Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Tessier-Cloutier B. Undifferentiated / dedifferentiated carcinoma (endometrium / ovary). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusundifferentiated.html. Accessed May 12th, 2024.
Definition / general
- This topic covers tumors of the endometrium and ovary
- Undifferentiated carcinomas are characterized by a patternless, sheet-like growth of discohesive cells with an aggressive clinical course (Am J Surg Pathol 2005;29:1316, Mod Pathol 2010;23:781, J Pathol Clin Res 2021;7:144)
- Tumors are often associated with mismatch repair and SWI / SNF protein deficiency (Mod Pathol 2016;29:302, Histopathology 2016;69:560, Mod Pathol 2016;29:1586)
- If areas of a differentiated carcinoma are found, the tumor is called dedifferentiated carcinoma (Int J Gynecol Pathol 2006;25:52)
Essential features
- At least focal sheet-like growth pattern with lack of glandular or papillary differentiation
- High mitotic and proliferation indices
- Absence or significantly diminished expression of markers of differentiation (e.g. CK7, PAX8, ER and claudin4)
- Presence of a differentiated component (usually low grade endometrioid adenocarcinoma) is necessary for the diagnosis of dedifferentiated carcinoma
- Loss of a core SWI / SNF protein is helpful in confirming the diagnosis but not essential to make the diagnosis
ICD coding
- ICD-O:
- ICD-11:
- 2C73.Y & XH1YY4 - other specified malignant neoplasms of the ovary & carcinoma, undifferentiated, NOS
- 2C73.Y & XH5R16 - other specified malignant neoplasms of the ovary & dedifferentiated carcinoma
- 2C76.Y & XH1YY4 - other specified malignant neoplasms of the corpus uteri & carcinoma, undifferentiated, NOS
- 2C76.Y & XH5R16 - other specified malignant neoplasms of the corpus uteri & dedifferentiated carcinoma
Epidemiology
- Median age is 55 years; range 21 - 76 years (Mod Pathol 2010;23:781)
- 2% of endometrial carcinomas (Mod Pathol 2010;23:781)
- 0.5% of ovarian carcinomas (Int J Gynecol Pathol 2010;29:203)
Sites
- Endometrium
- Ovary
Pathophysiology
- Likely evolves from a differentiated endometrial or ovarian carcinoma secondary to disrupted epigenetic modulation
- Inactivation of the SWI / SNF chromatin remodeling complex is common
- Often associated with mismatch repair deficiency
Etiology
- No associated environmental risk factors
Clinical features
- Usually presents at advanced stage
- Abdominal pain and swelling
Diagnosis
- There are no established tests to screen for endometrial and ovarian malignancies where the vast majority of undifferentiated and dedifferentiated carcinomas occur
- When clinical suspicion arises, abdominal ultrasound and computed tomography scans are useful adjunct
- Definitive diagnosis requires biopsy tissue
Radiology description
- Large, solid adnexal mass with variable hemorrhage and necrosis
Prognostic factors
- Undifferentiated and dedifferentiated tumors have a poor prognosis (Int J Gynecol Pathol 2006;25:52, Mod Pathol 2010;23:781, J Pathol Clin Res 2021;7:144)
- Presence of undifferentiated component in a differentiated tumor carries similar prognosis as pure undifferentiated carcinoma
Case reports
- 54 year old woman with dedifferentiated carcinoma of the ovary (Cesk Patol Spring 2018;54:33)
- 55 year old woman with BRG1 deficient dedifferentiated endometrioid adenocarcinoma of the ovary (Pathology 2016;48:82)
- 63 year old postmenopausal woman with an abdominal mass (Int J Clin Exp Pathol 2014;7:4422)
- 73 year old woman with ovarian undifferentiated carcinoma with voluminous mesenteric presentation (Int J Surg Case Rep 2012;3:551)
Treatment
- Primary ovarian tumor treated primarily with chemotherapy followed by surgery
- Total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH BSO) with or without lymphadenectomy
- Emerging evidence supports that SWI / SNF deficient undifferentiated and dedifferentiated carcinoma does not respond to the conventional platinum based regimens (J Pathol Clin Res 2021;7:144)
Gross description
- Large, solid mass with extensive tumor necrosis
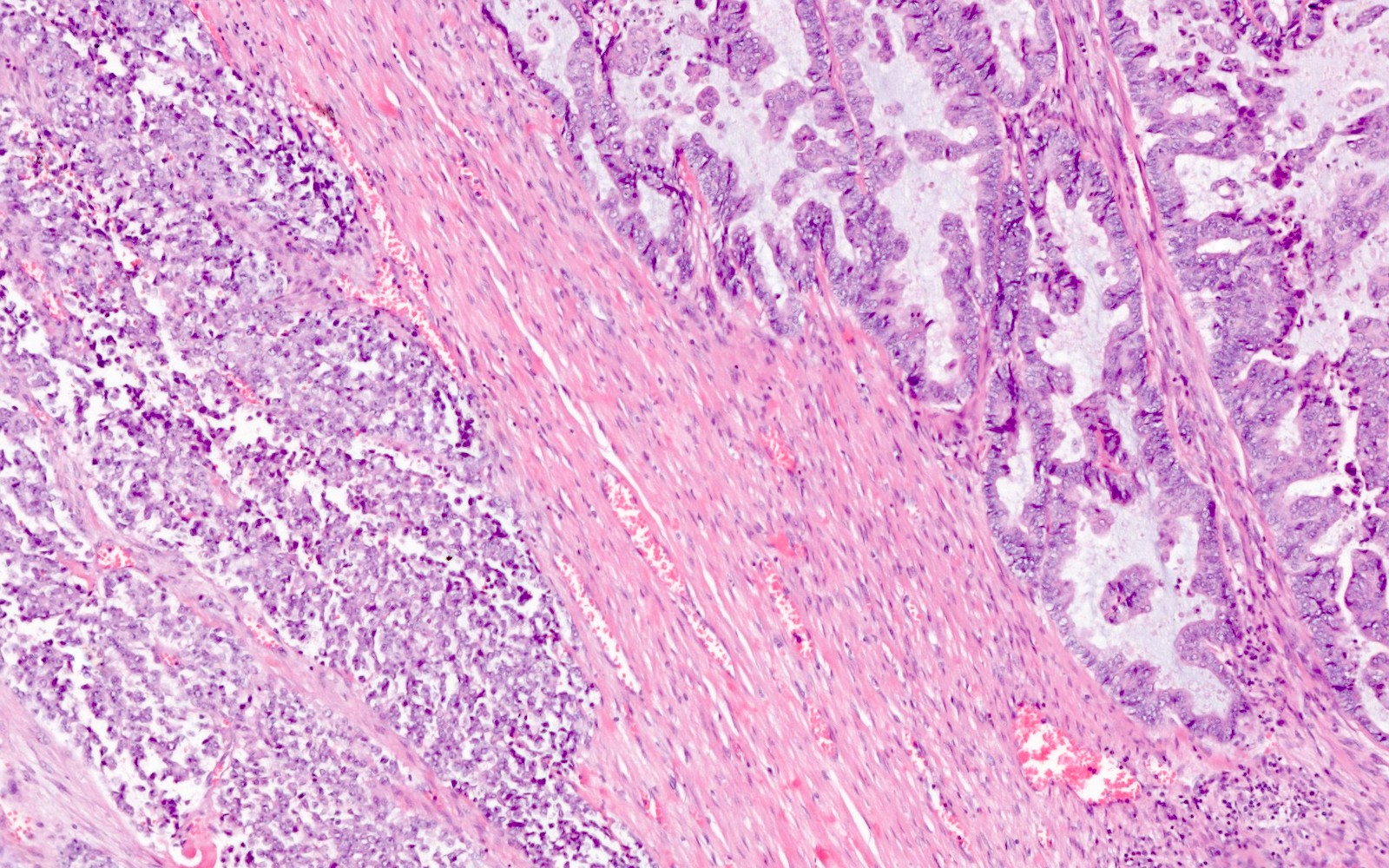
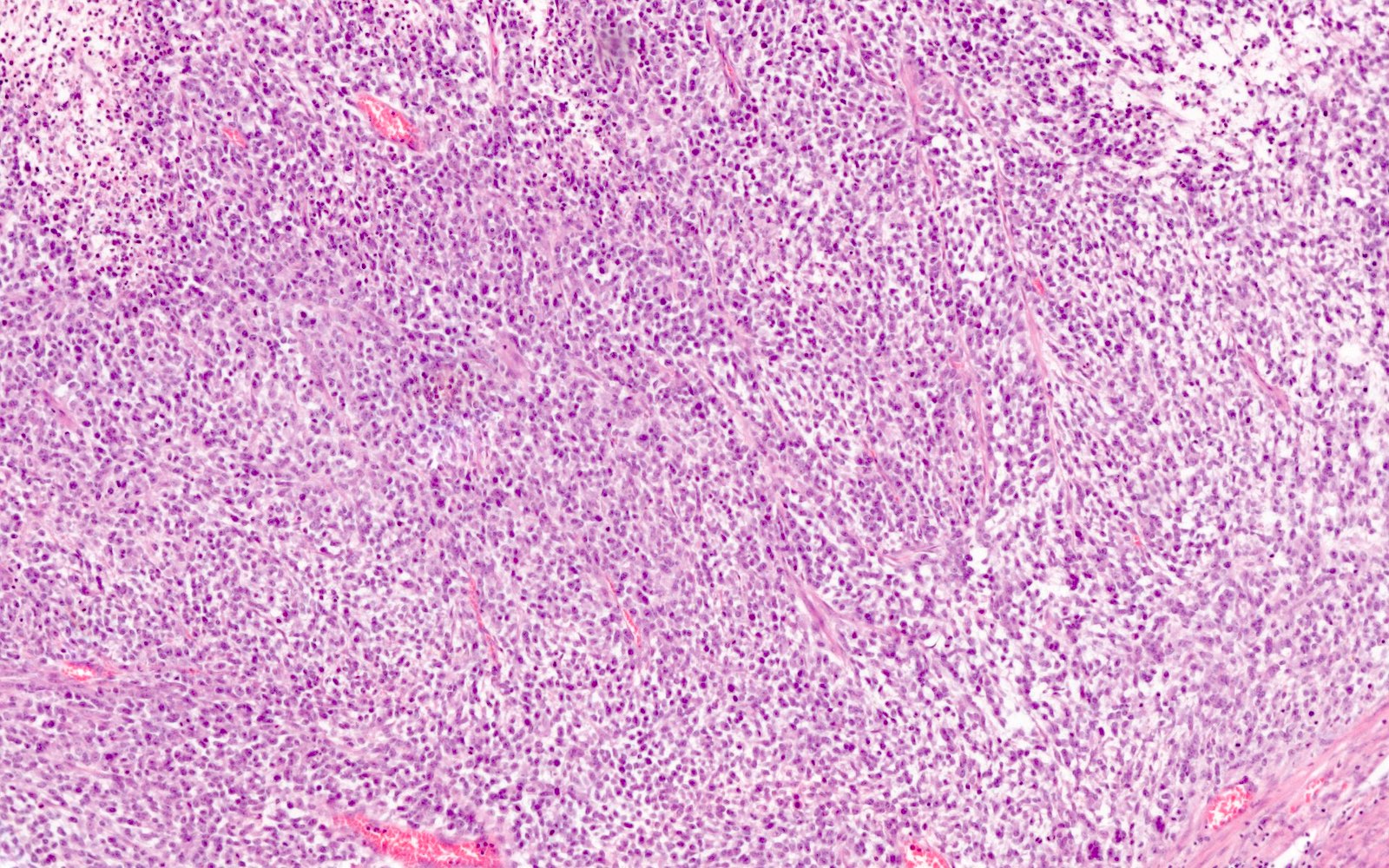
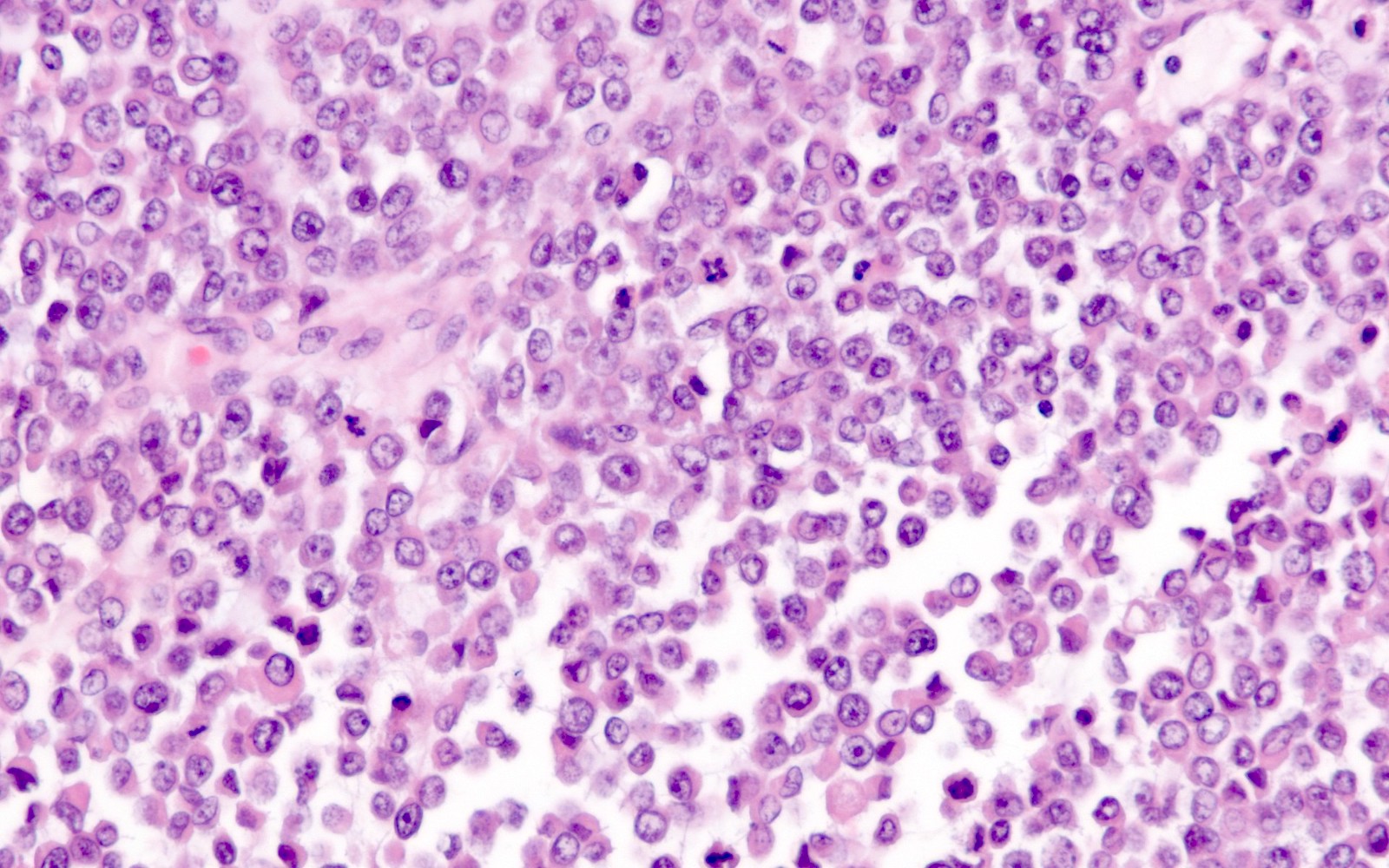
Microscopic (histologic) description
- Undifferentiated component:
- Diffuse, sheet-like growth pattern with lack of glandular or papillary architecture (occasionally glandular or papillary structures from the differentiated component may be entrapped)
- Comprised of a proliferation of monomorphic, medium sized cells
- Abrupt keratinization may be seen
- Cells frequently show discohesion (at least focally)
- Variable amount of cytoplasm, sometimes with rhabdoid features
- Brisk mitoses
- Necrosis frequently present
- Some cases may show focal spindling and myxoid changes
- Differentiated component:
- Typically low grade endometrioid adenocarcinoma
- Rarely high grade endometrioid adenocarcinoma, high grade serous carcinoma or clear cell carcinoma
Microscopic (histologic) images
Positive stains
- In the differentiated component (if present): CK7, PAX8, ER / PR, E-cadherin
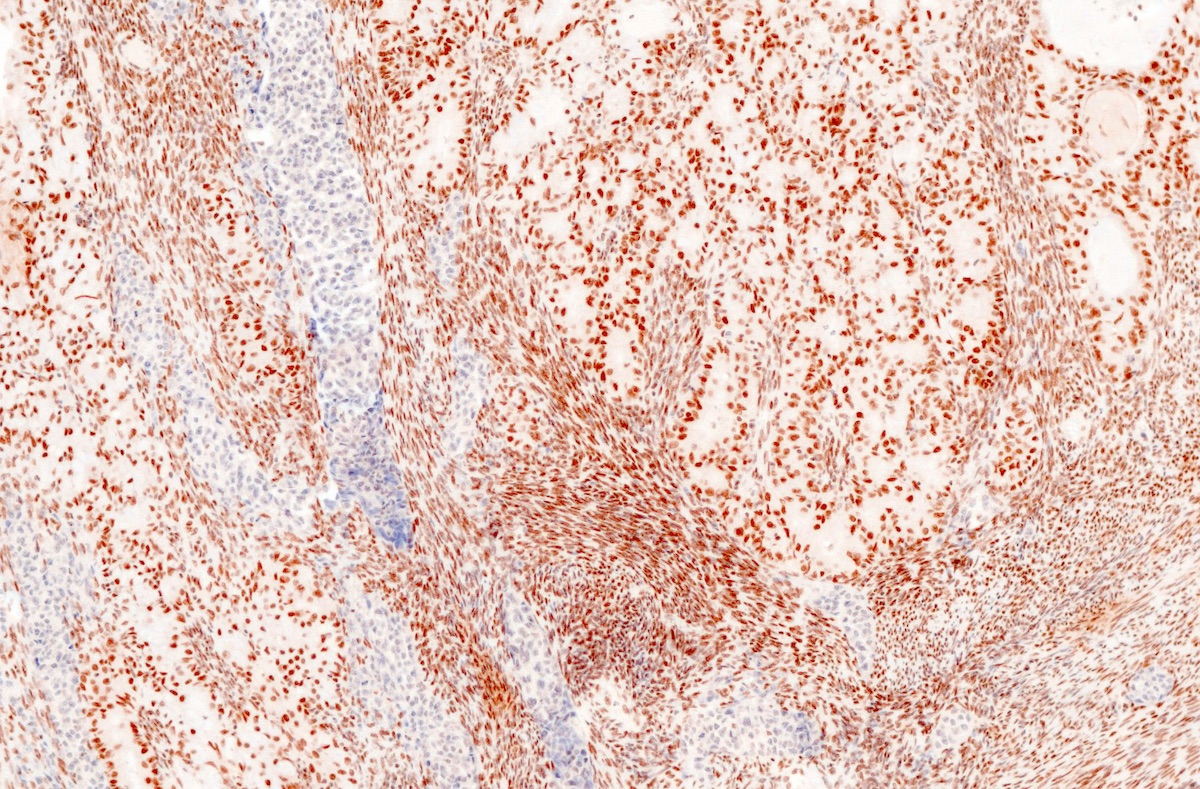
- In the undifferentiated component:
- Elevated Ki67 index
- EMA, keratins, CK18 or PAX8, ER / PR may be focally expressed but can be completely absent (Mod Pathol 2010;23:781)
- Neuroendocrine markers (chromogranin, INSM1, synaptophysin and CD56) are frequently expressed; most cases show only focal expression in 1 or 2 neuroendocrine markers
Negative stains
- In the differentiated component (if present):
- p53 wild type pattern (patchy negative to intermediate nuclear staining)
- Uncommonly the differentiated component may be high grade, which can be associated with a mutant p53 IHC pattern
- p16 (patchy negative to weak nuclear and cytoplasmic staining)
- Uncommonly the differentiated component may be high grade, which can be associated with a diffuse p16 IHC pattern
- Mismatch repair deficiency (MLH1, PMS2, MSH2, MSH6) is common (~50%)
- p53 wild type pattern (patchy negative to intermediate nuclear staining)
- In the undifferentiated component:
- CK7, PAX8, ER, WT1, claudin4 (may have weak or focal expression)
- SWI / SNF complex loss of protein expression (BRG1, INI1 or co-loss of ARID1A and ARID1B)
- Usually p53 wild type (negative to patchy intermediate nuclear staining)
- p16 (negative to patchy weak nuclear and cytoplasmic staining)
- Mismatch repair deficiency (MLH1, PMS2, MSH2, MSH6) is common (~50%)
Molecular / cytogenetics description
- Commonly shows an inactivating mutation in one of the core SWI / SNF complex genes, resulting in the loss of expression of BRG1 (SMARCA4 gene), INI1 (SMARCB1 gene) or co-loss of ARID1A and ARID1B
- Most cases are associated with mismatch repair deficiency and of those with MLH1 deficiency, almost all show MLH1 promoter hypermethylation
- Mutations in other genes associated with endometrioid carcinoma, such as PTEN, PIK3CA, KRAS and CTNNB1, are often reported
Sample pathology report
- Uterus, fallopian tubes and ovaries, hysterectomy and bilateral salpingo-oophorectomy:
- Endometrial dedifferentiated carcinoma (see synoptic report)
- Associated with an endometrioid adenocarcinoma FIGO grade 1
- Mismatch repair status: abnormal (MLH1 lost)
- Uterus, fallopian tubes and ovaries, hysterectomy and bilateral salpingo-oophorectomy:
- Undifferentiated carcinoma of the ovary (see synoptic report)
- Mismatch repair status: abnormal (MLH1 lost)
Differential diagnosis
- Endometrial undifferentiated and dedifferentiated carcinoma:
- Endometrial serous carcinoma, solid variant:
- Tumor cells have a cohesive growth pattern
- Mutated p53 IHC pattern
- Intact SWI / SNF immunohistochemistry
- High grade endometrial endometrioid adenocarcinoma:
- Tumor cells have a cohesive growth pattern
- Intact SWI / SNF immunohistochemistry
- May show isolated ARID1A loss of expression
- Carcinosarcoma of the endometrium (malignant mixed Müllerian tumor):
- Homologous or heterologous sarcoma
- Frequently mutated p53 IHC pattern
- SMARCA4 deficient uterine sarcoma:
- Endometrial small cell neuroendocrine carcinoma:
- Neuroendocrine nuclear features (fine chromatin, nuclear molding)
- Diffuse expression of 2 or more neuroendocrine markers (chromogranin, synaptophysin, INSM1, CD56)
- Intact SWI / SNF immunohistochemistry
- Lymphoma:
- CD45 positive
- Intact SWI / SNF immunohistochemistry
- Endometrial serous carcinoma, solid variant:
- Ovarian undifferentiated and dedifferentiated carcinoma:
- High grade serous carcinoma of the ovary, solid variant:
- Tumor cells have a cohesive growth pattern
- Mutated p53 IHC pattern
- Intact SWI / SNF immunohistochemistry
- High grade endometrioid adenocarcinoma of the ovary:
- Tumor cells have a cohesive growth pattern
- Intact SWI / SNF immunohistochemistry
- May show isolated ARID1A loss of expression
- Carcinosarcoma of the ovary (malignant mixed Müllerian tumor):
- Homologous or heterologous sarcoma
- Frequently mutated p53 IHC pattern
- Small cell carcinoma of the ovary hypercalcemic type:
- Small cell neuroendocrine carcinoma (small cell carcinoma of the ovary, pulmonary type):
- Neuroendocrine nuclear features (fine chromatin, nuclear molding)
- Diffuse expression of 2 or more neuroendocrine markers (chromogranin, synaptophysin, INSM1, CD56)
- Intact SWI / SNF immunohistochemistry
- Lymphoma:
- CD45 positive
- Intact SWI / SNF immunohistochemistry
- High grade serous carcinoma of the ovary, solid variant:
Additional references
Board review style question #1
This is a representative image from a solitary ovarian tumor in a 75 year old woman. The tumor shows absent INI1 immunohistochemistry expression. What is the most likely diagnosis?
- Carcinosarcoma
- High grade serous carcinoma of the ovary
- Primary diffuse large B cell lymphoma of the ovary
- Small cell carcinoma of the ovary hypercalcemic type
- Undifferentiated carcinoma of the ovary
Board review style answer #1
E. Undifferentiated carcinoma of the ovary
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Board review style question #2
Board review style answer #2
C. Endometrial dedifferentiated carcinoma
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)
Comment Here
Reference: Undifferentiated / dedifferentiated carcinoma (endometrium / ovary)