Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Balbin-Cuesta G, Skala SL. Serous carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusserous.html. Accessed September 29th, 2025.
Definition / general
- High grade endometrial carcinoma with complex papillary, solid or glandular architecture and marked cytologic atypia, similar to tubo-ovarian high grade serous carcinoma
- High risk of extrauterine spread / distant metastasis at diagnosis (Am J Obstet Gynecol 2008;198:218.e1, Int J Gynecol Cancer 2025;35:101672)
Essential features
- High grade carcinoma, usually arising in postmenopausal women
- Often arises in the background of atrophic endometrium with or without endometrial polyp
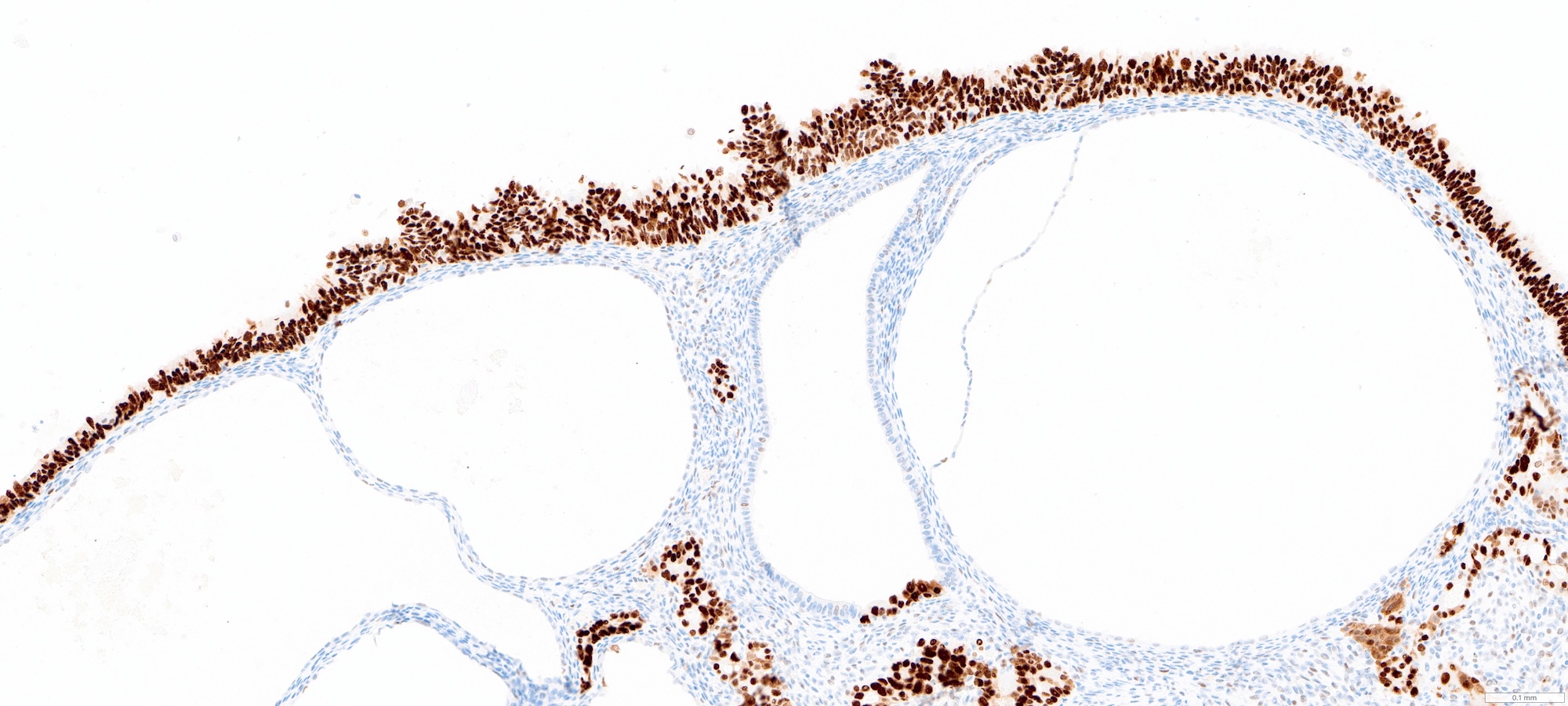
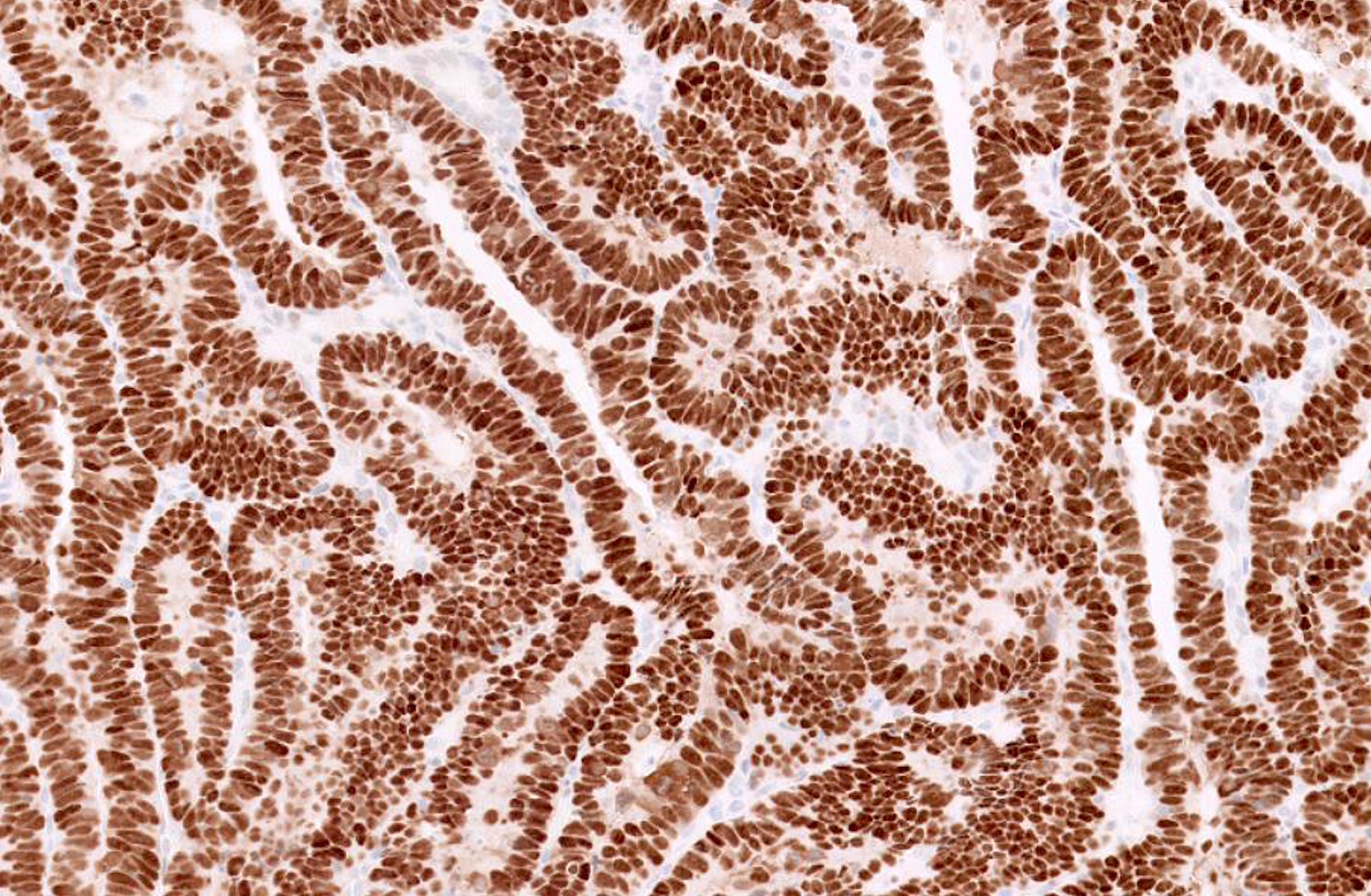
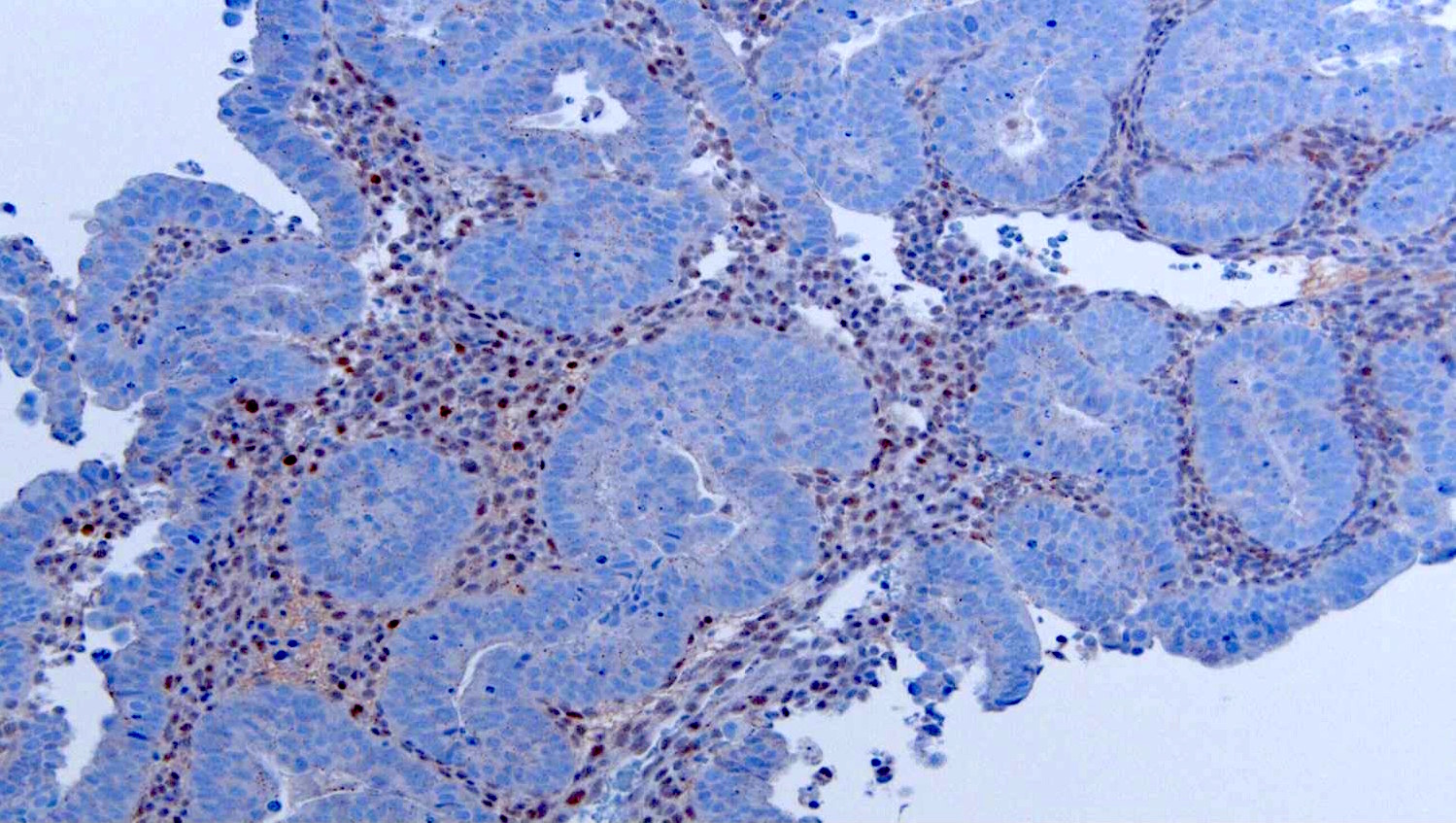
- Vast majority of tumors harbor TP53 mutations and corresponding aberrant p53 expression by immunohistochemistry (strong and diffuse, completely absent or cytoplasmic staining)
- Associated with a worse prognosis compared to endometrioid carcinoma and may present at a high stage
Terminology
- Endometrial serous carcinoma
- Uterine serous carcinoma
- Serous endometrial intraepithelial carcinoma (SEIC) terminology is discouraged; this is due to risk of metastasis despite its intraepithelial appearance as well as the possibility of confusion with endometrioid intraepithelial neoplasia (EIN) (Am J Surg Pathol 2000;24:797, Int J Gynecol Pathol 2001;20:214)
- Historical: uterine papillary serous carcinoma
ICD coding
Epidemiology
- Accounts for ~10% of endometrial carcinomas but ~40% of endometrial cancer related deaths (Curr Opin Obstet Gynecol 2010;22:21, Gynecol Oncol 2021;162:226)
- Patients are typically postmenopausal and nonobese
- Higher incidence in women who are black, have undergone pelvic irradiation or have a history of breast cancer and tamoxifen use (Am J Obstet Gynecol 2019;221:318.e1, Gynecol Oncol 2013;129:277)
- Possible link to BRCA mutations (Gynecol Oncol 2013;130:127, Clin Cancer Res 2019;25:7517, Clin Cancer Res 2019;25:1087, JAMA Oncol 2016;2:1434, Gynecol Oncol Rep 2024;55:101498)
Sites
- Uterine corpus
Pathophysiology
- Frequently arises on the surface of an endometrial polyp in a background of atrophic endometrium
- High likelihood of deep invasion, lymphatic or vascular invasion and early metastasis (even with minimal primary tumor) (Int J Gynecol Cancer 2025;35:101672)
- Recent data suggest that occasionally endometrial serous carcinoma can arise in conjunction with endometrial hyperplasia (Am J Surg Pathol 2025;49:753)
Etiology
- Mutations in TP53
- Possible association with BRCA1 / BRCA2 mutations (Gynecol Oncol 2013;130:127, Clin Cancer Res 2019;25:7517, Clin Cancer Res 2019;25:1087, JAMA Oncol 2016;2:1434, Gynecol Oncol Rep 2024;55:101498)
Clinical features
- Postmenopausal bleeding is common
- May present as extrauterine metastasis involving lymph nodes, peritoneal sites or omentum (Curr Opin Obstet Gynecol 2010;22:21)
Diagnosis
- Diagnosis can be made via endometrial biopsy, curettage or polypectomy during workup for postmenopausal bleeding
- Serous carcinoma may be an incidental finding in endometrial polyps or uteri removed for benign conditions
- WHO diagnostic criteria
- Essential: a cytologically high grade endometrial carcinoma with complex papillary or glandular architecture
- Desired: abnormal p53 and diffuse p16 immunohistochemistry
- Reference: Am J Surg Pathol 2007;31:979
Laboratory
- Tumor marker CA 125 may be elevated
Radiology description
- Heterogeneous signal on diffusion weighted MRI with or without peritoneal dissemination (Eur Radiol 2022;32:4128)
- Thickened endometrial stripe
Prognostic factors
- Aggressive subtype of endometrial carcinoma
- Higher stage, age > 60 years, race (African ancestry), CCNE1 and MYC amplification are associated with increased mortality (Int J Gynecol Cancer 2017;27:85, Front Oncol 2024;14:1445128, Gynecol Oncol 2022;164:558)
- Prognosis is more favorable for endometrium or polyp confined disease after complete surgical staging
Case reports
- 68 year old woman with history of endometrial serous carcinoma and metastasis to ureter and renal pelvis (Urol Case Rep 2021;39:101776)
- 68 year old woman with uterine choriocarcinoma arising from serous carcinoma (Diagn Pathol 2022;17:79)
- 69 and 73 year old women with HER2 positive uterine serous carcinoma received combined trastuzumab and pelvic radiation therapy without significant toxicity (Gynecol Oncol Rep 2023;49:101250)
- 70 year old woman with dedifferentiated endometrial carcinoma arising from serous carcinoma (Gynecol Oncol Rep 2023;47:101188)
Treatment
- Treatment includes total hysterectomy / bilateral salpingo-oophorectomy and surgical staging with consideration of maximal tumor debulking for gross disease
- Depending on tumor stage, vaginal brachytherapy, external beam radiation therapy and systemic chemotherapy (including carboplatin and paclitaxel) may be recommended
- Patients with recurrent or advanced stage HER2 positive endometrial serous carcinoma benefit from addition of trastuzumab (J Clin Oncol 2018;36:2044)
Gross description
- No unique gross findings to separate serous from nonserous carcinoma
- Endometrial polyp may be present
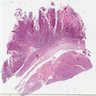
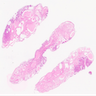
Microscopic (histologic) description
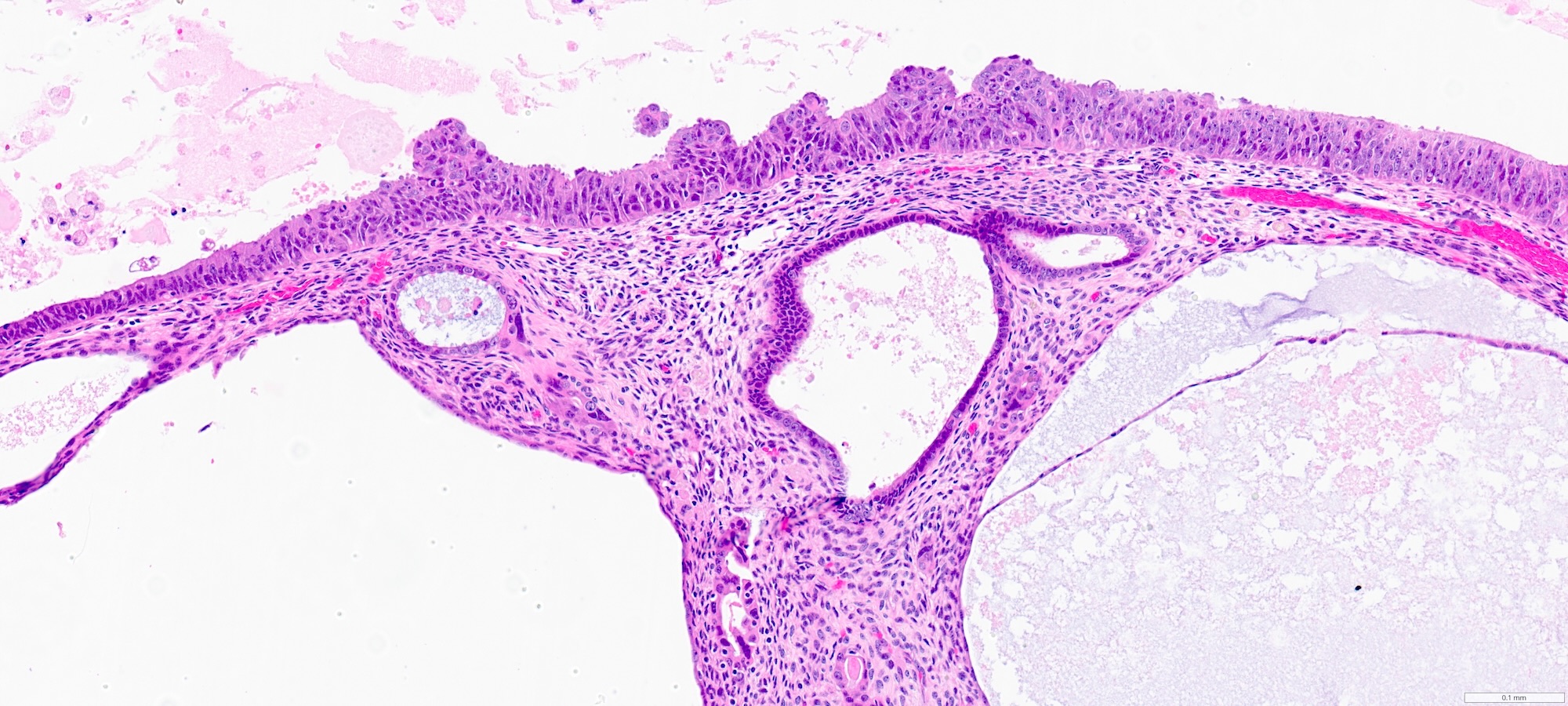
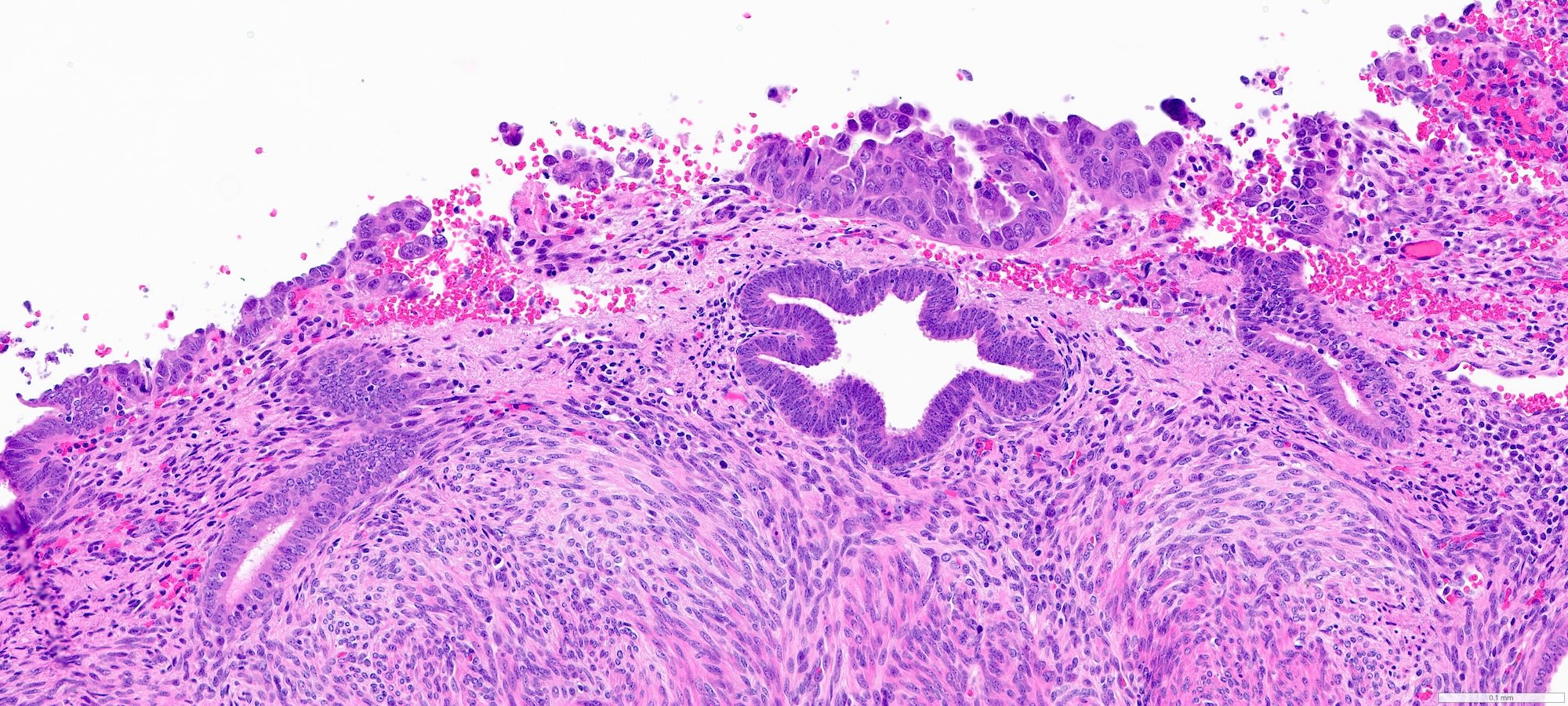
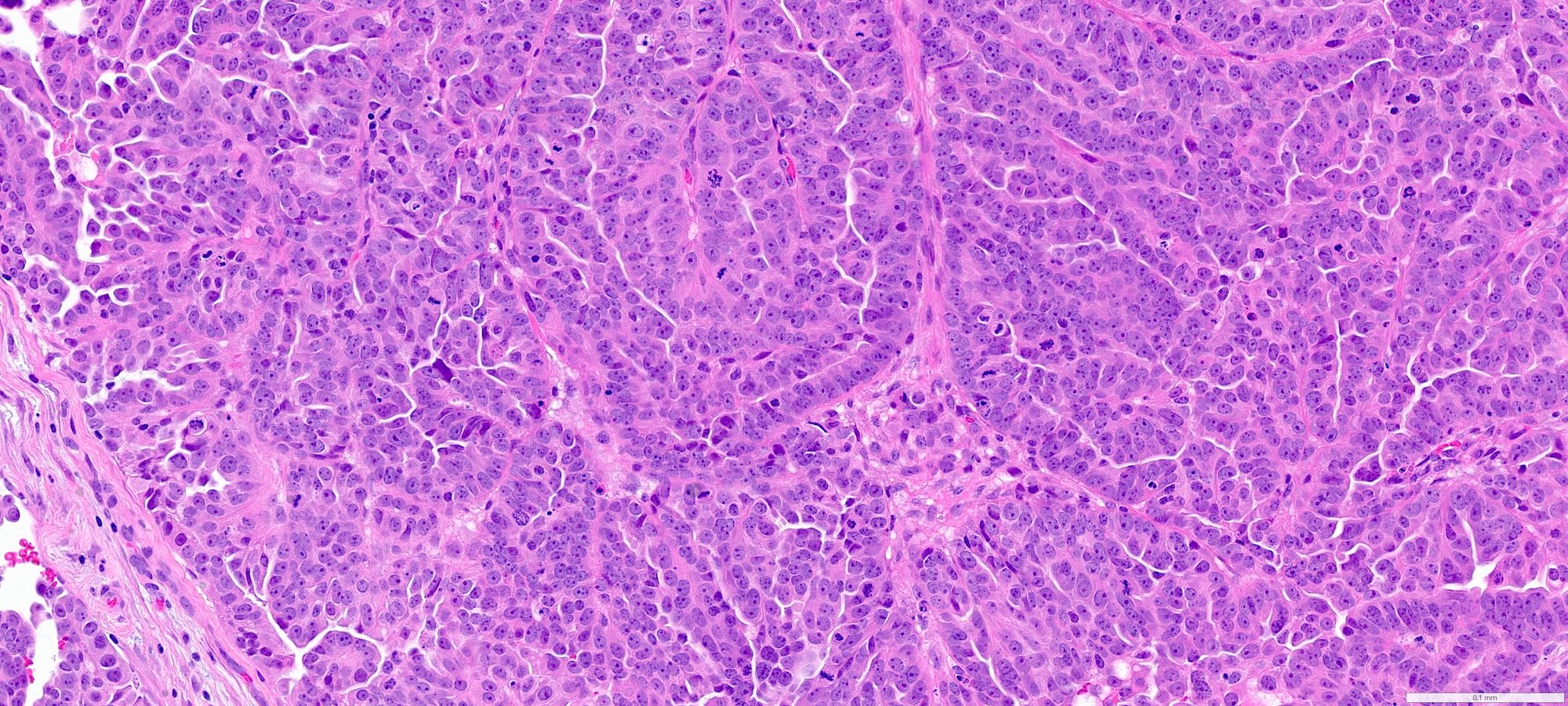
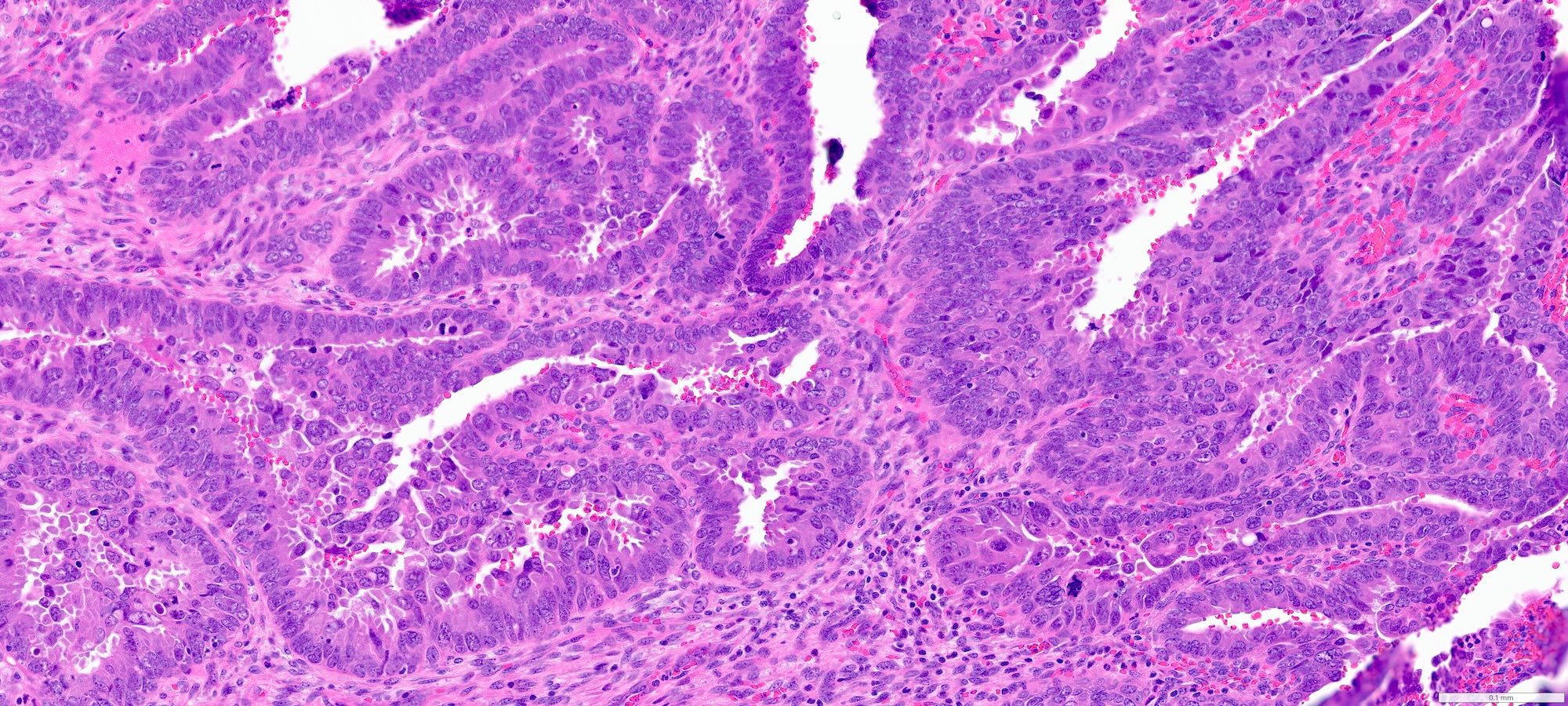
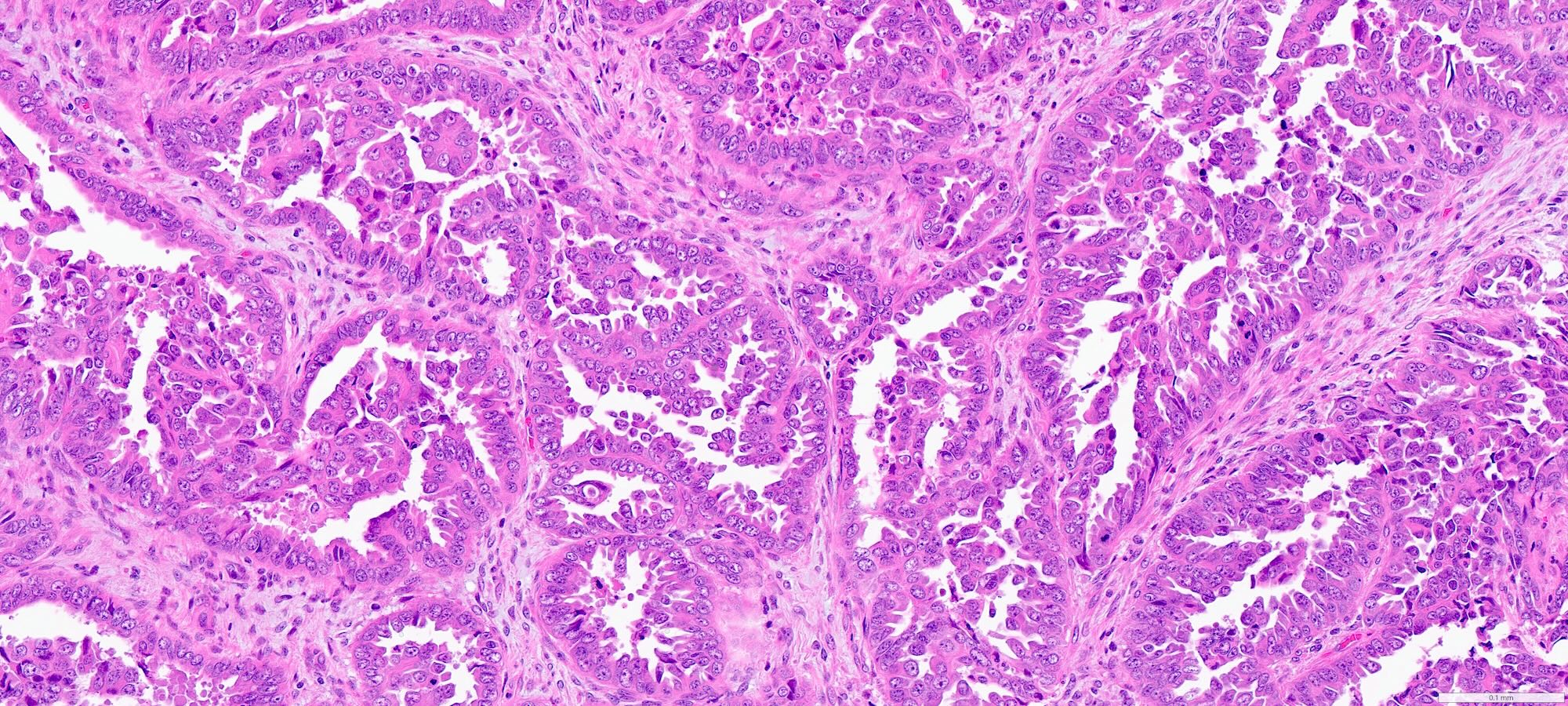
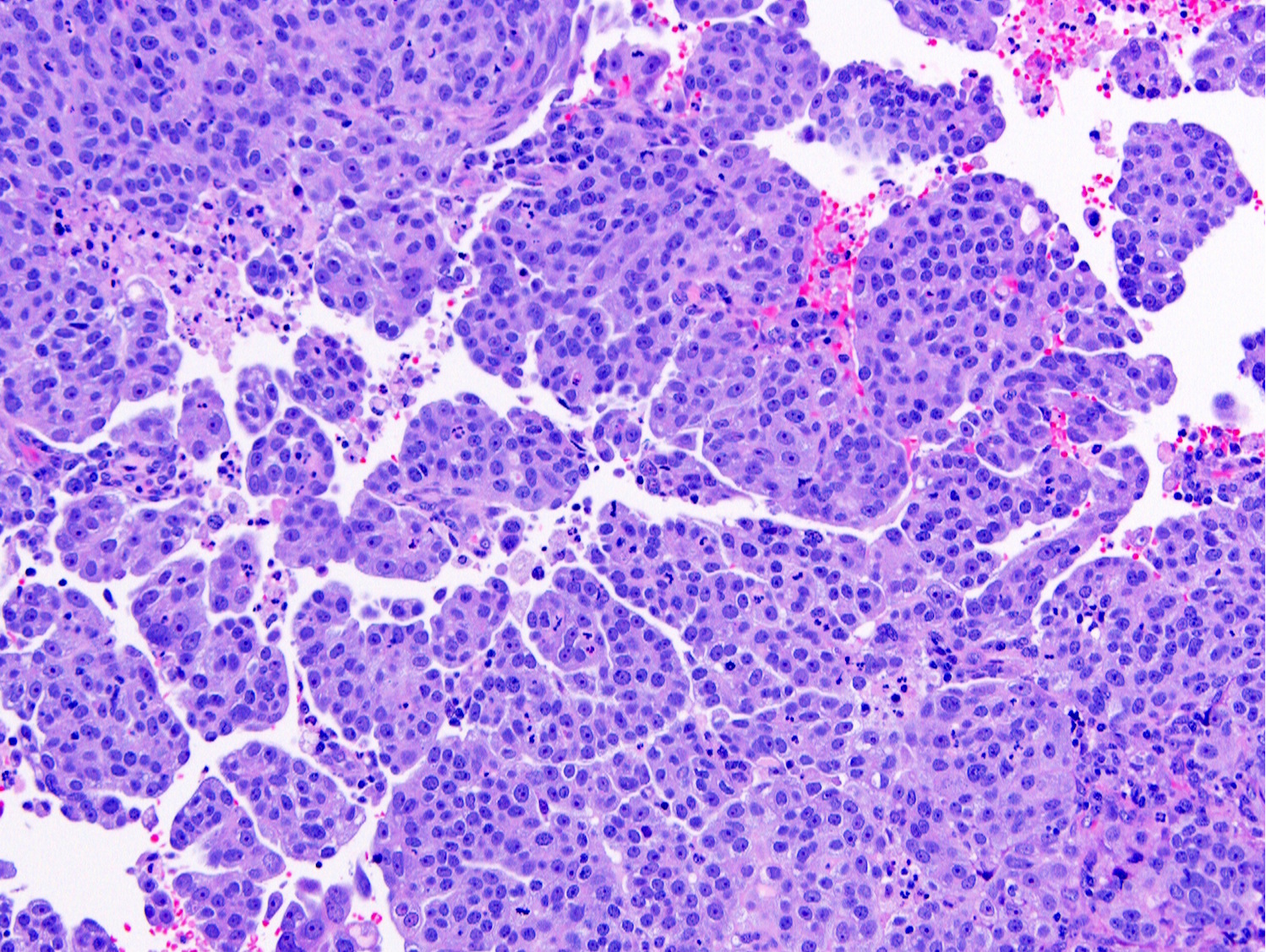
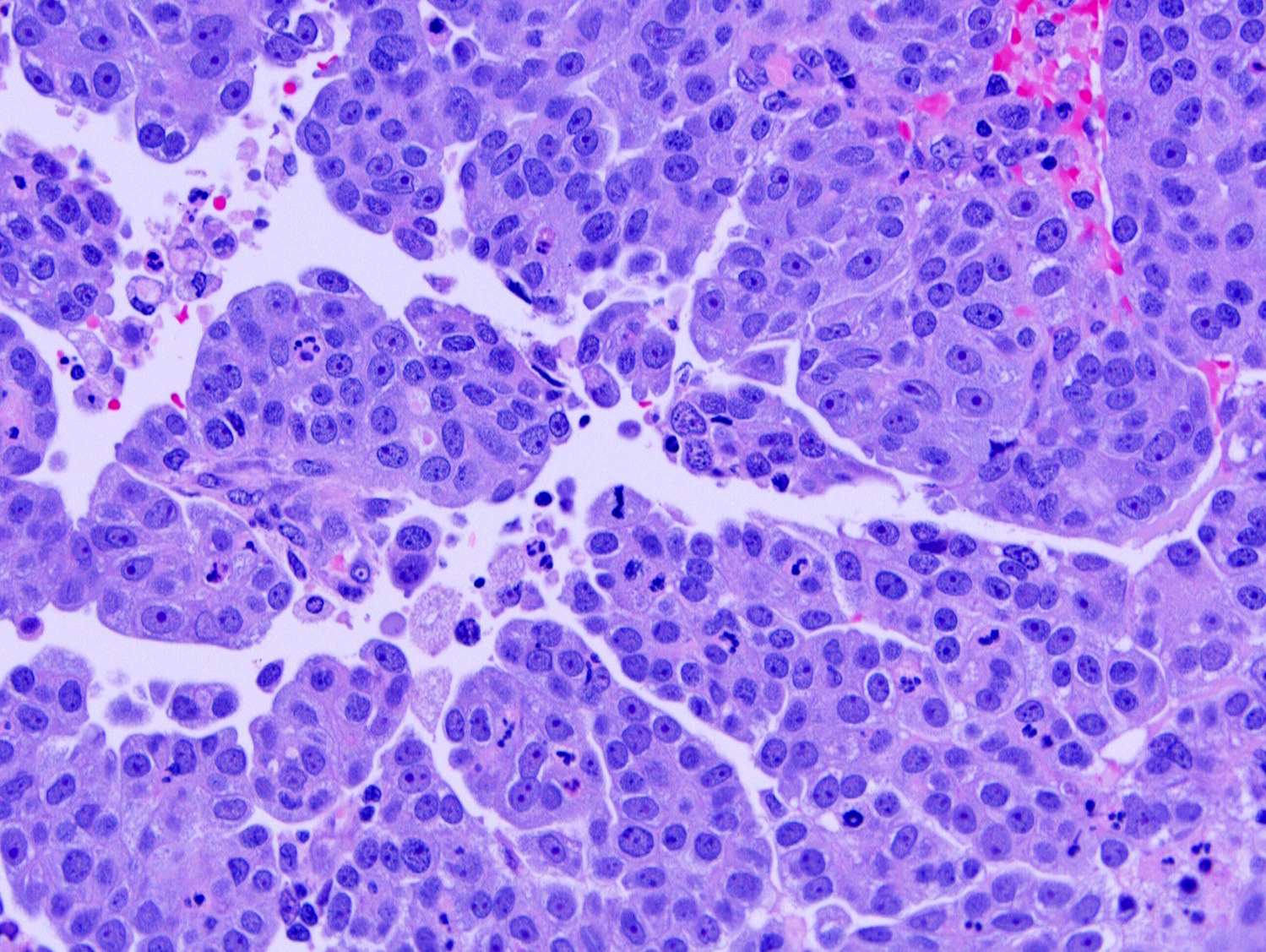
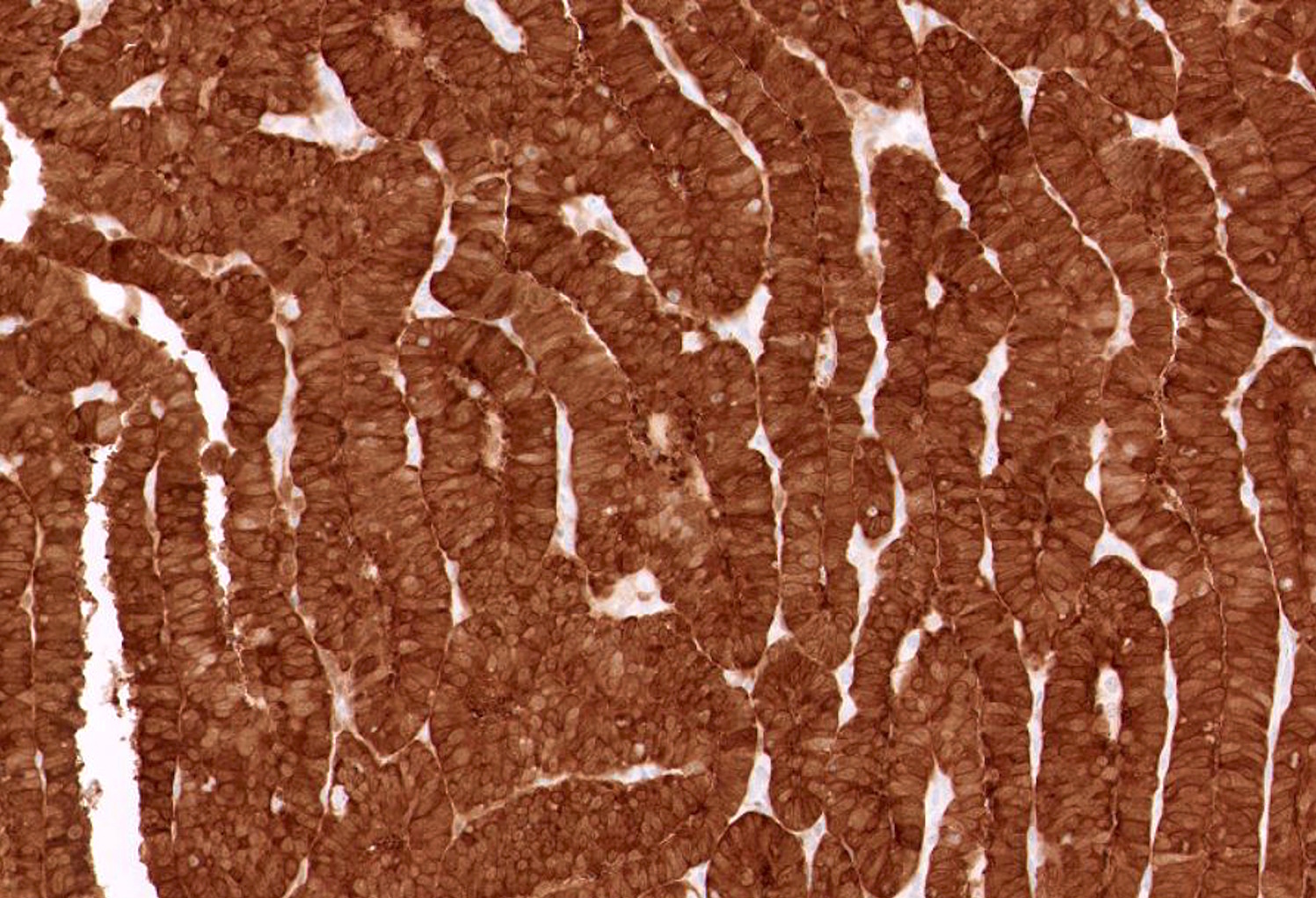
- Complex papillary or glandular growth is seen in most cases
- Glands are typically irregular with slit-like luminal spaces, though some cases have smooth luminal borders like those of endometrioid carcinoma
- Solid growth can be seen
- Marked nuclear pleomorphism, prominent nucleoli, brisk mitotic activity
- Cytoplasm is usually scant but can be abundant with eosinophilia or clearing
- Psammomatous calcifications may be seen
- Solid, pseudoendometrioid and transitional morphology is more commonly seen in endometrial serous carcinoma with homologous recombination deficiency via genomic loss of heterozygosity (Gynecol Oncol 2022;164:558)
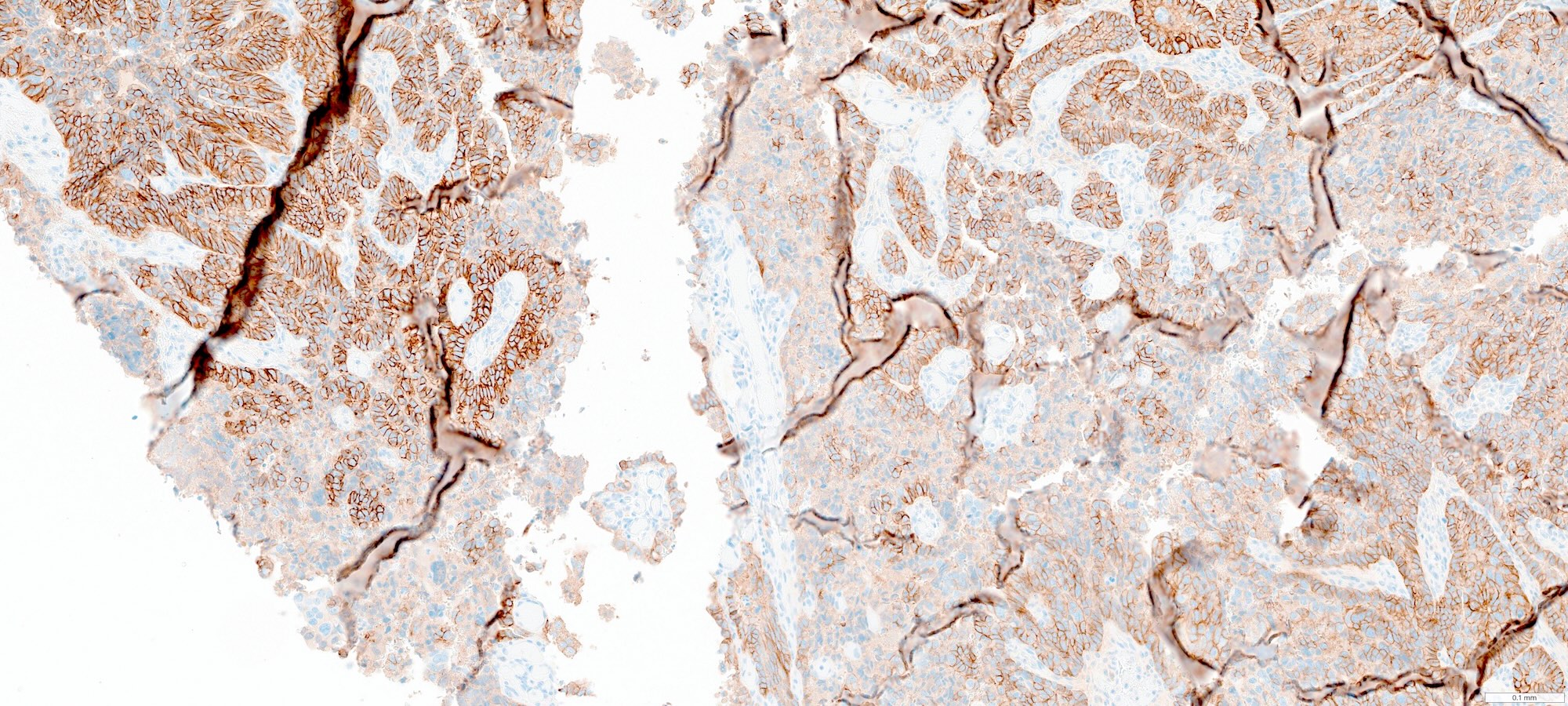
Microscopic (histologic) images
Contributed by Stephanie L. Skala, M.D. and Ricardo Lastra, M.D.
Virtual slides
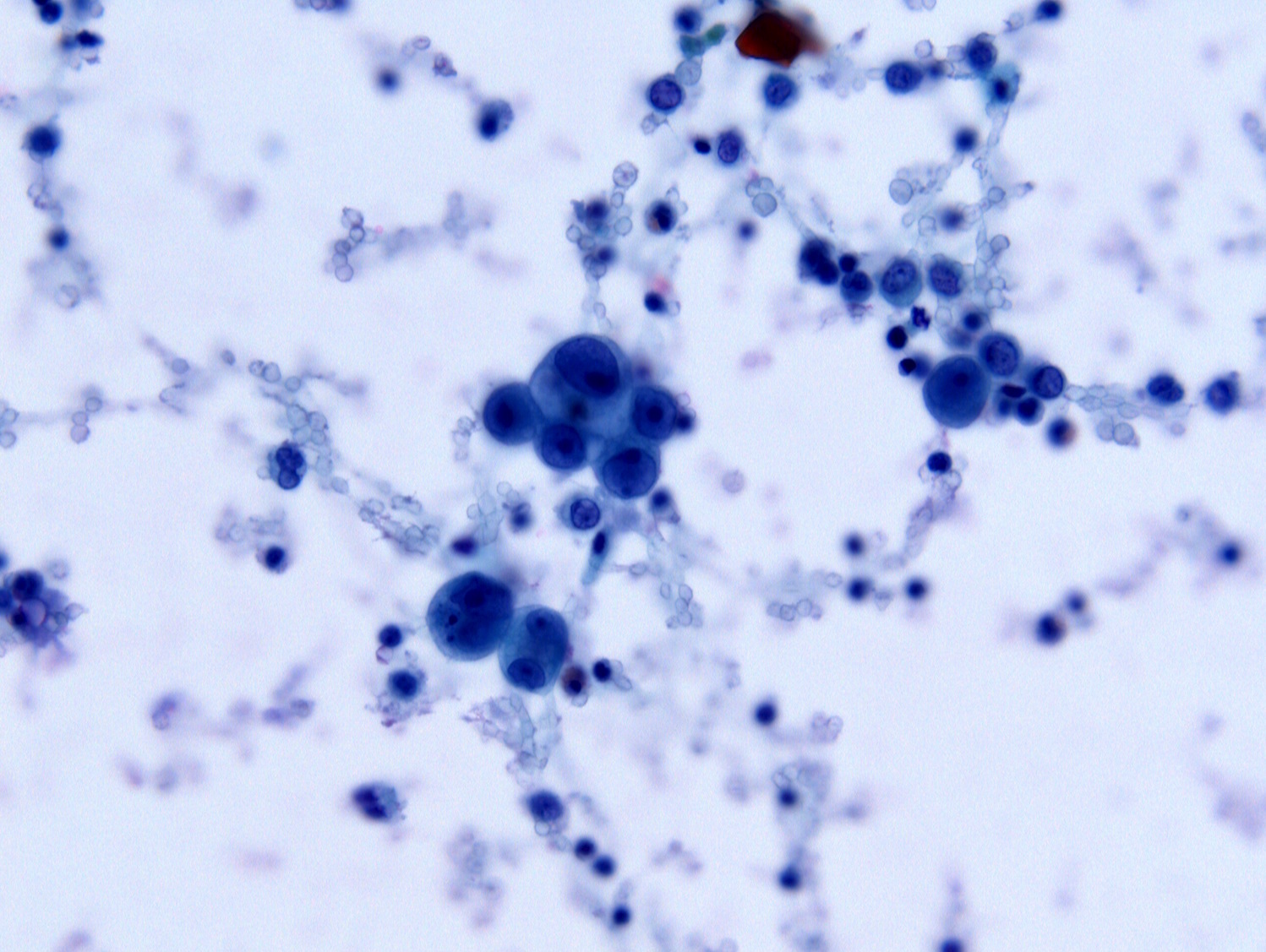
Cytology description
- Nuclear hyperchromasia, irregular nuclear contours, coarse chromatin, prominent cherry red nucleoli and variable presence of cytoplasmic vacuoles (Diagn Cytopathol 2016;44:1039)
Positive stains
Negative stains
- HER2: often basolateral or lateral staining with intratumoral heterogeneity; positive (3+) in ~16 - 47% (Int J Gynecol Cancer 2025;35:101672)
- Scoring for trastuzumab use is different from scoring for trastuzumab deruxtecan use (Clin Cancer Res 2020;26:3928, J Clin Oncol 2024;42:47)
- CK20
- ER / PR: variable
- WT1: may be focally positive in ~30% of cases; if strong and diffuse, consider extrauterine high grade serous carcinoma (Int J Gynecol Pathol 2019;38:S40)
Molecular / cytogenetics description
- Vast majority of tumors harbor TP53 mutations
- May also show alterations of PI3K / AKT / mTOR and MAPK pathways (J Surg Oncol 2015;112:188)
- PIK3CA (35 - 48%), FBXW7 (17 - 30%), PPP2R1A (25 - 39%), CHD4 (16%)
- HER2 gene amplification and HER2 / neu expression are more frequent in endometrial serous carcinoma than ovarian serous carcinoma (J Surg Oncol 2015;112:188)
- Amplification of CCNE1 (19 - 45%) and MYC (12 - 24%) (Mod Pathol 2014;27:1014)
- Mutations in PTEN and ARID1A are uncommon
- Copy number high (Nature 2013;497:67, Int J Gynecol Cancer 2025;35:101672, Gynecol Oncol 2022;164:558)
Sample pathology report
- Endometrium, polypectomy and curettage:
- Endometrial serous carcinoma involving endometrial polyp (see comment)
- Comment: Immunohistochemical stains show that the tumor is positive for p53 (aberrant overexpression) and p16 (strong and diffuse), supporting the above diagnosis.
- Uterus with bilateral fallopian tubes and ovaries, total hysterectomy and bilateral salpingo-oophorectomy:
- Endometrial serous carcinoma (1.2 cm), invading the inner half of the myometrium (0.6 / 1.8 cm) (see comment and synoptic report)
- Comment: Substantial lymphovascular invasion is present. Myoinvasive carcinoma involves the lower uterine segment; cervix is uninvolved. Bilateral fallopian tubes and ovaries with no significant histologic abnormality.
Differential diagnosis
- POLE ultramutated endometrial carcinoma:
- Endometrial carcinoma harboring pathogenic mutation in the exonuclease domain of the POLE gene
- Good prognosis despite high grade appearance
- Predominantly endometrioid or ambiguous / mixed morphology but represented across endometrial carcinoma histotypes
- MMR deficiency and p53 abnormalities may be seen in diffuse or subclonal patterns in multiple classifier tumors
- Atypical hyperplasia / endometrioid intraepithelial neoplasia (AH / EIN):
- Precursor lesion to endometrial endometrioid carcinoma
- Crowded glands without significant confluent epithelial growth
- Glands may show loss of polarity, hyperchromasia, nuclear enlargement, nuclear rounding or distinct morphologic features from background endometrium
- Variably associated with mutations in PTEN, PIK3CA, KRAS, CTNNB1
- Supportive immunohistochemical findings include loss of PTEN or PAX2 expression and nuclear localization of beta catenin; p53 is typically nonaberrant / wild type
- Endometrial endometrioid carcinoma:
- Areas with well defined glands and smooth luminal borders should be present in lower grade tumors
- Squamous and mucinous differentiation common
- Often less cytologic atypia than serous carcinoma
- ER / PR are often strongly positive
- p53 and p16 are typically nonaberrant
- Nuclear beta catenin expression and MMR deficiency are more common in endometrioid carcinoma
- Endometrial clear cell carcinoma:
- Typically shows multiple architectural patterns (papillary, tubulocystic, solid)
- Papillae are typically small with hyalinized cores lined by a single layer of polygonal hobnail cells
- Uniform nuclear enlargement and atypia (overall less pleomorphic than serous carcinoma)
- Frequent prominent nucleoli
- Clear to eosinophilic (oxyphilic) cytoplasm
- Often positive for HNF-1B, Napsin A, AMACR
- With or without abnormal p53 or p16
- Postradiotherapy serous-like endometrial change:
- Benign reactive epithelial atypia following pelvic radiation (Virchows Arch 2024;485:989)
- Minimal to absent mitotic activity
- No abrupt transition between areas with high grade atypia and areas with low grade or no atypia
- May show increased but not aberrant p53 expression by immunohistochemistry
- Lower Ki67 proliferation index; cutoff is not well defined (mean Ki67 is 26.9% in serous carcinoma versus 8.16% in postradiotherapy serous-like endometrial change)
- Mixed endometrial carcinoma:
- Defined by WHO as a tumor with at least 2 components (each encompassing at least 10% of the tumor volume)
- Different tumor components have distinct morphologies with or without immunophenotypic features
- Secondary involvement (drop metastasis) by tubo-ovarian high grade serous carcinoma:
- Clinical / imaging evidence of adnexal or peritoneal mass(es)
- May involve the surface of polyp
- WT1 is often strong and diffuse
- Metaplastic changes:
- Endometrial glands with tubal metaplasia are ciliated and lack significant cytologic atypia
- Papillary syncytial metaplasia is associated with endometrial stromal breakdown and often demonstrates neutrophilic microabscesses; p53 expression may be somewhat increased (Int J Gynecol Pathol 2012;31:206)
- Metaplastic processes in general should show relatively low Ki67 proliferation index and nonaberrant p53 expression
Additional references
Practice question #1
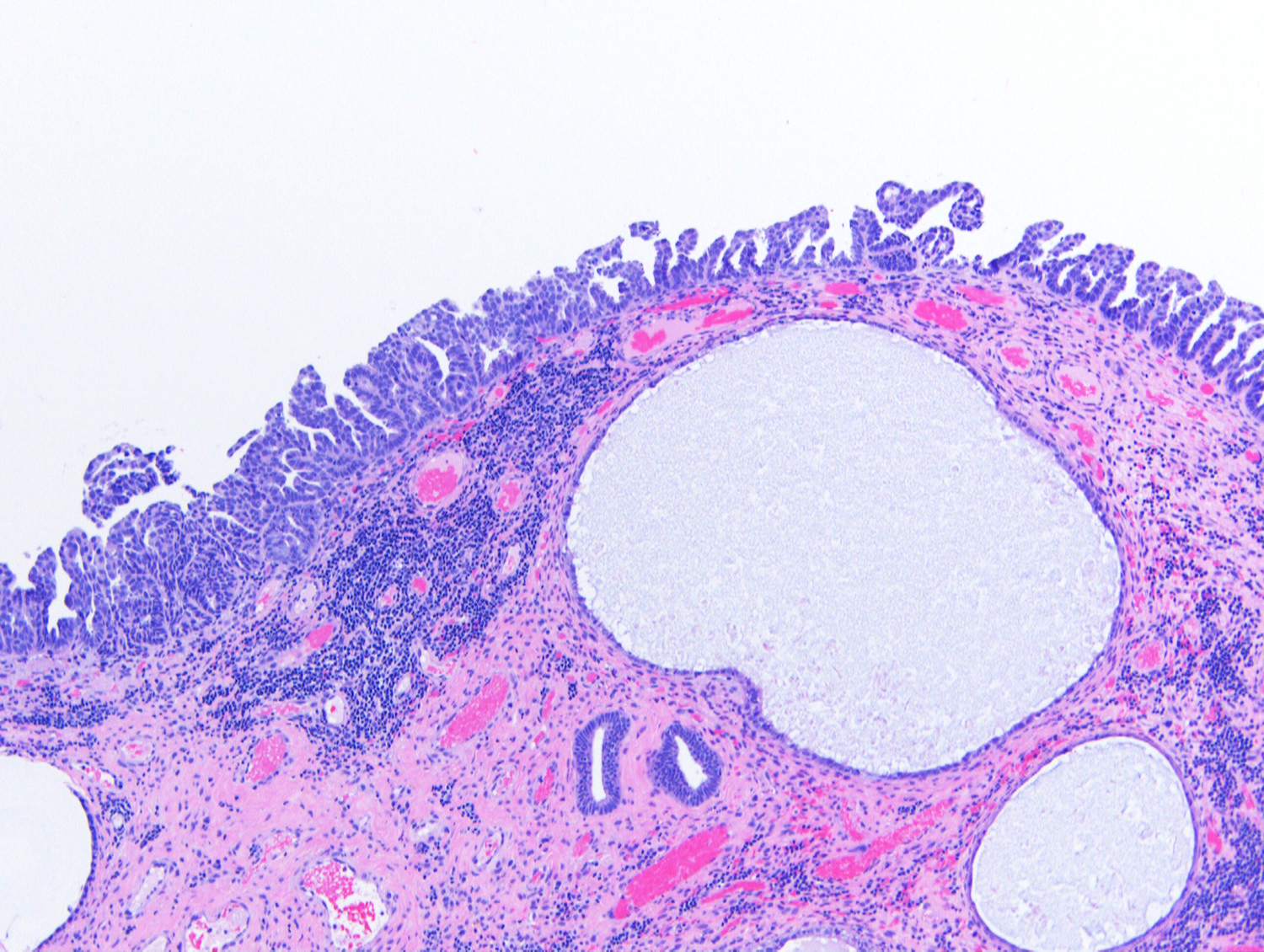
The carcinoma shown in the image above involves the surface of an endometrial polyp and shows strong and diffuse positivity for p53 and p16 by immunohistochemistry. Which of the following is true regarding this lesion?
- Can present at high stage even without definitive invasion
- Likely arose in a patient < 30 years of age
- Likely arose in an obese patient
- Often shows monotonous nuclei with low proliferation index
Practice answer #1
A. Can present at high stage even without definitive invasion. This is endometrial serous carcinoma involving the surface of an endometrial polyp. Even without definitive invasion, serous carcinoma can present at high stage with peritoneal metastases. Answer B is incorrect because endometrial serous carcinoma is typically diagnosed in postmenopausal patients. Answer C is incorrect because endometrial serous carcinoma is not associated with obesity or estrogen excess. Answer D is incorrect because endometrial serous carcinoma shows marked pleomorphism and high proliferation.
Comment Here
Reference: Serous carcinoma
Comment Here
Reference: Serous carcinoma
Practice question #2
Which of the following is true regarding endometrial serous carcinoma?
- Mutations in TP53 are the most common molecular driving event
- p16 overexpression indicates an etiologic role for HPV infection
- Well formed glands with smooth luminal borders are typical
- WT1 is often strongly and diffusely positive
Practice answer #2
A. Mutations in TP53 are the most common molecular driving event. The vast majority of endometrial serous carcinomas harbor mutations in TP53. Answer D is incorrect because endometrial serous carcinomas are typically negative for WT1 (in contrast to tubo-ovarian high grade serous carcinomas, which are typically positive for WT1). Answer C is incorrect because smooth luminal contours and well formed glands are associated with endometrioid carcinoma, while complex luminal contours are seen in endometrial serous carcinoma. Answer B is incorrect because HPV infection does not have an etiologic role in endometrial serous carcinoma; rather, p16 expression is the result of a dysregulated cell cycle.
Comment Here
Reference: Serous carcinoma
Comment Here
Reference: Serous carcinoma