Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Ammendola S. Melanocytic tumors / melanoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumormelanocytictumor.html. Accessed September 18th, 2025.
Definition / general
- Rare melanocytic tumors that originate from leptomeningeal melanocytes
- Classified according to their growth pattern (circumscribed or diffuse) and histological features (benign versus malignant)
Essential features
- Circumscribed forms include benign lesions (melanocytomas), intermediate grade lesions (intermediate grade melanocytomas) and their malignant counterpart (melanomas)
- Diffuse forms, often occurring in the setting of neurocutaneous melanosis, are classified as meningeal melanocytosis (benign) or meningeal melanomatosis (malignant) according to histological features
- Histological and molecular similarities of primary leptomeningeal melanomas to blue nevus-like melanoma and uveal melanoma
- Immunohistochemical positivity for melanocytic markers (S100, SOX10, MART1, HMB45)
- Mutations in GNAQ, GNA11, PLCB4 and CYSLTR; NRAS somatic mutations in diffuse tumors in children
Terminology
- Not recommended: melanoma / melanocytoma without specifying the site
ICD coding
- ICD-O
- ICD-11
- 2A0Z - primary melanoma of the central nervous system
- 2A01.0Y & XH8974 - other specified meningiomas & meningeal melanocytosis
- 2A01.0Y & XH1BP7 - other specified meningiomas & meningeal melanomatosis
- 2A01.0Y & XH2RY7 - other specified meningiomas & meningeal melanocytoma
- 2A01.0Y & XH3DN1 - other specified meningiomas & melanoma, meningeal
Epidemiology
- Diffuse melanocytosis
- Rare, with difficult to establish incidence
- Strongly associated with neurocutaneous melanosis (NCM), a rare congenital syndrome characterized by giant congenital pigmented skin nevi and neurological symptoms due to diffuse leptomeningeal melanocytic proliferation (Semin Cutan Med Surg 2004;23:138)
- Diffuse melanomatosis
- Bimodal age distribution: onset in childhood in the setting of neurocutaneous melanosis or in adulthood in the fourth decade (Brain Pathol 2015;25:209)
- Patients with NCM can develop meningeal melanomas in childhood (Pediatr Blood Cancer 2024;71:e30859)
- Meningeal melanocytoma (J Clin Neurosci 2010;17:1227)
- Incidence of 1/10,000,000 person years
- Arises at any age, mostly affects adults
- Meningeal melanoma (J Clin Neurosci 2010;17:1227)
- Incidence of 0.005/100,000 person years
- Reported in children and adults, with a peak in the fifth and sixth decades
Sites
- Diffuse meningeal melanocytosis and melanomatosis
- Can involve intracranial or spinal leptomeninges
- Highest frequency in cerebellum, brain stem, temporal lobes and spine (J Am Acad Dermatol 1991;24:747)
- Meningeal melanocytomas
- Can occur at any site; most common in cervical and thoracic spine (Am J Surg Pathol 1999;23:745)
- Meningeal melanomas
- Can occur anywhere but has a predilection for spinal cord and posterior fossa (Am J Surg Pathol 1999;23:745)
- Tumors that are entirely intraparenchymal are highly suggestive of metastatic melanoma rather than primary melanocytic neoplasm of the CNS
Pathophysiology
- Thought to arise from melanocyte precursor cells (melanoblasts) of the neural crest that colonize the leptomeninges (Brain Pathol 2015;25:209)
- Circumscribed forms
- Mutations in GNAQ, GNA11, PLCB4 or CYSLTR2 are considered the primary oncogenic event in most cases (J Invest Dermatol 2017;137:2033, J Neurooncol 2016;127:435)
- Mutations in GTPase domains of GNAQ and GNA11 lead to constitutive activation of RAS / RAF / MEK / ERK pathways (Annu Rev Pathol 2014;9:239)
- Shared GNAQ and GNA11 mutations in uveal melanomas and blue nevus-like melanoma suggest a common pathogenetic mechanism (Neuropathol Appl Neurobiol 2014;40:794)
- Diffuse forms
- NRAS mutations are frequent in children with neurocutaneous melanosis (J Invest Dermatol 2013;133:2229, Cancer Discov 2013;3:458)
- BRAF mutations are rare (Pediatr Dev Pathol 2015;18:1)
Etiology
- Diffuse forms arise from melanocyte precursor cells with postzygotic somatic mutations in the NRAS gene, mostly in the context of neurocutaneous melanosis (Cancer Discov 2013;3:458)
- ~23% of patients with neurocutaneous melanosis develop a primary meningeal melanocytic neoplasm (Neurosurg Focus 2022;52:E8)
- Circumscribed forms are characterized by somatic mutations in GNAQ, GNA11, PLCB4 or CYSLTR2 (Pathol Oncol Res 2015;21:439)
- Patients with BAP1 tumor predisposition syndrome have a higher risk of developing meningeal melanomas (Acta Neuropathol 2015;129:921)
Clinical features
- Mostly dependent on tumor location
- Diffuse melanocytosis and melanomatosis: hydrocephalus, seizures, intracranial hypertension, cranial nerve palsies and motor and sensory deficits (Neurosurg Focus 2022;52:E8)
- Melanocytoma and melanoma: mass effect / cord compression symptoms (J Clin Neurosci 2010;17:1227)
Diagnosis
- Based on neuroimaging and histological analysis on biopsy or resection specimen
- Metastatic melanoma must be excluded
- Molecular analysis is useful to differentiate metastatic melanoma from primary CNS melanocytic neoplasms (Neuropathol Appl Neurobiol 2014;40:794)
Laboratory
- Cerebrospinal fluid (CSF) analysis reveals high protein content in diffuse meningeal melanomatosis (Neuroradiol J 2018;31:42, Acta Neurol Belg 2011;111:228, Biomed Res Int 2015;2015:948497)
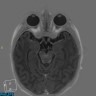
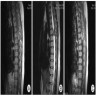
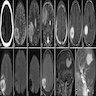
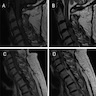
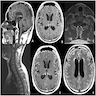
Radiology description
- Magnetic resonance imaging (MRI): depending on melanin content, hyperintensity in T1 and isointensity / hypointensity in T2 weighted images and contrast enhancing (Cancers (Basel) 2024;16:2508)
- MRI does not differentiate between benign and malignant forms (Cancers (Basel) 2024;16:2508)
- In diffuse melanocytosis, MRI reveals small linear foci of T1 shortening within the meninges and the brain parenchyma (Radiographics 2009;29:1503)
- Diffuse melanomatosis: meningeal thickening with or without discrete nodules and contrast enhancement (Radiographics 2009;29:1503)
Radiology images
Prognostic factors
- Diffuse melanocytosis (Radiographics 2009;29:1503)
- When symptomatic, poor prognosis even without malignant progression
- Diffuse melanomatosis (Br J Cancer 2017;116:990)
- Dismal prognosis
- Increased number of copy number alterations compared to its benign counterpart (Acta Neuropathol 2017;133:333)
- Melanocytoma
- Prognosis is dependent on complete tumor resection, especially in intermediate grade tumors with recurrence rates varying according to the extent of resection from 20% for gross total resection to 42% for subtotal resection (Zentralbl Neurochir 2004;65:146, World Neurosurg 2022;168:298)
- Rarely transforms into malignant melanoma (Neurosurgery 2010;67:E867, J Neurooncol 2011;102:323)
- SF3B1, EIF1AX and BAP1 mutations and chromosome 3 monosomy or complex copy number variations associated with aggressive behavior (Acta Neuropathol Commun 2016;4:5, J Neurooncol 2016;127:435)
- Meningeal melanoma
- Poor prognosis (overall survival [OS]: 6 years in patients with spinal tumors), yet better than metastatic melanoma to CNS (6 months) (Brain Pathol 2015;25:209, J Clin Oncol 2011;29:e499, J Neurosurg 1987;66:47)
Case reports
- 19 year old woman with a large parieto-occipital lesion (J Surg Case Rep 2024;2024:rjae332)
- 20 year old woman with insidious onset of holocranial headaches (Neurology 2023;101:e576)
- 41 year old man with hearing loss and back pain (J Neurosurg Case Lessons 2021;2:CASE21444)
- 68 year old woman with progressive mental deterioration (AJNR Am J Neuroradiol 2003;24:115)
Treatment
- Circumscribed tumors: gross total resection with or without adjuvant chemoradiation therapy (Cancers (Basel) 2024;16:2508)
- Diffuse or multifocal forms: palliative / symptomatic treatment (World Neurosurg 2019;122:648, Neurosurg Focus 2022;52:E8)
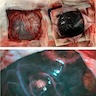
Gross description
- Circumscribed melanocytic neoplasms
- Usually solitary, well demarcated, intradural, extra axial mass that may be black, reddish-brown, blue or macroscopically nonpigmented, depending on the melanin content (Am J Surg Pathol 1999;23:745, J Clin Neurosci 2010;17:1227)
- Diffuse melanocytic neoplasms
- Depending on melanin content, blue to dark brown / black discoloration of the leptomeninges (J Neurooncol 2011;102:323, Radiographics 2009;29:1503)
Frozen section description
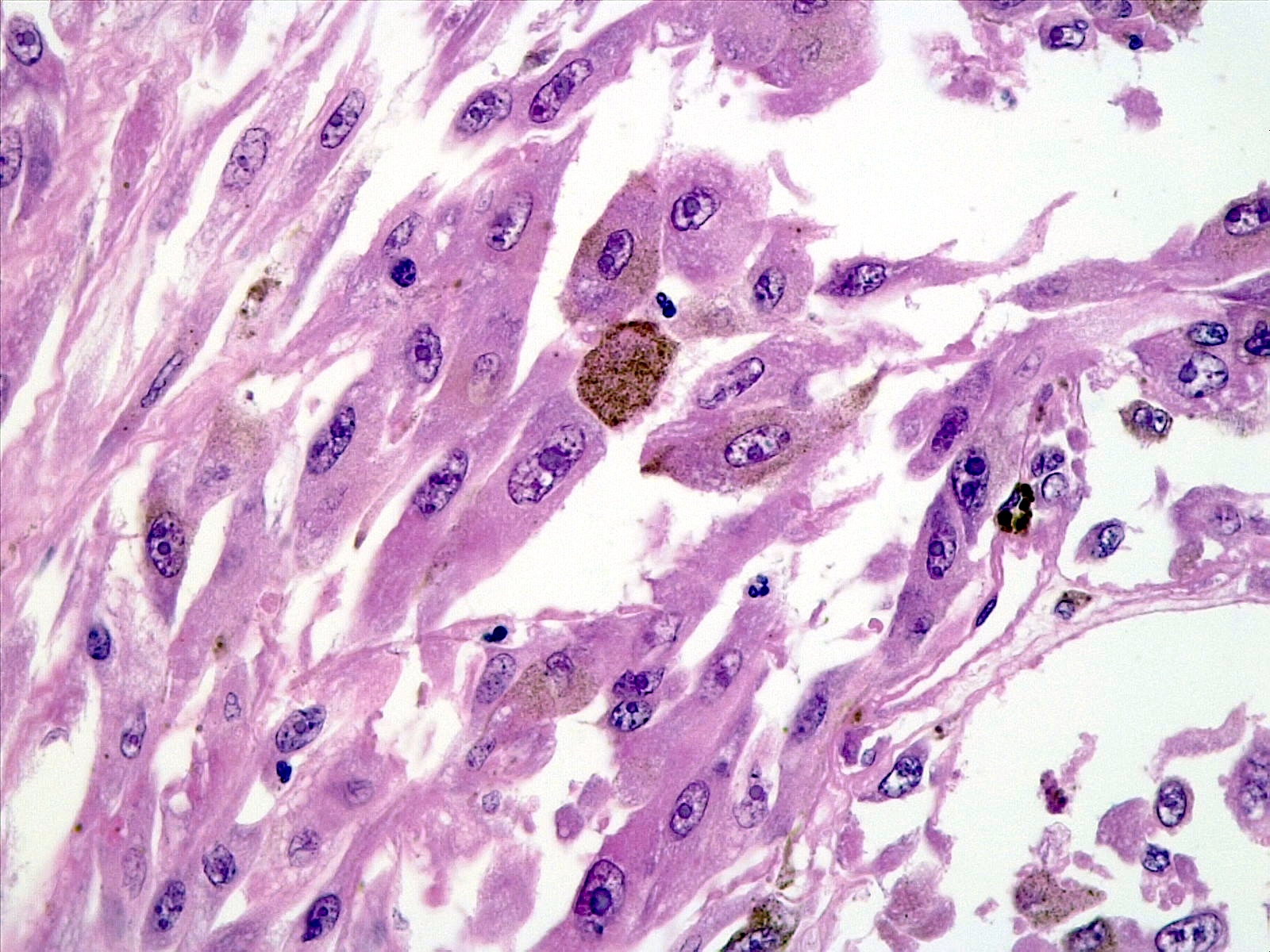
- Melanocytic nature can be suggested by the presence of brown intracellular pigment
- Differential diagnosis with nerve sheath tumors and with metastatic melanoma is not feasible intraoperatively
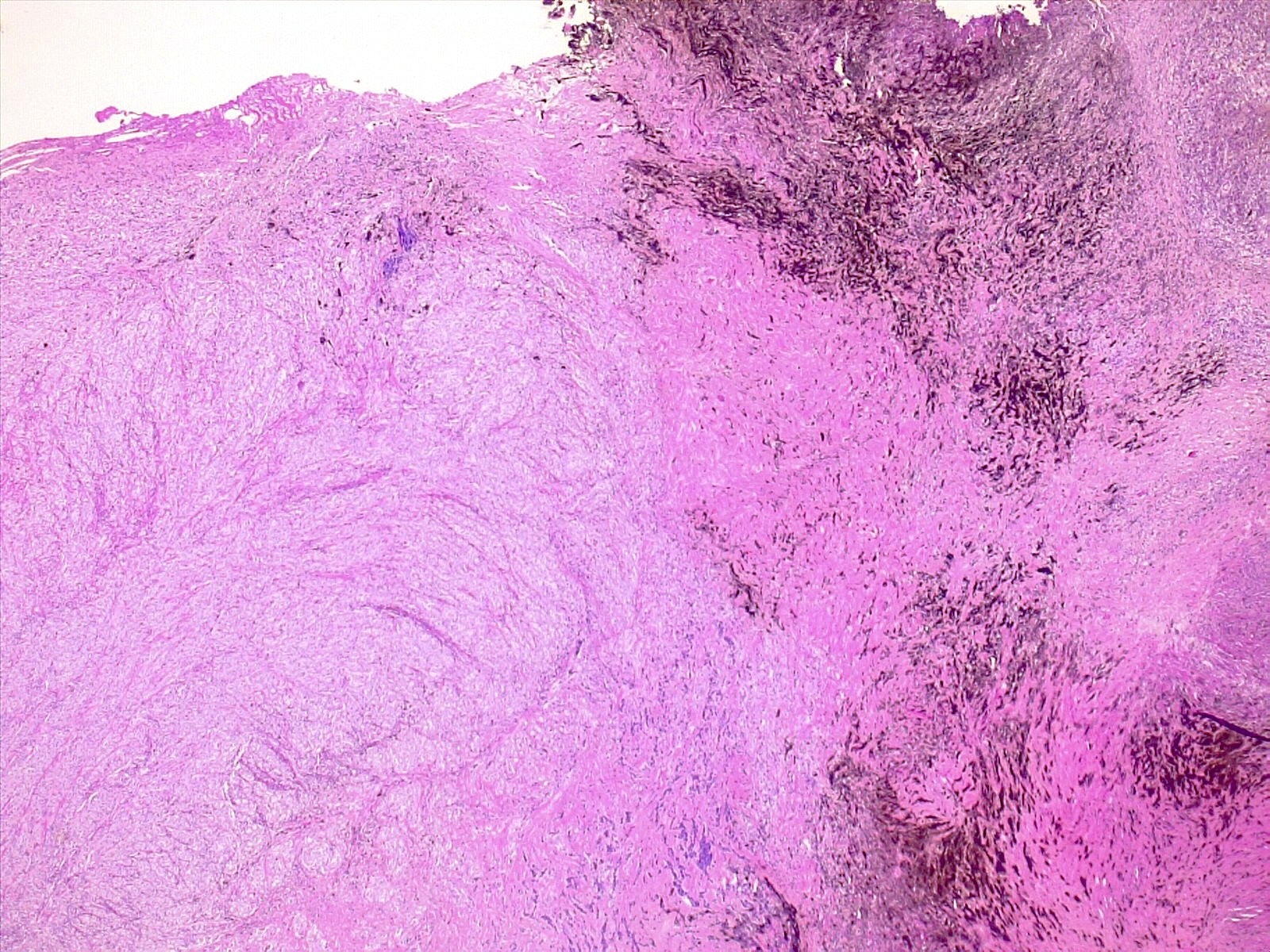
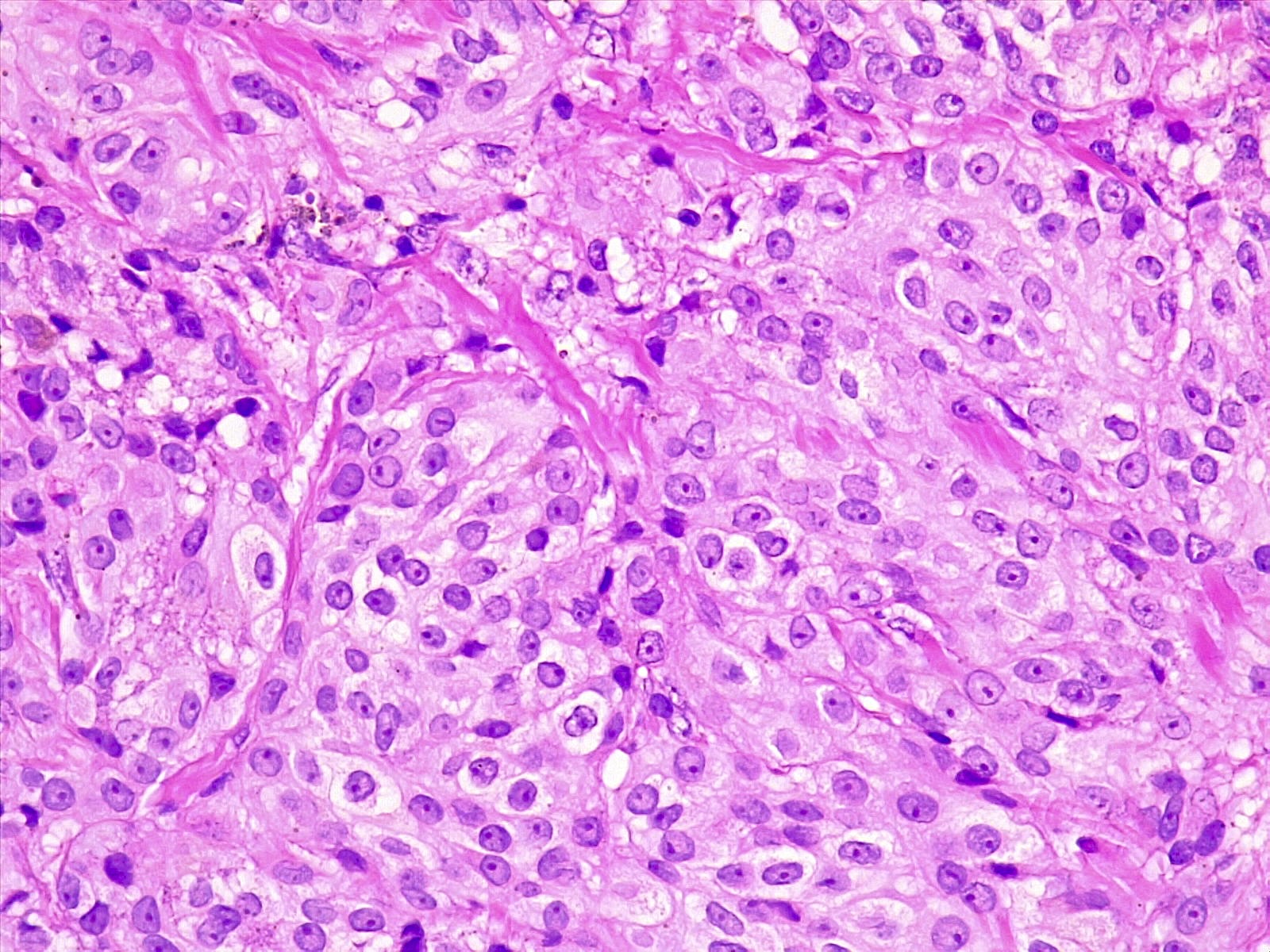
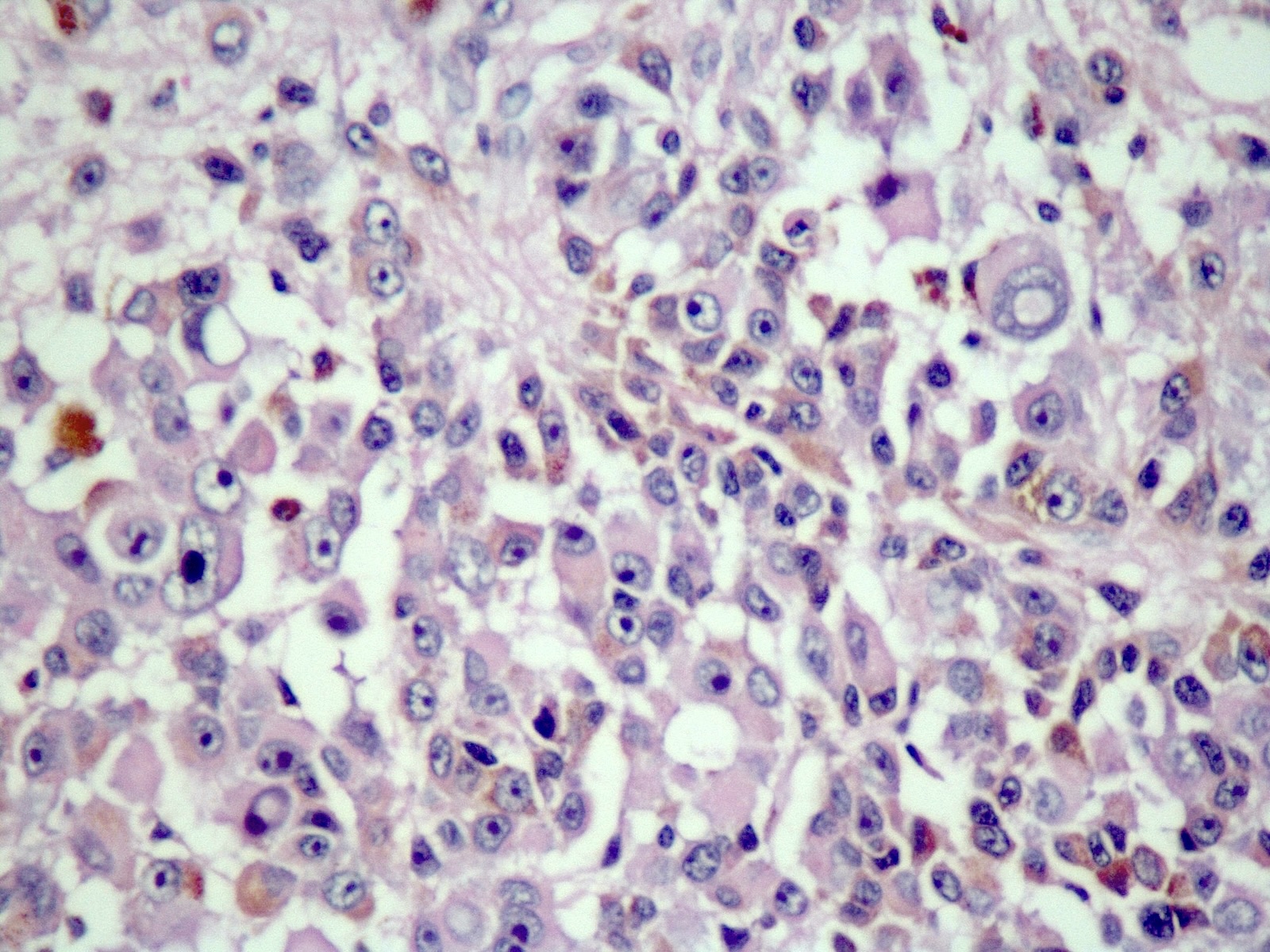
Microscopic (histologic) description
- Melanocytoma
- Solitary, circumscribed lesions; do not invade adjacent structures
- Densely packed nests of relatively uniform cells with variable amount of cytoplasmic brown pigment
- Tumor cells can form structures reminiscent of meningothelial whorls in meningiomas
- Bland, oval to bean shaped nuclei with small eosinophilic nucleoli, occasionally showing nuclear grooves
- Mitoses almost absent (on average < 0.5 mitoses/mm2, equating to < 1 mitosis/10 HPF of 0.5 mm in diameter and 0.2 mm2 in area) (Am J Surg Pathol 1999;23:745)
- Intermediate grade melanocytic neoplasms
- 0.5 - 1.5 mitoses/mm2 or CNS invasion
- Limited cytological atypia
- Necrosis is absent
- MIB1 labeling index: 1 - 4% (Am J Surg Pathol 1999;23:745)
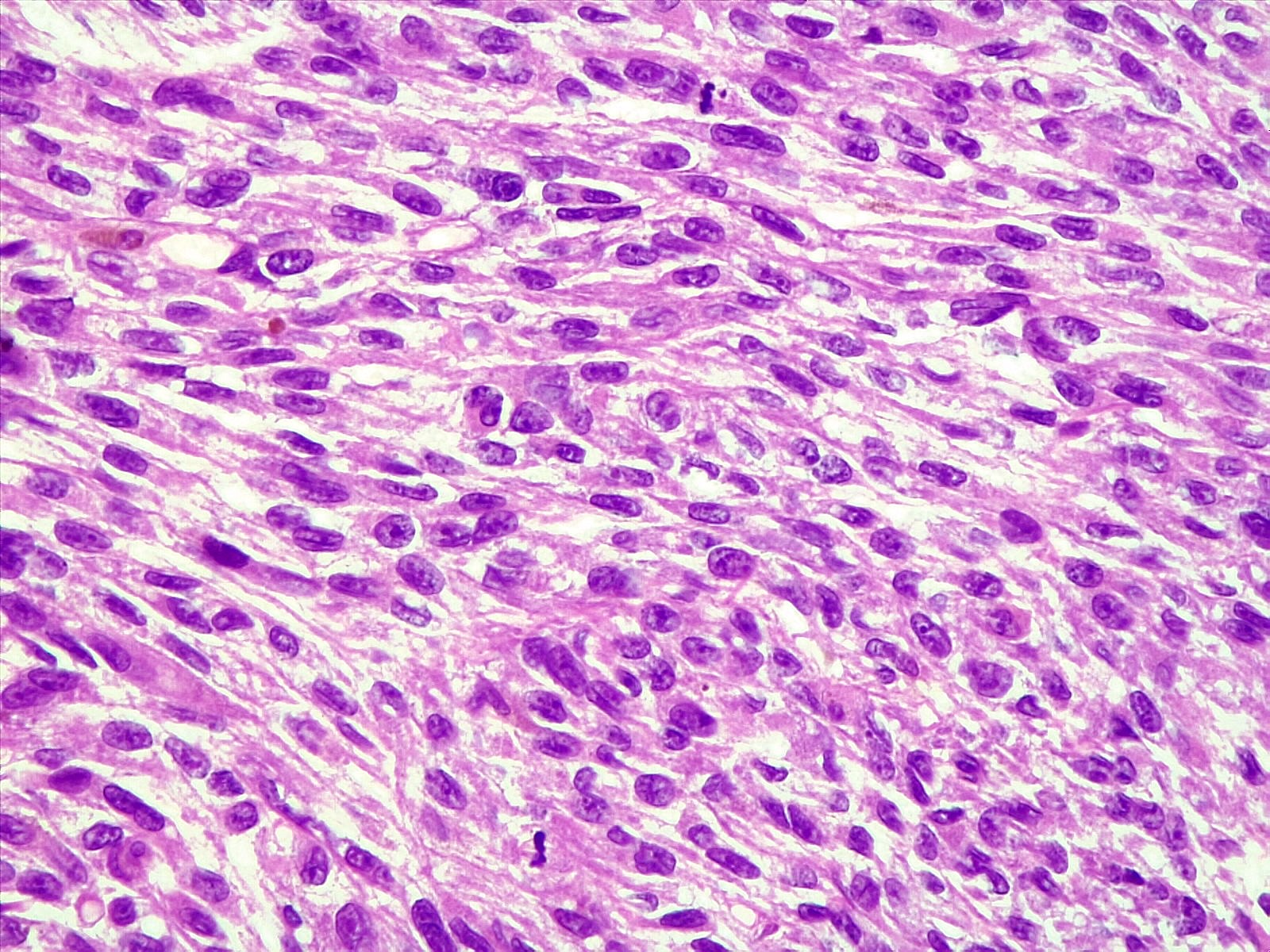
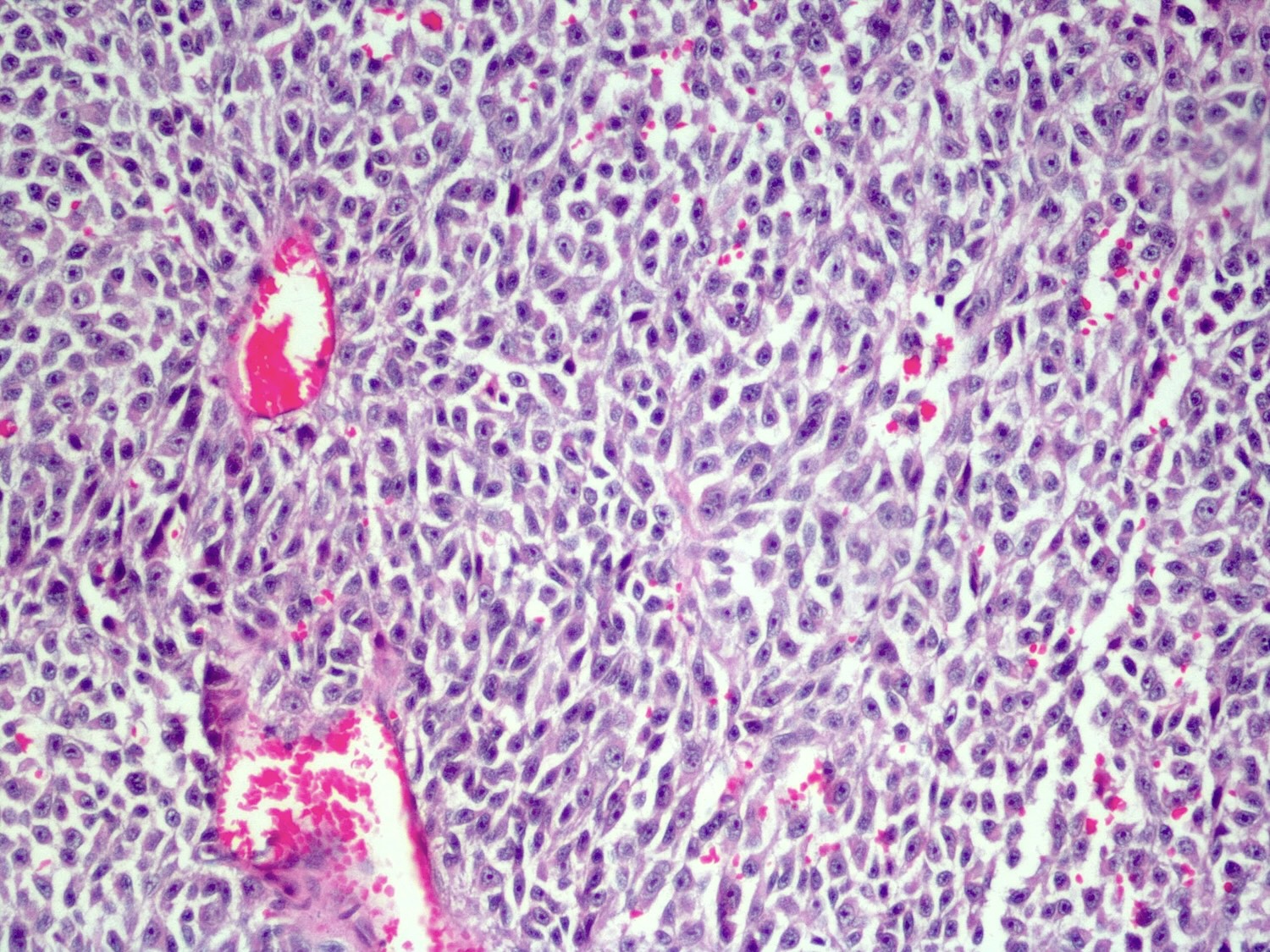
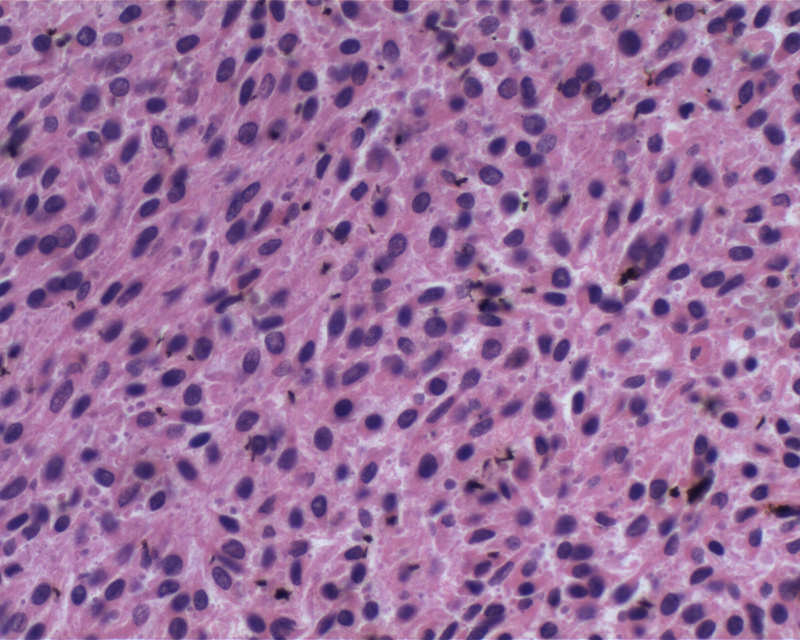
- Malignant melanoma
- Hypercellular sheets or nests of spindled to epithelioid cells
- May have marked cytological atypia
- > 1.5 mitoses/mm2 or necrosis
- Prominent nucleoli
- Invasion of adjacent structures may be seen
- Rarely amelanotic (World Neurosurg 2019;122:229)
Microscopic (histologic) images
Cytology description
- Monotonous proliferation of bland melanocytic cells with round to oval nuclei with inconspicuous nucleoli in benign lesions (Am J Surg Pathol 1999;23:745)
- Large atypical melanomatous cells with prominent nucleoli and pleomorphic nuclei in malignant neoplasm (Am J Surg Pathol 1999;23:745)
- Variable amount of intracellular melanin granules (Am J Surg Pathol 1999;23:745)
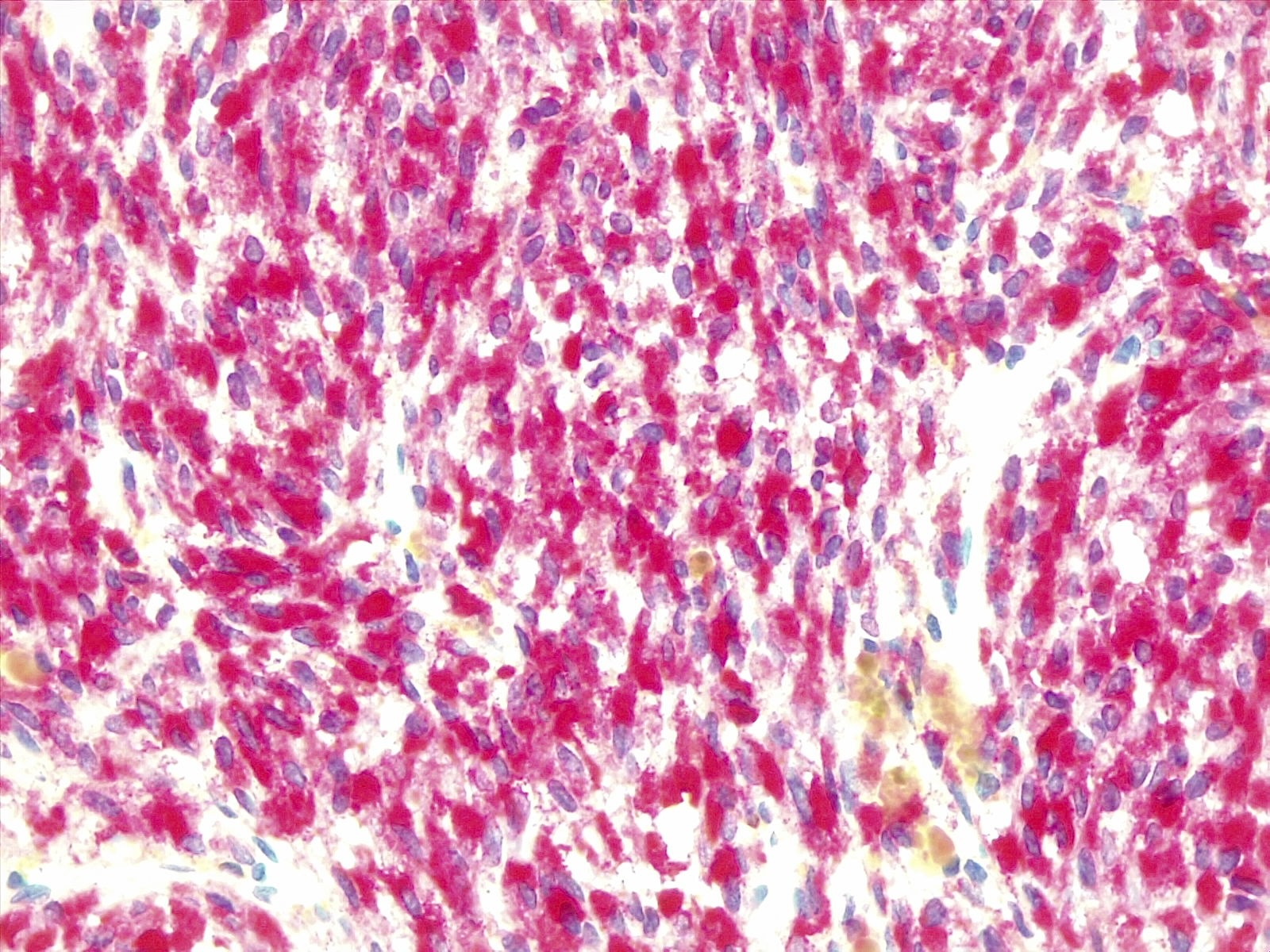
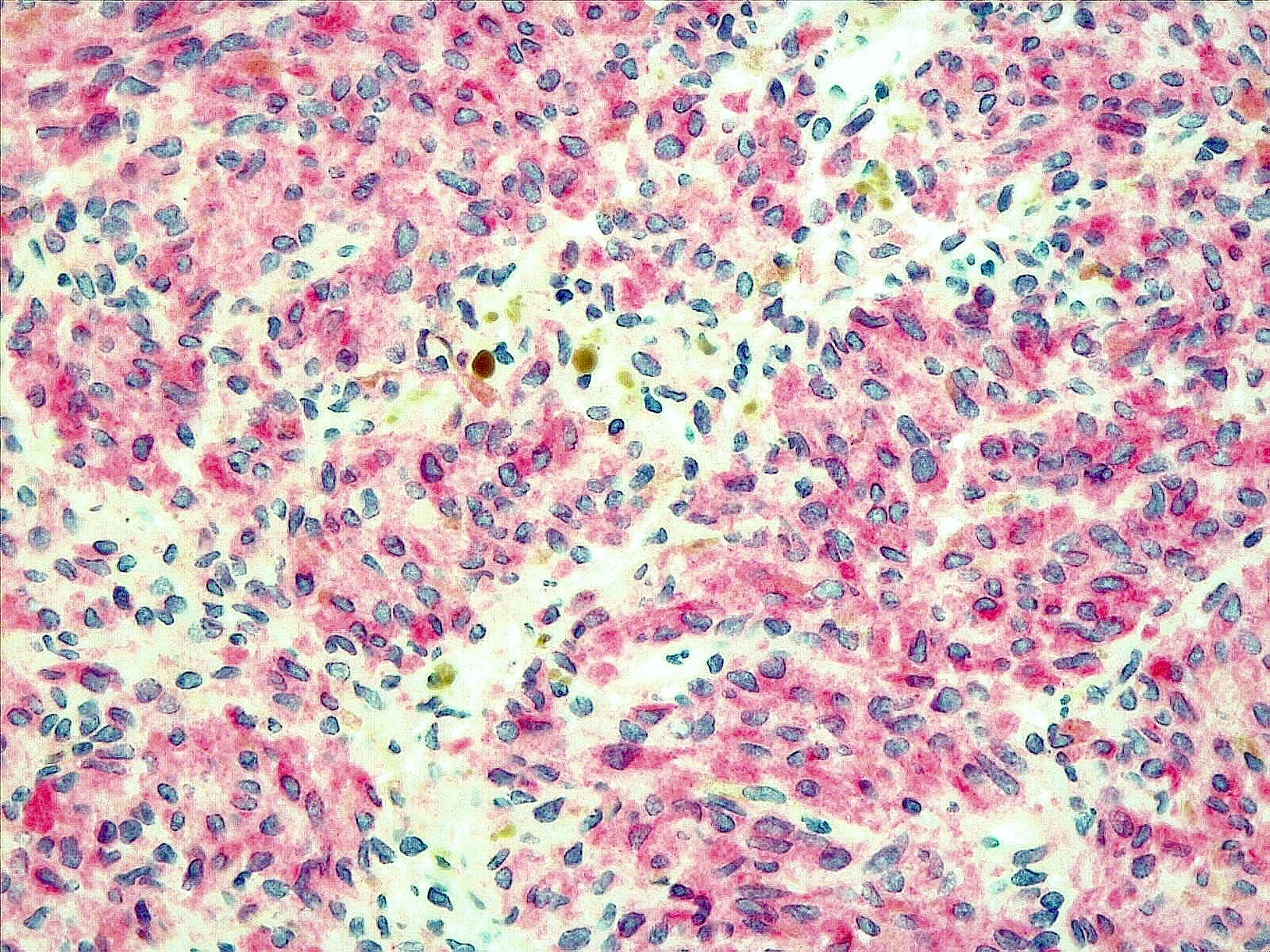
Positive stains
- MelanA, S100, HMB45, MITF (Am J Surg Pathol 1999;23:745)
- SOX10 (Pathol Oncol Res 2024;29:1611482)
- Ki67: < 2% for melanocytomas, ~8% for melanomas (Am J Surg Pathol 1999;23:745)
- Fontana-Masson: stains melanin black
Negative stains
Electron microscopy description
- Melanosomes are present (Cancer 1972;30:1286)
Molecular / cytogenetics description
- Mutually exclusive mutations in GNAQ, GNA11, PLCB4 and CYSLTR2
- GNA11 is the most frequently mutated gene (60 - 70% of cases)
- BAP1, SF3B1 and EIF1AX mutations are correlated with aggressive behavior, even in cases without worrisome histological features (Acta Neuropathol Commun 2016;4:5)
- Copy number abnormalities are absent or limited in melanocytomas and frequent in melanomas (Pathol Oncol Res 2015;21:439)
- Monosomy of chromosome 3 has been found in intermediate grade tumors (Acta Neuropathol 2015;129:921)
- TERT promoter mutations are usually absent (Neuropathol Appl Neurobiol 2014;40:794)
- BRAF mutations are rare (Brain Pathol 2015;25:209)
- NRAS mutations, especially at codon 61 in tumors in the context of neurocutaneous melanocytosis (Brain Pathol 2015;25:209)
- DNA methylation profiling identifies 2 clusters, 1 including cutaneous melanomas and the other composed of primary leptomeningeal tumors, uveal and blue nevus-like melanomas (Clin Cancer Res 2018;24:4494)
- At DNA methylation analysis primary leptomeningeal tumors are indistinguishable from uveal and blue nevus-like melanomas (Clin Cancer Res 2018;24:4494)
Videos
Malignant amelanotic meningeal melanoma
Sample pathology report
- Cervical spine, intradural mass:
- Meningeal melanocytoma (see comment)
- Comment: Fascicles and nests of pigmented spindle to epithelioid cells with abundant brown intracellular pigment. Necrosis, marked cytological atypia and mitoses are absent. CNS invasion is absent. Neoplastic cells are diffusely positive for S100, MART1 and HMB45. Ki67 labelling index is < 1%.
Differential diagnosis
- Circumscribed meningeal melanocytic neoplasms
- Malignant melanotic nerve sheath tumor (MMNST):
- Classical features of schwannian differentiations such as Antoni A and Antoni B, Verocay bodies are less evident (Acta Neuropathol 2010;120:755)
- MMNSTs are S100, SOX10, MART1 and HMB45 positive (Genes Chromosomes Cancer 2015;54:463)
- GNAQ / GNA11 mutations are usually absent (Acta Neuropathol 2010;120:755)
- PRKAR1A alterations in most cases of malignant melanotic nerve sheath tumors (Acta Neuropathol 2010;120:755, Am J Surg Pathol 2014;38:94, Acta Neuropathol 2010;120:755)
- Recurrent monosomy of 22q (Brain Pathol 2015;25:202)
- Metastatic cutaneous melanoma:
- Clinical history positive for extra CNS melanoma favors this diagnosis
- Purely intraparenchymal lesions are highly suspicious of metastatic disease from extra CNS primary melanoma
- Molecular analysis useful to differentiate metastatic melanoma from primary CNS melanocytic neoplasms (Neuropathol Appl Neurobiol 2014;40:794, Cancers (Basel) 2024;16:2508)
- Mutations commonly found in in primary cutaneous melanocytic lesions such as HRAS, BRAF, KRAS and KIT are usually absent in primary meningeal melanocytic lesions (Acta Neuropathol 2010;119:317, Neuropathol Appl Neurobiol 2013;39:417)
- Medulloblastoma with melanotic differentiation:
- Positive for neural markers such as NSE, synaptophysin and NeuN, sometimes with focal GFAP expression (Pediatr Blood Cancer 2009;52:875)
- HMB45 positivity is limited to pigmented cells (J Neurooncol 2011;103:759, Neuropathol Appl Neurobiol 2002;28:257)
- Malignant melanotic nerve sheath tumor (MMNST):
Additional references
Practice question #1
A 48 year old woman with no prior oncological history presented with a intradural extra-axial lesion of the cervical spine. Histological examination revealed a neoplasm composed of atypical spindled cells, seldom containing intracellular brown pigment, with up to 3 mitoses/mm2. At immunohistochemical analysis, neoplastic cells are negative for GFAP, EMA, SSTR2A, pancytokeratins and synaptophysin, while diffusely positive for S100, MelanA and HMB45. What is the most likely diagnosis?
- Melanocytoma
- Meningeal carcinomatosis
- Meningioma
- Primary meningeal melanoma
Practice answer #1
D. Primary meningeal melanoma shows histological features of a malignant neoplasm with an immunohistochemical profile consistent with melanocytic origin. GNAQ and GNA11 mutations are typically found in primary meningeal melanocytic tumors and help differentiate between primary CNS tumors and metastatic melanoma.
Answer B is incorrect because epithelial neoplasms are typically pancytokeratin positive.
Answer C is incorrect because meningioma is typically EMA positive.
Answer A is incorrect because in melanocytomas, mitoses are almost absent or < 0.5/mm2.
Comment Here
Reference: Melanocytic tumors / melanoma
Comment Here
Reference: Melanocytic tumors / melanoma
Practice question #2
Which of the following genes is commonly mutated in meningeal melanocytomas?
- BRAF
- GNA11
- PRKAR1A
- TERT (promoter)
Practice answer #2
B. GNA11 and GNAQ are the most frequent mutated genes in circumscribed meningeal melanocytic neoplasms.
Answer A is incorrect because BRAF mutations are usually absent in primary meningeal melanocytoma and found in metastatic melanoma.
Answer D is incorrect because pTERT mutations are usually absent in primary meningeal melanocytic neoplasms.
Answer C is incorrect because PRKAR1A alterations are associated with malignant melanotic nerve sheath tumors and Carney complex.
Comment Here
Reference: Melanocytic tumors / melanoma
Comment Here
Reference: Melanocytic tumors / melanoma