Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Martinez Ciarpaglini C. Carcinoma-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stomachcarcinomageneral.html. Accessed May 13th, 2024.
Definition / general
- More than 90% of gastric carcinomas are adenocarcinomas (Ann Oncol 2016;27:v38)
- Gastric cancer is a heterogeneous disease with different phenotypes, genotypes and clinical progress, including sensitivity to treatments and prognoses
Essential features
- Most sporadic gastric cancers are now considered to be inflammation driven, usually related to Helicobacter pylori infection (Histopathology 2020;76:182)
- Distal portion of the stomach is the most common location of gastric cancer (noncardia gastric cancer subtype) (Prz Gastroenterol 2019;14:26)
- In recent years, the incidence of intestinal noncardia gastric cancer (associated with H. pylori) has decreased, while cardia and diffuse gastric cancer is on the rise (Prz Gastroenterol 2019;14:26, Gastric Cancer 2019;22:1, Arch Pathol Lab Med 2004;128:765)
- The most common and worldwide histological classifications for gastric cancer are the Lauren and the WHO classifications; however, their prognostic relevance is still controversial (Gastric Cancer 2019;22:1, J Gastrointest Oncol 2017;8:1026, Anticancer Res 2001;21:617)
Epidemiology
- Gastric cancer was the fifth most commonly diagnosed cancer type worldwide in 2018 (5.7% of all cancer cases diagnosed) (CA Cancer J Clin 2018;68:394)
- Gastric cancer was the third leading cause of cancer related death and was responsible for 8.2% of all deaths from cancer in 2018 (CA Cancer J Clin 2018;68:394)
- Gastric cancer incidence rates have increased in patients aged between 55 and 80; it is rare in those under 50 years (Clin Gastroenterol Hepatol 2020;18:534)
- Incidence rates are 2 to 3 times higher for men than women (Clin Gastroenterol Hepatol 2020;18:534, CA Cancer J Clin 2018;68:394)
- Success in preventing and treating H. pylori infections in much of the developing world has modulated the incidence and the epidemiology of gastric cancer (Prz Gastroenterol 2019;14:26)
- In recent years, the incidence of intestinal noncardia gastric cancer (associated with H. pylori) has decreased, while cardia and diffuse gastric cancer is on the rise, especially in the developed world (Prz Gastroenterol 2019;14:26, Gastric Cancer 2019;22:1, Arch Pathol Lab Med 2004;128:765)
Sites
- Most frequent location of gastric adenocarcinoma is the incisura angularis
- Due to its epidemiological, etiological and clinical differences, cardia and noncardia (distal) gastric cancer are distinguished (Prz Gastroenterol 2019;14:26)
- Cardia gastric cancer arises in the region adjoining the gastroesophageal junction and shares epidemiological characteristics with esophageal adenocarcinoma
- Noncardia gastric cancer is more common and arises in the lower portion of the stomach
- More than 90% of cases of the noncardia subtype are associated with H. pylori infection
Etiology
- Gastric cancer demonstrates familial aggregation in around 10% of cases (Ann Oncol 2016;27:v38)
- 1 - 3% of all gastric cancers are associated with inherited genetic predisposition disorders, such as familial adenomatous polyposis (APC mutation), hereditary diffuse gastric cancer (CDH1 mutations / loss of heterozygosity) and gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) (Prz Gastroenterol 2019;14:26)
- Most sporadic gastric cancers are now considered to be inflammation driven and their etiology is characteristically environmental, usually related to H. pylori infection (Histopathology 2020;76:182)
- Chronic H. pylori infection is recognized as the main cause of noncardia (distal) gastric cancer (odds ratio 5.9 within 10 years of infection) (Int J Cancer 2015;136:487)
- Patients with gastric intestinal metaplasia (GIM) may have a greater than tenfold increased risk of gastric cancer than the general population
- More extensive GIM correlates with increased gastric adenocarcinoma risk (especially if it extends to the body) (Gastrointest Endosc 2015;82:1, Gut 2019;68:1545)
- Gastric dysplasia is linked to an increased risk of adenocarcinoma (6% of patients with high grade dysplasia progress to cancer within 5 years) and should alert to the possibility of synchronous gastric cancer (Gut 2019;68:1545)
- 5 - 10% of gastric carcinomas are associated with Epstein-Barr virus (EBV) infection, especially in tumors located in the cardia and gastric pouch (ESMO Open 2019;4:e000470, Appl Immunohistochem Mol Morphol 2017;25:12)
- Smoking is associated with an increased risk of both gastric cardia (HR, 2.86; 95% CI, 1.73e4.70) and noncardia (HR, 2.04; 95% CI, 1.32e3.16) cancers (Best Pract Res Clin Gastroenterol 2017;31:509)
- Heavy alcohol drinking has been pointed to as a risk factor for gastric cancer; however, the available information on the association between alcohol consumption and risk of gastric cancer is contradictory (Int J Cancer 2017;141:1950, Best Pract Res Clin Gastroenterol 2017;31:509)
- Other proposed risk factors are diet (salt, preserved meats, coffee), low socioeconomic status, obesity (body mass index over 25 kg/m is associated with 1.13 odds ratio of developing cancer), pernicious anemia (risk of gastric cancer 6.9%), previous gastric surgery, race / ethnicity (higher risk in Asians and African Americans) and blood group A, among others (Prz Gastroenterol 2019;14:26)
Clinical features
- Symptoms of gastric cancer are often nonspecific, frequently leading to diagnosis at an advanced stage (> 70%) (Tumour Biol 2017;39:1010428317714626)
- 35% of gastric cancer patients in the U.S. have metastasis at diagnosis (clinical stage 4) (Clin Gastroenterol Hepatol 2020;18:534)
- It is not uncommon for patients to have undergone several months of therapy for peptic ulcer disease before gastric cancer diagnosis
- Most common symptoms are abdominal pain (50 - 65%) and weight loss (40%) (Kufe: Holland-Frei Cancer Medicine, 6th edition, 2003)
- Although anemia is a frequent finding among patients with gastric cancer, upper gastrointestinal bleeding is less common and occurs in 16 - 17% of patients (Kufe: Holland-Frei Cancer Medicine, 6th edition, 2003)
- Endoscopically, according to the Paris system, early gastric cancer is classified as follows (Gastrointest Endosc 2003;58:S3)
- 0-I: polypoid - pedunculated (0-Ip) or sessile (0-Is)
- 0-II: nonpolypoid - superficial elevated (0-IIa), flat (0-IIb) or superficial shallow, depressed (0-IIc)
- 0-III: nonpolypoid - excavated (ulcerated)
- Paris type 0-I and 0-III lesions have often submucosal infiltration and are not an ideal indication for endoscopic treatment (Endoscopy 2015;47:829)
Diagnosis
- Diagnosis is routinely made on endoscopic biopsy
- In the case of a malignant gastric ulcer, at least 7 biopsies of the heaped up edges of the ulcer and base should be performed (Gastrointest Endosc 2015;82:1)
- Obtaining representative biopsies from cases with linitis plastica growth may be difficult because this condition is associated with infiltration of the submucosa or muscularis propria of the stomach, reducing the yield of mucosal biopsies (Gastrointest Endosc 2015;82:1)
- In the absence of metastatic disease, endoscopic ultrasound (EUS) with or without FNA is indicated for local regional staging: tumor depth (pT1 - 2, pT3 - 4) and regional lymph node assessment (pN) (Gastrointest Endosc 2015;82:1)
- Laparoscopy with or without peritoneal washings for malignant cells is recommended in all clinical stage IB - III gastric cancers which are considered potentially resectable, to exclude radiologically occult metastatic disease (Ann Oncol 2016;27:v38)
Prognostic factors
- Most important prognostic factor is clinical stage
- 5 year survival rate of early gastric cancer can reach > 95% (Tumour Biol 2017;39:1010428317714626)
- For patients undergoing resectional surgery, the 5 year survival rate is around 20 - 40%, whereas in nonoperated patients, it is 3 - 5% (Ann Surg Oncol 2018;25:2693)
- Microsatellite instability (MSI) in gastric cancer is associated with a better outcome (ESMO Open 2019;4:e000470, Appl Immunohistochem Mol Morphol 2017;25:12, Histopathology 2020;76:182)
- Prognostic relevance of the morphological classifications of gastric cancer is still controversial, probably because of the high heterogeneity, lack of standardization or the low reproducibility of the histopathological criteria (Gastric Cancer 2019;22:1, J Gastrointest Oncol 2017;8:1026, Anticancer Res 2001;21:617)
- Modified Lauren classification according to tumor location seems to improve the prognostic value (Ann Surg Oncol 2018;25:3257)
Case reports
- 49 year old man with increasing serum alpha fetoprotein levels (Scand J Gastroenterol 2016;51:646)
- 70 year old man with gastric submucosal mass (Medicine (Baltimore) 2018;97:e12341)
- 74 year old woman with calcified gastric carcinoma presenting with ocular metastasis (J Med Case Rep 2019;13:64)
- 74 year old man presenting with acanthosis nigricans (World J Surg Oncol 2017;15:208)
- 78 year old man with gastric carcinoma with osteoclast-like giant cells (J Zhejiang Univ Sci B 2009;10:237)
Treatment
- Early gastric cancer can be treated through endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) if they are confined to the mucosa, well differentiated, ≤ 2 cm and nonulcerated (Gastrointest Endosc 2015;82:1, Ann Oncol 2016;27:v38)
- Early gastric cancer Paris 0-IIa, 0-IIb and 0-IIc show a low probability of advanced histology; therefore, EMR is acceptable for treatment in these cases (Endoscopy 2015;47:829)
- For stage pT2 tumors and beyond, with no evidence of distant metastasis (stage IB - III), radical gastrectomy is indicated (Ann Oncol 2016;27:v38)
- Perioperative (pre and postoperative) chemotherapy with a platinum / fluoropyrimidine combination is recommended for patients with resectable gastric cancer (Ann Oncol 2016;27:v38)
- Doublet or triplet platinum / fluoropyrimidine combinations are recommended for fit patients with advanced gastric cancer (Ann Oncol 2016;27:v38)
- Anti-HER2 drugs are currently added to the first line platinum based chemotherapy for the treatment of advanced gastric cancer cases showing HER2 amplification (Ann Oncol 2019;30:1254, Ann Oncol 2016;27:v38)
- Immune checkpoint blockade inhibitors are recently approved for gastric cancer treatment
- Utility of predictive biomarkers including EBV, MSI, PDL1 expression and H. pylori infection has been preliminarily confirmed (Chin J Cancer Res 2020;32:287, Histopathology 2020;76:182)
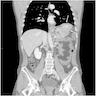
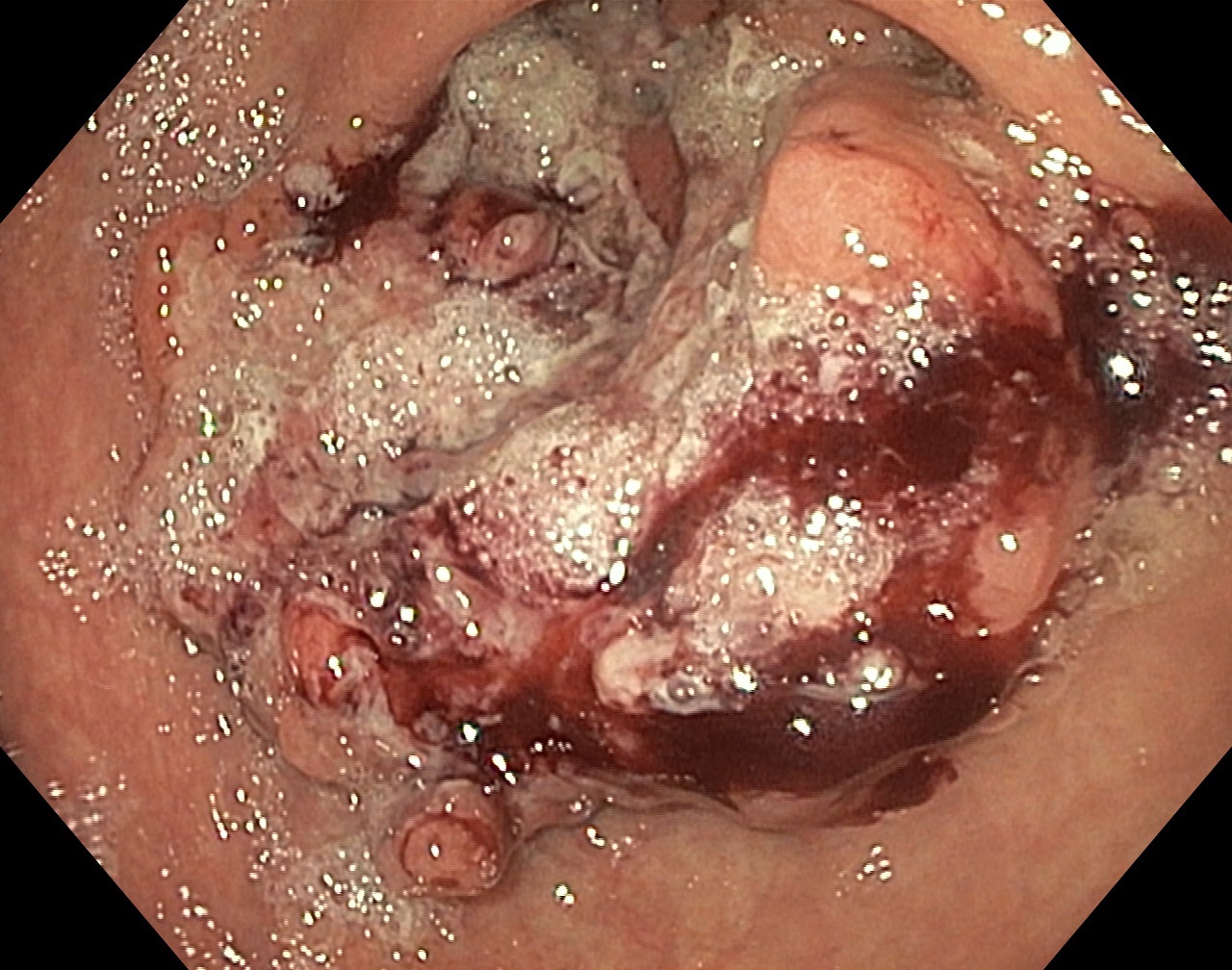
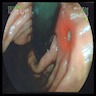
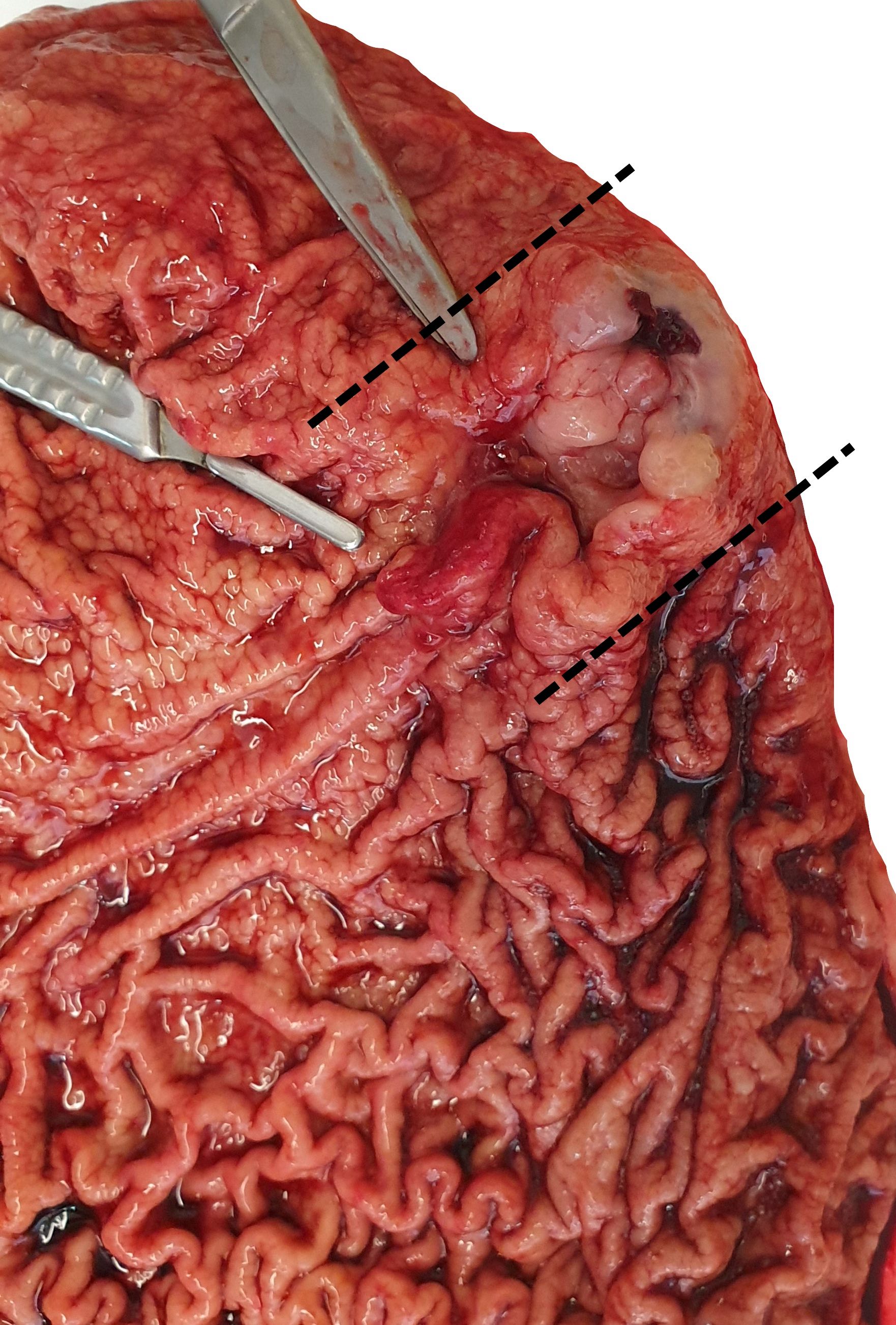
Clinical images
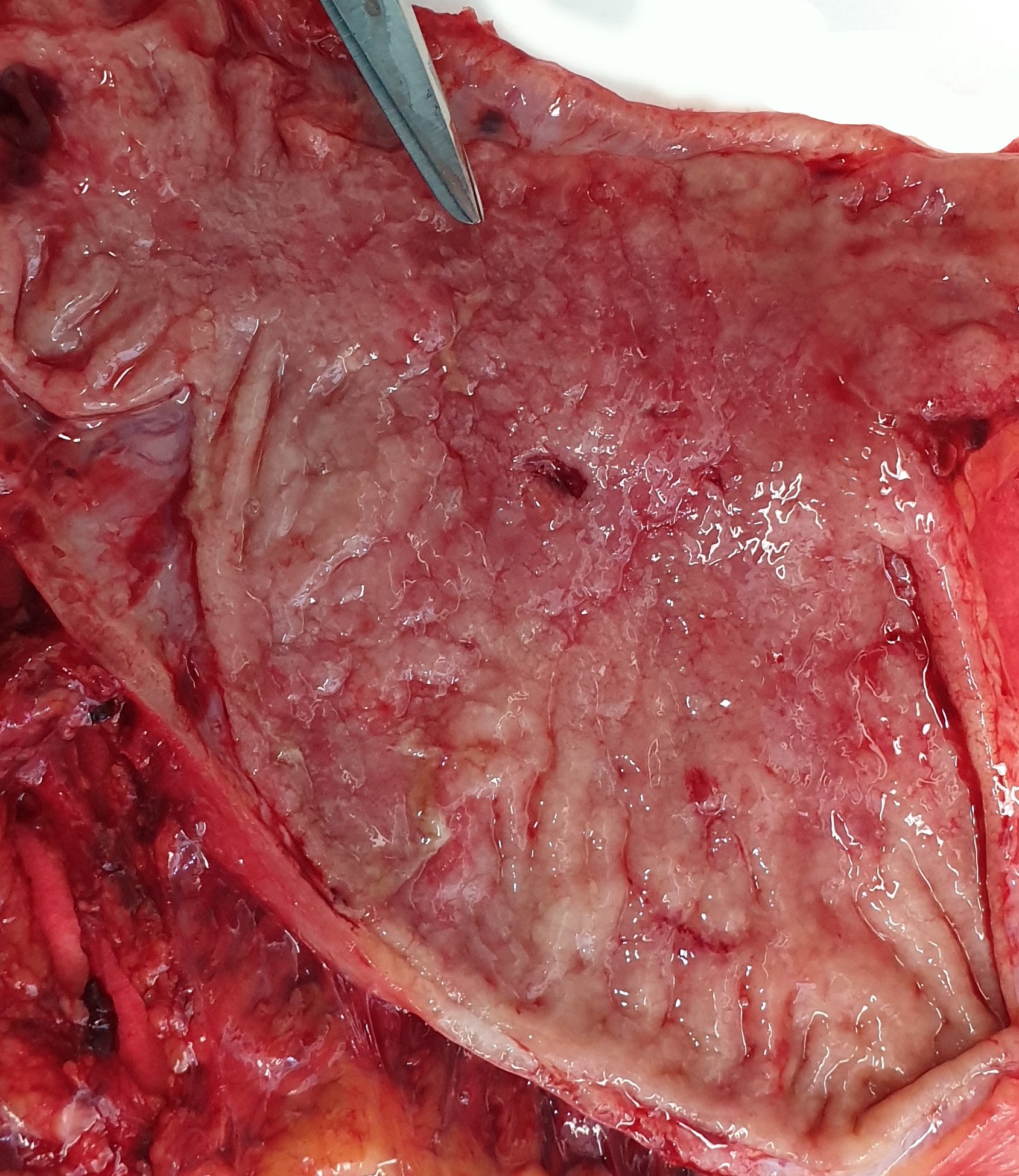
Gross description
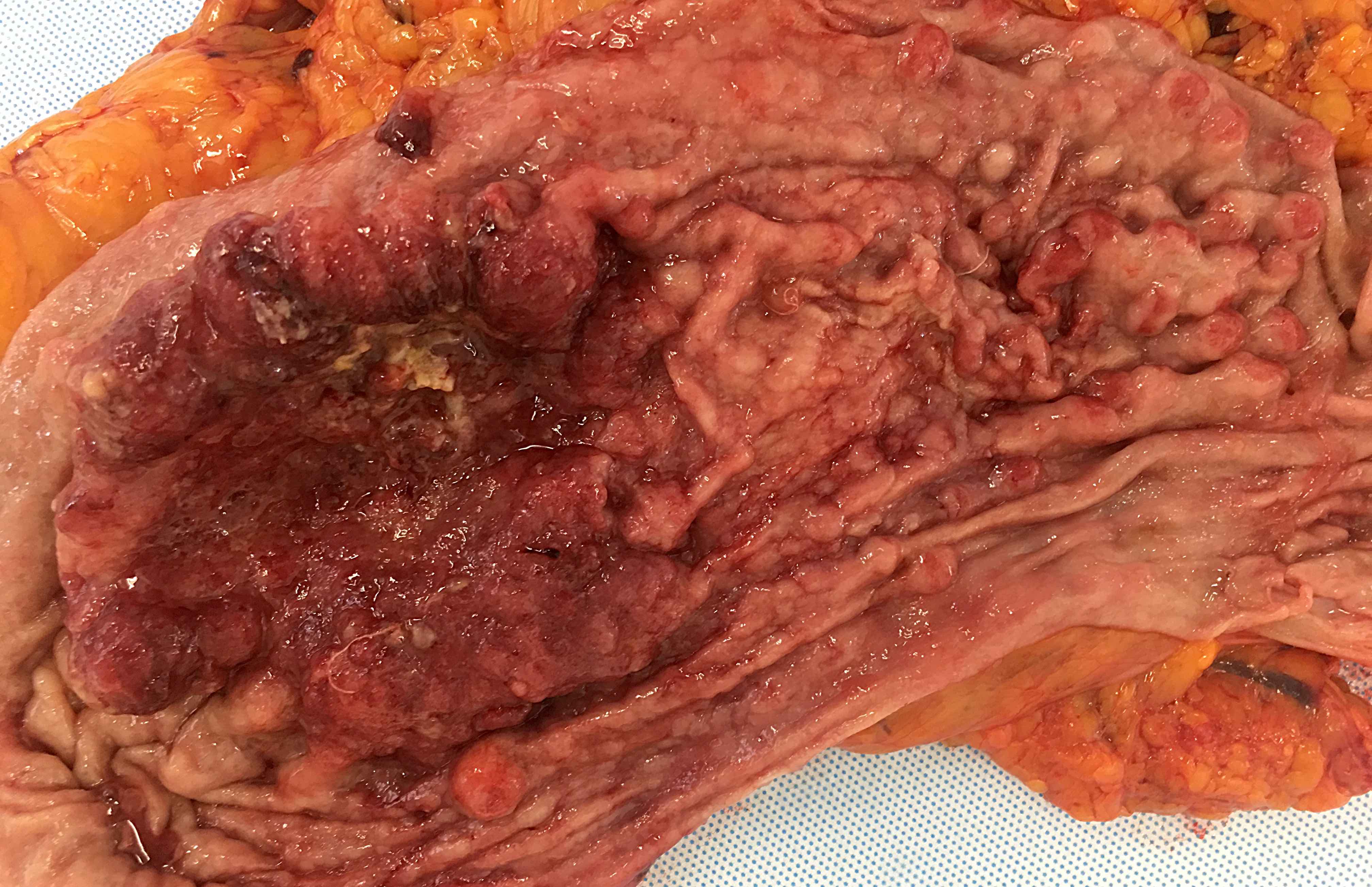
- Typically, gastric cancer presents as a mass lesion; in other cases, a nonhealing gastric ulcer may be the presenting form (Gastrointest Endosc 2015;82:1)
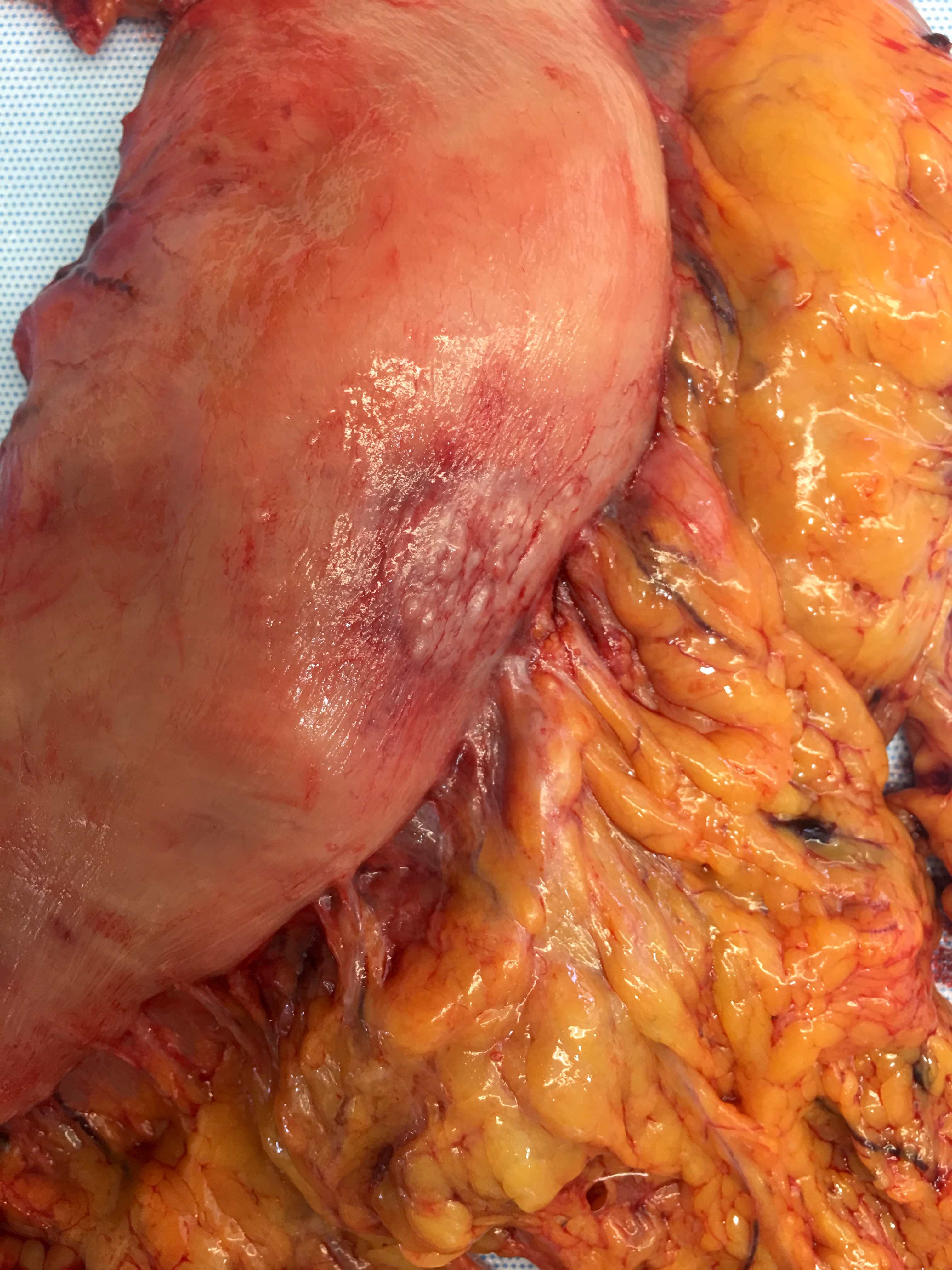
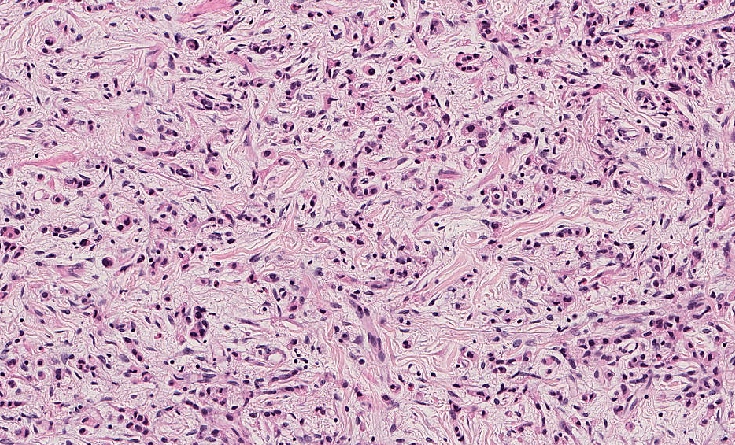
- Diffuse gastric cancer is characterized by an infiltrative growth pattern knows as linitis plastica
- In these cases, neoplastic cells permeate deeply through the gastric wall, evoking an intense desmoplastic reaction causing diffuse rugal flattening and a rigid thickened wall
- The pattern of linitis plastica is highly correlated with the presence of signet ring cells (J Gastrointest Surg 2020;24:1018)
- The term linitis plastica should only be used for the description of the macroscopic characteristics of the tumor (Gastric Cancer 2019;22:1)
Gross images
Microscopic (histologic) description
- The most common and worldwide histological classifications for gastric cancer are the Lauren and the WHO classifications
- Lauren classification (Acta Pathol Microbiol Scand 1965;64:31)
- Diffuse type:
- Lack of or poor cohesion between the neoplastic cells
- Composed of scattered, small clusters or rows of cells with little or no gland formation
- Neoplastic cells usually show an atypical morphology with irregular nuclear contours and variable amounts of eosinophilic cytoplasm
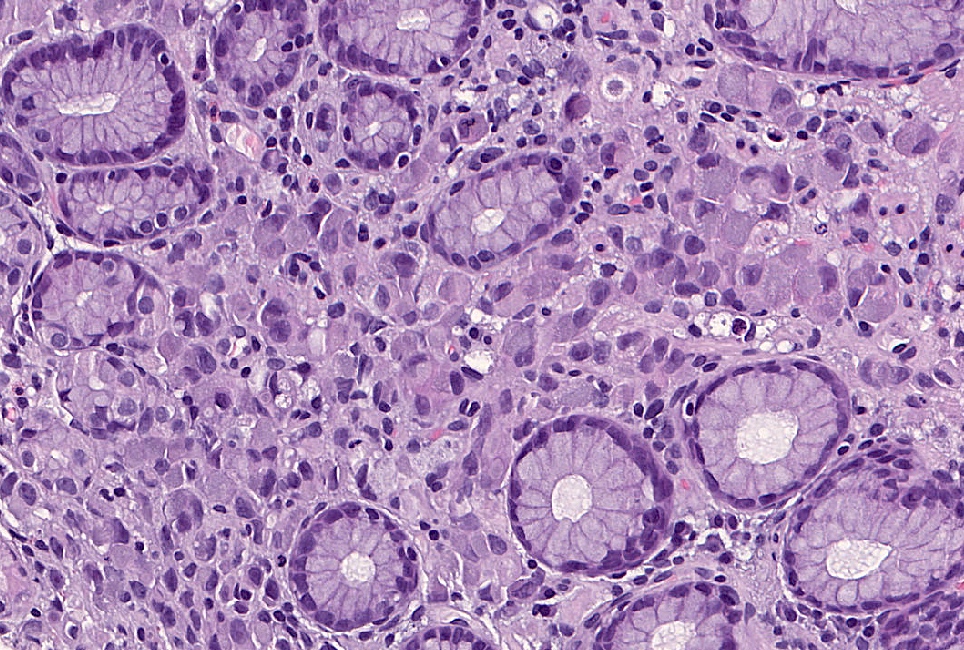
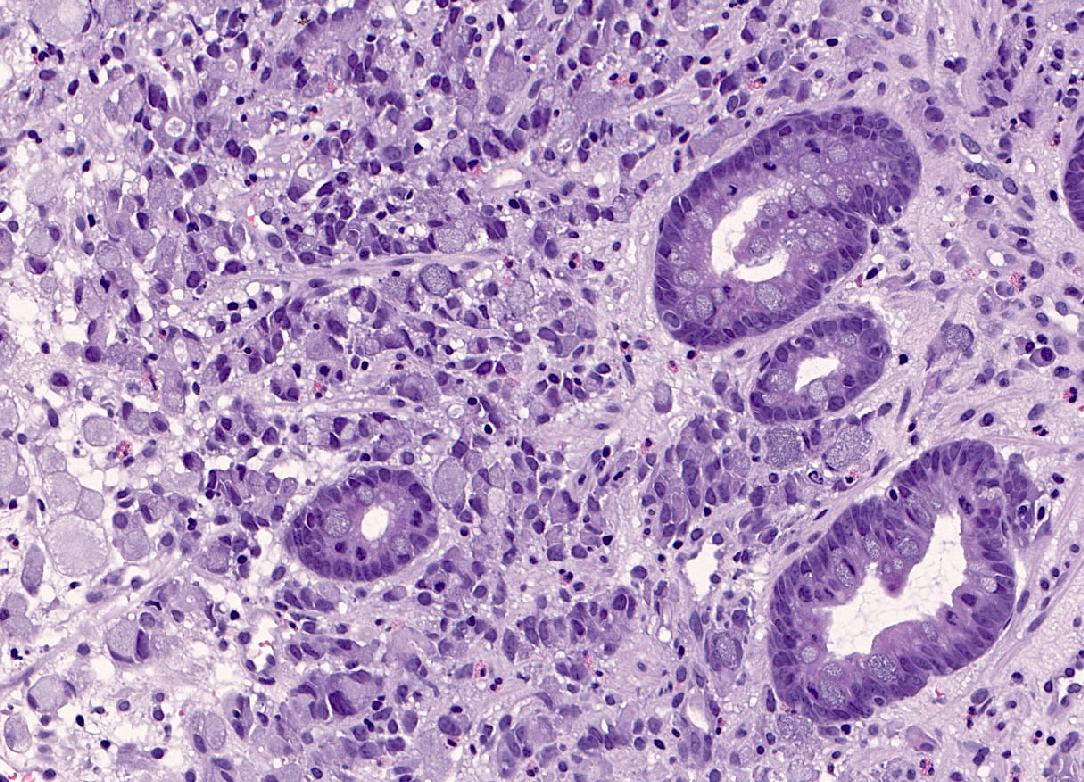
- In some cases, there is a variable component of cells showing the so called signet ring morphology
- Signet ring cells show ample cytoplasmic mucin, which appears optically clear on H&E staining and an eccentrically placed nucleus (Gastric Cancer 2019;22:1)
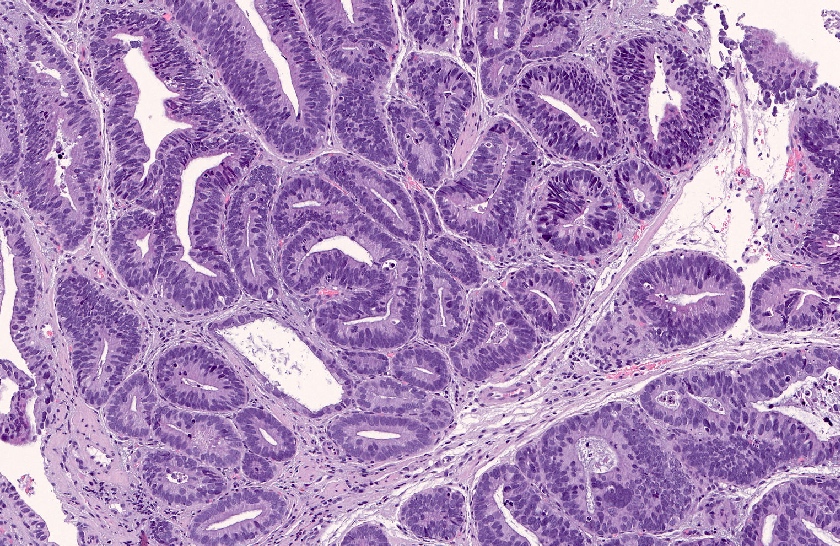
- Intestinal type:
- Composed of tubular or glandular structures similar to intestinal adenocarcinoma
- Neoplastic cells usually contain apical mucin vacuoles
- Unlike diffuse gastric cancer, intestinal tumors grow along broad cohesive fronts to form an exophytic mass
- Mixed
- Undifferentiated
- Diffuse type:
- WHO classification of gastric carcinomas comprises a more detailed classification based on morphological and in some cases immune expression profile of tumors, as listed here
- Main similarities and differences between the 2 classifications are
- WHO classification separates tubular gastric carcinoma into 3 categories: well, moderately and poorly differentiated, based on tubular formation
- Lauren intestinal subtype corresponds with the papillary and the well and moderately differentiated tubular categories of the WHO classification
- Lauren diffuse subtype corresponds with the poorly cohesive category of the WHO classification (Gastric Cancer 2019;22:1)
- WHO classification separates poorly cohesive carcinoma into 2 categories (Gastric Cancer 2019;22:1):
- Poorly cohesive, signet ring cell phenotype: composed only or predominately (more than 90%) of signet ring cells
- Poorly cohesive, not otherwise specified (NOS): all other cases that do not display signet ring cell morphology (Gastric Cancer 2019;22:1)
- Both classifications recognize a mixed subtype, usually composed of cases with intestinal / tubular or papillary and diffuse / poorly cohesive components
- There is currently no cutoff concerning the percentage of each component for a tumor to be classified as mixed adenocarcinoma (Gastric Cancer 2019;22:1)
- Most of the undifferentiated cases in the Lauren classification comprise cases classified as poorly differentiated tubular adenocarcinomas according to the WHO classification
- WHO classification includes a series of less frequent variants, such as papillary, hepatoid, lymphoid stroma rich, among others, which are not defined in the Lauren classification (Gastric Cancer 2019;22:1)
- Modified Lauren classification established 3 scenarios based not only on the histological appearance of the lesion but also on tumor location and association with pre-existing atrophy in the surrounding mucosa (Clin Cancer Res 2011;17:2693)
- Proximal nondiffuse gastric cancer:
- Comprises tumors located in the gastric cardia or gastroesophageal junction
- Includes intestinal tumors with any grade of differentiation or mixed tumors with a minor component of diffuse carcinoma
- Surrounding mucosa usually shows evidence of glandular dysplasia in the setting of chronic inflammation without atrophy
- Diffuse gastric cancer:
- Includes tumors composed entirely of diffuse morphology located anywhere in the stomach
- In these cases, the remaining nontumoral mucosa shows no apparent gastritis or atrophy
- Distal nondiffuse gastric cancer:
- In these cases, the bulk of the tumor is usually located in the distal stomach and may extend to the mid body or the pylorus
- This category includes intestinal tumors with any grade of differentiation or mixed tumors with a minor component of diffuse carcinoma
- Proximal nondiffuse gastric cancer:
Microscopic (histologic) images
Positive stains
- CK7 (50 - 70%), CK20 (30 - 48%), CDX2 (60%) (J Gastrointest Oncol 2012;3:262, Hum Pathol 2004;35:576)
- MUC2 (30%) (J Gastrointest Oncol 2012;3:262)
- MUC5AC (71%) (Arch Pathol Lab Med 2005;129:338)
- CK5/6 (14%) (Arch Pathol Lab Med 2005;129:338)
Negative stains
- Estrogen receptor (ER), progesterone receptor (PR) and GCDFP-15 help distinguish primary gastric carcinomas (consistently negative) from metastatic breast carcinomas (usually positive) (Arch Pathol Lab Med 2005;129:338)
- GATA3 is rarely expressed (around 10% of cases) (Am J Surg Pathol 2014;38:13)
- E-cadherin or cytokeratin subtyping does not aid in the identification of signet ring cells (Gastric Cancer 2019;22:1)
Molecular / cytogenetics description
Sample pathology report
- Gastric mass, antrum, endoscopic biopsy:
- Diffuse gastric carcinoma (poorly cohesive, NOS, per WHO classification)
- Gastric mass, antrum, endoscopic biopsy:
- Diffuse gastric carcinoma (poorly cohesive, signet ring cell type, per WHO classification)
- Gastric mass, body, endoscopic biopsy:
- Intestinal type gastric carcinoma (tubular low grade, per WHO classification)
Differential diagnosis
- Metastatic lobular breast carcinoma:
- Breast carcinoma is the third most frequent source of metastasis to the stomach (14%) (Gut Liver 2015;9:615)
- Interestingly, the vast majority of breast carcinomas metastatic to the stomach belong to the lobular subtype (97%) (Dig Liver Dis 2011;43:823)
- The most common macroscopic growth pattern of presentation is linitis plastica, indistinguishable from primary diffuse carcinoma (Cureus 2020;12:e11920)
- Morphologically, metastatic lobular breast carcinoma shows poorly cohesive, round tumor cells arranged in linear cords between the normal gastric glands, very similar to primary diffuse carcinoma (Cureus 2020;12:e11920)
- Estrogen receptor (ER), progesterone receptor (PR) and GCDFP-15 are highly specific for metastatic breast carcinomas, whereas CK20 and CDX2 are highly specific for primary gastric carcinomas (Arch Pathol Lab Med 2005;129:338, Cureus 2020;12:e11920)
- Loss of E-cadherin is not useful for the differential diagnosis, as it occurs in both invasive lobular carcinoma of the breast and primary diffuse gastric carcinoma (Cureus 2020;12:e11920)
- Melanoma:
- Malignant melanoma is the tumor that most frequently metastasized to the stomach (27% of all metastatic tumors in the stomach), followed by lung cancer (19%) (Gut Liver 2015;9:615)
- Most melanomas found in the gastrointestinal tract are metastatic and a small proportion (around 4%) are primary mucosal melanomas (Int J Clin Exp Pathol 2014;7:6826)
- Microscopic preoperative diagnosis may be a challenge and relies on the identification of melanin pigment (Melanoma Manag 2020;7:MMT51)
- Some cases may present poorly cohesive epithelioid cells resembling a primary diffuse carcinoma
- High pleomorphism, prominent nucleoli and nuclear inclusions may suggest a melanoma, especially in the absence of melanic deposition (Melanoma Manag 2020;7:MMT51)
- S100, tyrosinase, MelanA, HMB45 and SOX10 are useful to demonstrate the melanic nature of tumor cells, especially in nonpigmented cases (Int J Clin Exp Pathol 2014;7:6826, Appl Immunohistochem Mol Morphol 2013;21:506)
- Epithelial changes:
- There are benign lookalikes that can mimic signet ring cells (Gastric Cancer 2019;22:1)
- Changes associated with ulceration and ischemia: e.g. hyperplastic polyp with globoid change
- Macrophages: e.g. gastric xanthoma
- Mesothelial cells in cytology preparations
- There are benign lookalikes that can mimic signet ring cells (Gastric Cancer 2019;22:1)
Board review style question #1
Select the correct histopathological diagnosis for this type of gastric tumor
- Intestinal gastric carcinoma (Lauren classification)
- Poorly cohesive carcinoma, NOS (WHO classification)
- Poorly cohesive carcinoma with signet ring cell phenotype (WHO classification)
- Tubular carcinoma (WHO classification)
Board review style answer #1
Board review style question #2
Which of the following is true about signet ring cells in gastric cancer?
- Definition of signet ring cells relies purely on morphological criteria
- E-cadherin staining is useful to recognize them
- If more than 30% are present, the tumor is called poorly cohesive signet ring cell type according to the WHO definition
- Pattern of linitis plastica is not correlated with the presence of signet ring cells
Board review style answer #2
A. Definition of signet ring cells relies purely on morphological criteria: ample cytoplasmic mucin which appears optically clear on H&E staining and an eccentrically placed nucleus.
Comment Here
Reference: Carcinoma-general
Comment Here
Reference: Carcinoma-general