Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Differential diagnosisCite this page: Perrino C, Zynger DL. Ganglioneuroblastoma, intermixed and nodular. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/adrenalganglioneuroblastoma.html. Accessed September 9th, 2025.
Definition / general
- Neoplasm of neuroectodermal origin comprised of mixture of neuroblasts and ganglion cells in varying proportions
- Divided into stroma rich (well differentiated, intermixed, nodular) and stroma poor categories depending on amount of Schwannian, spindle cell stroma
- Intermixed: composite tumor in the stroma rich category
- Ganglioneuromatous tissue with interspersed, sharply defined, unencapsulated nests of variably differentiated neuroblastic cells
- Nodular: composite tumor in the stroma rich category
- Predominance of differentiated Schwannian type stroma associated with ≥ 1 macroscopically visible nodule(s) of neuroblasts showing neuropil formation but lacking Schwannian stroma (stroma poor nodule) (Mod Pathol 2015;28:166)
- Grossly identifiable mature and immature components may either both be in primary tumor or one may be in primary and one in metastasis (Lack: Tumors of the Adrenal Glands and Extraadrenal Paraganglia, AFIP, 2007))
Epidemiology
- 4th most common tumor in childhood
- 75 - 85% occur within first 4 years of life
- M = F
Sites
- Occur anywhere in anatomic distribution of sympathoadrenal neuroendocrine system
- ~80% arise within abdomen or adrenal gland
- ~20% within thoracic cavity
Etiology
- Clonal proliferation of immature cells of neural crest origin
Clinical features
- Asymptomatic
- Abdominal / back mass
- Watery diarrhea due to production of vasoactive intestinal polypeptide (Lack: Tumors of the Adrenal Glands and Extraadrenal Paraganglia, Volume 8, AFIP, Series 4)
- Patients with favorable histology lesions usually present with localized disease (stage I, II, III) (Pediatr Blood Cancer 2009;53:563)
- Patients with unfavorable histology lesions present with distant metastases in > 50% of cases (stage IV) (Pediatr Blood Cancer 2009;53:563)
Diagnosis
- 60 - 70% are advanced stage (III, IV) or have metastases at diagnosis (J Clin Oncol 1993;11:1466)
Laboratory
- Increased urine catecholamine metabolites (homovanillic acid, vanillylmandelic acid)
- Increased urine / serum dopamine as adjunct laboratory test
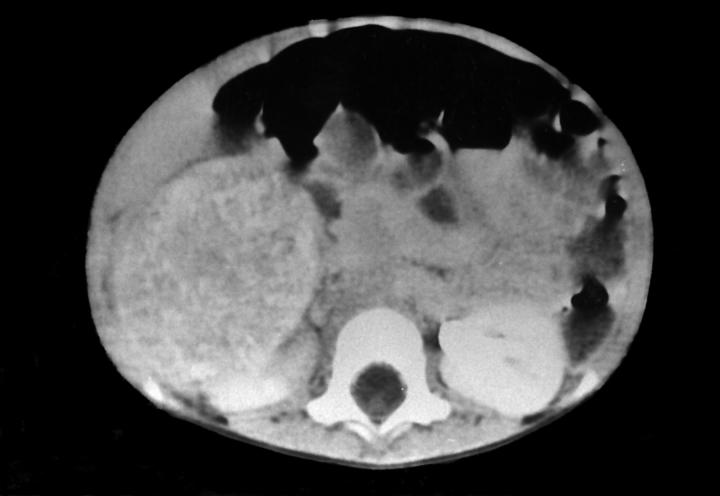
Radiology description
- MRI: hypointensity on T1 weighted image with rapid enhancement and hyperintensity on T2 weighted image (Intern Med 1995;34:1168)
Prognostic factors
- Multiple classification systems have been developed with the goal of stratifying patients into prognostic groups (see staging - neuroblastic tumors)
- In Shimada Classification, intermixed type is favorable histology
- Nodular subtype was initially categorized as unfavorable histology and in 2003, the INPC created 2 prognostically different subsets within this subtype (Cancer 2003;98:2274, Cancer 2000;89:1150)
- Favorable subset: composed of Schwannian rich, stroma dominant component favorable nodule(s)
- Poorly differentiated or differentiating neuroblastoma: mitosis karyorrhexis index ([MKI] count of cells undergoing mitosis or karryhorexis, based on 5,000 cell count from random fields) ≤ 200, age < 1.5 years
- Differentiating neuroblastoma: MKI < 100, age 1.5 - 5 years
- Unfavorable subset: composed of unfavorable nodule(s)
- Any neuroblastoma, MKI > 200, any age
- Any neuroblastoma, MKI 100 - 200, > 1.5 years
- Undifferentiated neuroblastoma, any age
- Poorly differentiated neuroblastoma, age > 1.5 years
- Any neuroblastoma, age > 5 years
Treatment
- Depends on prognostic stage (Pediatr Blood Cancer 2009;53:563, UpToDate: Treatment and prognosis of neuroblastoma)
- Low risk
- Surgical resection alone is mainstay
- Chemotherapy only if tumor is unresectable or symptoms of spinal cord / respiratory / bowel compromise
- Expectant observation in some infants with small adrenal masses, localized neuroblastoma or asymptomatic stage 4S disease
- Intermediate risk
- Surgical resection
- Moderate chemotherapy
- Radiation only if disease progresses despite surgery / chemotherapy
- High risk
- Induction: intensive chemotherapy
- Local control: surgical resection, radiation
- Consolidation: chemotherapy, myeloablative therapy, autologous stem cell transplant
- Maintenance: cis-retinoic acid or immunotherapy
- Low risk
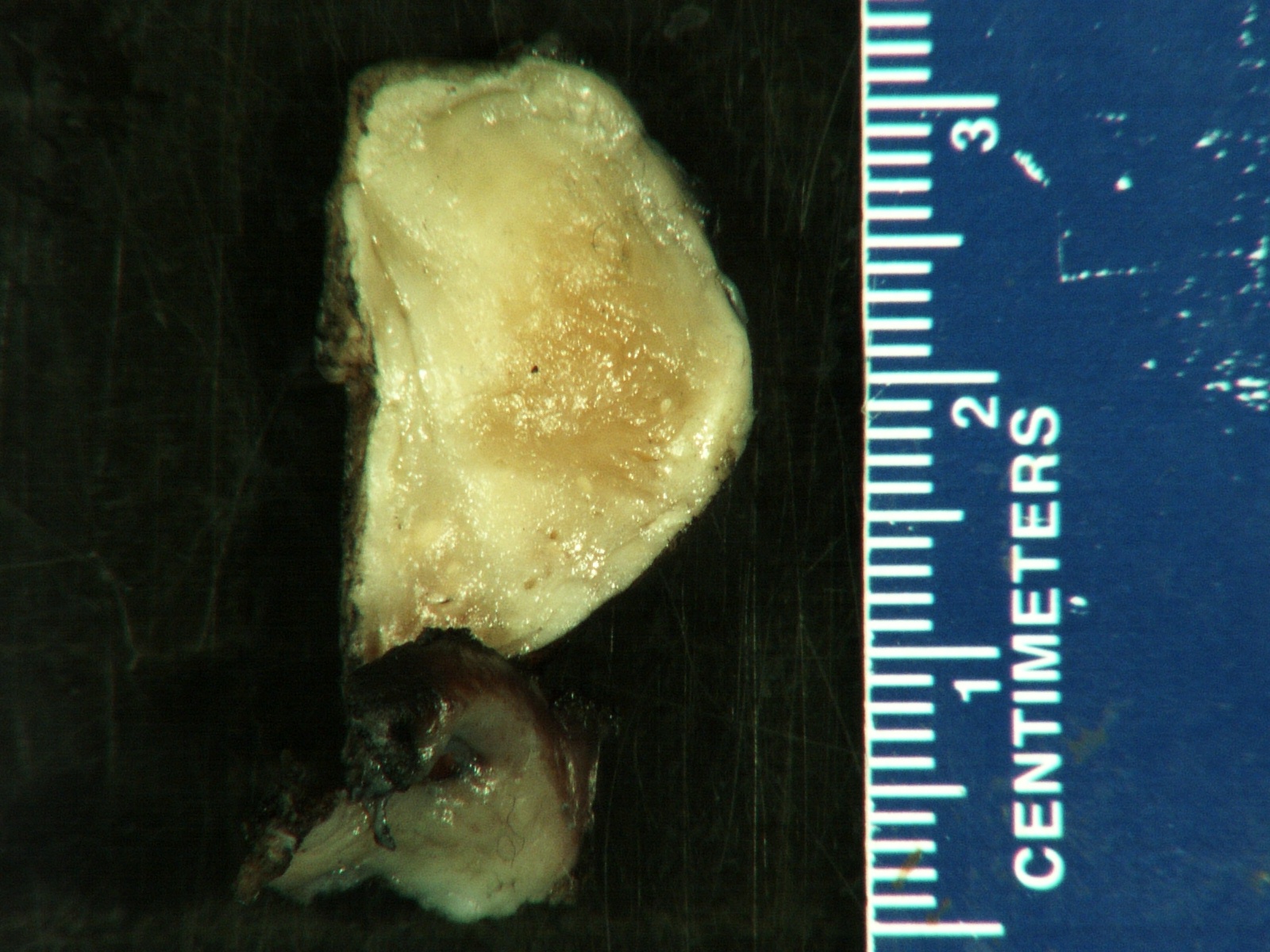
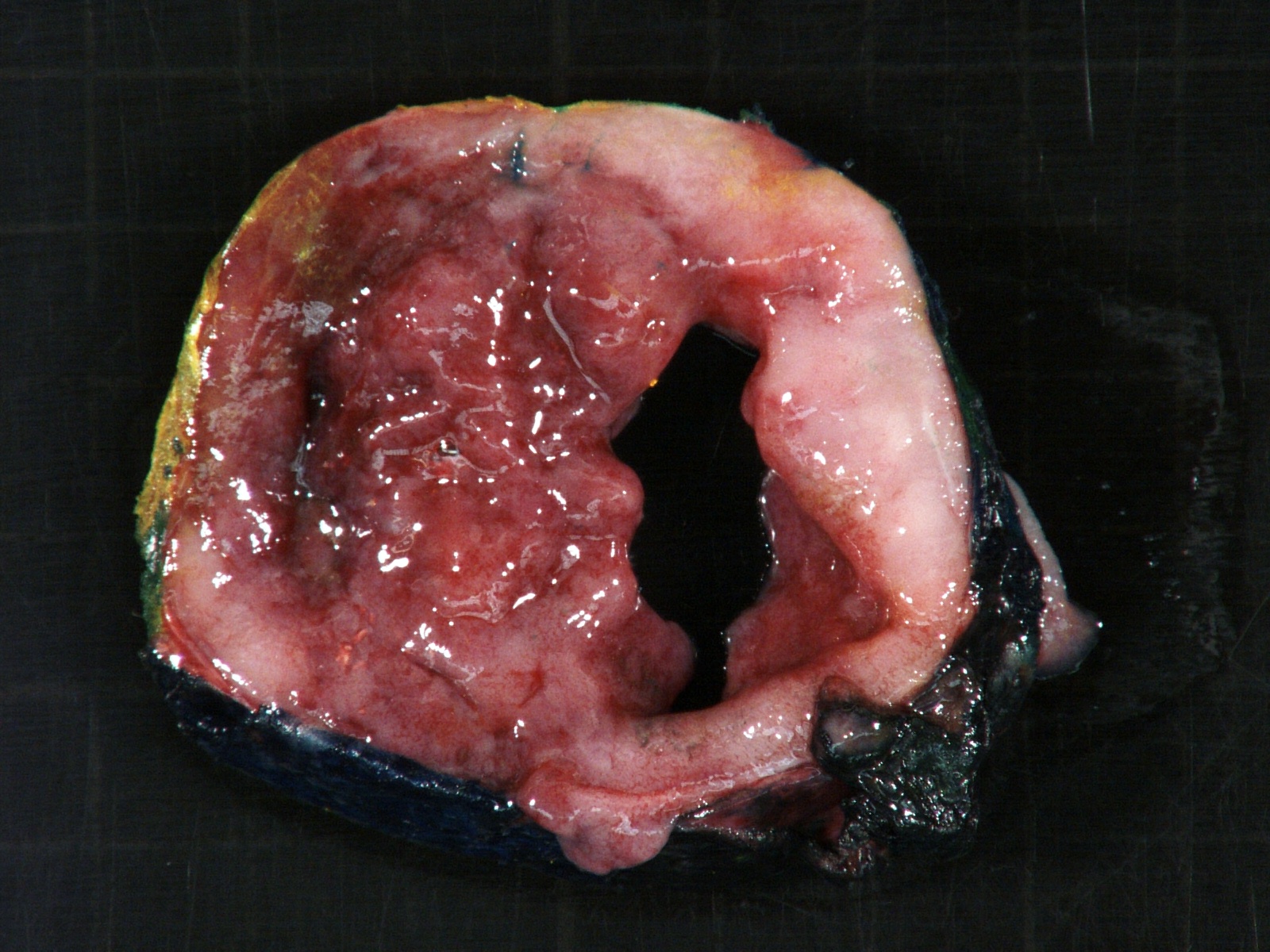
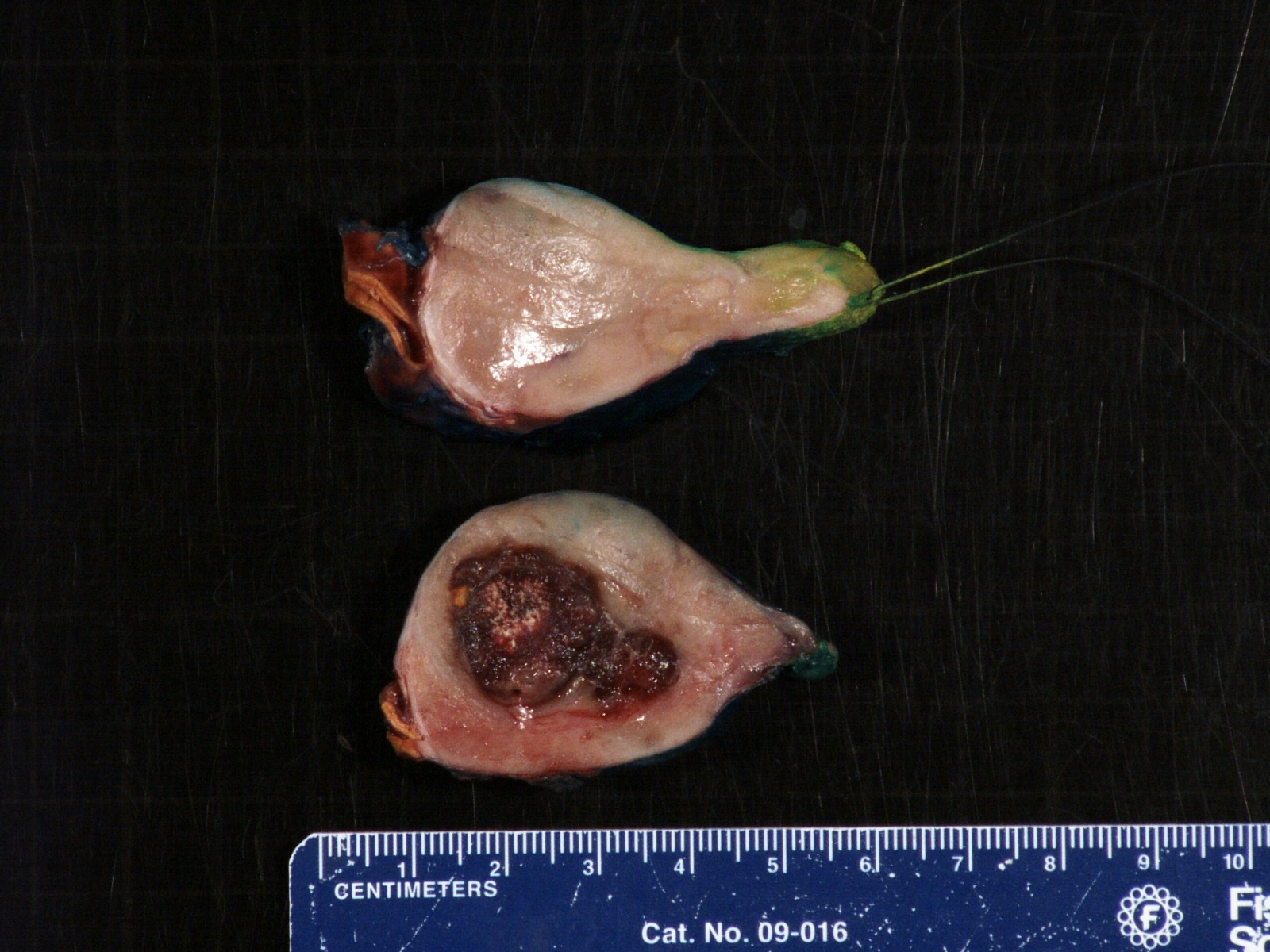
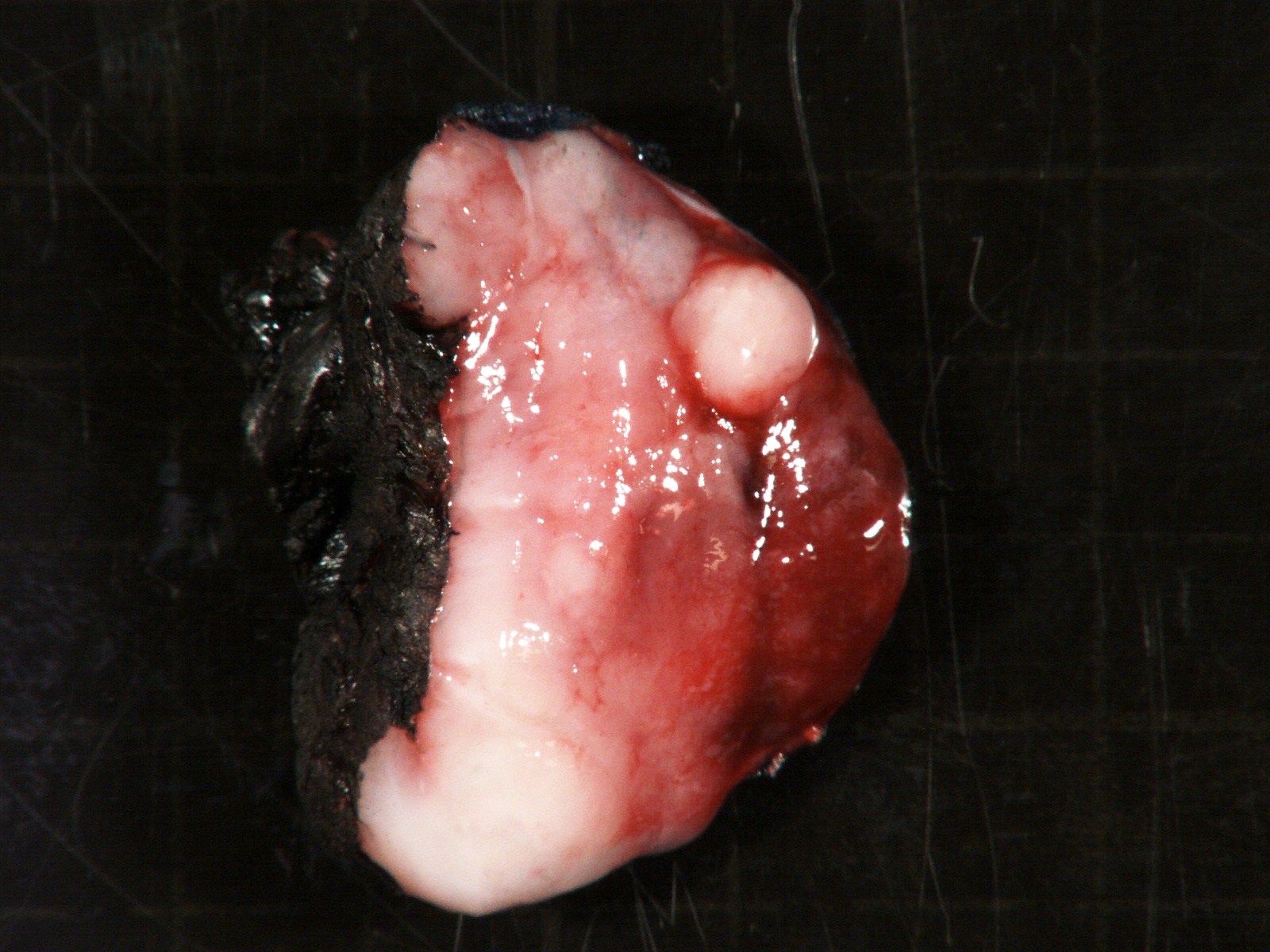
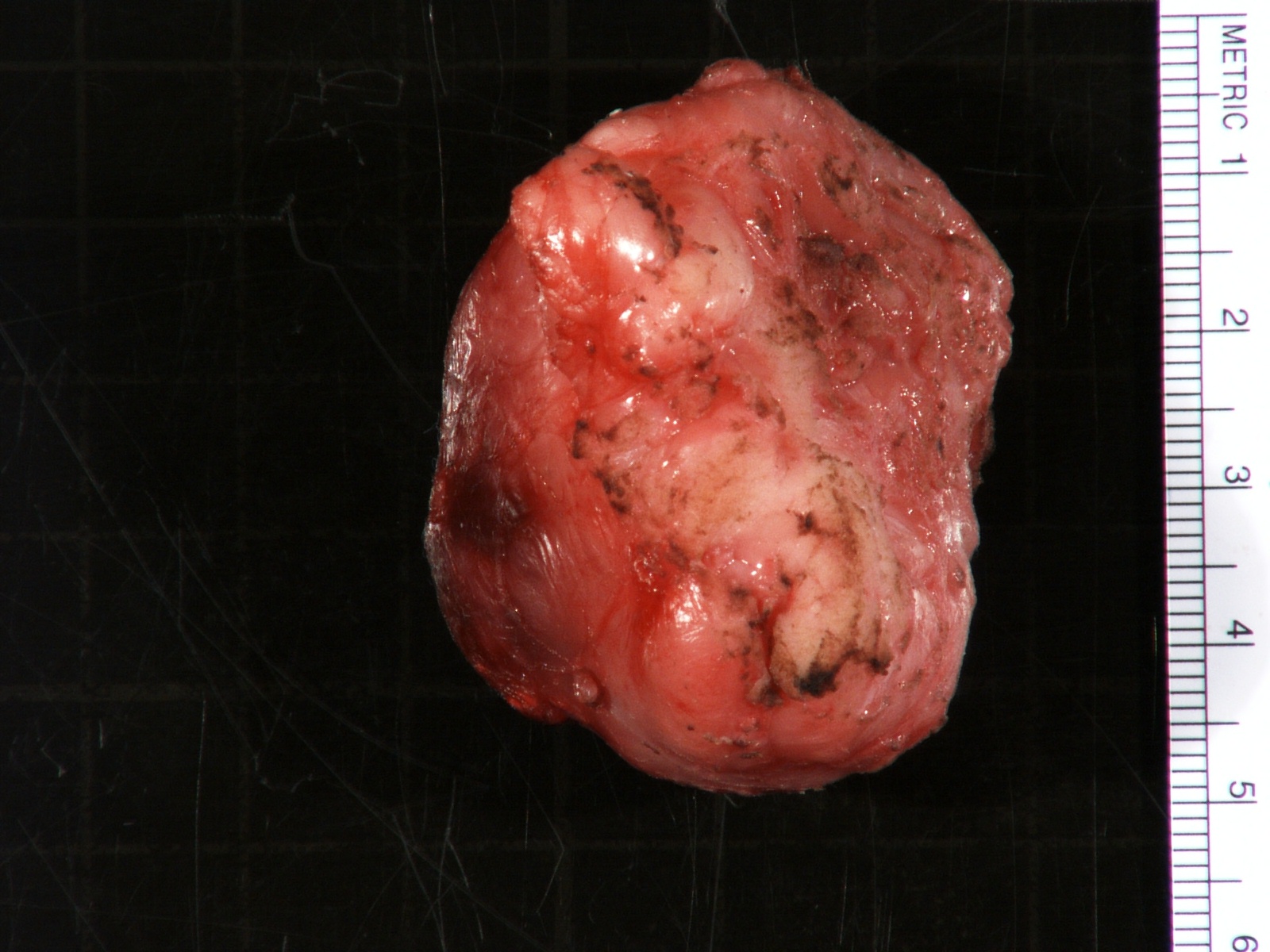
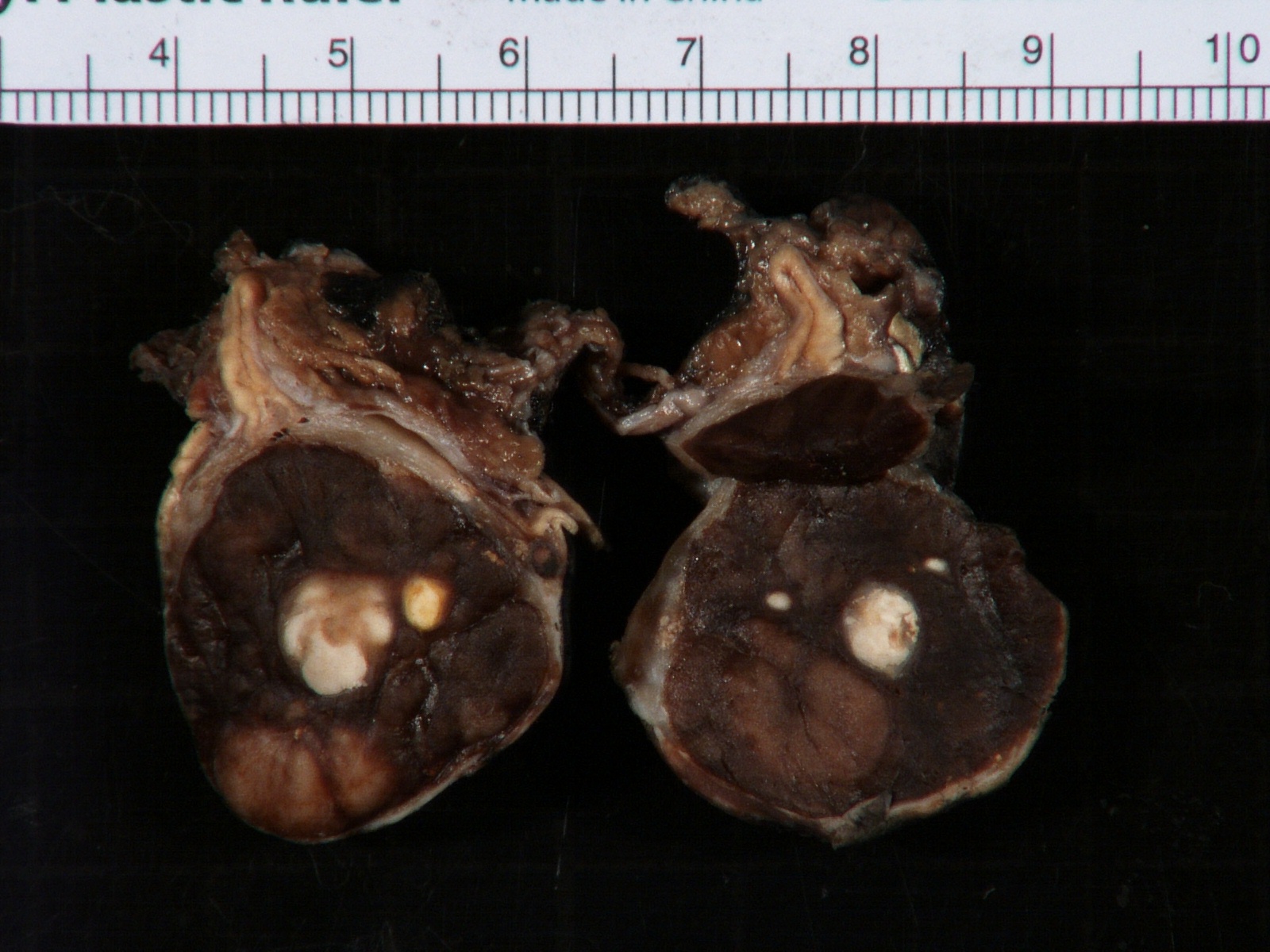
Gross description
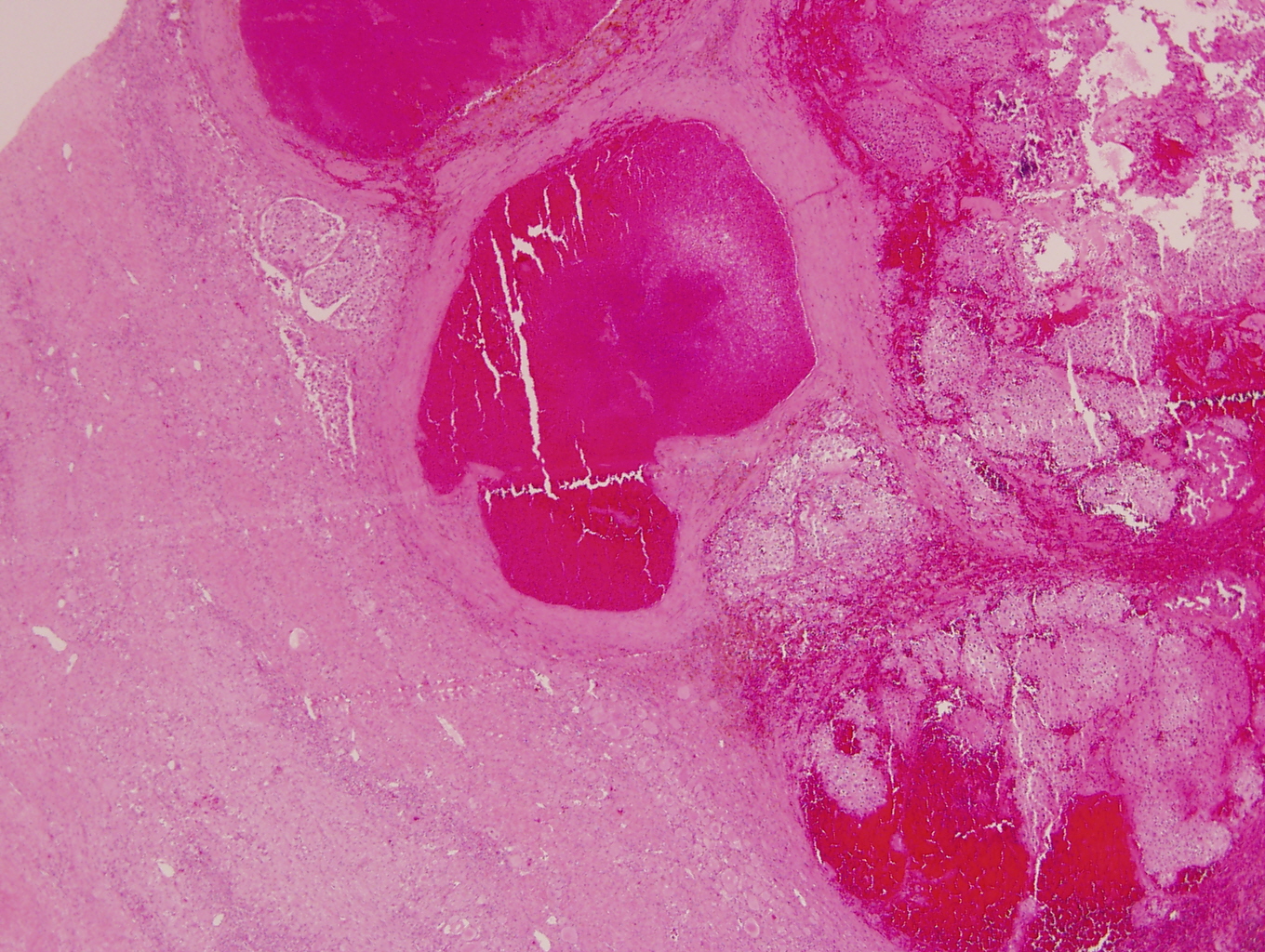
- More homogeneous and mature appearance than neuroblastoma
- Varies by subtype, from circumscribed ovoid mass to large multilobulated tumor
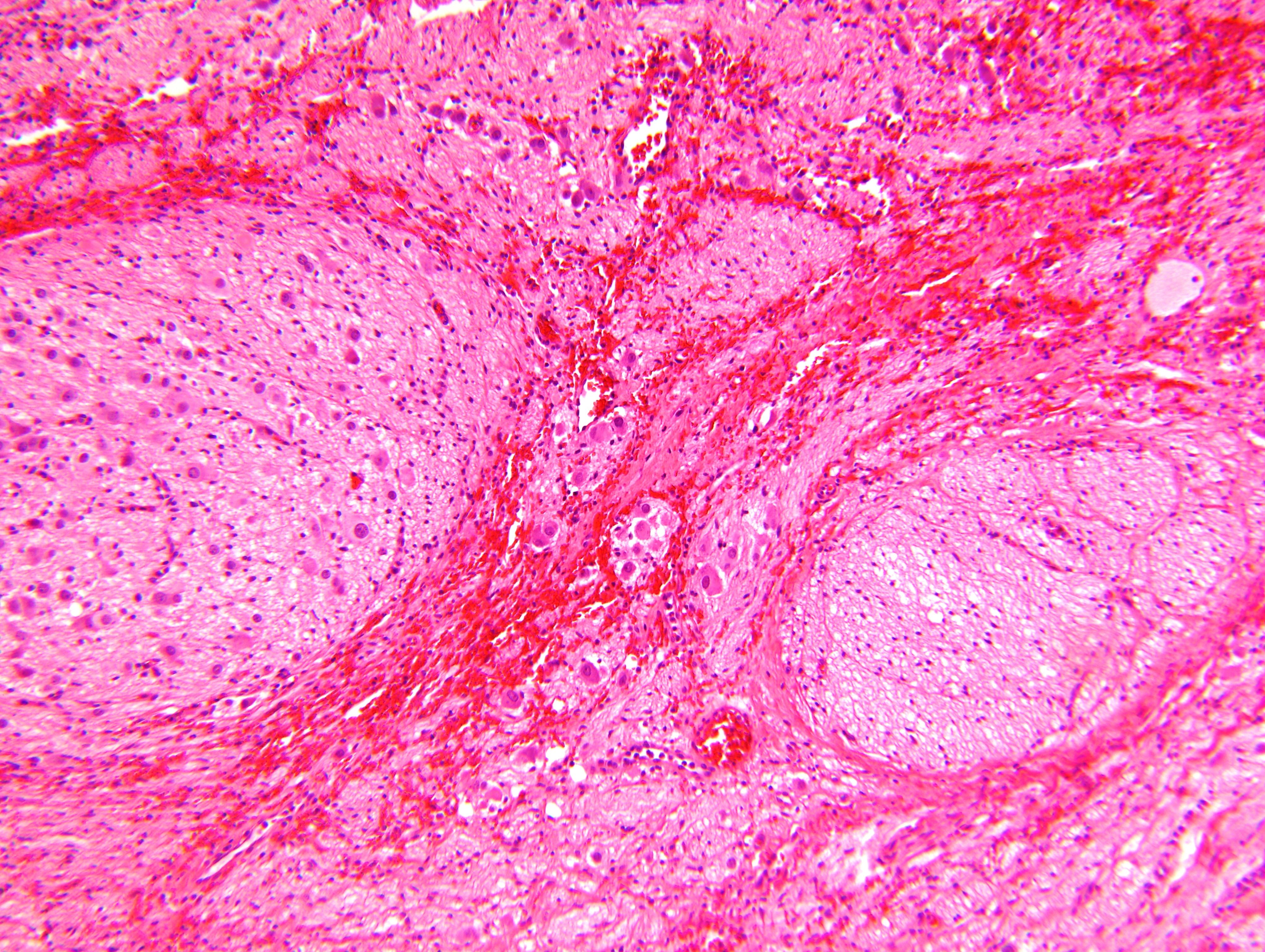
- Stroma rich, nodular subtype: area(s) of stroma poor, immature tumor are usually hemorrhagic with well defined borders (J Natl Cancer Inst 1984;73:405)
- Calcification (chalky white, yellow areas) and cystic degeneration may occur
- If large, adrenal gland may be difficult to identify
Gross images
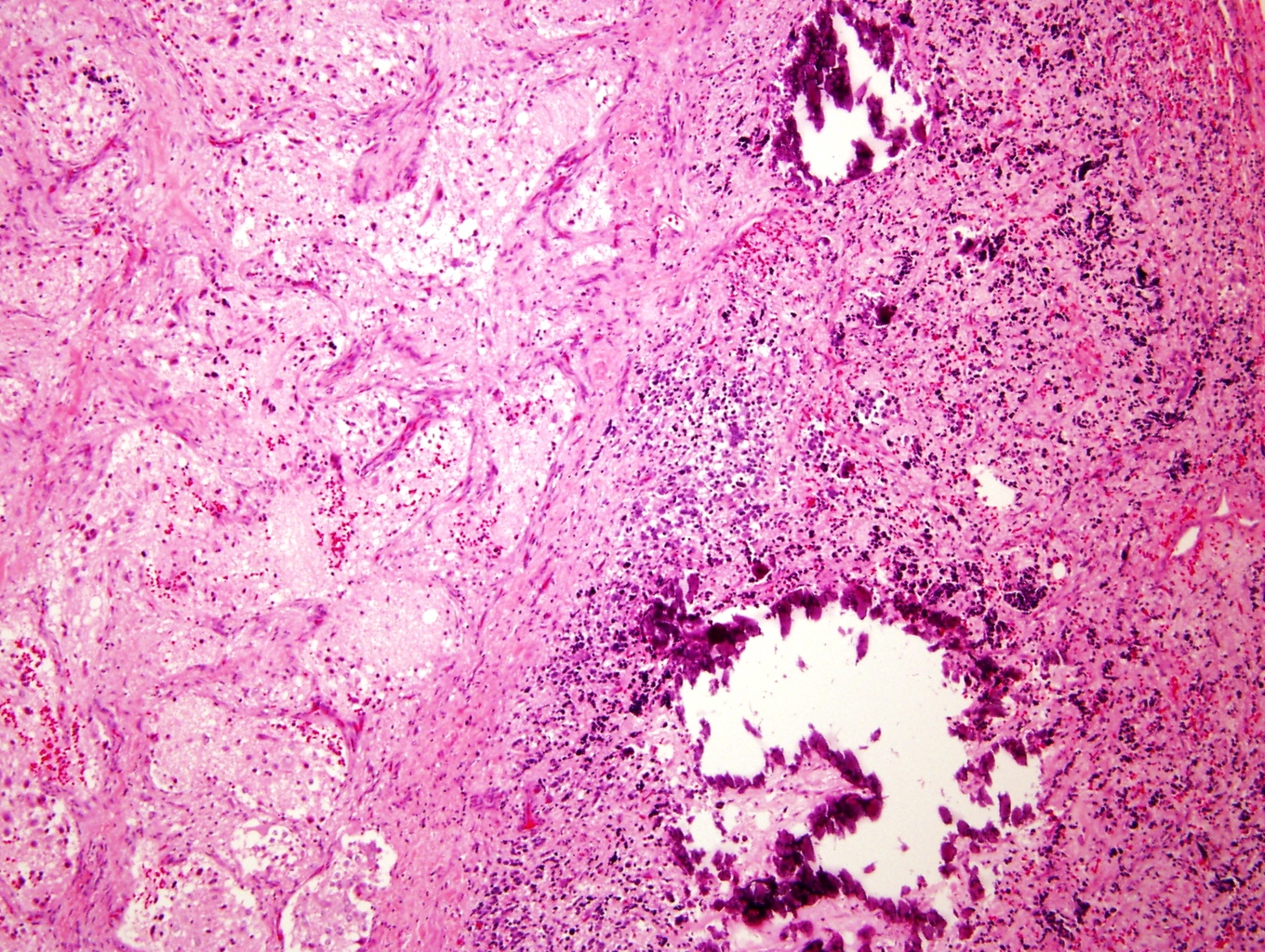
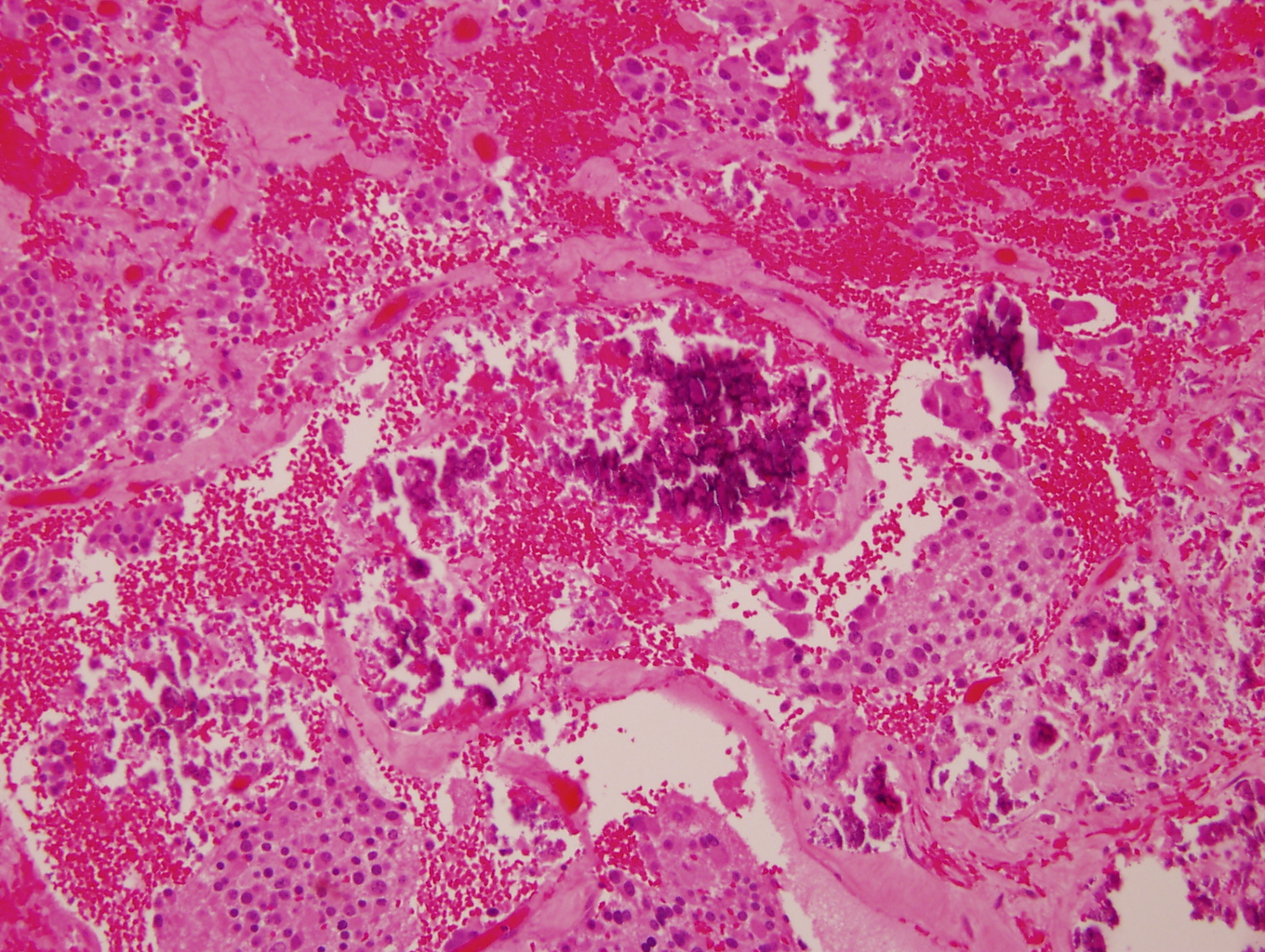
Microscopic (histologic) description
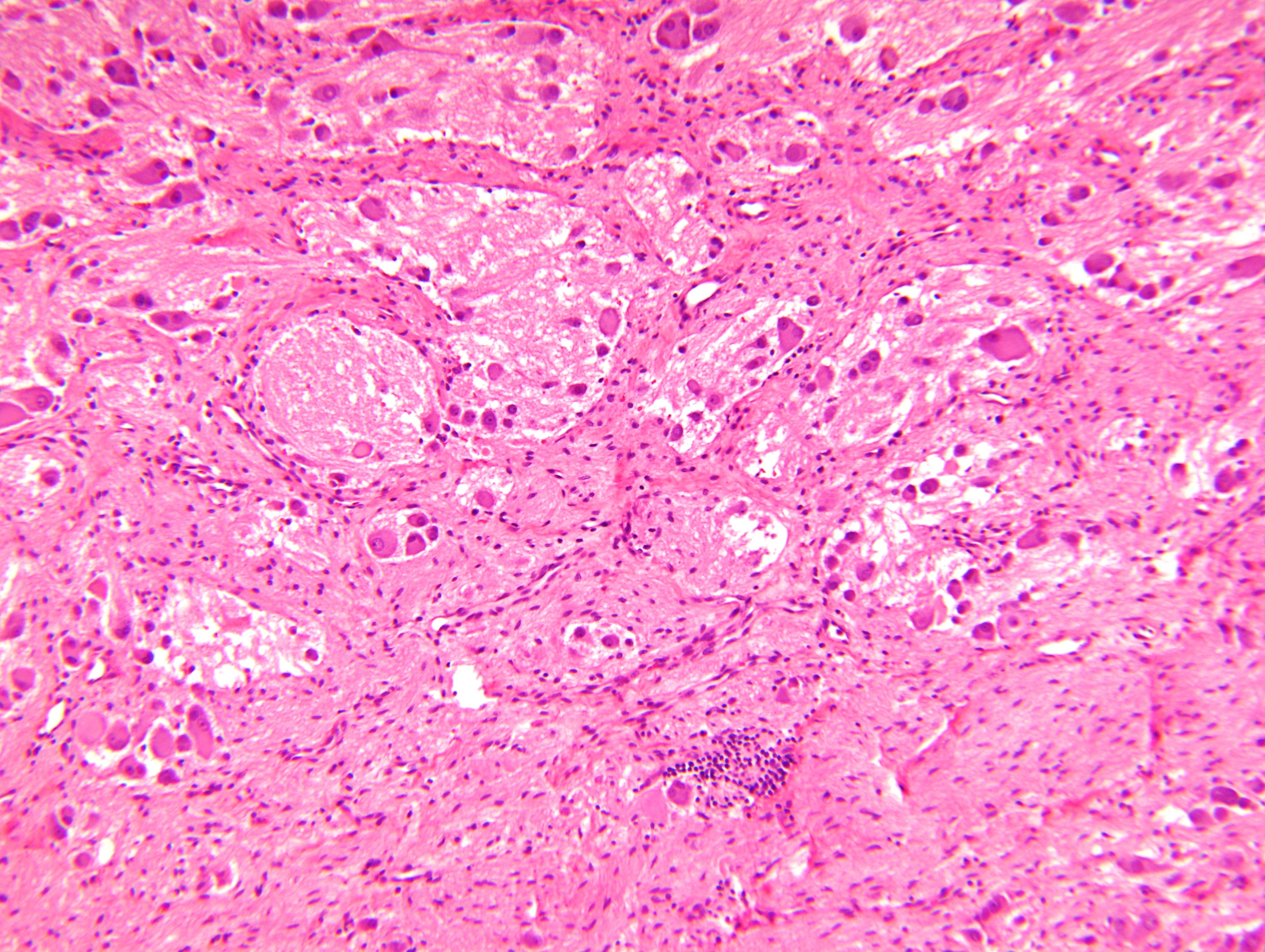
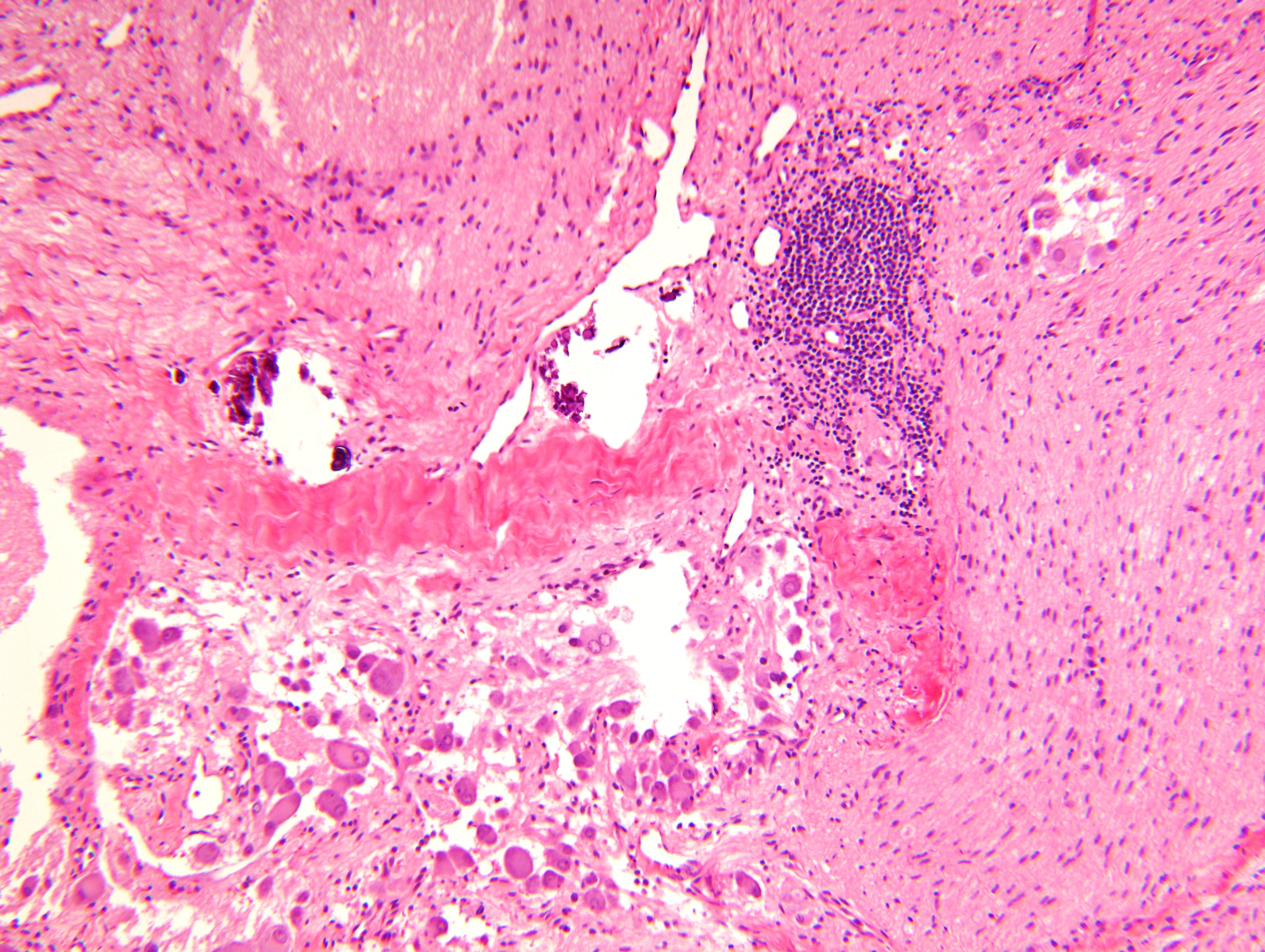
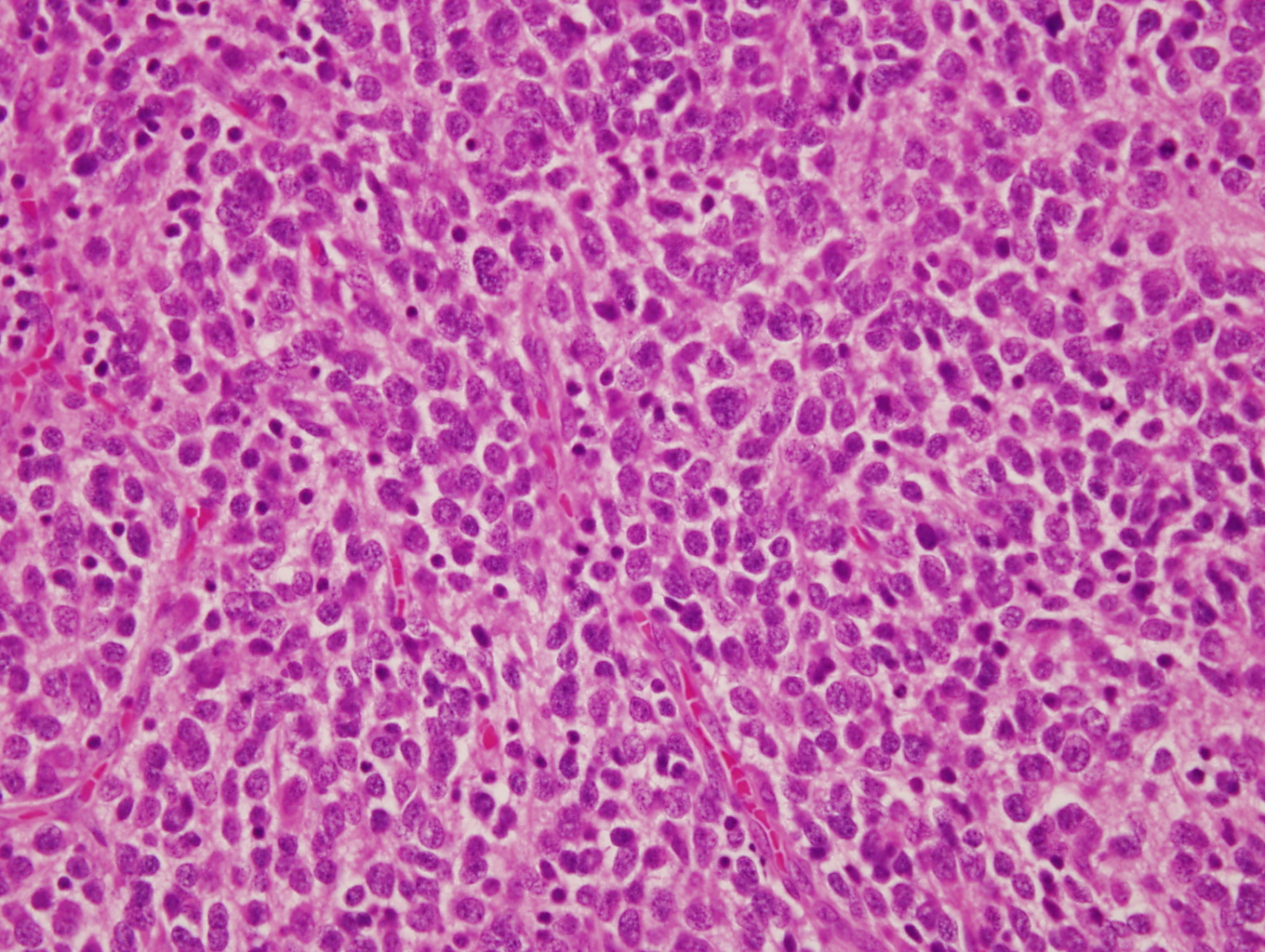
- Architecture: lobular, diffuse / solid, organoid
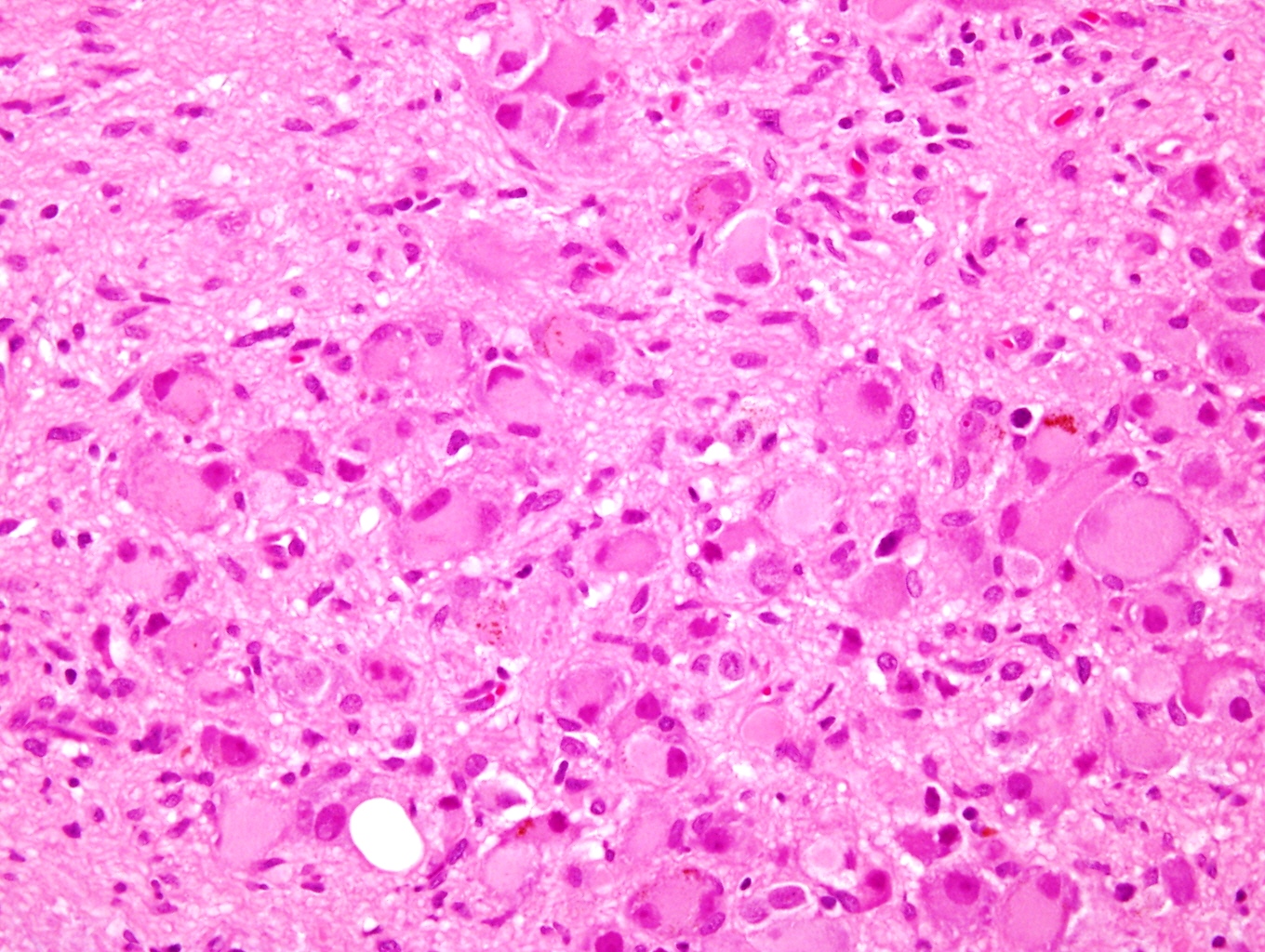
- Neuroblasts
- Homer Wright pseudorosettes = circular, ovoid, angular zones of pale staining neuritic cell processes surrounded by tumor cell nuclei; may rarely palisade
- Minimal cytoplasm, may have cytoplasmic tail
- Round to ovoid nuclei with stippled salt and pepper chromatin, inconspicuous nucleoli
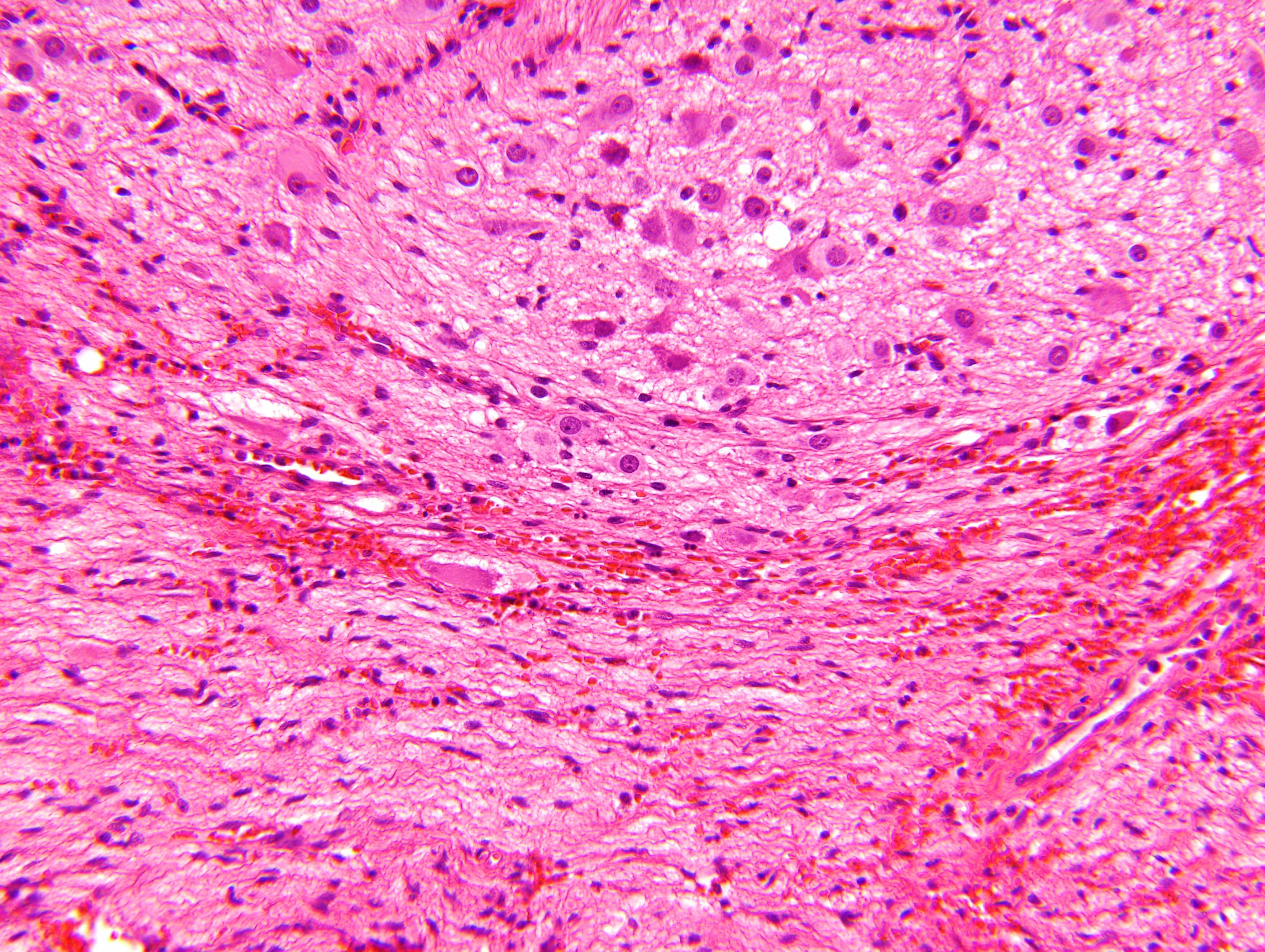
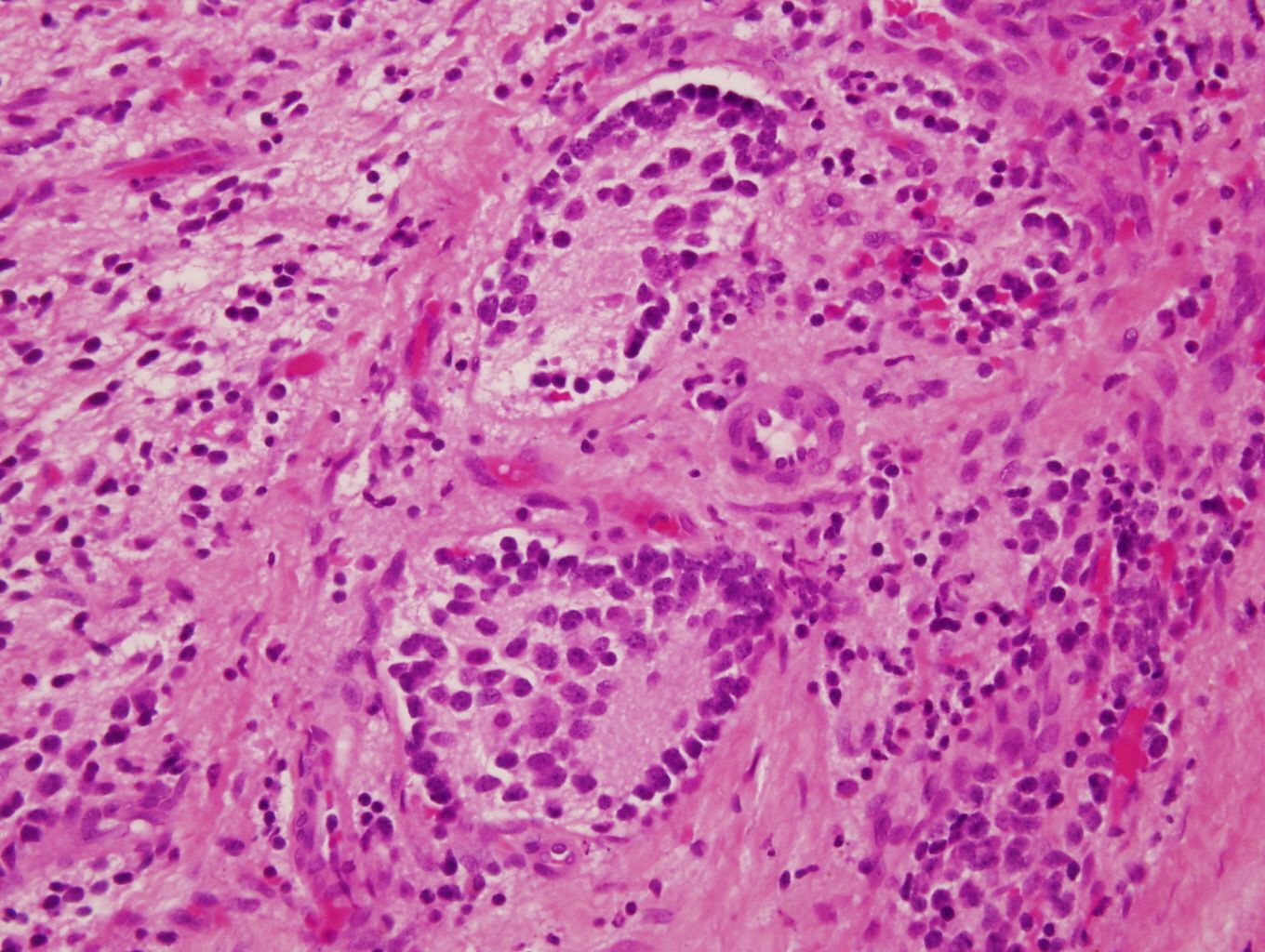
- Ganglion cells
- Abundant granular eosinophilic cytoplasm (Nissl substance = rough endoplasmic reticulum)
- Distinct cell borders
- Nuclear enlargement, eccentric nuclei, prominent nucleoli
- May see neuromelanin pigment (brown, finely granular; rarely present), cystic degeneration, hemorrhage, dystrophic calcification
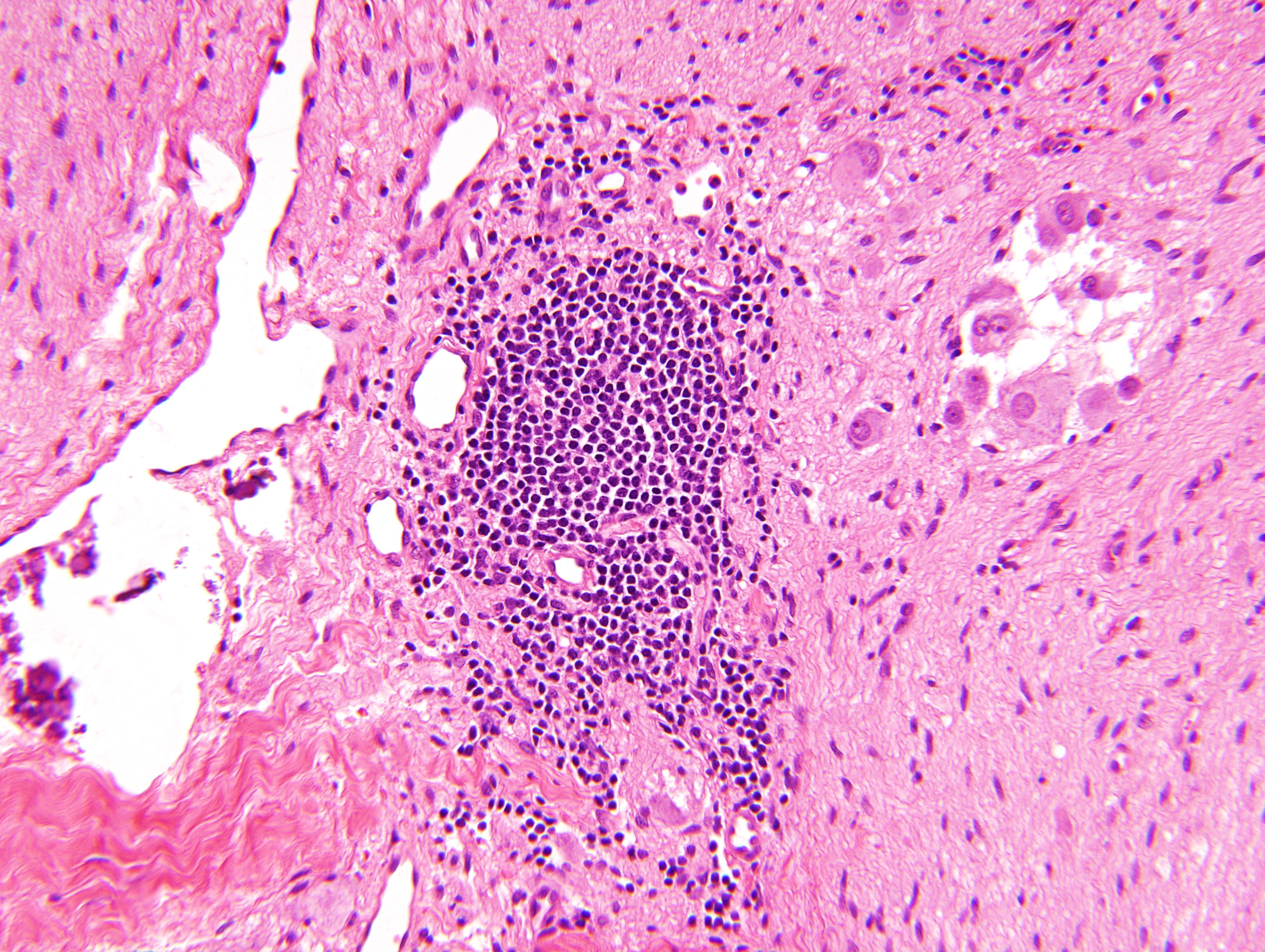
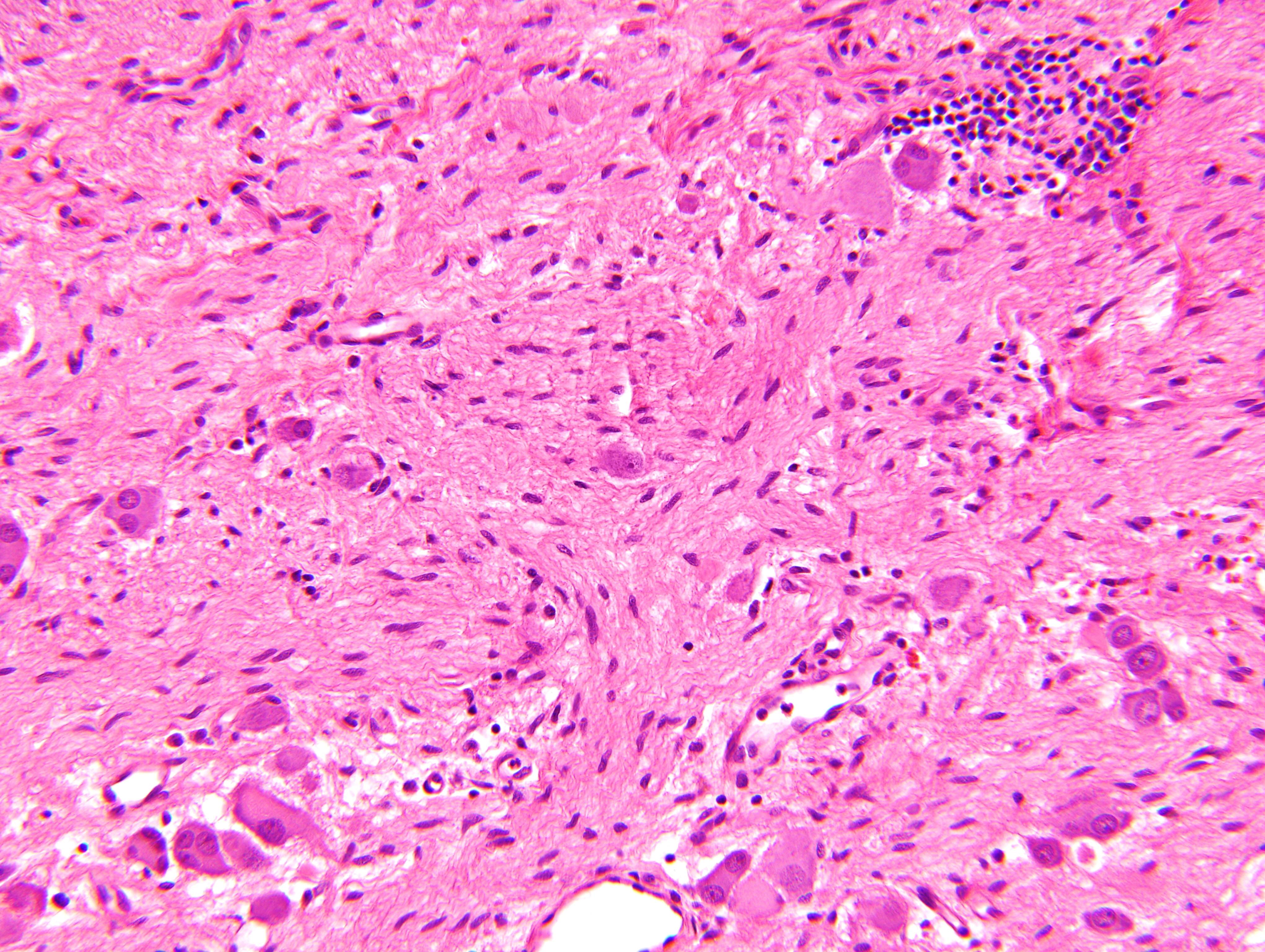
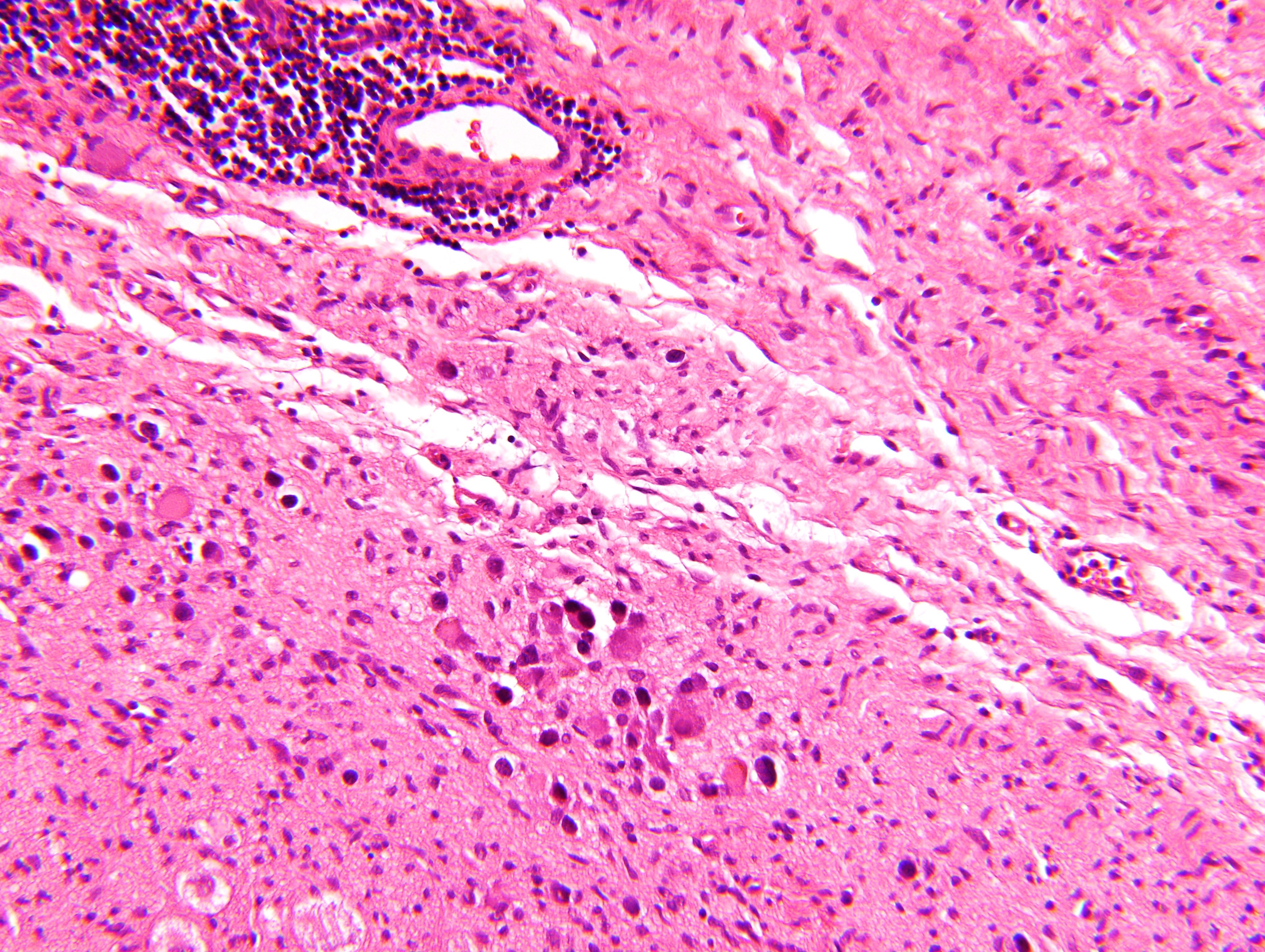
Microscopic (histologic) images
Contributed by Carmen Perrino, M.D. and Debra L. Zynger, M.D.
Intermixed type
Nodular type
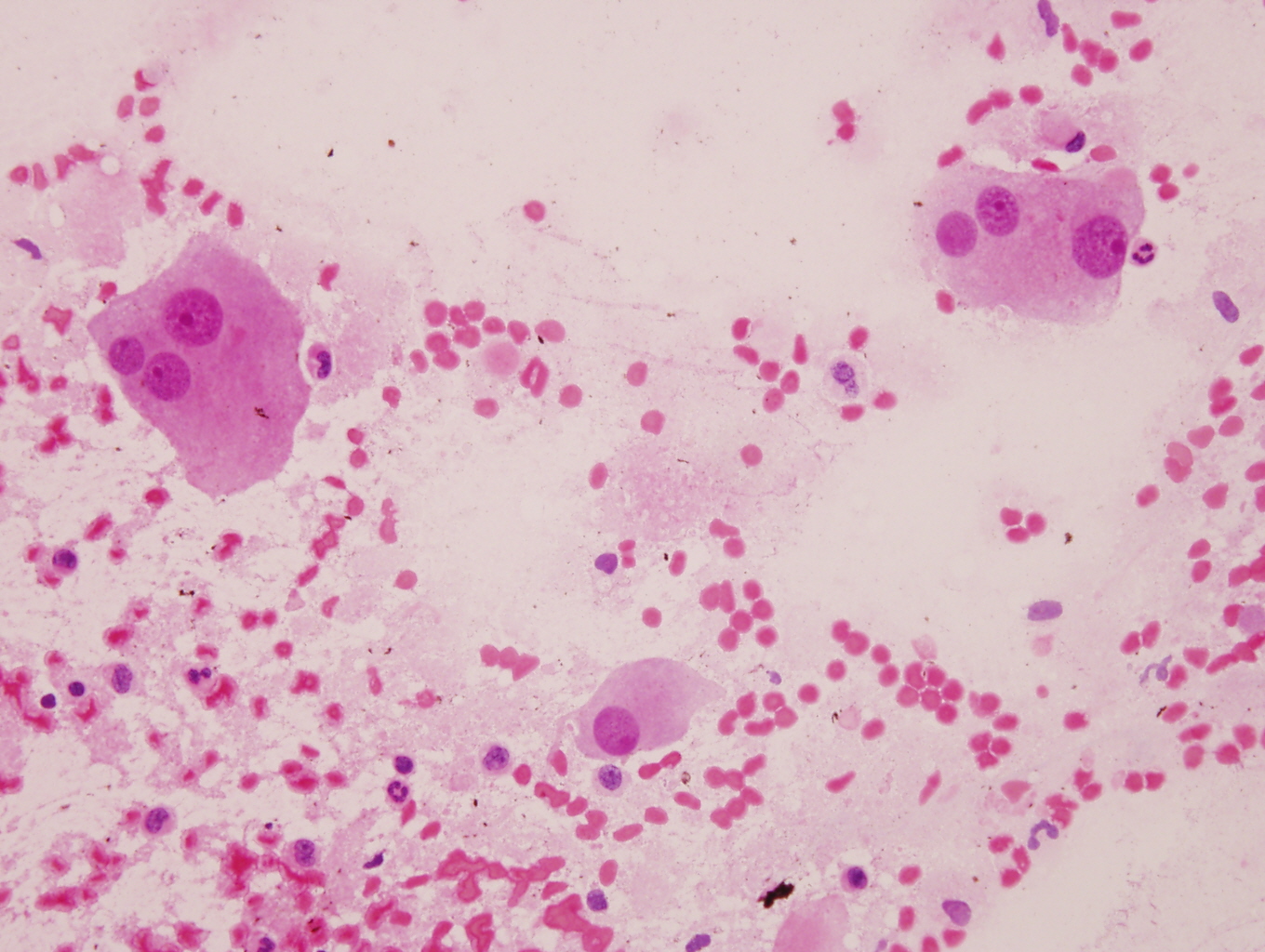
Cytology description
- Ganglion cells: larger cells, abundant cytoplasm, fine chromatin, prominent nucleoli
- Neuroblasts: uniform, small, blue cells with scant, eosinophilic, fibrillary cytoplasm; hyperchromatic to vesicular chromatin
- May form Homer Wright pseudorosettes
Cytology images
Positive stains
- Neuroblasts:
- Neuron specific enolase (NSE), CD57 / Leu7, CD56, protein gene product 9.5 (PGP 9.5), synaptophysin, chromogranin, neurofilament protein, ALK1 (> 90%), PHOX2B (Am J Pathol 2012;180:1223, Am J Surg Pathol 2012;36:1141)
- Schwannian stroma:
- Ganglion cells:
Negative stains
Electron microscopy description
- Neuritic processes with neurotubules and neurofilaments, regular / uniform dense core granules measuring < 200 nm in diameter (Lack: Tumors of the Adrenal Glands and Extraadrenal Paraganglia, Volume 8, AFIP, Series 4)
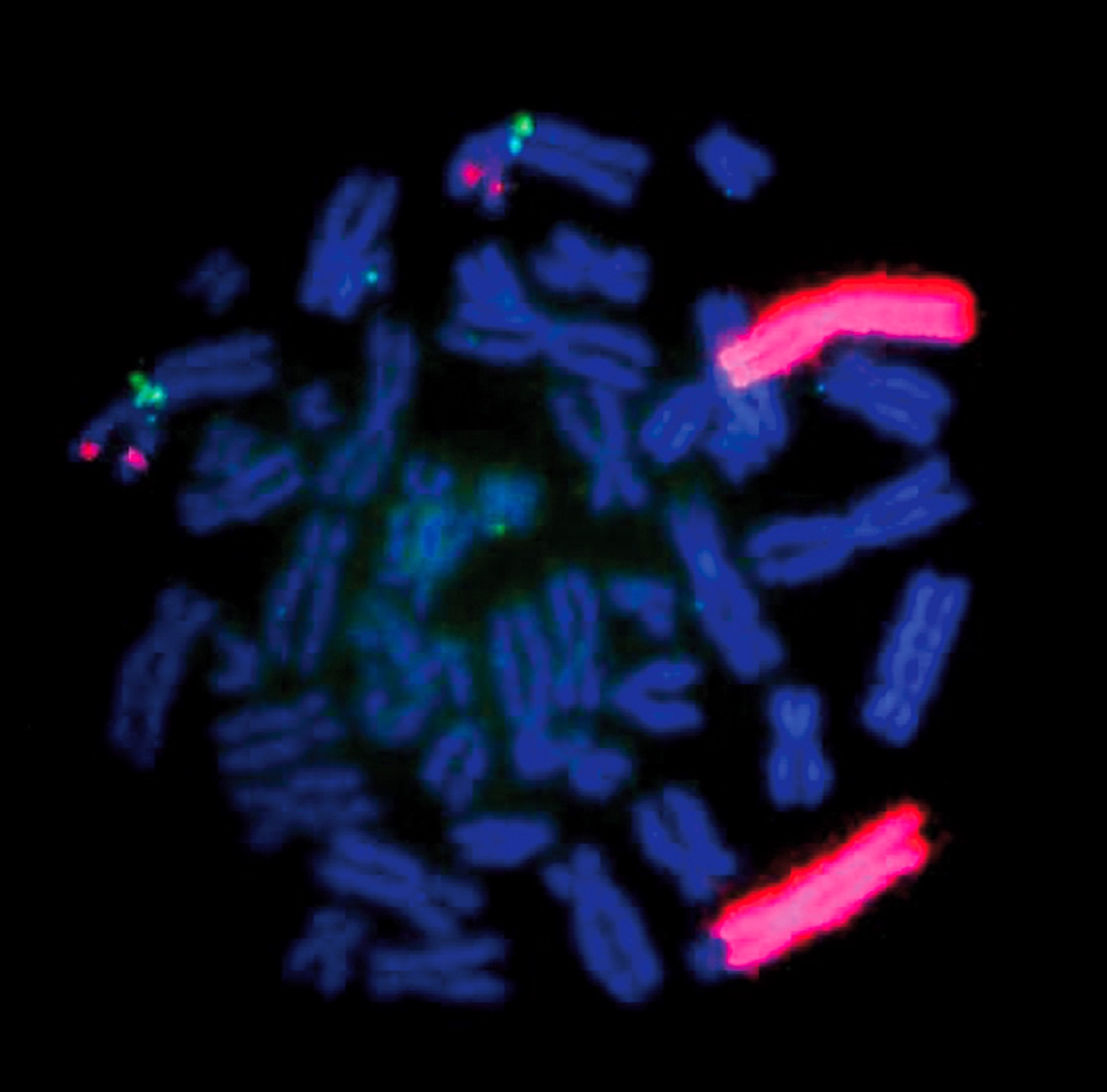
Molecular / cytogenetics description
- Considered molecularly heterogeneous but much of genetic basis remains unexplained (Cancer 2003;98:2274)
- ALK gene mutations have been implicated in some cases of ganglioneuroblastoma (Am J Pathol 2012;180:1223)
- Ganglioneuroblastoma, stroma rich, nodular subtype, is considered a composite tumor consisting of separate clones (less aggressive stroma rich component; nodular component consisting of a favorable / unfavorable / both clones) (Cancer 2003;98:2274)