Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Tam I, Rohr BR. Pediatric melanoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticmelanomapediatric.html. Accessed April 26th, 2024.
Definition / general
- Pediatric melanoma is defined as melanoma occurring in patients younger than or equal to 19 years of age (Pediatrics 2013;131:846)
Essential features
- Pediatric melanoma is exceedingly rare with distinct biologic, clinical and histopathologic features compared to adult melanoma (J Eur Acad Dermatol Venereol 2023;37:1758)
- Pediatric melanoma is classified into 3 main categories: conventional melanoma, melanoma arising in congenital melanocytic nevi and Spitz and spitzoid melanoma (J Eur Acad Dermatol Venereol 2023;37:1758)
- Pediatric melanoma, particularly Spitz and spitzoid melanoma, is usually greater in thickness at initial presentation and has a higher occurrence of amelanotic lesions, higher frequency of positive sentinel nodes and an overall less aggressive clinical progression as compared to adult melanoma (J Eur Acad Dermatol Venereol 2023;37:1758, Cancers (Basel) 2023;15:1835)
Terminology
- Juvenile melanoma (J Eur Acad Dermatol Venereol 2023;37:1758)
- Childhood melanoma
ICD coding
Epidemiology
- Pediatric melanoma is rare, accounting for 1 - 3% of all pediatric malignancies (J Eur Acad Dermatol Venereol 2023;37:1758)
- ~1 - 4% of all melanomas occur in pediatric patients
- Conventional melanomas (i.e., superficial spreading and nodular) are most common
- 58.1% in children < 11 years and 86.7% in children 12 - 19 years
- Spitz and spitzoid melanomas are second most common
- 35.5% in children < 11 years and 12.4% in children 12 - 19 years
- Melanoma arising in giant congenital nevi are least common
- 6.5% in children < 11 years and 0.9% in children 12 - 19 years
- Genetic factors including (J Eur Acad Dermatol Venereol 2023;37:1758)
- Inherited deoxyribonucleic acid (DNA) repair defects
- Family history of melanoma
- Light skinned individuals
- Increased total number of nevi
- Congenital melanocytic nevi with risks correlating with size (> 40 cm) and number
- Cumulative sun damage (J Eur Acad Dermatol Venereol 2023;37:1758)
- Extensive sun exposure and sunburns
- Indoor tanning
Sites
- Childhood melanomas are more common on the extremities and trunk (J Eur Acad Dermatol Venereol 2023;37:1758)
- Adolescent melanomas are more common on the trunk (J Eur Acad Dermatol Venereol 2023;37:1758)
Pathophysiology
- Biologically distinct from melanoma in adults
- Conventional pediatric melanomas are histologically alike to adult melanoma with a majority demonstrating somatic single nucleotide mutations such as those seen with ultraviolet radiation (J Eur Acad Dermatol Venereol 2023;37:1758)
- Mutations
- BRAF
- TERT
- CDKN2A
- PTEN
- TP53
- RB1
- Mutations
- Melanomas arising from congenital nevi are associated with NRAS mutation (J Eur Acad Dermatol Venereol 2023;37:1758)
- Spitz and spitzoid melanomas are associated with kinase fusion mutations (J Eur Acad Dermatol Venereol 2023;37:1758)
- MAP3K8
- ALK
- NTRK1
- NTRK3
- MET
- RET
- ROS1
- BRAF
Etiology
- Immunosuppression and immunodeficiency (J Eur Acad Dermatol Venereol 2023;37:1758)
- History of retinoblastoma (J Eur Acad Dermatol Venereol 2023;37:1758)
- History of certain genetic disorders such as xeroderma pigmentosum or Werner syndrome (J Eur Acad Dermatol Venereol 2023;37:1758)
- Giant melanocytic nevi (> 40 cm) (N Engl J Med 1995;332:656)
- Cumulative sun damage (N Engl J Med 1995;332:656)
- Numerous nevi
- Family history (N Engl J Med 1995;332:656)
- Dysplastic nevus syndrome
- Melanoma
Clinical features
- Childhood melanomas, particularly in prepubertal cases, deviate from conventional ABCDE criteria (asymmetry, border irregularity, color variegation, diameter ≥ 6 mm, evolving lesion) (N Engl J Med 1995;332:656)
- Pink or reddish, dome shaped papule
- Amelanotic, nodular lesion
- Often mimics benign lesions (i.e., symmetric, pigmented lesions with regular borders)
- Modified ABCD criteria: amelanotic, bleeding / bump, color uniformity, de novo / any diameter (N Engl J Med 1995;332:656)
- Melanoma arising in congenital melanocytic nevi features (Br J Dermatol 2006;155:1, J Eur Acad Dermatol Venereol 2023;37:32)
- Giant congenital melanocytic nevi (> 40 cm)
- Arising in deeper nevi
- Predominantly observed on the trunk region
- Giant congenital melanocytic nevi that extend across the midline of the spine
- Presence of satellite nevi
- Melanomas may also arise within the central nervous system
Diagnosis
- Excisional biopsy deep to subcutaneous fat with surrounding 2 mm of normal appearing skin
- Histopathologic diagnosis is gold standard (Ital J Dermatol Venerol 2021;156:300)
- Conventional pediatric melanomas (NCCN: NCCN Guidelines - Melanoma: Cutaneous [Accessed 7 February 2024])
- Sentinel lymph node biopsy (SLNB) is recommended for lesions with Breslow depth of 1 mm or deeper
- Indeterminate or spitzoid pediatric melanoma indications for sentinel lymph node biopsy have not been defined (J Pediatr Surg 2022;57:425)
- Sentinel lymph node biopsy should take into consideration patient's age, histopathologic and molecular diagnosis and anatomic location
- Prognosis of positive SNLB is unclear in spitzoid pediatric melanoma (J Am Acad Dermatol 2013;68:913)
- Additional molecular testing with comparative genomic hybridization (CGH), fluorescence in situ hybridization (FISH) and alterations in TERT promoter to aid in accurate pathologic classification of pediatric melanoma (Pediatr Blood Cancer 2018;65:10.1002/pbc.26792, Sci Rep 2015;5:11200)
Prognostic factors
- Adverse prognostic indicators (J Am Acad Dermatol 2023;88:609)
- Breslow thickness > 4 mm
- Presence of ulceration
- Localization on head and neck
- Diagnosis in adolescence
- Spitz and spitzoid melanomas have a high risk of nodal progression but are associated with low mortality (J Eur Acad Dermatol Venereol 2023;37:1758)
- Sentinel lymph node positivity does not predict prognosis or outcome (Ann Surg 2011;253:1211)
Case reports
- Newborn girl with a giant congenital nodular melanoma (BMC Pediatr 2021;21:121)
- 7 year old boy with amelanotic conjunctival melanoma (Am J Ophthalmol Case Rep 2022;29:101735)
- 11 year old boy with Fitzpatrick type VI skin type with an ulcerated amelanotic melanoma of the ear (Pediatr Dermatol 2021;38:106)
- 14 year old Japanese girl with metastatic juvenile spitzoid melanoma on the buttock expressing PRAME (Case Rep Oncol 2020;13:1141)
- 15 year old boy with primary intracranial malignant melanoma (Pediatr Neurosurg 2023;58:223)
Treatment
- First line treatment is wide local excision to the deep fascia
- Margins for excision specific to pediatric melanoma have not been defined but in most cases follow adult melanoma guidelines
- Wide local excision 1 - 2 cm depending on the thickness of melanoma
- Margins for excision specific to pediatric melanoma have not been defined but in most cases follow adult melanoma guidelines
- SNLB should be individualized, considering factors such as the patient's age, clinical circumstances, melanoma subtype, diagnostic certainty, location on the body, lymph drainage pattern and desired prognostic information
- SNLB for pediatric melanomas typically follows adult melanoma guidelines
- Consider SNLB if < 0.8 mm thick with ulceration or 0.8 - 1.0 mm thick with or without ulceration
- Targeted adjuvant therapy
- BRAF and MEK inhibitors
- Immunotherapy with checkpoint inhibitor
- PD-1 and CTLA4 inhibitors
- Chemotherapy
- Radiotherapy
- Surveillance with regular total body skin and lymph node examinations
- References: PDQ Cancer Information Summaries: Childhood Melanoma Treatment (PDQ®) - Health Professional Version [Accessed 7 February 2024], NCCN: NCCN Guidelines - Melanoma: Cutaneous [Accessed 7 February 2024], N Engl J Med 2017;377:1824, Lancet Oncol 2020;21:121, Target Oncol 2022;17:283
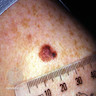
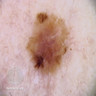
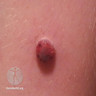
Clinical images
Microscopic (histologic) description
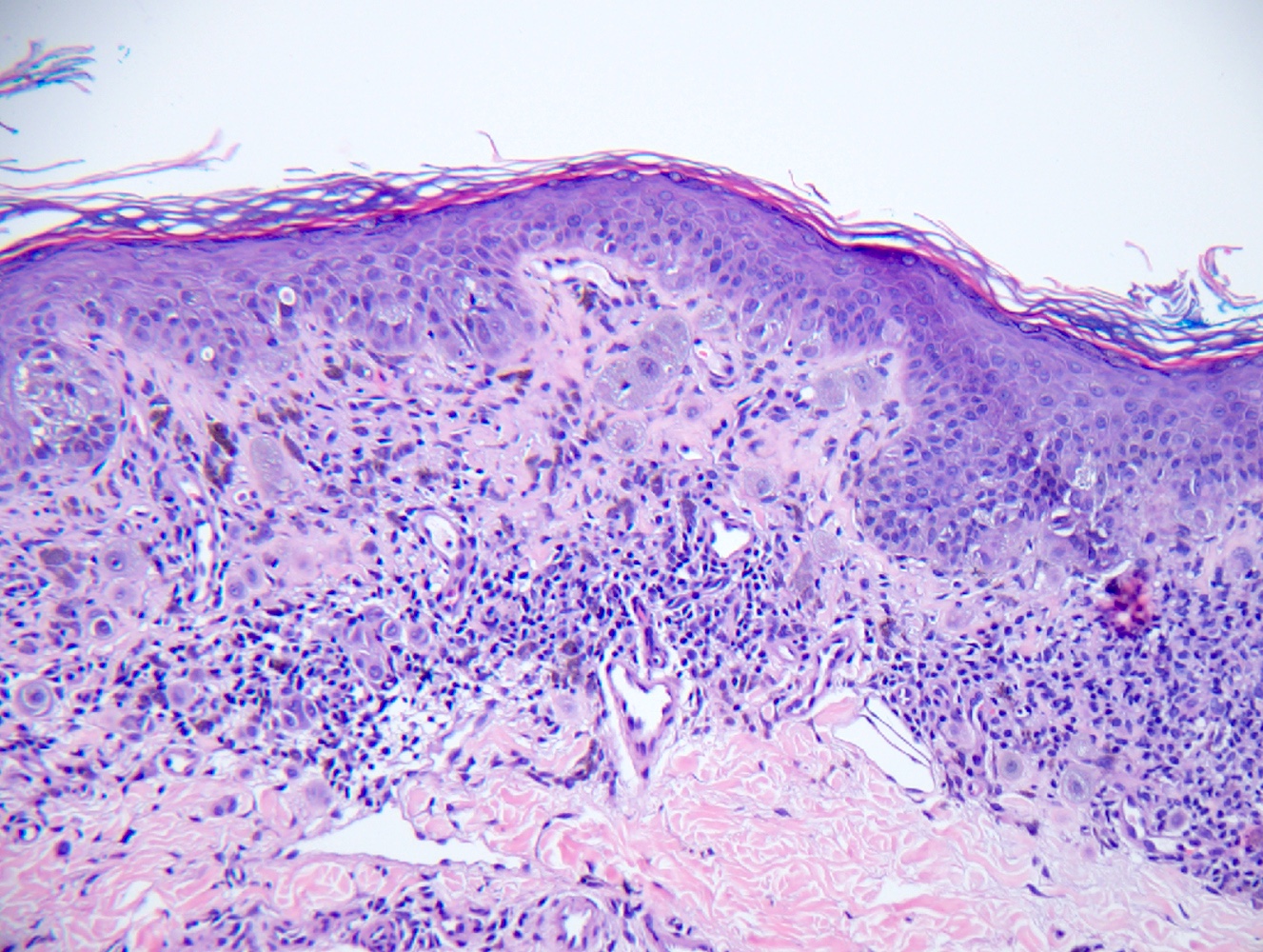
- Conventional melanoma
- Superficial spreading (Arch Pathol Lab Med 2020;144:500)
- Radial followed by horizontal growth phase
- Pagetoid growth scattered in the epidermis
- Nests vary in size, shape and distribution
- Not evenly spaced and not confined to tips of rete
- Asymmetrical
- Fails to mature from superficial to deep
- Cytological atypia and mitoses
- Can have lymphoid infiltrate at the base
- With or without ulceration
- Nodular (Arch Pathol Lab Med 2020;144:500, Ital J Dermatol Venerol 2021;156:300)
- May be polypoid and exophytic
- Lacks radial growth
- Tumor size often > 10 mm
- Cytologic atypia and mitoses
- Necrosis
- With or without ulceration
- Pathologic stage classification American Joint Committee on Cancer (AJCC) guidelines, eighth edition (2018) (Ital J Dermatol Venerol 2021;156:300)
- Tumor (Breslow) depth is the strongest predictor of clinical outcome, used for staging
- Measure vertically from the top of granular layer of the epidermis to the deepest invasive melanoma cell(s)
- If ulcerated, measure from base of ulcer
- Round to nearest 0.1 mm using ocular micrometer
- Avoid measuring vascular invasion, microsatellites, involvement of skin appendages
- Tumor (Breslow) depth is the strongest predictor of clinical outcome, used for staging
- Superficial spreading (Arch Pathol Lab Med 2020;144:500)
- Melanoma arising in congenital nevi (Am J Dermatopathol 2013;35:e16)
- Dermal lesions composed of epithelioid cells, spindle cells or round cells resembling a malignant small round blue cell tumor
- Proliferative nodules may be present
- Generally, little to no necrosis, cytological atypia or increased mitotic activity
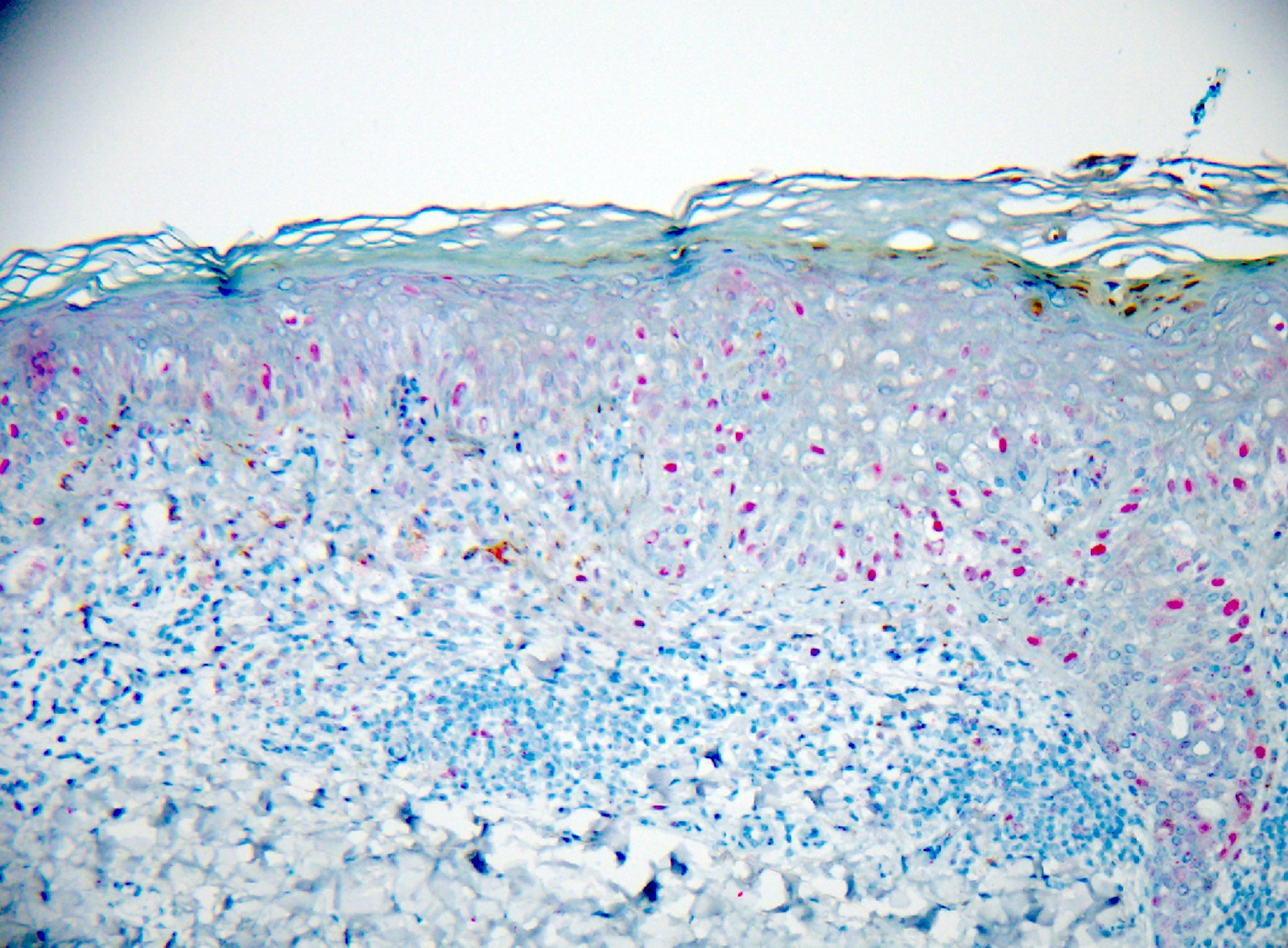
- Spitz and spitzoid melanoma (Pediatr Blood Cancer 2018;65:10.1002/pbc.26792, Diagnostics (Basel) 2023;13:2380)
- Presence of enlarged epithelioid or spindled cells
- Dome shaped configuration with epidermal hyperplasia
- Vertical disposition of melanocytes
- Asymmetry, absence of dermal maturation
- May reach Breslow > 10 mm without ulceration
- Frequent mitoses in the dermis
- High grade of cellular atypia
- High N:C ratio
- Absence of Kamino bodies
Microscopic (histologic) images
Positive stains
- SOX10 (nuclear)
- MART1 (MelanA)
- HMB45
- S100
- MITF
- PRAME
- Tyrosinase
- Ki67 / MIB1 (elevated)
- BRAF (J Cutan Pathol 2023;50:223)
- References: Pediatr Blood Cancer 2018;65:10.1002/pbc.26792, Semin Diagn Pathol 2022;39:239
Negative stains
- Loss of melanocytic markers is possible (Dermatopathology (Basel) 2021;8:359)
- p16 (nuclear loss in melanoma) (J Cutan Pathol 2023;50:763)
- Cytokeratins (Dermatopathology (Basel) 2021;8:359)
- CD34, CD31 (Dermatopathology (Basel) 2021;8:359)
Molecular / cytogenetics description
- Fluorescence in situ hybridization (FISH) to identify copy number alterations of chromosomes in melanoma and Spitz tumors (Am J Surg Pathol 2013;37:676)
- Interpret in context with clinical and histopathologic findings
- 6p25
- 6q23
- 11q13
- 9p21
- 8q24
- Interpret in context with clinical and histopathologic findings
- Next generation sequencing to identify melanoma related gene mutations (Am J Pathol 2002;161:1163)
- Interpret in context with clinical and histopathologic findings
- BRAF
- TERT
- MAP3K8
- RET
- ALK
- NTRK
- ROS1
- Interpret in context with clinical and histopathologic findings
- Comparative genomic hybridization can detect amplifications and deletions of smaller DNA regions along the chromosome (Am J Pathol 2002;161:1163, Pathology 2004;36:458)
- Can detect copy number variations of melanomas arising in giant congenital nevi
- Melanomas show frequent aberrations in chromosomes 6, 7, 9 and 10
Molecular / cytogenetics images
Sample pathology report
- Skin, biopsy:
- Invasive malignant melanoma, superficial spreading subtype (Breslow depth to the nearest 0.1 mm) (see comment)
- Comment: The sections demonstrate a highly atypical compound melanocytic proliferation consisting of irregular epithelioid melanocytes. These melanocytes exhibit poor nesting along the dermal epidermal junction, showing both confluence and pagetoid scatter. In the dermis, melanocytes fail to mature and disperse with descent. Dermal mitotic activity is observed.
Differential diagnosis
- Benign nevi:
- Well circumscribed and symmetric
- Well nested junctional component without significant pagetoid scatter or confluence
- Dermal component matures and disperses with descent
- Dysplastic nevi:
- Can resemble melanoma clinically with asymmetry, border irregularity, color variegation and large diameters
- Generally, lacks severe cytologic and architectural atypia
- Dermal component matures and disperses with descent
- Lacks dermal cytologic atypia, pleomorphism and mitotic activity
- Histologically may resemble early melanoma, especially in severely dysplastic nevi
- Spitz nevi:
- Often presents as pink papules that primarily affect pediatric patients
- Histologically lacks severe cytologic atypia and has rare mitotic figures
- Should be well demarcated
- Usually lacks deep dermal to subcutaneous extension
- Presence of Kamino bodies
- Blue nevi:
- Blue to dark gray homogenous macules or papules
- Histologically lacks cytologic atypia and mitotic figures
- Sclerotic stroma
- Various subtypes
- Pyogenic granulomas:
- Red, friable, bleeding papule
- May clinically resemble Spitz or spitzoid melanoma and amelanotic melanoma
- Histologically, this is a vascular tumor with lobular thin capillaries with surrounding fibrous stroma
- Deep penetrating nevus:
- Dark dome shaped papule
- Histologically, it is wedged shaped or plexiform growth pattern with melanin pigment
- Well circumscribed and symmetrical
- Can have occasional mitotic figure and cytologic atypia
Board review style question #1
A 7 year old child presents with a new, evolving, reddish, dome shaped papule that is symmetric with regular borders. What are the ABCD criteria of childhood melanoma?
- Amelanotic, bleeding / bump, color uniformity, de novo / any diameter
- Amelanotic, bleeding / bump, color variegation, diameter > 6 mm
- Asymmetry, bleeding / bump, color uniformity, diameter > 6 mm, evolution over time
- Asymmetry, irregular borders, color variegation, diameter > 6 mm, evolution over time
Board review style answer #1
A. Amelanotic, bleeding / bump, color uniformity, de novo / any diameter. Childhood melanomas often do not adhere to the conventional ABCDE criteria because often spitzoid melanomas occur. The deviation from the typical criteria includes lesions that are amelanotic, symmetric, pigmented or bleeding with color uniformity. Answers B - D are incorrect because these lesions tend to be de novo and can be of any size. In addition, the evolution of lesions over time is not a good indicator for pediatric melanoma because at this age group, new onset and evolution of common nevi is expected.
Comment Here
Reference: Pediatric melanoma
Comment Here
Reference: Pediatric melanoma
Board review style question #2
What is the recommended method for biopsy when there is clinical suspicion of melanoma in a child?
- Excisional biopsy of entire lesion
- Incisional biopsy of thickest portion of the lesion
- Punch biopsy of thickest portion of the lesion
- Superficial shave biopsy of the entire lesion
Board review style answer #2
A. Excisional biopsy of entire lesion. Excision biopsy is the preferred approach for suspected malignant melanoma. Answer B is incorrect because incision biopsy can be considered for large lesions in areas where cosmetic concerns are heightened, such as on the face or in cases of acral melanoma. In certain instances, incision biopsy may also be justified in regions undergoing recent changes within a giant congenital nevus. However, partial sampling of the lesion will be inadequate for accurate staging. Answers C and D are incorrect because other biopsy methods, including punch and superficial shave techniques, are not recommended as they lack the ability to provide comprehensive histological staging. At times, deeper scoop shave biopsies may be utilized to accurately sample melanoma and determine the lesion's true depth.
Comment Here
Reference: Pediatric melanoma
Comment Here
Reference: Pediatric melanoma
Board review style question #3
What is the role of fluorescence in situ hybridization (FISH) in the molecular characterization of pediatric melanoma?
- Analysis of TERT promoter alterations
- Assessment of chromosomal copy number variations
- Detection of BRAF mutations
- Sequencing of melanoma specific gene panels
Board review style answer #3
B. Assessment of chromosomal copy number variations. FISH is used to identify copy number variations of chromosomes in melanoma and spitz tumors. Answers A, C and D are incorrect because they do not support the purpose of FISH.
Comment Here
Reference: Pediatric melanoma
Comment Here
Reference: Pediatric melanoma