Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Azhar AF, Asadbeigi SN, McBride J. Common acquired nevus. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticacquirednevus.html. Accessed May 10th, 2024.
Definition / general
- Common acquired nevus is a benign proliferation of melanocytes which is classified by its location in the epidermis, the dermis or both
- Common acquired nevi consist of junctional nevi, compound nevi and intradermal nevi (Clin Cosmet Investig Dermatol 2014;7:89)
Essential features
- Common acquired nevi are typically diagnosed clinically
- Histologically they may be characterized as junctional, compound or intradermal based on the location of melanocytes
- Approximately 33% of melanomas are derived from benign melanocytic nevi; therefore, understanding of the common acquired nevus is necessary
Terminology
- Acquired melanocytic nevus
- Nevocellular nevus
- Mole
ICD coding
Epidemiology
- Arises at 6 months of age and reaches peak incidence during the third decade of life
- Exhibits a particularly rapid rate of development at puberty and may decrease in quantity with increasing age
- More common in people of Caucasian descent or in those with Fitzpatrick skin phenotype I and II
- Incidence is increased in association with sun exposure and sun burns
- Associated with familial melanoma syndromes
- Acral and conjunctival nevi are more common in African, African American and Asian populations (Arch Dermatol 1999;135:47, Pigment Cell Melanoma Res 2011;24:879, Clin Cosmet Investig Dermatol 2014;7:89, J Natl Cancer Inst 1986;77:329, Arch Dermatol 2005;141:579)
- Eruptive melanocytic nevi can be associated with different conditions such as immunodeficiency or immune suppression and targeted therapies in malignancies (Expert Opin Drug Metab Toxicol 2017;13:293)
Sites
- May occur on any site, including acral skin, mucosal surfaces, within the nail matrix or within lymph nodes
- Most commonly occur on sun exposed sites
- May favor the trunk in males and the lower extremities in females (Arch Dermatol 1999;135:47, Arch Dermatol 2010;146:1085)
Pathophysiology
- During embryogenesis pluripotent neural crest cells migrate through paraspinal ganglia and undergo differentiation into melanocytes of the basal layer of the epidermis and dermis
- Melanocytes organize into interspersed single cells in the basal layer
- Single melanocytes then proliferate into a lentiginous proliferation of contiguous individual melanocytes
- Subsequently, melanocytes undergo a morphological transition into epithelioid type melanocytes in nests of 3 or more melanocytes at the dermoepidermal junction (junctional nevus)
- With further cellular proliferation and differentiation, junctional nests may follow the dropping off model (or Abtropfung phenomenon) and some nests migrate downward to the dermis (compound nevus)
- When nests are completely within the dermis, they are considered intradermal nevi (Clin Cosmet Investig Dermatol 2014;7:89)
Etiology
- Risk factors:
- Childhood UV radiation
- Male gender
- Genetic susceptibility / genetic syndromes (familial atypical mole and melanoma syndrome [associated with CDKN2A mutations], BAP1 cancer syndrome, Carney complex, Turner syndrome, Noonan syndrome, EEC syndrome [ectrodactyly, ectodermal dysplasia and cleft lip / palate syndrome], Goeminne syndrome, Kuskokwim syndrome, Mulvihill-Smith syndrome, Langer-Giedion syndrome, tricho-odonto-onychial dysplasia)
- Individuals with fair skin phenotypes (Fitzpatrick types I and II) (Dermatol Clin 1995;13:595, J Am Acad Dermatol 1993;29:373)
Clinical features
- Junctional nevi are typically flat or macular, brown to dark brown in color, < 6 mm, with regular borders and an even pigment network under dermoscopy
- Compound nevi are typically elevated or papular, light tan to dark brown in color, < 6 mm and occasionally with hairs egressing from within it
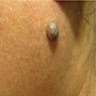
- Intradermal nevi are often round, well defined, amelanotic, pink to reddish brown or flesh colored, soft and raised papules, some of which may appear warty, papillomatous, framebesiform, pedunculated or dome shaped
- Approximately 33% of melanomas are derived from a benign melanocytic nevus, especially from the junctional melanocytes (Clin Cosmet Investig Dermatol 2014;7:89, Oncogene 2017;36:5771)
- Favorable prognosis for malignant melanoma arising from acquired melanocytic nevi compared to de novo melanomas (Arch Dermatol 1983;119:455)
Diagnosis
- Diagnosis can often be made clinically through characteristic features and age of onset
- If there are concerning or suspicious features such as asymmetry, irregular or indistinct borders, color variegation, diameter > 6 mm or evolution of the lesion, it should be biopsied and examined by a dermatopathologist to rule out dysplasia or melanoma
- Dermoscopy may be utilized to assess pigment network and vessels for benign patterns (J Am Acad Dermatol 2009;60:508)
Case reports
- 5 year old girl with acquired compound melanocytic nevus on the hard palate (J Oral Maxillofac Res 2022;13:e5)
- 24, 41 and 55 year old men with abrupt development of melanocytic nevi with different stages of disease (Dermatol Online J 2018;24:13030)
- 57 year old woman with melanocytic nevus in the external auditory canal with keratin build up presenting with hearing loss (Case Rep Otolaryngol 2021;2021:5539286)
- 59 year old woman with multiple eruptive melanocytic nevi and change in pre-existing nevi secondary to encorafenib (In Vivo 2020;34:441)
Treatment
- No treatment is necessary
- Excisions can be performed for cosmetic purposes or for definitive diagnosis
- Shave biopsies may not result in complete removal if residual melanocytes are left within the skin
- Laser removal is controversial, as one should confident it is a benign nevus being treated (Acta Derm Venereol 2006;86:44)
Clinical images
Gross description
- Flat tan to dark brown skin ellipse, size is usually < 1 cm or well circumscribed, pink, dome shaped papule
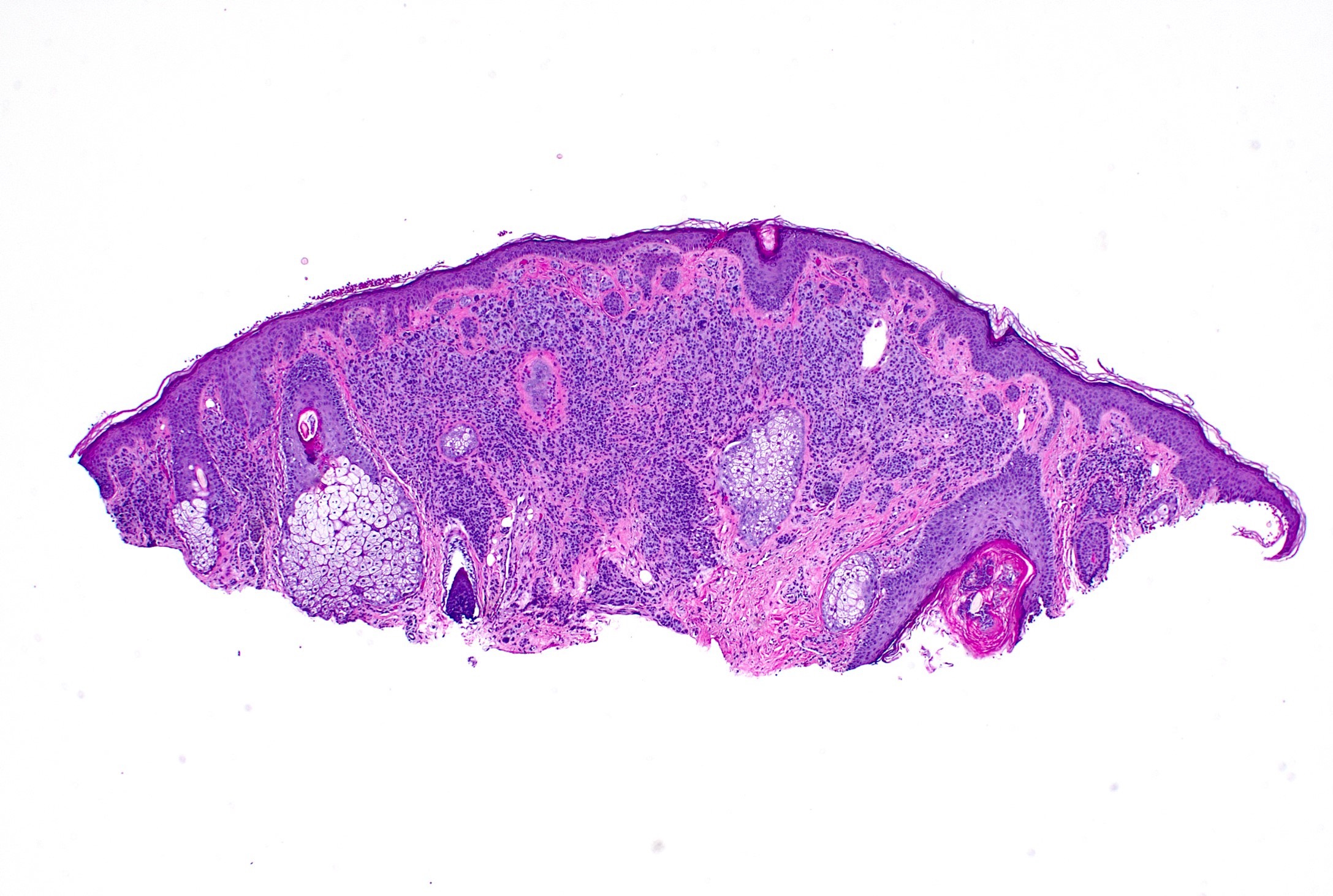
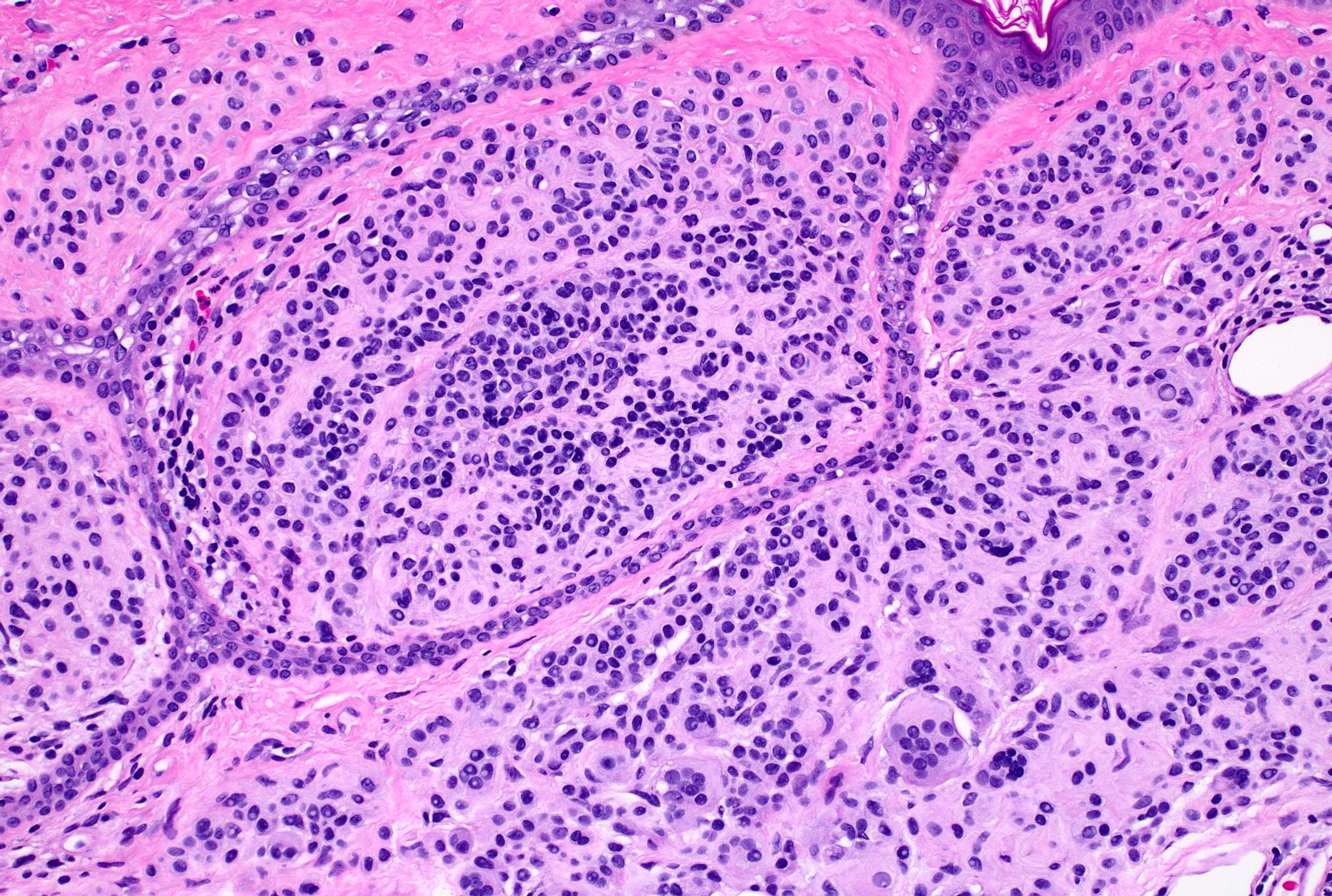
Microscopic (histologic) description
- Well nested melanocytic proliferation of round to oval nests or theques at the dermoepidermal junction; no pagetoid spread (exception: ~33% of benign acral nevi may show scattered pagetoid upward spread) (J Am Acad Dermatol 1994;31:740)
- Symmetrical from right to left, asymmetrical from top to bottom
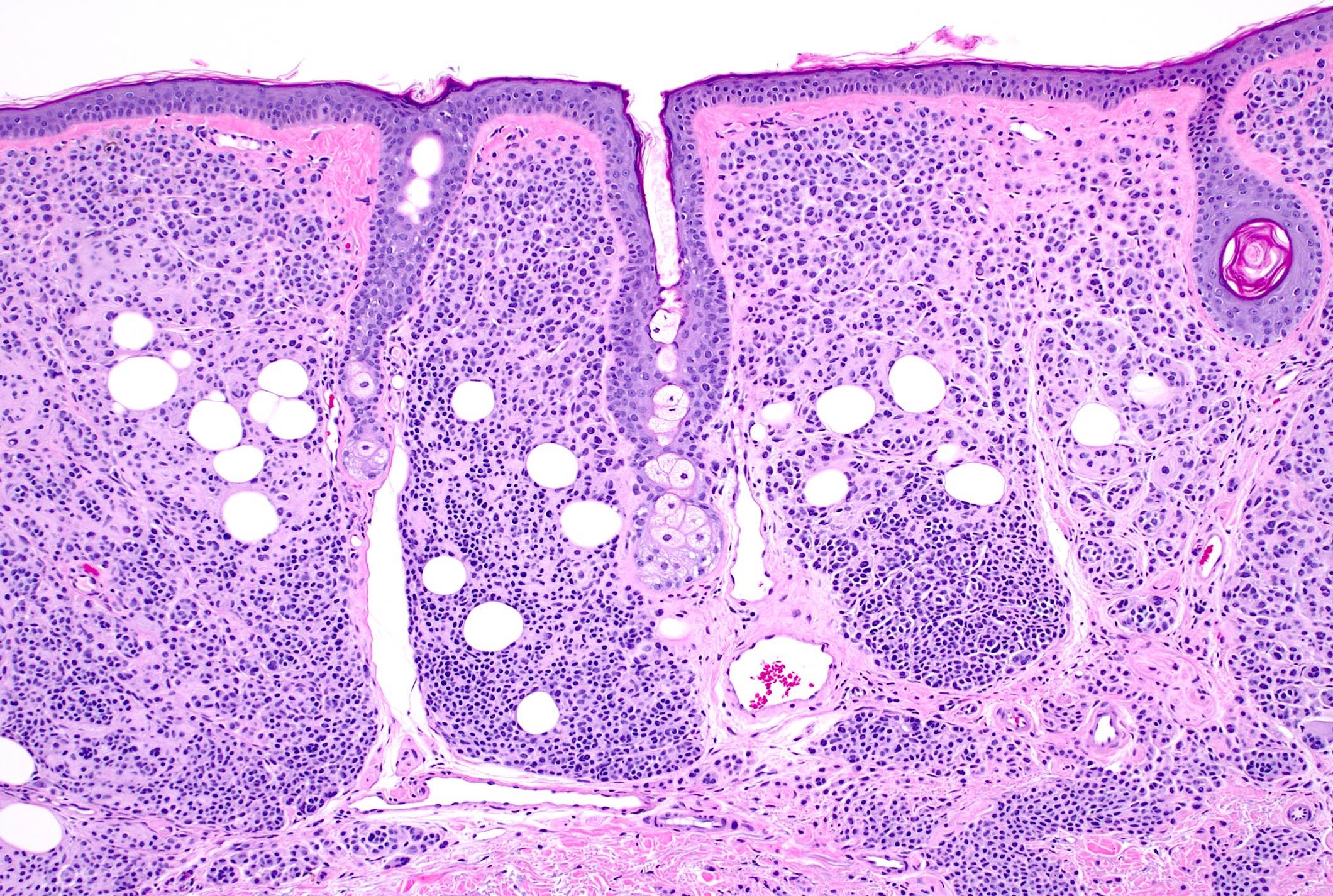
- Junctional nests are located at the tips and sides of rete ridges with regular spacing
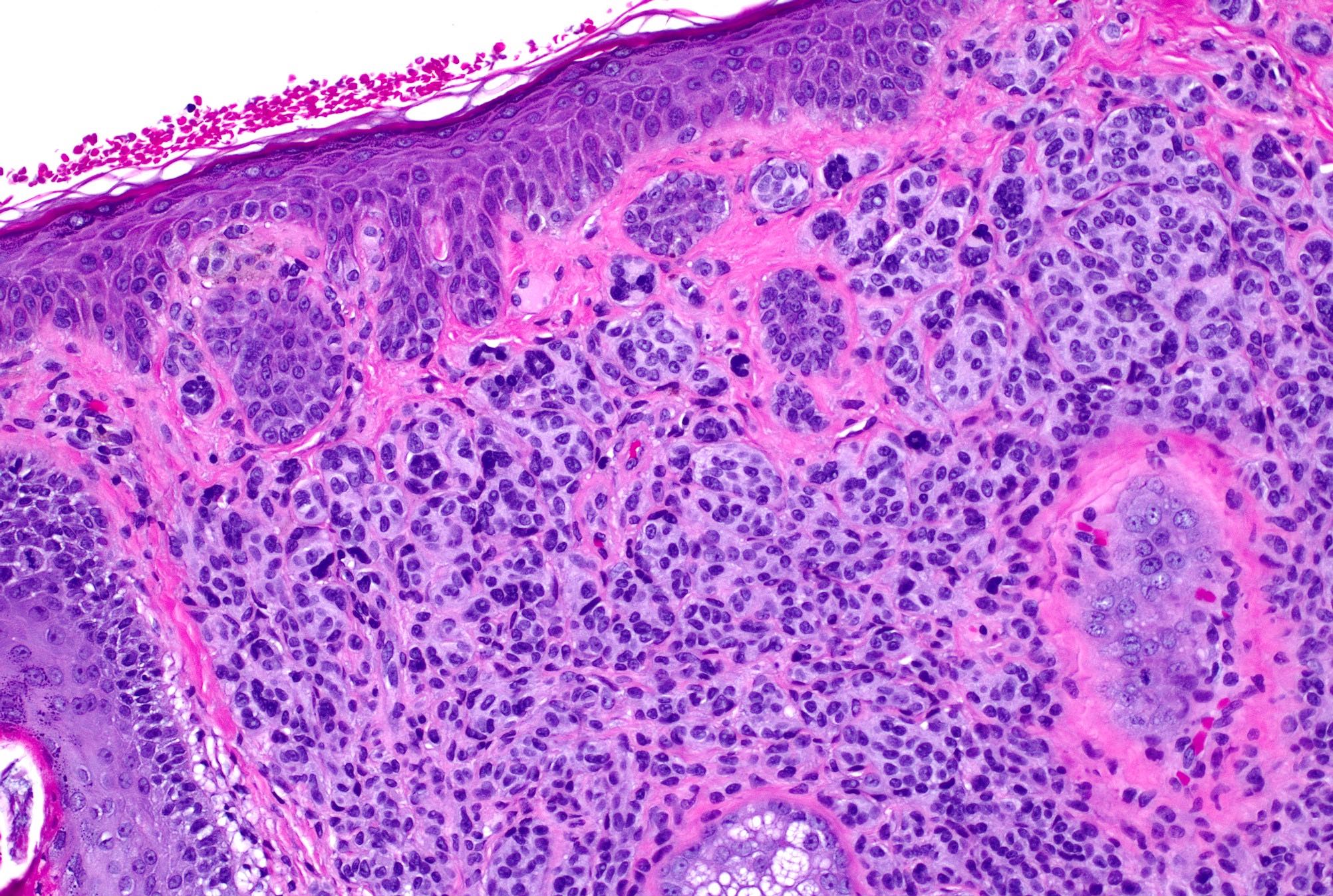
- As migration downward occurs, melanocytes disperse to single units and melanocytes undergo normal maturation from epithelioid (type A) to lymphocyte-like (type B), then neurotized (type C) morphologies
- Multinucleated melanocytes can be present
- Deep mitoses and deep pigment in melanocytic nests are rare
- No destruction of surrounding structures
- No significant nuclear atypia or mitotic activity
- Intradermal nevus: melanocytic nests within the dermis only
- Compound nevus: melanocytic nests at the dermal - epidermal junction and within the dermis
Microscopic (histologic) images
Positive stains
- MelanA / MART1
- SOX10
- S100
- HMB45 (intraepidermal and superficial dermal components)
- p16
- MITF1
- Tyrosinase (Int J Clin Exp Pathol 2015;8:9742, J Cutan Pathol 2008;35:433, Am J Surg Pathol 2018;42:1456)
Negative stains
- Ki67 (low expression, usually < 5% staining of cells)
- PRAME
- Scattered positive cells are possible
- Majority of melanocytes are completely negative
- Some benign nevi can have scattered positive melanocytes but are negative or only 1+ positive (e.g., 1 - 25% = 1+; 26 - 50% = 2+; 51 - 75% = 3+)
- Exceptions are rare
- p53
- BCL2
- Cyclin D (Int J Clin Exp Pathol 2015;8:9742, J Cutan Pathol 2008;35:433, Am J Surg Pathol 2018;42:1456, J Cutan Pathol 2022;49:220, Surg Pathol Clin 2021;14:165)
Molecular / cytogenetics description
- Estimates are that 70 - 80% of common acquired nevi harbor activating BRAF mutations (e.g., BRAF V600E)
- NRAS mutations are less common but still present in a small minority of acquired melanocytic nevi (5.9 - 18.2%) (Pigment Cell Melanoma Res 2015;28:661)
- Estimated overall frequency of activating KIT gene mutations is 15% (Mod Pathol 2009;22:1446)
Videos
Microscopic description of junctional, compound and intradermal melanocytic nevi
Microscopic differences between solar lentigines and junctional nevi
Comprehensive clinical and histopathologic review of benign pigmented and melanocytic lesions including common acquired nevi
Microscopic clues to the diagnosis of melanoma versus benign nevi
Sample pathology report
- Skin, left upper back, shave biopsy:
- Compound nevus (see comment)
- Comment: Sections reveal junctional and dermal melanocytes arranged in nests at the dermoepidermal junction and reticular dermis. The lesion is well circumscribed and symmetrical from left to right. No mitotic activity or cytological atypia is noted.
Differential diagnosis
- Melanoma:
- Cytological atypia: absence of maturation, abundant pagetoid upward migration of melanocytes, nuclear pleomorphism, dermal mitoses, prominent and eosinophilic nucleoli, variably dusty pigmented cytoplasm
- Architectural atypia: asymmetry, epidermal consumption, ill defined borders, confluent growth of junctional melanocytes, expansile dermal growth pattern, irregular nest distribution
- Stromal change: variable inflammatory reaction, dermal fibrosis
- Retention of HMB45 in dermal nests, strong and diffuse PRAME positivity (> 75% positive melanocytes = 4+ positive)
- Dysplastic nevus:
- Cytological atypia: pleomorphism (pleomorphism is less than melanoma)
- Architectural atypia: shouldering, bridging, lentiginous hyperplasia, irregular nesting
- Stromal change: lamellar fibroplasia, possible epidermal change
- Minor architectural atypia can be present in benign nevus; cytological abnormality should not be present
- Small congenital melanocytic nevus:
- Tends to involve reticular dermis and nevomelanocytes involve the lower dermis
- Extension of nevomelanocytes between collagen bundles and around the skin adnexa
- Café noir macule:
- Hyperpigmentation of basal cells without nesting of melanocytes
- Neurofibroma:
- Proliferation of nerve fibers in dermis with background mast cells
- Pigmentation might be present
Board review style question #1
Board review style answer #1
Board review style question #2
What is the most common mutation in common acquired nevi?
- BRAF V600E
- GNAQ
- RAS
- TERT
Board review style answer #2