Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Lin W, Zheng W. Small cell neuroendocrine carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cervixsmallcell.html. Accessed September 24th, 2025.
Definition / general
- Small cell neuroendocrine carcinoma (SCNEC) is a high grade malignant neuroendocrine tumor characterized by small to medium sized cells with scant cytoplasm
Essential features
- Rare in cervix but represents the most common neuroendocrine neoplasm at this site with aggressive behavior and poor prognosis
- Cervix is the most common site for small cell neuroendocrine carcinoma in the gynecologic tract
- Almost always human papillomavirus (HPV) associated
- Commonly coexists with in situ or invasive squamous carcinoma, adenocarcinoma or (rarely) mesonephric carcinoma
- Composed of high grade small cells with scant cytoplasm, hyperchromatic (salt and pepper) nuclei and prominent nuclear molding
- Typically immunoreactive for neuroendocrine markers, although immunohistochemical confirmation is not strictly required
- Diagnosis is based predominantly on morphology
Terminology
- WHO acceptable terminology: small cell carcinoma, neuroendocrine carcinoma, small cell type
- Not recommended by WHO: high grade neuroendocrine carcinoma
ICD coding
- ICD-O: 8041/3 - small cell neuroendocrine carcinoma
- ICD-11
- 2C7Z & XH0U20 - malignant neoplasms of female genital organs, unspecified & neuroendocrine carcinoma, NOS
- 2C7Z & XH0YB0 - malignant neoplasms of female genital organs, unspecified & small cell carcinoma, NOS
Epidemiology
- Women with a mean age of 48 years old (Front Cell Infect Microbiol 2022;12:916506)
- Cervix is the most common site for small cell neuroendocrine carcinoma in the gynecologic tract (Gynecol Oncol 2014;134:410, Semin Oncol 2007;34:57)
- Small cell neuroendocrine carcinoma is the most common neuroendocrine neoplasm of the cervix; from 1977 to 2003, the average annual incidence in the U.S. was 0.06 per 100,000 women, compared to 6.6 for squamous cell carcinoma and 1.2 for adenocarcinoma, according to Surveillance, Epidemiology and End Results (SEER) data (Obstet Gynecol 2008;111:1394)
- 55% of cases are HPV 16 positive; 41% are HPV 18 positive; 4% are positive for other types (Papillomavirus Res 2018;5:134)
Sites
- Cervix
Pathophysiology
- Neuroendocrine cells present in the cervical epithelium (particularly at the squamocolumnar junction) and are thought to be the cells of origin for small cell neuroendocrine carcinoma (Autops Case Rep 2023;13:e2023452)
- Endocrine cell hyperplasia of the uterine cervix may be a precursor lesion (Am J Clin Pathol 1989;92:825)
Etiology
- Most cases are strongly linked to high risk HPV infection, especially types 16 and 18 (Int J Gynecol Cancer 2014;24:S102)
Clinical features
- Patients often present with abdominopelvic symptoms (e.g., vaginal bleeding, discharge, pelvic pain or pressure), though some are asymptomatic or detected via abnormal Pap smear (Am J Obstet Gynecol 2010;203:347.e1)
- Pelvic mass is frequently noted and most patients have bulky disease at diagnosis
- In a review of 188 cases, 135 were early (stage I to IIA), 45 advanced (stage IIB to IVA) and 8 metastatic (stage IVB); among those with recorded tumor sizes, 80% were ≥ 2 cm (Am J Obstet Gynecol 2010;203:347.e1)
Diagnosis
- Tumor location: uterine cervix
- Surgical specimen: biopsy, gross and microscopic examination
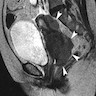
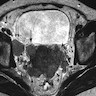
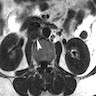
Radiology description
- Morphology: overall tumor shape and margins can be nonspecific (round / oval or lobulated), so magnetic resonance imaging (MRI) appearance alone may not distinguish small cell neuroendocrine carcinoma from other cervical cancers (AJR Am J Roentgenol 2004;182:1255)
- Signal characteristics: typically displays low signal intensity on T1 weighted images and high signal intensity on T2 weighted images, often with a homogeneous internal appearance (AJR Am J Roentgenol 2004;182:1255)
- Parametrial invasion: frequently observed (up to 71% in the study), even in tumors measuring ≤ 4 cm (AJR Am J Roentgenol 2004;182:1255)
- Lymphadenopathy: common and often extensive, reflecting the aggressive behavior of small cell neuroendocrine carcinoma (86% in the study), including paraaortic nodes in larger tumors (AJR Am J Roentgenol 2004;182:1255)
Radiology images
Prognostic factors
- Pathological stage is the most critical prognostic factor
- Lymph node metastases, depth of invasion and lymphovascular space invasion increase recurrence risk in early stage disease (Zheng: Gynecologic and Obstetric Pathology, 2nd Edition, 2025)
- ~67% of patients experience relapse at a median of 8 months (Gynecol Oncol 2004;93:27)
- In patients with limited stage disease, the 5 year survival rate is ~30 - 50%, while few with more extensive disease survive beyond 2 years (Int J Gynecol Cancer 2019;29:986)
Case reports
- 29 year old woman with synchronous small cell neuroendocrine carcinoma of the cervix and immature ovarian teratoma (Oncol Lett 2024;28:313)
- 33 year old woman with small cell neuroendocrine carcinoma and leptomeningeal metastasis (Surg Neurol Int 2024;15:310)
- 47 year old woman with a history of breast cancer manifesting with abnormal vaginal bleeding (Caspian J Intern Med 2024;15:546)
- 55 year old woman with syndrome of inappropriate antidiuretic hormone secretion (J Int Med Res 2021;49:300060520985657)
- 73 year old woman with a metastatic spinal cord tumor (Clin Med Insights Case Rep 2020;13:1179547620920170)
- 19 cases of small cell neuroendocrine carcinomas (Front Cell Infect Microbiol 2022;12:916506)
Treatment
- Consensus guidelines (SGO) recommend multimodal therapy (surgery, chemotherapy, radiotherapy) for early stage tumors (Gynecol Oncol 2011;122:190)
- Historically, 5 year survival was 14 - 29%; recent data suggest improved survival (up to 45%) with aggressive multimodal therapy (Gynecol Oncol 2016;141:247)
- Ongoing studies involve angiogenesis inhibitors, mTOR inhibitors, VEGF inhibitors and tyrosine kinase inhibitors (Zheng: Gynecologic and Obstetric Pathology, 2nd Edition, 2025)
- Have worse prognosis than squamous cell carcinoma, with hazard ratios of 2.96 for early stage and 1.70 for late stage disease (Int J Gynecol Cancer 2014;24:272)
Gross description
- Often presents as friable cervical masses that tend to ulcerate easily (Zheng: Gynecologic and Obstetric Pathology, 2nd Edition, 2025)
- Gross tumor necrosis and focal hemorrhage are commonly present on cutting sections
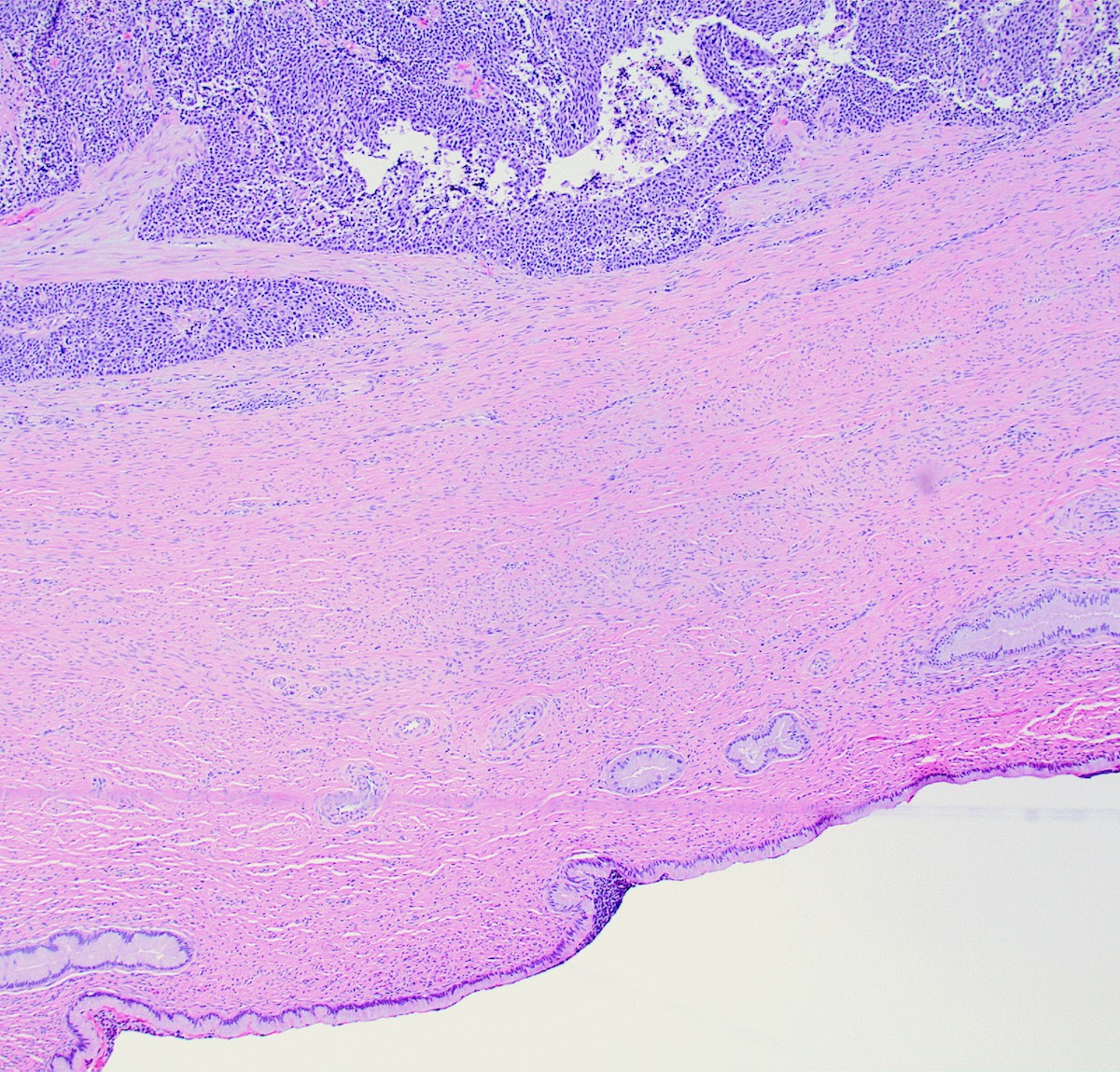
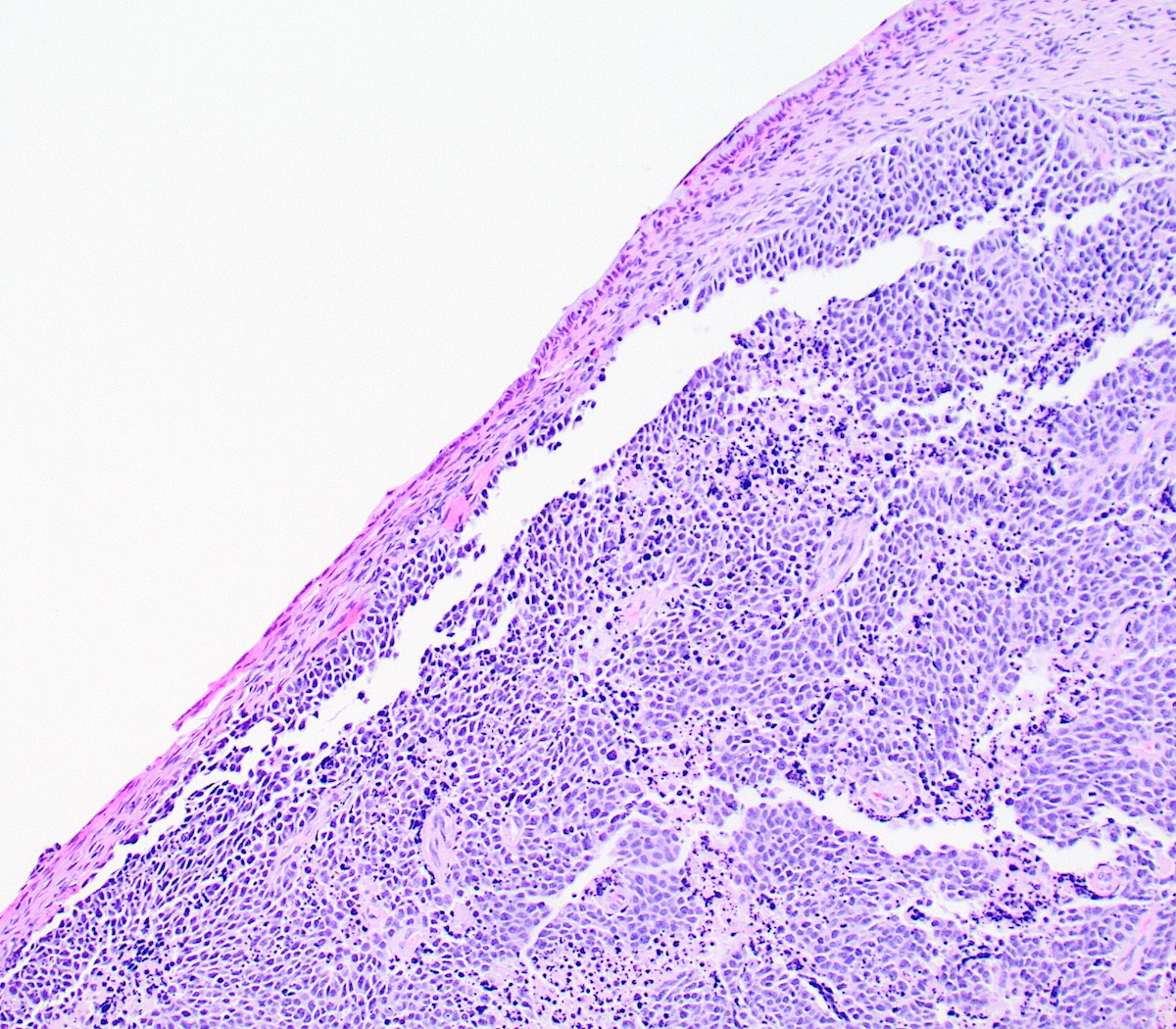
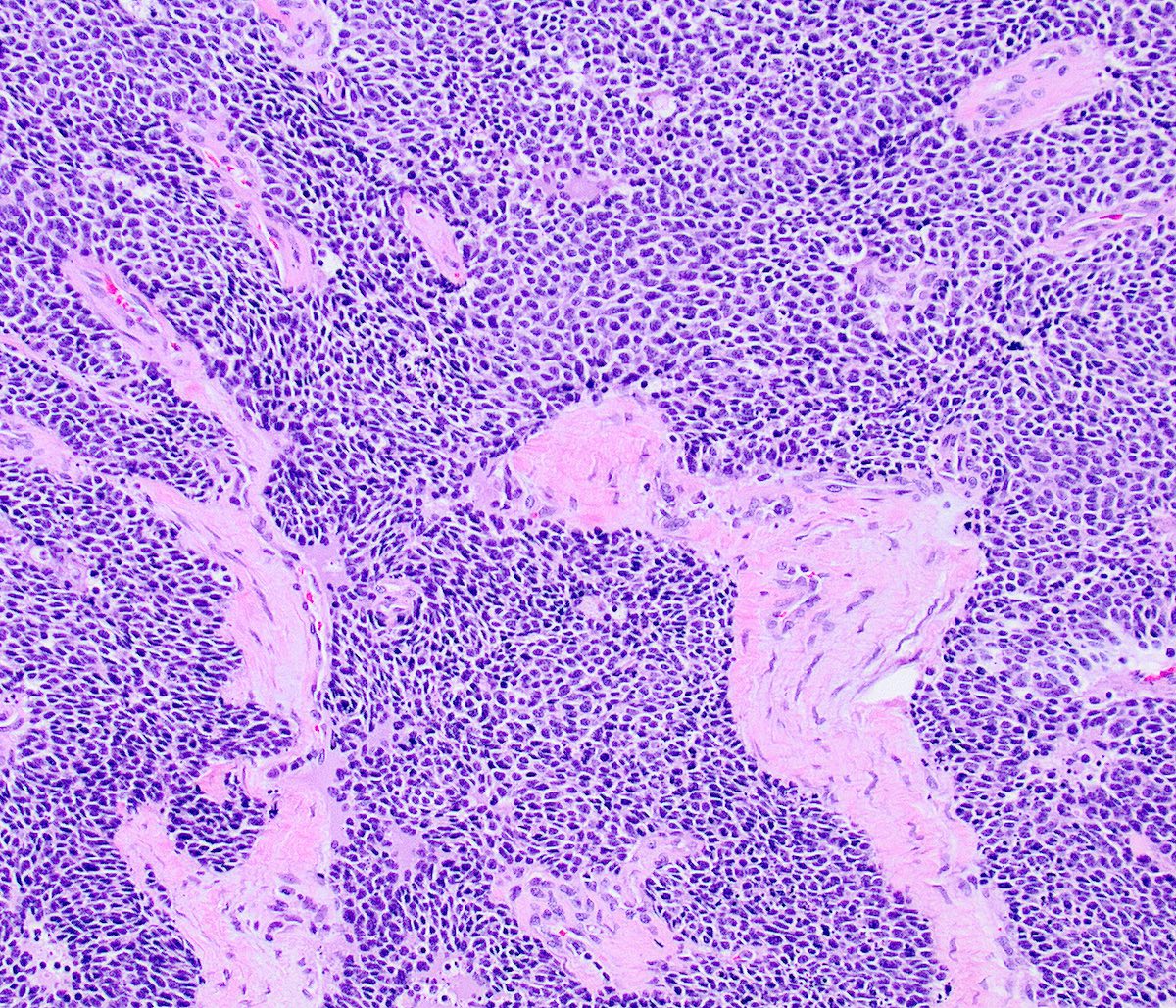
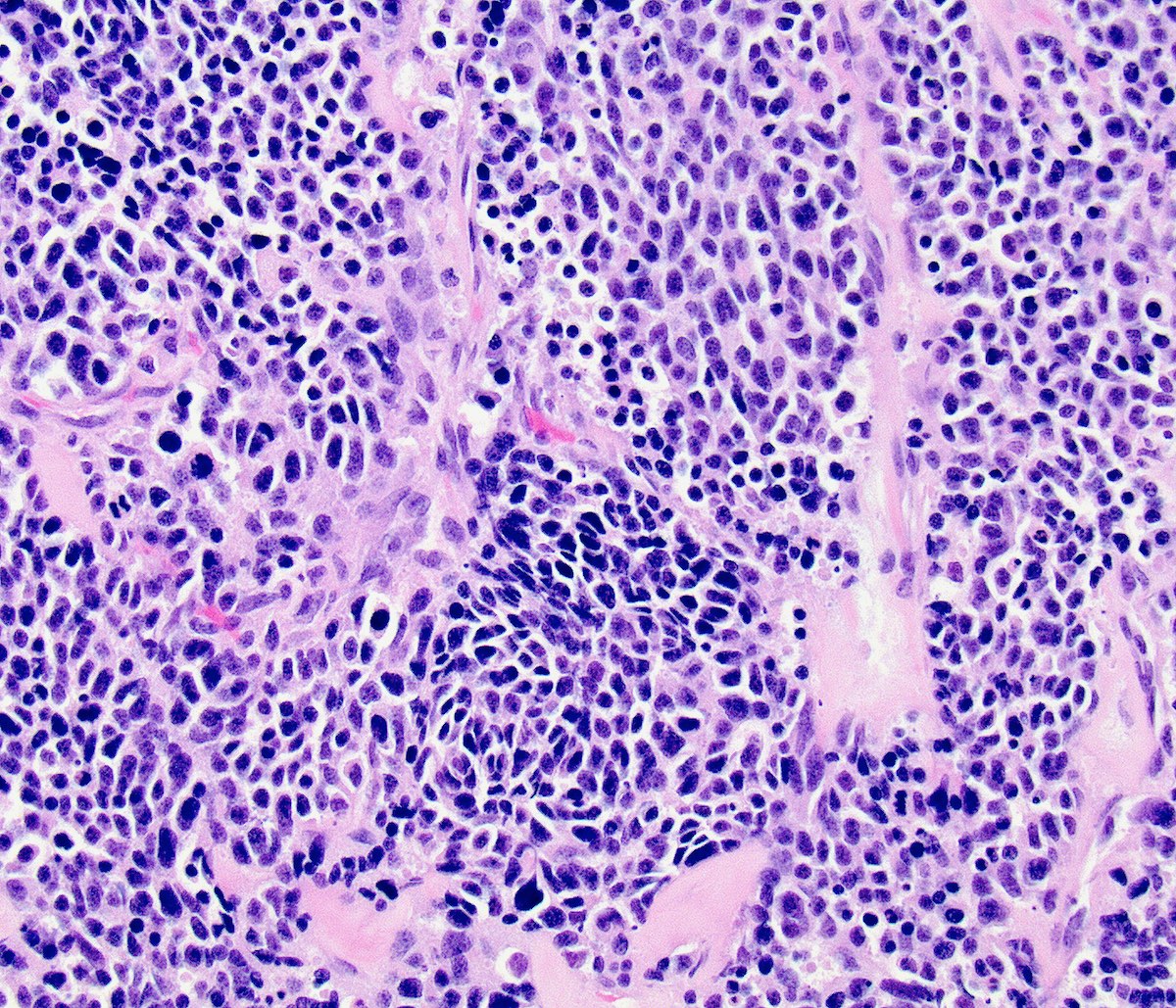
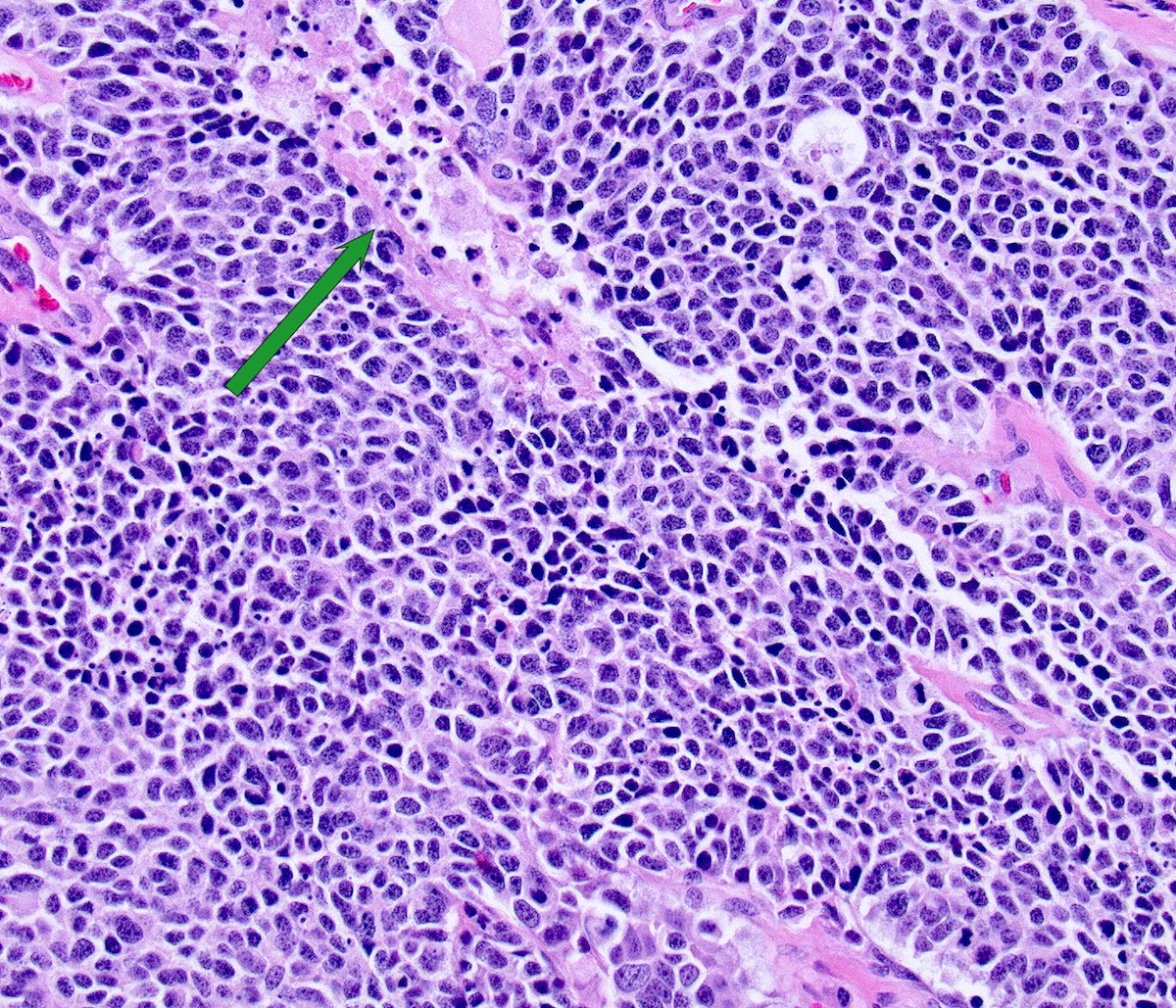
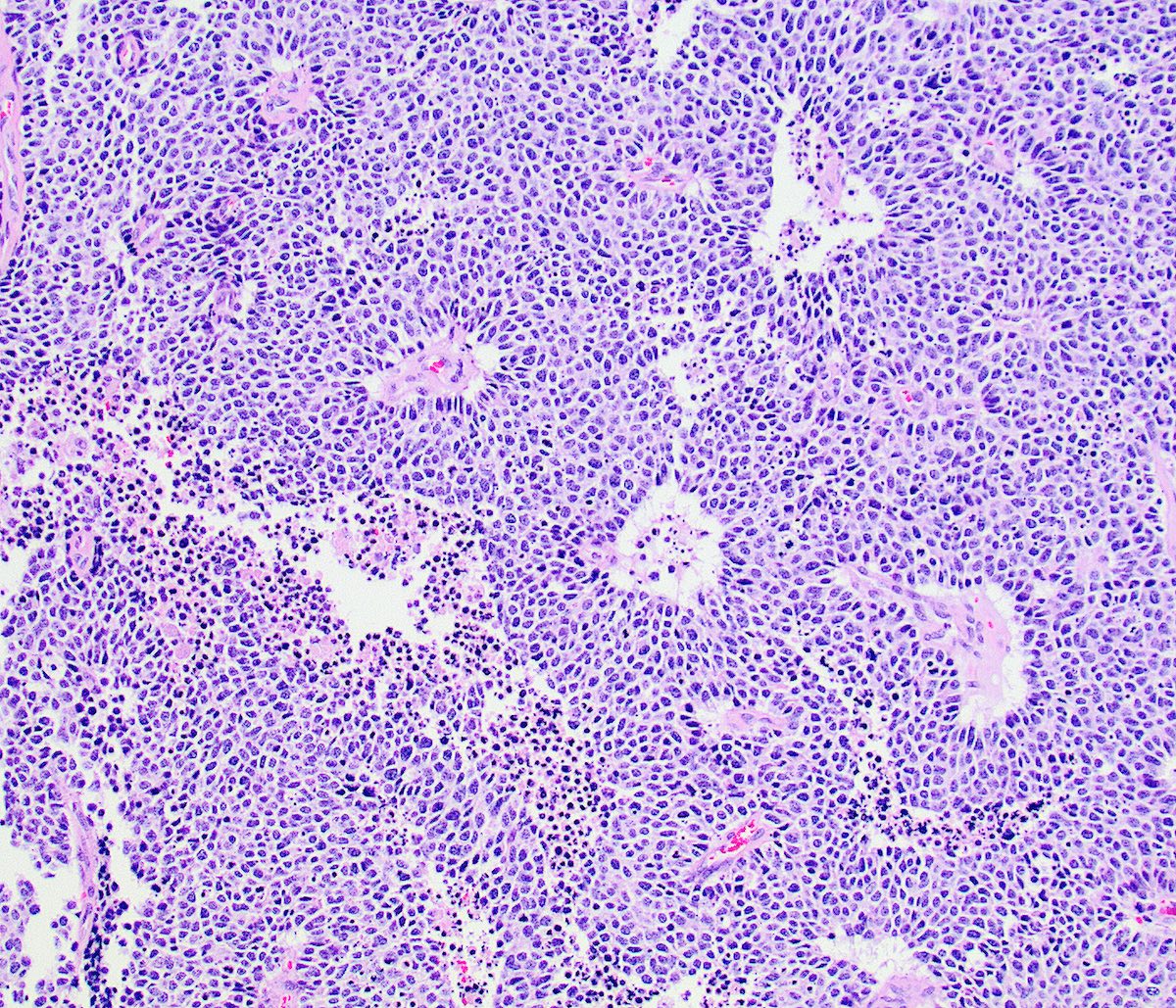
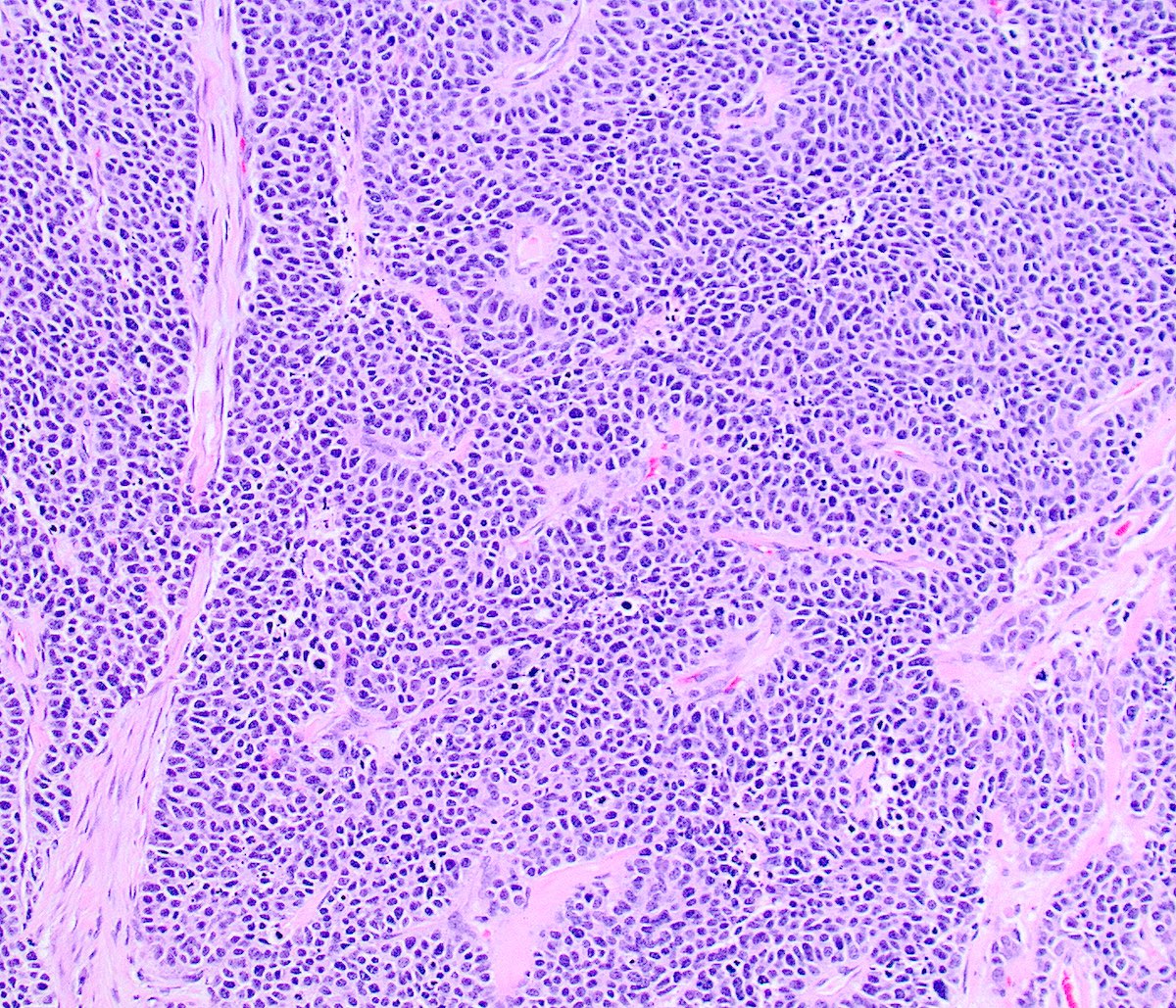
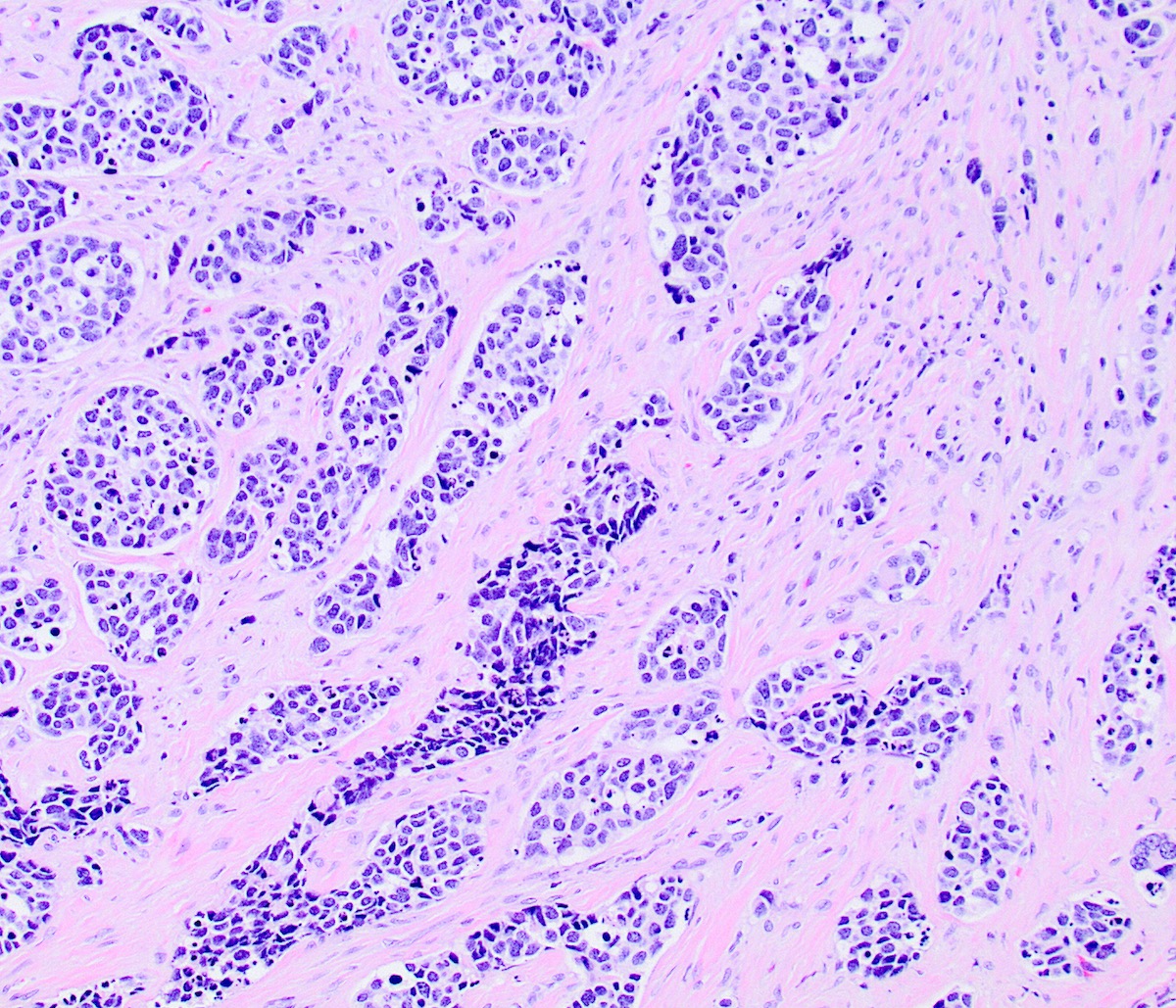
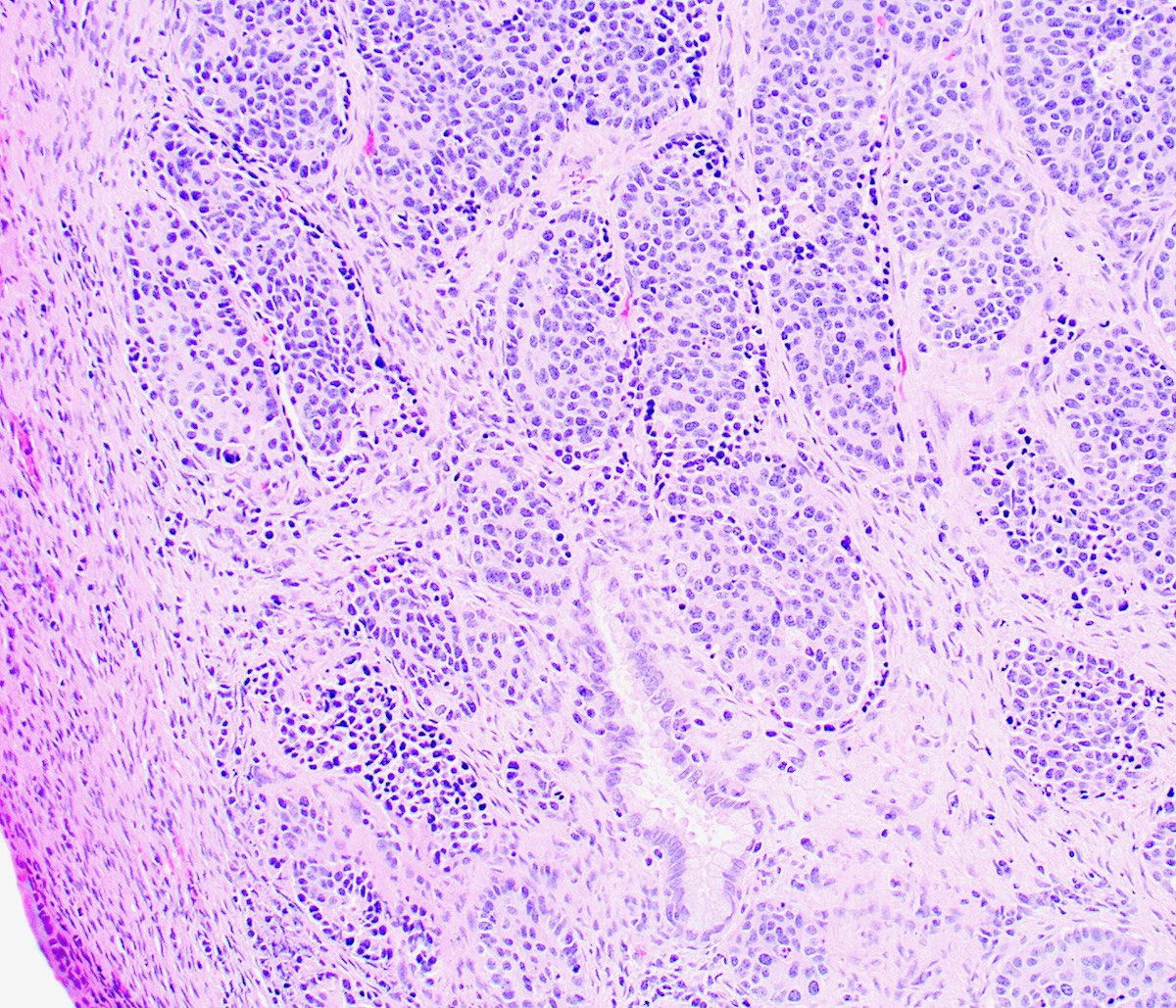
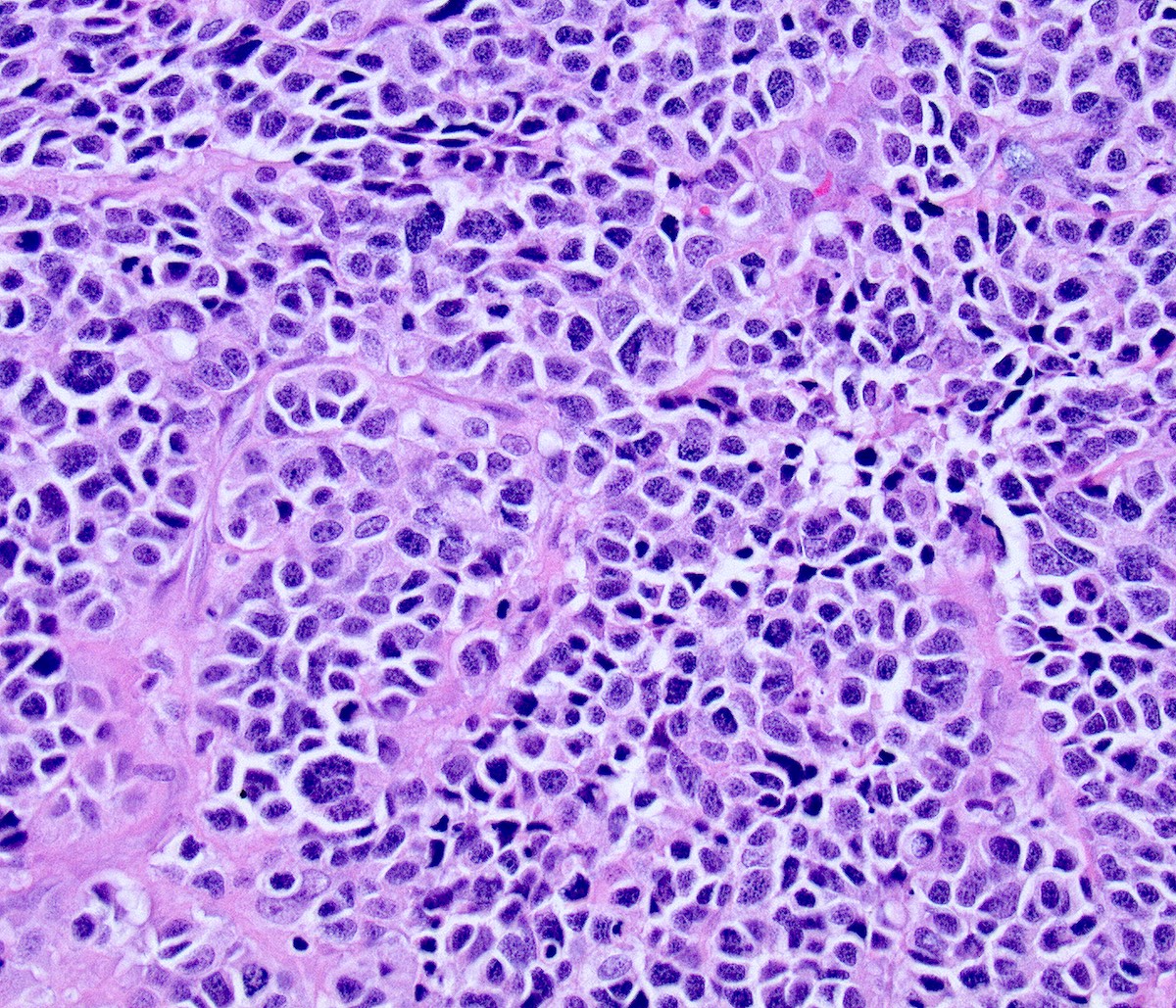
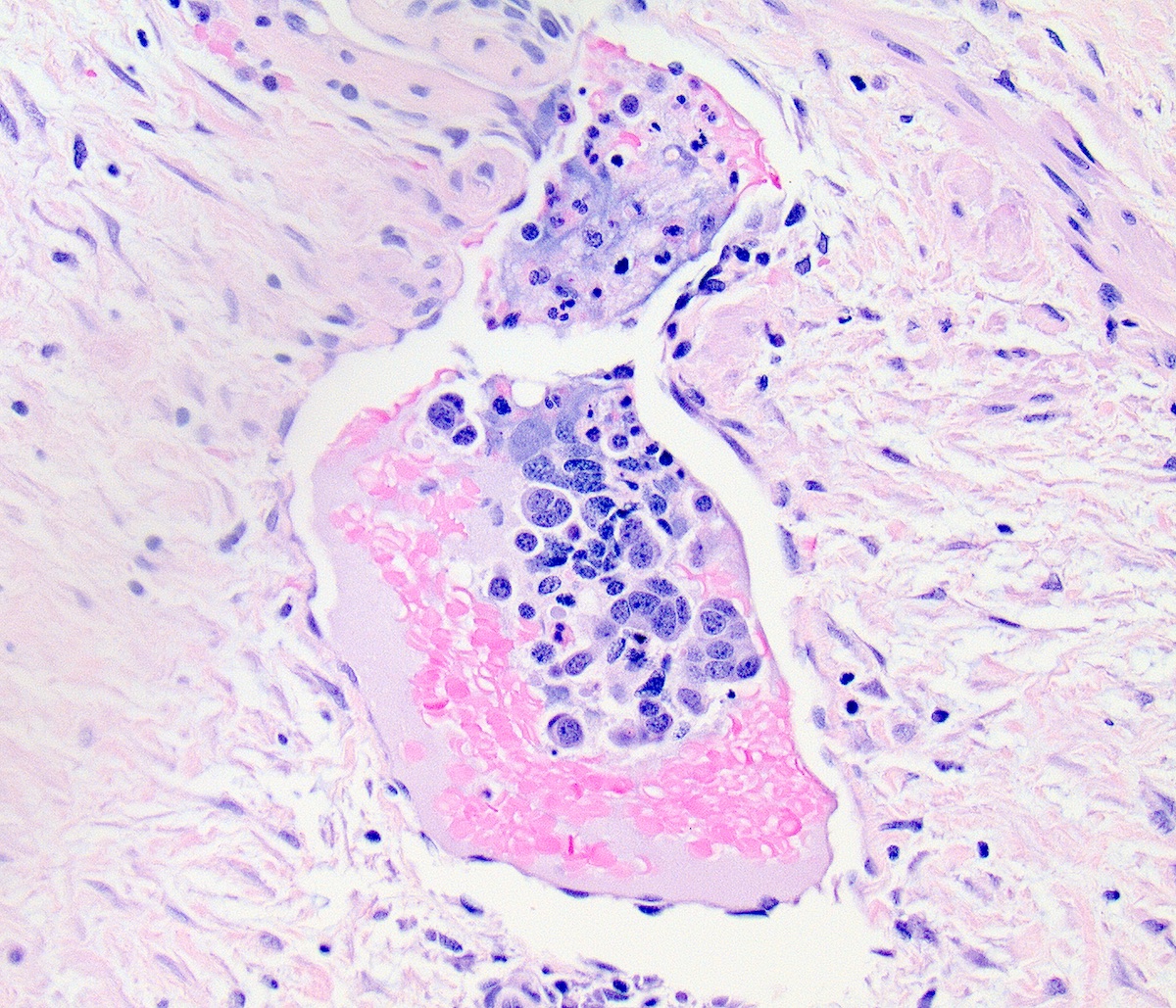
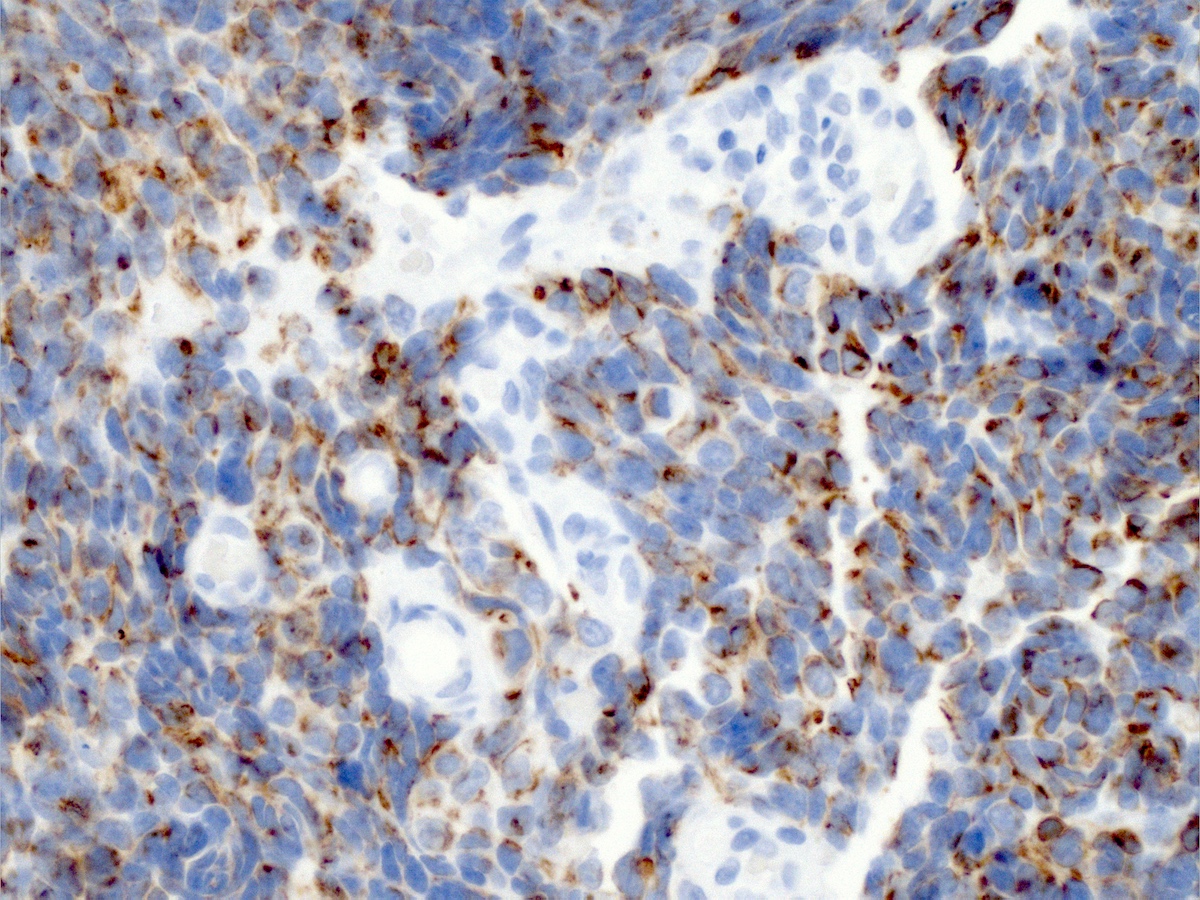
Microscopic (histologic) description
- Growth is seen in diffuse sheets, nests, trabeculae, cords or insular patterns; pseudoglandular, rosette-like or acinar structures can be present
- Highly atypical small cells with scant cytoplasm, round, ovoid or spindled hyperchromatic nuclei, dispersed (salt and pepper) chromatin and inconspicuous nucleoli
- Nuclear molding and high N:C ratio
- Frequent mitoses and apoptotic bodies
- Prominent necrosis, crush artifact and vascular / lymphovascular invasion (Ann Diagn Pathol 2013;17:1, Int J Surg Pathol 2016;24:490)
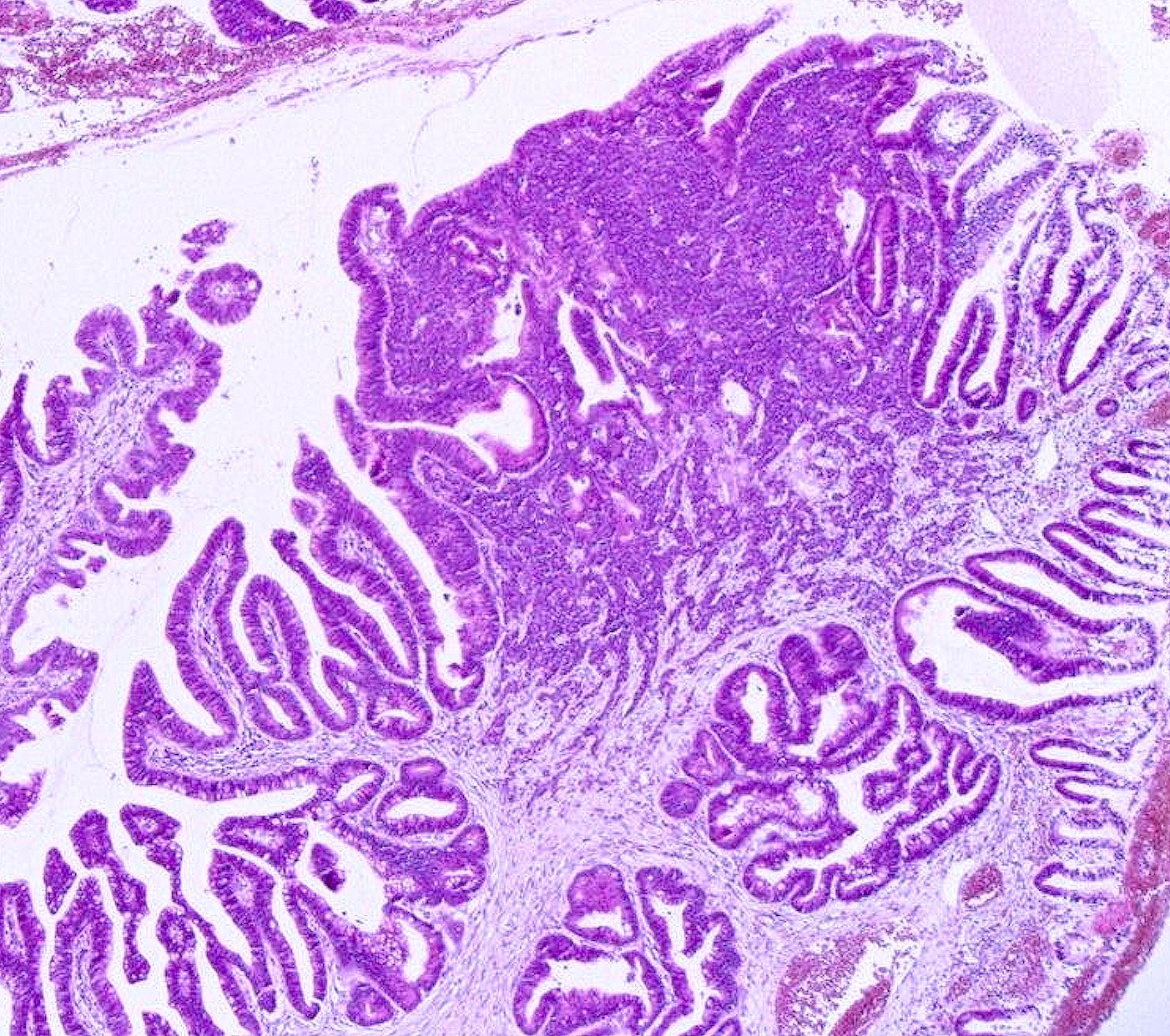
- Focal squamous or glandular differentiation are often seen; commonly coexists with in situ or invasive squamous carcinoma, adenocarcinoma or (rarely) mesonephric carcinoma (Zheng: Gynecologic and Obstetric Pathology, 2nd Edition, 2025)
- Pure small cell neuroendocrine carcinoma is rare
- Unlike neuroendocrine carcinomas from other sites, there are no established criteria for Ki67 labeling index or mitotic count in small cell neuroendocrine carcinoma of the cervix (Ann Diagn Pathol 2017;29:11)
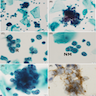
Microscopic (histologic) images
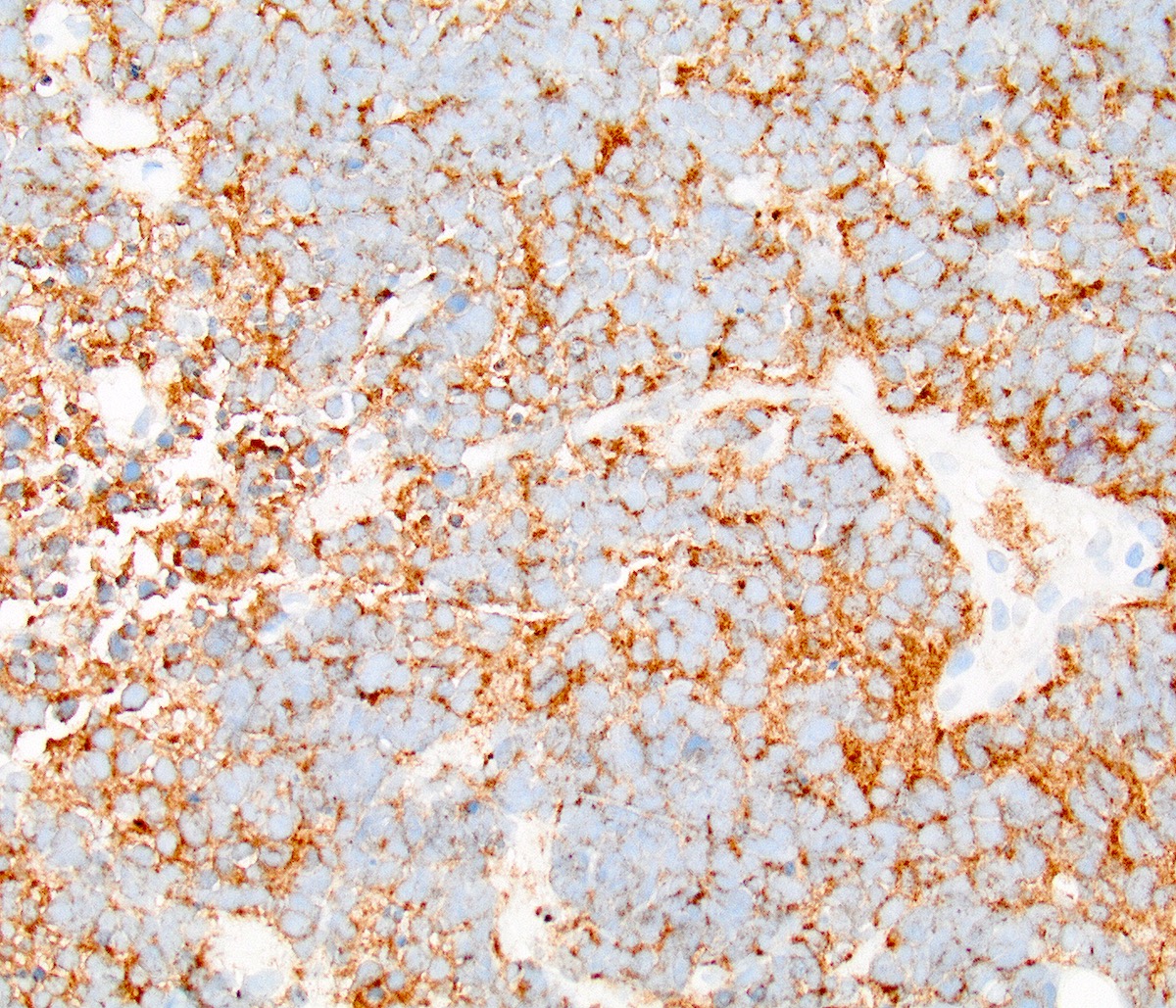
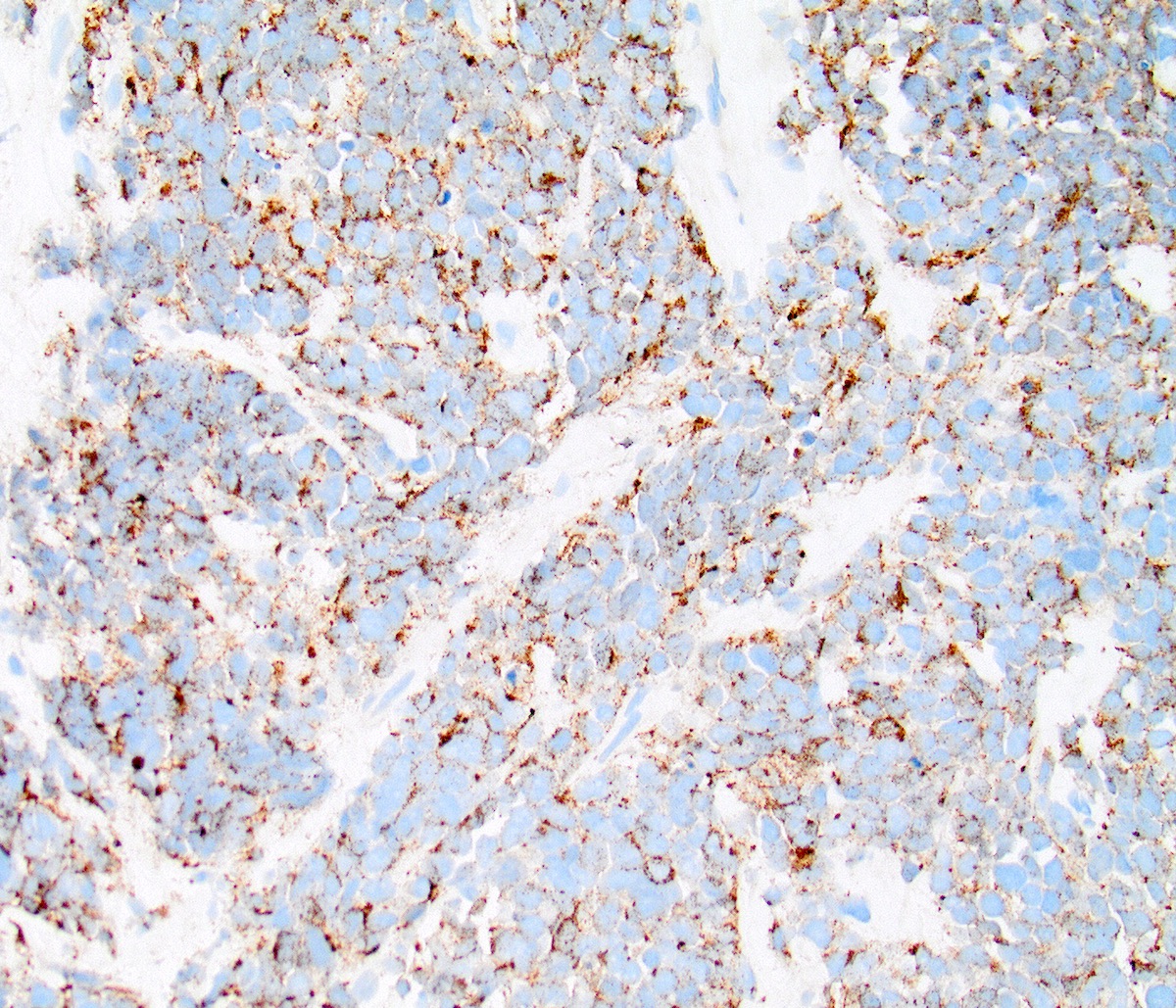
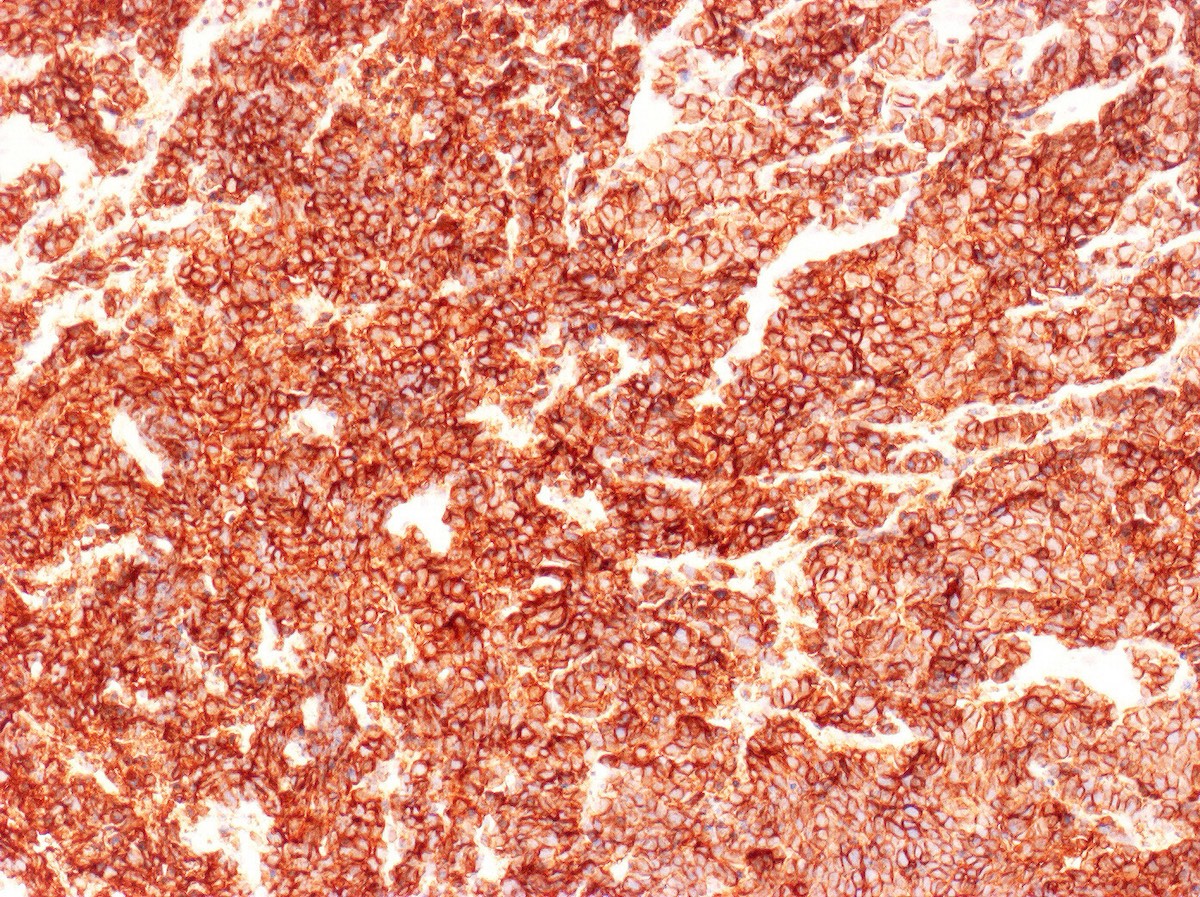
Contributed by Seyed Salehi, M.D., Wanrun Lin, M.D., Ph.D. and Wenxin Zheng, M.D.
Cytology description
- Typically moderate to high cellularity
- Arranged in sheets, clusters and as single dispersed cells
- Small, round, oval or spindle shaped cells with scant cytoplasm and a high N:C ratio
- Nuclei with finely granular (salt and pepper) chromatin or hyperchromasia, inconspicuous nucleoli, frequent nuclear molding and occasional chromatin streak artifact (Onco Targets Ther 2024;17:557, Cytopathology 2021;32:813)
- Marked brisk mitotic activity (> 10 mitoses per 10 high power fields [HPF]) and numerous apoptotic bodies
- Crush artifact is common due to cellular fragility
- Occasional intermediate type cells show larger, more uniform nuclei with coarser chromatin
Positive stains
- Chromogranin (60%; relative specific) (Am J Surg Pathol 2010;34:525)
- Synaptophysin (70%) (Am J Surg Pathol 2010;34:525)
- CD56 (88%; sensitive but less specific) (Int J Gynecol Pathol 2005;24:113)
- INSM1 (93%; 27/29) (Gynecol Oncol 2017;144:384)
- Neural cell adhesion molecule (NCAM) (68%) (Am J Surg Pathol 2010;34:525)
- NSE (80%; cytoplasmic)
- p16 (diffuse block type, due to the association with high risk HPV) (Am J Surg Pathol 2016;40:577, Gynecol Oncol 2018;148:422)
- PGP9.5 (cytoplasmic)
- AE1 / AE3 (cytoplasmic)
- TTF1 (variable positive, up to 85%; 11/13) (Am J Surg Pathol 2010;34:525)
- No neuroendocrine marker is expressed in 100% of cases; a diagnosis of small cell neuroendocrine carcinoma can be made on morphology alone (Zheng: Gynecologic and Obstetric Pathology, 2nd Edition, 2025)
Negative stains
- CDX2 (rarely positive; does not entirely exclude a gynecological origin) (Am J Surg Pathol 2004;28:1169)
- CK5/6, p63, p40 (to distinguish from small cell squamous cell carcinoma)
- CD45, CD3, CD20 (to distinguish from lymphoma)
- S100, HMB45 (to distinguish from melanoma)
- MyoD1, myogenin, desmin (to distinguish from rhabdomyosarcoma)
Molecular / cytogenetics description
- Some cervical small cell neuroendocrine carcinomas show aberrations in the MAPK, PI3K / AKT / mTOR and p53 / BRCA pathways, along with 3p loss of heterozygosity (Am J Surg Pathol 2018;42:750)
Videos
SCNEC digital slide review
Sample pathology report
- Uterus, cervix, bilateral tubes and ovaries, radical hysterectomy and bilateral salpingo-oophorectomy:
- Small cell neuroendocrine carcinoma of the cervix (see comment)
- Focal area of HPV associated well differentiated adenocarcinoma present
- Tumor size: 3.5 x 2.5 x 2 cm
- Depth of cervical stromal invasion: > one - third cervical wall thickness
- Lymphovascular space invasion: focally identified (< 3)
- Frank tumor necrosis seen
- Parametrial invasion: not identified
- Vaginal cuff involvement: not identified
- Resection margins: negative
- Inactive endometrium
- Unremarkable myometrium, ovaries and fallopian tubes
- Comment: This cervical mass histologically exhibits sheets and clusters of tumor cells with small, round and oval shaped nuclei, scant cytoplasm and a high N:C ratio. The tumor cell nuclei display salt and pepper chromatin with inconspicuous nucleoli. Nuclear molding is observed. Immunohistochemically, the tumor cells are positive for INSM1 and synaptophysin but negative for chromogranin. Additionally, a 5 mm area of well differentiated adenocarcinoma is identified adjacent to the small cell carcinoma. Both cancer components are diffusely positive for p16 and positive for high risk HPV by RNA in situ hybridization. Overall, these findings are diagnostic of cervical small cell neuroendocrine carcinoma admixed with adenocarcinoma, HPV associated.
Differential diagnosis
- Squamous carcinoma with small or basaloid cells:
- Typically lacks trabecular or cord-like growth, nuclear molding or crush artifact
- Displays cohesive cells with intercellular bridges, often nonkeratinizing; cytoplasm usually surrounds the nucleus rather than it being eccentrically located
- Lymphovascular invasion is less common than small cell neuroendocrine carcinoma
- Immunohistochemically negative (or only focally positive) for neuroendocrine markers; positive for CK5/6, p63 and p40
- Prognosis is better than small cell neuroendocrine carcinoma
- Metastatic small cell carcinoma:
- Often widely metastatic at diagnosis
- Usually HPV negative
- Consider relevant clinical history and imaging
- Lymphoma / leukemia:
- History often indicates hematologic malignancy
- Noncohesive cells lacking insular or nested growth patterns; B or T cell markers (lymphomas) or myelocytic markers (leukemias) positive
- Negative for neuroendocrine markers
- Adenoid basal carcinoma:
- Rhabdomyosarcoma:
- Rare in the cervix, especially in adolescents or young adults; more commonly occurs in children
- Embryonal rhabdomyosarcoma commonly arises in the vagina of children and the cervix or uterine corpus in adolescents / adults, occasionally showing rhabdomyoblasts (strap or tadpole cells)
- Alveolar rhabdomyosarcoma typically affects the vulva and has nests of discohesive tumor cells separated by fibrous septa
- Positive for myogenin, MyoD1 and desmin; negative for AE1 / AE3 and neuroendocrine markers
- Undifferentiated carcinoma:
- Melanoma:
- Coexisting epithelial malignancies:
- Neuroendocrine carcinoma can coexist with adenocarcinoma or squamous cell carcinoma
- Recommended terminology for distinct components: adenocarcinoma (or squamous cell carcinoma) admixed with neuroendocrine carcinoma, to guide high grade NEC management
Additional references
Practice question #1
A uterine cervical biopsy exhibits the features shown in the images above. What is the most likely diagnosis?
- Adenocarcinoma of the uterine cervix
- Large cell neuroendocrine carcinoma of the uterine cervix
- Small cell neuroendocrine carcinoma of the uterine cervix
- Squamous cell carcinoma of the uterine cervix
Practice answer #1
C. Small cell neuroendocrine carcinoma of the uterine cervix. The H&E images display classic features of small cell neuroendocrine carcinoma, namely sheets of small cells with high N:C ratio, hyperchromatic nuclei and nuclear molding.
Answer D is incorrect because squamous cell carcinoma typically shows keratinization and intercellular bridges.
Answer A is incorrect because adenocarcinoma usually forms glands and produces mucin.
Answer B is incorrect because large cell neuroendocrine carcinoma presents with larger cells and more abundant cytoplasm.
Comment Here
Reference: Small cell neuroendocrine carcinoma
Comment Here
Reference: Small cell neuroendocrine carcinoma
Practice question #2
A 42 year old woman presents with an aggressive cervical tumor. In addition to the classic histologic features seen on H&E, immunohistochemical studies reveal diffuse positivity for synaptophysin and chromogranin, patchy p16 expression and a markedly high Ki67 proliferation index. Which of the following statements best explains the diagnostic significance of this immunoprofile in differentiating small cell neuroendocrine carcinoma from other high grade cervical carcinomas?
- Combination of these immunostains is nonspecific and cannot reliably differentiate small cell neuroendocrine carcinoma from adenocarcinoma of the cervix
- Diffuse positivity for neuroendocrine markers (synaptophysin and chromogranin) supports the diagnosis of small cell neuroendocrine carcinoma, distinguishing it from high grade squamous cell carcinoma, which typically lacks this expression
- High Ki67 proliferation index is unusual for small cell neuroendocrine carcinoma, suggesting an alternative high grade neoplasm
- Patchy p16 expression excludes small cell neuroendocrine carcinoma, as these tumors uniformly exhibit diffuse p16 positivity due to high risk HPV infection
Practice answer #2
B. Diffuse positivity for neuroendocrine markers (synaptophysin and chromogranin) supports the diagnosis of small cell neuroendocrine carcinoma, distinguishing it from high grade squamous cell carcinoma, which typically lacks this expression. The immunoprofile in small cell neuroendocrine carcinoma is characterized by diffuse expression of neuroendocrine markers such as synaptophysin and chromogranin. This finding, together with the classic H&E features (small cells with scant cytoplasm, nuclear molding and high mitotic activity) and a high Ki67 index, reinforces the diagnosis of small cell neuroendocrine carcinoma. Although p16 is often diffusely positive in HPV related cervical carcinomas, its patchy expression in this context does not exclude the diagnosis.
Answer D is incorrect because small cell neuroendocrine carcinomas may not always show diffuse p16 positivity and patchy expression does not rule out the diagnosis. Answer C is incorrect because a high Ki67 proliferation index is a well recognized feature of small cell neuroendocrine carcinoma, reflecting its aggressive nature. Answer A is incorrect because the combination of diffuse neuroendocrine marker positivity and the described histologic features is highly specific for small cell neuroendocrine carcinoma and aids in distinguishing it from other high grade cervical neoplasms.
Comment Here
Reference: Small cell neuroendocrine carcinoma
Answer D is incorrect because small cell neuroendocrine carcinomas may not always show diffuse p16 positivity and patchy expression does not rule out the diagnosis. Answer C is incorrect because a high Ki67 proliferation index is a well recognized feature of small cell neuroendocrine carcinoma, reflecting its aggressive nature. Answer A is incorrect because the combination of diffuse neuroendocrine marker positivity and the described histologic features is highly specific for small cell neuroendocrine carcinoma and aids in distinguishing it from other high grade cervical neoplasms.
Comment Here
Reference: Small cell neuroendocrine carcinoma