Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Bárcena C, de Leval L. CHL mixed cellularity. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomanonBmixed.html. Accessed April 26th, 2024.
Definition / general
- Subtype of classic Hodgkin lymphoma (CHL) with Hodgkin / Reed-Sternberg (HRS) cells in a nonneoplastic mixed inflammatory background (Blood 2022;140:1229, Leukemia 2022;36:1720)
Essential features
- B cell lymphoma derived from germinal center B cells
- HRS cells in a reactive mixed inflammatory microenvironment consisting of small lymphocytes, eosinophils, plasma cells, neutrophils and histiocytes
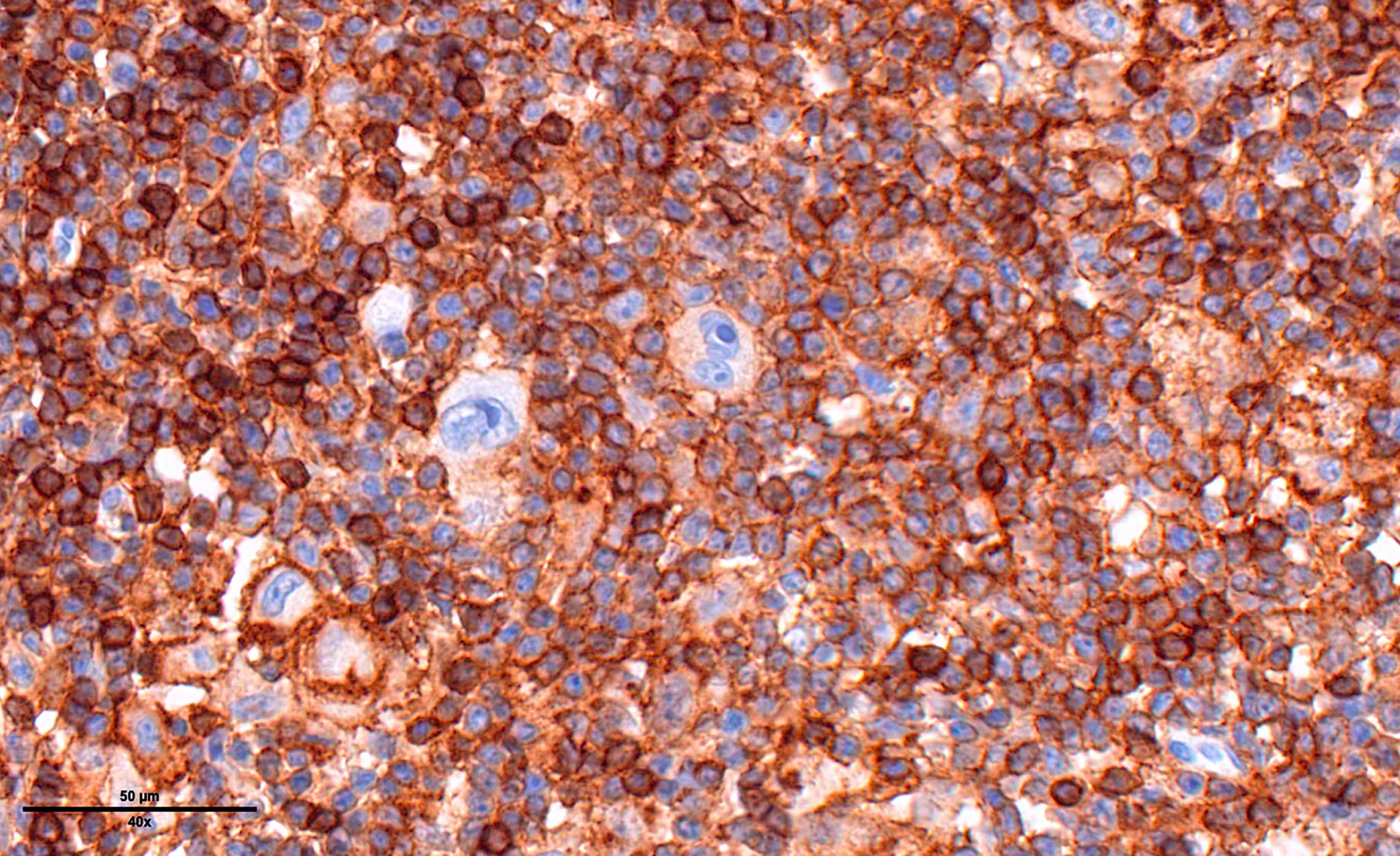
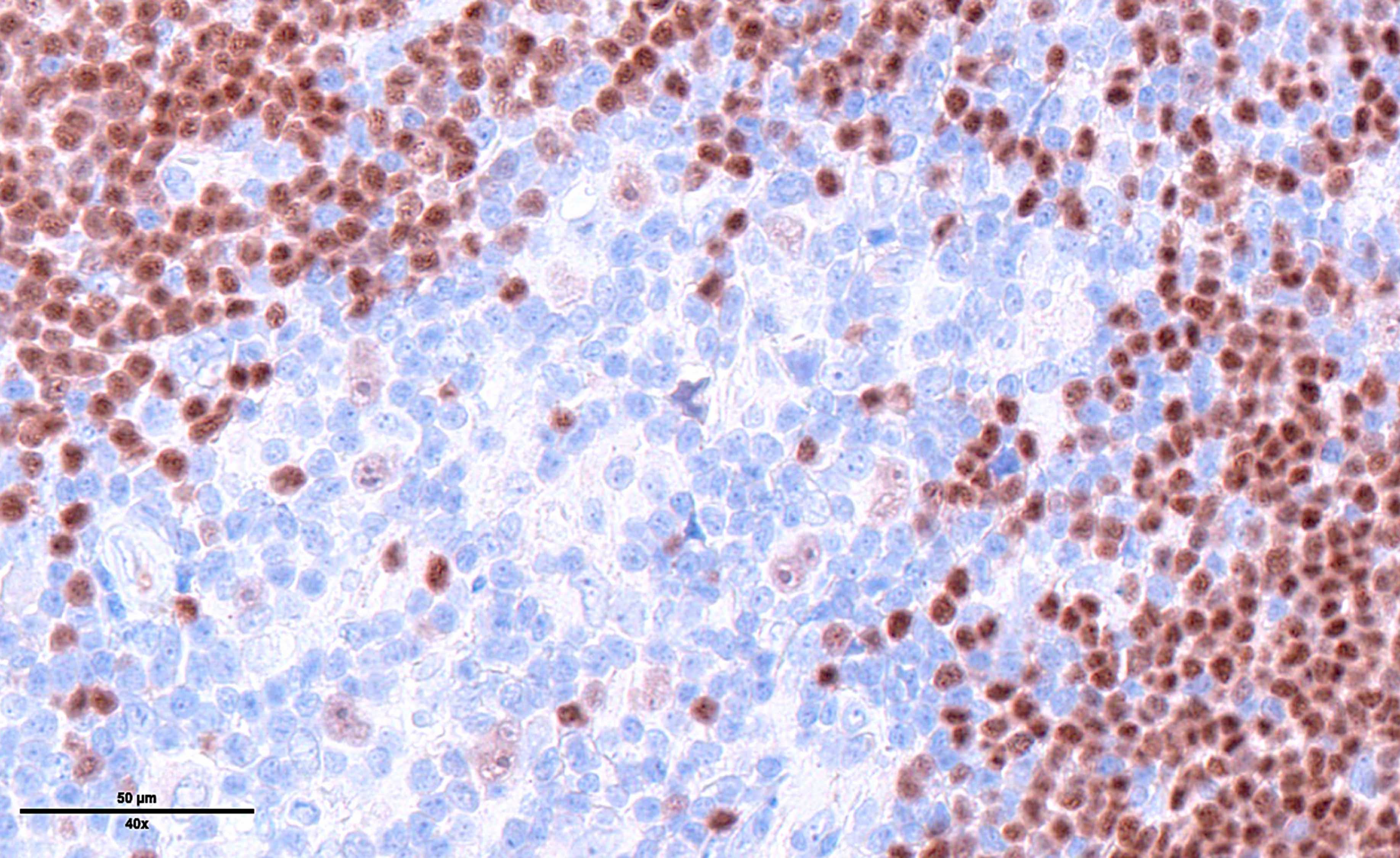
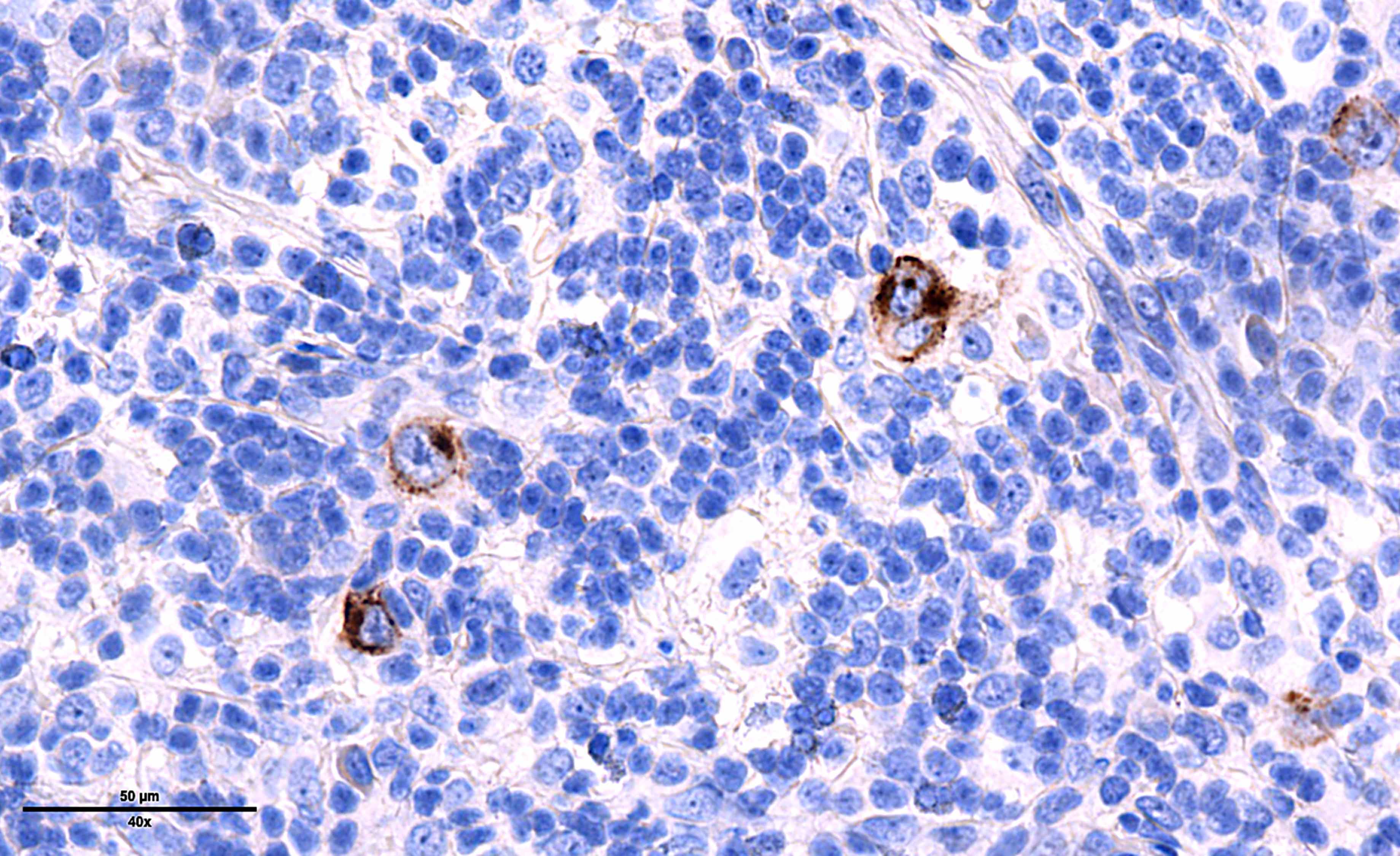
- Immunophenotype of HRS cells: PAX5+ (dim), CD30+, CD15 variable, CD20 variable
- EBER+ in 75% of cases
ICD coding
Epidemiology
- Second most common subtype of classic Hodgkin lymphoma
- Accounts for 20 - 25% of classic Hodgkin lymphoma
- Bimodal incidence: pediatric population and elderly patients
- M > F
- More common in patients with immunodeficiency and in developing countries (Expert Rev Hematol 2021;14:547)
- EBV infection in 75% of cases; virus is located within the HRS cells and usually shows a type II latency pattern of infection (N Engl J Med 1989;320:502)
Sites
- Lymph nodes (70%) > spleen (30%) > bone marrow (10%) > liver (3%) > other organs (1 - 3%) (Leuk Lymphoma 2019;60:3426)
- Rarely limited to a single region and is more likely to involve extralymphatic tissues compared to other classic Hodgkin lymphoma subtypes
- Mediastinal involvement is rare
Pathophysiology
- Pathophysiology incompletely understood
- Hodgkin / Reed-Sternberg cells derived from preapoptotic germinal center B cells with a disrupted B cell program (Leukemia 2021;35:968)
- HRS harbor monoclonal IgH rearrangements with somatic mutations of their immunoglobulin (Ig) variable region genes, losing the capacity of Ig expression (Leukemia 2021;35:968)
- Genetic lesions that promote HRS cell proliferation, survival, immune evasion and interaction with their microenvironment, including alterations in NFκB and JAK-STAT signaling pathways (Leukemia 2021;35:968)
- EBV could play a crucial role by infecting and rescuing Hodgkin / Reed-Sternberg cells with a crippled B cell receptor in the germinal centers from apoptosis and thus inducing lymphomagenesis (Blood 2005;106:4339)
- EBV infection activates the NFκB pathway, promoting survival and growth (Histopathology 2021;79:451)
Clinical features
- B symptoms are common
- May be diagnosed in all clinical stages (more commonly with stage II and III disease)
Diagnosis
- Excisional lymph node biopsy or tissue biopsy with ancillary techniques (immunohistochemistry)
Laboratory
- Nonspecific findings: anemia, leukocytosis, lymphocytopenia, elevated C reactive protein (N Engl J Med 1998;339:1506)
Prognostic factors
- About 90 - 95% of patients with early stage CHL and 80 - 85% with advanced stage CHL are cured (Am J Hematol 2020;95:978)
- Treatment is individualized according to defined risk groups, based on the following prognostic factors:
- Ann Arbor stage (most important predictor of outcome) (J Clin Oncol 2014;32:3059)
- Bulky (mediastinal) disease
- Extranodal involvement
- B symptoms
- International Prognostic Score (N Engl J Med 1998;339:1506)
Treatment
- Intensive polychemotherapy (ABVD or BEACOPP), with or without radiotherapy (Am J Hematol 2020;95:978)
- For refractory cases: stem cell transplant, brentuximab vedotin, checkpoint inhibitors (N Engl J Med 2018;378:331)
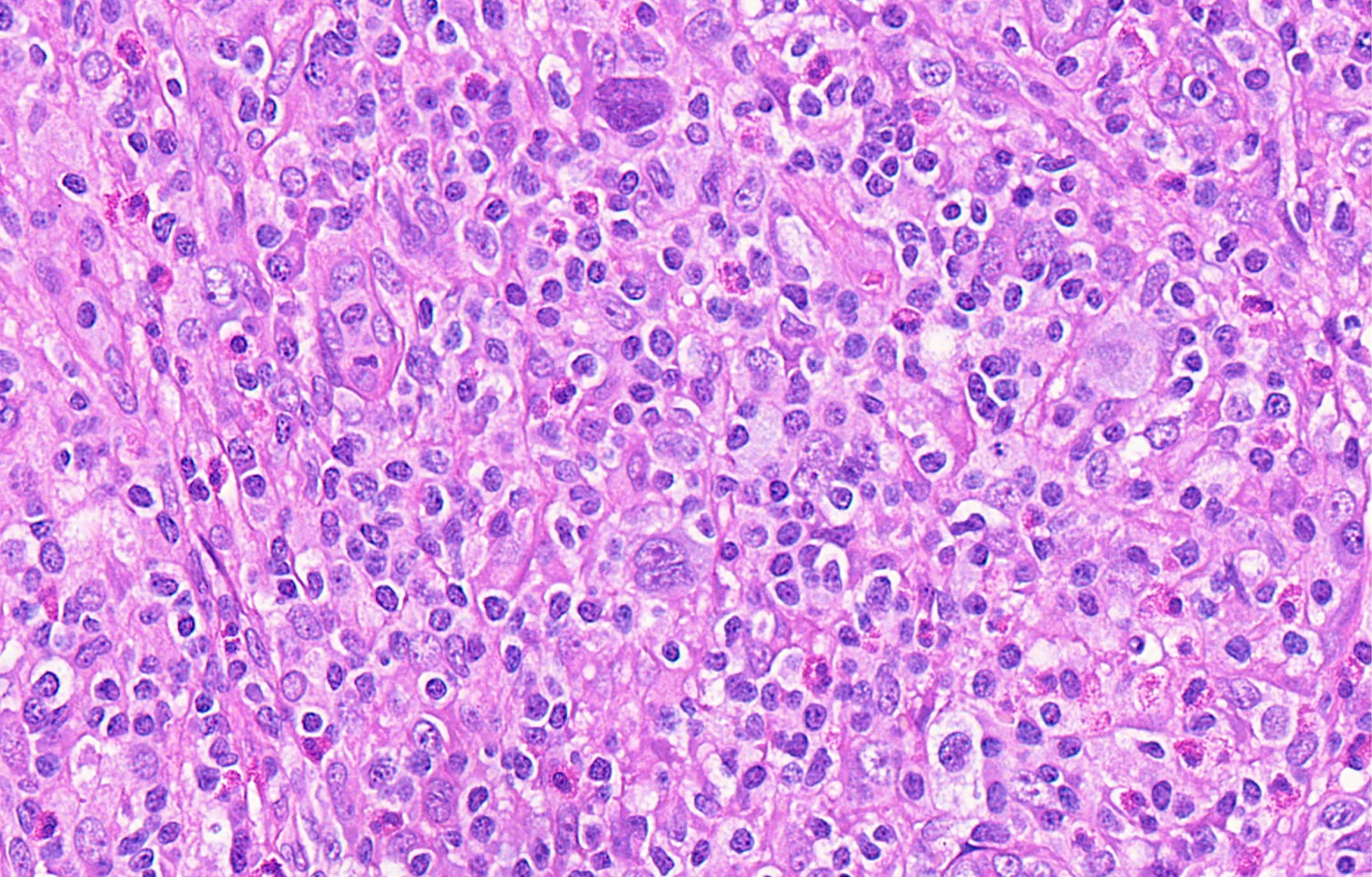
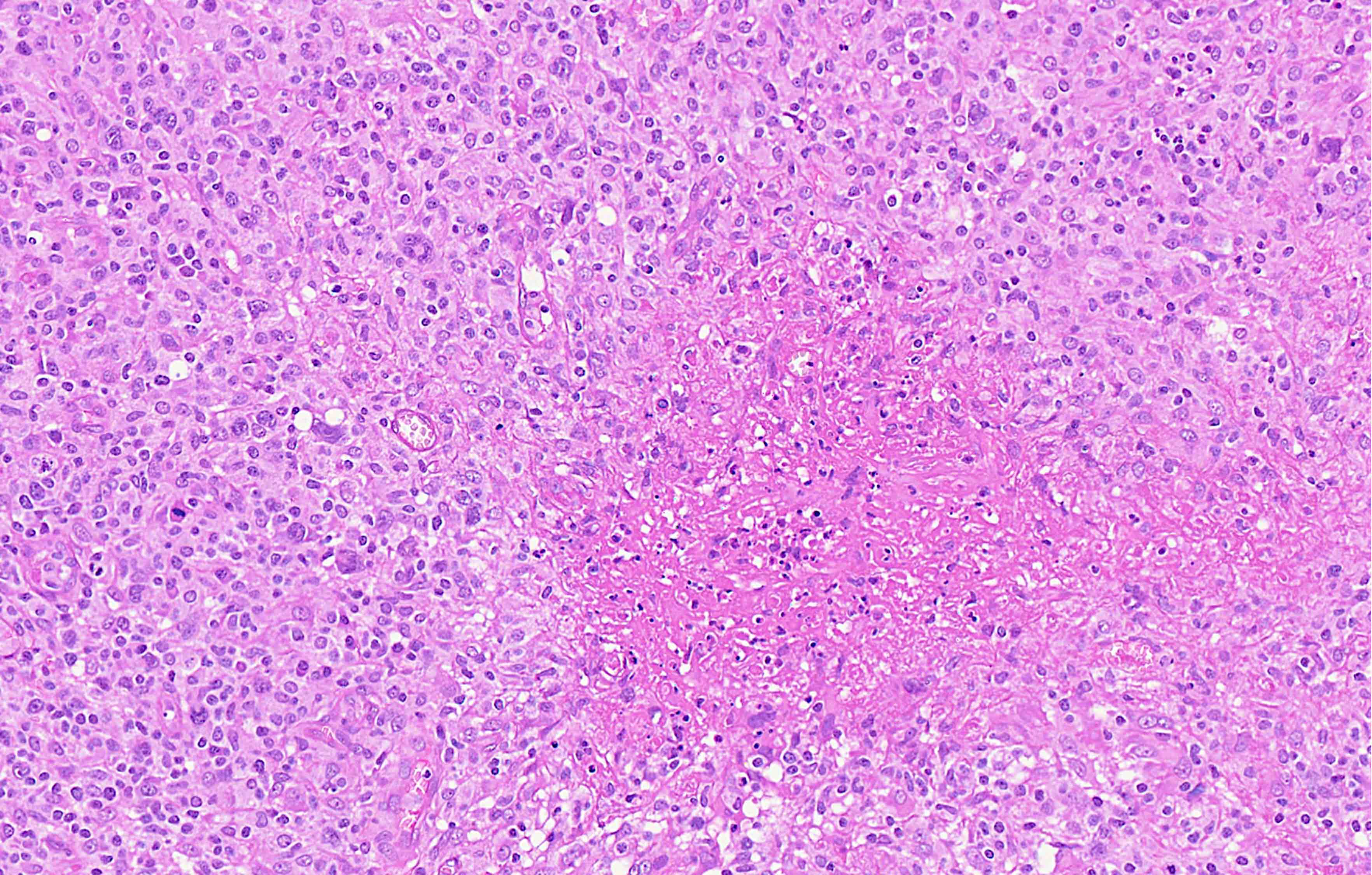
Microscopic (histologic) description
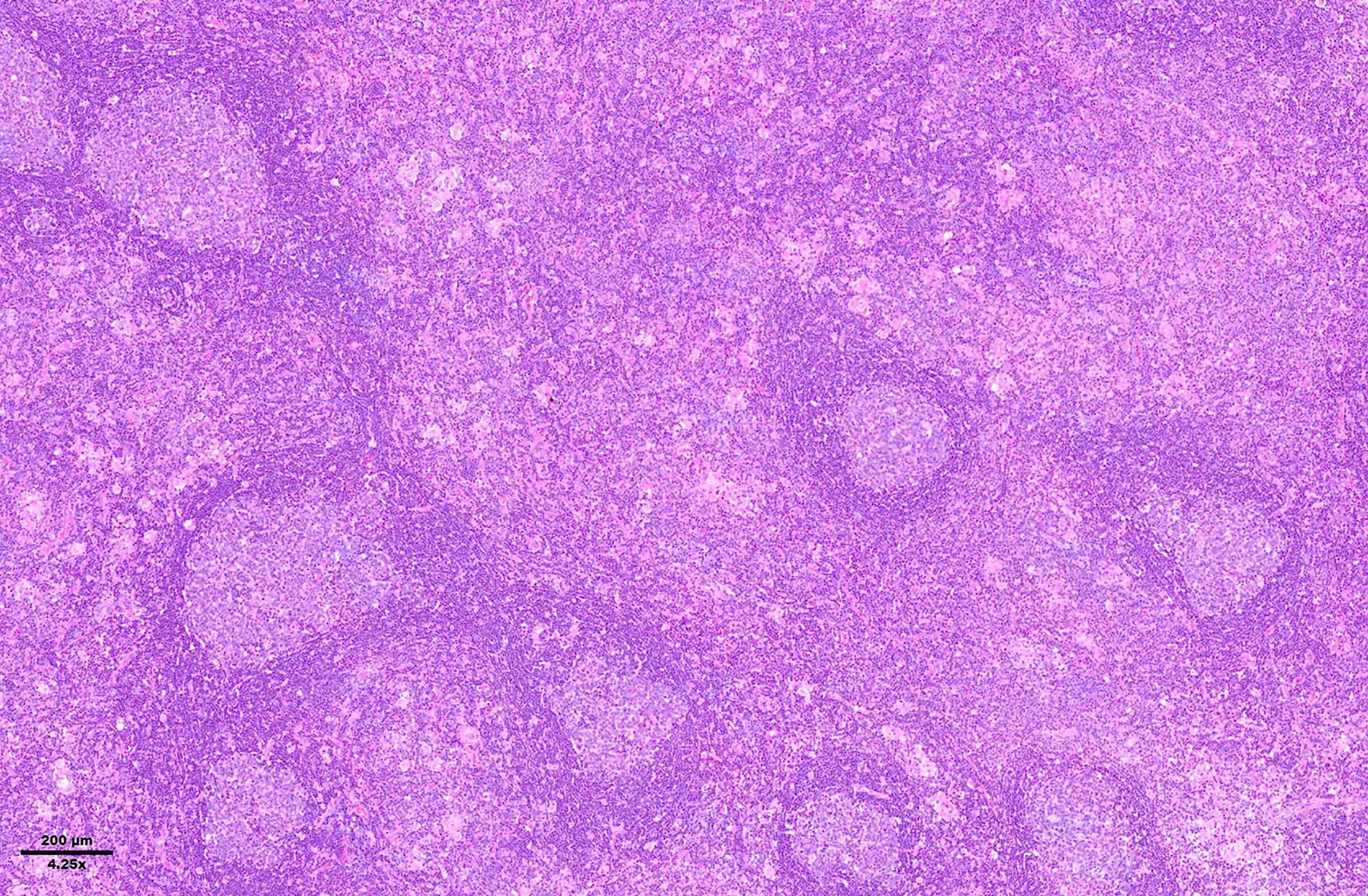
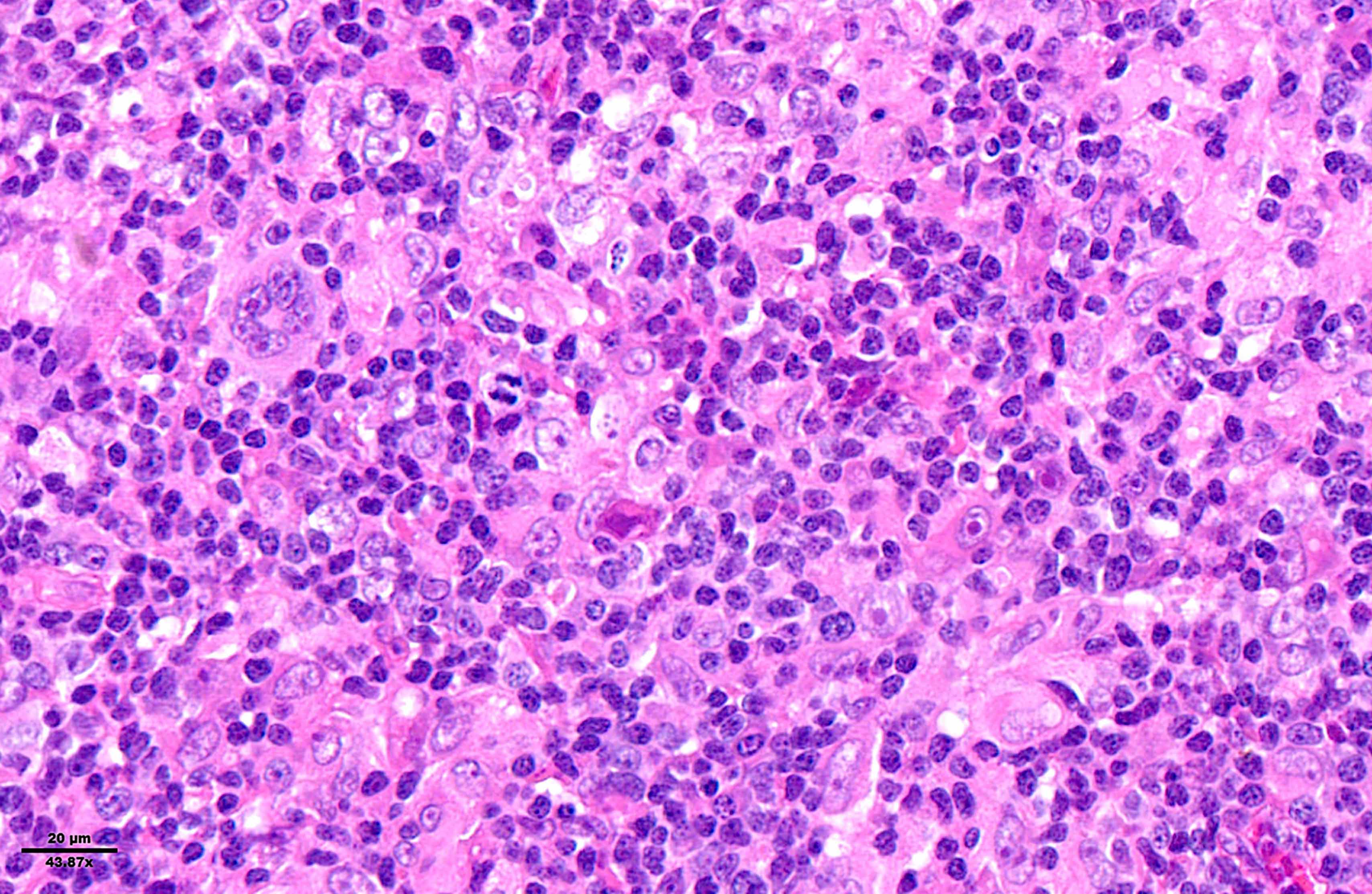
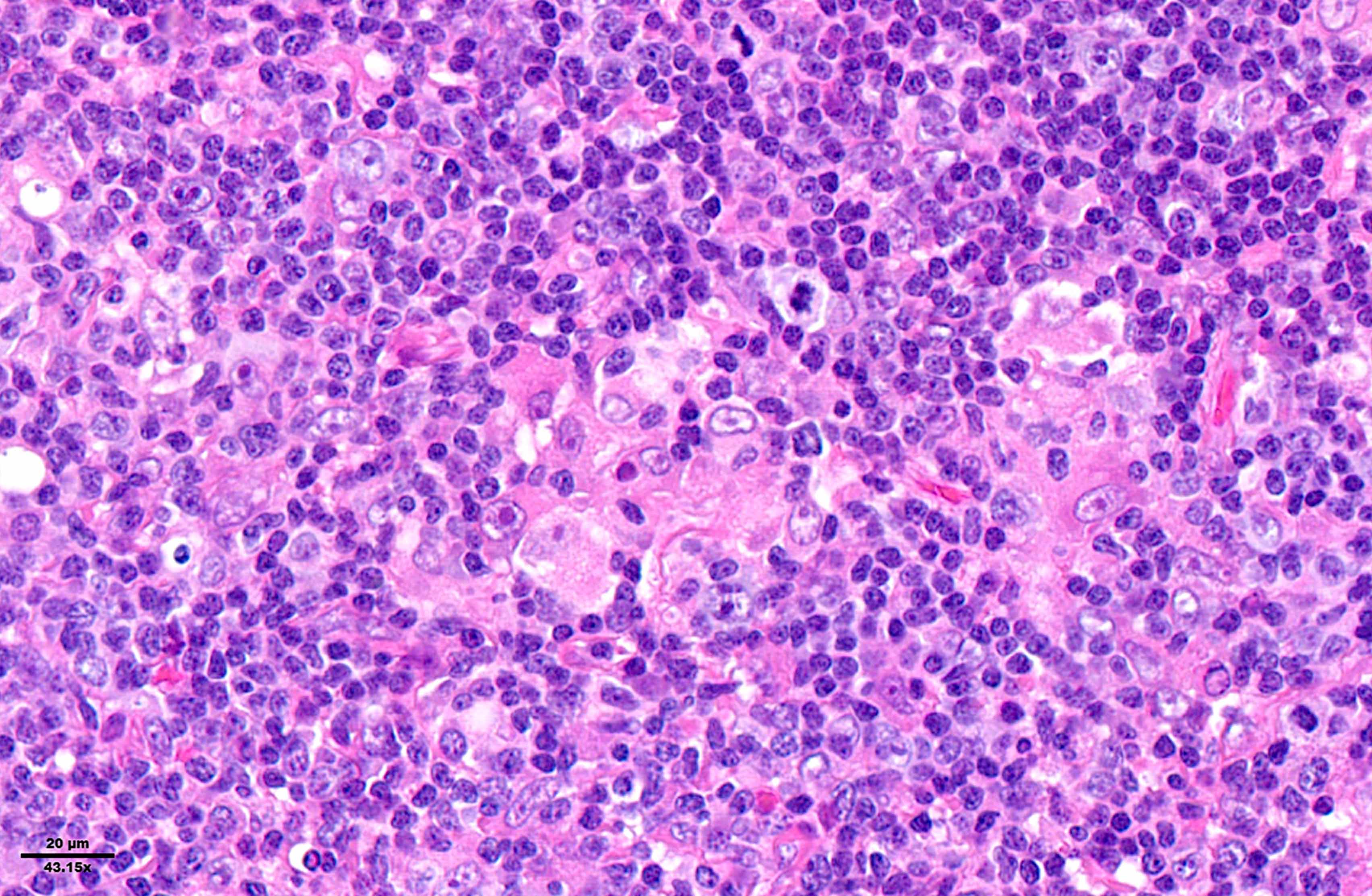
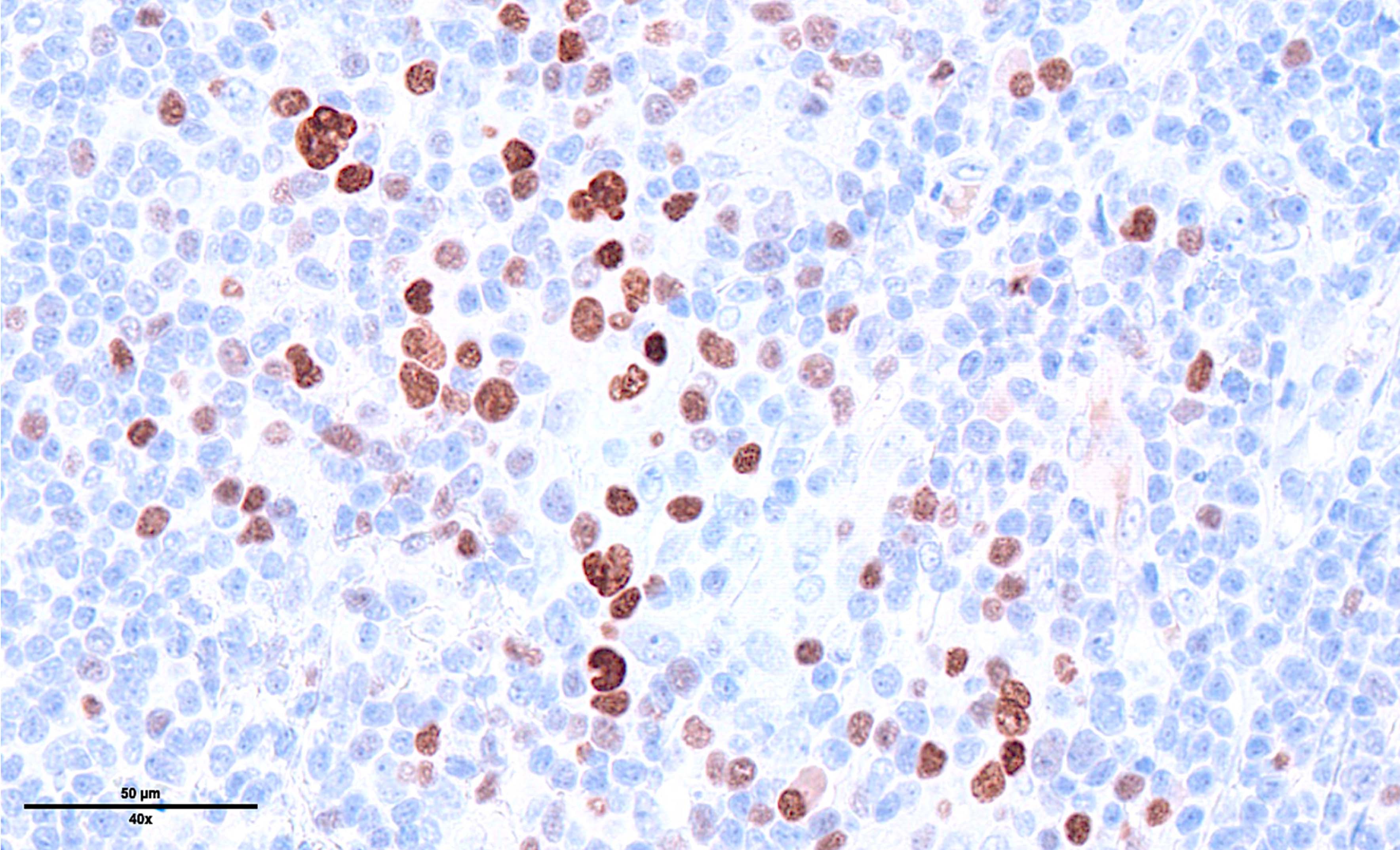
- Diffuse or interfollicular proliferation of HRS cells (< 10% of the cellularity) in a reactive microenvironment composed of lymphocytes, eosinophils, neutrophils, plasma cells, histiocytes, fibroblasts (Leukemia 2022;36:1720)
- CD4+ T cells are the most abundant immune cells in the CHL microenvironment (Cytometry B Clin Cytom 2008;74:1, Blood 2004;103:1755, Lab Invest 2008;88:482)
- In EBV+ cases, there may be numerous epithelioid histiocytes and even granulomas
- Fine interstitial fibrosis may be seen but without collagen broad bands and with no capsular thickening
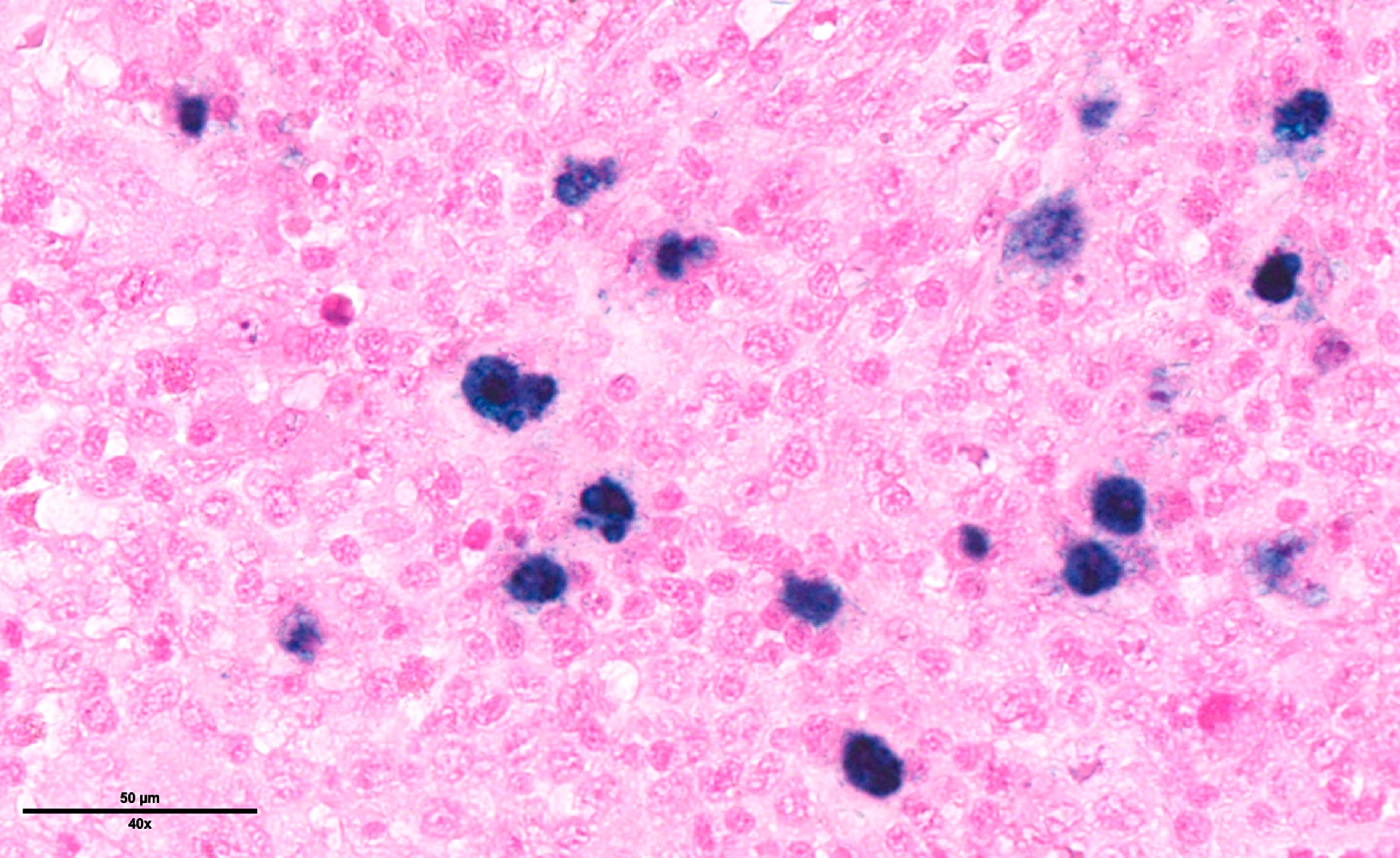
- Bone marrow is involved in 10% of cases and shows scattered HRS cells in a background that is rich in histiocytes with ill defined granulomas merging with inflammatory cells and normal hematopoiesis
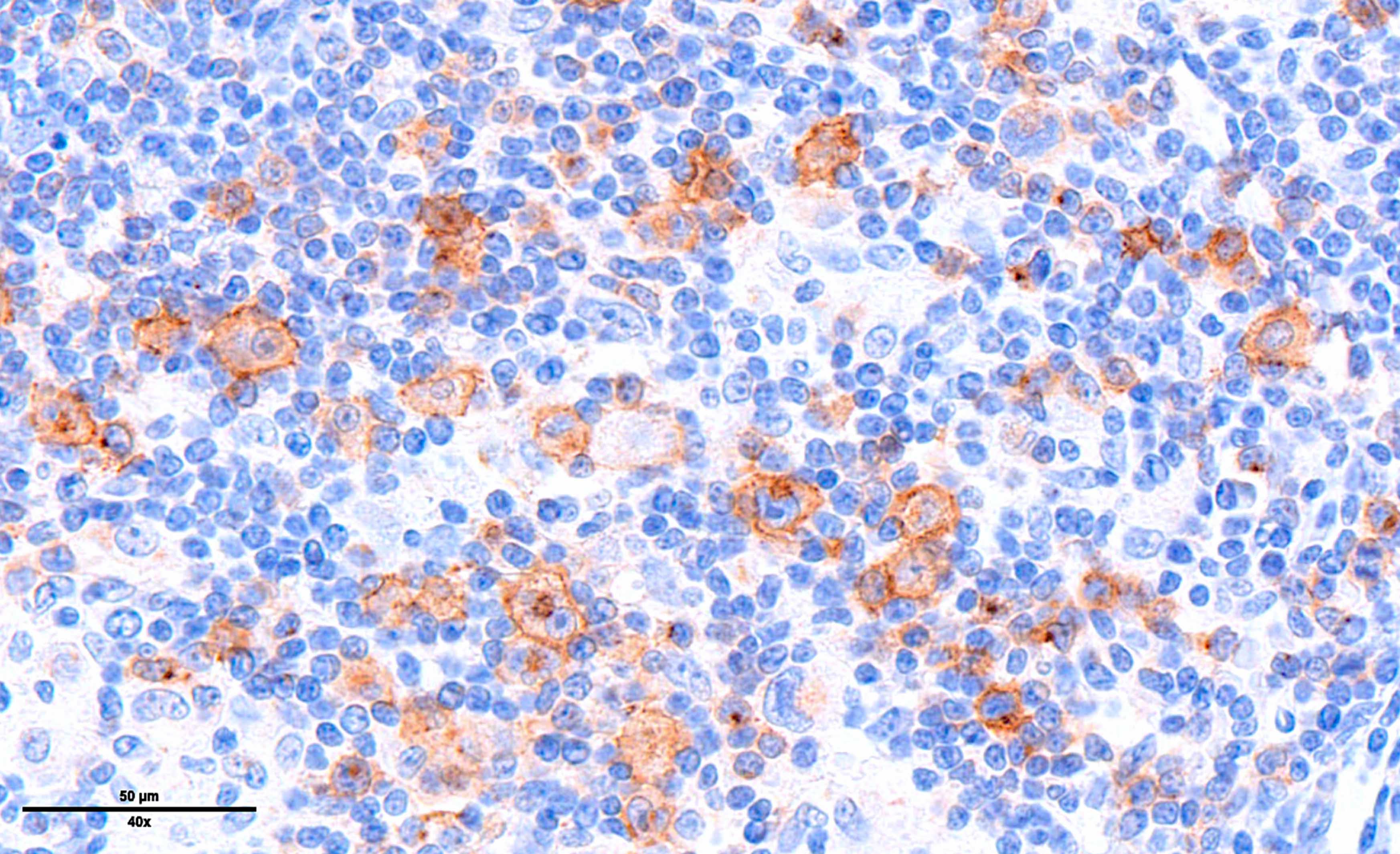
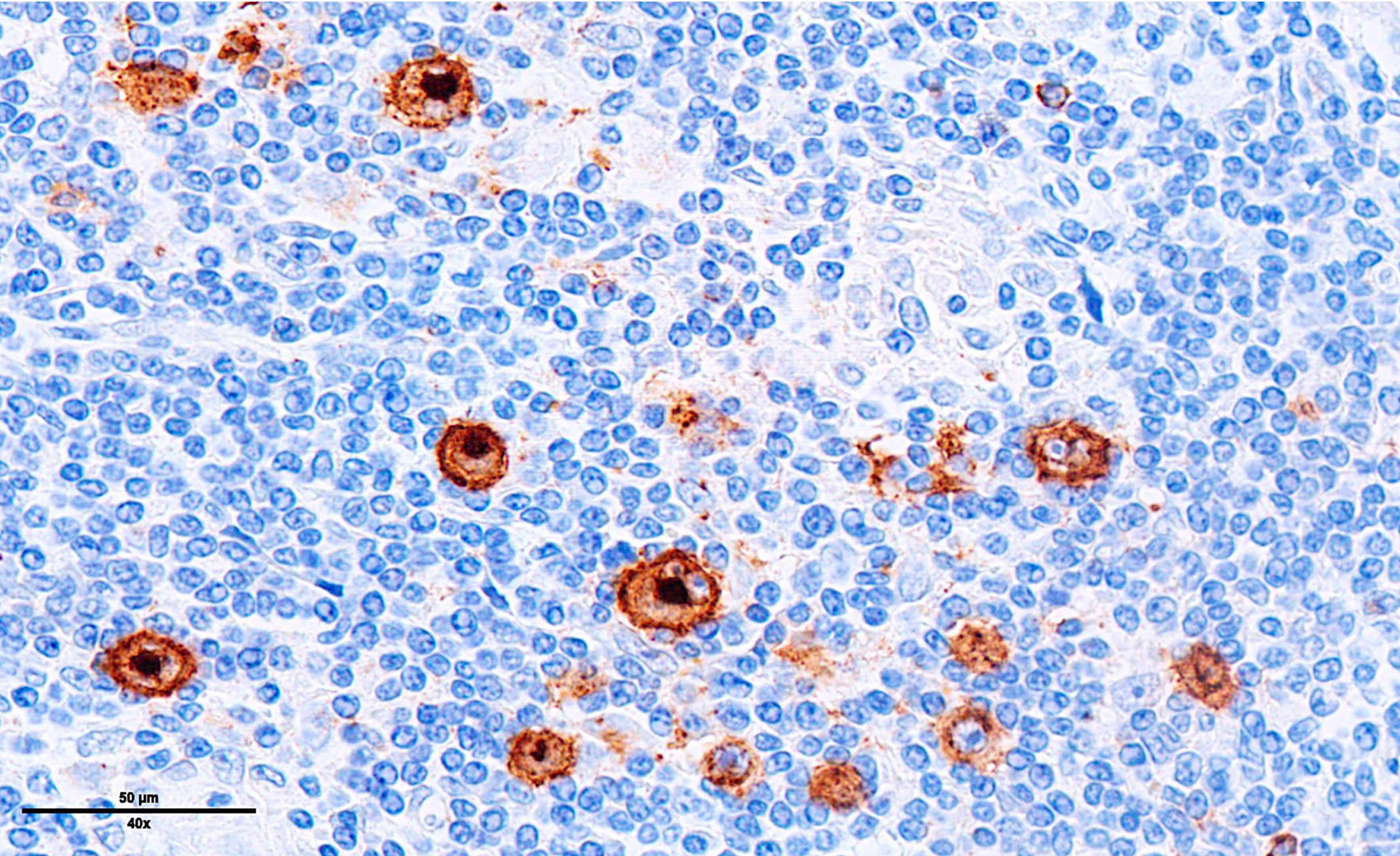
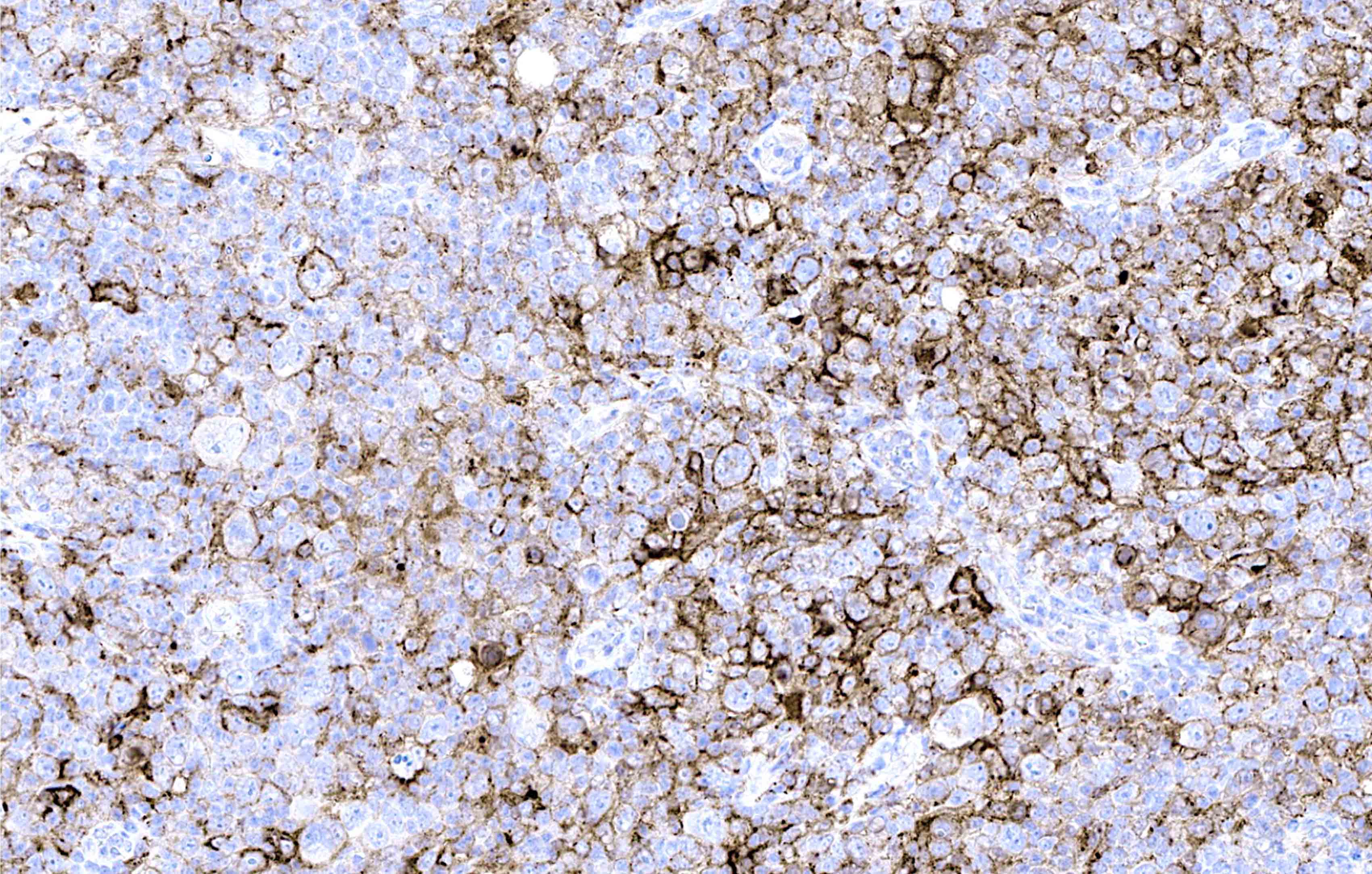
Microscopic (histologic) images
Contributed by Laurence de Leval, M.D., Ph.D. and Carmen Bárcena, M.D.
Positive stains
- PAX5 (dim), CD30 (strong and uniform), CD15 variable (75% of cases), MUM1 (Ann Diagn Pathol 2019;39:105)
- STAT6YE361, PDL1, PDL2 (Histopathology 2018;72:1156, Mod Pathol 2020;33:834, Blood 2018;131:68)
- EBV positive cases: EBV LMP1 variable, EBER
Negative stains
- Variable: CD20 (weak), CD79a, OCT2, BOB1 (Leukemia 2022;36:1720, J Pathol 2004;202:60)
- BCL2, BCL6 (Leukemia 2022;36:1720)
- CD45, ALK, EMA, CD23, EBNA2 (Int J Surg Pathol 2010;18:121, Ann Diagn Pathol 2019;40:72, Proc Natl Acad Sci U S A 1991;88:4766)
- Immunoglobulins, light and heavy chains, are negative (Leukemia 2022;36:1720)
Molecular / cytogenetics description
- Aneuploidy is seen in nearly all cases (Blood 1995;86:1464, Cancer Res 2006;66:10332, Cancers (Basel) 2018;10:91)
- High constitutive activity of the NFκB pathway is the hallmark of HRS cells, which is essential for HRS survival (Semin Cancer Biol 2016;39:32)
- Mutations in members of the JAK-STAT signaling pathway, main mediator of cytokine signaling (Nat Genet 2014;46:329)
- Mutations in ITPKB, PI3K-AKT and MAP-ERK pathways (Leukemia 2020;34:151)
- Lesions in genes involved in immune evasion of HRS: PDL1, PDL2, B2M, CIITA, CD58 (Blood 2010;116:3268, J Clin Oncol 2016;34:2690)
- Mutations in additional genes: ARID1A, TNFRS14, XPO1 (Leukemia 2021;35:968)
Sample pathology report
- Lymph node, right neck, excision:
- Mixed cellularity classic Hodgkin lymphoma (see comment)
- Comment: H&E stained sections show a lymph node with architectural effacement by an interfollicular mixed inflammatory infiltrate comprising small lymphocytes, eosinophils, neutrophils, plasma cells, with scattered admixed large atypical lymphoid cells. The neoplastic cells have monolobated / bilobated / multilobated nuclei, vesicular chromatin, prominent nucleoli and amphophilic cytoplasm, consistent with Hodgkin and Reed-Sternberg cells.
- Immunophenotypically, the large atypical cells are positive for PAX5 (weak), CD30 (strong), CD15 and MUM1. These cells are negative for CD20 and CD45.
- T cell markers reveal a rich background composed of small lymphocytes.
- In situ hybridization for EBV encoded RNA (EBER) is positive on the large atypical cells.
Differential diagnosis
- EBV+ diffuse large B cell lymphoma, NOS:
- EBV+ mucocutaneous ulcer:
- Lymphomatoid granulomatosis:
- Angiocentric and angiodestructive lymphoproliferation, composed of EBV positive B cells, may include HRS-like cells (CD20+, CD30+, CD15-)
- Pulmonary involvement is mandatory for the diagnosis (Blood 2020;135:1344)
- T cell / histiocytic rich large B cell lymphoma (THRLBCL):
- Posttransplant lymphoproliferative disorder, polymorphic type:
- A spectrum of maturation of lymphoid cells, including small lymphocytes, lymphoplasmocytoid cells, plasma cells, immunoblasts and large HRS-like cells
- HRS-like cells are usually CD45+, CD20+, PAX5+ (strong), CD30+, CD15-
- Most EBV positive cases reveal a type III EBV latency pattern
- Classic Hodgkin lymphoma is uncommon in the posttransplant setting
- Angioimmunoblastic T cell lymphoma (AITL) and other subtypes of follicular helper T cell lymphoma:
- Proliferation of atypical neoplastic T follicular helper (TFH) cells
- Proliferation of high endothelial venules
- Expansion of the follicular dendritic cell meshwork
- HRS-like cells are usually CD20+, CD30+, CD15- (or sometimes CD15+)
- Rosettes of neoplastic TFH cells around HRS-like cells
- Monoclonal T cell receptor gene rearrangement
- Reactive conditions:
- Infectious mononucleosis:
- Lymph node architecture may be partially effaced and may reveal a mottled appearance
- Hyperplasic follicles with prominent germinal centers
- Paracortical expansion with polymorphic lymphoid cells, including immunoblasts, polytypic plasma cells and HRS-like cells (CD30+, CD15-), numerous mitoses and a high proliferative index (Ki67)
- Monocytoid cells and sinus histiocytosis may be present
- Infectious mononucleosis:
Board review style question #1
Board review style answer #1
D. CD45-, PAX5+, CD30+, CD15+. This is the characteristic immunophenotype of Hodgkin / Reed-Sternberg cells. The immunophenotype in answer A is diagnostic of ALK positive anaplastic large cell lymphoma. The lymphocyte predominant (LP) cells in nodular lymphocyte Hodgkin lymphoma (WHO) / nodular lymphocyte predominant B cell lymphoma (ICC) typically express the immunophenotype in answer C. The immunophenotype in answer E is not specific. T cells could show this immunophenotype.
Comment Here
Reference: Mixed cellularity classic Hodgkin lymphoma
Comment Here
Reference: Mixed cellularity classic Hodgkin lymphoma
Board review style question #2
Which subtype of classic Hodgkin lymphoma is most frequently encountered in patients with HIV / AIDS?
- Lymphocyte depleted
- Lymphocyte rich
- Mixed cellularity
- Nodular sclerosis
Board review style answer #2
C. Mixed cellularity. Mixed cellularity classic Hodgkin lymphoma is the most common subtype of CHL associated with immunodeficiency states, including HIV infection / AIDS, immunodeficiency secondary to immunosenescence and immunodeficiency secondary to solid organ transplantation.
Comment Here
Reference: Mixed cellularity classic Hodgkin lymphoma
Comment Here
Reference: Mixed cellularity classic Hodgkin lymphoma