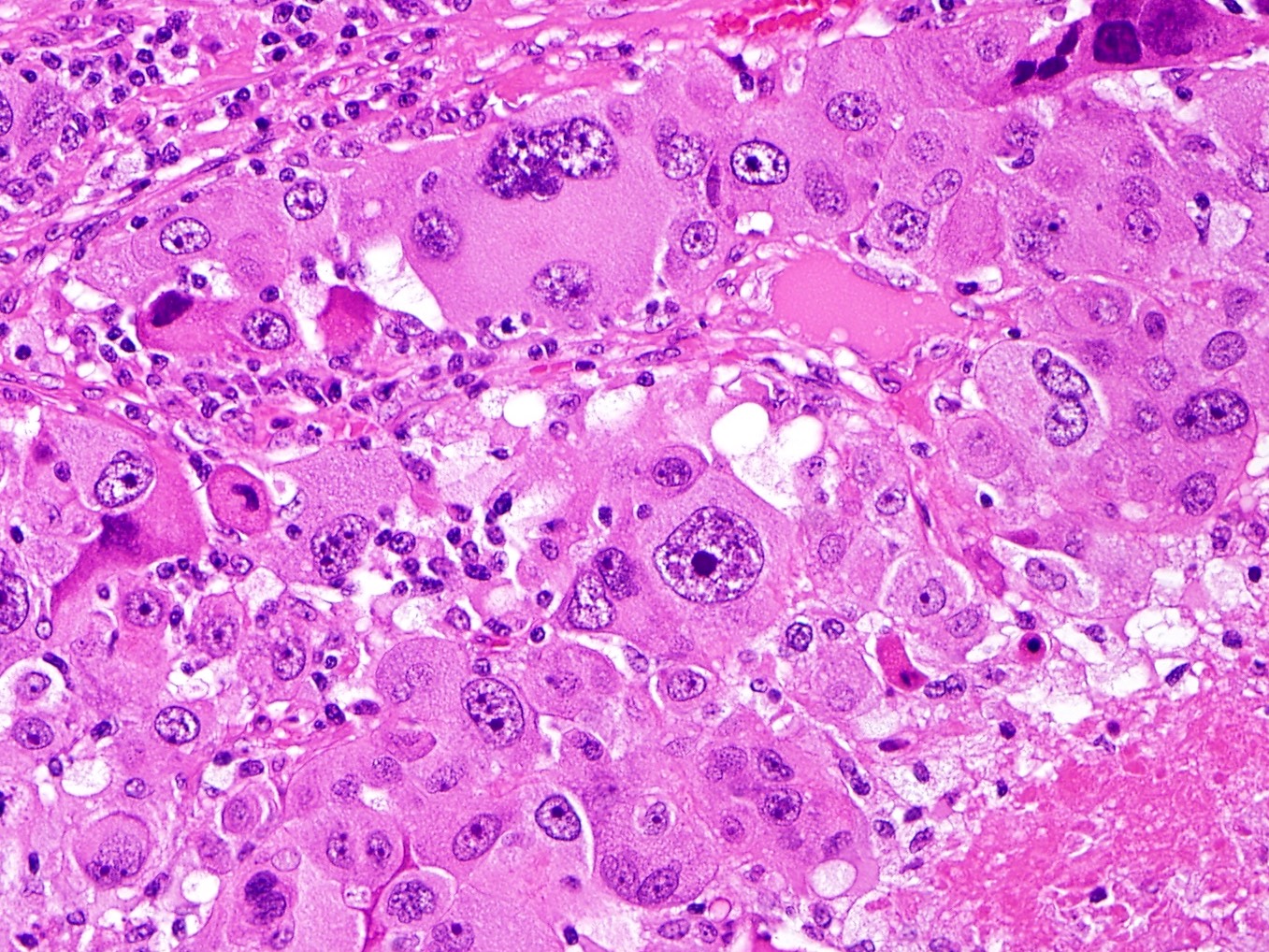
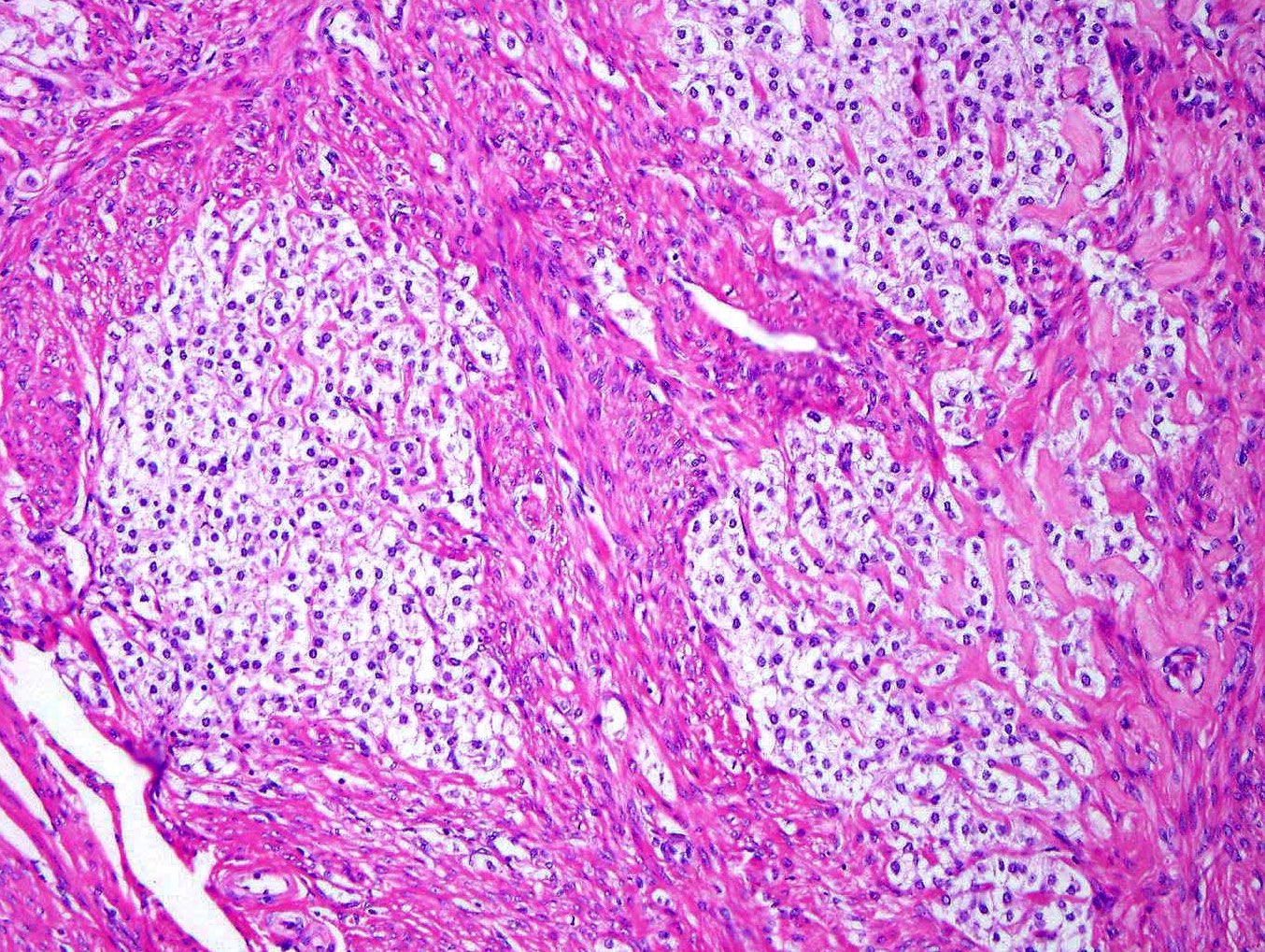
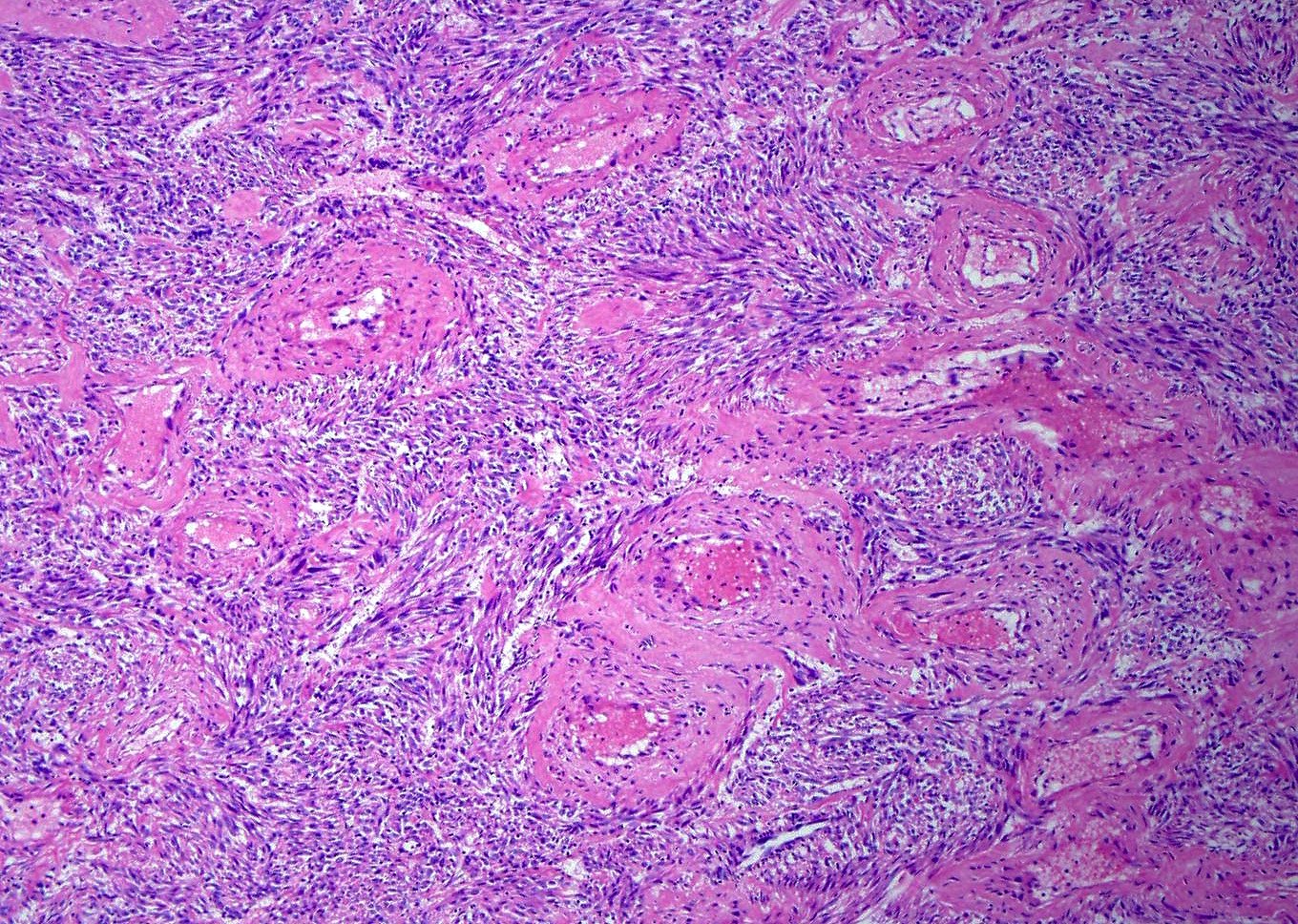
Microscopic (histologic) description
- Well circumscribed or infiltrative growth
- Numerous growth patterns, with sheets and nests being the most common
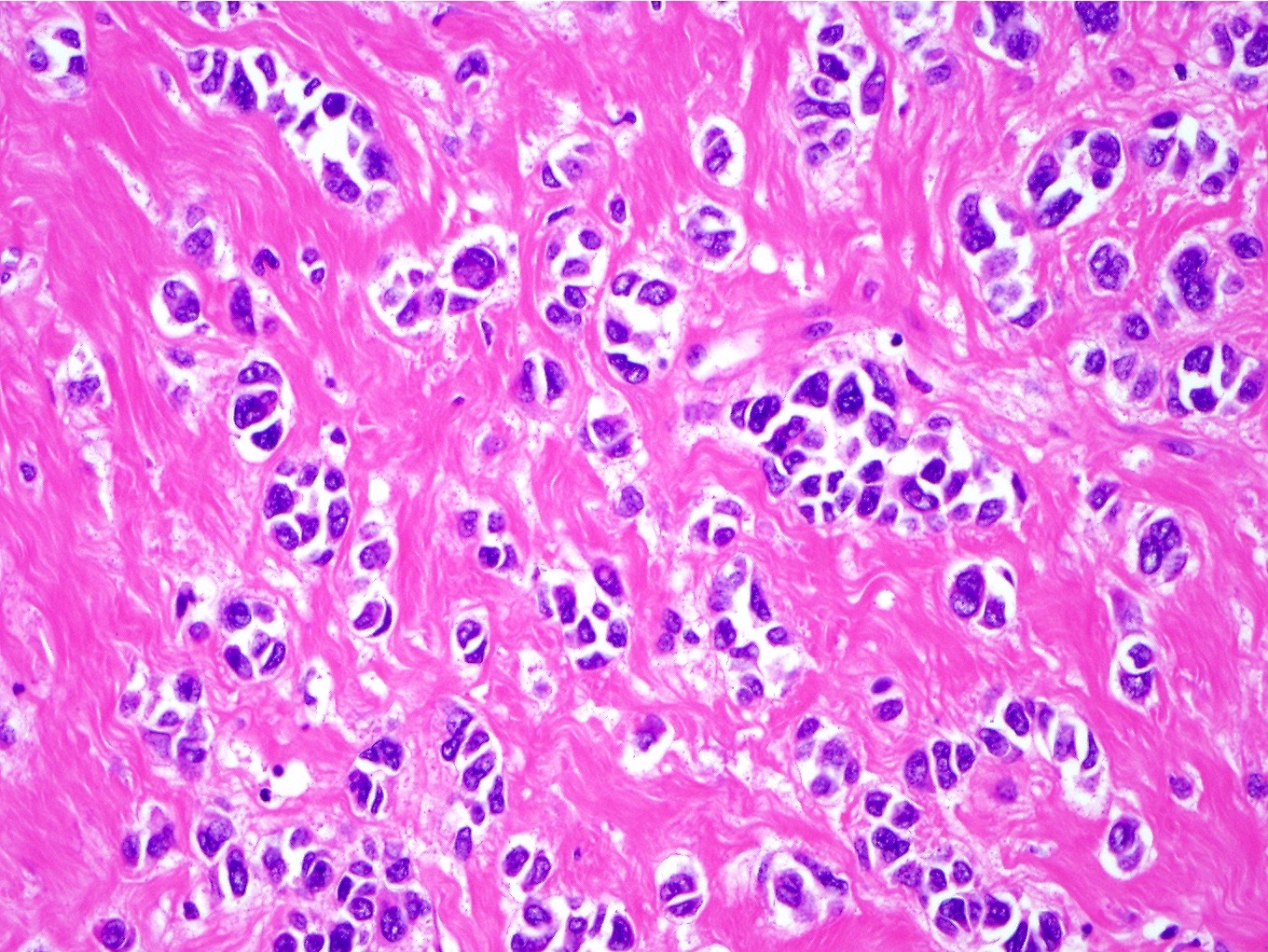
- Noncohesive epithelioid cells with clear to eosinophilic granular cytoplasm
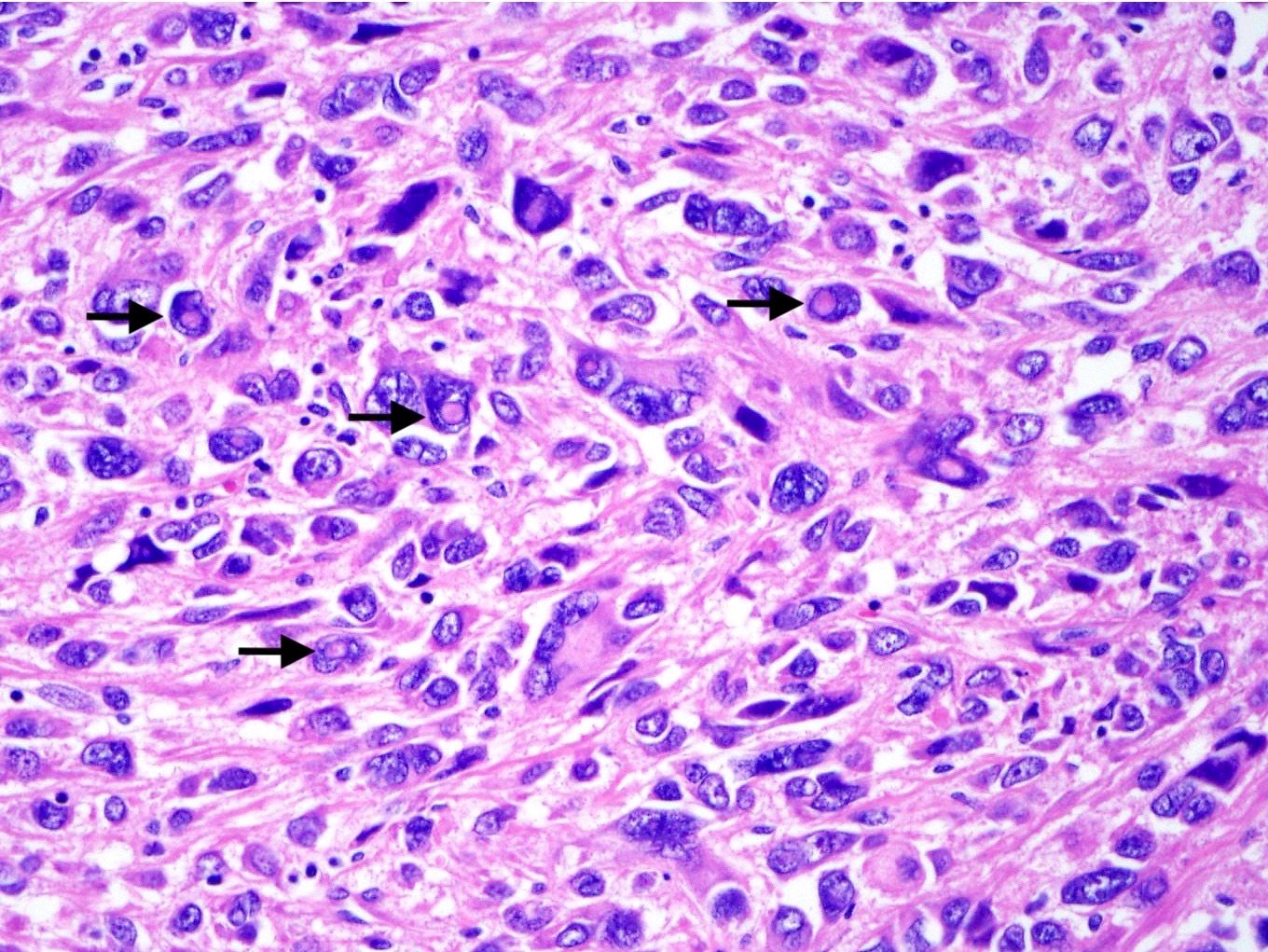
- May have a component of spindled cells (usually minor)
- Subset have dense eosinophilic (rhabdoid) or foamy cytoplasm
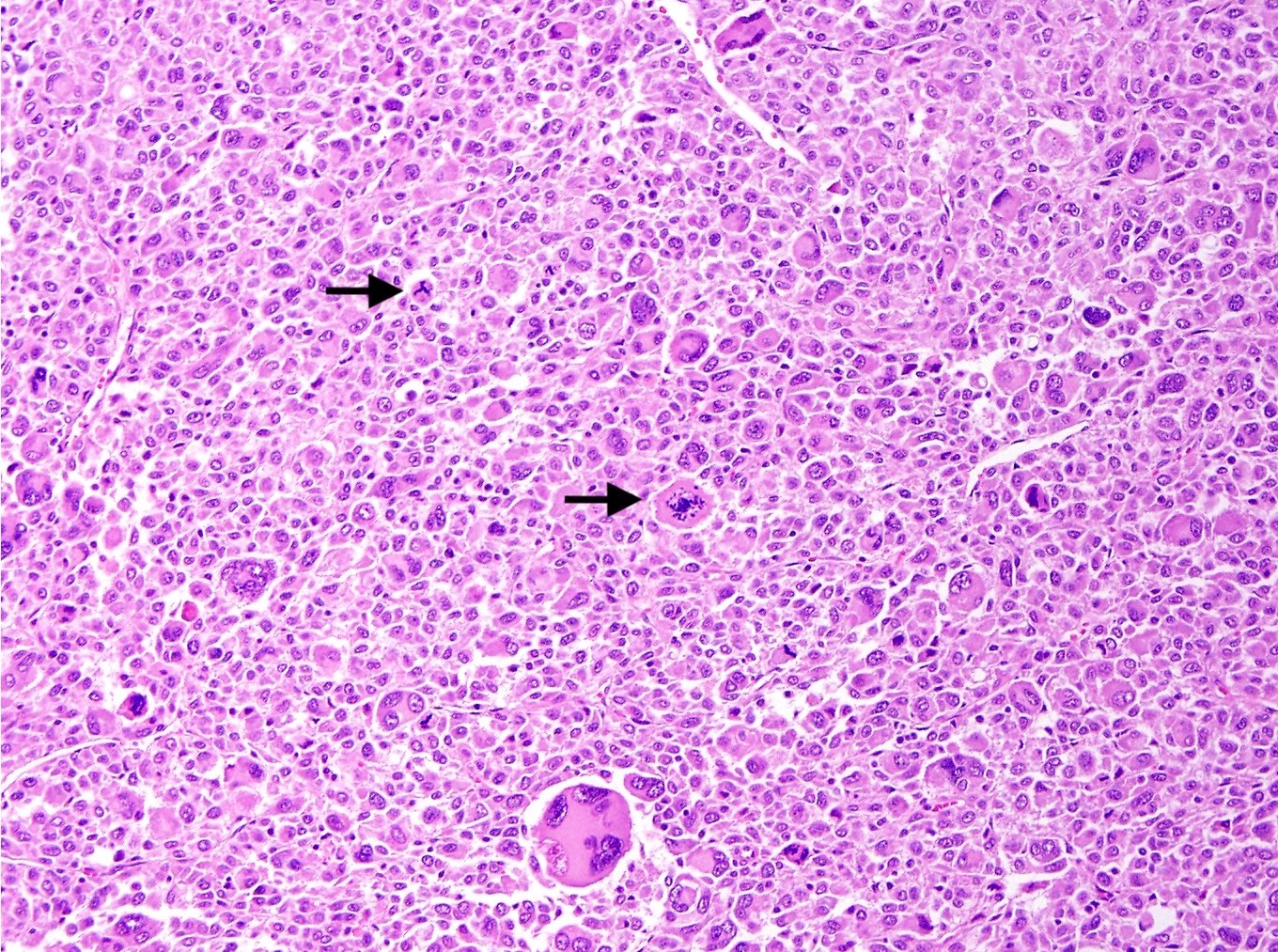
- Variable cytologic atypia and mitotic index
- Melanoma-like nucleoli, intranuclear pseudoinclusions, multinucleated cells, Touton giant cells and melanin pigment (rare) may be present
- Characterized by thin and delicate vessels but may also have thick walled (generally peripherally located) and staghorn vessels
- Radial / perivascular distribution of tumor cells identified in < 25% (Am J Surg Pathol 2018;42:1370)
- Stromal hyalinization is common
- Sclerosing PEComa is rare in the gynecologic tract (Int J Gynecol Pathol 2011;30:121, Pathol Int 2011;61:768)
- TFE3 translocation associated PEComas (Am J Surg Pathol 2010;34:1395, Am J Surg Pathol 2015;39:394):
- Typically epithelioid with clear cells in a nested / alveolar growth
- Low grade atypia and rare mitoses
- Lymphangioleiomyomatosis (LAM)-like PEComas are rare, predominantly spindled with thick walled blood vessels, cleft / slit-like spaces, low grade atypia and infrequent mitoses (Am J Surg Pathol 2018;42:1370)
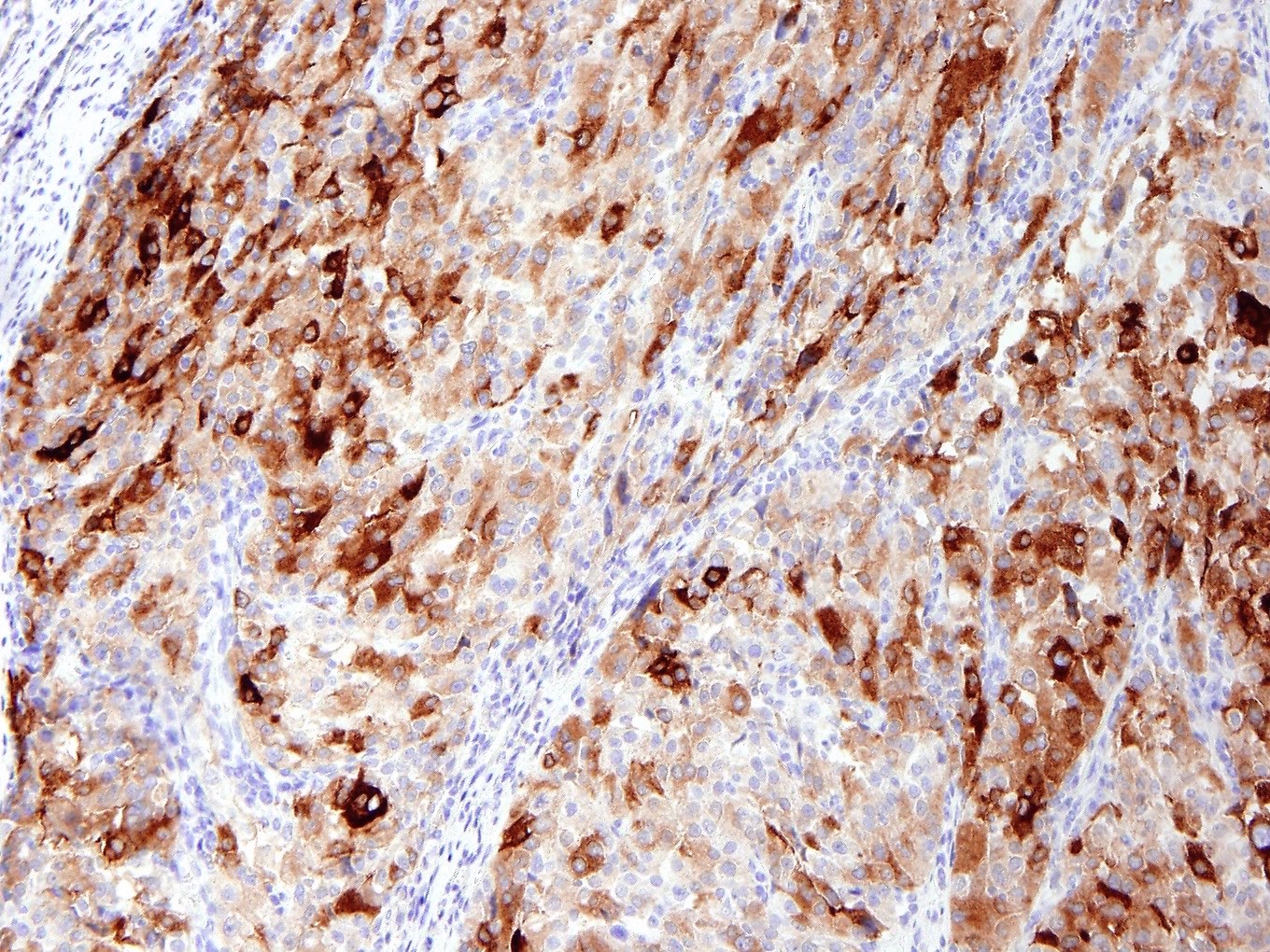
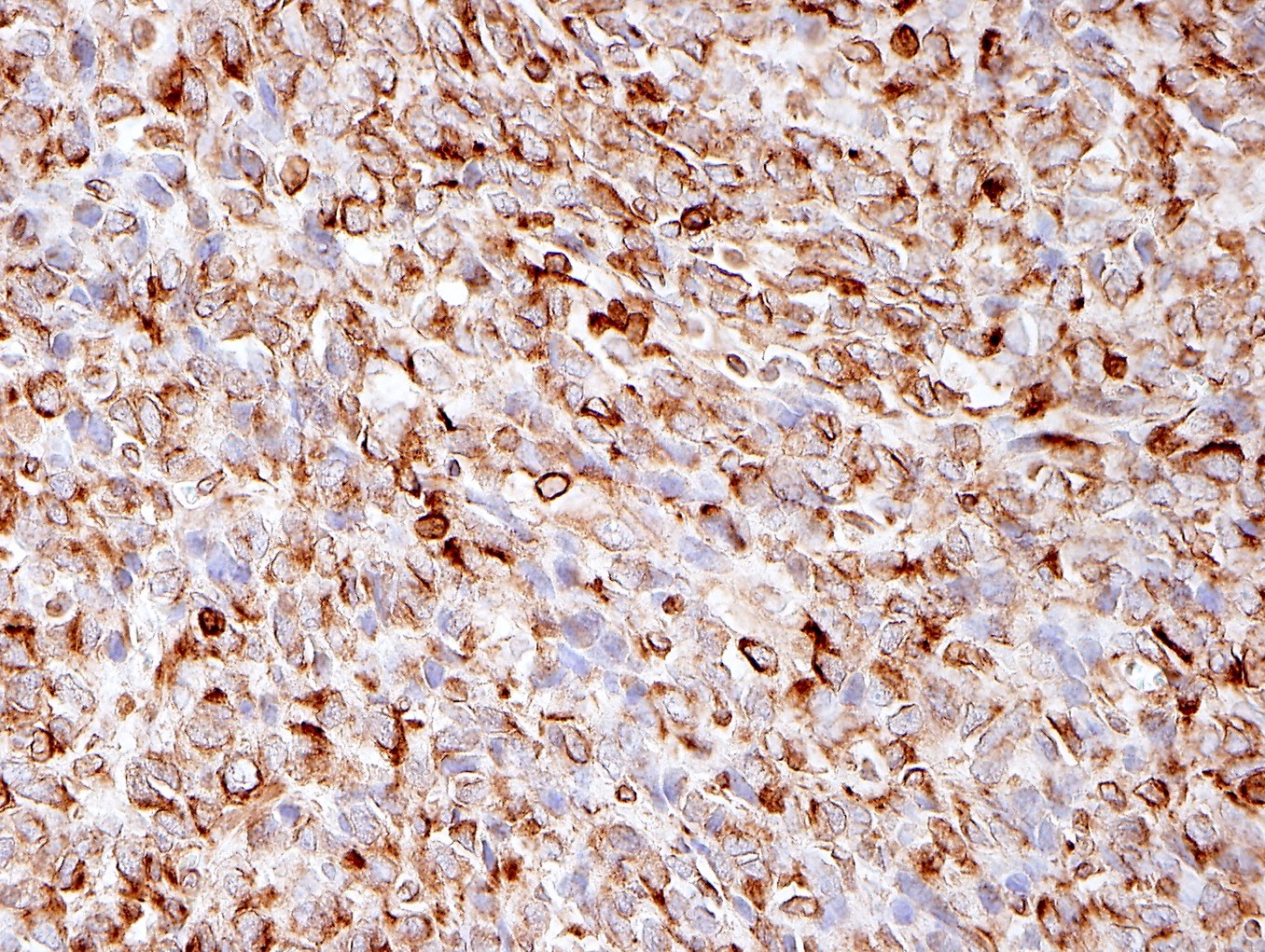
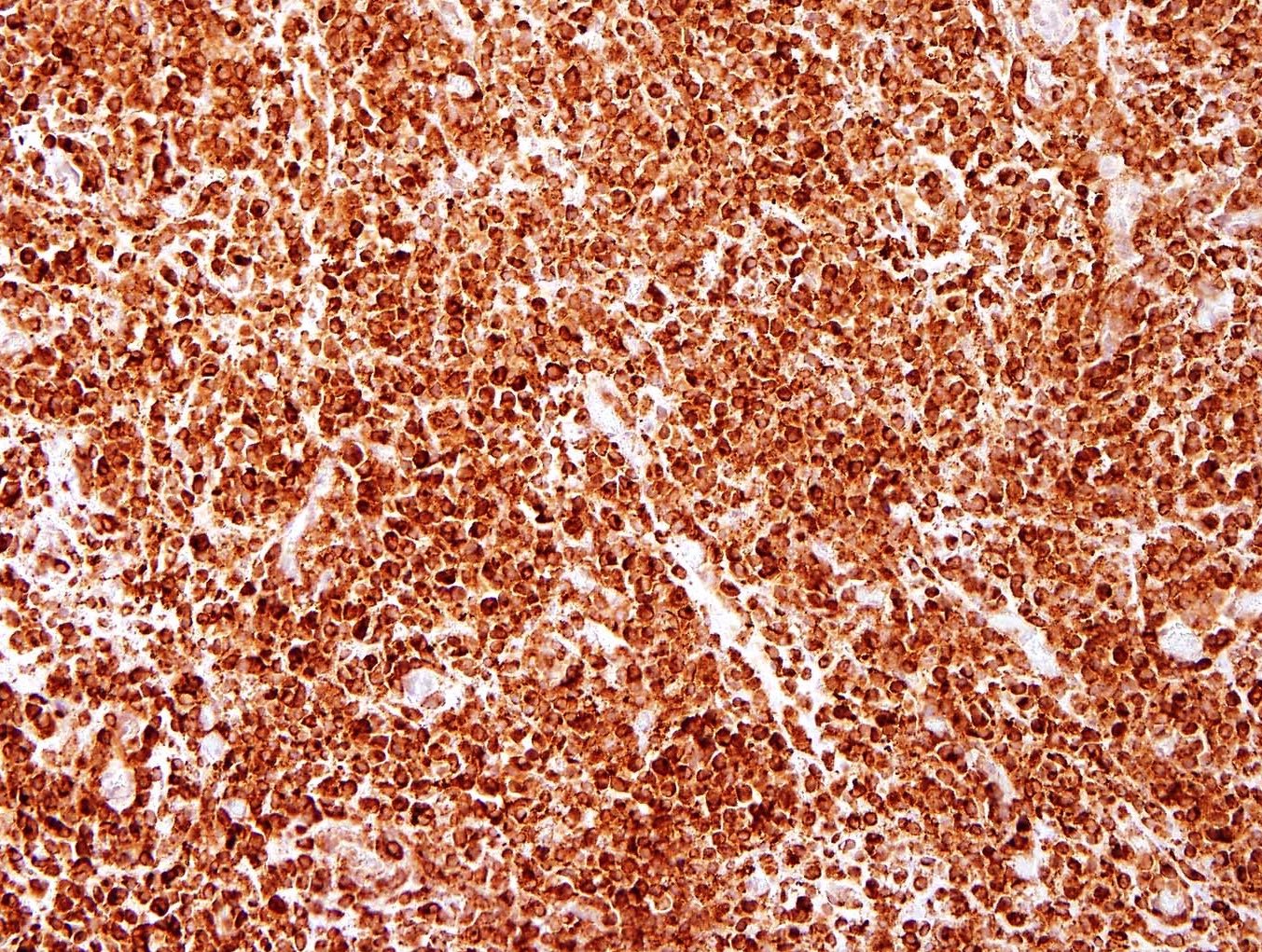
Microscopic (histologic) images
Contributed by Jennifer Bennett, M.D.
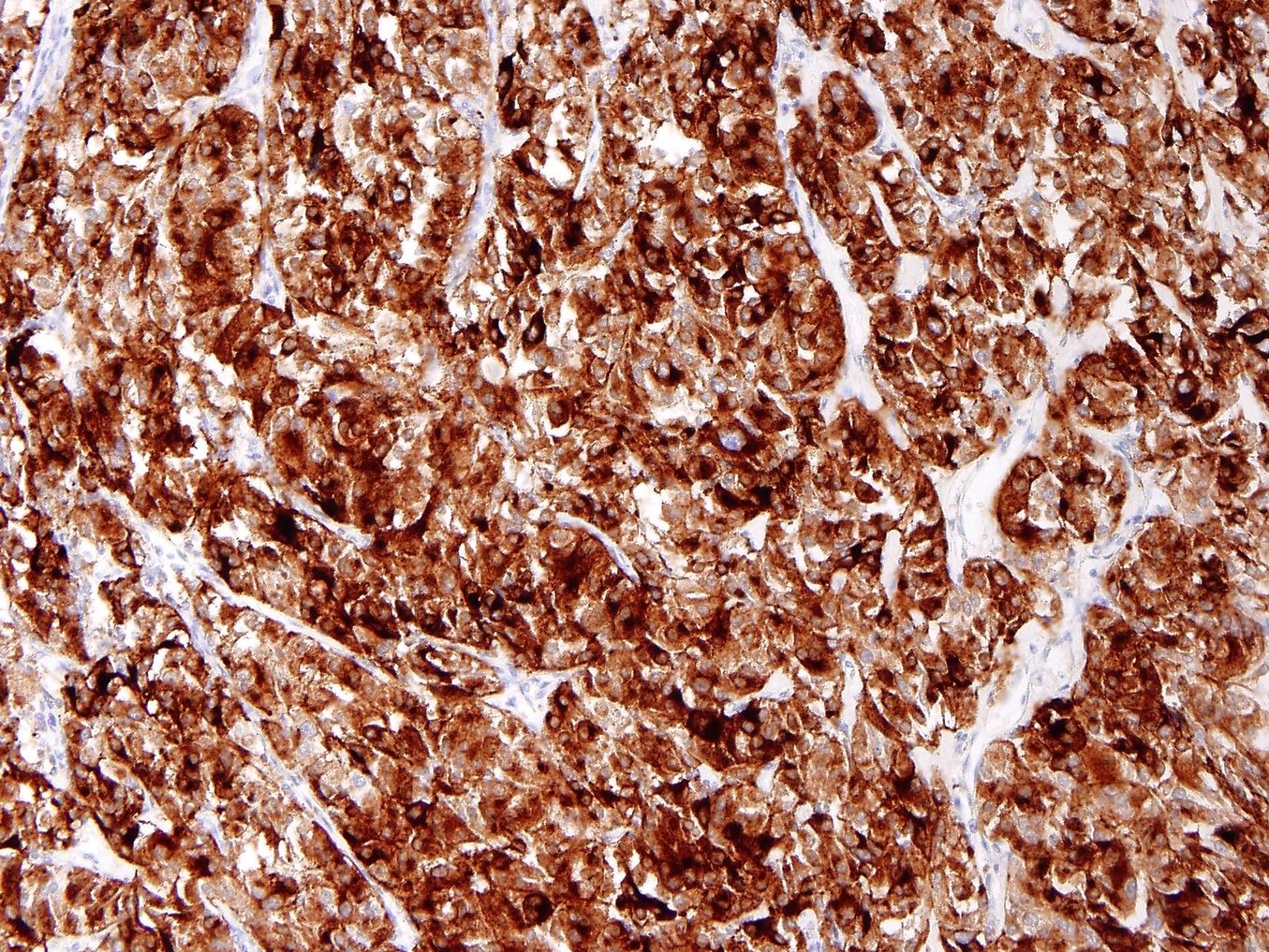
Positive stains
- Melanocytic markers - variable intensity and distribution (Genes Chromosomes Cancer 2021;60:168):
- HMB45 (99%)
- PNL2 (86%)
- MiTF (83%)
- MelanA / MART1 (67%)
- Myogenic markers - variable intensity and distribution (Genes Chromosomes Cancer 2021;60:168):
- Cathepsin K: usually strong and diffuse
- ER (53%), PR (85%) (J Clin Pathol 2015;68:418)
- TFE3 may show focal staining but typically is nonspecific unless strong and diffuse (Am J Surg Pathol 2010;34:1395)
- TFE3 translocation associated PEComa: HMB45 and TFE3 strong and diffuse
Negative stains
- S100 (focally positive in 20%) (Am J Surg Pathol 2014;38:176)
- AE1 / AE3 (focally positive in 11%) (Am J Surg Pathol 2014;38:176)
- PAX8
- TFE3 translocation associated PEComa: MelanA / MART1 and smooth muscle markers (focal / negative), MiTF negative
Molecular / cytogenetics description
- 2 main molecular subgroups that are mutually exclusive:
- TSC1 / TSC2 alterations (Am J Surg Pathol 2015;39:813, Am J Surg Pathol 2021;45:77, Oncology 2020;98:905, Mod Pathol 2022;35:515)
- TFE3 fusions (Am J Surg Pathol 2010;34:1395, Am J Surg Pathol 2015;39:394)
- RAD51B and HTR4-ST3GAL1 fusions are rare (Am J Surg Pathol 2015;39:813, Am J Surg Pathol 2018;42:1370)
- Concurrent TP53, ATRX or RB1 alterations in malignant PEComas (Am J Surg Pathol 2015;39:813, Am J Surg Pathol 2021;45:77, Oncology 2020;98:905, Mod Pathol 2022;35:515)
- Tumors with hybrid PEComa and endometrial stromal sarcoma histologic features harbor TSC2 mutations and JAZF1-SUZ12 fusions (Mod Pathol 2022;35:117)
Sample pathology report
- Uterus, hysterectomy:
- PEComa of uncertain malignant potential (4.5 cm) (see comment)
- Chronic cervicitis
- Proliferative endometrium
- Leiomyomas
- Comment: 1 of the myometrial nodules is comprised of sheets of epithelioid cells with clear to eosinophilic and granular cytoplasm. Cytologic atypia, mitoses and necrosis are not appreciated. This nodule is positive for HMB45 (50%, strong), MelanA (focal), desmin, caldesmon and SMA. The morphologic and immunoprofile supports the diagnosis of PEComa. Based on the WHO 2020 criteria for predicting behavior in PEComas, this tumor would classify as being of uncertain malignant potential. Most tumors in this category have a benign clinical course; however, as a small subset recur, close clinicoradiological follow up is recommended.
Differential diagnosis
- Epithelioid smooth muscle tumor (Am J Surg Pathol 2022;46:464):
- No thin or delicate vessels
- Perinuclear vacuoles and diffuse eosinophilic cytoplasm with cytoplasmic granularity
- Typically negative or focally / weakly positive for melanocytic markers and cathepsin K
- No known association with TSC1 / TSC2 alterations or TFE3 fusions
- Subset with PGR fusions (Am J Surg Pathol 2019;43:810)
- Malignant melanoma:
- Alveolar soft part sarcoma (Am J Surg Pathol 2017;41:622):
- Poorly differentiated endometrial carcinoma:
- Focal gland formation may be present
- Cytokeratin and PAX8 positive
- Negative for myomelanocytic markers
- Placental site trophoblastic tumor:
- Fibrinoid material surrounds groups of tumor cells and replaces vessel walls
- Endometrium may show decidual / Arias-Stella changes
- Inhibin, hPL, cytokeratin positive
- Negative for myomelanocytic markers
- No TSC1 / TSC2 alterations or TFE3 fusions
Additional references
Practice question #1
A 32 year old woman with a history of pulmonary lymphangioleiomyomatosis, seizures and angiofibromas presents with a myometrial mass. Which molecular / cytogenetic abnormality does this patient likely have?
- JAZF1-SUZ12 fusion
- RAD51B fusion
- TFE3 fusion
- TP53 mutation
- TSC2 mutation
Practice answer #1
Practice question #2
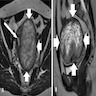
A 50 year old woman presents with a myometrial mass with lung metastases, as seen in the image. The myometrial tumor is characterized by noncohesive epithelioid and spindled cells surrounded by a delicate vasculature. The tumor cells are strongly and diffusely positive for HMB45, desmin, SMA and caldesmon with focal MelanA expression. What type of tumor does this likely represent?
- Alveolar soft part sarcoma
- Leiomyosarcoma
- Low grade endometrial stomal sarcoma
- Melanoma
- Perivascular epithelioid cell tumor (PEComa)
Practice answer #2
E. Perivascular epithelioid cell tumor (PEComa)
Comment Here
Reference: Perivascular epithelioid cell tumor (PEComa)
Comment Here
Reference: Perivascular epithelioid cell tumor (PEComa)