Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Anderson D, Tretiakova M. SMARCB1 deficient renal medullary carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneytumormalignantmedullarycar.html. Accessed September 16th, 2025.
Definition / general
- Rare, highly aggressive, typically medulla centered carcinoma with SMARCB1 (INI1) deficiency
- Associated with sickle cell trait or other sickle hemoglobinopathies
Essential features
- Rare carcinoma arising in the renal medulla
- Occurs predominately in young patients with sickle cell trait
- Variety of histologic patterns
- Sickled erythrocytes (drepanocytes) may be pathognomonic
- Shows loss of SMARCB1 / INI1
- Poor prognosis
Terminology
- SMARCB1 deficient renal medullary carcinoma, renal medullary carcinoma
ICD coding
- ICD-O: 8510/3 - medullary carcinoma, NOS
- ICD-10:
- ICD-11: 2C90.Y & XH2YP5 - other specified malignant neoplasm of kidney, except renal pelvis & medullary carcinoma, NOS
Epidemiology
- Rare, < 0.5% of all renal carcinomas; ~200 cases described, first in 1995 (Am J Surg Pathol 1995;19:1, J Oncol Pract 2017;13:414)
- Arises almost exclusively in patients with sickle cell trait (hemoglobin AS), although it has been reported rarely in (J Oncol Pract 2017;13:414):
- Homozygous SS disease (sickle cell anemia) (Arch Pathol Lab Med 2003;127:e135, Pediatr Blood Cancer 2014;61:567)
- HbS / beta thalassemia (BJU Int 2017;120:782)
- HbSC (Am J Surg Pathol 2014;38:871)
- Rare reports of tumors that are morphologically similar to renal medullary carcinoma in the absence of sickle cell trait, sickle cell disease or other hemoglobinopathy (Nat Rev Urol 2010;7:110, Hum Pathol 2017;67:134)
- These tumors have been previously termed unclassified renal cell carcinoma with medullary phenotype
- Mean age: 26 years (wide age range: 5 - 69 years) (J Oncol Pract 2017;13:414, Am J Surg Pathol 2013;37:368)
- M:F = 2:1 (Am J Surg Pathol 2013;37:368, Mod Pathol 2007;20:914)
- 75 - 89% occur in the right kidney (Urology 2007;70:878)
Sites
- Involves the kidney, more often the right kidney, in any location of the renal medulla and with or without cortex
Pathophysiology
- Arises in the renal medulla from the distal segment of the collecting ducts (Mod Pathol 2007;20:914, Hansel: The Kidney, 1st Edition, 2016)
- Believed to arise from the renal papillae or calyceal epithelium and may be triggered by the chronic medullary hypoxia and hypertonic environment resulting from sickled red cells and microvascular occlusion (Urology 2002;60:1083, Clin Cancer Res 2018;24:2044)
- Environment promotes double strand DNA breaks
- Chronic hypoxia also leads to repression of the RAD51 and BRCA1 pathways associated with high fidelity homologous recombination and induces a switch to nonhomologous end joining (NHEJ) repair pathways, which are more likely to produce deletions and translocations
- Translocations and deletions are the most common causes of SMARCB1 inactivation / loss of expression, a chromatin remodeler and tumor suppressor gene
- Right side predominance may be due to differences in vascular anatomy, particularly the greater length of the right renal artery, which results in less blood flow and therefore relative hypoxia
- Considered to be the seventh sickle cell nephropathy (others: unilateral hematuria, papillary necrosis, renal infarct, nephrotic syndrome, pyelonephritis, inability to concentrate urine) (Afr Health Sci 2016;16:490, Am J Surg Pathol 1995;19:1)
Clinical features
- Symptoms including hematuria, flank / abdominal pain, dysuria and weight loss in virtually all patients (J Oncol Pract 2017;13:414)
- Fever, nausea, vomiting and a palpable abdominal mass may be present
- Aggressive; usually advanced disease at presentation with metastases to lung, liver, lymph nodes, adrenal gland, peritoneum, retroperitoneum, inferior vena cava, etc.
- 90% of patients present with metastasis at the time of diagnosis
Diagnosis
- Essential: SMARCB1 deficient, high grade, infiltrating adenocarcinoma
- Desirable: clinical / laboratory evidence of sickle hemoglobinopathy
- Consideration of other carcinomas with SMARCB1 deficiency (see Differential diagnosis)
Radiology description
- May show an infiltrative tumor, more often on the right side, with necrosis, caliectasis and regional adenopathy (AJR Am J Roentgenol 2005;185:268)
Prognostic factors
- Poor prognosis with a median overall survival of 6 - 13 months (J Oncol Pract 2017;13:422)
- Metastatic stage at diagnosis increases the risk of death about threefold (Can Urol Assoc J 2015;9:E172)
Case reports
- 15 year old black girl with sickle cell trait, lymphadenopathy and both pleural and pericardial effusions (Arch Pathol Lab Med 2003;127:e288)
- 20 year old pregnant black woman with sickle cell trait (Arch Pathol Lab Med 2002;126:627)
- 21 year old black man with sickle cell disease and 40 year old black man with HbSC disease (Arch Pathol Lab Med 2003;127:e135)
- 22 year old man with renal medullary metastasis to the orbit and temporal fossa (Ophthalmic Plast Reconstr Surg 2019;35:e149)
- 29 year old man with renal medullary carcinoma clinically masquerading as renal infection (BMC Nephrol 2020;21:79)
- 29 year old woman with renal medullary carcinoma with an aggressive clinical course (Case Rep Oncol 2017;10:1)
- 32 year old African American man with sickle cell trait with multiple lung nodules and 11 cm right kidney mass (Cytojournal 2018;15:21)
- 33 year old black man with sickle cell trait (Arch Pathol Lab Med 2000;124:1561)
Treatment
- Radical nephrectomy
- Chemotherapy is usually administered with platinum based regimens (Clin Genitourin Cancer 2021;19:e401)
- Few data regarding the role of other therapies and immunomodulatory drugs (UptoDate: The Treatment of Advanced Non-Clear Cell Renal Carcinoma [Accessed 10 November 2022], Int J Mol Sci 2022;23:7097, Clin Genitourin Cancer 2021;19:103)
- Ongoing clinical trials (ClinicalTrials: Medullary Carcinoma [Accessed 10 November 2022])
- SMARCB1 loss may be target for proteasome inhibition (Elife 2019;8:e44161)
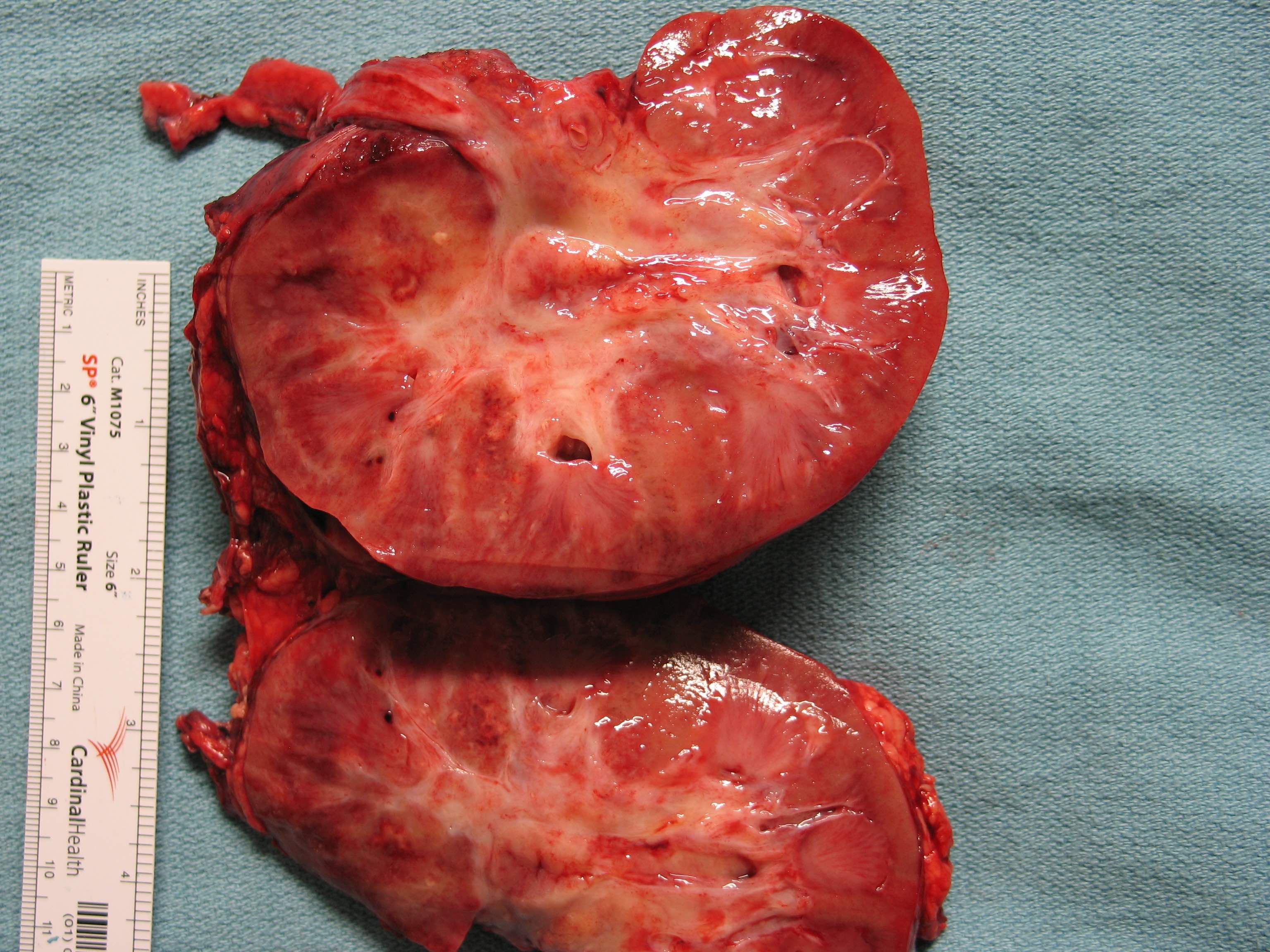
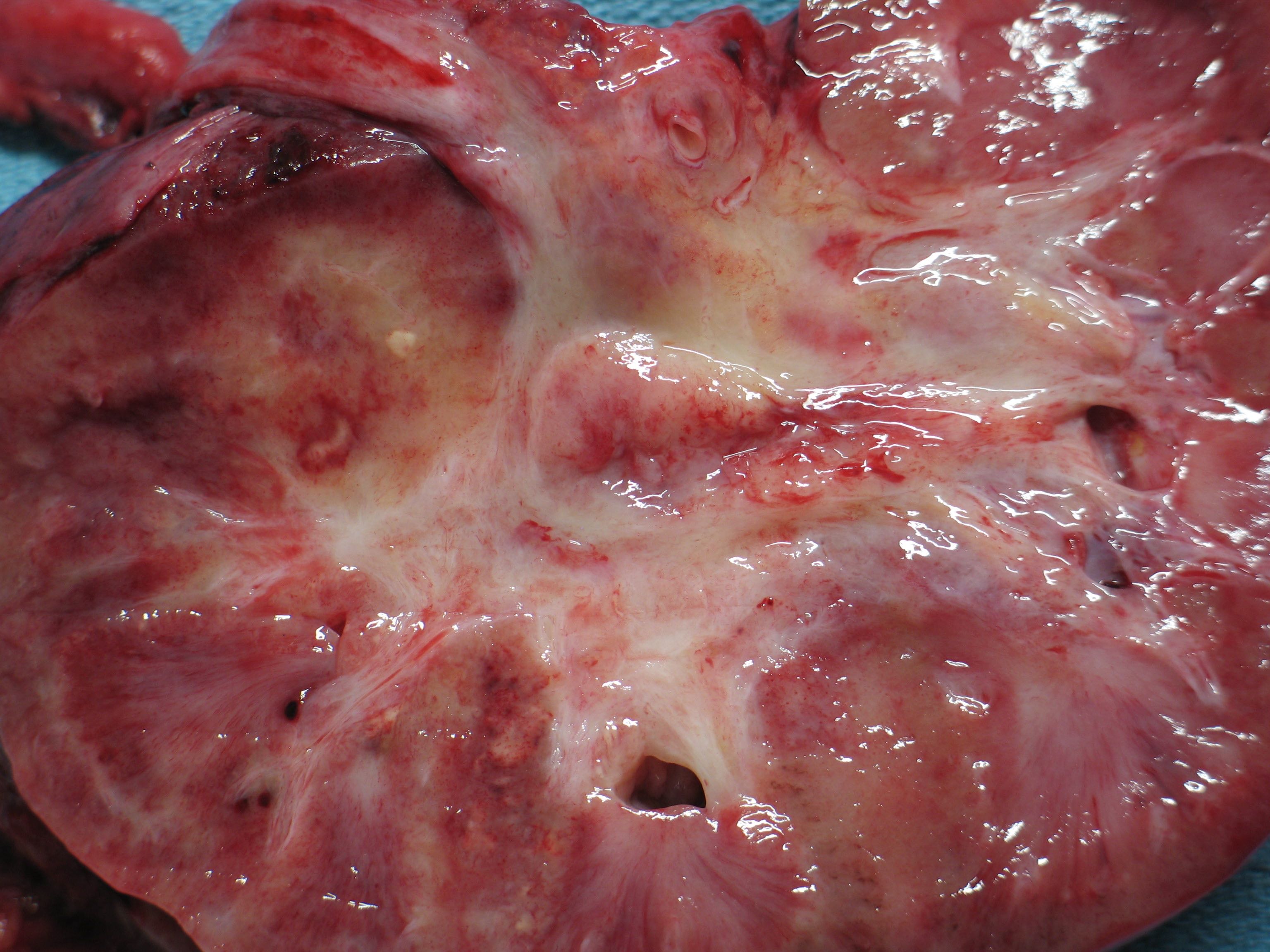
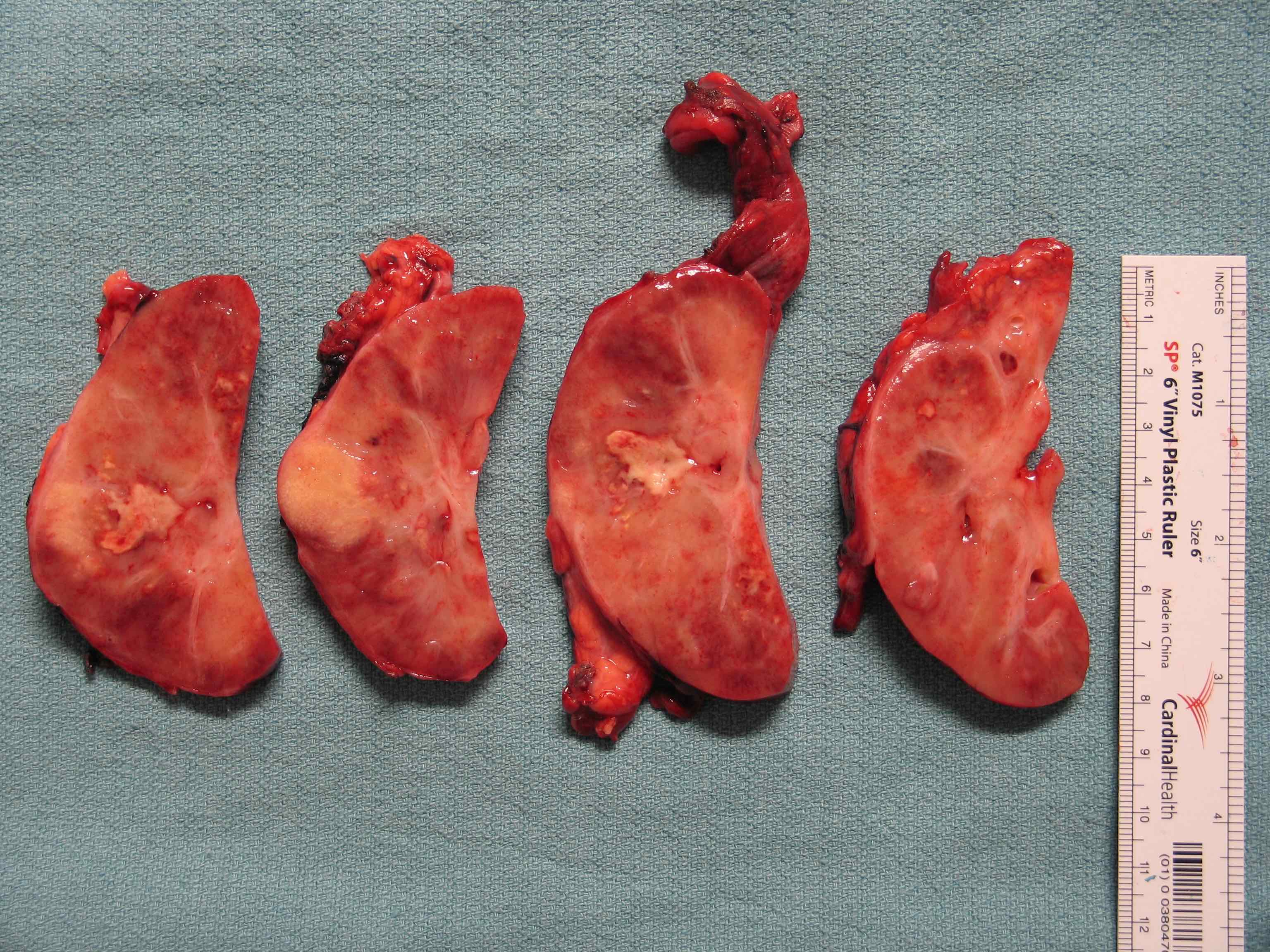
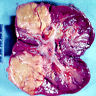
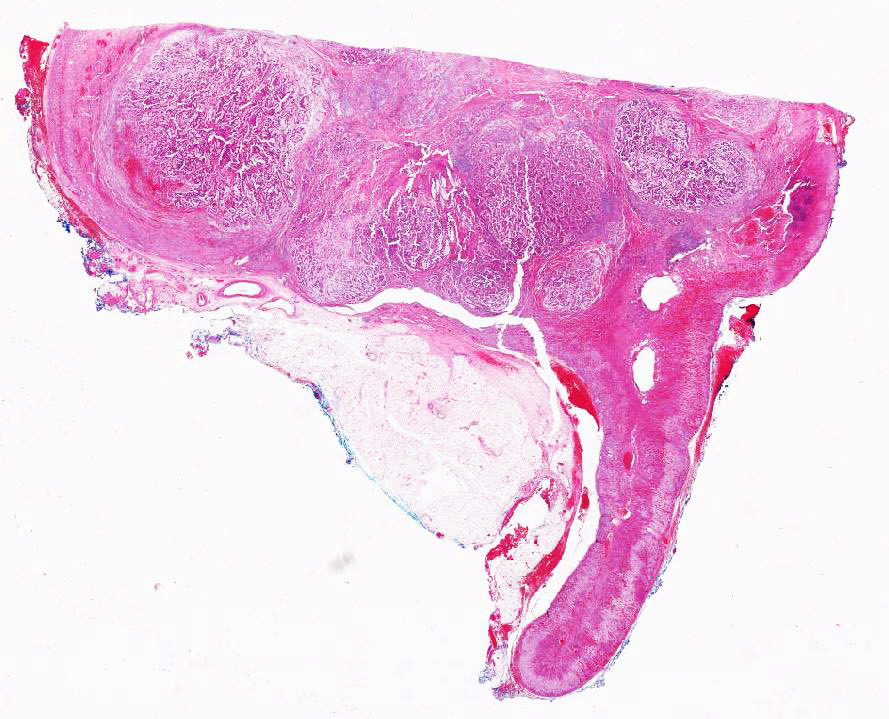
Gross description
- Poorly circumscribed, lobulated, firm, rubbery, gray-white tumor centered in renal medulla
- Size ranges from 4 to 12 cm, mean is 5.9 cm (Am J Surg Pathol 2013;37:368)
- Hemorrhage and necrosis are common
- Typically extends into the calyces and pelvis
- Satellite nodules in the cortex, extension into the perinephric and sinus fat can be present
Gross images
Microscopic (histologic) description
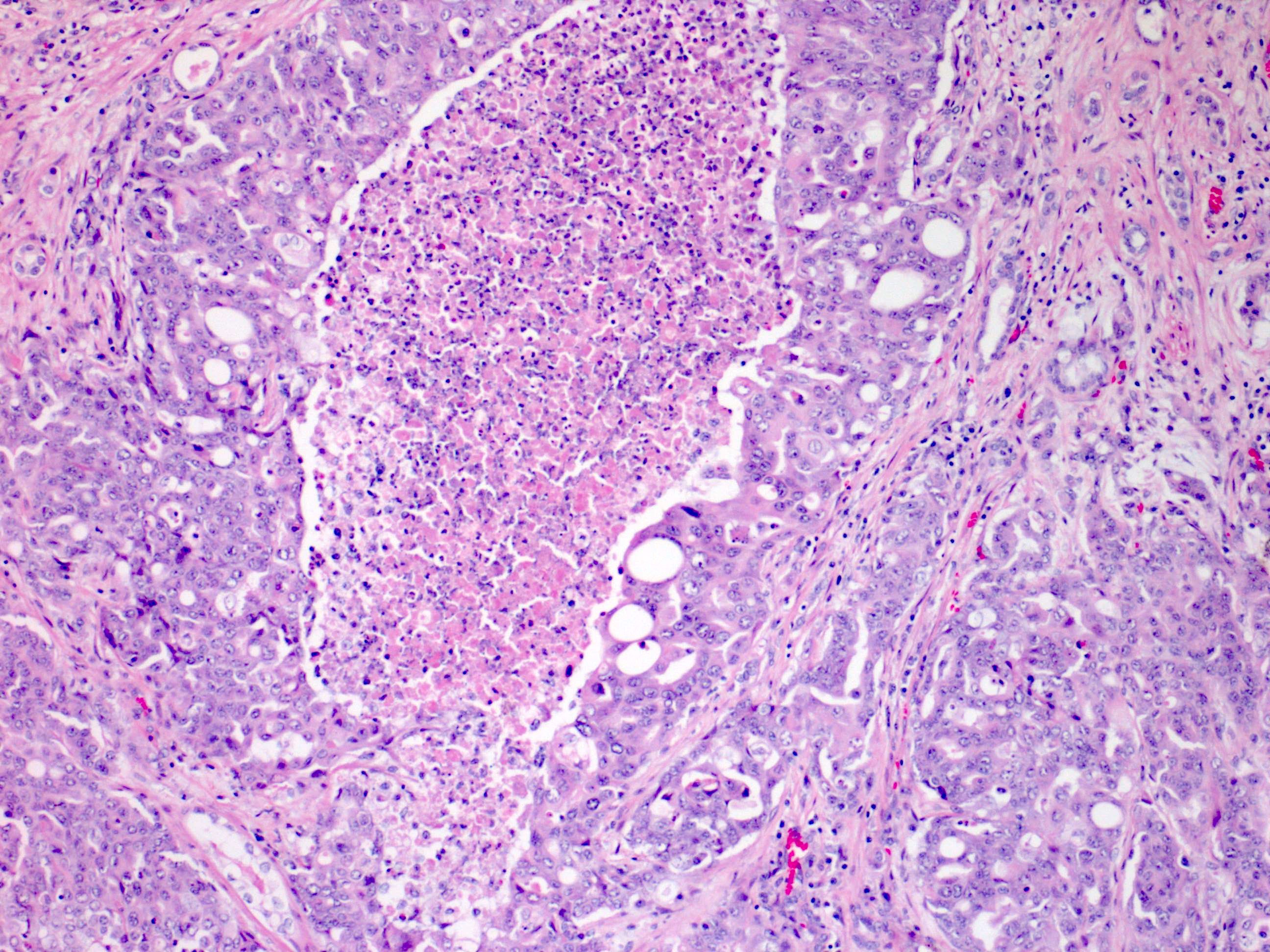
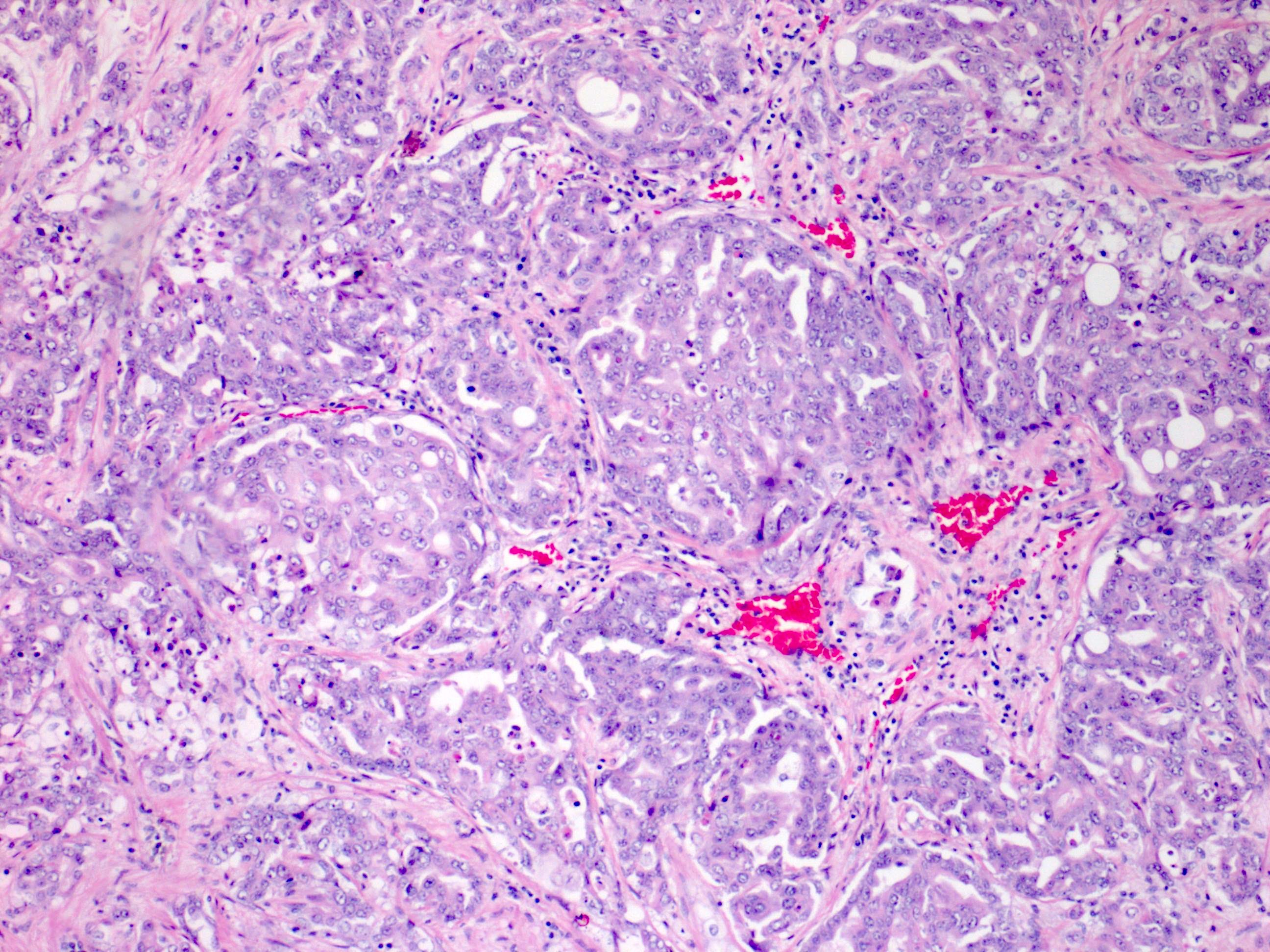
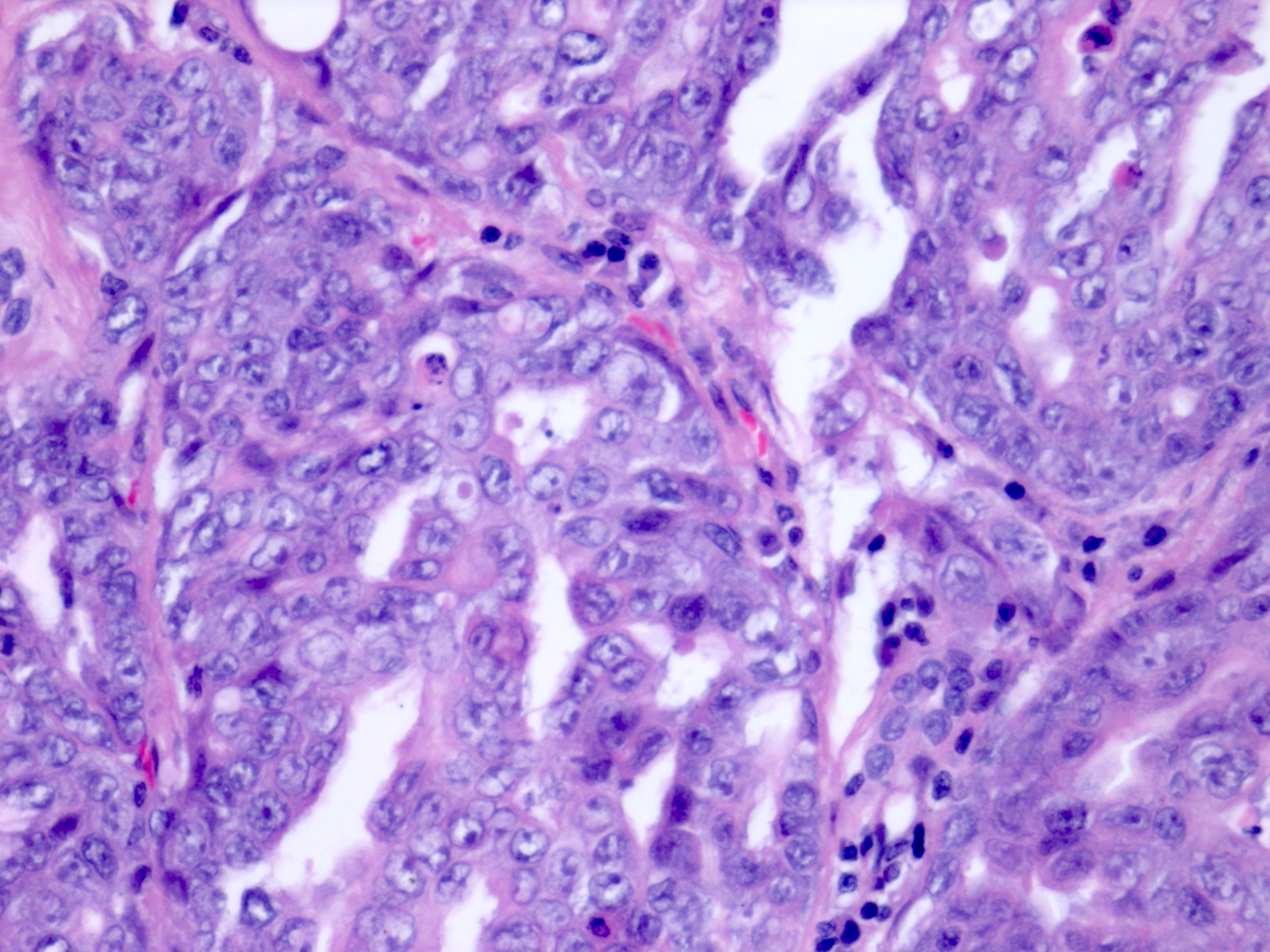
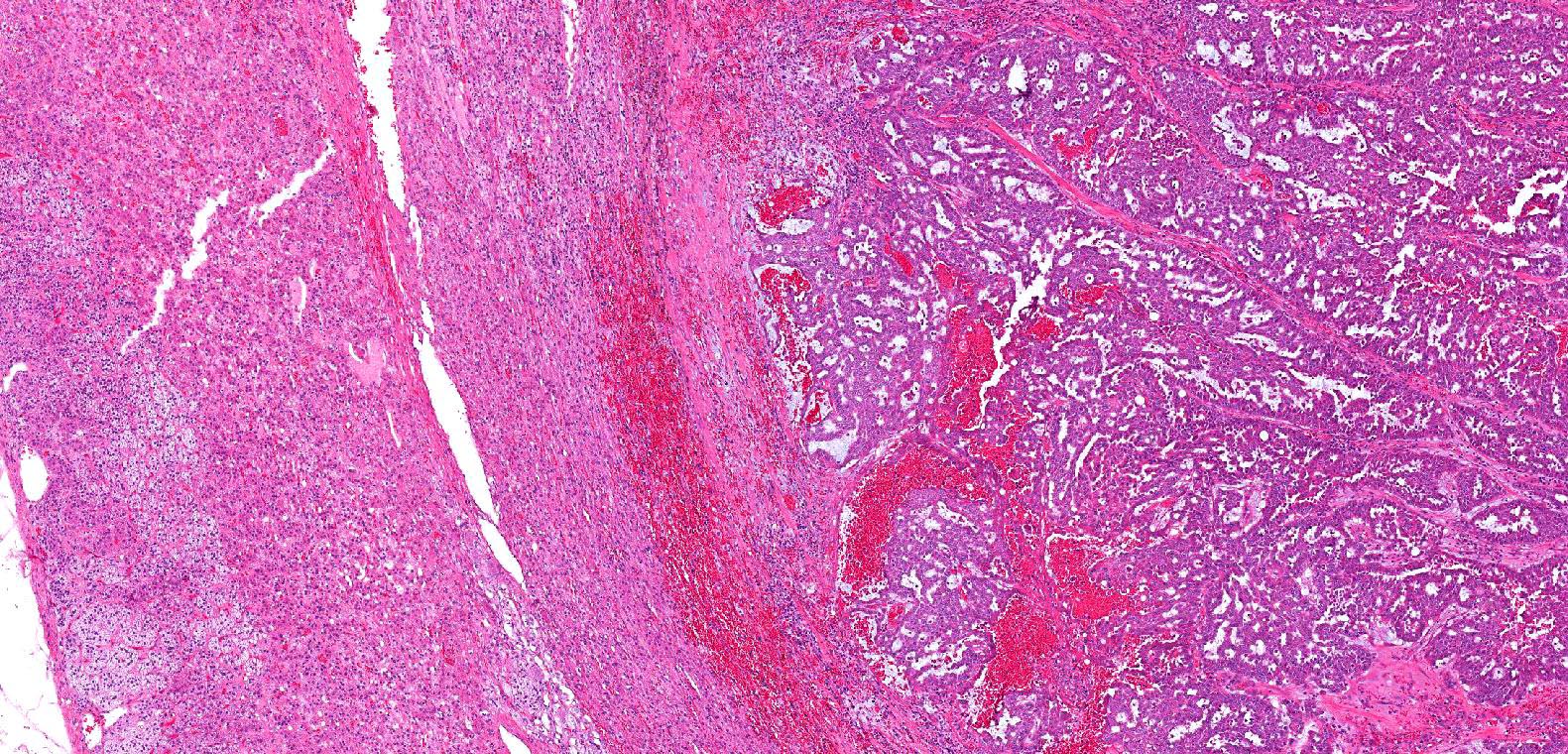
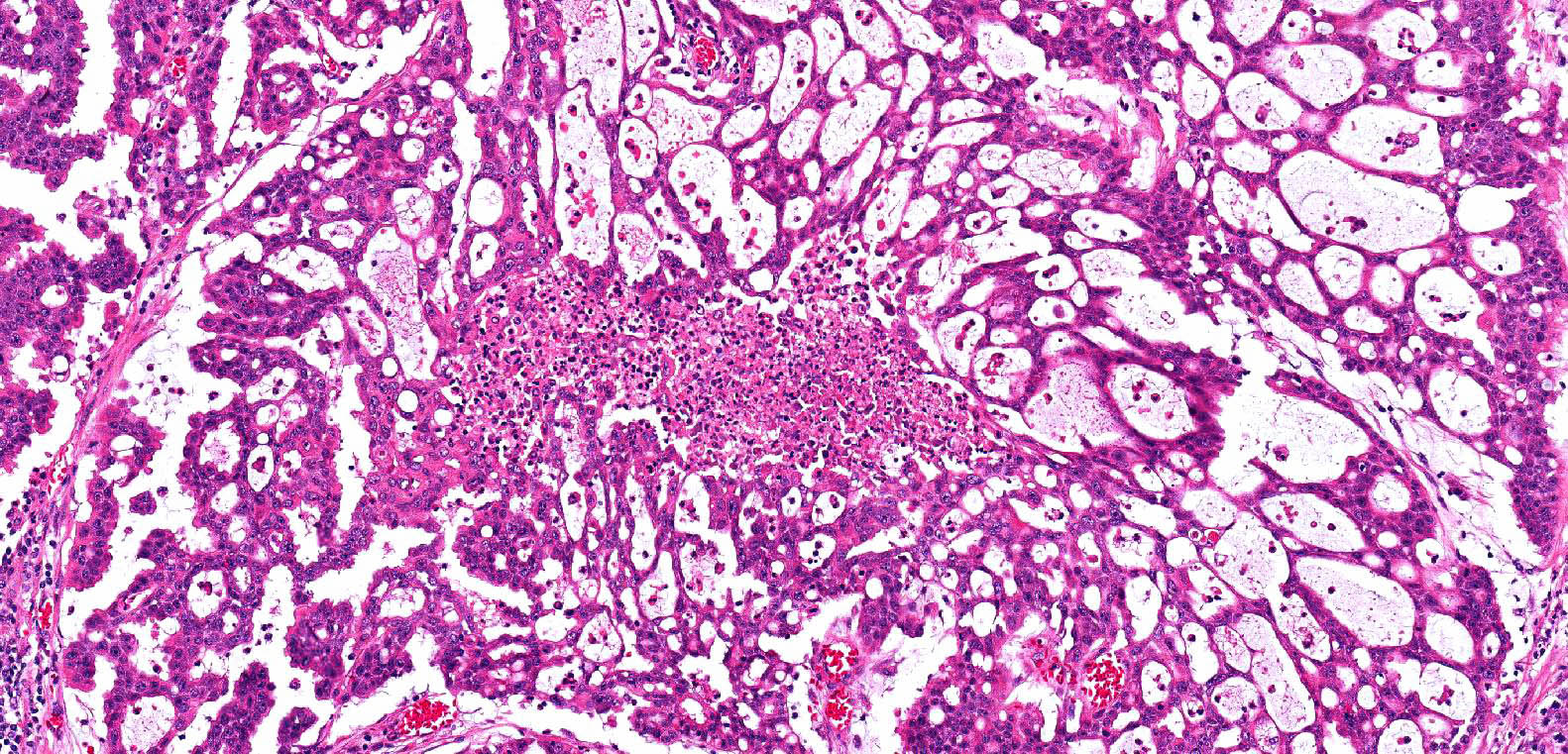
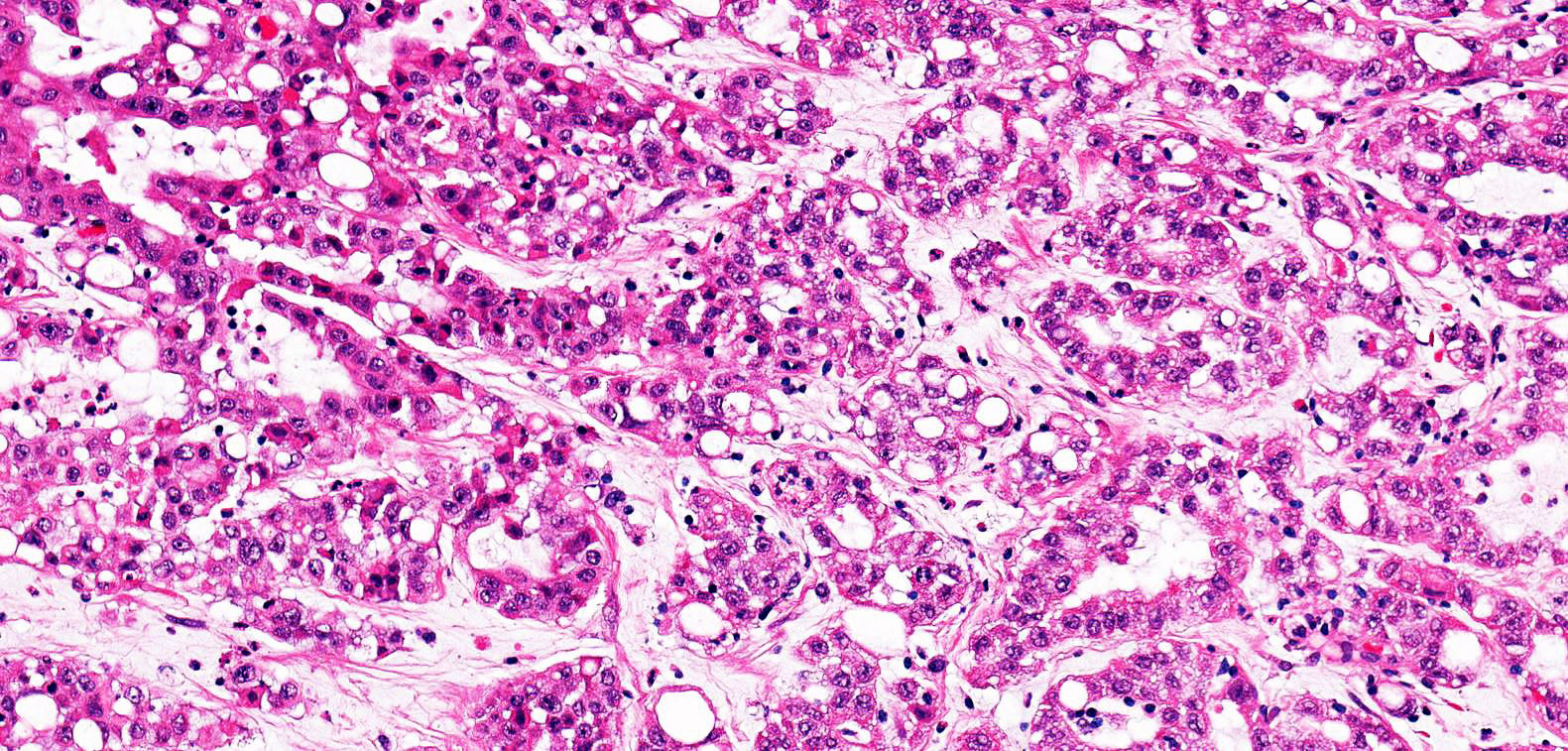
- Variety of morphologic patterns:
- Distinctive patterns include reticular / yolk sac tumor-like and sieve-like / cribriform / microcystic / adenoid cystic-like
- Cords, nests, tubular, glandular, tubulopapillary and solid / sheet-like patterns overlaps with collecting duct carcinoma and fumarate hydratase (FH) deficient renal cell carcinoma (Am J Surg Pathol 2018;42:279)
- Tumor cells are pleomorphic with hyperchromatic enlarged nuclei, vesicular chromatin, prominent nucleoli, nuclear grooves, eosinophilic cytoplasm and may have rhabdoid features
- Hemorrhage, necrosis (may be ischemic, geographic or central / comedo type)
- Frequent mitotic figures
- Angiolymphatic invasion, desmoplastic stroma and infiltrative borders
- Abundant intratumoral neutrophils (sometimes abscess forming) and lymphocytes at tumor rim
- Abundant sickled erythrocytes (drepanocytes) may be pathognomonic
Microscopic (histologic) images
Cytology description
- Loosely cohesive groups, sheets or single cells
- High grade / pleomorphic cells with vacuolated fine pale cytoplasm that often displace nuclei
- Nuclei often have irregular membranes, coarse or vesicular chromatin, smooth borders, membrane grooves and prominent nucleoli (Cancer 2005;105:28, J Am Soc Cytopathol 2021;10:187)
Positive stains
- CAM 5.2, AE1 / AE3, CK7 and CK20 (CK7 and CK20 may be variable), vimentin, EMA and CEA (Urology 2002;60:1083, Am J Surg Pathol 2013;37:368)
- p53 and PAX8 (Urology 2002;60:1083, Mod Pathol 2009;22:1218)
- OCT 3/4 in 50% of cases (Am J Surg Pathol 2012;36:583)
- Ulex may be focally positive in a minority of cases (Urology 2002;60:1083)
- May be strongly positive for vascular endothelial growth factor (VEGF) and hypoxia inducible factor (HIF) (Urology 2002;60:1083)
Negative stains
- Loss of expression of SMARCB1 (also known as INI1, SNF5 or BAF47), colloidal iron, PAS, desmin (Mod Pathol 2008;21:647)
- CK 34 beta E12 (Urology 2002;60:1083)
Electron microscopy description
- Cells contain tight junctions and large intracytoplasmic lumina, which contain long slender microvilli
- Condensed fibrillary electron dense filamentous luminal deposits are also seen (Ultrastruct Pathol 2008;32:252)
Molecular / cytogenetics description
- Molecular:
- Inactivation of SMARCB1 / INI1 (22q11.2) from chromosomal translocations or deletions is a major driver mutation
- SMARCB1 protein expression loss arises through concurrent hemizygous loss and translocation or by homozygous loss
- Loss of INI1 has been linked to downregulation of p16INK4a and upregulation of cyclin D1, promoting unregulated progression through the cell cycle (J Oncol Pract 2017;13:414, Am J Surg Pathol 2013;37:368, Adv Anat Pathol 2017;24:65)
- Hypoxia inducible factor and VHL abnormalities (Hum Pathol 2011;42:1979)
- DNA topoisomerase II amplification (BJU Int 2010;106:62)
- Chromosome 8q gain, where c-MYC is located, was noted in 46.7% of renal medullary carcinoma tumors and contains recurrent focal copy number alterations (CNA) in regions of genes related to cell proliferation including a distinct CNA pattern that results in Notch pathway activation (Cancer Cell 2020;37:720)
- Inactivation of SMARCB1 / INI1 (22q11.2) from chromosomal translocations or deletions is a major driver mutation
- Cytogenetics (Arch Pathol Lab Med 2019;143:1556, Eur Urol 2016;69:1055, Histopathology 2012;61:428, Am J Surg Pathol 2011;35:e47):
- Rearrangement of chromosome 8 resulting in a loss of 8p and a gain of 8q can be observed
- Gains of chromosomes 7, 8, 10 and 11 can be seen along with losses of chromosomes 9 and 13
- Additional unknown material on 10p, a deletion of 7q and a dicentric chromosome composed of 13q and 21q
- Rare instances of ABL gene amplification or translocation have also been described
Videos
Pathology mini tutorial of renal medullary carcinoma
Cytomorphologic study of renal medullary carcinoma involving serous cavity fluids
Sample pathology report
- Right kidney, radical nephrectomy:
- SMARCB1 deficient renal medullary carcinoma (X cm), extends into the renal sinus, margins are negative (see comment and synoptic report)
- Comment: The patient's history of sickle cell trait is noted. Immunohistochemistry for SMARCB1 (INI1) is performed and shows loss of expression within the tumor cells. The clinical, morphologic and immunohistochemical findings support the diagnosis.
Differential diagnosis
- Viniculin (VCL)::ALK fusion renal cell carcinoma:
- Also occurs in young patients with sickle cell trait, usually younger (mean age of 9 years)
- Shows VCL::ALK fusion, retained (positive) INI1 and much lower proliferative activity (Am J Surg Pathol 2014;38:858)
- Rhabdoid tumor of the kidney:
- Occurs in very young patients (mean / median patient age of 1 year), nearly all cases occurring in patients < 3 years of age; clinically lacks sickle cell trait
- Shows loss of SMARCB1 (INI1)
- Diverse pattern of immunoreactivity may include cytokeratins, vimentin, SMA, synaptophysin, GFAP and CD99
- Many show eosinophilic intracytoplasmic inclusions
- High grade urothelial carcinoma:
- Collecting duct carcinoma:
- Older patients (mean age of 55 years, whereas renal medullary carcinoma is uncommon in patients older than 40 years of age)
- Not associated with sickle cell trait
- Retained SMARCB1 (collecting duct carcinomas have been reported to have secondary loss of SMARCB1 / INI1 in up to 15% of cases)
- Negative for OCT 3/4
- Multinodular, infiltrating papillary morphologic patterns may be favored (Am J Surg Pathol 2018;42:279)
- Metastatic germ cell neoplasm (e.g., embyronal carcinoma) (Am J Surg Pathol 2012;36:583):
- Fumarate hydratase (FH) deficient renal cell carcinoma:
- Often viral inclusion-like (CMV-like) macronucleoli with perinucleolar halos
- Tubulocystic and intracystic papillary with or without hyalinization patterns may favor FH deficient RCC (Am J Surg Pathol 2018;42:279)
- Loss of fumarate hydratase, nuclear and cytoplasmic expression of 2SC
- Negative for OCT 3/4 (Am J Surg Pathol 2018;42:279)
- Rare secondary SMARCB1 loss has been reported (Hum Pathol 2018;77:139)
- SMARCB1 deficient renal cell carcinoma with medullary-like features or phenotype, also known as renal cell carcinoma, unclassified, with medullary phenotype (RCCU MP) (Hum Pathol 2017;67:134):
- Morphologically similar to renal medullary carcinoma, lacking SMARCB1 expression by immunohistochemistry but without evidence of a hemoglobinopathy (Hum Pathol 2017;67:134)
- Other renal cell carcinoma subtype with secondary SMARCB1 (INI1) deficiency
- If the size of the tumor obscures the site of origin, infiltrative growth that extends between normal structures, such as glomeruli and tubules, can be seen as a clue that the tumor is medullary based
- This pattern can be seen in collecting duct carcinoma, medullary carcinoma and invasive urothelial carcinoma; similar patterns can also be seen in metastatic tumors and lymphoma (Surg Pathol Clin 2015;8:587)
Additional references
Practice question #1
A 24 year old man with sickle cell trait presents with hematuria and flank pain. Imaging shows a 6 cm medulla centered renal mass. A radical nephrectomy is performed. What will the pathologic workup likely show?
- Inactivation of 12q11.2 due to deletion or translocation
- Papillary growth with pale to eosinophilic, flocculent cytoplasm
- Reticular or cribriform growth pattern with high grade cells
- TFE3 (on Xp11) translocation with ASPSCR1 (ASPL)
Practice answer #1
C. Reticular or cribriform growth pattern with high grade cells. SMARCB1 deficient renal medullary carcinoma, also known as renal medullary carcinoma, often shows a reticular / yolk sac tumor-like and sieve-like / cribriform / microcystic / adenoid cystic-like morphology on a desmoplastic stroma with an associated inflammatory infiltrate. The tumor is found in patients with sickle cell trait and is defined by loss of SMARCB1 / INI1 (found on chromosome 22q11.2). TFE3 rearranged renal cell carcinoma often shows solid to papillary growth with psammoma bodies and can show voluminous clear to eosinophilic cytoplasm. TFE3 rearranged renal cell carcinomas are molecularly defined with TFE3 fusions with one of multiple genes including ASPSCR1 (ASPL), PRCC, NONO (P54NRB), SFPQ (PSF), RBM10, etc.
Comment here
Reference: SMARCB1 deficient renal medullary carcinoma
Comment here
Reference: SMARCB1 deficient renal medullary carcinoma
Practice question #2
A 1 year old boy with hypercalcemia and without evidence of any hemoglobinopathy presents with a right renal mass. Histologically, the tumor displays a diffuse growth pattern of rhabdoid cells with prominent nucleoli and intracytoplasmic inclusions. What similarities will this tumor show to renal medullary carcinoma?
- Both tumors have an indolent prognosis
- CD20 positivity
- May show positive staining for GFAP, SMA, synaptophysin, CD99
- Will show SMARCB1 (INI1) loss / inactivation by immunohistochemistry
Practice answer #2
D. Will show SMARCB1 (INI) loss / inactivation by immunohistochemistry. The scenario is describing rhabdoid tumor of the kidney. This entity will show biallelic inactivation of the SMARCB1 or SMARCA4 genes, leading to a lack of SMARCB1 / INI1 immunostaining, a feature shared with renal medullary carcinoma. Both tumors share a dismal prognosis. Rhabdoid tumors of the kidney have a reported 5 year overall survival rate ranging from < 15% to 25% and show a diverse range of immunoreactivity including cytokeratins, GFAP, SMA, synaptophysin and CD99 staining. CD20 positivity has not been described in either tumor.
Comment here
Reference: SMARCB1 deficient renal medullary carcinoma
Comment here
Reference: SMARCB1 deficient renal medullary carcinoma