Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Bourgeau M, Falzarano SM. Liposarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/testisliposarcoma.html. Accessed August 21st, 2025.
Definition / general
- Well differentiated and dedifferentiated liposarcomas are characterized by MDM2 amplifications and represent the vast majority of paratesticular liposarcomas
- Other liposarcomas (myxoid liposarcoma, pleomorphic liposarcoma) are very rare in this location and are driven by other mechanisms of tumorigenesis
Essential features
- Well differentiated liposarcoma (WDLPS)
- Mature adipocytes and stromal cells with at least focal cytologic atypia
- Adipocytic component may be limited in tumors with predominant sclerosing or inflammatory morphology
- Giant ring / marker chromosomes; MDM2 gene amplification
- Dedifferentiated liposarcoma (DDLPS)
- Undifferentiated pleomorphic or spindle cell sarcoma that arises in association with WDLPS
- Typically nonlipogenic and high grade; rare heterologous (osteosarcomatous, rhabdosarcomatous, or leiomyosarcomatous) differentiation reported
- Well differentiated component can be minimal or absent
- Myxoid liposarcoma (MLPS)
- Small primitive mesenchymal cells and univacuolated (signet ring) lipoblasts
- Myxoid stroma with delicate, thin walled arborizing capillaries
- > 5% high grade features are associated with a worse prognosis
- DDIT3 (12q13) gene rearrangement
- Pleomorphic liposarcoma (PLPS)
- Variable amount of pleomorphic lipoblasts, admixed with a high grade sarcoma
- Epithelioid subtype can be positive for keratins
- Important to rule out DDLPS with homologous dedifferentiation
Terminology
- Well differentiated liposarcoma
- Deep, centrally located tumors have a significant risk of recurrence and dedifferentiation and should be designated as WDLPS
- Atypical lipomatous tumor (ALT) is used for tumors of the extremities and trunk that are amenable to complete resection
- Subtypes: lipoma-like (adipocytic), sclerosing, inflammatory
- Not recommended: atypical lipoma
- Dedifferentiated liposarcoma
- Using FNCLCC criteria, DDLPS should be given a differentiation score of 3
- Low grade differentiation (grade 2 by FNCLCC) is officially recognized but there is no consensus regarding diagnostic criteria and prognostic significance (Am J Surg Pathol 2007;31:1, Am J Surg Pathol 1997;21:271)
- Myxoid liposarcoma
- High grade MLPS: > 5% high grade morphology is associated with more aggressive behavior (Ann Surg Oncol 2012;19:1081, Sarcoma 2011;2011:538085)
- Not recommended: round cell liposarcoma
- Pleomorphic liposarcoma
- Subtype: epithelioid
ICD coding
- Well differentiated liposarcoma
- ICD-O: 8851/3 - liposarcoma, well differentiated
- ICD-10: C49.9 - liposarcoma, well differentiated type
- ICD-11: 2B5H & XH7Y61 - well differentiated lipomatous tumor, primary site & liposarcoma, well differentiated
- Dedifferentiated liposarcoma
- ICD-O: 8858/3 - dedifferentiated liposarcoma
- ICD-10: C49.9 - dedifferentiated liposarcoma
- ICD-11: 2B59.Y & XH1C03 - liposarcoma, other specified primary site & dedifferentiated liposarcoma
- Myxoid liposarcoma
- ICD-O: 8852/3 - myxoid liposarcoma
- ICD-10: C49.9 - myxoid liposarcoma
- ICD-11: 2B59.Y & XH3EL0 - liposarcoma, other specified primary site & myxoid liposarcoma
- Pleomorphic liposarcoma
- ICD-O: 8854/3 - pleomorphic liposarcoma
- ICD-10: C49.9 - pleomorphic liposarcoma
- ICD-11: 2B59.Y & XH25R1 - liposarcoma, other specified primary site & pleomorphic liposarcoma
Epidemiology
- Most common paratesticular malignancy in adults but overall exceedingly rare, with < 1 case per million in the United States (Urol Oncol 2014;32:52.e19)
- Most common in middle aged and older adults (Am J Surg Pathol 2003;27:40)
- Third most common location for WDLPS and DDLPS after retroperitoneum and limbs (10 - 15% of cases) (Am J Surg Pathol 1992;16:1051, Am J Surg Pathol 1997;21:271)
- Other liposarcomas are exceedingly rare in this location, particularly as primary tumors; must rule out metastasis and DDLPS
Sites
- Majority of cases arise from spermatic cord; less commonly testicular tunica and epididymis (Am J Surg Pathol 2003;27:40)
Pathophysiology
Etiology
- Mostly sporadic; rarely associated with Li-Fraumeni syndrome (p53 positive, MDM2 negative) (Pediatr Dev Pathol 2010;13:218, Hum Pathol 2024;147:82)
Clinical features
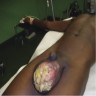
- Painless paratesticular swelling or mass, usually longstanding
- Rapidly enlarging mass suggestive of a high grade tumor
- Rarely presents as inguinal hernia, inflammatory process or testicular tumor (Case Rep Med 2014;2014:735380, J Clin Diagn Res 2014;8:165)
- DDLPS can occur in the primary tumor (90%) or recurrences (10%) of WDLPS (Am J Surg Pathol 1992;16:1051)
Diagnosis
- In conjunction with imaging findings, initial diagnosis can be made on targeted biopsy
- Due to intratumoral heterogeneity, surgical resection is recommended for definitive classification (AJR Am J Roentgenol 2021;216:997)
- Ancillary testing may be needed in difficult cases (see Molecular / cytogenetics description)
Laboratory
- No specific laboratory abnormality is known
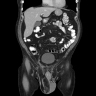
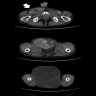
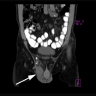
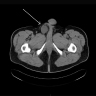
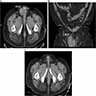
Radiology description
- Well differentiated liposarcoma
- Variable fatty component
- Septal and nodular enhancement, corresponding to areas of stromal fibrosis / sclerosis (Radiographics 2005;25:1371)
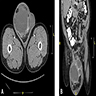
- Dedifferentiated liposarcoma
- Enhancing nonfatty mass, in association with lipomatous mass or abnormal fat (Radiographics 2005;25:1371, Sarcoma 2020;2020:8363986)
- Myxoid liposarcoma
- Subtle fatty component in lacy / linear pattern (Radiographics 2000;20:1007)
- Nodular enhancement represents high grade areas (Radiol Case Rep 2017;12:811)
- Pleomorphic liposarcoma
- Heterogeneous enhancing mass with necrosis and hemorrhage; may have small fatty component (Radiographics 2005;25:1371)
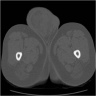
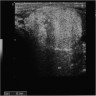
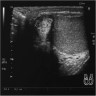
Radiology images
Prognostic factors
- Well differentiated liposarcoma
- Local recurrence is common (60%) but time to recurrence is highly variable (Am J Surg Pathol 2003;27:40)
- No metastatic potential (unless dedifferentiation occurs)
- Progression to DDLPS is the most important prognostic factor and a time dependent phenomenon (Am J Surg Pathol 1992;16:1051)
- Dedifferentiated liposarcoma
- Relatively better prognosis compared to other high grade sarcomas (Am J Surg Pathol 1994;18:1213, Am J Surg Pathol 1997;21:271)
- Rhabdomyoblastic differentiation associated with significantly higher mortality (5 year survival is 29%; 75% without myogenic differentiation) (Am J Surg Pathol 2015;39:383)
- Very few confirmed cases of paratesticular MLPS and PLPS in the literature
Case reports
- 23 year old man with paratesticular myxoid liposarcoma (Rare Tumors 2010;2:e23)
- 46 year old man with paratesticular myxoid liposarcoma (Pathol Res Pract 2013;209:124)
- 50 year old man with paratesticular well differentiated liposarcoma mimicking a testicular tumor (J Clin Diagn Res 2014;8:165)
- 50 year old man with recurrent paratesticular well differentiated liposarcoma (World J Surg Oncol 2014;12:276)
- 59 year old man with paratesticular dedifferentiated liposarcoma (Int J Surg Case Rep 2020;72:418)
- 61 year old man with paratesticular dedifferentiated liposarcoma with osseous metaplasia (Case Rep Urol 2015;2015:965876)
- 61 year old man with bilateral paratesticular dedifferentiated liposarcoma with homologous differentiation (J Surg Tech Case Rep 2014;6:15)
- 70 year old man with paratesticular dedifferentiated liposarcoma with osteosarcomatous differentiation mimicking a complex hydrocele (J Surg Case Rep 2020;2020:rjaa342)
- 70 year old man with paratesticular dedifferentiated liposarcoma presenting as inguinal hernia (Case Rep Med 2014;2014:735380)
- 75 year old man with paratesticular dedifferentiated liposarcoma and lung metastases (J Med Case Rep 2020;14:86)
- 77 year old man with paratesticular dedifferentiated liposarcoma with leiomyosarcomatous differentiation (Diagn Pathol 2013;8:142)
Treatment
- Well differentiated liposarcoma
- Surgical resection (en bloc excision, including radical orchiectomy, involving high ligation of the spermatic cord) for primary and recurrent tumors (Int J Clin Oncol 2020;25:2099)
- Wide re-resection, including hemiscrotectomy, may be performed to prevent local recurrence in cases with inadequate resection margins (J Surg Oncol 2018;117:1464)
- Radiation therapy for widely positive margins and unresectable tumors (Cancer Radiother 2017;21:16)
- Dedifferentiated liposarcoma
- Surgical resection with negative margins, particularly for dedifferentiated component (Int J Clin Oncol 2020;25:2099)
- Neoadjuvant radiation or palbociclib (CDK4 inhibitor) if unresectable or high risk of recurrence (Curr Treat Options Oncol 2020;21:15)
- Myxoid liposarcoma
- Surgical resection with negative margins (Int J Clin Oncol 2020;25:2099)
- Neoadjuvant or adjuvant radiation therapy in certain clinical situations (Cancer 2009;115:3254)
- Unresectable tumors can also be treated with trabectedin (Lancet Oncol 2007;8:595)
- Pleomorphic liposarcoma
- Surgical resection with negative margins, with adjuvant radiation or chemotherapy (Oncol Res Treat 2022;45:525)
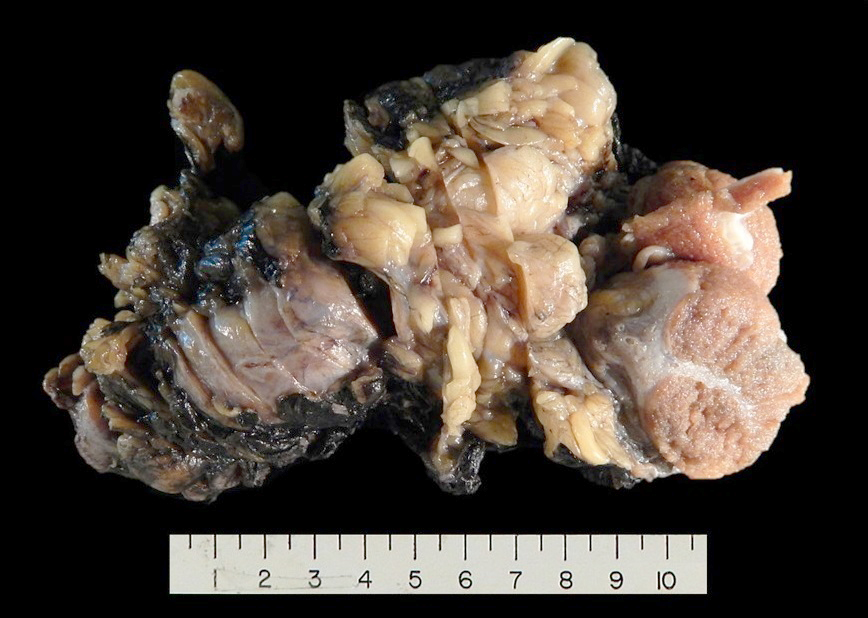
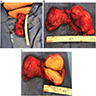
Gross description
- Well differentiated liposarcoma
- Well defined, multilobulated
- Adipocytic areas are marbled and yellow-white; fibrotic / sclerotic areas are firm and white-gray; myxoid areas are gelatinous (Virchows Arch 2010;456:167)
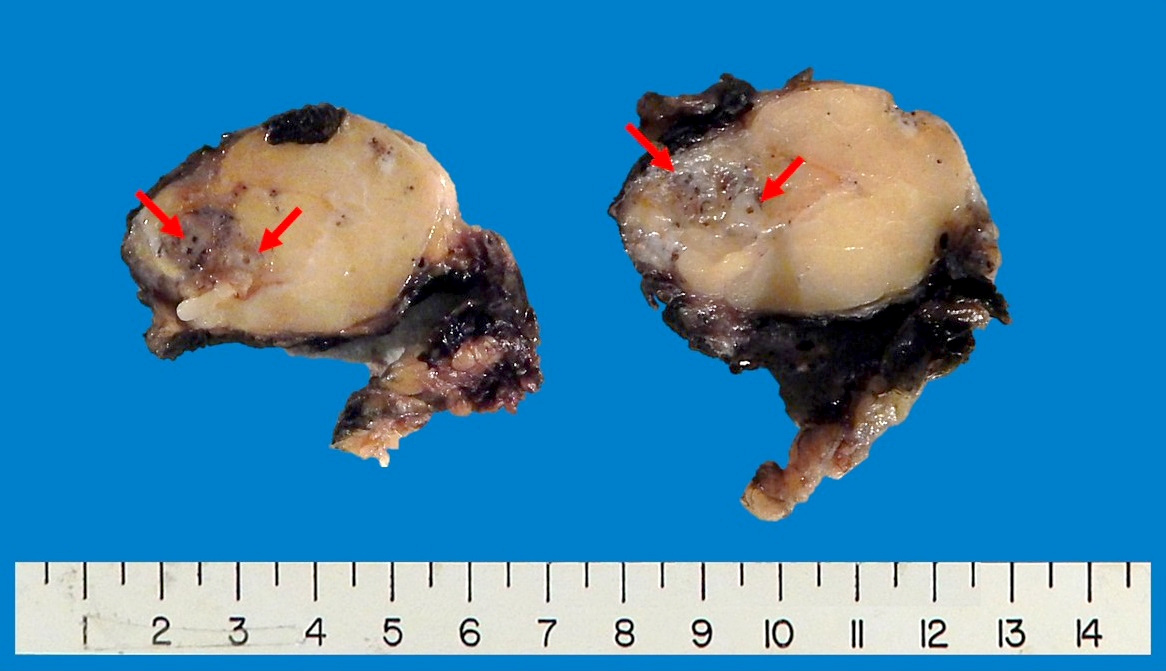
- Dedifferentiated liposarcoma
- Firm, fleshy, tan-gray nodules; occasionally gradual transition or infiltrative (Virchows Arch 2010;456:167)
- Well differentiated component may be limited or absent
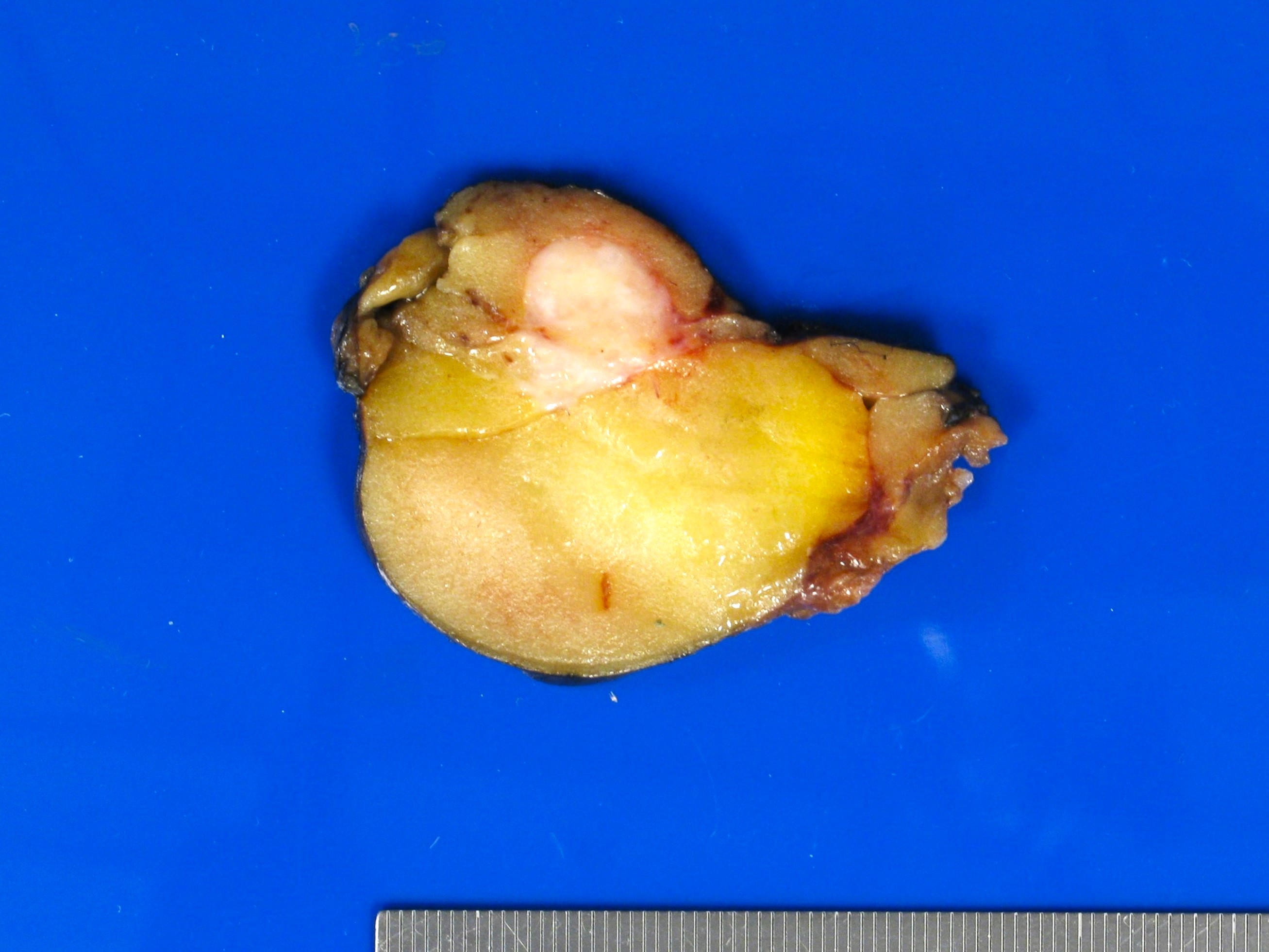
- Myxoid liposarcoma
- Well circumscribed, multinodular, gelatinous
- High grade areas are tan-gray, firm, fleshy
- Pleomorphic liposarcoma
- Variable growth (well defined, infiltrative, multinodular)
- Firm, white-yellow cut surface; often areas of necrosis and hemorrhage
Gross images
Frozen section description
- Not routinely used for diagnosis; may be helpful to evaluate for high grade sarcoma if no prior imaging or biopsy
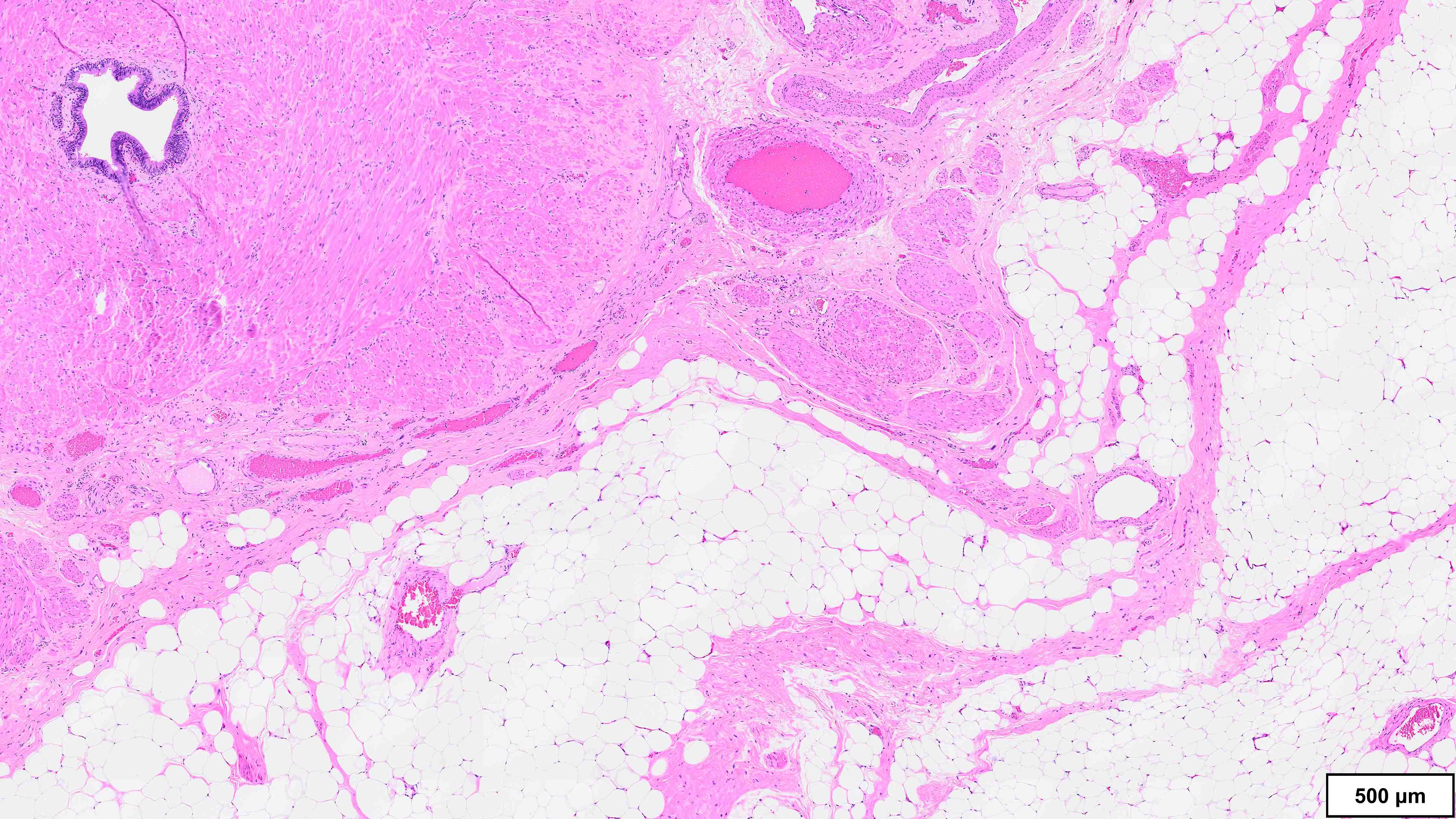
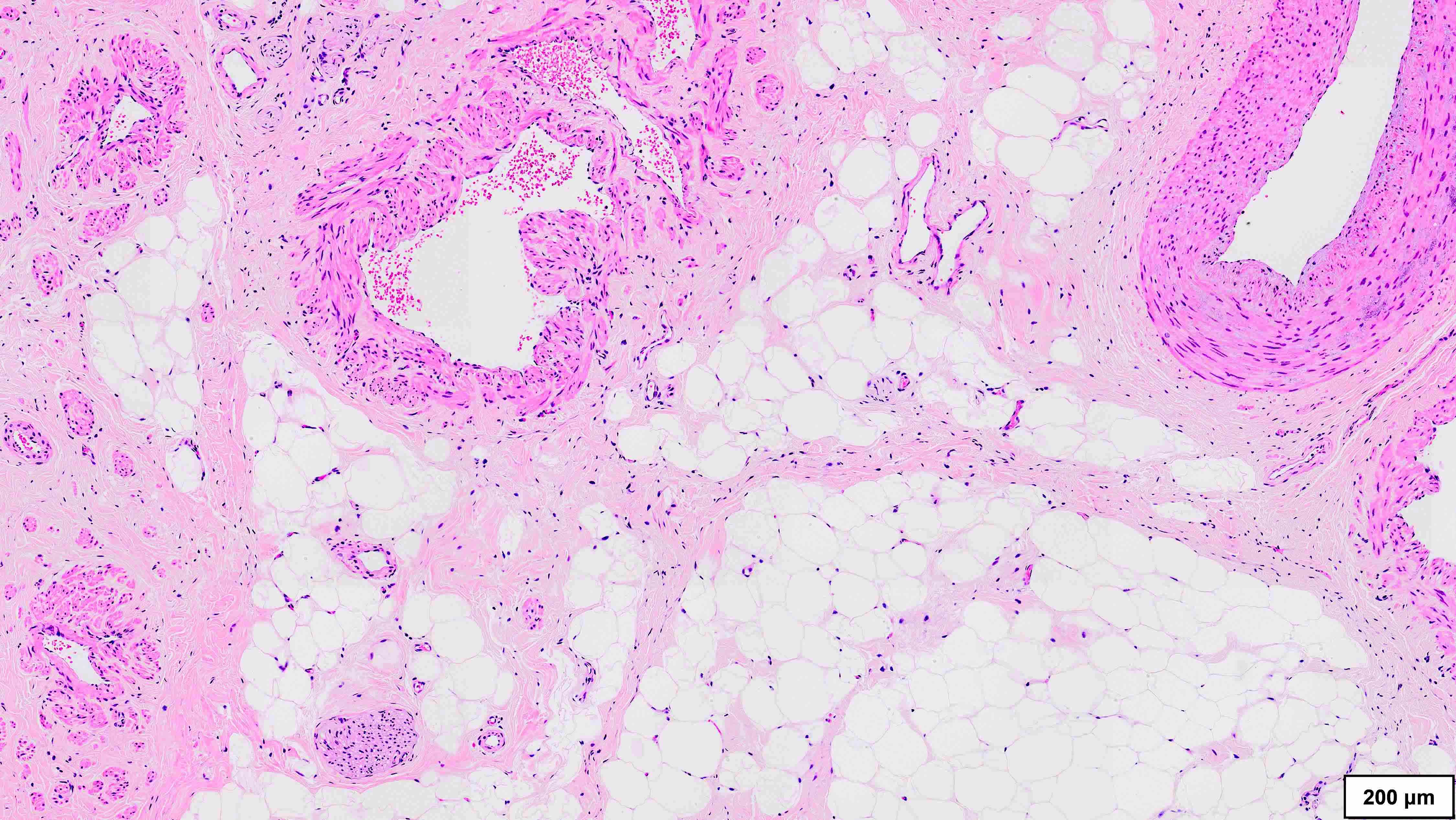
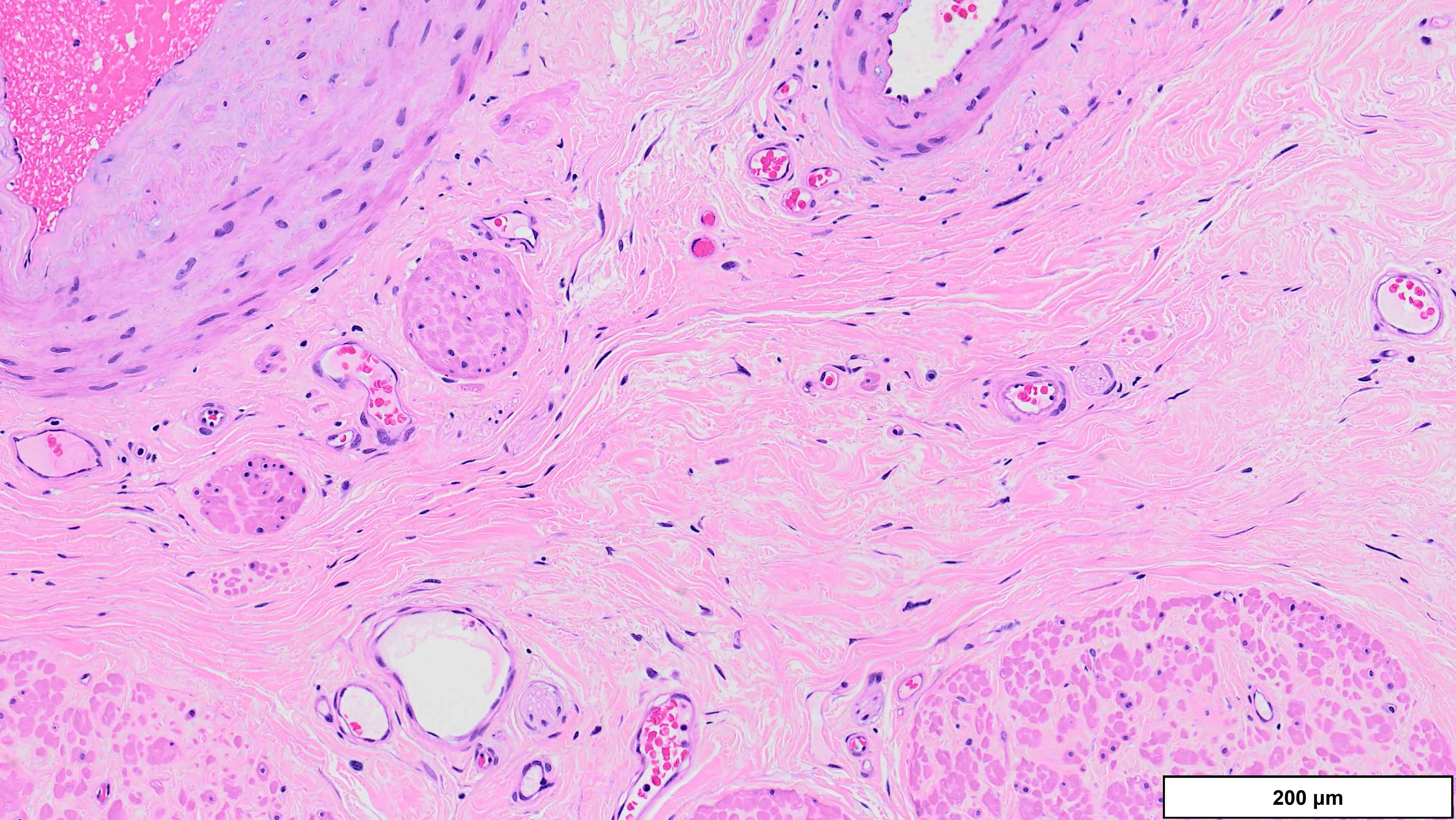
Microscopic (histologic) description
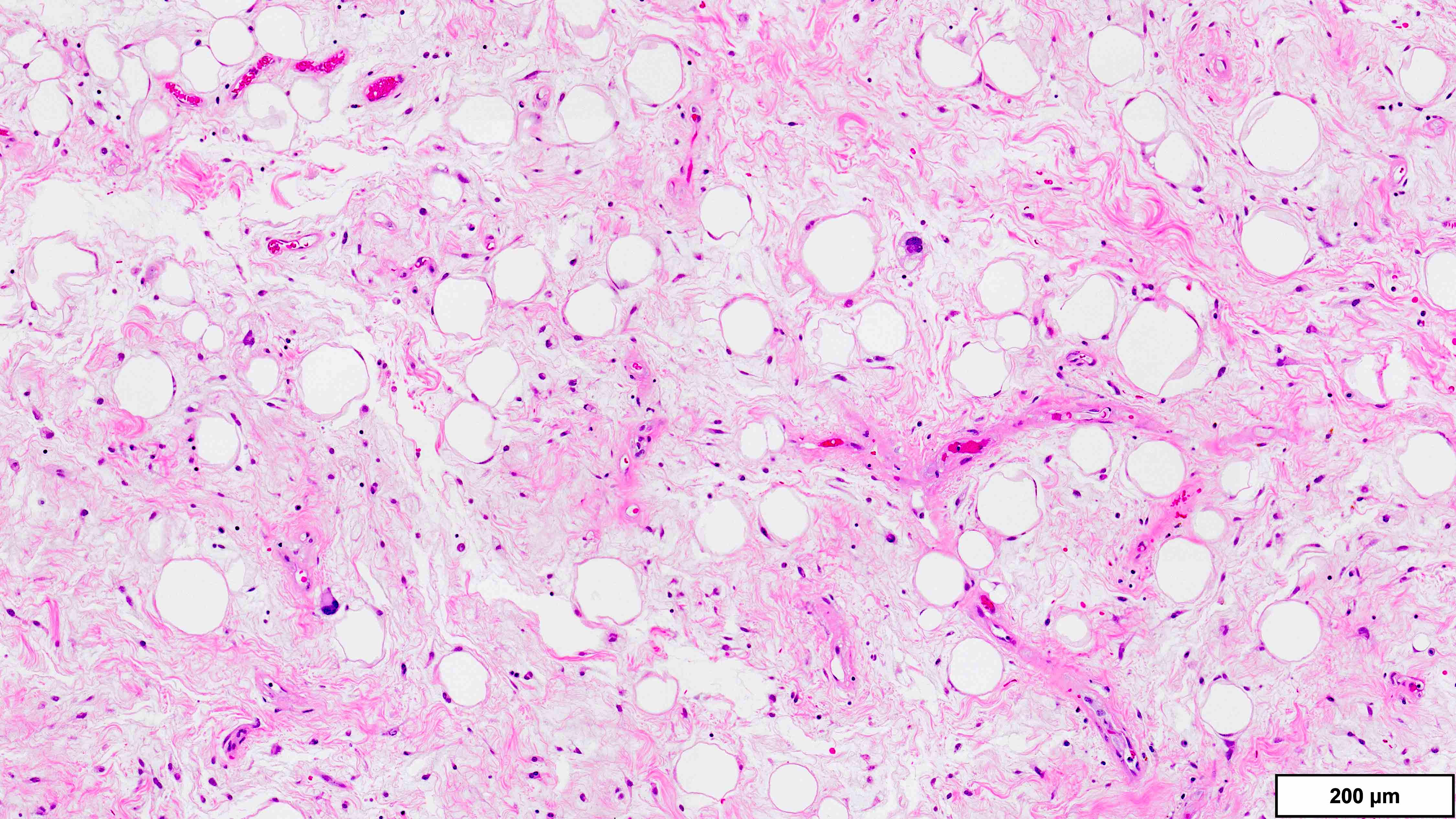
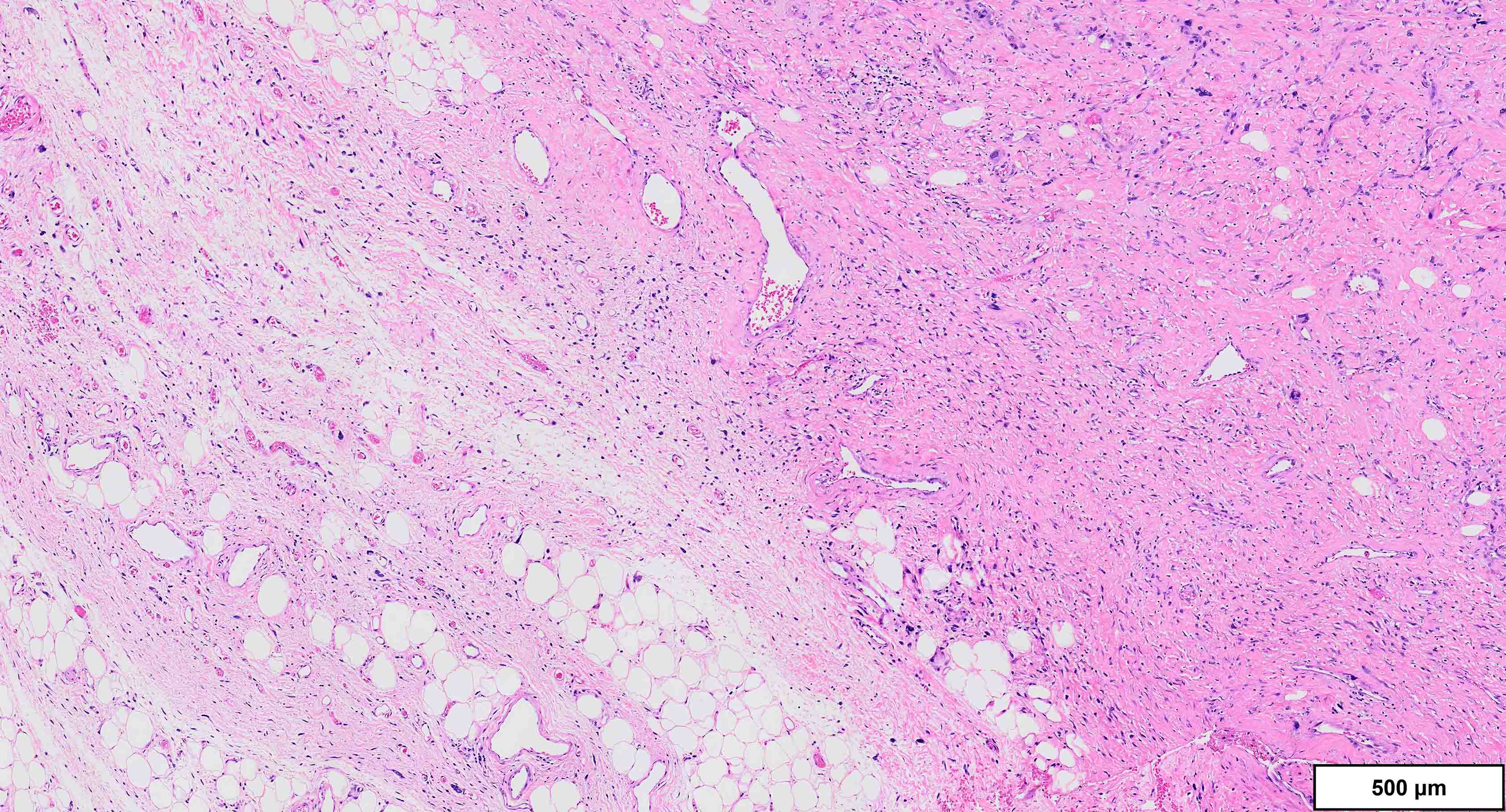
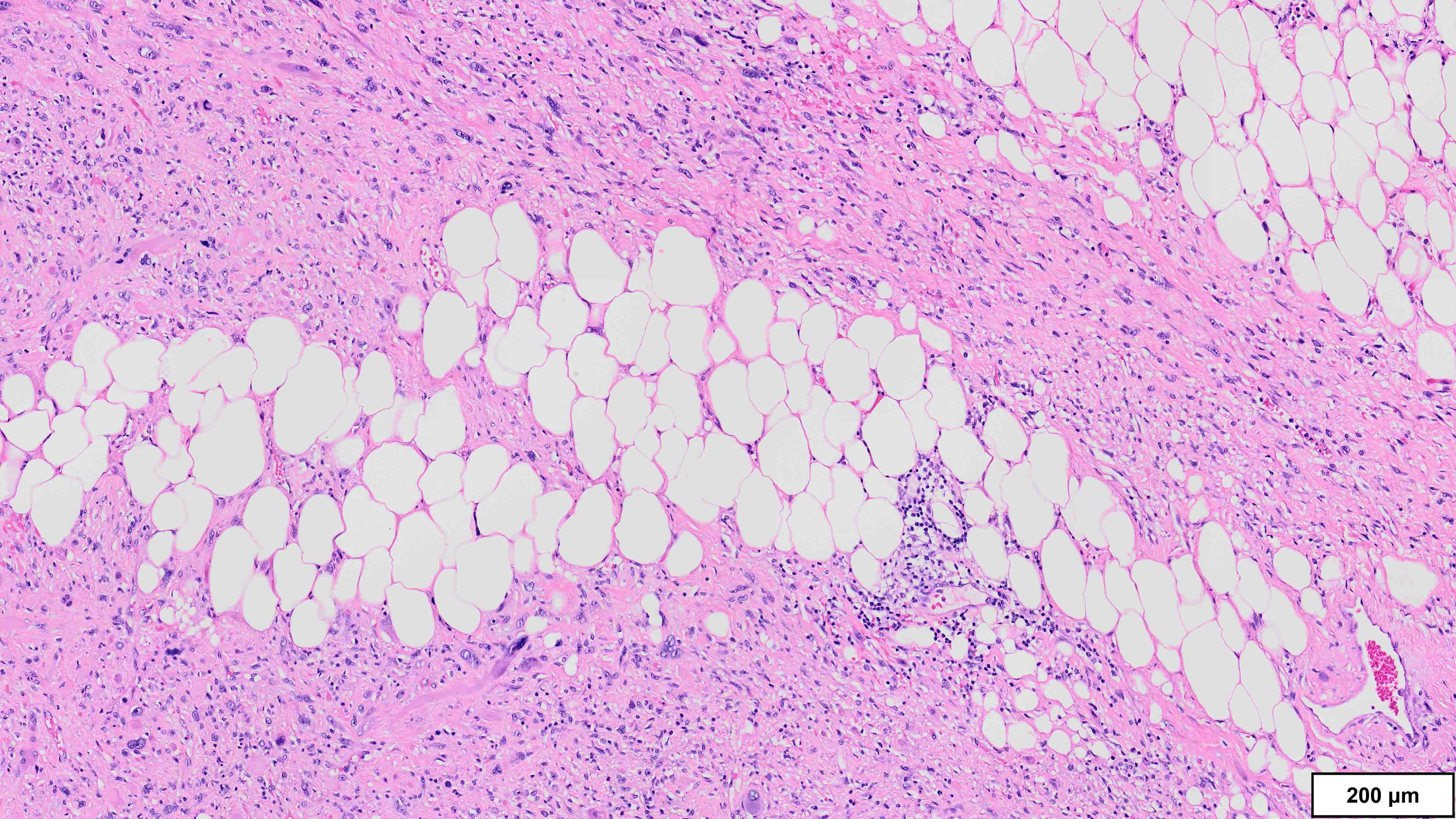
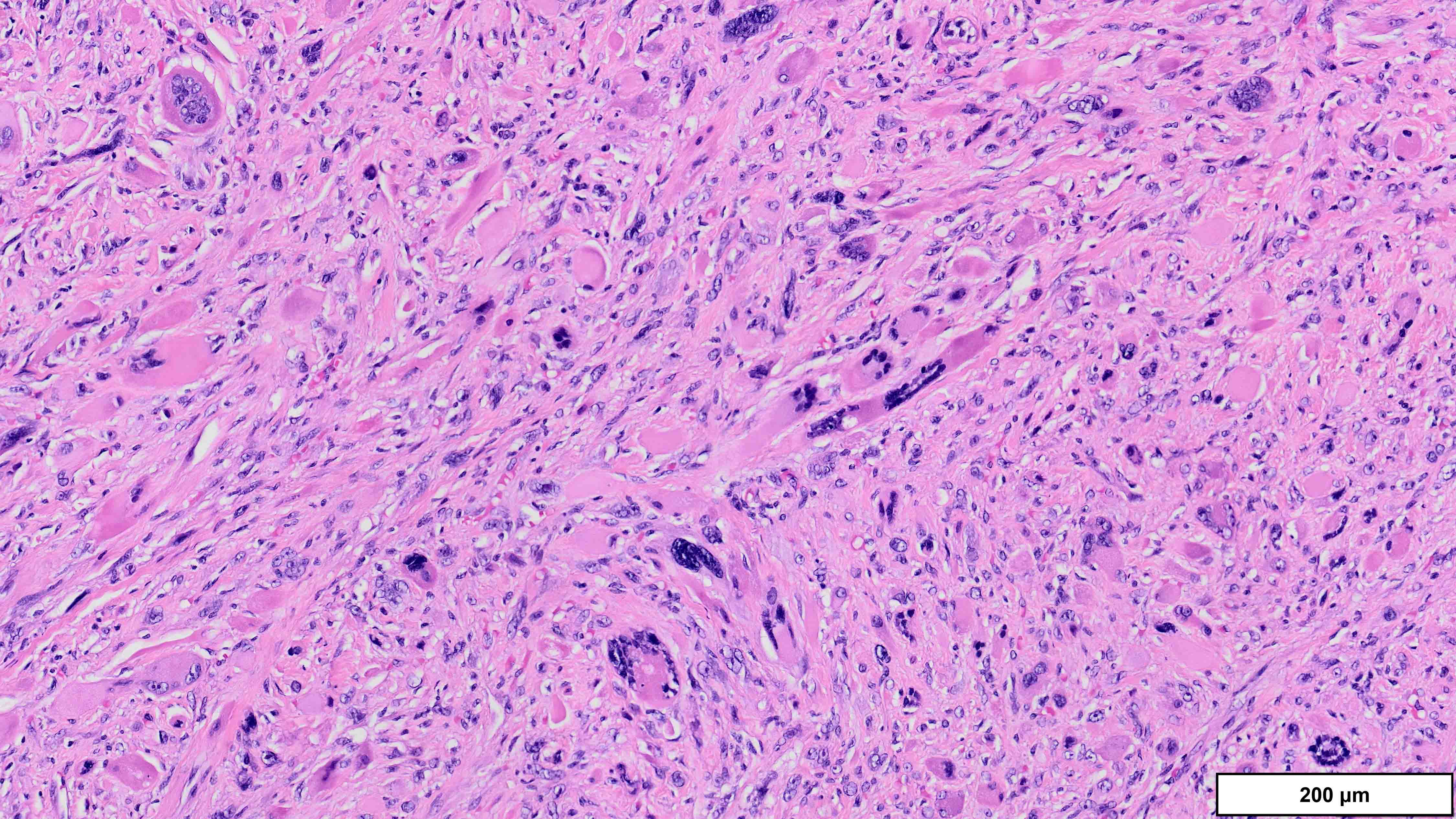
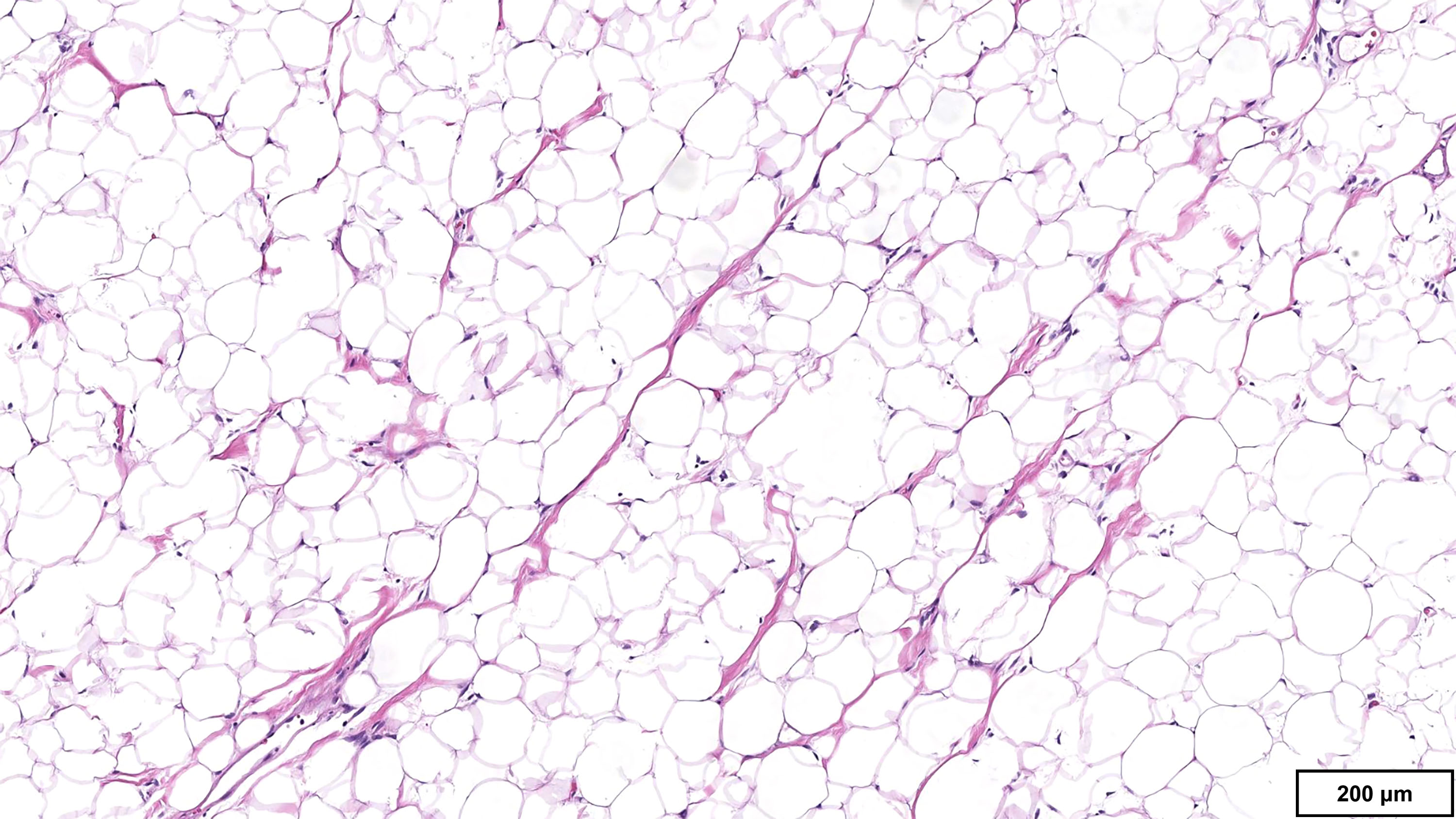
- Well differentiated liposarcoma
- 3 main morphologic subtypes, often multiple / mixed patterns; no prognostic significance (Virchows Arch 2010;456:167)
- Lipoma-like
- Lobules of variably sized mature adipocytes
- Lipoblasts; not required for diagnosis
- Thickened fibrous septa containing atypical and multinucleated stromal cells
- Areas may be indistinguishable from lipoma
- Sclerotic
- Common pattern in paratesticular tumors
- Hypocellular dense collagenous stroma with scattered atypical cells
- Inflammatory
- Prominent chronic mixed inflammatory infiltrate; obscures atypical stromal cells (Am J Surg Pathol 1997;21:518)
- Can be superimposed on another morphologic subtype
- Other patterns: myxoid and spindle cell (resembling MLPS), metaplastic bone / cartilage
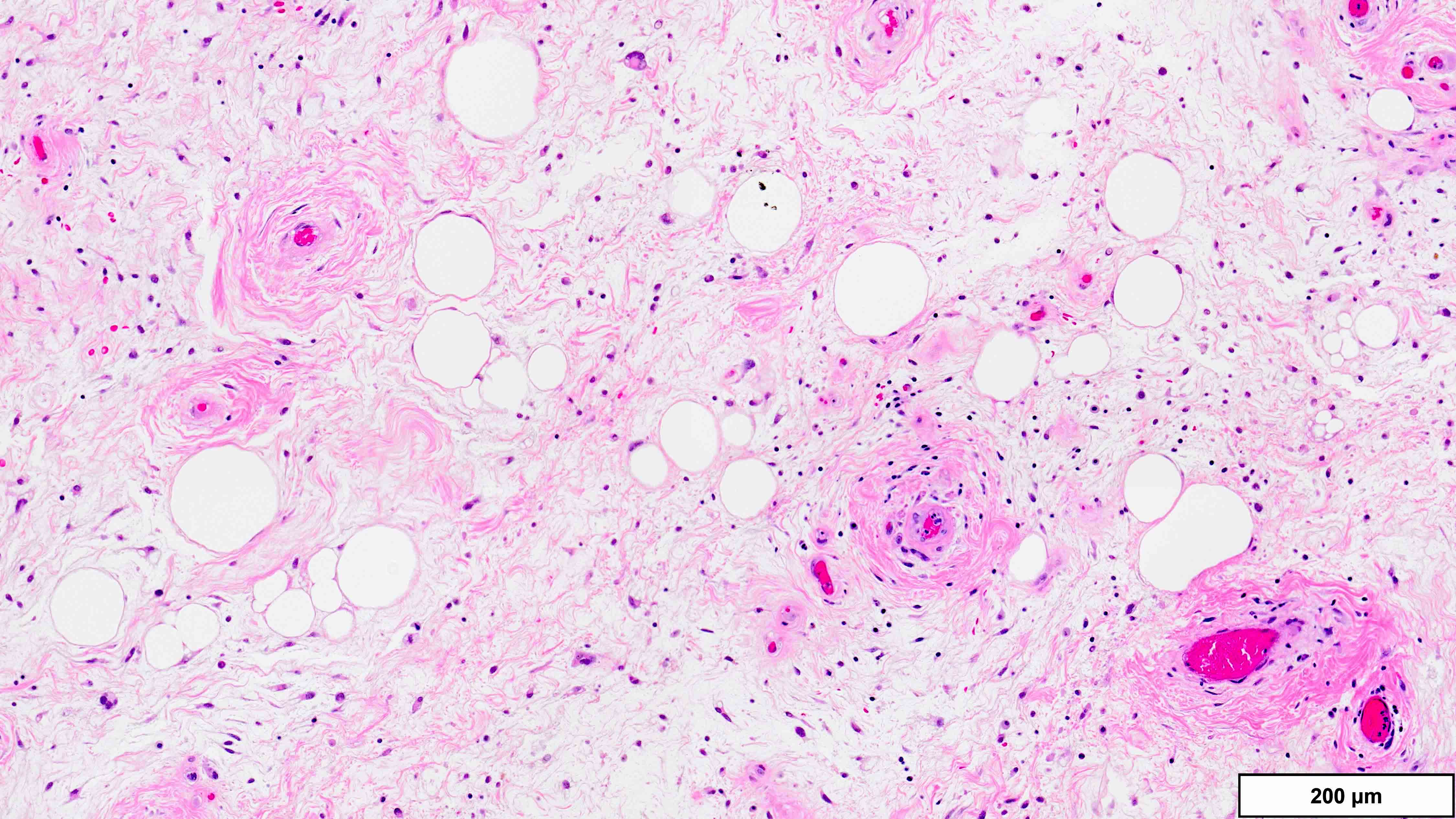
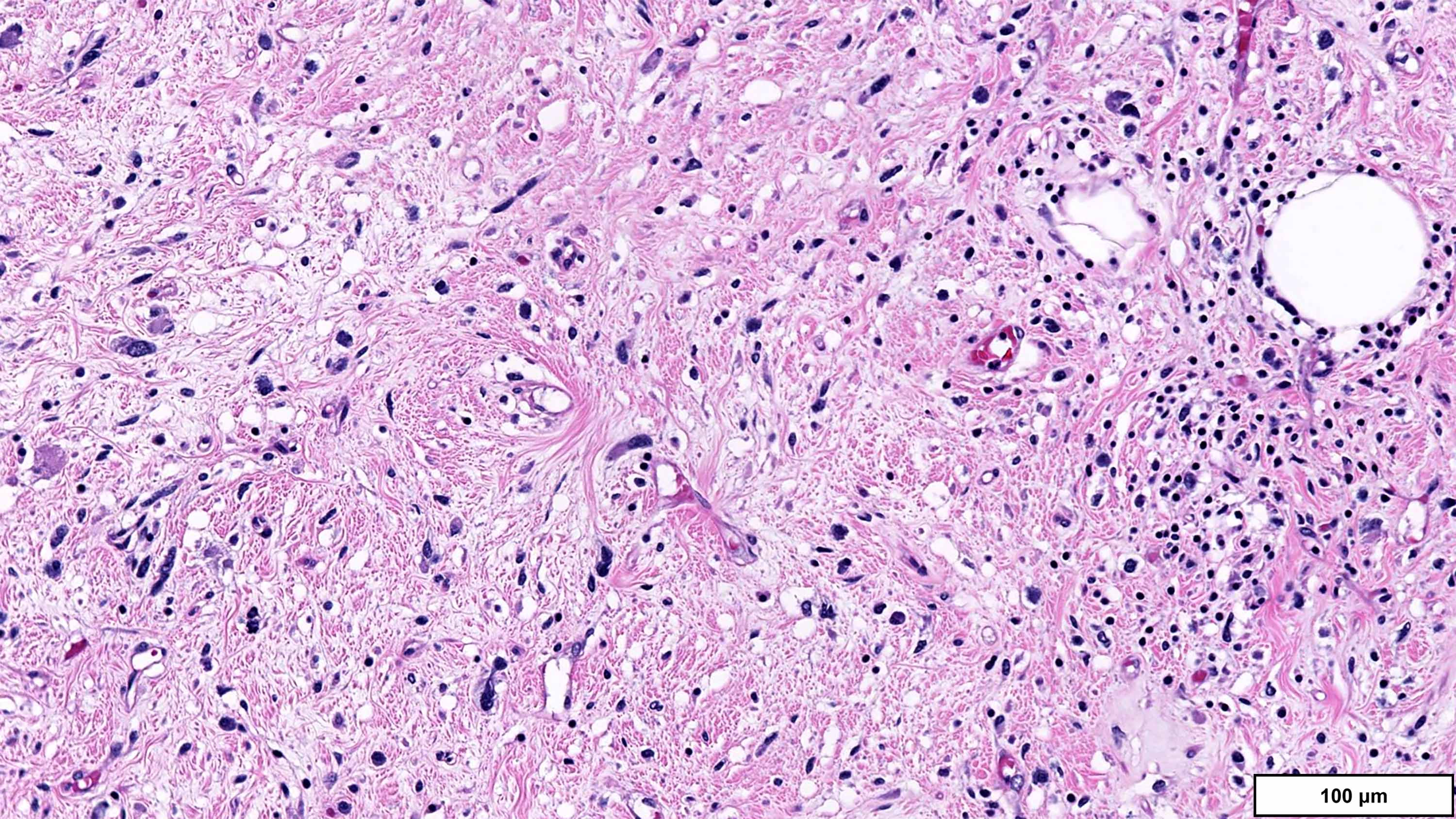
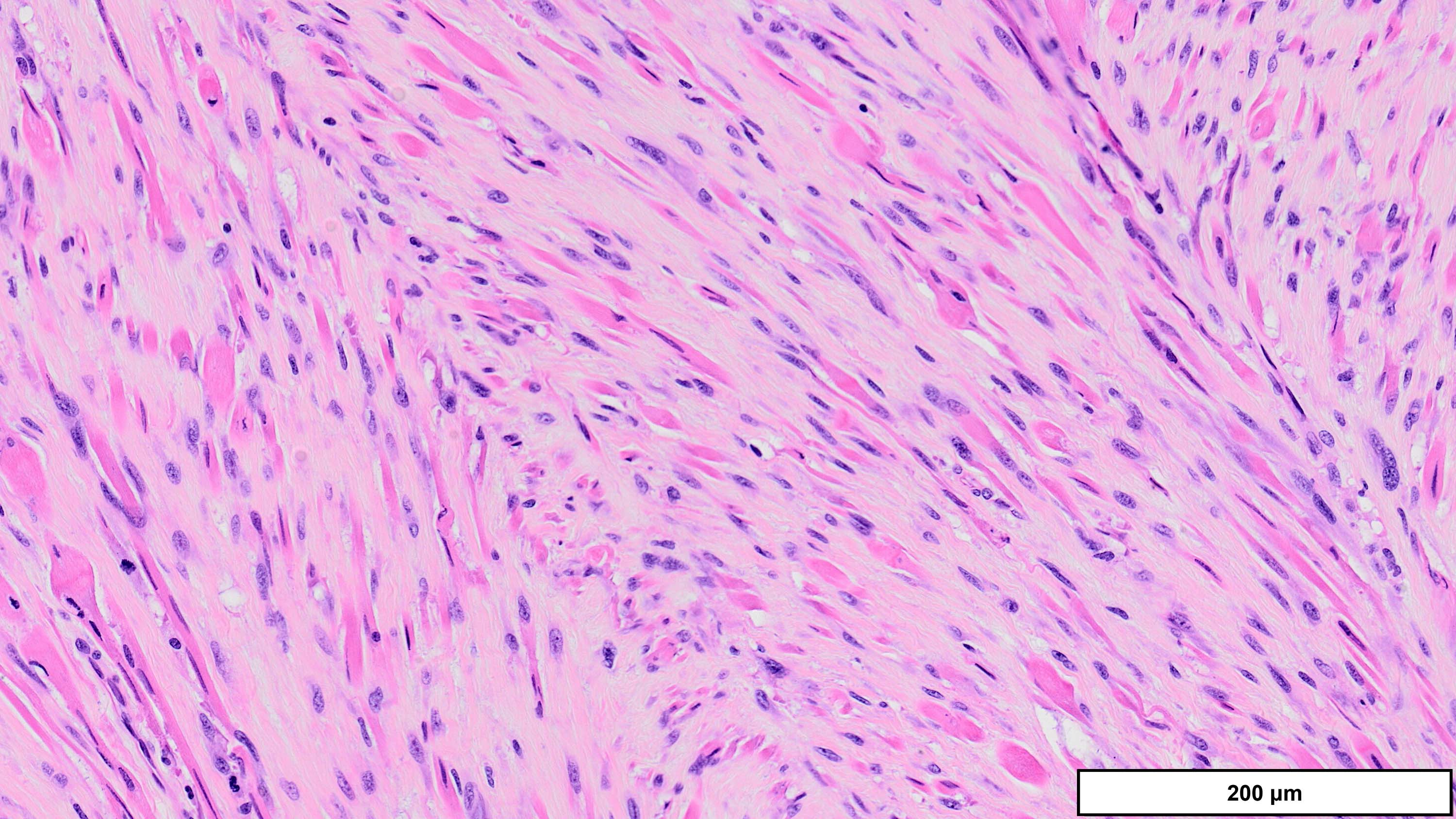
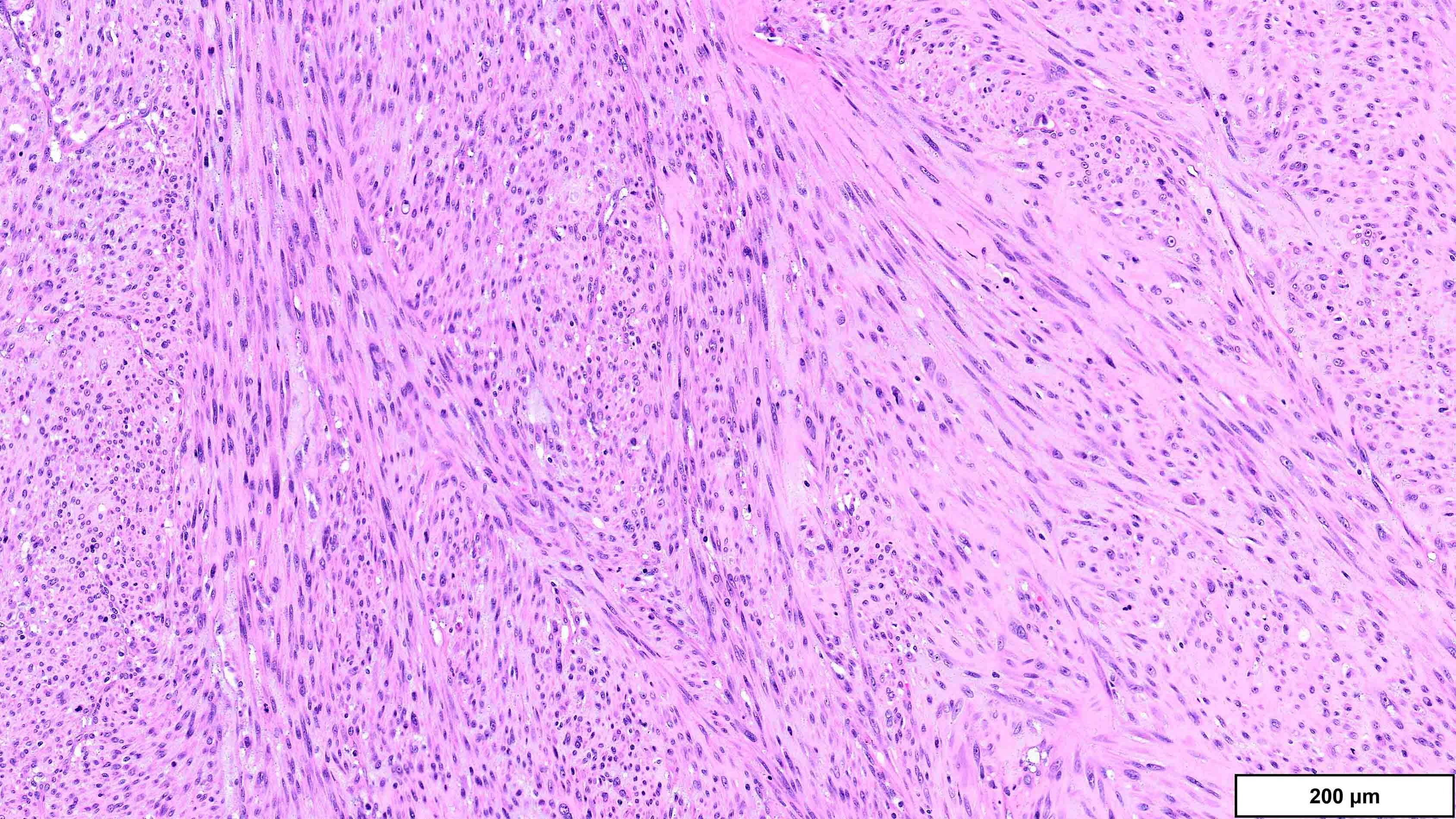
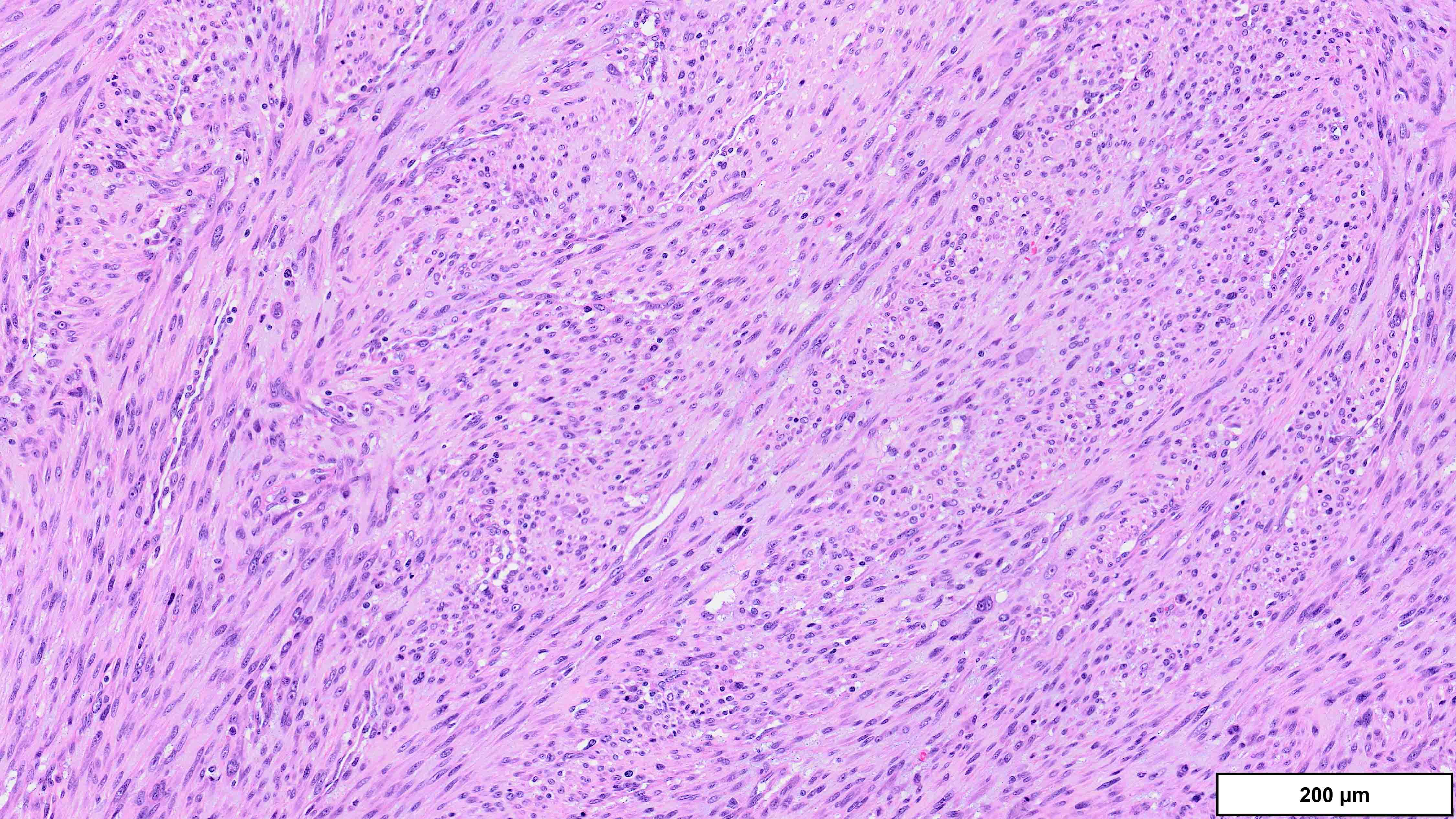
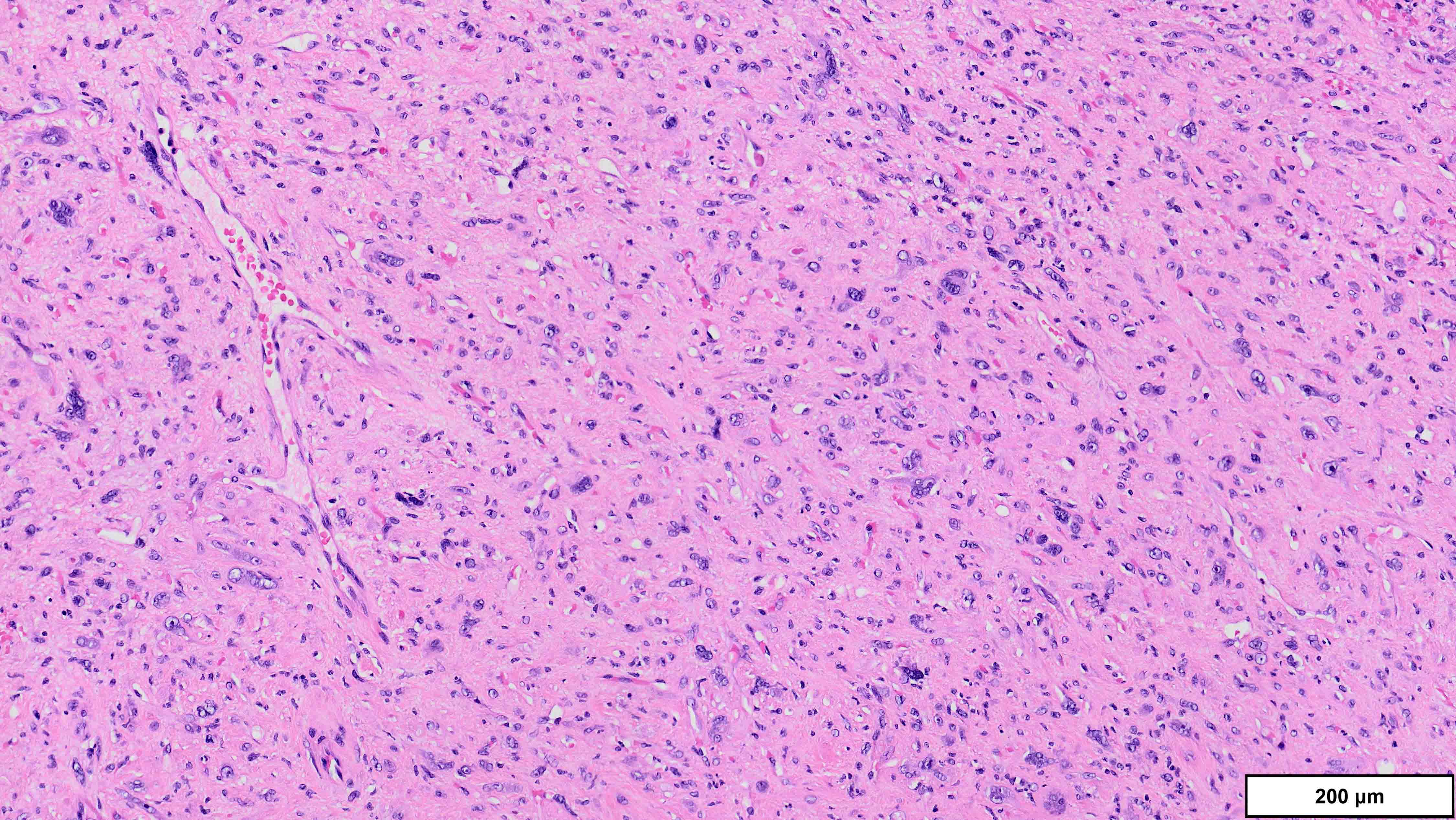
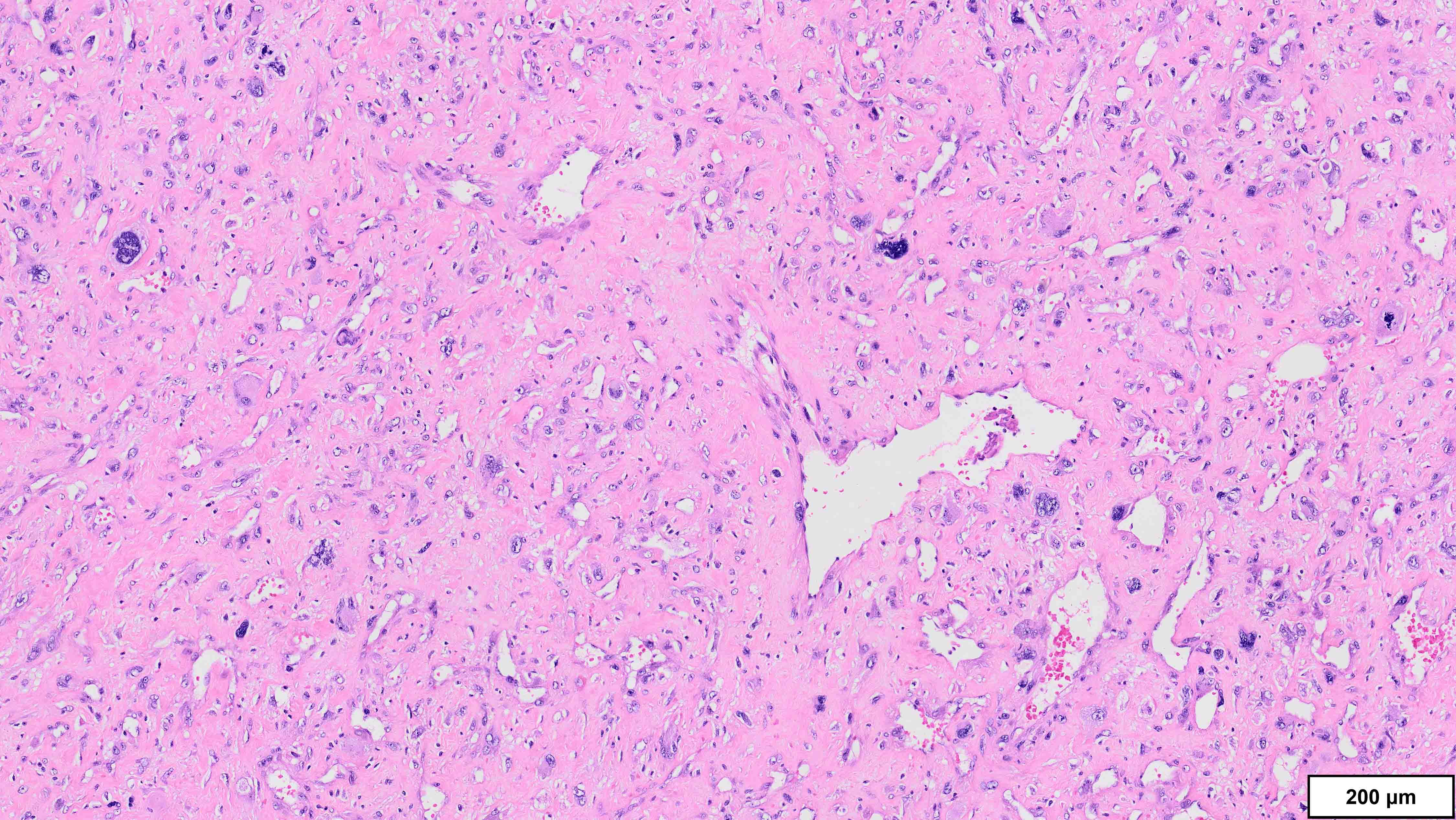
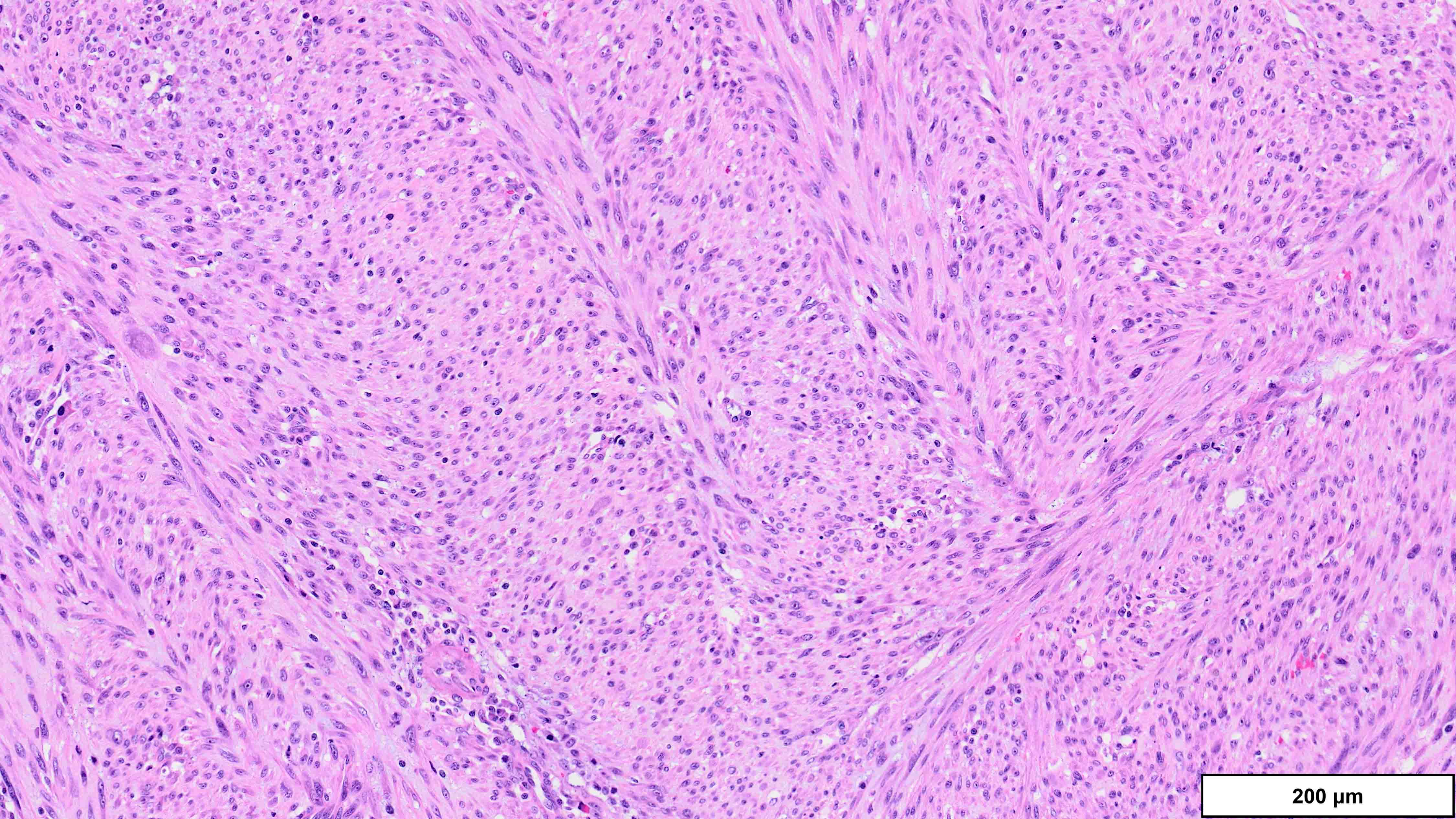
- Dedifferentiated liposarcoma
- Wide morphologic spectrum and intratumoral heterogeneity (Virchows Arch 2010;456:167)
- Most commonly high grade with low grade foci; rarely entirely low grade
- High grade
- Most often resembles undifferentiated pleomorphic sarcoma (UPS)
- Can also mimic other pleomorphic and spindle cell sarcomas (myxofibrosarcoma, solitary fibrous tumor, leiomyosarcoma and malignant peripheral nerve sheath tumor)
- Deep tumors previously diagnosed as inflammatory UPS (formerly malignant fibrous histiocytoma); now favored to represent the inflammatory variant of DDLPS (J Pathol 2004;203:822)
- Low grade
- Usually resembles a low grade spindle cell tumor (fibromatosis, nodular fasciitis, perineurioma / meningioma, low grade fibromyxoid sarcoma)
- Distinction between cellular WDLPS and low grade DDLPS is controversial (Am J Surg Pathol 2023;47:649)
- Distinct clonal proliferations with a mitotic rate > 5/10 high power fields are generally considered low grade dedifferentiation
- Heterologous differentiation in 5 - 10% of cases
- Rare cases with homologous differentiation, pleomorphic lipoblasts (Am J Surg Pathol 2010;34:1122)
- Myxoid liposarcoma
- Hypocellular lobules with increased cellularity at the periphery
- Prominent myxoid stroma; may form mucin pools (pulmonary edema-like pattern)
- Delicate, thin walled branching capillaries (chicken wire or chicken feet appearance)
- Small spindled to stellate cells with uniform, bland nuclei, scant eosinophilic cytoplasm
- Lipoblasts; often univacuolated (signet ring)
- No significant cytologic atypia or mitotic activity
- Mature adipocytic component in some cases; rarely metaplastic bone or cartilage (Am J Clin Pathol 2012;137:229)
- High grade areas composed of hypercellular sheets of round cells; increased nuclear grade and mitotic activity
- Pleomorphic liposarcoma
- Variable number of pleomorphic lipoblasts admixed with high grade sarcoma, most often resembling undifferentiated pleomorphic sarcoma or myxofibrosarcoma (Am J Surg Pathol 2002;26:601, Am J Surg Pathol 2004;28:1257)
- Univacuolated (signet ring) lipoblasts; can be extensive
- Epithelioid morphology in 25%; contains nonlipogenic cells with cytoplasmic vacuolization (Mod Pathol 1999;12:722)
Microscopic (histologic) images
Cytology description
- Not routinely performed on paratesticular tumors
Positive stains
- Well differentiated liposarcoma
- MDM2 (89 - 100%), CDK4 (68 - 91%) (Am J Surg Pathol 2005;29:1340, Hum Pathol 2017;59:34)
- Lower sensitivity (41%) in lipoma-like WDLPS (Am J Surg Pathol 2016;40:1647)
- Can also stain endothelial cells and histiocytes in areas of fat necrosis (Am J Surg Pathol 2016;40:1647)
- HMGA2 (87%); can be easier to interpret (strong, more diffuse staining versus MDM2) but positive in most lipomas (Mod Pathol 2010;23:1657)
- Variable S100 (adipocytes), CD34 (spindle cells)
- MDM2 (89 - 100%), CDK4 (68 - 91%) (Am J Surg Pathol 2005;29:1340, Hum Pathol 2017;59:34)
- Dedifferentiated liposarcoma
- MDM2 (95 - 100%), CDK4 (83 - 92%) (Am J Surg Pathol 2005;29:1340, Hum Pathol 2017;59:34)
- Cases without myogenic differentiation can be focally positive for desmin (Am J Surg Pathol 2007;31:1557)
- Myxoid liposarcoma
- S100 (89%): primitive mesenchymal cells and lipoblasts (Am J Clin Pathol 1995;103:20)
- SOX11 (90%): not specific (Histopathology 2019;74:391)
- DDIT3 (98%): 96 - 100% specific but not widely available (Histopathology 2021;79:106)
- Rare focal staining (up to 40% of cells) in other liposarcomas
- Pleomorphic liposarcoma
- Focal S100 (33 - 54%, lipoblasts)
- Focal MelanA in some cases of epithelioid PLPS (Semin Diagn Pathol 2019;36:122)
Negative stains
- Well differentiated liposarcoma
- Cytokeratins, melanocytic markers (HMB45) (Case Rep Nephrol 2012;2012:374107)
- Myxoid liposarcoma
- Pleomorphic liposarcoma
- MDM2, CDK4 (Am J Surg Pathol 2005;29:1340)
- Focal SMA (45% positive), desmin (13% positive) (Am J Surg Pathol 2004;28:1257, Mod Pathol 1999;12:722)
- Focal cytokeratin (21% positive): more common in epithelioid subtype (38 - 45% positive) (Mod Pathol 1999;12:722)
Molecular / cytogenetics description
- Well differentiated liposarcoma
- Supernumerary ring or giant marker chromosomes with material from 12q13-15 region (Genes Chromosomes Cancer 1999;24:30)
- MDM2 amplification is main driver of tumorigenesis; other genes coamplified (CDK4, HMGA2) (Clin Cancer Res 2009;15:5696, Int J Cancer 2008;122:2233)
- MDM2, CDK4 amplifications detected by FISH or real time PCR (Am J Surg Pathol 2007;31:1476)
- Dedifferentiated liposarcoma
- Often gains additional cytogenetic alterations
- More complex karyotype with high copy number variation but low rate of mutation (Oncotarget 2015;6:42429)
- Myxoid liposarcoma
- Alterations involving 12q13 breakpoint, most commonly t(12;16)(q13;p11.2), resulting in FUS::DDIT3 fusion (95% of cases)
- Rarely, t(12;22)(q13;q12.2) EWSR1::DDIT3 or more complex rearrangements (Oncogene 1996;12:489)
- DDIT3 FISH is very sensitive and specific; rare false negatives for non-FUS::DDIT3 fusions (Genes Chromosomes Cancer 2023;62:167)
- Pleomorphic liposarcoma
- Complex karyotype, with high intratumoral heterogeneity and copy number variations; similar to undifferentiated pleomorphic sarcoma and high grade myxofibrosarcoma (Diagnostics (Basel) 2021;11:430)
Sample pathology report
- Testicle, partial orchiectomy:
- Well differentiated liposarcoma (4 cm) (see comment and synoptic report)
- No evidence of dedifferentiation
- Surgical margins are negative for tumor
- Comment: Histologic sections demonstrate a well differentiated adipocytic tumor and fibrous septa containing atypical multinucleated stromal cells. Areas with sclerotic and inflammatory morphology are also seen. Immunohistochemistry for MDM2 is strongly positive in lesional cells, consistent with the above diagnosis.
- Note: if resection, the soft tissue synoptic template should be used in addition, with staging performed according to retroperitoneum
- Testicle, radical orchiectomy:
- Dedifferentiated liposarcoma (10 cm) (see comment and synoptic report)
- Well differentiated liposarcoma component is present
- Surgical margins are negative for tumor
- Comment: Histologic sections demonstrate a high grade nonlipogenic sarcoma with pleomorphic and spindle morphology, in association with a well differentiated adipocytic tumor. The dedifferentiated (nonlipogenic) component constitutes ~60% of the tumor. Immunohistochemistry for MDM2 is strongly positive in lesional cells, consistent with the above diagnosis.
- Testicle, partial orchiectomy:
- Myxoid liposarcoma, low grade (3 cm) (see comment and synoptic report)
- Surgical margins are negative for tumor
- Comment: Histologic sections demonstrate a hypocellular myxoid lesion composed of small, bland, primitive mesenchymal cells and delicate, branching capillaries. There are occasional univacuolated and multivacuolated lipoblasts. No high grade features are identified. FISH is positive for DDIT3 rearrangement, confirming the above diagnosis.
- Testicle, radical orchiectomy:
- Myxoid liposarcoma, high grade (5 cm) (see comment and synoptic report)
- Surgical margins are negative for tumor
- Comment: Histologic sections demonstrate characteristic features of a low grade myxoid liposarcoma as well as areas with high grade morphology, which constitutes ~30% of the tumor. FISH is positive for DDIT3 rearrangement, confirming the above diagnosis.
- Testicle, radical orchiectomy:
- Pleomorphic liposarcoma (6 cm) (see comment and synoptic report)
- Surgical margins are negative for tumor
- Comment: Histologic sections demonstrate a high grade pleomorphic and spindle cell sarcoma with focal / extensive pleomorphic lipoblasts. FISH is negative for MDM2 amplification, consistent with the above diagnosis.
Differential diagnosis
- Well differentiated liposarcoma, lipoma-like subtype
- Lipoma:
- Most fatty spermatic cord lesions are benign; incidental liposarcomas are exceedingly rare (< 0.1%) (J Surg Oncol 1999;71:50)
- MDM2 immunohistochemistry has lower sensitivity in lipoma-like WDLPS with minimal atypia (Am J Surg Pathol 2016;40:1647)
- MDM2 FISH is recommended in large tumors (> 10 cm) with equivocal atypia, concerning imaging findings or recurrent tumors (Histopathology 2022;80:369, Am J Surg Pathol 2015;39:1433)
- Spindle cell / pleomorphic lipoma and atypical spindle cell / pleomorphic lipomatous tumor (ASPLT):
- Ropey collagen, myxoid change and floret giant cells can also be seen in some cases of WDLPS
- Lipoblasts can be seen in ASPLT and WDLPS
- Typically do not occur in the paratesticular region
- RB1 loss, 13q14 deletions (Am J Surg Pathol 2012;36:1119, Am J Surg Pathol 2021;45:1282)
- Rare focal staining for MDM2 or CDK4 in ASPLT; MDM2 not amplified (Am J Surg Pathol 2021;45:1282)
- More atypia in WDLPS but can be subtle
- Lipoma:
- Well differentiated liposarcoma, sclerotic subtype
- Lipoblastoma:
- Can demonstrate fatty maturation and stromal fibrosis
- Occurs in infants and young children
- PLAG1 gene rearrangements (8q12) (Cancer Res 2000;60:4869)
- Lipoblastoma:
- Well differentiated liposarcoma, inflammatory subtype
- Inflammatory myofibroblastic tumor, chronic inflammatory processes (IgG4 related disease) and lymphoproliferative disorders:
- MDM2 IHC can be positive in areas of fat necrosis but not MDM2 FISH (no amplification)
- Stromal cells in WDLPS have greater atypia but may be focal and obscured by inflammation
- IgG4 positive plasma cells not increased in WDLPS (Mod Pathol 2012;25:1181)
- ALK rearrangements in ~50% of inflammatory myofibroblastic tumors (Mod Pathol 2001;14:569)
- Inflammatory myofibroblastic tumor, chronic inflammatory processes (IgG4 related disease) and lymphoproliferative disorders:
- Dedifferentiated liposarcoma
- Leiomyosarcoma and rhabdomyosarcoma:
- DDLPS with extensive heterologous differentiation may be difficult to differentiate from primary myogenic sarcomas
- No well differentiated component or MDM2 amplification
- DDLPS has more morphologic heterogeneity
- Solitary fibrous tumor:
- Subset has mature adipocytic component (lipomatous variant)
- Diffusely positive for CD34 and STAT6
- Rare focal staining for STAT6 in DDLPS (Mod Pathol 2014;27:1231)
- No MDM2 amplification
- Dermatofibrosarcoma protuberans (DFSP):
- Infiltrative growth and extensive entrapped nonneoplastic fat may falsely suggest lipomatous differentiation
- Diffusely positive for CD34
- t(17;22)(q22;q13) COL1A1::PDGFB gene fusion
- Malignant peripheral nerve sheath tumor (MPNST):
- MDM2 gene amplification reported in small minority of cases (Arch Pathol Lab Med 2012;136:95)
- Heterologous differentiation in a subset of tumors, typically rhabdomyosarcomatous (malignant triton tumor); there have been rare reports of liposarcomatous differentiation (Pathol Res Pract 2010;206:138)
- Unlike DDLPS, typically has a relatively uniform appearance throughout
- Loss of staining for H3K27me3 in conventional (spindle cell) MPNST
- Diffuse S100, SOX10 positivity, loss of INI1 in epithelioid MPNST
- Sarcomatoid carcinoma and mesothelioma:
- At least focal positivity for keratins or EMA; limited staining for mesothelial markers in sarcomatoid mesothelioma
- No WDLPS component
- Rare instances of MDM2 amplification reported in some carcinomas, although none in the paratestis (Hum Pathol 2019;87:28)
- Clinical history, imaging and presence of nonsarcomatoid component may be helpful
- Leiomyosarcoma and rhabdomyosarcoma:
- Myxoid liposarcoma, low grade
- Extraskeletal myxoid chondrosarcoma:
- Primitive mesenchymal cells and matrix appear similar to MLPS
- No lipoblasts or prominent capillary vasculature
- t(9;22)(q22;q12) EWSR1::NR4A3 fusion
- Negative for DDIT3 overexpression and DDIT3 gene rearrangement
- Lipoblastoma:
- Occurs in infants and young children
- Primitive myxoid areas resemble MPLS
- PLAG1 gene rearrangements (8q12) (Cancer Res 2000;60:4869)
- Extraskeletal myxoid chondrosarcoma:
- Myxoid liposarcoma, high grade
- Undifferentiated round cell sarcomas (Ewing, BCOR rearranged, CIC::DUX):
- May be indistinguishable if no low grade MLPS component
- DDIT3 immunostain is highly sensitive and specific for MLPS in this context (Histopathology 2021;79:106)
- All have characteristic gene rearrangements; FISH probes available for most
- Undifferentiated round cell sarcomas (Ewing, BCOR rearranged, CIC::DUX):
- Pleomorphic liposarcoma
- Undifferentiated pleomorphic sarcoma:
- Pleomorphic lipoblasts may be limited / focal in PLPS; may require extensive sampling
- Epithelioid variant can resemble melanoma or carcinoma:
- Undifferentiated pleomorphic sarcoma:
Additional references
Practice question #1
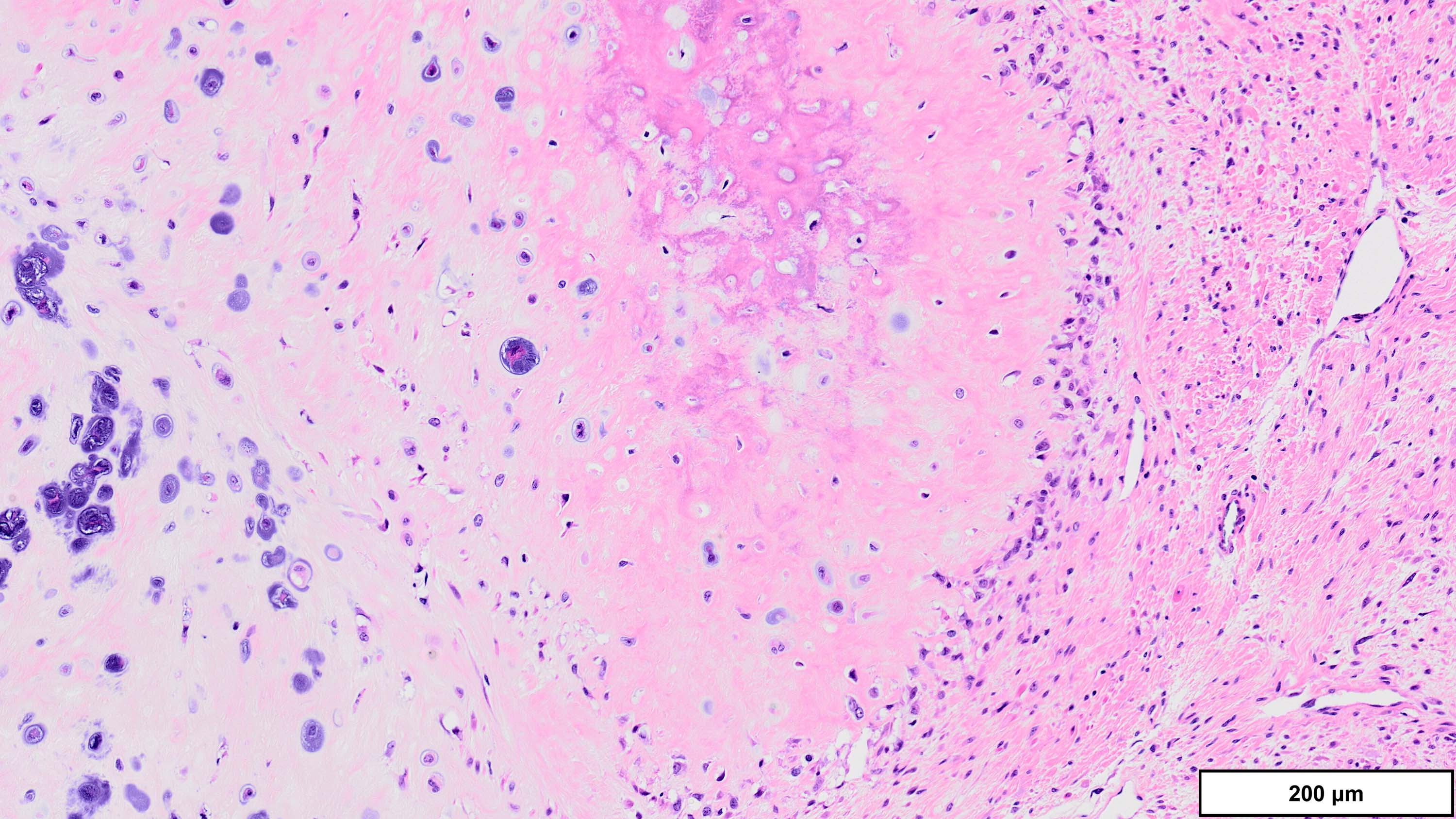
A 70 year old man is found to have an 11 cm cord lipoma during elective surgery for inguinal hernia repair. Representative histologic findings are shown in the image provided above. Which of the following tests would be most helpful in this context?
- FISH for DDIT3 rearrangement
- FISH for MDM2 amplification
- Immunohistochemistry for HMGA2
- Immunohistochemistry for MDM2
- Immunohistochemistry for RB1
Practice answer #1
B. FISH for MDM2 amplification. The microscopic image demonstrates variably sized mature adipocytes and small areas of fibrous bands containing spindle cells without significant atypia. MDM2 FISH is recommended for deep / centrally located or large tumors (> 10 cm) with equivocal atypia, concerning clinical / imaging findings and recurrent tumors. Answer D is incorrect because MDM2 IHC has low sensitivity in distinguishing between lipoma and lipoma-like well differentiated liposarcoma (WDLPS). Answer C is incorrect because HMGA2 is positive in both lipomas and WDLPS. Answer E is incorrect because RB1 loss is seen in spindle cell / pleomorphic lipoma and atypical spindle cell pleomorphic lipomatous tumor (ASPLT) and is not a feature of conventional lipomas or WDLPS. Answer A is incorrect because although DDIT3 (12q13) may be amplified in some cases of WDLPS, DDIT3 rearrangements are characteristic of myxoid liposarcoma and do not occur in lipomas or WDLPS.
Comment Here
Reference: Liposarcoma
Comment Here
Reference: Liposarcoma
Practice question #2
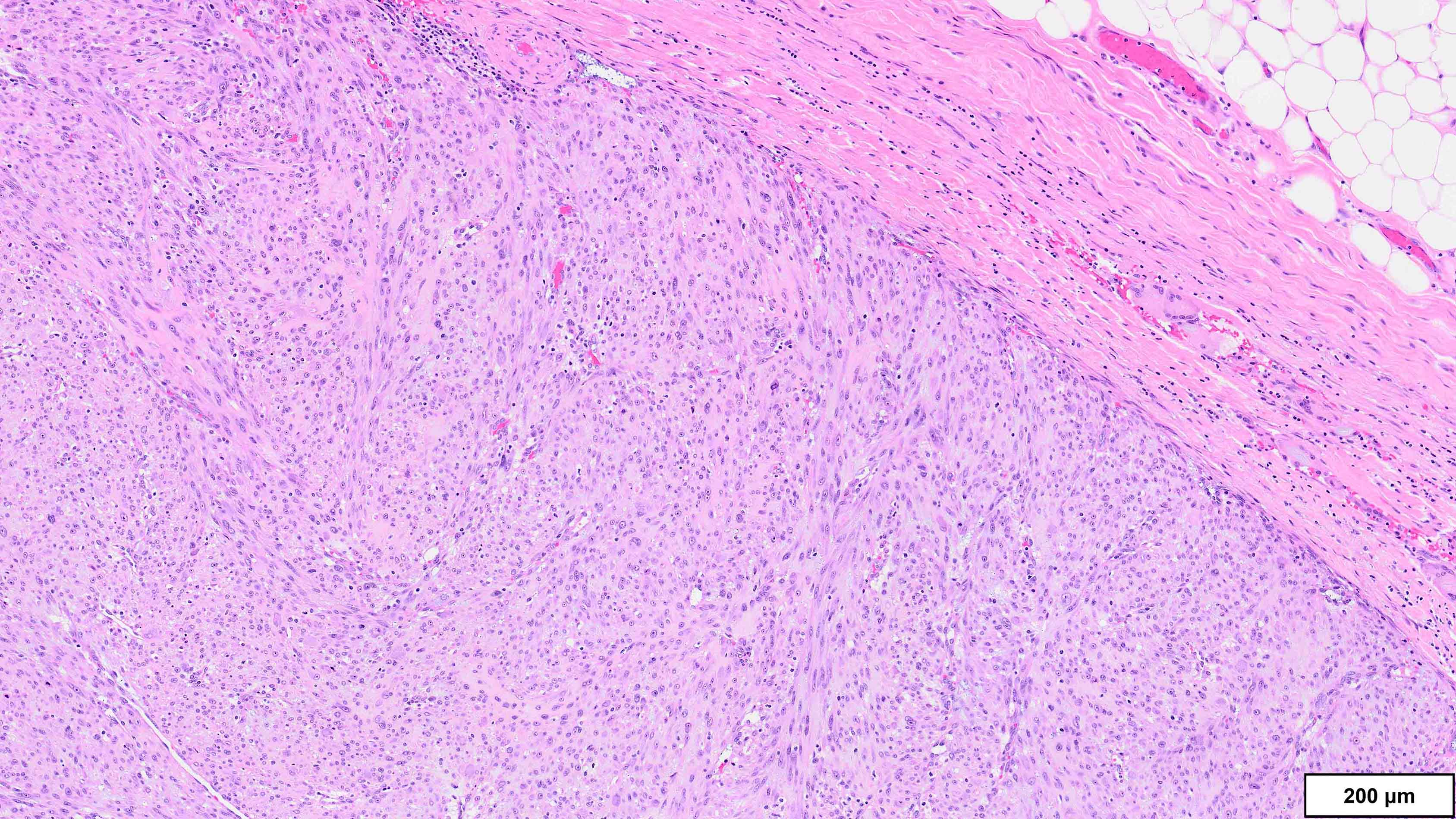
A 75 year old man presents with worsening scrotal swelling. On exam, there is a grossly apparent, large, right inguinal mass with no skin changes or signs of infection. A computerized tomography (CT) scan reveals a right inguinal canal ovoidal soft tissue mass, measuring 6 cm in greatest dimension, that was not present on previous imaging performed 12 months prior for chronic back pain. No other abnormalities are noted. Clinical history is otherwise unremarkable. Representative histologic findings from the resected mass are shown in the provided image. Tumor cells are focally positive for desmin and STAT6. FISH is positive for MDM2 amplification. What is the most likely diagnosis?
- Dedifferentiated liposarcoma
- Leiomyosarcoma
- Sarcomatoid carcinoma
- Solitary fibrous tumor
- Undifferentiated pleomorphic sarcoma
Practice answer #2
A. Dedifferentiated liposarcoma (DDLPS). The microscopic image demonstrates atypical spindled and multinucleated cells arranged in short fascicles. In this setting, DDLPS is the most likely diagnosis. Identification of a well differentiated liposarcoma (WDLPS) component is the most specific feature but is not identified in all cases. Answer B is incorrect because leiomyosarcomas do not have MDM2 amplification; in addition, they usually demonstrate more extensive staining for at least 2 myogenic markers. Focal staining for desmin is seen in DDLPS with and without myogenic differentiation. Answer D is incorrect because solitary fibrous tumors are characterized by NAB2::STAT6 fusions and do not have MDM2 amplifications. Focal staining for STAT6 may be related to the close proximity of the STAT6 gene (12q13) to the region amplified in WDLPS / DDLPS (12q13-15). Answer E is incorrect because undifferentiated pleomorphic sarcoma is a diagnosis of exclusion and therefore should be negative for MDM2 amplification. Answer C is incorrect because the lack of any significant clinical history or other imaging findings, together with the typical location and molecular alteration, makes DDLPS the most likely diagnosis in this case.
Comment Here
Reference: Liposarcoma
Comment Here
Reference: Liposarcoma