Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Potter A. Metastatic melanoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticmetastaticmelanoma.html. Accessed September 13th, 2025.
Definition / general
- Metastatic melanoma is the spread of melanoma beyond the primary site of disease (cutaneous or noncutaneous primary melanoma)
- Melanoma can metastasize to the skin or to sites beyond the skin / subcutaneous fat, including lymph nodes, soft tissue / bone compartments and visceral organs
Essential features
- Presence of histologically confirmed deposit(s) of melanoma beyond the primary site of disease
- Confirmation of diagnosis may require ancillary tests including immunohistochemistry and molecular studies
- Clinical correlation is required to ascertain relationship to the culprit primary tumor (in the setting of in transit / satellite cutaneous metastases) or the possibility of a regressed primary tumor (metastatic melanoma of occult primary)
Terminology
- In transit melanoma metastasis, epidermotropic melanoma, satellitosis / microsatellitosis
ICD coding
- ICD-O: 8720/6 - melanoma metastatic to the skin / melanoma metastatic to other organs
- ICD-11: 2E2Z & XH4846 - malignant neoplasm metastasis, unspecified & malignant melanoma, NOS
Epidemiology
- Age and gender distributions reflect those of the primary melanoma site and type
Sites
- Cutaneous sites of metastasis are commonly within the vicinity of a primary tumor on the scalp and limbs (Am J Dermatopathol 2010;32:129, J Surg Oncol 2019;119:232)
- Common noncutaneous sites of melanoma metastasis are lymph nodes, lung, liver, central nervous system and bone (J Clin Oncol 1983;1:126)
- Less frequent metastatic sites include intestines, pancreas, gallbladder, kidney, adrenals and thyroid gland
- Primary uveal melanoma has a propensity to metastasize to liver (Arch Ophthalmol 2005;123:1639)
Pathophysiology
- Arises as lymphatic, hematogenous or perineural spread from the primary site of disease (J Invest Dermatol 2002;119:705, Cancer Med 2018;7:583)
- Driver mutations of oncogenesis at the cutaneous primary site of disease, including BRAF (40 - 50%), NRAS (20 - 30%) and NF1 (10%), are also present within the metastatic deposit, with additional genetic abnormalities that contribute to tumor progression (J Transl Med 2019;17:289, Pathology 2016;48:147, Cancer Metastasis Rev 2016;35:93)
Etiology
- May develop in anyone with a primary invasive melanoma (of cutaneous or noncutaneous site)
- More likely in patients with thick cutaneous melanoma (≥ 1.0 mm T2 - 4) than thin (< 1 mm T1) (Amin: AJCC Cancer Staging Manual, 8th Edition, 2018, CA Cancer J Clin 2017;67:472)
Clinical features
- Most commonly presents as a palpable lymph node in the draining nodal basin from the primary site of disease (CA Cancer J Clin 2017;67:472)
- May arise as a solitary metastasis or multifocal disease
- Can be diagnosed in patients who have no known prior melanoma and no clinically detected culprit primary lesion on thorough clinical examination (occult primary), due to complete regression of the primary melanoma (J Surg Oncol 2019;119:232)
Diagnosis
- Clinical examination and radiological investigation of suspected sites of disease, including the use of modalities such as computed tomography (CT), positron emission tomography (PET) and magnetic resonance imaging (MRI) (J Surg Oncol 2019;119:232)
- Histological assessment is required for definitive diagnosis, obtained through biopsy techniques or surgical excision (J Surg Oncol 2019;119:232)
Laboratory
- Serum lactate dehydrogenase (LDH), S100B or melanoma inhibitory activity (MIA) protein testing is not required or recommended for screening or diagnosis of metastatic melanoma (Surg Oncol Clin N Am 2011;20:181)
Radiology description
- Involved lymph nodes can have an irregular hypoechoic and lobulated appearance on ultrasound
- When combined with computed tomography, positron emission tomography (PET / CT) is the most sensitive modality for detection of metastatic deposits (Clin Radiol 2011;66:224)
- MRI is more sensitive for detection of cerebral metastases than CT alone (J Neurooncol 1999;44:275)
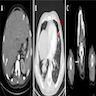
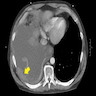
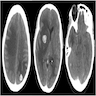
Radiology images
Prognostic factors
- Prognosis is dependent on stage of disease, number of sites involved, disease burden and primary melanoma site (cutaneous versus noncutaneous) (CA Cancer J Clin 2017;67:472)
- Metastasis to the regional lymph node basin (5 year survival stage III: 77%) has a better prognosis than visceral organ involvement (5 year survival stage IV: 27%)
- Tumor biology, including driver mutations and the tumor inflammatory microenvironment, may alter outcomes and predict response to therapies (Front Immunol 2021;12:663495)
Case reports
- 39 year old man with metastatic melanoma to the parietal cortex (CA Cancer J Clin 2020;70:78)
- 46 year old man with multiorgan involvement by metastatic melanoma (World J Clin Cases 2022;10:10136)
- 55 and 57 year old women and 84 year old man with melanoma with divergent differentiation, presenting as sarcoma (Pathologica 2022;114:217)
- 57 year old man with solitary lung nodule (J Med Case Rep 2021;15:347)
- 67 and 69 year old men with metastatic melanoma to the gastrointestinal tract (Int J Surg Case Rep 2023;103:107907)
Treatment
- Solitary metastases can be treated by complete surgical resection
- Isolated limb perfusion / infusion with chemotherapeutic agents can be used for multiple in transit metastases (Ann Surg Oncol 2016;23:2330)
- Macroscopic lymph node involvement may be managed with regional lymph node dissection, with longterm control in 50% of patients (Ann Surg Oncol 2014;21:292)
- Patients with extensive stage III or IV disease may be treated with systemic therapies, including targeted therapy with BRAF / MEK inhibitors or immunotherapies that target PD1 and CTLA4 immune checkpoints (N Engl J Med 2014;371:1867, N Engl J Med 2015;373:23)
- Unresectable cutaneous, subcutaneous or nodal metastases can be treated with intralesional talimogene laherperepvec (T-VEC), an engineered oncolytic herpesvirus that induces a local and systemic immunological antitumor response and can be used in combination with systemic therapies (Cancers (Basel) 2021;13:1383)
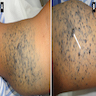
Clinical images
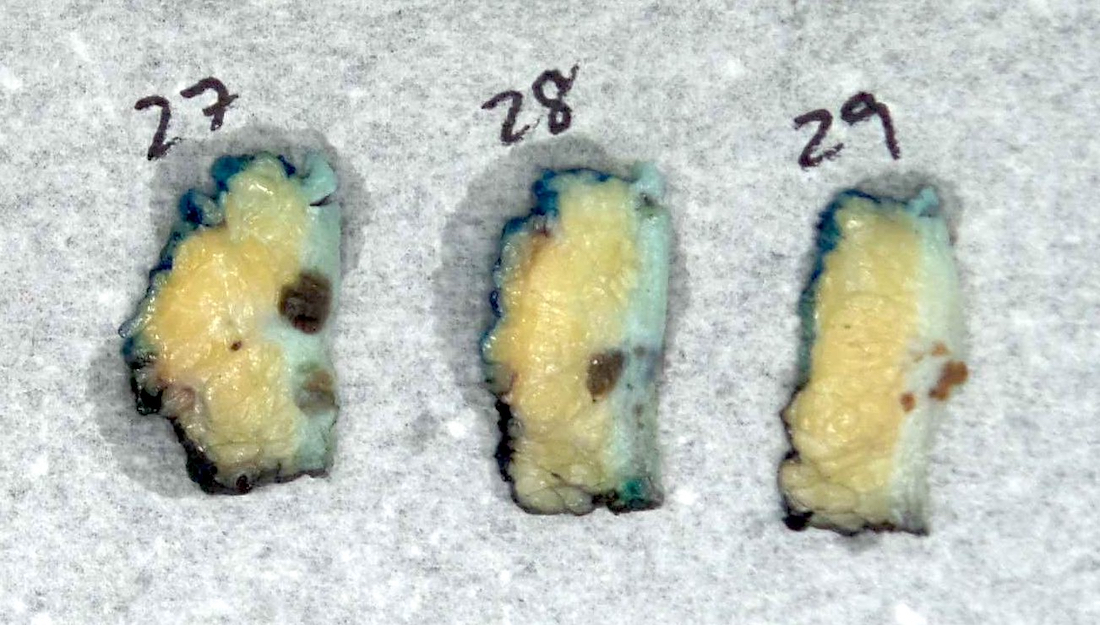
Gross description
- Numerous and distinct dermal or subcuticular nodules, seen either within 2 cm (satellite) or beyond 2 cm (in transit) of the primary tumor without appreciable connection (needs to be confirmed microscopically) (Ann Surg Oncol 2018;25:2105)
- Cutaneous, nodal or visceral deposits may be circumscribed, poorly defined or encapsulated and may show pigmentation (brown discoloration), hemorrhage or evidence of necrosis
Microscopic (histologic) description
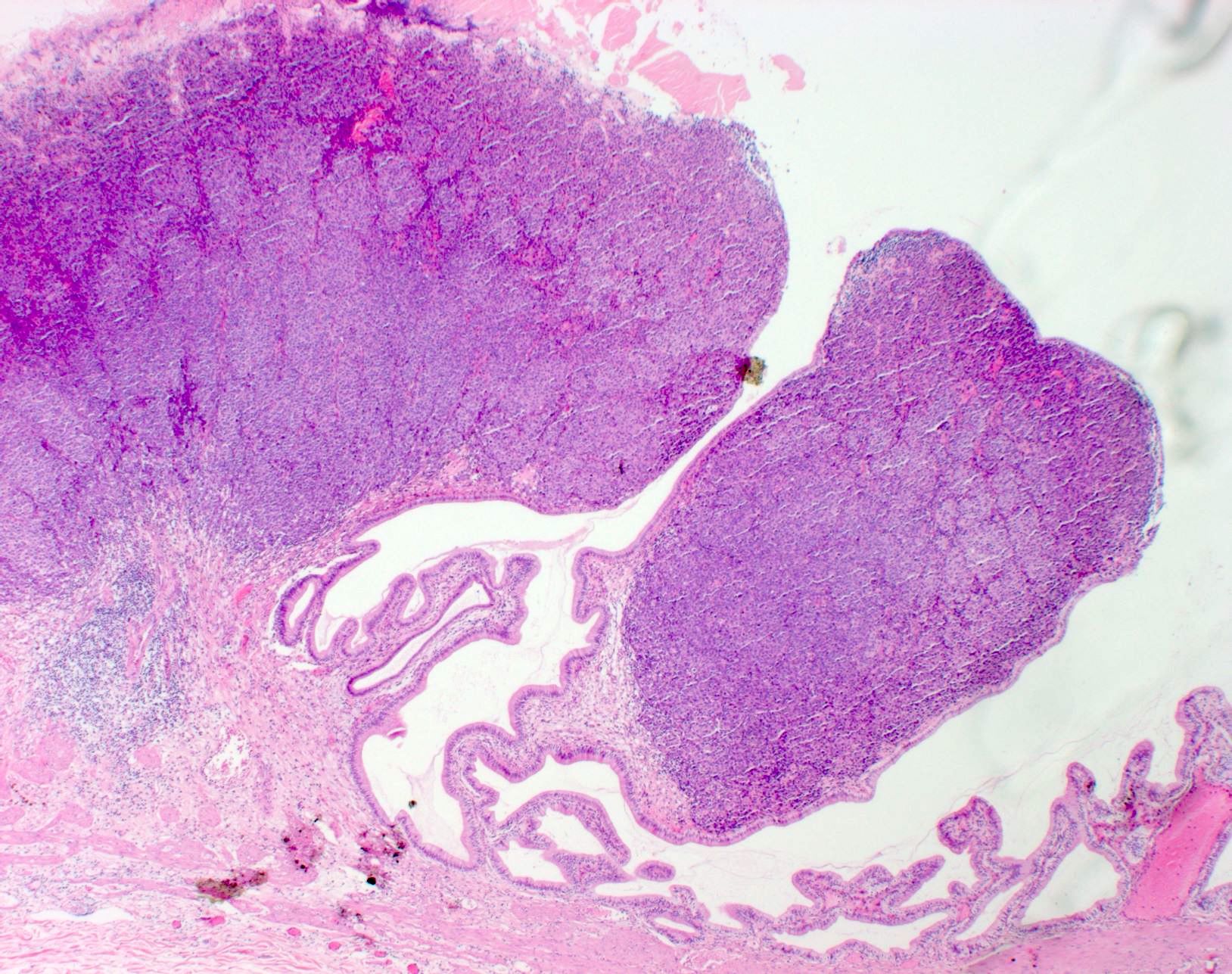
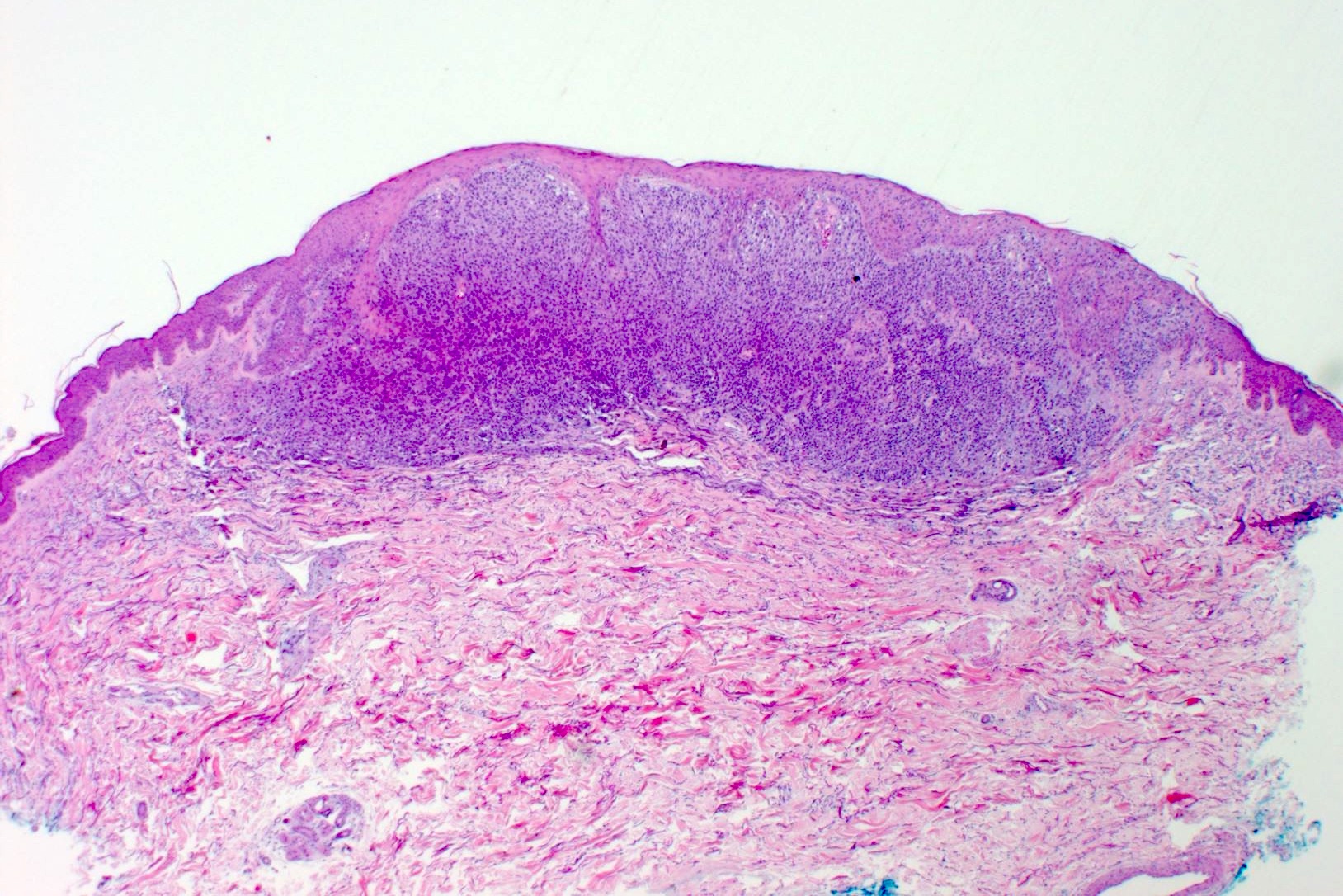
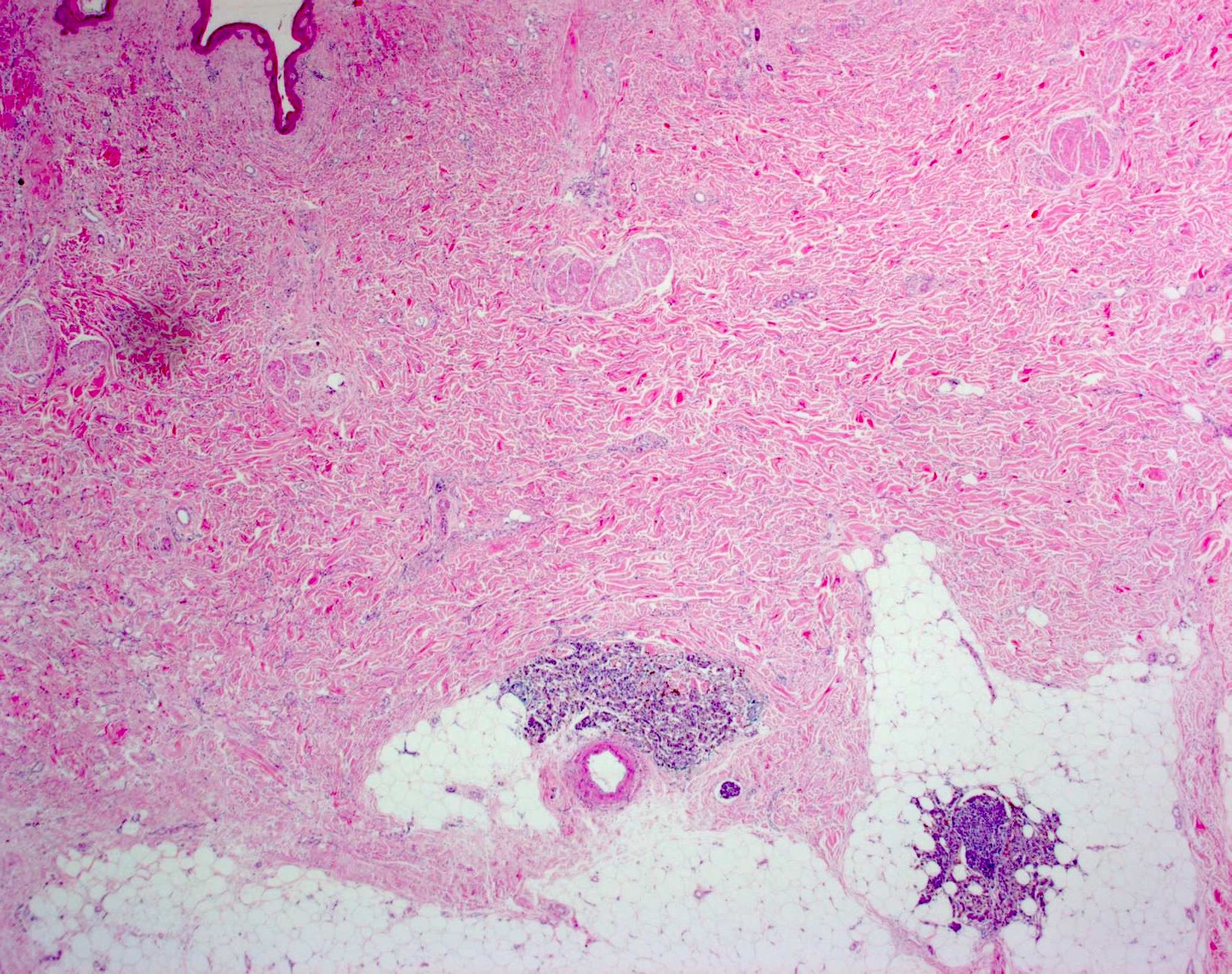
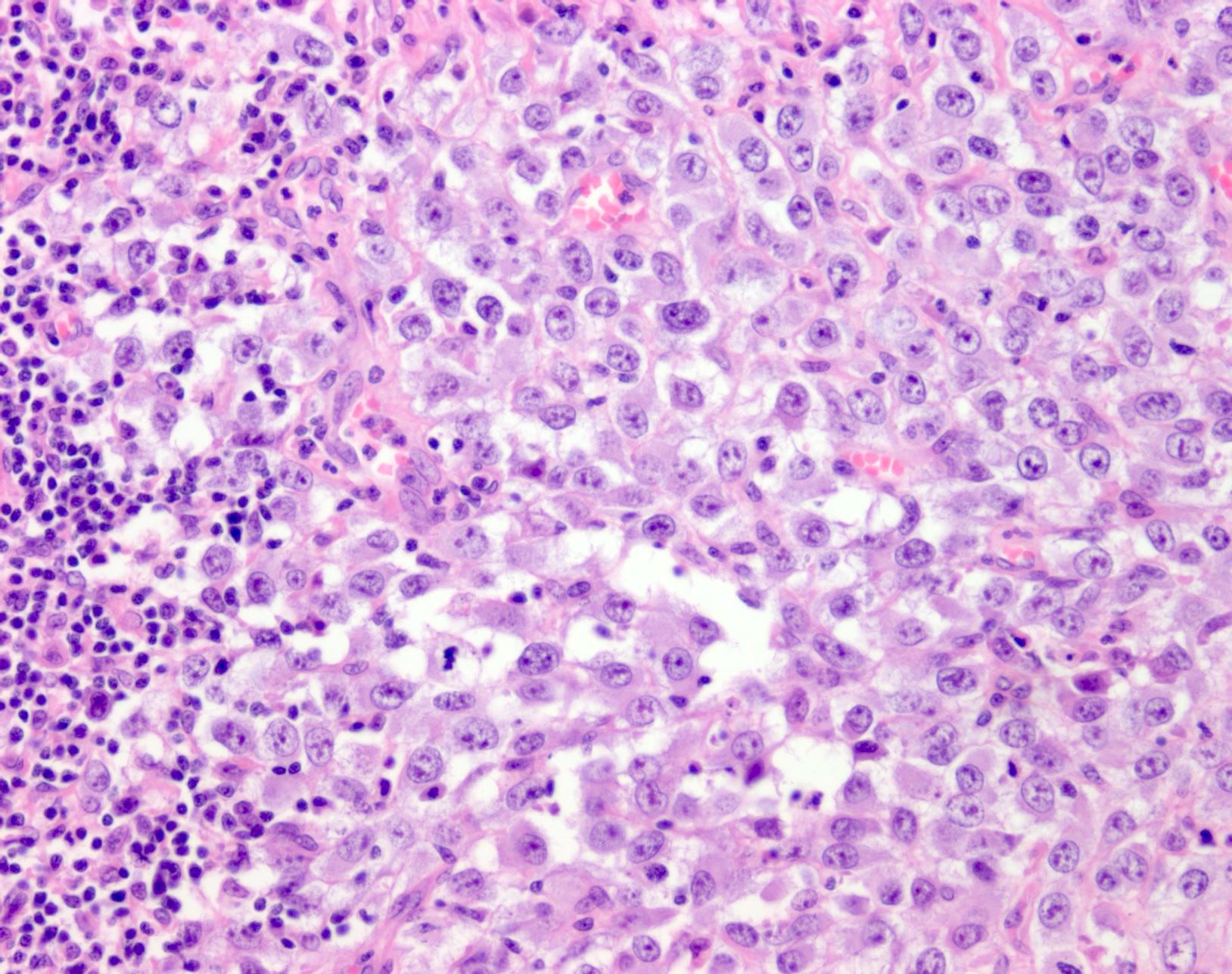
- Within skin, are often well circumscribed nodules within the dermis or the subcutis
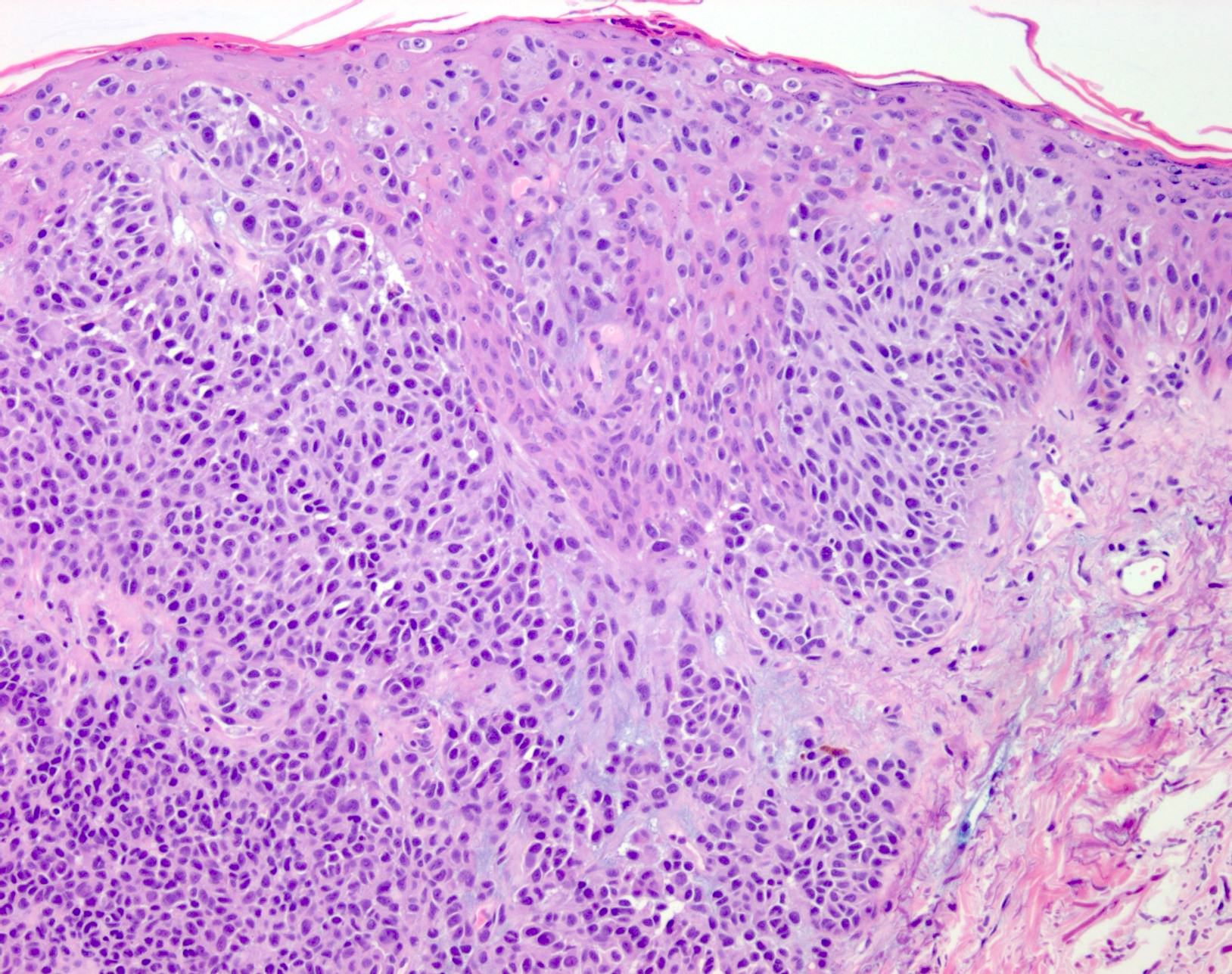
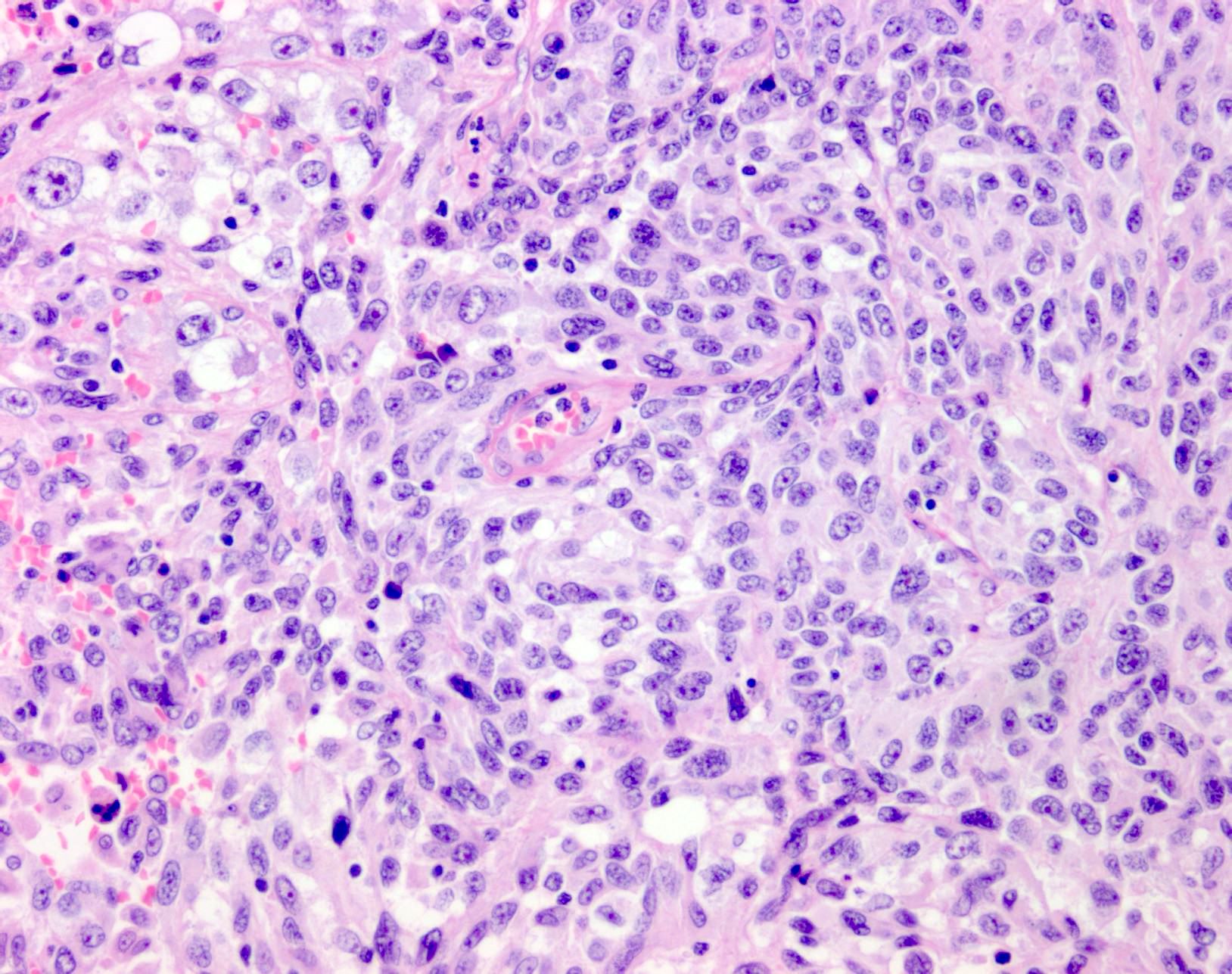
- Display overt malignant melanocytic features, including cytological atypia, pleomorphism, sheet-like growth and proliferative activity
- Cytomorphology is frequently similar to that seen in the primary tumor; however, there can be variation or dedifferentiation at the metastatic site (Histopathology 2012;61:889)
- Presence of an intraepidermal component does not exclude a metastatic deposit, as up to 10% of cutaneous metastasis can show epidermotropism (Am J Dermatopathol 2010;32:129, Histopathology 2018;72:472, Am J Surg Pathol 1994;18:1140)
- Microsatellites (0.05 - 0.1 mm) are confirmed microscopic foci of metastasis, which are most likely lymphovascular in nature (Histopathology 2012;61:889)
- Satellites occur within 2 cm of the primary lesion and in transit melanoma > 2 cm from the primary tumor, often towards the draining nodal basin; cutaneous deposits at a distant site from a culprit primary are considered stage IV disease
- Metastasis within the lymph node most frequently involves the nodal sinus and can extend into parenchyma, show extracapsular extension or complete node replacement (Eur J Cancer 2009;45:2736, J Clin Oncol 2004;22:3345)
- Isolated tumor cells within a lymph node are classified as nodal disease, stage III (Amin: AJCC Cancer Staging Manual, 8th Edition, 2018, CA Cancer J Clin 2017;67:472)
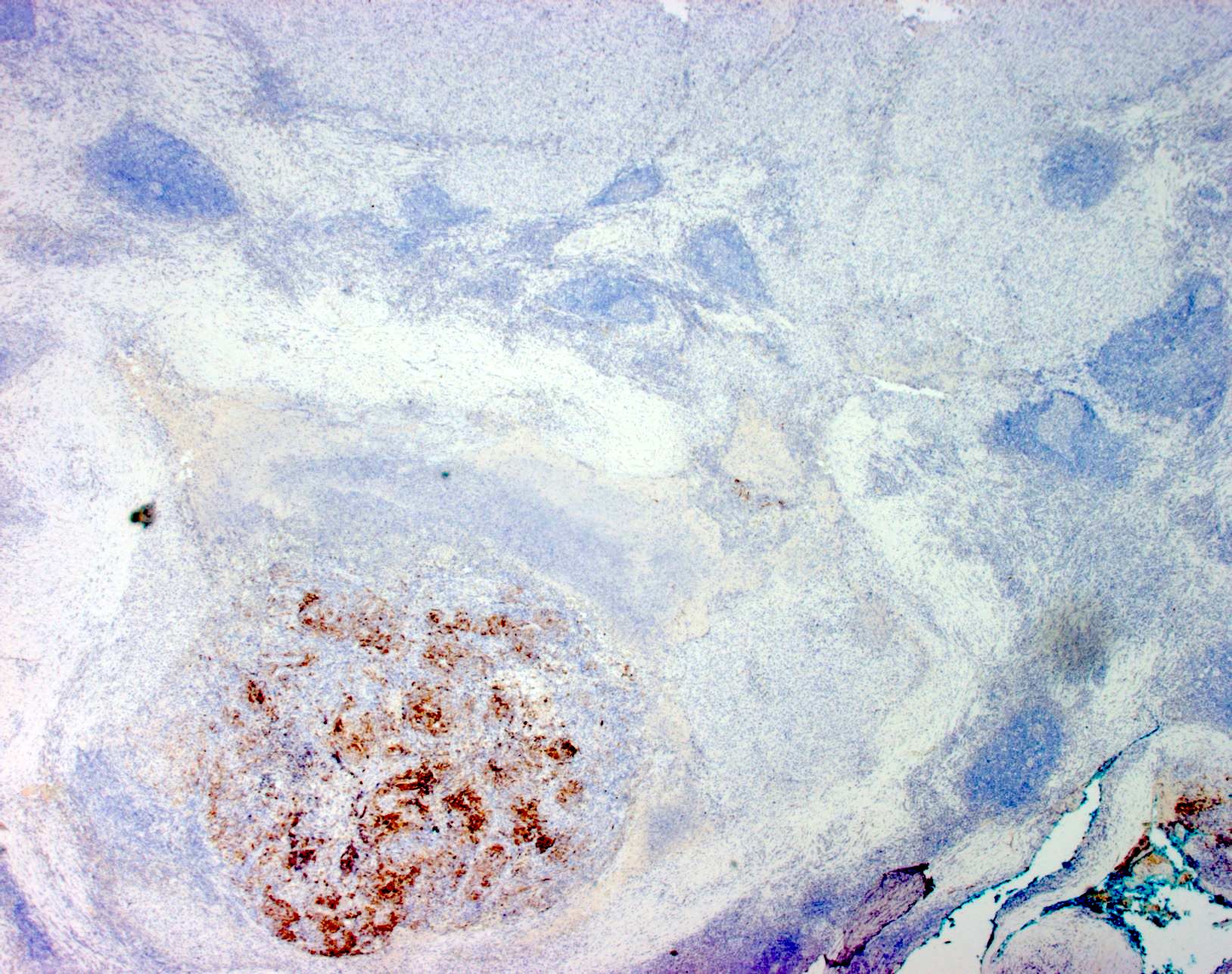
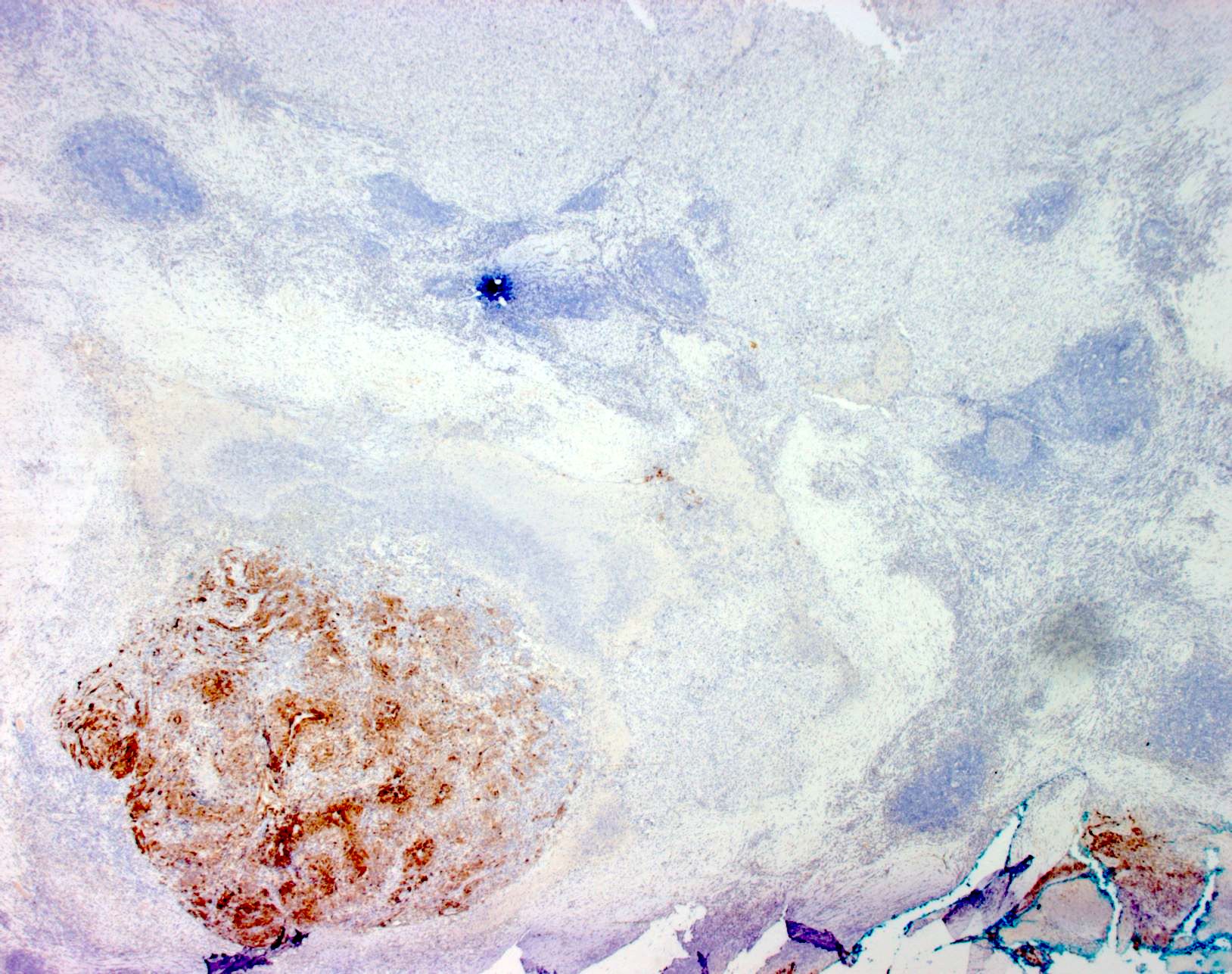
- SOX10 and S100 immunohistochemistry highlights nerve fibers, with S100 detecting interdigitating dendritic cells within normal lymph node
- Metastases in visceral organs form solid expansile nodules or infiltrate in a nondestructive pattern around native structures; perivascular (peritheliomatous) accentuation of tumor growth can be noted, especially in intracranial metastases (Angiogenesis 2020;23:27, J Cutan Pathol 2019;46:570)
- Dedifferentiated and undifferentiated melanoma can occur, whereby cytomorphological and immunohistochemical features of melanoma are lost
- Thorough sampling and immunohistochemical investigation is required
- Identification of a transition between conventional and undifferentiated components can aid in establishing the correct diagnosis (Am J Surg Pathol 2021;45:240)
Microscopic (histologic) images
Contributed by Alison Potter, M.B.B.S.
Virtual slides
Cytology description
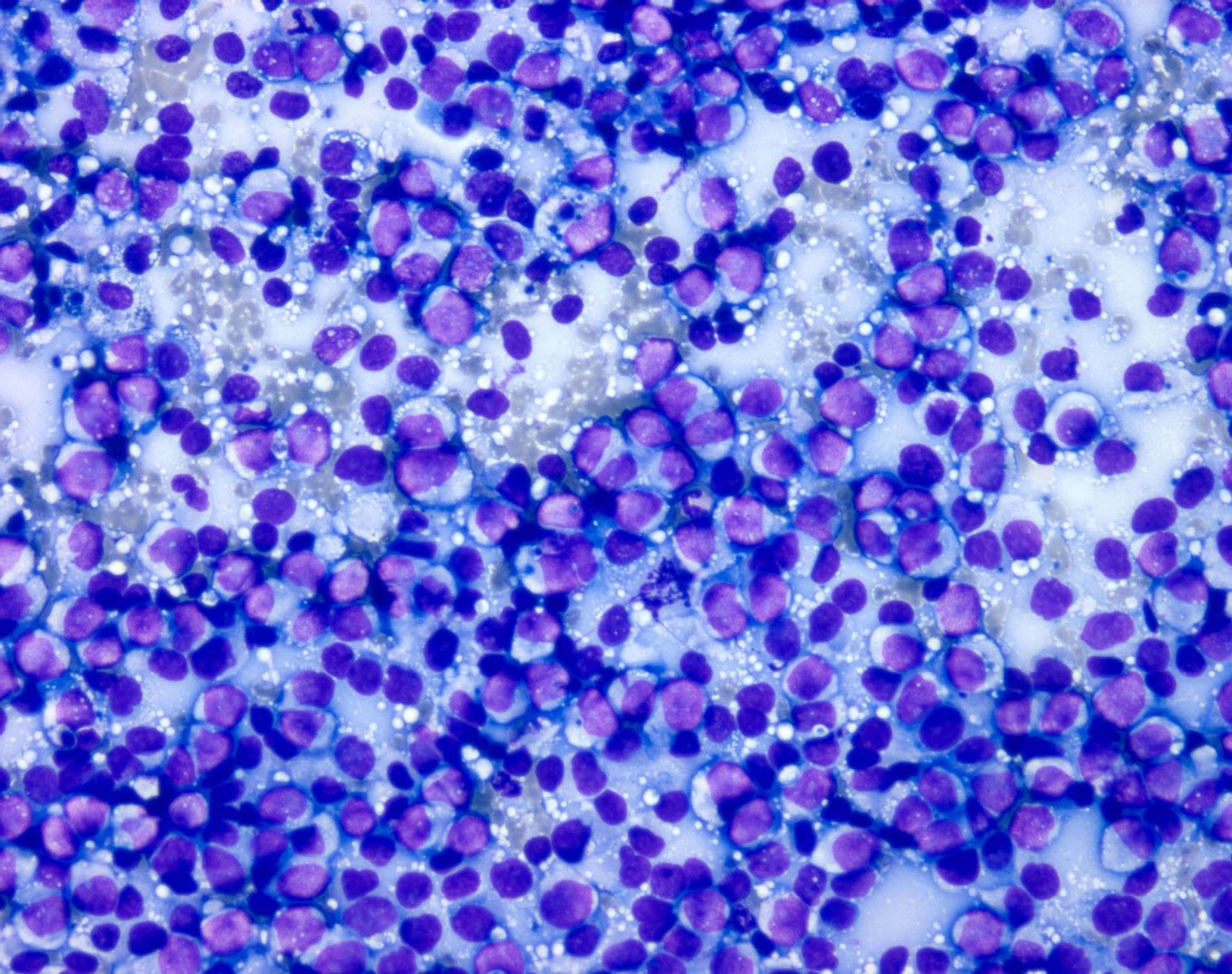
- Preparations are usually highly cellular, with loosely cohesive and singly dispersed malignant cells with overt features of malignancy, including pleomorphism, nuclear membrane irregularities, coarse chromatin, prominent nucleoli and mitotic activity (Semin Diagn Pathol 2016;33:198)
- Binucleation and multinucleation is a common feature, as are intranuclear pseudoinclusions
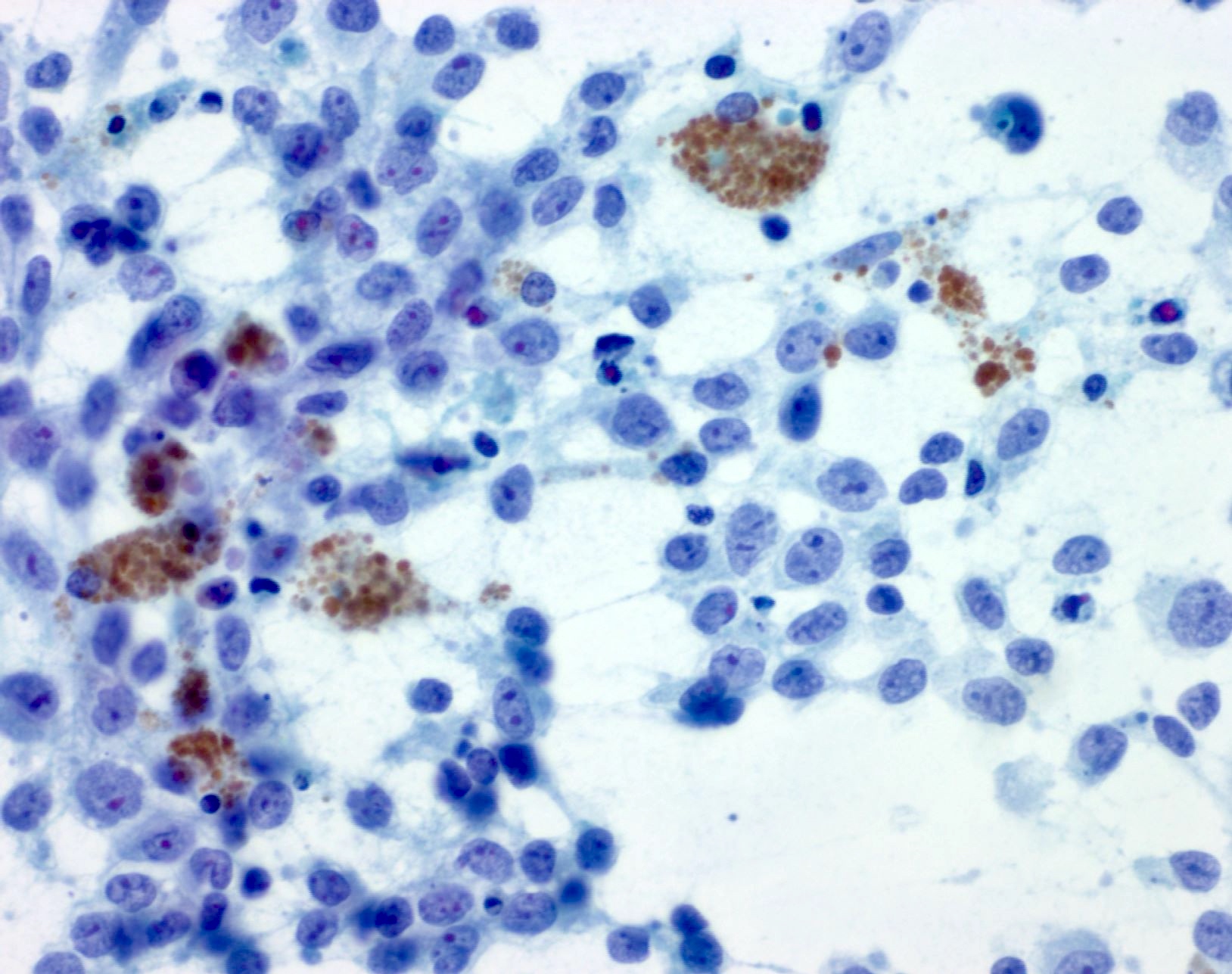
- Melanin pigment can be sparse at metastatic sites but when present is of a blue-black (petroleum blue) quality on Romanowsky based stains and brown-black on Papanicolaou stain
- Presence of numerous pigmented histiocytes may provide a clue to the diagnosis
- Limitations
- Broad spectrum of morphologies from epithelioid to spindled to bizarre forms
- Morphological overlap with many primary and metastatic malignancies, especially in nodal and visceral sites
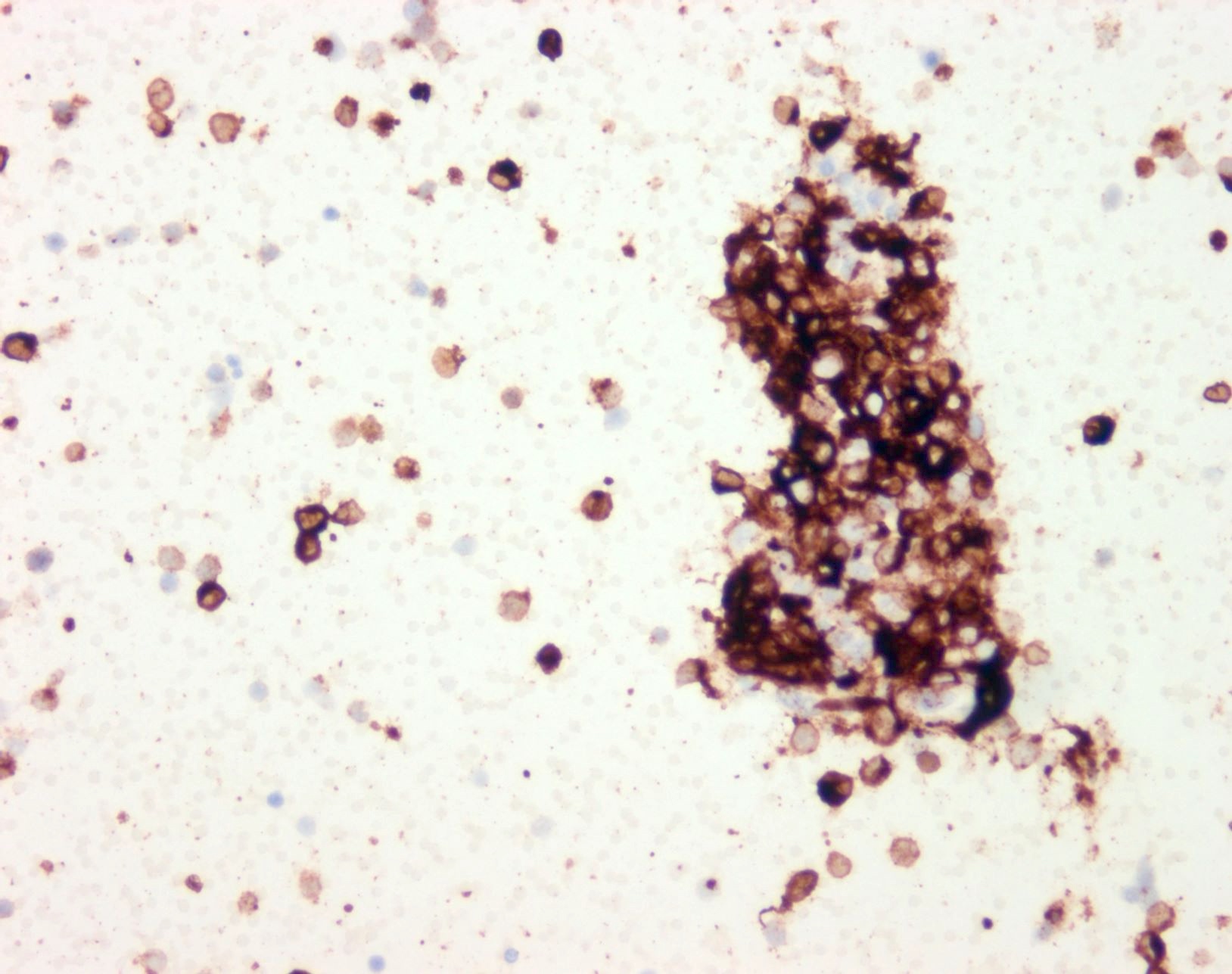
Cytology images
Positive stains
- S100 protein, MelanA (MART1), SOX10, HMB45 (J Cutan Pathol 2008;35:433)
- Tyrosinase and MITF are less sensitive, with MITF showing only moderate specificity
- PRAME may be positive but should not be used as confirmation of melanocytic lineage, as expression can occur in other entities, including numerous carcinomas and sarcomas (Am J Surg Pathol 2022;46:1467)
- Aberrant expression of markers of epithelial, mesenchymal and hematopoietic lineage may occur in dedifferentiated tumors (Am J Surg Pathol 2021;45:240)
Negative stains
- Cytokeratins
- CD45
- SMA, desmin, myogenin, MyoD1
- Reference: J Cutan Pathol 2008;35:433
Molecular / cytogenetics description
- Melanoma driver mutations (most frequently seen in BRAF or NRAS) are carried through from the primary to the metastatic site of disease (J Transl Med 2019;17:289)
- Identification of a common driver mutation can support the link between the metastatic tumor and the culprit primary site, in cases where numerous possible primary sites are clinically identified
- New diagnosis of metastatic melanoma (stage III or IV disease) warrants characterization of melanoma associated targetable mutations, in order to facilitate systemic treatment strategies (Amin: AJCC Cancer Staging Manual, 8th Edition, 2018, Pathology 2022;54:6)
- BRAF V600E mutation or NRAS Q61R mutation can be identified by immunohistochemistry; alternative mutations can be detected on polymerase chain reaction (PCR) based sequencing modalities
- Demonstration of a melanoma associated mutation (e.g., in BRAF and NRAS) may aid in the diagnosis of metastatic melanoma in cases where the morphology is dedifferentiated / undifferentiated (J Cutan Pathol 2023;50:223, Am J Surg Pathol 2016;40:181)
Sample pathology report
- Skin, left proximal thigh, excision:
- Dermal melanoma, favor cutaneous / in transit metastasis (see comment)
- Comment: Sections of the left proximal thigh lesion show a dermal nodule of malignant epithelioid and spindled cells in keeping with melanoma. The tumor is located within the papillary dermis and attenuates an overlying epidermis, without evidence of a junctional melanocytic component. No significant surrounding inflammation or fibrosis is seen. Adjacent lymphatic spaces contain small tumor nests, in keeping with lymphovascular space invasion. Central tumor necrosis is present as well as numerous mitoses (6 per mm2). No background nevus component is appreciated. Margins are clear of tumor by 4 mm (both peripheral and deep). BRAF V600E immunohistochemistry is negative and tissue has been sent for formal molecular testing for melanoma associated targetable mutations; a separate molecular report will be issued.
- Small bowel, excision:
- Metastatic melanoma (see comment)
- Comment: Sections show multiple deposits of metastatic melanoma, between 10 and 150 mm in size. Several deposits involve the mucosal surface with extension into lamina propria and secondary erosion of the overlying epithelium. There is evidence of patchy tumor necrosis and a sparse infiltrate of lymphocytes. Most tumor appears viable and is highlighted on immunohistochemistry for S100, SOX10, MelanA, HMB45 and BRAF V600E. 22 lymph nodes are identified, none of which show metastatic melanoma (0/22). The melanoma deposits appear completely excised. The closest margin is 2 mm (gastric margin).
- Right axilla lymph node / mass, excision:
- Metastatic poorly differentiated / dedifferentiated melanoma (see comment)
- Comment: Sections of the left axillary mass show a poorly differentiated epithelioid malignancy with cohesive sheet-like growth and large areas of necrosis. There is no evidence of glandular or squamoid differentiation and no melanin pigment is seen. A large range of immunostains (performed on block A8) show the tumor is negative for a large range of melanocytic, epithelial and lymphoid markers. Due to the poorly differentiated morphology, additional stains were performed (on block A1) and these demonstrate a small focus of viable tumor with positivity for SOX10, S100, HMB45, MelanA, tyrosinase, MITF and PRAME. The tumor cells are also positive for BRAF V600E. Repeated staining for SMA, desmin, MNF116, p40, myogenin and MyoD1 are negative. Overall, despite being focal in nature, the expression of a panel of melanocytic markers would favor this nodal disease to represent poorly differentiated / dedifferentiated metastatic melanoma.
Differential diagnosis
- Primary cutaneous melanoma (versus satellite melanoma metastasis):
- Multiple step sections are required to exclude contiguous soft tissue or periadnexal / perineural tracking from the primary tumor
- Primary dermal melanoma (versus in transit melanoma metastasis):
- Presence of an inflammatory infiltrate, surrounding fibrosis, junctional activity or adjacent benign nevus are all features which favor a primary (dermal) nodular melanoma, over a metastatic deposit (Histopathology 2018;72:472)
- Capsular nevus (versus lymph node metastatic melanoma):
- Seen in lymph nodes draining the skin, capsular nevi are commonly present within the fibrous capsule and trabeculae (rather than sinus and parenchyma)
- Nevus cells lack cytological atypia, pleomorphism and mitotic activity
- Immunohistochemistry for S100 and SOX10 are strongly positive but HMB45 shows negative to weak expression, compared to the strong diffuse expression of most melanomas
- Lack of PRAME expression may also support a benign nevus if a culprit primary melanoma is known to be PRAME positive
- Primary tumor of the visceral site of disease (versus visceral metastatic melanoma):
- Melanoma can mimic tumors from a range of organs and cell lineages.
- Clinicopathological correlation and knowledge of primary tumors within the particular visceral site is required to aid in distinction
- Alterations and premalignant change in the adjacent organ may provide diagnostic clues
- Judicious use of a panel of immunohistochemical stains and molecular studies may assist in the correct diagnosis
- Undifferentiated pleomorphic sarcoma (versus undifferentiated / dedifferentiated melanoma):
- Identification of transition between conventional and dedifferentiated / undifferentiated components can aid in the diagnosis of melanoma
- Factors favoring a dedifferentiated / undifferentiated melanoma include (Am J Surg Pathol 2021;45:240)
- Previous history of melanoma
- Metastatic disease in a regional nodal basin or site uncommon for sarcomas (including neck, axilla, inguinal, viscera)
- Multifocal disease
- Identification of a melanoma associated mutation or molecular signature concordant with that of a culprit primary tumor
- Absence of a bona fide primary tumor at another site with alternative histogenesis
- Clear cell sarcoma (versus dermal or soft tissue melanoma metastasis):
- These tumors show overlap in architecture, cytology, immunohistochemistry (S100, HMB45 and MelanA) and ultrastructure
- Presence of wreath-like multinucleated tumor cells would favor clear cell sarcoma, with definitive diagnosis by FISH or PCR to detect EWSR1 rearrangement, with common fusion partners ATF1 or CREB1
- Intraepidermal involvement can be seen in both melanoma metastasis and cutaneous clear cell sarcoma (Am J Surg Pathol 2020;44:21, Pathology 2022;54:369)
- PEComa (versus soft tissue metastatic melanoma):
- Cellular blue nevus / malignant blue nevus (versus dermal metastasis):
- These tumors have distinct morphological features and demonstrate aberrations in GNAQ or GNA11
- Cutaneous tumor with CRTC1::TRIM11 rearrangement (versus dermal melanoma metastasis)
- These tumors frequently show intersecting bundles and nests of tumor cells with cytological uniformity and expression of melanocytic markers (S100, HMB45)
- Definitive distinction can be made on molecular studies (Am J Surg Pathol 2022;46:1457)
Practice question #1
This undifferentiated epithelioid malignancy is identified within the right groin of a 76 year old man. The patient was diagnosed with a 1.2 mm Breslow thickness superficial spreading melanoma on the right lateral ankle 3 years ago. A broad panel of immunohistochemical stains is negative. Which of the following modalities would be most helpful in establishing a diagnosis of metastatic dedifferentiated / undifferentiated melanoma?
- Investigations for melanoma associated mutations in BRAF or NRAS
- Morphological comparison to the primary ankle melanoma
- Performing immunohistochemistry for PRAME on multiple different regions of tumor
- Performing special stains (e.g., Fontana-Masson / Schmorl stain) to identify intracytoplasmic pigmentation
Practice answer #1
A. Investigations for melanoma associated mutations in BRAF or NRAS. A dedifferentiated tumor in a nodal basin in a patient with a history of melanoma is suspicious for metastatic melanoma. There may only be focal conventional melanocytic differentiation present within dedifferentiated melanoma and sampling of numerous areas of the tumor may be required to identify these foci. A broad panel of immunohistochemistry is required to exclude other lines of differentiation and may be able to identify small foci of tumor that retain immunohistochemical features of melanoma. Identification of a melanoma associated mutation such as BRAF or NRAS may assist in rendering the diagnosis, especially if the mutational status of the primary tumor is known. Answer D is incorrect because dedifferentiated melanomas lack the morphological features of melanoma and are likely to lack evidence of pigmentation, with special stains negative. Answer B is incorrect because primary melanomas are rarely dedifferentiated at their primary site and morphological overlap is insufficient to render a diagnosis in an undifferentiated tumor. Answer C is incorrect because PRAME immunohistochemistry is not specific for a melanocytic lineage and can be positive in a range of high grade mesenchymal tumors; therefore, it cannot be relied upon to establish a diagnosis of dedifferentiated melanoma.
Comment Here
Reference: Metastatic melanoma
Comment Here
Reference: Metastatic melanoma
Practice question #2
Which of the following presentations of melanoma is considered metastatic in nature?
- Dermal nodule of melanoma seen within the perineural space, which on deeper levels is identified to be in continuity with the primary tumor invasive front
- Dermal nodule of melanoma with an adjacent dysplastic compound nevus
- Solitary subcuticular nodule of melanoma with adjacent lymphovascular space invasion
- Thin rim of bland melanocytes within the fibrous capsule of an axillary lymph node
Practice answer #2
C. Solitary subcuticular nodule of melanoma with adjacent lymphovascular space invasion. Subcuticular deposits of melanoma without continuity to the dermis / epidermis and with evidence of nearby lymphovascular space invasion are classic for in transit metastasis. Answers A and B are incorrect because a focus of melanoma that remains in direct continuity with the primary tumor is not regarded as metastatic disease, nor are tumors that arise within an adjacent nevus (even if there is an absence of in situ component). Answer D is incorrect because lymph nodes draining the skin, which can contain capsular nevi, are located within the fibrous capsule and should not be regarded as metastatic melanoma. Care should be taken to ensure that no continuity exists with a nearby primary melanoma prior to establishing a diagnosis of metastatic melanoma.
Comment Here
Reference: Metastatic melanoma
Comment Here
Reference: Metastatic melanoma