Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Differential diagnosis | Additional referencesCite this page: Perrino C, Zynger D. Adrenal cortical adenoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/adrenaladenomageneral.html. Accessed April 26th, 2024.
Definition / general
- Benign neoplasm arising from adrenal cortical cells
- May or may not be functional
Terminology
- Adrenal cortical adenoma (ACA)
- Incidentaloma: small adenoma discovered incidentally during workup of other conditions (Mod Pathol 2011;24:S58)
- Black (pigmented) adenoma: diffusely pigmented, brown-black ACA presumably due to lipofuscin
- Hypercortisolism: sometimes used synonymously for Cushing syndrome
- Pre-clinical / sub-clinical Cushing syndrome: hypercortisolism in the context of an incidentally discovered adrenal mass without overt clinical manifestations of Cushing syndrome Arq Bras Endocrinol Metabol 2007;51:1272)
- Primary hypercortisolism: due to secretion of cortisol by the adrenal gland versus secondary hypercortisolism: due to increased secretion of ACTH by pituitary or to secretion of cortisol by an ectopic tumor
Epidemiology
- F > M
- More common in adults, 5th - 7th decade
- Equal predilection for right and left adrenal glands
- True incidence unknown because many are not functional, estimates include 8.7% in autopsy series and 4% in radiology series (Mol Cell Endocrinol 2014;386:67)
- Incidence has been increasing due to increasing utilization of imaging, estimated 0.2 to 0.4% in general population (Endocrinol Metab (Seoul) 2014;29:5)
- Incidence increases with age, reported in <1% of patients under 30 years and in up to 7% of patients over 70 years (Indian J Endocrinol Metab 2013;17:S59)
- Children
- Generally uncommon in children; only ~25 cases annually in U.S. in those < 20 years (Braz J Med Biol Res 2000;33:1225)
- Bimodal age distribution; most commonly < age 5, second peak ages 9 - 16 years
- Incidence higher in females, M:F ratio 1:1.6 (J Clin Oncol 2004;22:838)
- Higher incidence in southern Brazil, associated with specific p53 mutation (R337H TP53)
- Associated with several genetic syndromes:
- Beckwidth-Wiedemann syndrome: hemihypertrophy, splanchnomegaly, macroglossia, intraabdominal neoplasms (i.e. adrenal cortical neoplasms, nephroblastoma, hepatoblastoma), due to alteration of 11p15 region
- Li-Fraumeni syndrome (SBLA syndrome): sarcoma (rhabdomyosarcoma), breast/brain tumors, leukemia, laryngeal carcinoma, lung cancer, adrenal cortical carcinoma; due to alteration of p53 on chromosome 17p
- Carney triad: malignant gastrointestinal stromal tumor, pulmonary chondroma, extra-adrenal paraganglioma, adrenal cortical adenoma
- Adrenogenital syndrome: adrenal cortical neoplasms, congenital adrenal hypertrophy
- No proven relationship with environmental factors
Sites
- Adrenal cortex, all 3 layers
- May be ectopic, reported sites include gastric wall (World J Gastroenterol 2013;19:778) and spinal cord (Am J Surg Pathol 1990;14:481)
Pathophysiology
- Approximately 90% of ACAs are nonfunctional
- When functional, may secrete one or more of the 3 major classes of adrenal steroids (from external to internal layers):
- Zona glomerulosa: mineralocorticoids (aldosterone)
- Zona fasciculata: glucocorticoids (cortisol)
- Zona reticularis: androgens (testosterone, dihydrotestosterone [DHT], androstenedione, dihydroepiandosterone [DHEA])
- Hyperaldosteronism/Conn's syndrome: ↑aldosterone → impacts distal tubules & collecting ducts of nephron → ↑ sodium and water retention, ↓ potassium retention → ↑ blood pressure
- Hypercortisolism/Cushing's syndrome: ↑cortisol → ↓ corticotropin releasing hormone (CRH), ↓ adrenocorticotropic hormone (ACTH), ↑ blood glucose
- Virilization: ↑ DHEA, ↑ DHEA-sulfate (DHEA-S), ↑ androstenedione, ↑ testosterone, ↑ DHT → ↑ urinary 17-ketosteroids (metabolic product)
- Feminization: ↑ androgens → aromatization → ↑ estrogen, ↑ estradiol → ↑ urinary 17-ketosteroids (metabolic product)
- Children: predisposing genetic factors are present in ~50% of children with adrenal cortical tumors, most commonly Li-Fraumeni syndrome and Beckwith-Wiedemann syndrome (see above); may arise due to defective apoptosis (J Clin Endocrinol Metab 2000;85:2048)
Etiology
- Neoplastic proliferation of adrenal cortical cells
- May arise from any of the 3 layers, but zona fasciculata most common (Mod Pathol 2011;24:S58)
Clinical features
- Minority are functional, may produce a pure or mixed endocrine syndrome (from most to least common):
- Hyperaldosteronism/Conn's syndrome: hypertension, proximal muscle weakness, headache, polyuria, tachycardia with/without palpitation, hypokalemia, hypocalcemia
- Hypercortisolism/Cushing's syndrome: central obesity, moon facies, plethora, striae, thin skin, easy bruising, hirsutism, telangiectasias, hyperhidrosis
- Virilization:
- Females: increased muscle mass (Herculean habitus), clitoromegaly, facial hair, deep voice, pubic hair
- Males: penile enlargement, pubic hair
- Feminization: gynecomastia, impotence
Diagnosis
- Adrenal lesion discovered with imaging used to work up unrelated clinical symptoms, therefore usually no detectable hormonal abnormalities or clinical symptoms (Pol J Radiol 2013;78:47)
- Work up may include observation (serial imaging, laboratory tests) or fine needle aspiration / core biopsy (rarely indicated, mainly for ruling out non-adrenal metastases) (J Clin Endocrinol Metab 2010;95:4106)
- Well circumscribed lesion comprised of cells resembling any of the 3 layers of the normal adrenal cortex
- Difficult to differentiate ACA from normal adrenal cortex in adrenal core needle biopsies
- Atypical histologic features are commonly found in children, making adrenal cortical adenomas difficult to distinguish from adrenal cortical carcinoma
Laboratory
- Battery of endocrine tests usually within normal limits, although a minority may have subclinical hormone production with slight abnormalities
- Suggested endocrine tests include: dexamethasone suppression test, ACTH levels, plasma free metanephrine/normetanephrine, 24 hour total urinary metanephrines, ratio plasma aldosterone:plasma renin (Indian J Endocrinol Metab 2013;17:S59)
- Adrenal tumors in patients with previously unrecognized clinical symptoms attributable to the tumor are not considered incidentalomas (Pol J Radiol 2013;78:47)
- Hyperaldosteronism/Conn's syndrome: ↑aldosterone, hypernatremia, hypokalemia
- Cushing's syndrome: ↑cortisol, ↓CRH, ↓ACTH, hyperglycemia
- Virilization: ↑DHEA, ↑DHEA-S, ↑androstenedione, ↑testosterone, ↑DHT, ↑urinary 17-ketosteroids
- Feminization: ↑estrogen, ↑estradiol, ↑urinary 17-ketosteroids
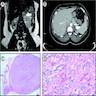
Radiology description
- Computed tomography (CT):
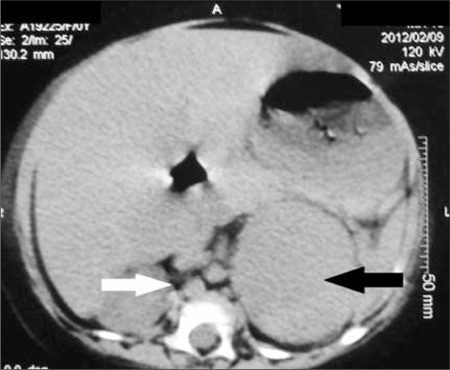
- Rounded, well delineated borders, homogeneous, clear separation from and no extension into surrounding structures, decreased attenuation compared to uninvolved adrenal parenchyma on non-contrast CT (≤10 HU), contrast enhancing (Theranostics 2012;2:516)
- Magnetic resonance imagining (MRI):
- Used to visualize microscopic fat (favoring ACA), "chemical shift" phenomenon (increased "in phase" signal intensity, decreased "out of phase" signal) (Theranostics 2012;2:516)
- 18FDG-PET:
- Malignant lesions have greater 18FDG uptake than liver (Theranostics 2012;2:516)
Radiology images
Prognostic factors
- Distinguishing ACA from adrenocortical carcinoma (ACC) is difficult and several systems have been proposed
- In general, most reliable factors include size, necrosis, mitotic activity, atypical mitoses (Mod Pathol 2011;24:S58)
- Weiss System (Am J Surg Pathol 1984;8:163): most widely used criteria
- Criteria (≥ 3 criteria indicates malignancy): high mitotic rate, atypical mitoses, high nuclear grade, low percentage of clear cells, necrosis, diffuse tumor architecture, capsular invasion, sinusoidal invasion, venous invasion
- Modified Weiss System (Am J Surg Pathol 2002;26:1612): > 5 mitoses per 50 high powered fields, < 25% clear cells, atypical mitotic figures, necrosis, and capsular invasion
- Calculation:
- 1 point each for the presence of atypical mitotic figures, necrosis, and capsular invasion
- 2 points each for the presence of > 5 mitoses per 50 high powered fields and < 25% clear cells
- Total score ranges from 0 to 7, and score of > 3 highly correlates with subsequent malignant behavior
- Calculation:
Case reports
- 24 year old woman with unusual presentation of Carney complex (Endocr J 2012;59:823)
- 33 year old woman with Cushing's syndrome during pregnancy secondary to adrenal adenoma (Acta Med Iran 2012;50:76)
- 34 year old woman with eplerenone use in primary aldosteronism during pregnancy (Hypertension 2012;59:e18)
- 35 year old man with primary adrenal angiosarcoma and functioning adrenocortical adenoma (Eur J Endocrinol 2012;166:131)
- 36 year old woman with cortisol producing adrenal adenoma associated with latent aldosteronoma (Intern Med 2012;51:395)
- 46 year old woman with black adrenal adenoma causing preclinical Cushing's syndrome (Tokai J Exp Clin Med 2010;35:57)
- 50 year old woman with myelolipomata arising within adrenocortical adenoma ipsilateral to synchronous clear cell renal cell carcinoma (Malays J Pathol 2010;32:123)
- 52 year old woman with adrenalectomy by retroperitoneal laparoendoscopic single site surgery (JSLS 2010;14:571)
- 56 year old woman with coexistence of Cushing syndrome from functional adrenal adenoma and Addison disease from immune-mediated adrenalitis (J Am Osteopath Assoc 2012;112:374)
- 56 year old man with generalized glucocorticoid resistance accompanied with an adrenocortical adenoma and caused by a novel point mutation of human glucocorticoid receptor gene (Chin Med J (Engl) 2011;124:551)
- 66 year old woman with laparoscopic adrenalectomy for bilateral metachronous aldosteronomas (JSLS 2011;15:100)
- 72 year old woman with ectopic adrenal cortical adenoma in the gastric wall (World J Gastroenterol 2013;19:778)
Treatment
- Most adrenal lesions > 4 cm should be removed regardless of imaging findings because of increased risk of ACC (BMC Surg 2013;13:57)
- If large (> 4 cm), functional, worrisome characteristics on imaging: surgical resection
- If small (< 4 cm), non-functional, benign features on imaging: clinical observation and follow up (exact guidelines not well established)
- Repeat laboratory studies to assess functional status, yearly for 4 years
- Repeat CT scan 6 - 12 months after diagnosis, if no increase in size then no further follow up needed
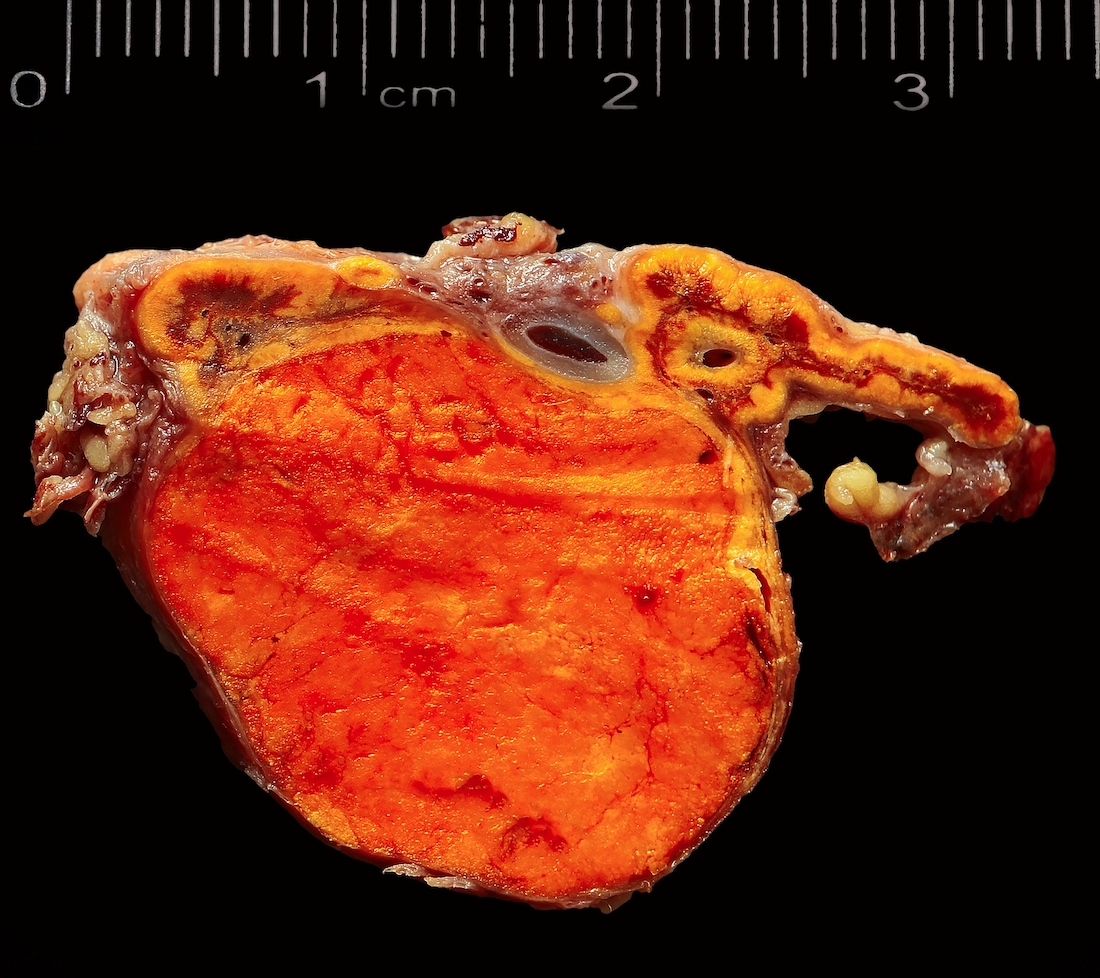
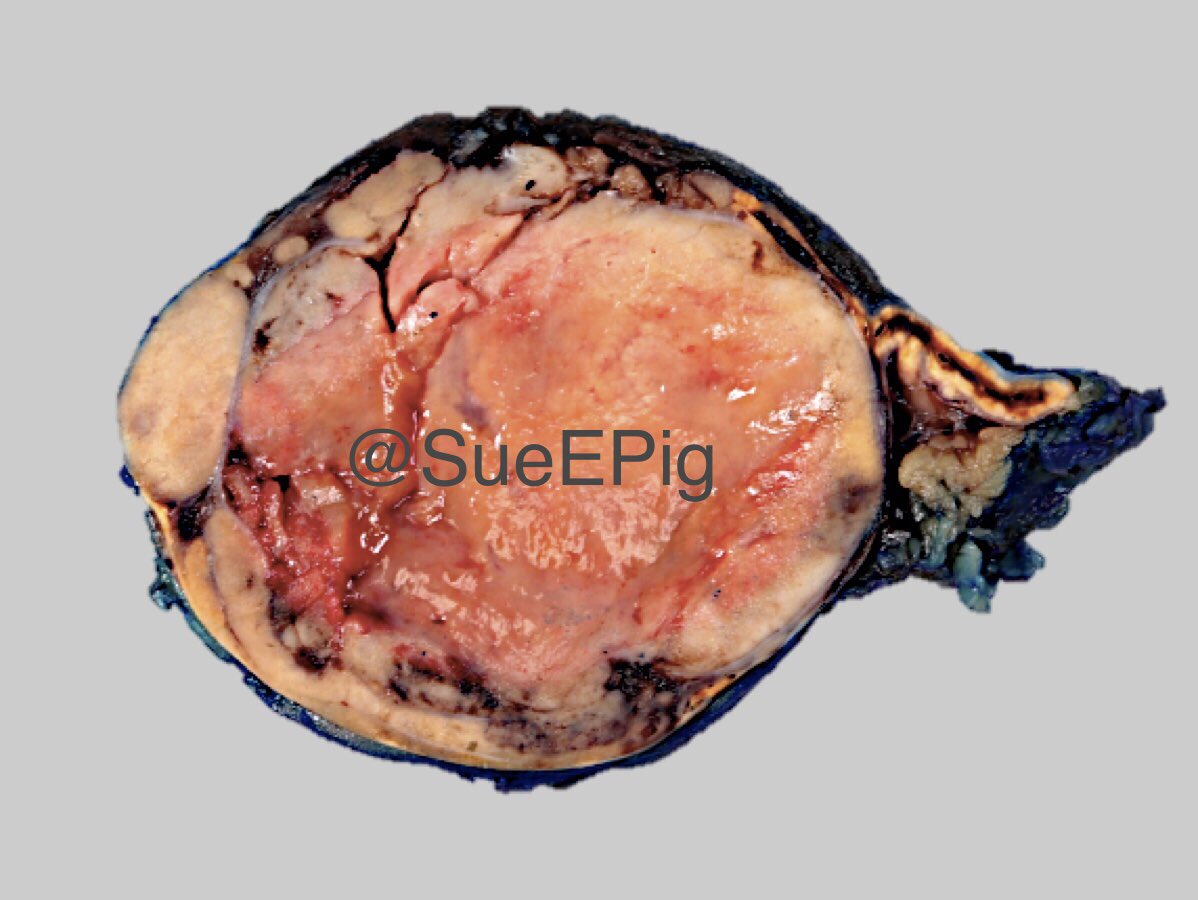
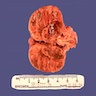
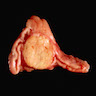
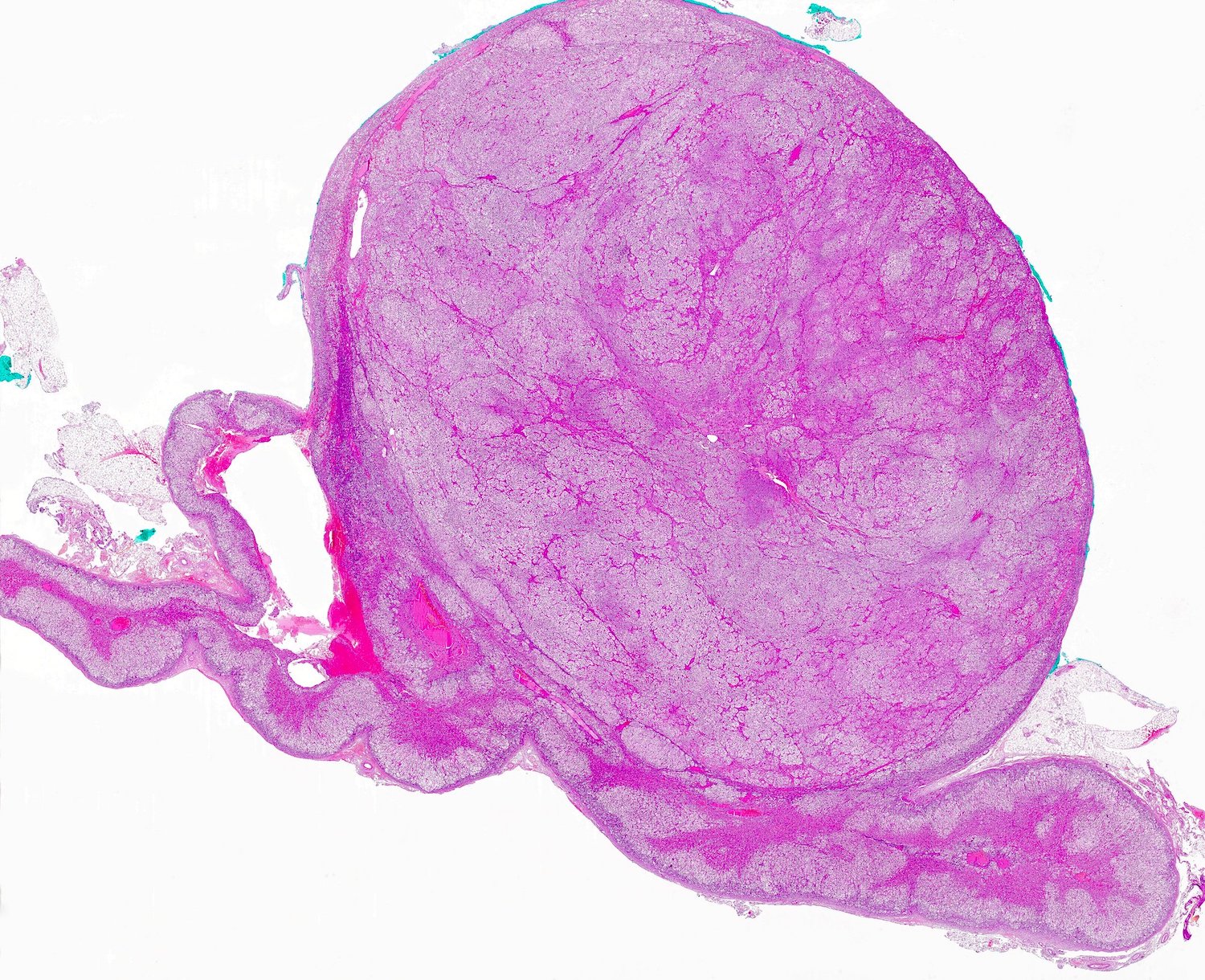
Gross description
- Weight usually < 50 grams (in pediatric patients may weight up to 500 grams) (Mod Pathol 2011;24:S58)
- Size usually < 5 cm
- Unilateral, solitary, golden yellow
- May have focal dark areas corresponding with hemorrhage, lipid depletion, increased lipofuscin
- Functional adenoma may result in atrophy of ipsilateral or contralateral adrenal cortex
Gross images
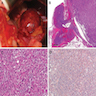
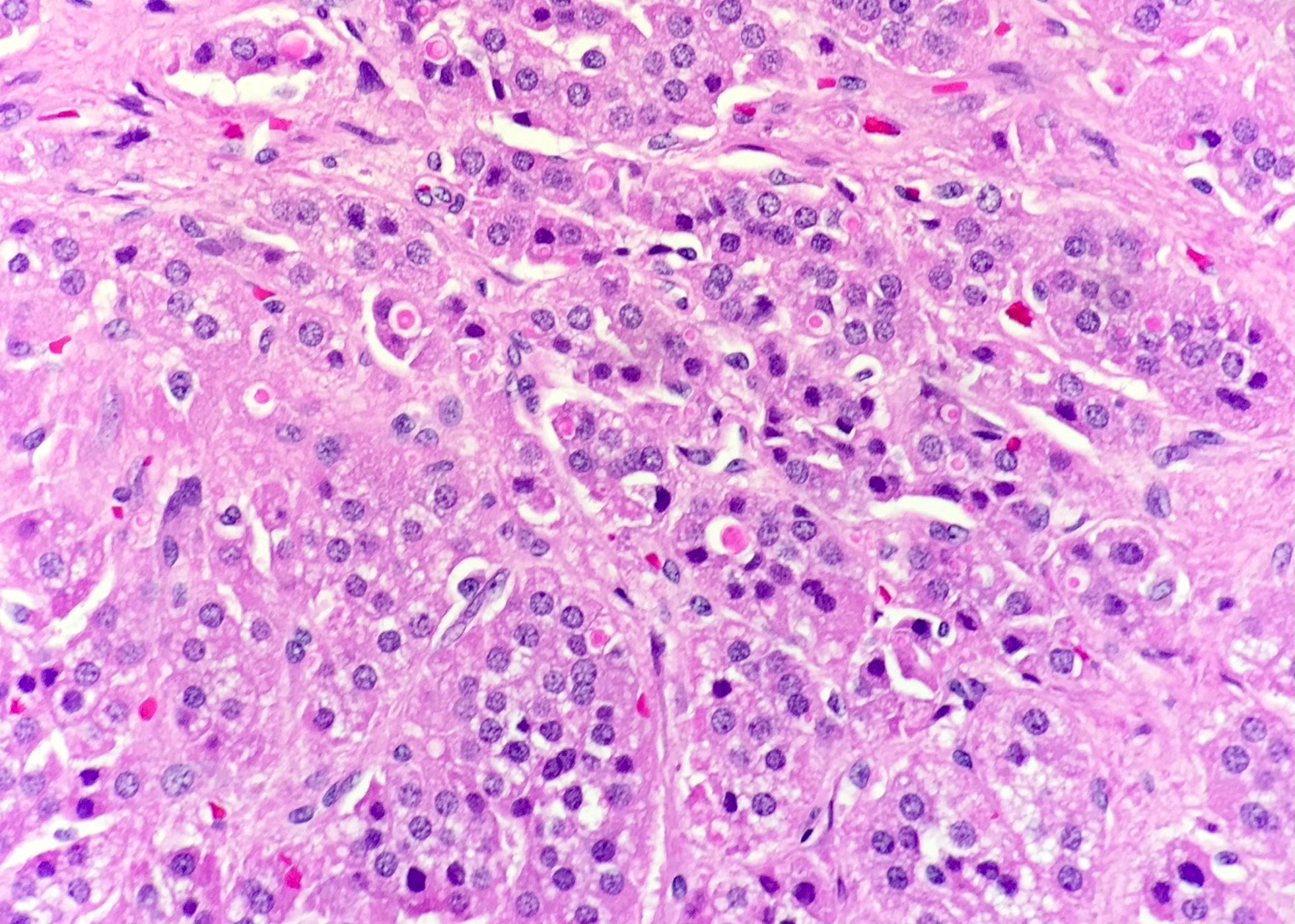
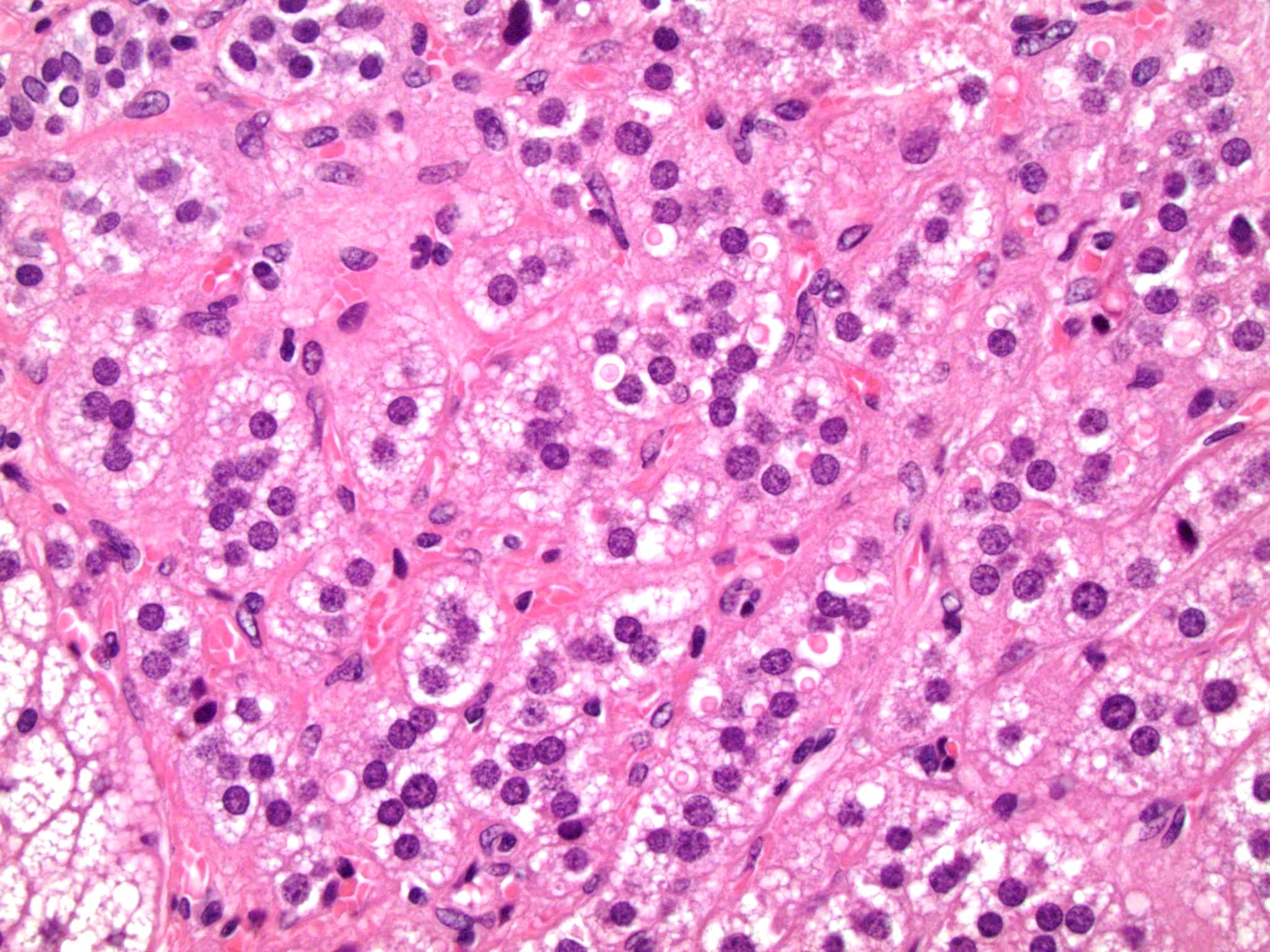
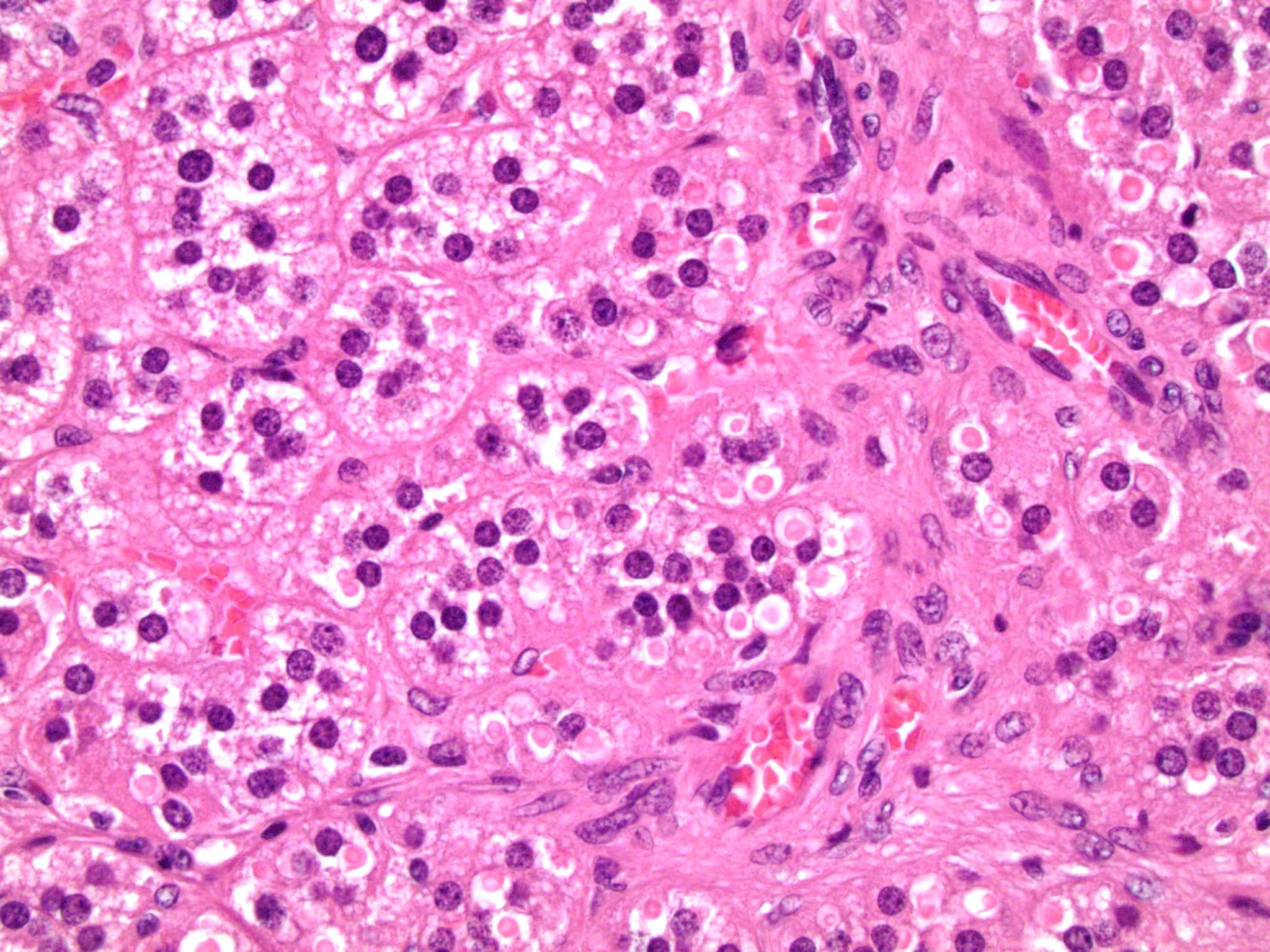
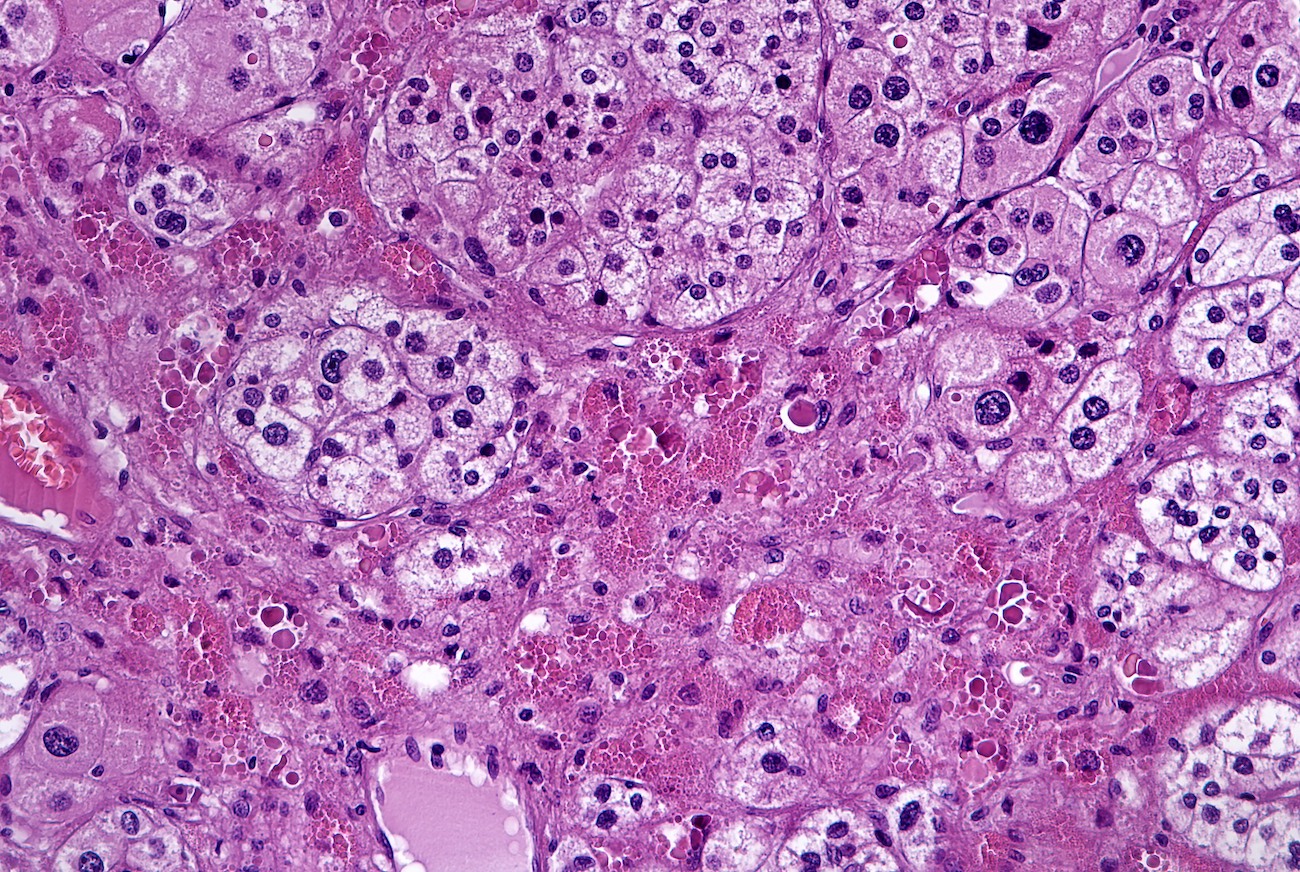
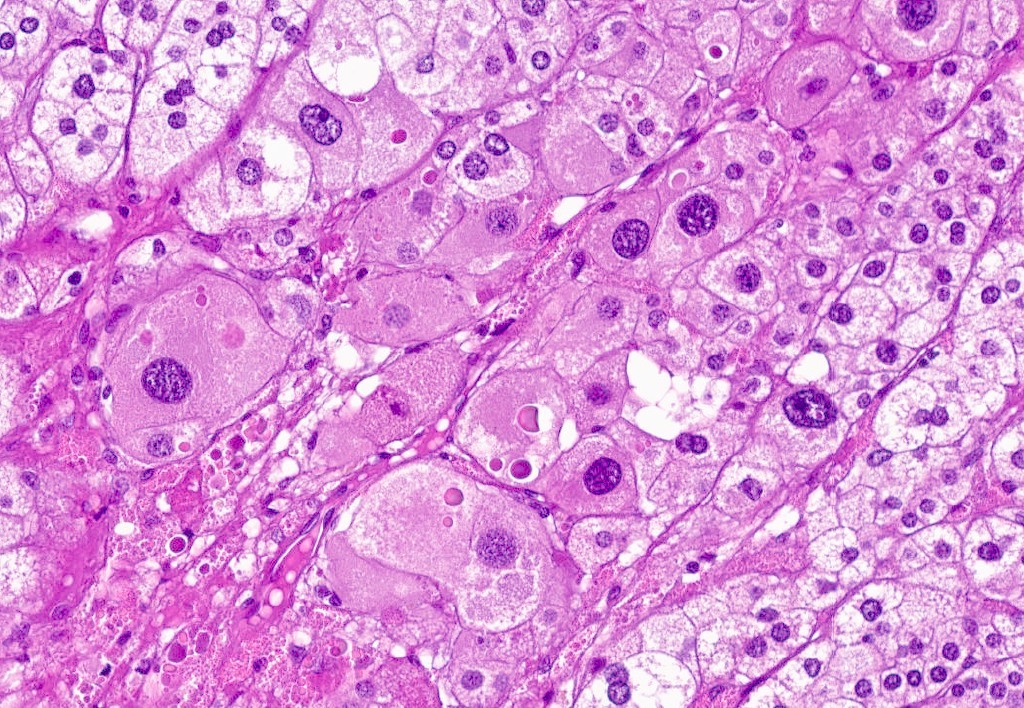
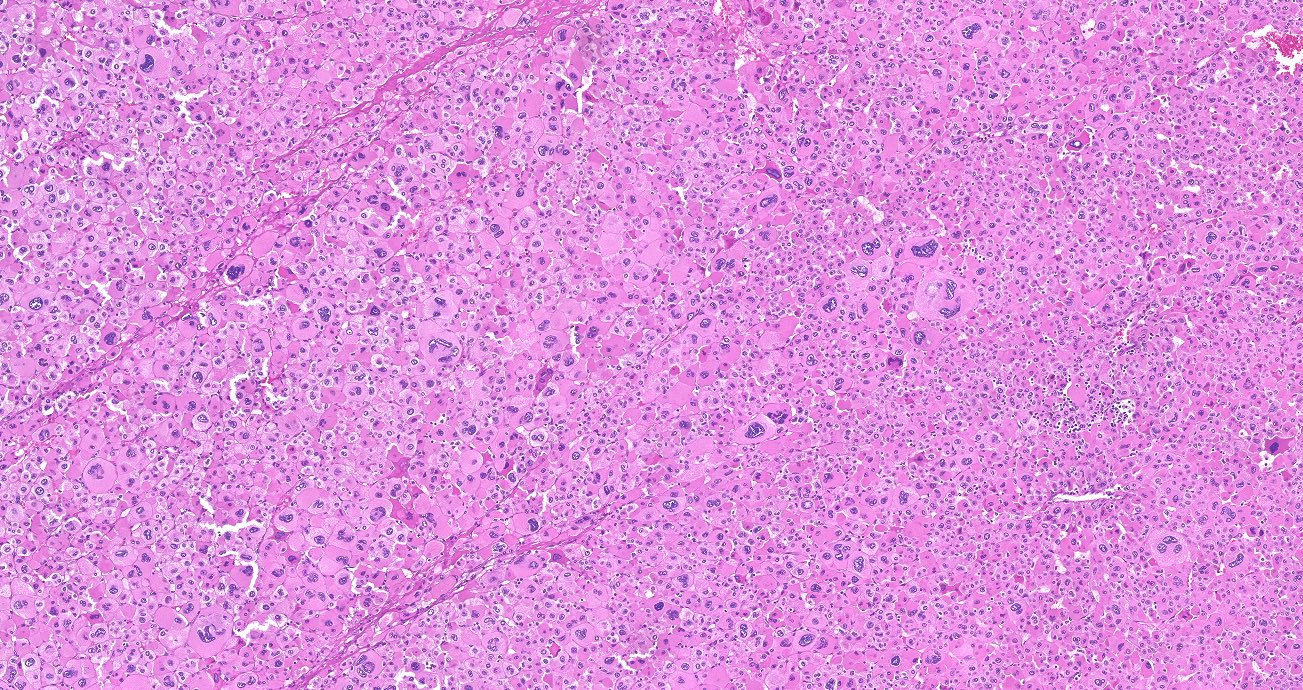
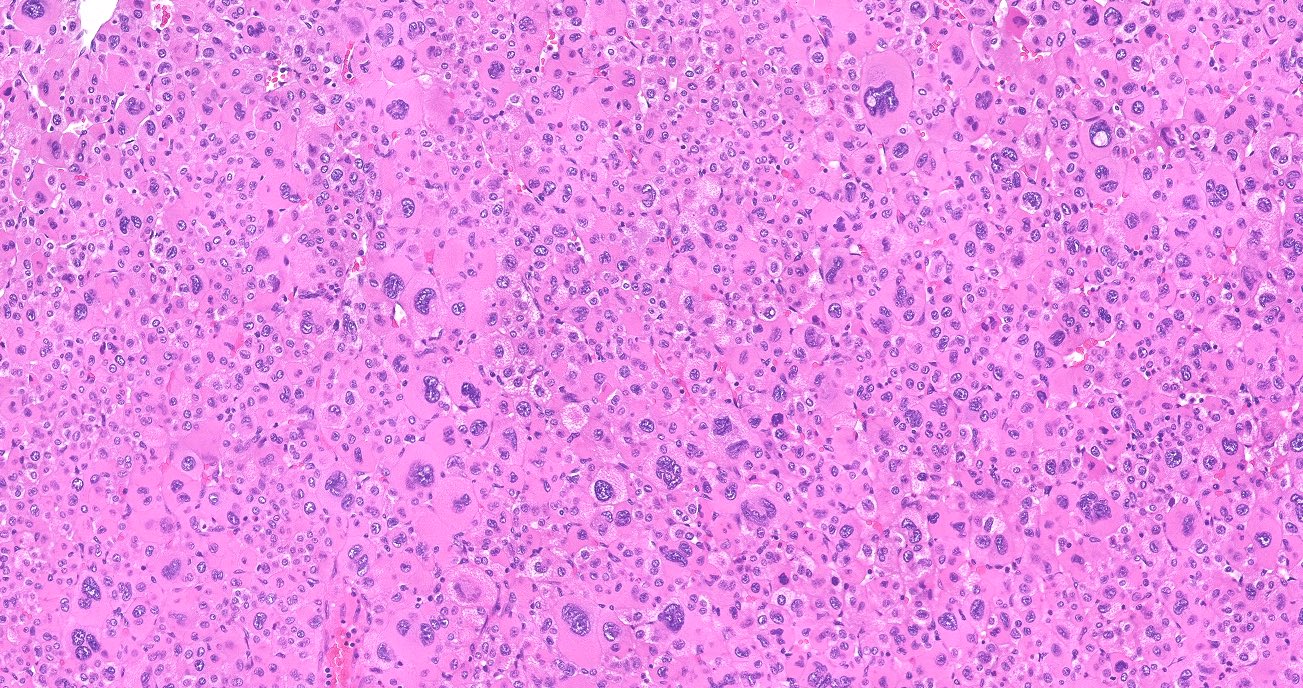
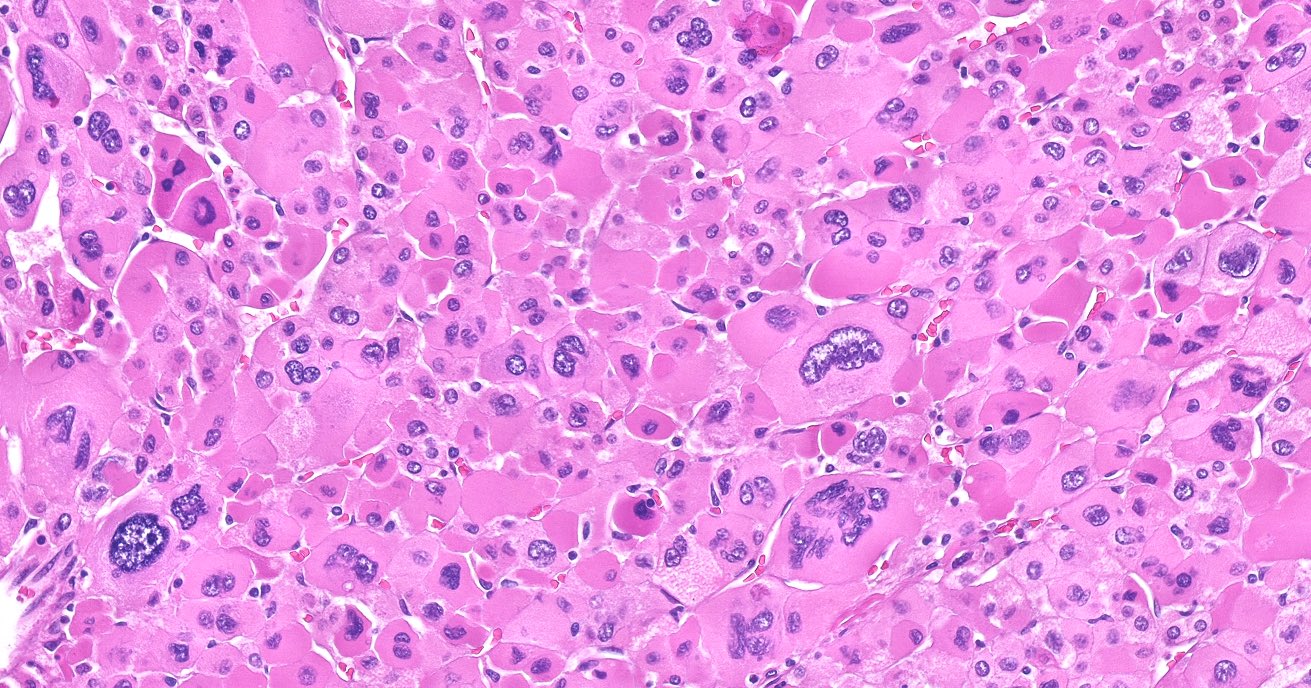
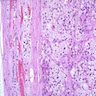
Microscopic (histologic) description
- In comparison to surrounding adrenal gland, adenoma cells are larger with different cytoplasm, increased variation in nuclear size
- Distinct cell borders, cells have abundant foamy cytoplasm reminiscent of zona fasciculata
- Balloon cells: clusters of cells with enlarged lipid-rich cytoplasm (seen in Cushing syndrome)
- Histologic variants: oncocytic, myxoid
Microscopic (histologic) images
Contributed by Xiaoyin "Sara" Jiang, M.D., Debra Zynger, M.D., @Andrew_Fltv on Twitter and @SueEPig on Twitter
Images hosted on other servers:
Cytology description
- Difficult to impossible to distinguish from unremarkable adrenal cortex (Diagn Cytopathol 2005;33:26)
- Cellularity varies, generally loose clusters of large cells (Diagn Cytopathol 2005;33:26, Diagn Cytopathol 1996;14:126)
- Foamy / vacuolated cytoplasm (Diagn Cytopathol 2005;33:26, Diagn Cytopathol 1996;14:126)
- Round to oval nuclei with smooth contours, may have naked nuclei, variation in nuclear size / shape has little significance (Diagn Cytopathol 2005;33:26, Diagn Cytopathol 1996;14:126)
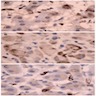
Positive stains
- Usually positive: α-inhibin, MelanA / Mart1, steroidogenic factor-1 (SF-1), calretinin, Oil Red O (fresh frozen tumor, highlights intracytoplasmic lipid), BCL2 (Mod Pathol 1998;11:716), D2-40 (J Clin Pathol 2008;61:293)
Negative stains
- EMA, CEA, B72.3, S100, chromogranin (stains adrenal medulla), vimentin, carbonic anhydrase IX (CAIX)
- Usually negative: synaptophysin, neuron specific enolase (NSE), low molecular weight cytokeratin (AE1 / AE3, CAM 5.2)
- Low Ki67 index (usually < 5%)
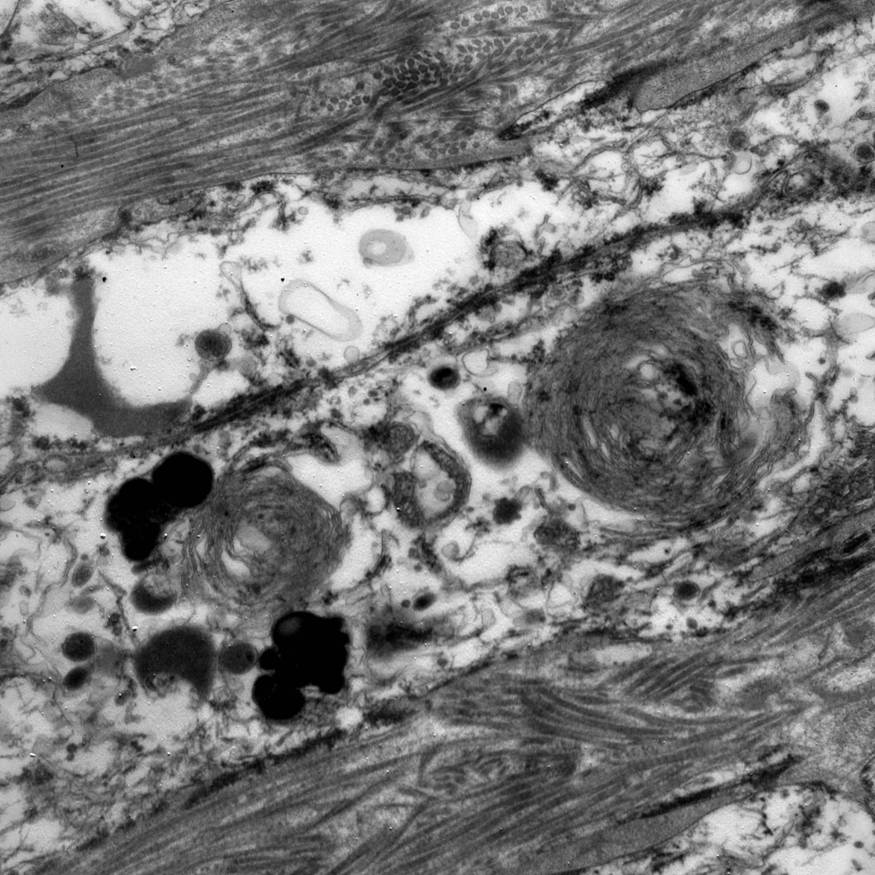
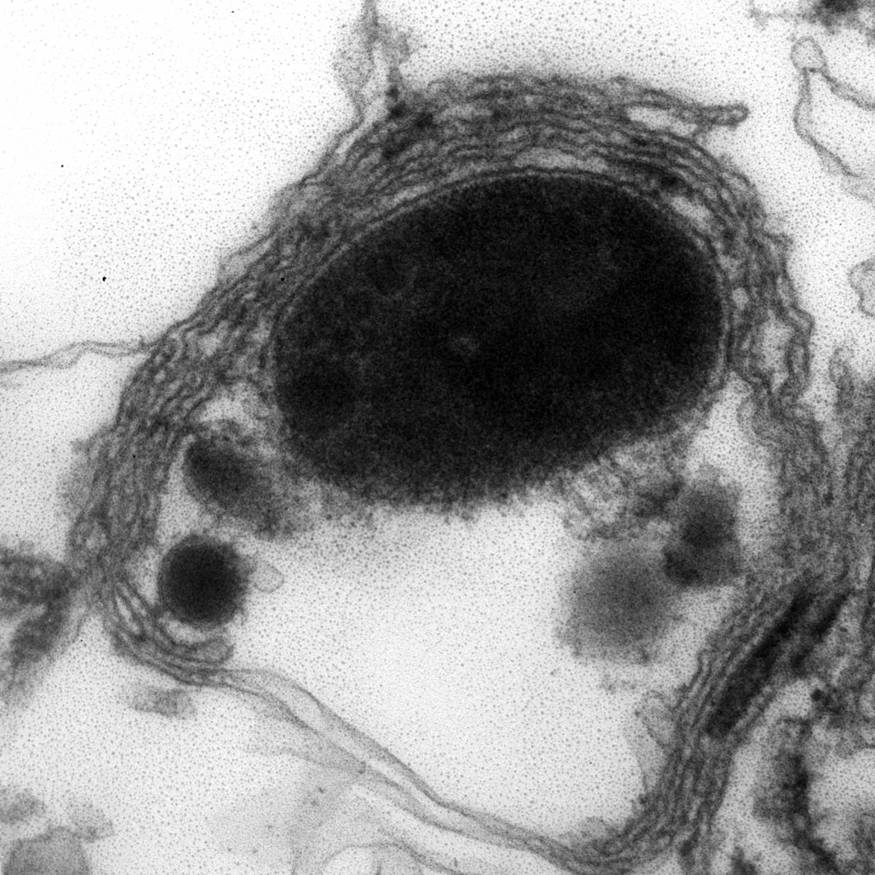
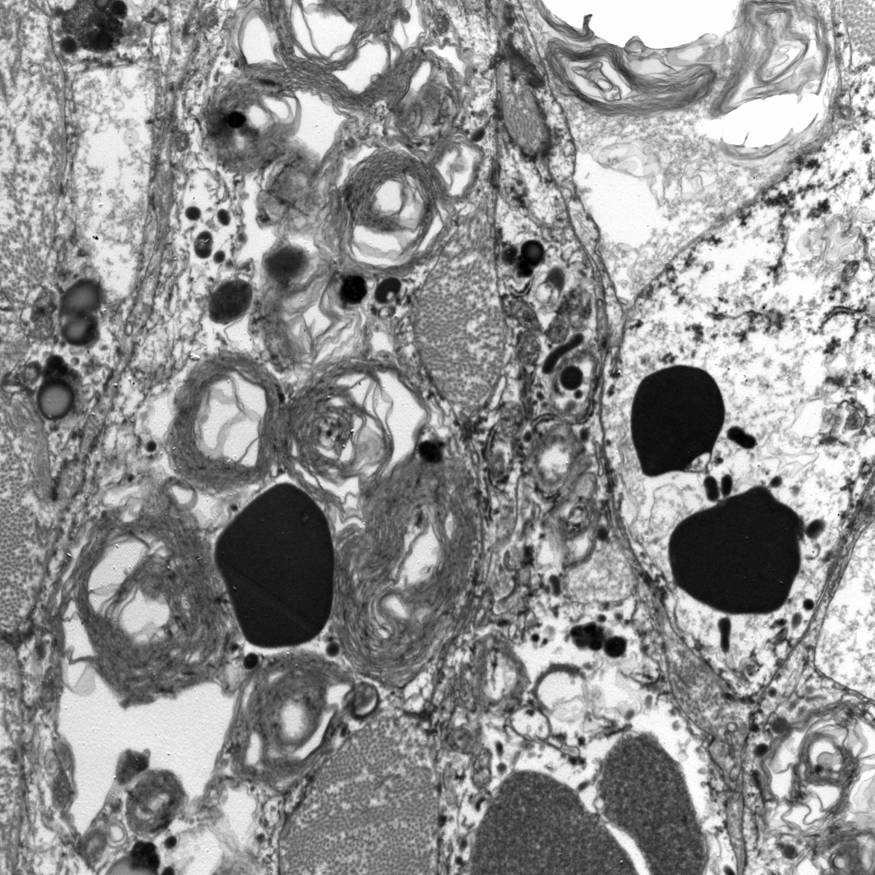
Electron microscopy description
- Abundant intracytoplasmic lipid droplets of varying sizes
- Prominent microvillous projections along cell borders
- Abundant smooth endoplasmic reticulum
- Prominent, round to oval mitochondria; cristae may have tubular to vesicular (zona fasciculata) or lamellar (zona reticularis) profile
Molecular / cytogenetics description
- Tumorigenesis not well understood
- Outside of immunohistochemistry for diagnosis, adjunct molecular studies not currently utilized for clinical purposes (i.e. treatment, prognosis, distinction from ACC)
- Usually monoclonal and diploid, versus ACC monoclonal and aneuploid/polyploid (Mol Cell Endocrinol 2014;386:67)
- Gene expression profiling shows decrease in expression of major histocompatibility complex (MCH) class II genes in ACC when compared to ACA in children (Cancer Res 2007;67:600)
- Usually sporadic, but may be associated with genetic syndrome
- Cortisol-producing ACA may be associated with McCune-Albright syndrome, primary pigmented nodular adrenocortical disease or Carney complex (Mol Cell Endocrinol 2014;386:67)
- Comparative genomic hybridization (CGH) studies showed adrenal tumors have complex pattern of chromosomal alterations, with ACCs having more more chromosomal gains/losses than ACAs (Mol Cell Endocrinol 2014;386:67)
- Single nucleotide polymorphism (SNP) arrays confirm high genetic variability in ACAs (Neoplasia 2012;14:206)
- Chromosomes with most frequent gains are #5, 3, 6, 11, 2
- Chromosomes with most frequent losses are #1, 6, 2
- Candidate genes include NOTCH1, CYP11B2, HRAS, IGF2
Differential diagnosis
- Adrenal cortical carcinoma:
- Weight usually > 100 grams (Ann Oncol 1997;8:423)
- Usually > 5 cm, median size 11 cm in one large case series (Eur J Endocrinol 2013;169:891)
- Variegated appearance (nodularity, fibrous bands) suggests carcinoma (Mod Pathol 2011;24:S58)
- See histologic criteria above
- Corticomedullary adenoma:
- Mixed adrenocortical adenoma-pheochromocytoma
- Hepatocellular carcinoma
- Melanoma
- Metastatic carcinoma
- Pheochromocytoma
- Renal cell carcinoma, clear cell type
Additional references