Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2 | Practice question #3 | Practice answer #3Cite this page: Chen Z, Aron M. Leydig cell tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/testisleydig.html. Accessed October 5th, 2025.
Definition / general
- Most common sex cord stromal tumor of the testis; comprised of cells resembling nonneoplastic Leydig cells
- A small minority (< 10%) of cases are clinically malignant (World J Urol 2020;38:2857, Int J Urol 2010;17:886)
Essential features
- Most common sex cord stromal tumor of the testis
- Histology: diffuse / nodular growth of polygonal cells with abundant eosinophilic cytoplasm, uniform round nuclei and prominent central nucleoli; Reinke crystals may be present
- Immunohistochemistry: inhibin A+, calretinin+, MelanA+, SF1+, AR+
- Features associated with malignant potential include: size > 5 cm, infiltrative borders, cytological atypia, frequent mitoses (> 3/10 high power fields), vascular invasion and necrosis
- Treatment: surgical resection, curative for nonmetastasizing tumors
- Prognosis: overall 5 year survival after orchiectomy > 90%
Terminology
- Interstitial cell tumor - obsolete term
ICD coding
- ICD-10: D40.10 - Leydig cell tumor of testis
Epidemiology
- 1 - 2% of testicular tumors in adults and 3 - 6% of testicular tumors in prepubertal males (J Urol 2009;181:2299, J Pediatr Hematol Oncol 2019;41:74)
- Mostly sporadic, rarely associated with hereditary leiomyomatous and renal cell carcinoma syndrome (J Clin Endocrinol Metab 2006;91:3071)
- Occurs at any age with 2 peaks: 5 - 10 years and 30 - 60 years (J Urol 2009;181:2299)
Sites
- Testicular parenchyma
- Rarely in ectopic rests of Leydig cells in the epididymis (Andrologia 2013;45:430)
Pathophysiology
- Produces androgen, mainly testosterone, which can cause symptoms described below
- Can also produce estrogen by either direct production of estradiol or by peripheral aromatization of testosterone (Arch Pathol Lab Med 2007;131:311)
Etiology
- Little is known
- Rare association with germline fumarate hydratase mutations in patients with hereditary leiomyomatosis and renal cell carcinoma syndrome and activating mutations of the luteinizing hormone receptor (N Engl J Med 1999;341:1731)
Clinical features
- > 90% benign
- Usually unilateral, rarely bilateral
- Painless testicular enlargement
- Children
- Precocious puberty caused by androgen production
- Gynecomastia and breast tenderness due to estrogen production (Arch Pathol Lab Med 2007;131:311)
- Adults
- Gynecomastia
- Infertility, loss of libido and erectile dysfunction (Hum Reprod 2019;34:1389)
- Rarely, Cushing syndrome (Urology 2000;56:153)
- Malignant Leydig cell tumors may rarely present with metastases, usually to the retroperitoneal lymph nodes or lungs (Am J Surg Pathol 1985;9:177, J Urol 2020;203:949)
Diagnosis
- Tumor histology and immunohistochemistry
Laboratory
- Serum testosterone and estrogen levels may be elevated
- Lower sperm concentration, lower total sperm count and motility (Hum Reprod 2019;34:1389)
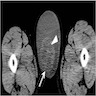
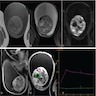
Radiology description
- Nonspecific
- On ultrasound, tumors are generally well defined, homogeneous hypoechoic, small solid masses with hypervascularity (Arch Pathol Lab Med 2007;131:311)
- May show cystic areas
Radiology images
Prognostic factors
- Benign Leydig cell tumors: excellent prognosis, curative by surgery
- Malignant Leydig cell tumors: poor survival, most develop metastatic disease leading to death (J Urol 2020;203:949)
Case reports
- 6 year old boy with precocious puberty and a left testicular mass (J Indian Assoc Pediatr Surg 2017;22:181)
- 32 year old man with gynecomastia and a small mass in the right testis (Case Rep Urol 2018;2018:7202560)
- 45 year old man with a large painless mass in the right testis (Urol Case Rep 2019;28:101064)
- 62 year old man with a huge painless mass in the right testis (Medicine (Baltimore) 2018;97:e11158)
- 91 year old man with a malignant Leydig cell tumor in the left testis (Int Braz J Urol 2019;45:1260)
Treatment
- Benign Leydig cell tumors
- Orchidectomy
- Testis sparing surgery in small tumors is a safe alternative (Hum Reprod 2019;34:1389, World J Urol 2020;38:2857)
- Malignant Leydig cell tumors
- Orchiectomy with retroperitoneal lymph node dissection can be curative
- No significant response to radiation or chemotherapy (Oncology (Williston Park) 2014;28:211,, World J Urol 2020;38:2857)
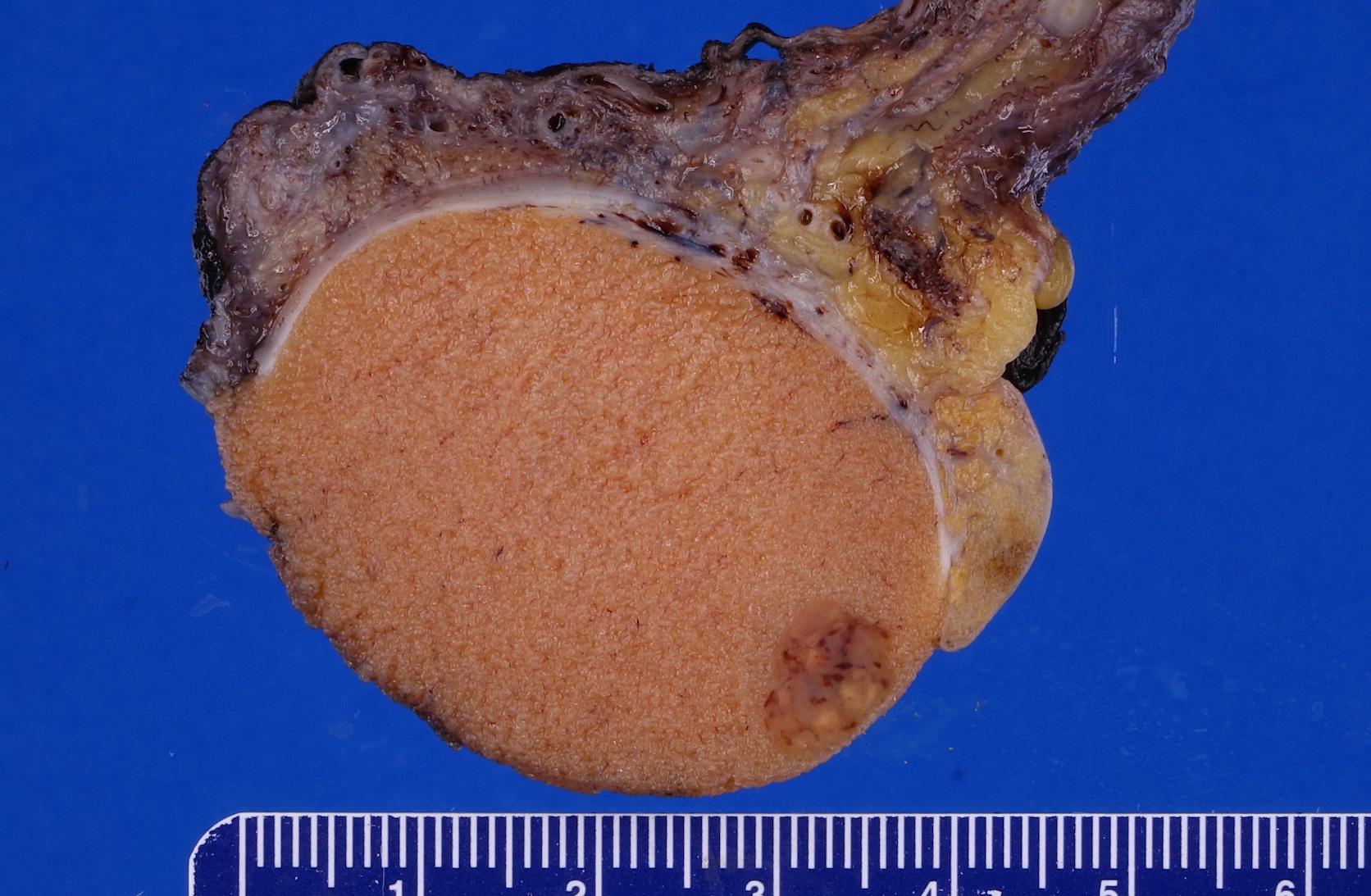
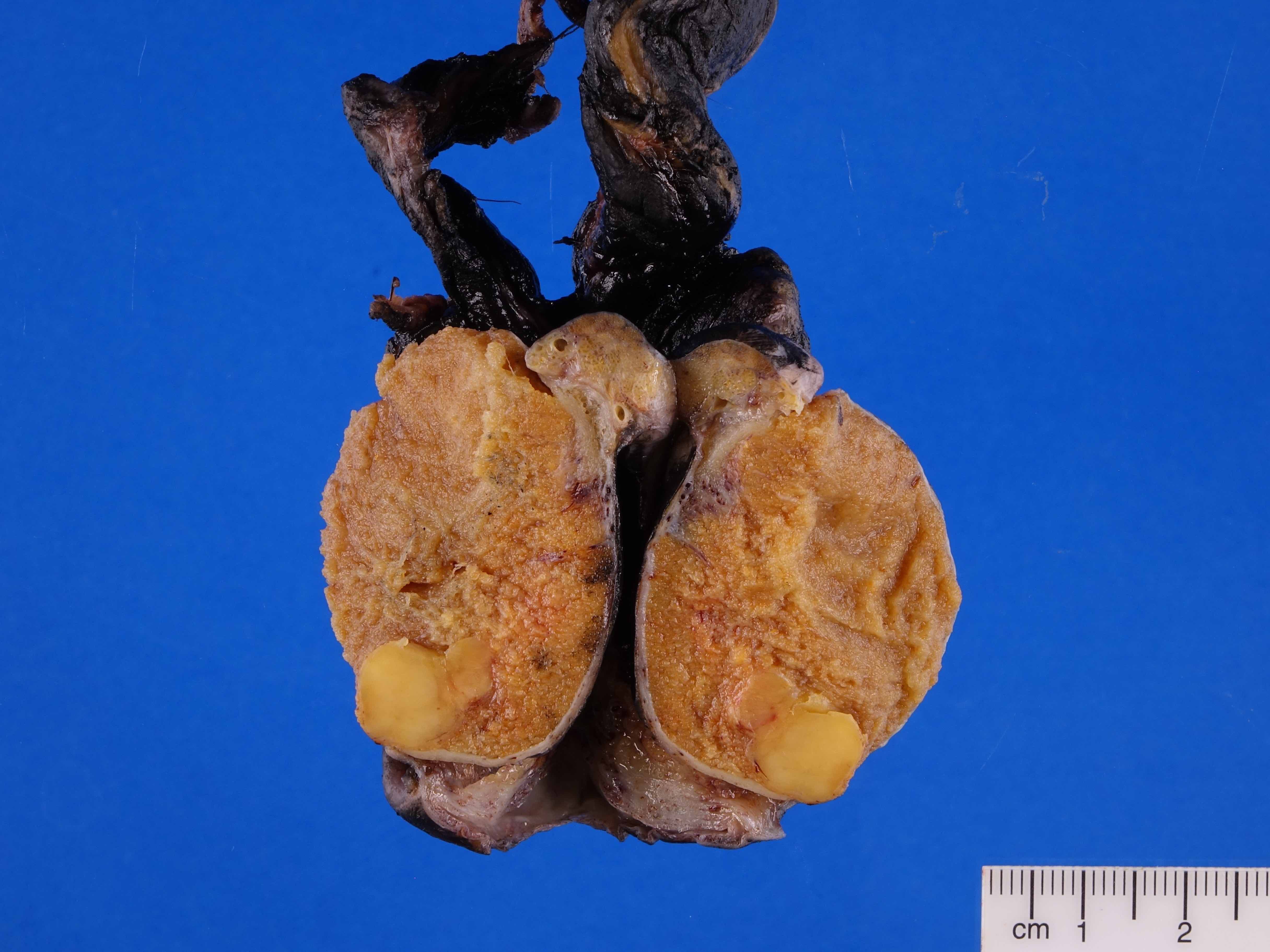
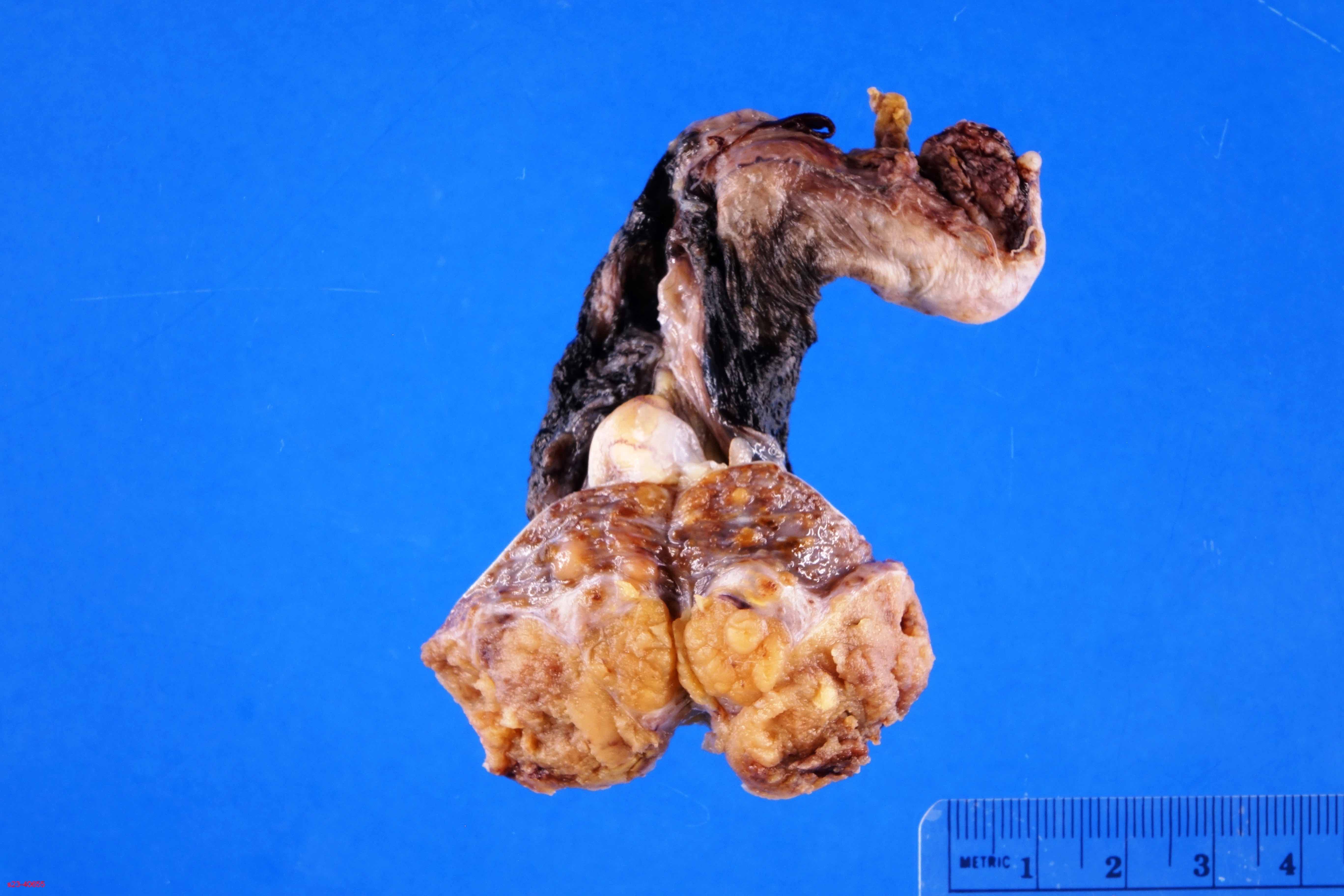
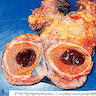
Gross description
- Well circumscribed, solid homogeneous mass
- Usually < 5 cm in size
- Golden brown or brownish green cut surface
- Hyalinization and calcification may be present
- Gross features suggestive of malignancy (Arch Pathol Lab Med 2007;131:311)
- Large size: > 5 cm
- Infiltrative margins
- Hemorrhage and necrosis
- Extratesticular extension
Gross images
Frozen section description
- Diffuse sheets of uniform polygonal cells with round nuclei, central nucleoli, abundant granular, eosinophilic cytoplasm and rectangular to club shaped Reinke crystals (Hum Pathol 2015;46:600)
- Touch imprint and scrape smear preparations are better to highlight Reinke crystals (Arch Pathol Lab Med 2005;129:e65)
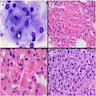
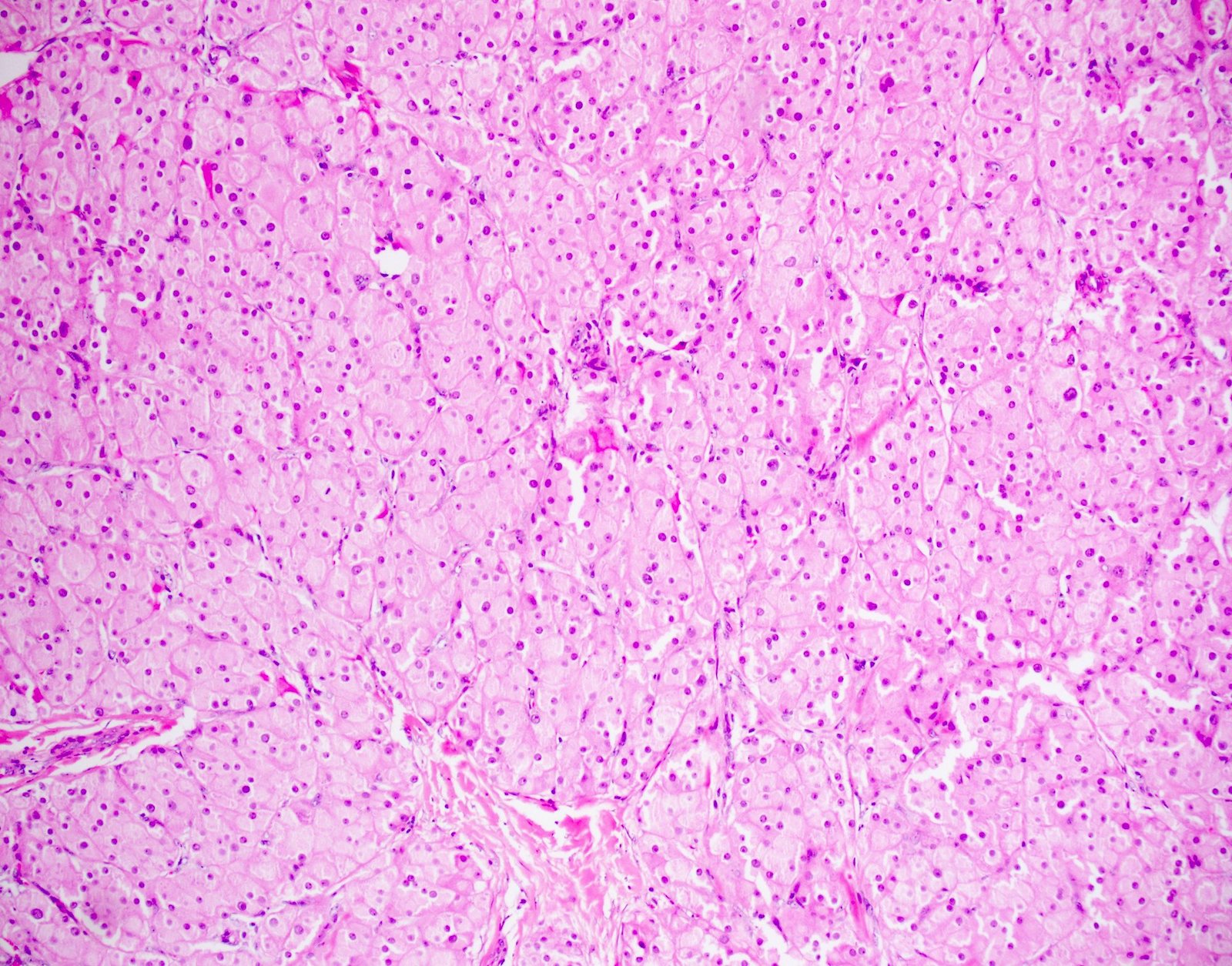
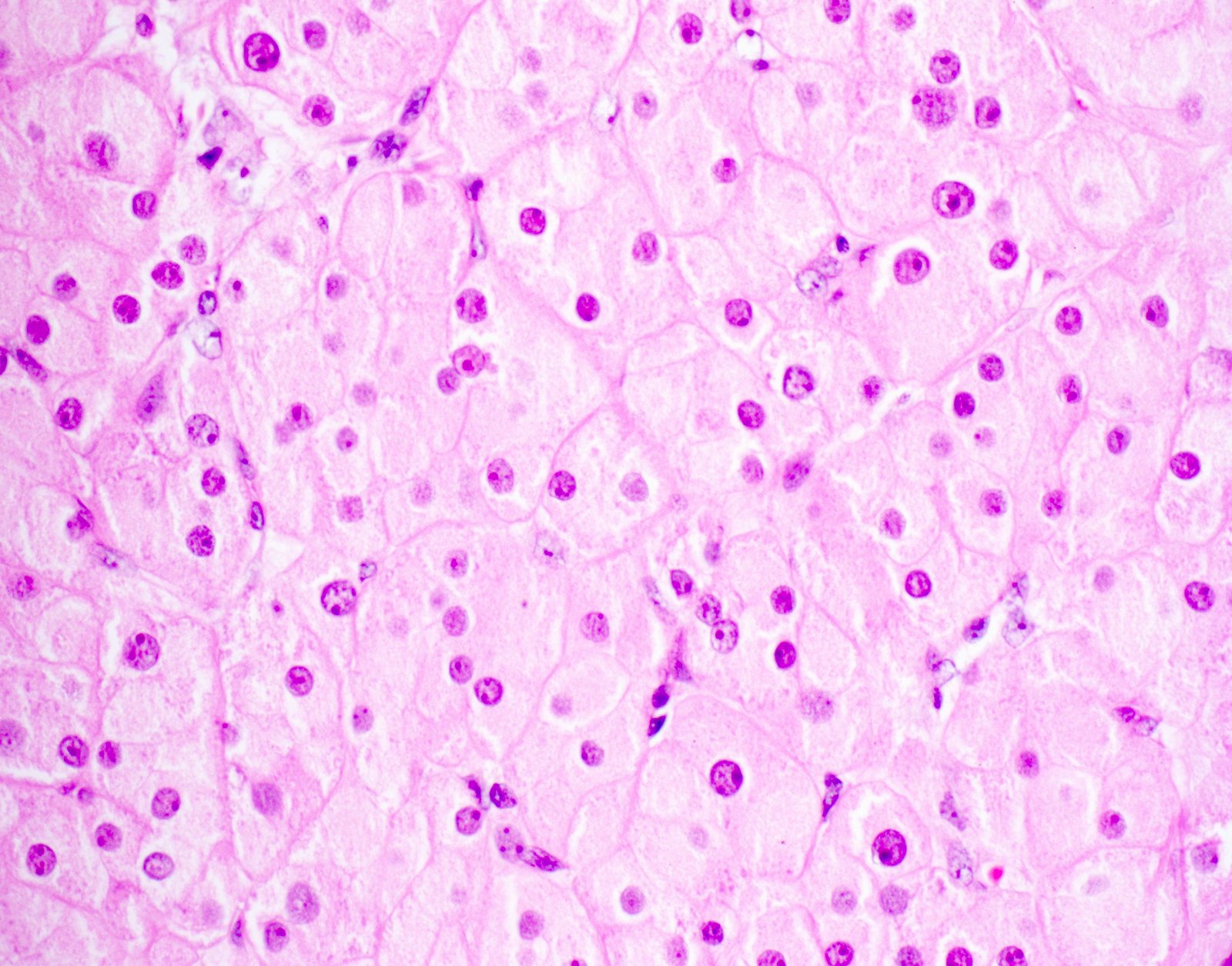
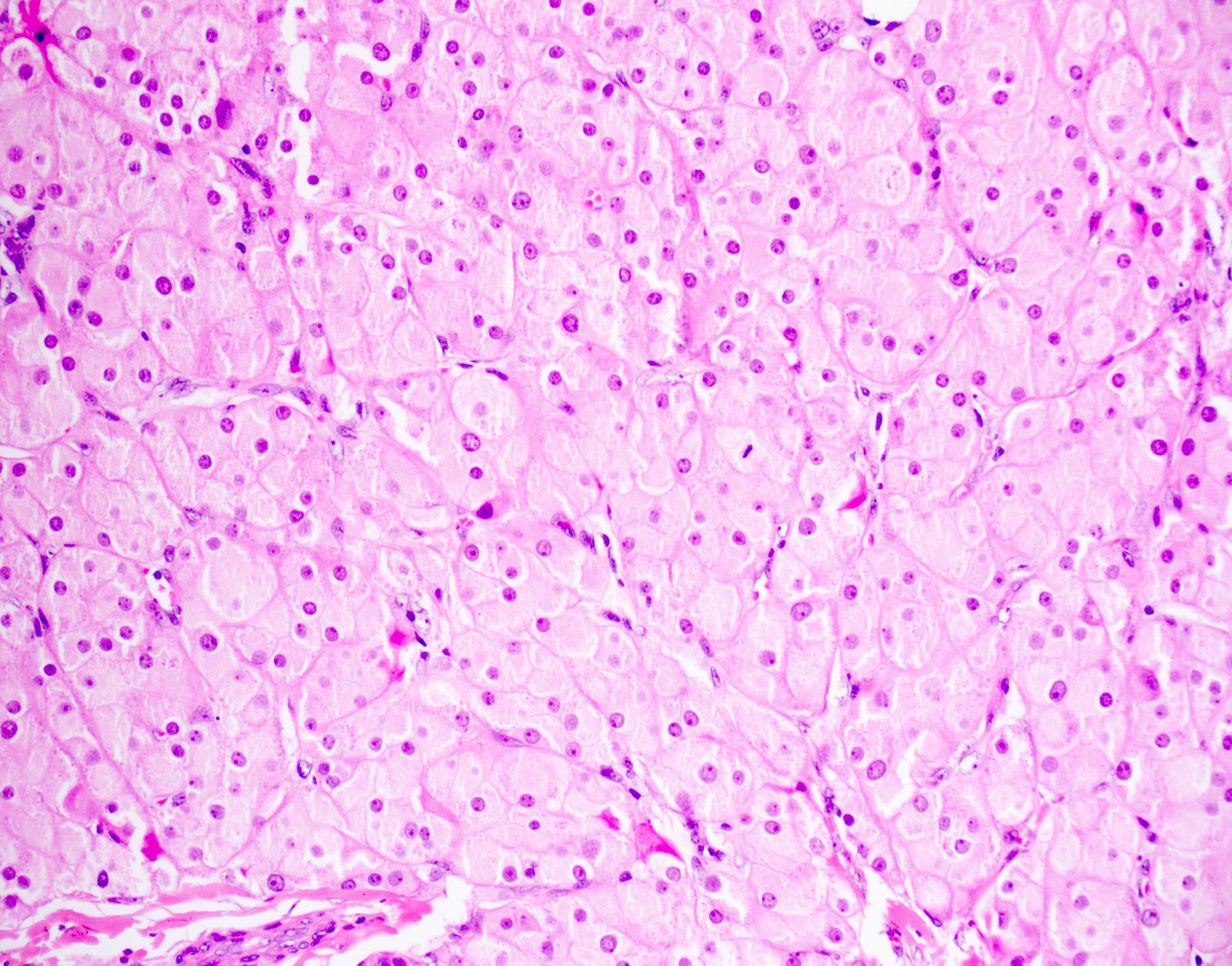
Microscopic (histologic) description
- Architecture:
- Diffuse or nodular with fibrous bands
- Uncommon patterns: insular, trabecular, pseudotubular, ribbon-like, trabecular, spindled and microcystic (Surg Pathol Clin 2018;11:739)
- Cytologic features:
- Polygonal cells with abundant eosinophilic granular cytoplasm, uniform round nuclei and prominent central nucleoli; rarely, nuclei may have a ground glass appearance
- Uncommon cell types: scant cytoplasm, foamy cytoplasm and spindling (Am J Surg Pathol 2002;26:1424)
- Lipofuscin pigment maybe present: golden yellow on H&E stain, red-purple granular appearance on PAS stain
- Binucleated and multinucleated cells may be present
- Reinke crystals: pathognomonic; identified in only up to 30% (degradation / dissolution by formalin fixation); intracytoplasmic, rarely extracellular
- Mitosis: rare
- Mild nuclear atypia permissible
- Occasionally, psammoma bodies, calcification, osseous and adipocytic metaplasia may be identified (Am J Surg Pathol 2002;26:1424)
- Microscopic features suggestive of malignancy (most malignant tumors will have more than 2 of these features) (Am J Surg Pathol 1985;9:177):
- > 5 cm
- Infiltrative borders
- Cytological atypia
- Frequent mitoses (> 3/10 high power fields)
- Vascular invasion
- Necrosis
Microscopic (histologic) images
Cytology description
- Fine needle aspiration is rarely performed unless in a metastatic lymph node
- Cellular smears with discohesive cells having eccentric round nuclei, evenly distributed chromatin, prominent nucleoli and abundant eosinophilic granular cytoplasm
- Naked nuclei are common
- Cytoplasm may be vacuolated due to lipid accumulation
- Nuclear grooves, binucleation and multinucleation may be identified
- Nuclear pseudoinclusions and Reinke crystals can be seen
- No cytological features to differentiate Leydig cell tumors from nodular Leydig cell hyperplasia (J Am Soc Cytopathol 2019;8:220)
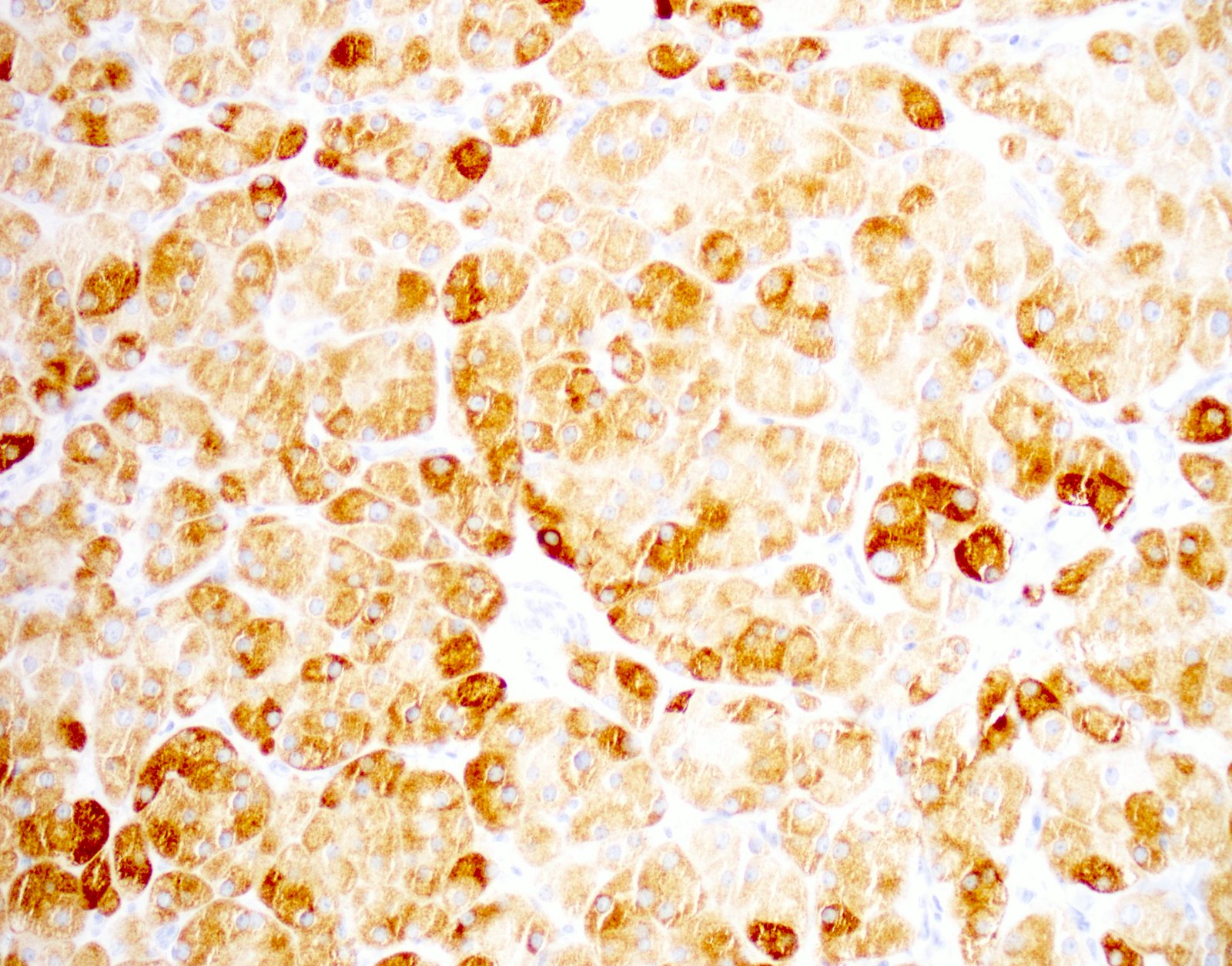
Positive stains
- Inhibin A, calretinin, MelanA, androgen receptor (AR) and steroidogenic factor 1 (SF1)
- Insulin-like 3 (INSL3) variably reported to be positive
- CD99 (MIC2) membranous staining (Histopathology 2021;78:290)
Negative stains
- SALL4, OCT4 and beta catenin (Am J Surg Pathol 2015;39:1390)
- Cytokeratin, chromogranin, synaptophysin, S100 and PLAP (rarely focally positive)
Electron microscopy description
- Reinke crystals are diagnostic
- Appearance depends on plane of sectioning: prismatics, hexagonal lattices or hexagonal microtubules with parallel lines
- Located in cytoplasm but can be seen in nucleus or interstitium
- Abundant smooth endoplasmic reticulum, mitochondria with tubulovesicular cristae, numerous lipid droplets and lipofuscin granules (Case Reports Histol Histopathol 1989;4:247)
Molecular / cytogenetics description
- DNA aneuploidy is associated with malignant Leydig cell tumors, benign Leydig cell tumors are diploid
- Gain of chromosome X, 19 or 19p and loss on chromosome 8 and 16 are most frequent findings (Oncol Rep 2007;17:585)
- Somatic GNAS (guanine nucleotide binding protein, alpha stimulating activity polypeptide 1)
- Activating mutation (R201S) has been reported (J Androl 2012;33:578)
Sample pathology report
- Right testicle, radical orchiectomy:
- Leydig cell tumor, 2 cm in greatest dimension (see synoptic report)
- Tumor limited to testis
- Resection margins uninvolved by tumor
- Note that benign tumors are not staged
Differential diagnosis
- Testicular tumor of adrenogenital syndrome or testicular adrenal rest tumors:
- Arise in males with congenital adrenal hyperplasia
- Hormonal profile with high 17-hydroxyprogesterone and adrenal androgen levels and frequent regression following dexamethasone treatment
- Usually bilateral, dark brown nodules
- Spotty cytological atypia, abundant cytoplasmic lipofuscin pigment but no Reinke crystals, broad bands of hyalinized collagenous stroma
- Androgen receptor and INSL3 negative
- More frequently positive for neuroendocrine markers (synaptophysin and CD56)
- Reference: Histopathology 2017;70:513
- Leydig cell hyperplasia:
- Interstitial growth pattern with small nodules < 0.5 cm; usually bilateral and multifocal
- Diffuse positivity for INSL3
- Reference: Appl Immunohistochem Mol Morphol 2019;27:203
- Granular cell tumor:
- Large cell calcifying Sertoli cell tumor:
- Associated with Carney complex
- Sertoli cells with abundant eosinophilic cytoplasm and extensive calcification, variable tubular or intratubular growth, stroma more myxoid and neutrophil rich, no Reinke crystals
- Positive for SMA and desmin, more diffuse S100 positivity
- Reference: Hum Pathol 2010;41:552
- Malakoplakia:
- Involves tubules and interstitium with xanthogranulomatous inflammation and Michaelis-Gutmann bodies
- Seminoma:
Practice question #1
Practice answer #1
Practice question #2
Malignant potential in Leydig cell tumor is associated with which of the following factors?
- Younger patient age
- Diffuse growth pattern
- Large tumor size (> 5 cm)
- Calcification
- Reinke crystals
Practice answer #2
Practice question #3
A 41 year old man was hit in the groin area by a baseball. An ultrasound showed a 3 cm tumor of the testis. On histology, the tumor showed diffuse sheet-like growth of cells with minimal stroma, shown above. The tumor cells were large and polygonal with abundant, slightly granular, eosinophilic cytoplasm. The tumor cell nuclei were round and contained a single prominent nucleolus. Rare intracytoplasmic eosinophilic crystals were identified. The most likely diagnosis of this tumor is which of the following?
- Adult granulosa cell tumor
- Juvenile granulosa cell tumor
- Leydig cell tumor
- Sertoli cell tumor
Practice answer #3