Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Liu YA, Cho WC. Cutaneous lymphoid hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticlymphoidhyperplasia.html. Accessed September 17th, 2025.
Definition / general
- Heterogeneous group of disorders with benign T or B cell lymphocytic infiltrates in the skin that mimic cutaneous lymphomas both clinically and histologically (J Am Acad Dermatol 1998;38:877)
Essential features
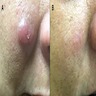
- Typically presents as a solitary nodule in the head and neck region
- Spontaneous regression is common; excellent prognosis
- Idiopathic; however, some cases are associated with specific antigenic stimuli or triggering factors (e.g., drugs, tattoos, vaccinations, arthropod bites and infections)
- Classically characterized by a dermal lymphoid infiltrate composed predominantly of reactive polyclonal lymphocytes with a variable admixture of other inflammatory cells
- Although typically absent, clonal T or B cell populations can occasionally be seen in cutaneous lymphoid hyperplasia
Terminology
- Pseudolymphoma, lymphocytoma cutis, lymphadenosis benigna cutis
ICD coding
Epidemiology
- Can affect all age groups
- Slight female predilection; M:F = 1:2 - 3 (Hum Pathol 2003;34:617)
Sites
- Can involve any area of the skin
- Most commonly arises in the head and neck regions (particularly the face), followed by the extremities and trunk
Pathophysiology
- Largely unknown but thought to be a reactive process to certain antigenic stimuli
Etiology
- Often idiopathic
- Various antigenic stimuli or triggering factors (e.g., medications, tattoos, vaccinations, arthropod bites, infections with various pathogens, especially with Borrelia burgdorferi) have been reported (Hum Pathol 2003;34:617, Acta Derm Venereol 2018;98:310, J Cutan Pathol 2020;47:76)
Clinical features
- Solitary nodule or localized cluster of red to violaceous papulonodules
Diagnosis
- Requires thorough clinical and histopathologic evaluation and acquisition of appropriate clinical history to identify possible triggering factors
- In the absence of an identifiable triggering factor, it may pose a diagnostic challenge to pathologists and may potentially be misdiagnosed as a neoplastic process, especially if clonality is found in the cutaneous infiltrate
- Historically divided into 3 categories based on the predominant cell type in the infiltrate (J Am Acad Dermatol 1998;38:877, Hum Pathol 2003;34:617):
- B cell predominant type
- T cell predominant type
- Mixed B cell / T cell type
- Newly proposed classification divides cutaneous lymphoid hyperplasia into 4 major groups, based on both histologic and clinical features (J Cutan Pathol 2020;47:76):
- Nodular cutaneous lymphoid hyperplasia
- Cutaneous lymphoid hyperplasia mimicking mycosis fungoides or other cutaneous T cell lymphomas
- Other cutaneous lymphoid hyperplasia with distinct clinical features (e.g., acral pseudolymphomatous angiokeratoma, T cell rich angiomatoid polypoid pseudolymphoma, primary cutaneous angioplasmocellular hyperplasia, lymphoplasmacytic plaque, cutaneous plasmacytosis)
- Intravascular cutaneous lymphoid hyperplasia
- Nodular type is the most common form and encompasses nodular B cell cutaneous lymphoid hyperplasia, Borrelia associated nodular B cell cutaneous lymphoid hyperplasia (also known as lymphocytoma cutis or lymphadenosis benigna cutis), nodular T cell and mixed cutaneous lymphoid hyperplasia and nodular CD30+ cutaneous lymphoid hyperplasia (J Cutan Pathol 2020;47:76)
Prognostic factors
- Benign clinical course
- Spontaneous regression is common following excision or topical / intralesional corticosteroids
- May rarely recur
- Almost never progresses to an overt lymphoma (although rare anecdotal examples of such progression have been described) (Am J Pathol 1989;135:13, Hum Pathol 2003;34:617)
Case reports
- 25 year old woman with pruritic papulovesicular lesions on the right shoulder (J Investig Allergol Clin Immunol 2018;28:199)
- 36 year old woman with a pruritic erythematous nodule on the left forearm (Indian J Dermatol Venereol Leprol 2021;87:748)
- 48 year old woman with a red erythematous nodule on the right medial cheek (CMAJ 2018;190:E398)
- 78 year old woman with a pink papule on the right arm (JAAD Case Rep 2022;20:26)
- 81 year old woman with a rapidly enlarging, solitary lesion on the left lateral trapezial neck (Am J Dermatopathol 2022;44:226)
Treatment
- Observation
- Antibiotics, topical or intralesional corticosteroids, surgical excision or localized radiotherapy
- Less commonly, intralesional rituximab or methotrexate (Clin Lymphoma Myeloma Leuk 2011;11:286, Dermatol Online J 2021;27)
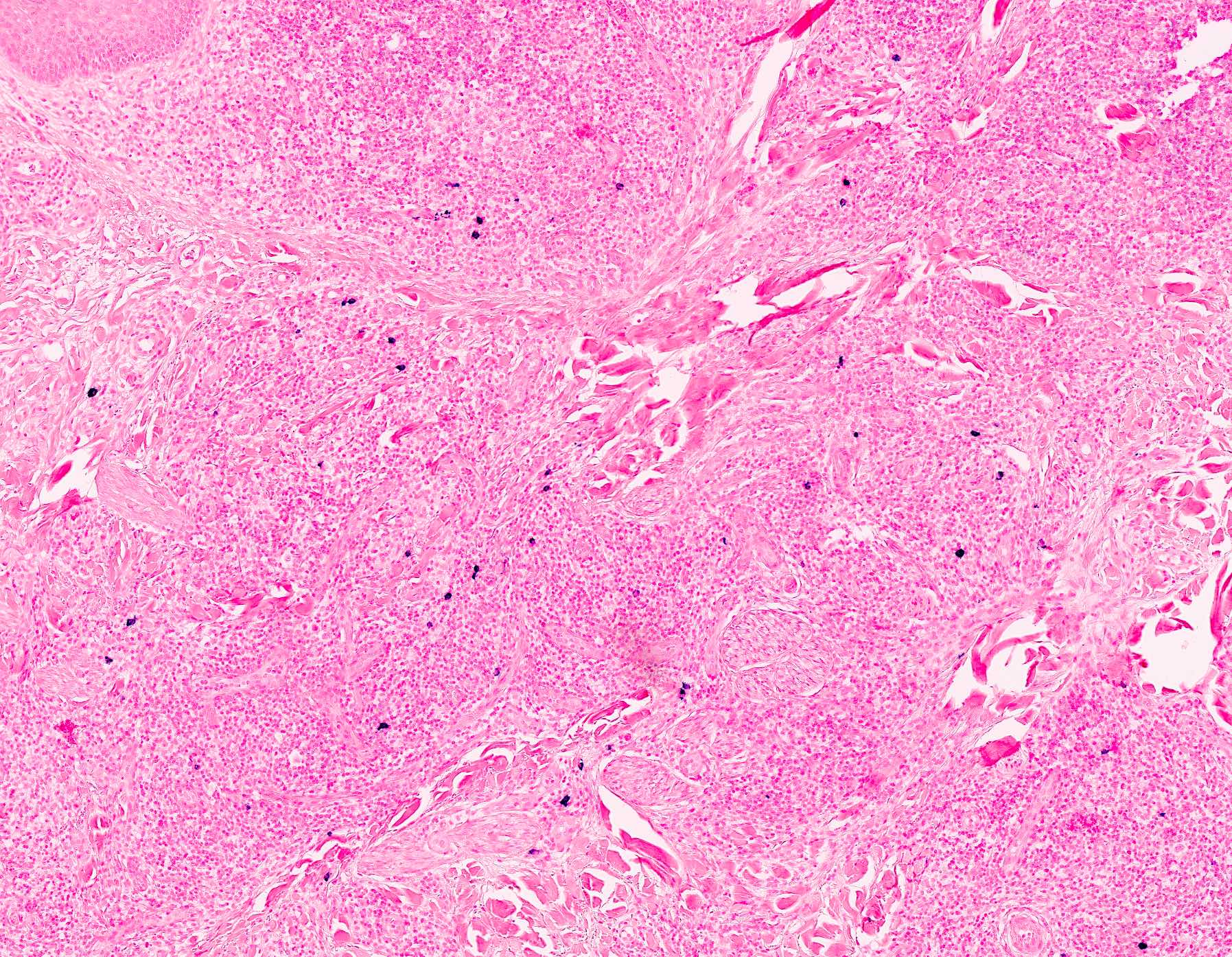
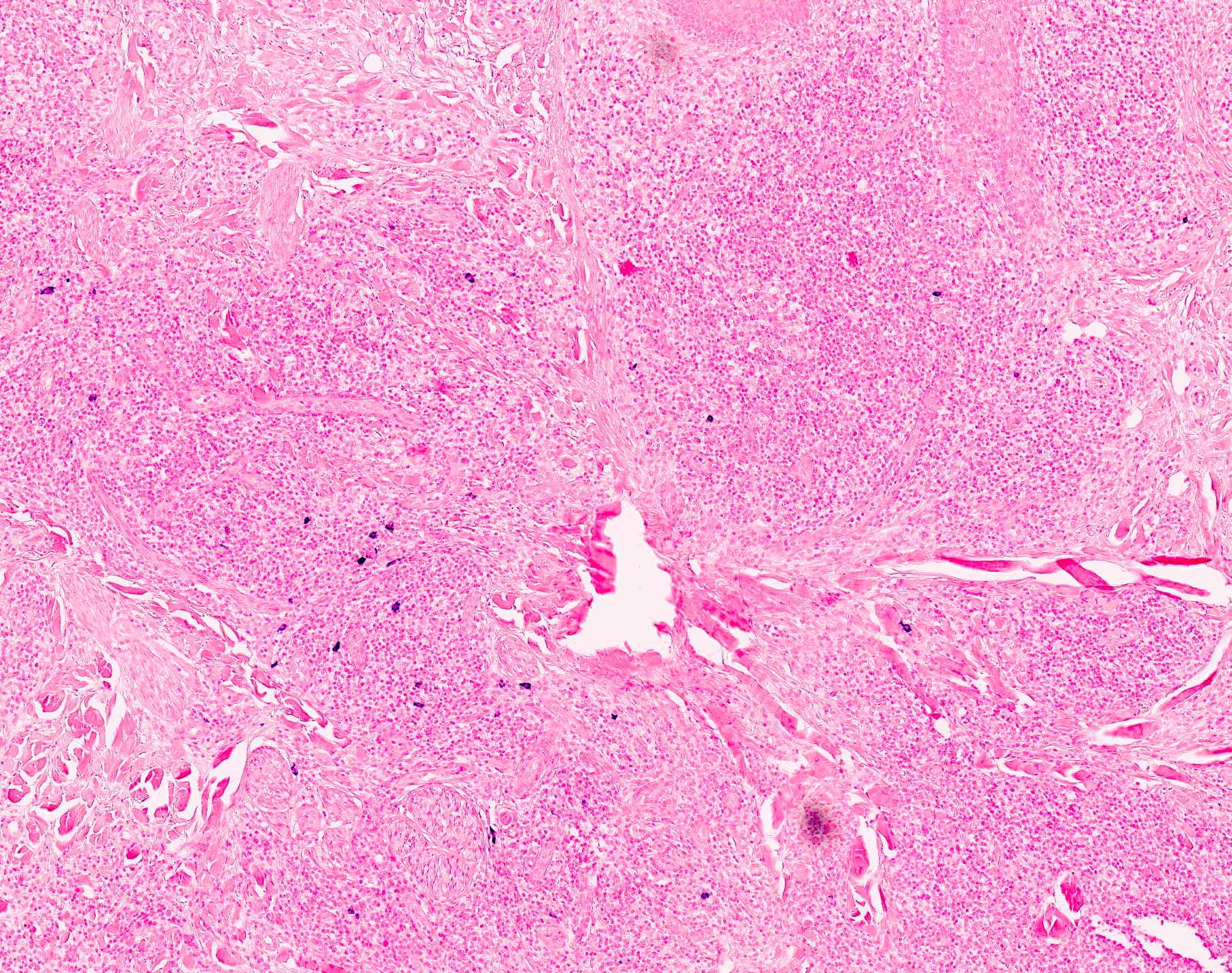
Microscopic (histologic) description
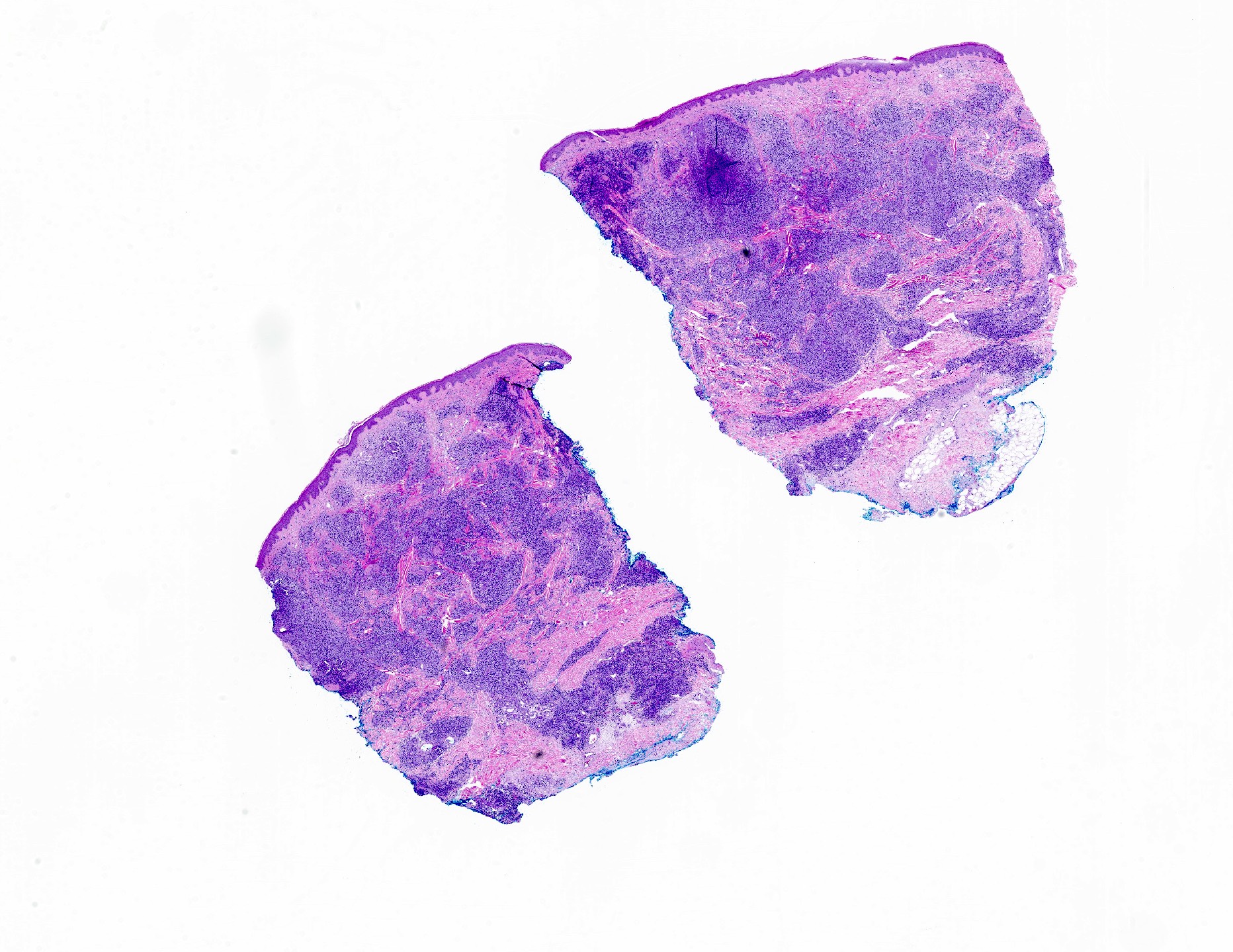
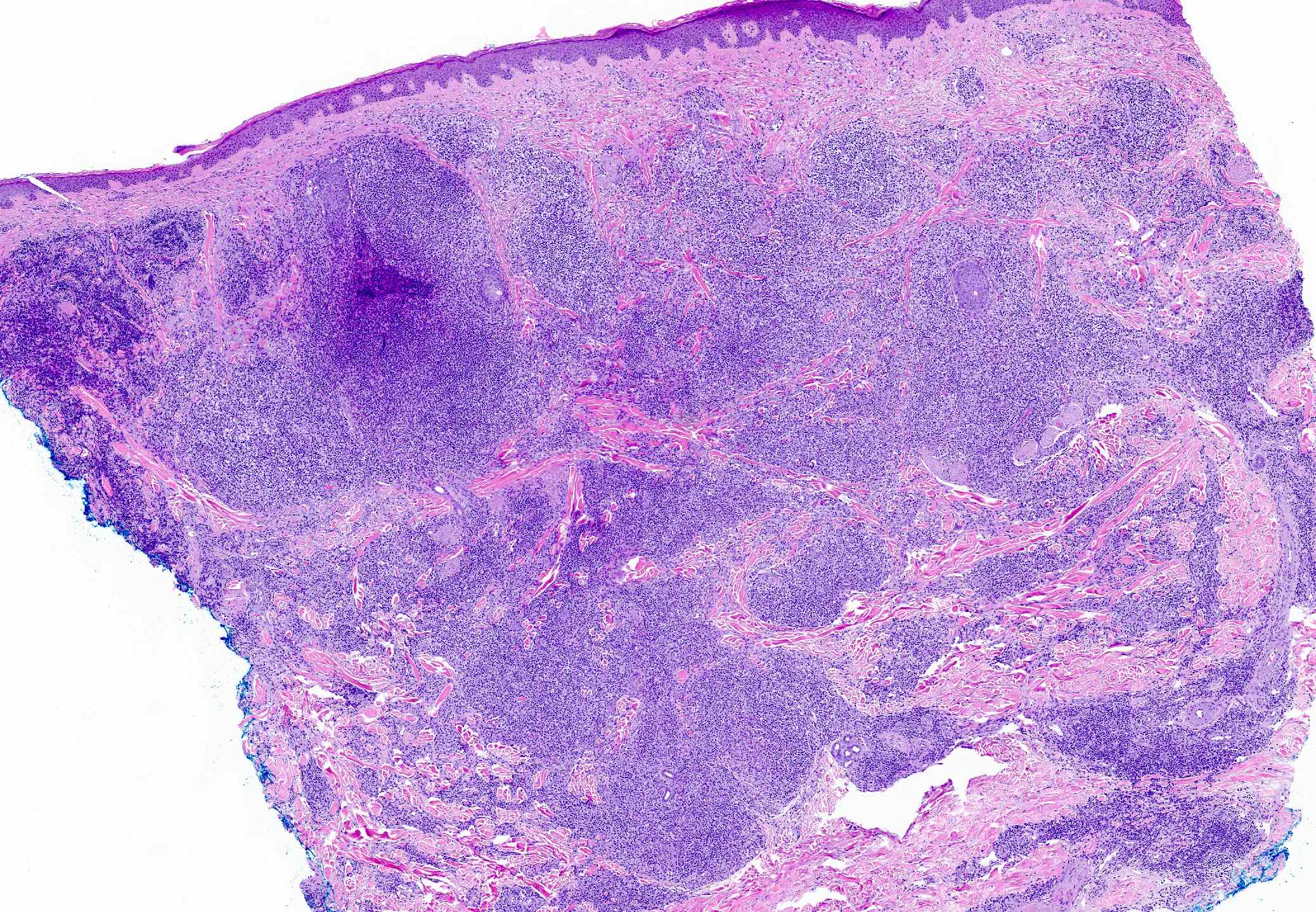
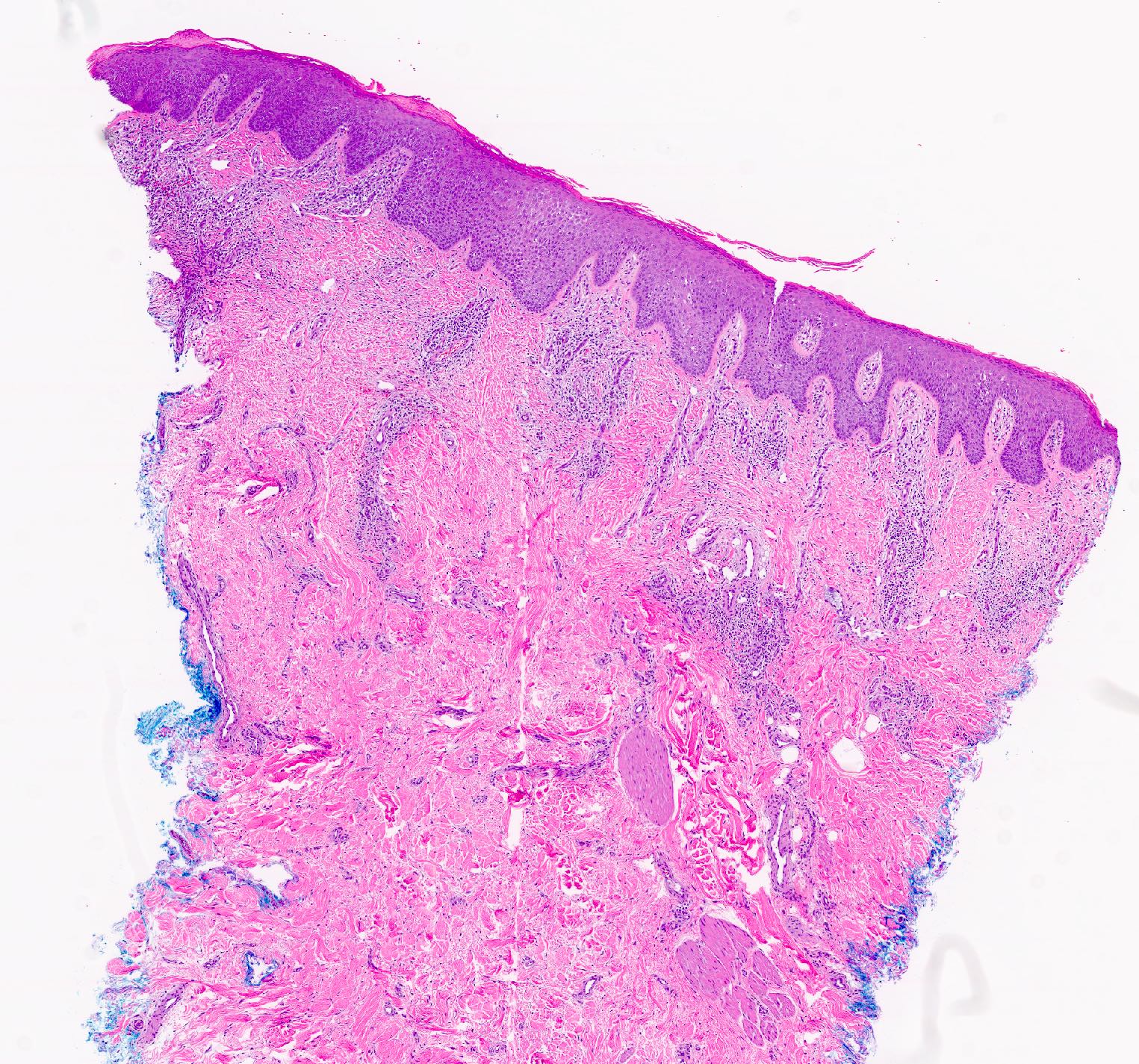
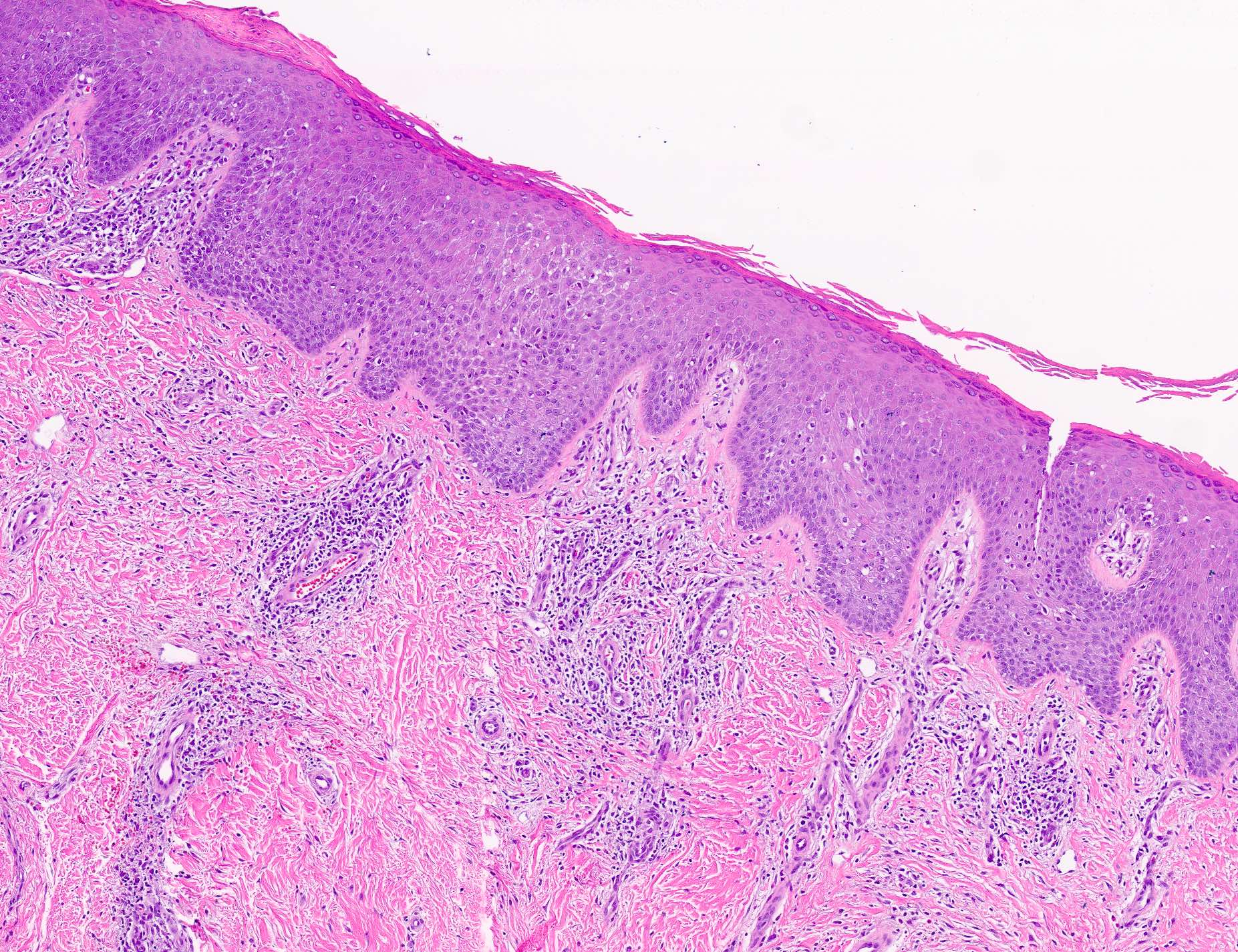
- Dermal lymphoid infiltrate, forming nodules or diffuse sheets (particularly in nodular cutaneous lymphoid hyperplasia), without significant epidermal involvement
- Grenz zone, between the dermal infiltrate and the overlying epidermis, is frequently found
- Perivascular and periadnexal distribution patterns may also be seen
- Although typically intradermal, the infiltrate may also occasionally extend into the superficial subcutis
- Prominent lymphocytic exocytosis may occasionally be seen mimicking other cutaneous T cell lymphomas, such as mycosis fungoides
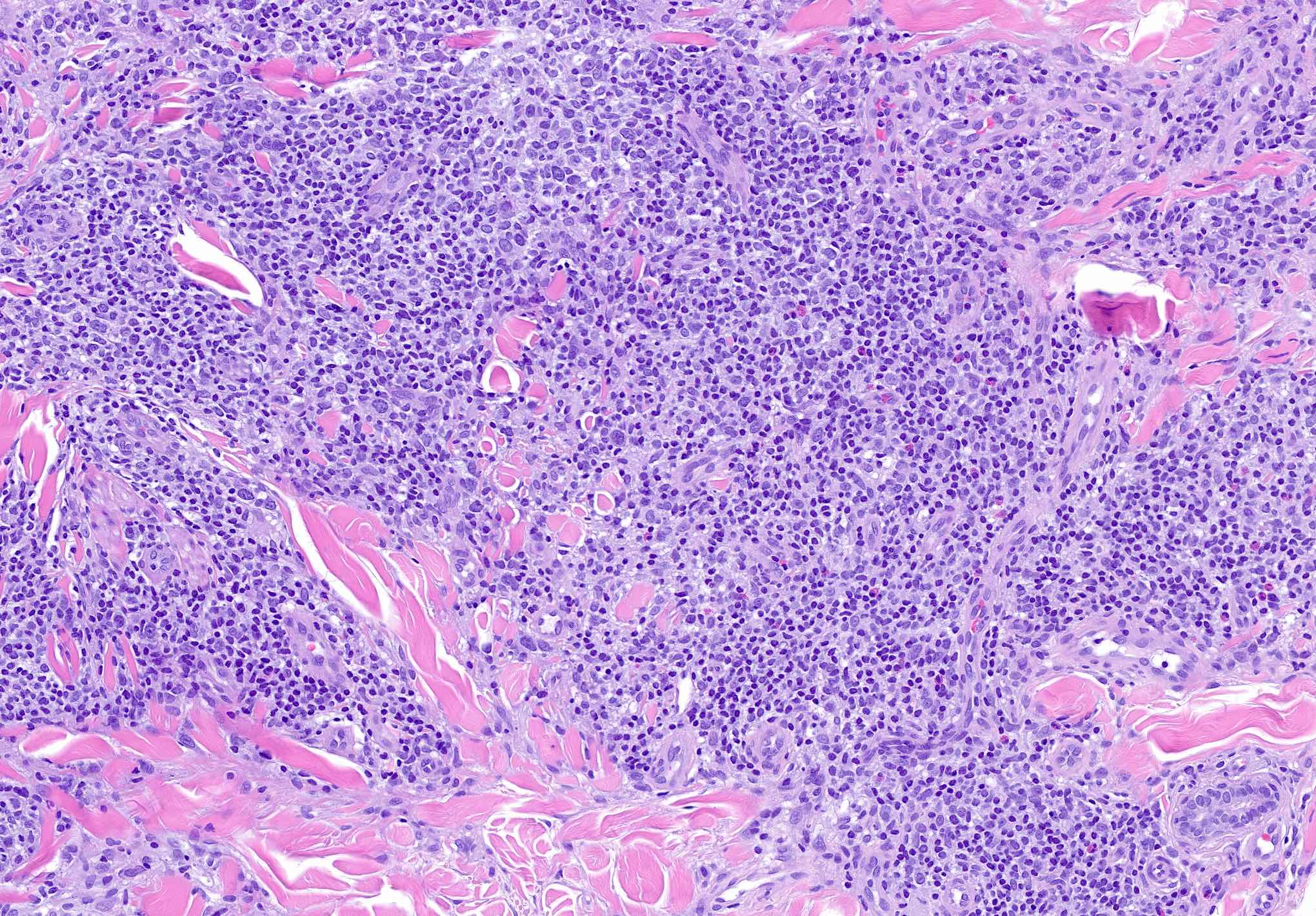
- Infiltrate predominantly composed of small to medium sized lymphocytes, often indistinguishable from those seen in primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder, with variable admixture of other inflammatory cells (e.g., histiocytes, eosinophils, plasma cells)
- Well formed reactive germinal centers may or may not be seen
- If present, reactive germinal centers often show tingible body macrophages and preservation of polarity and mantle zone
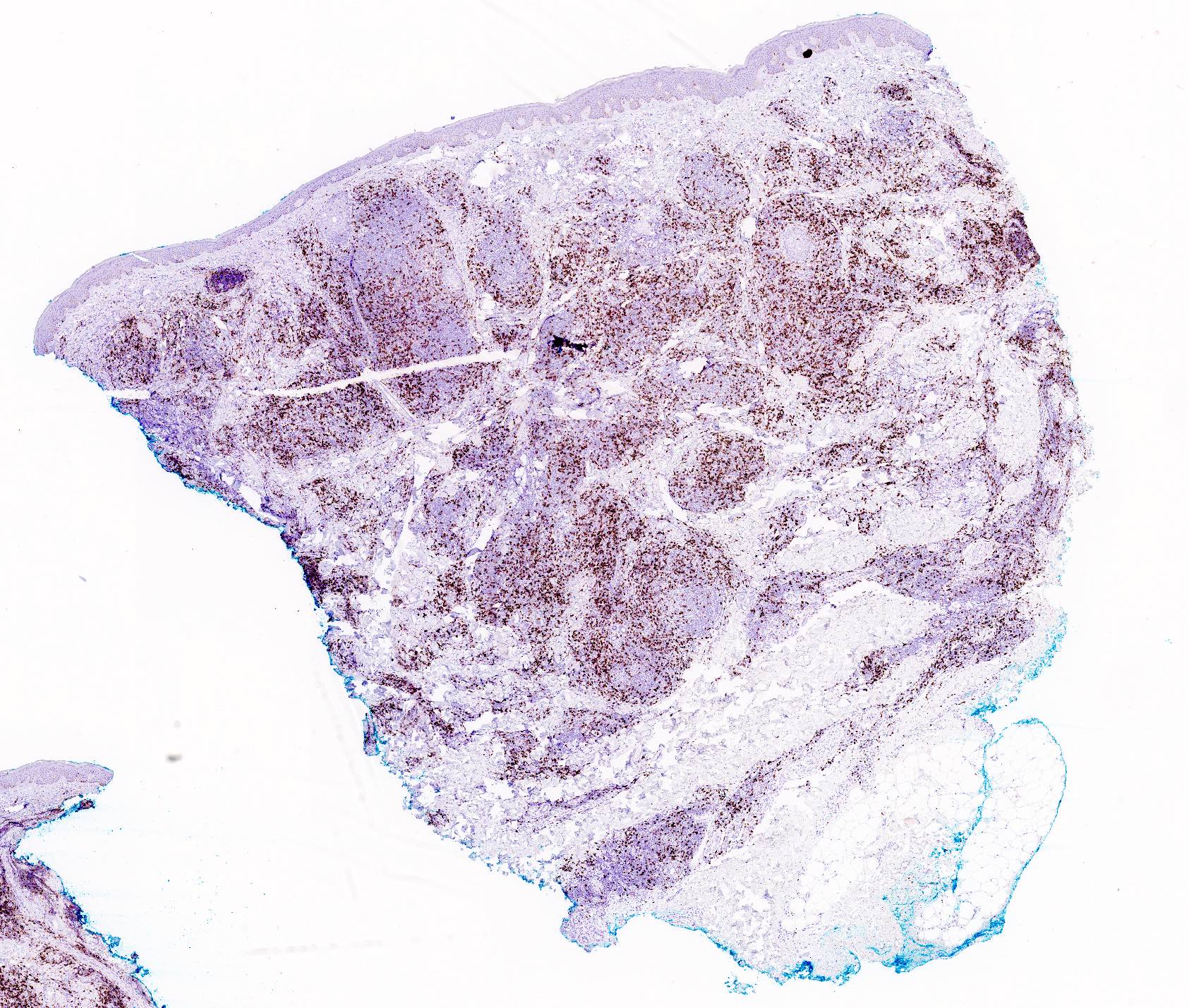
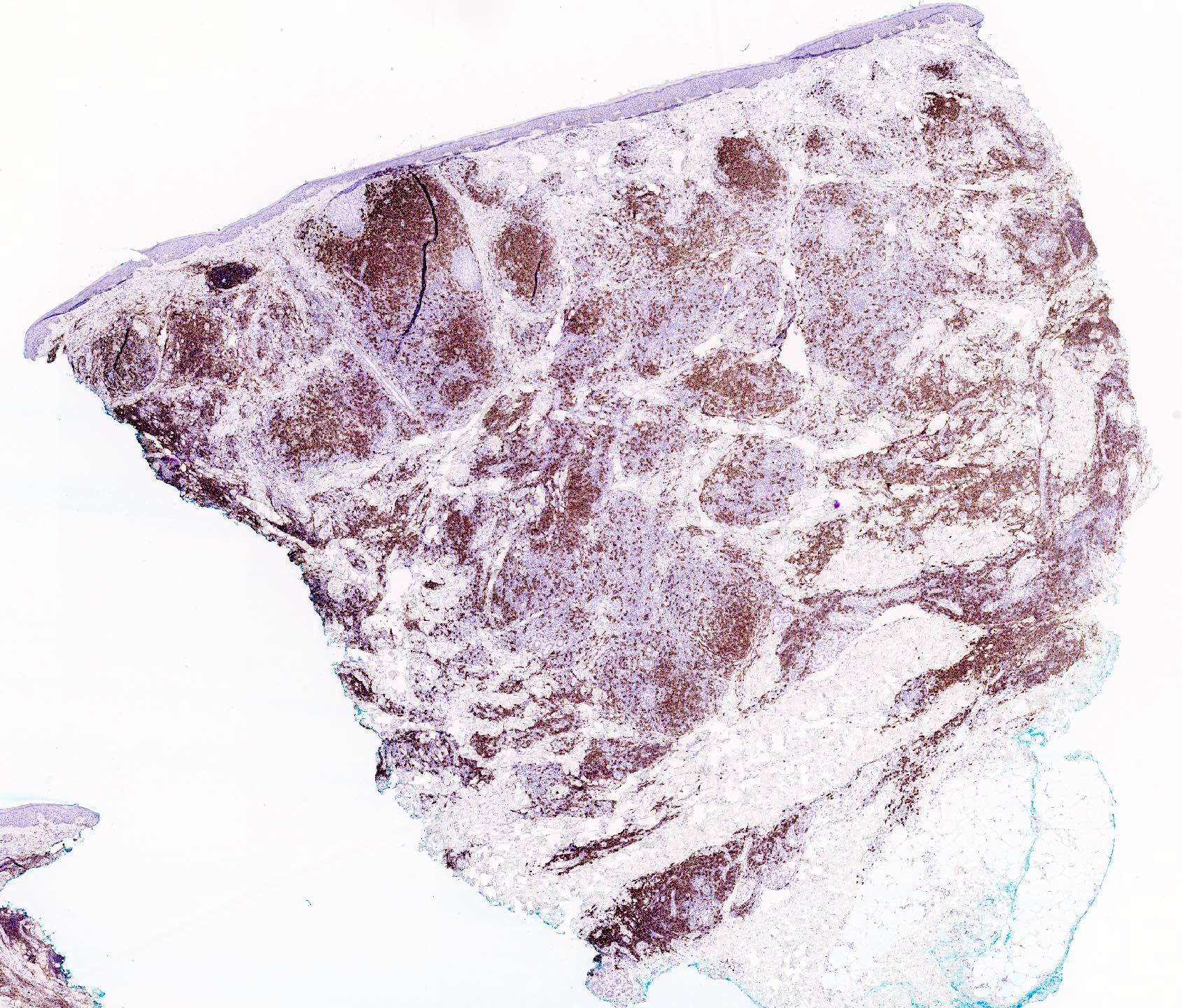
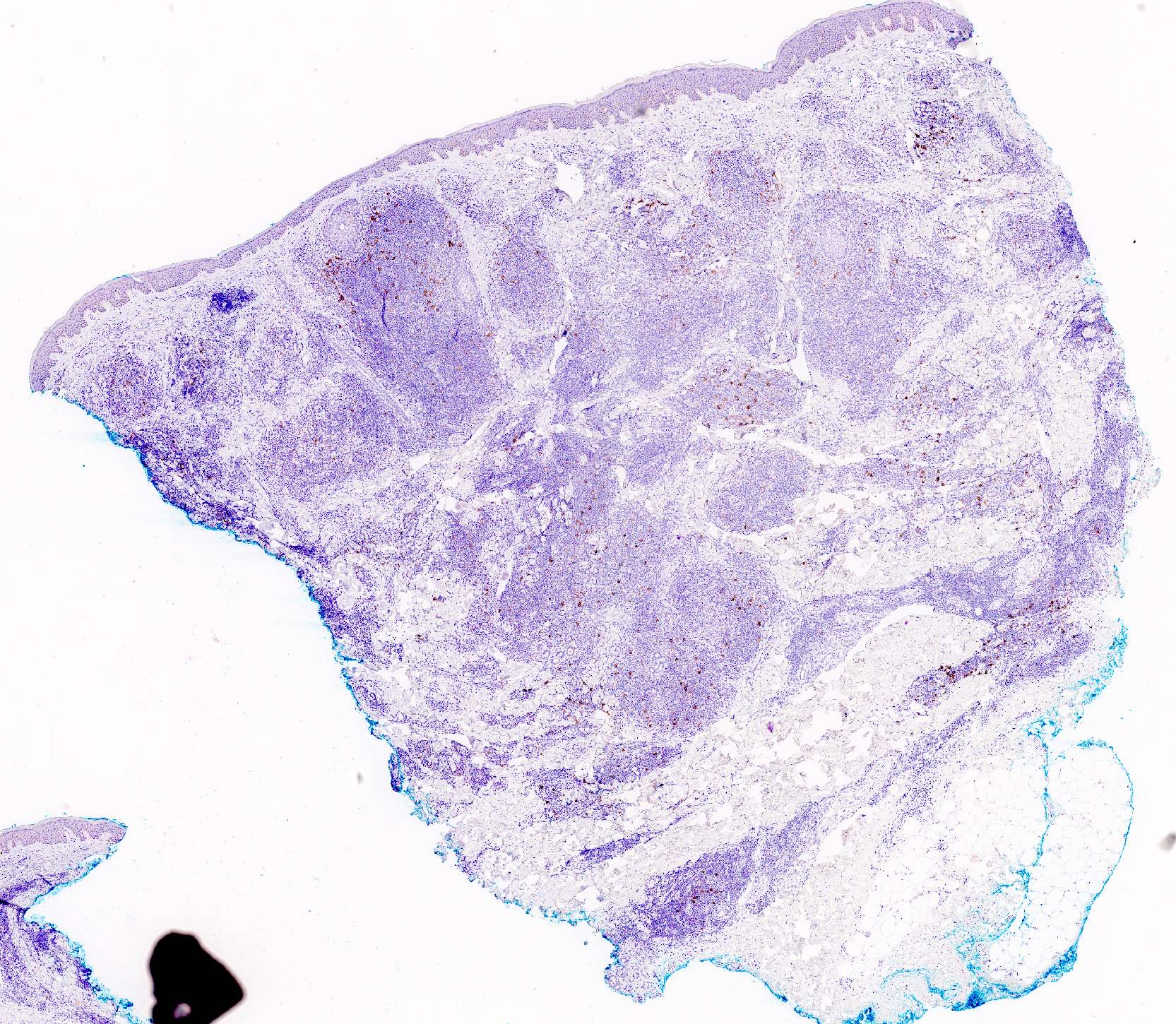
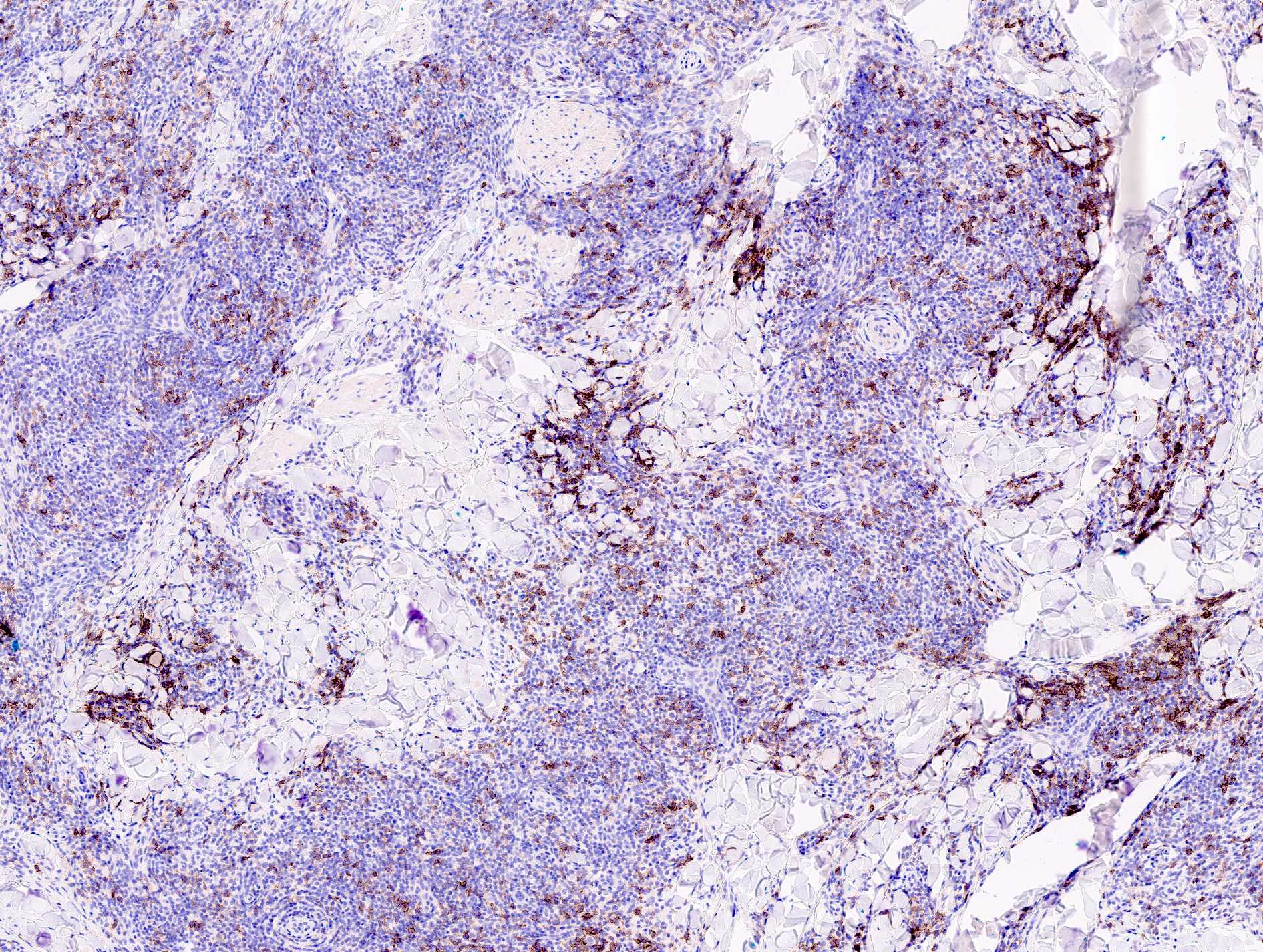
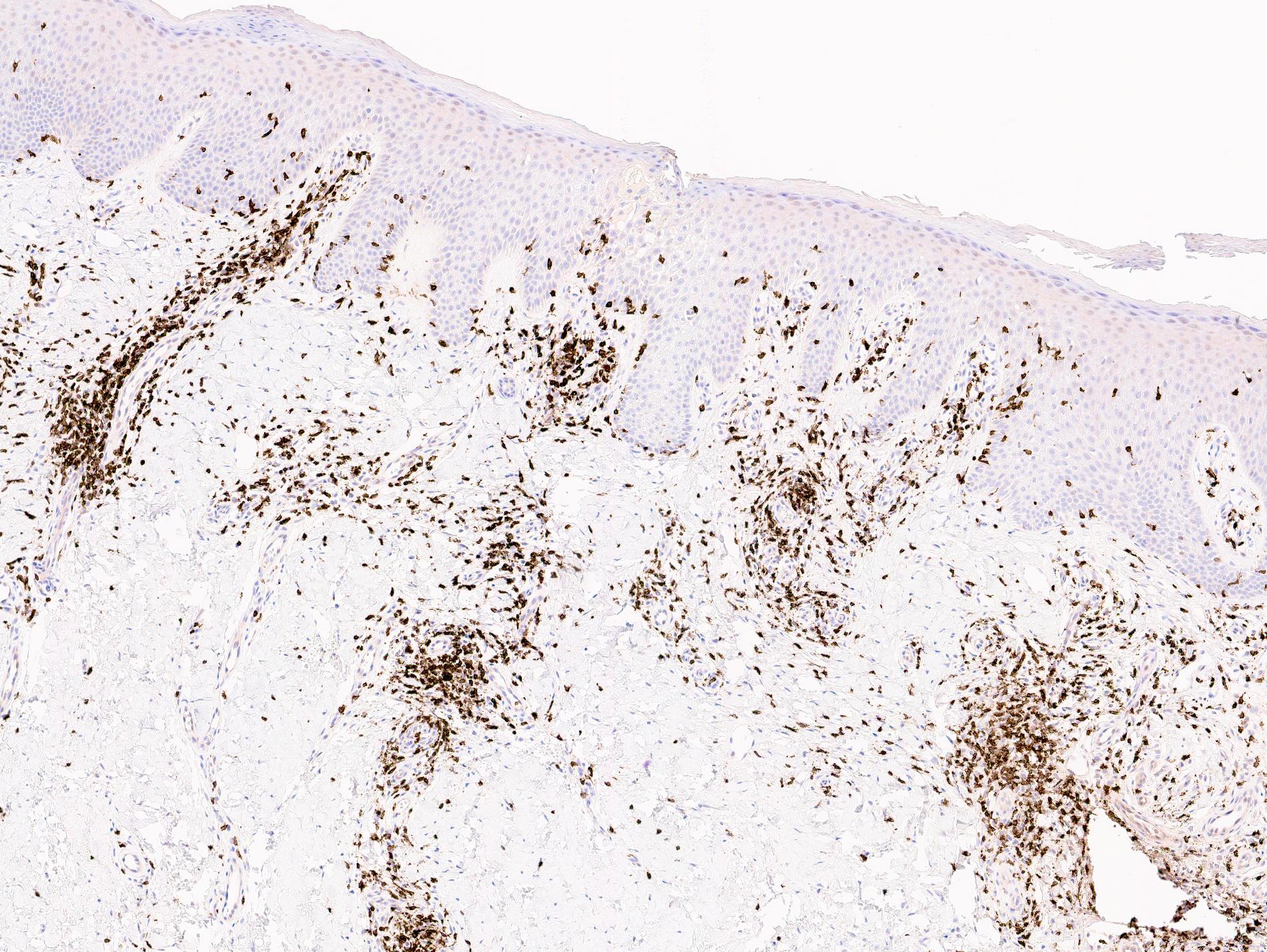
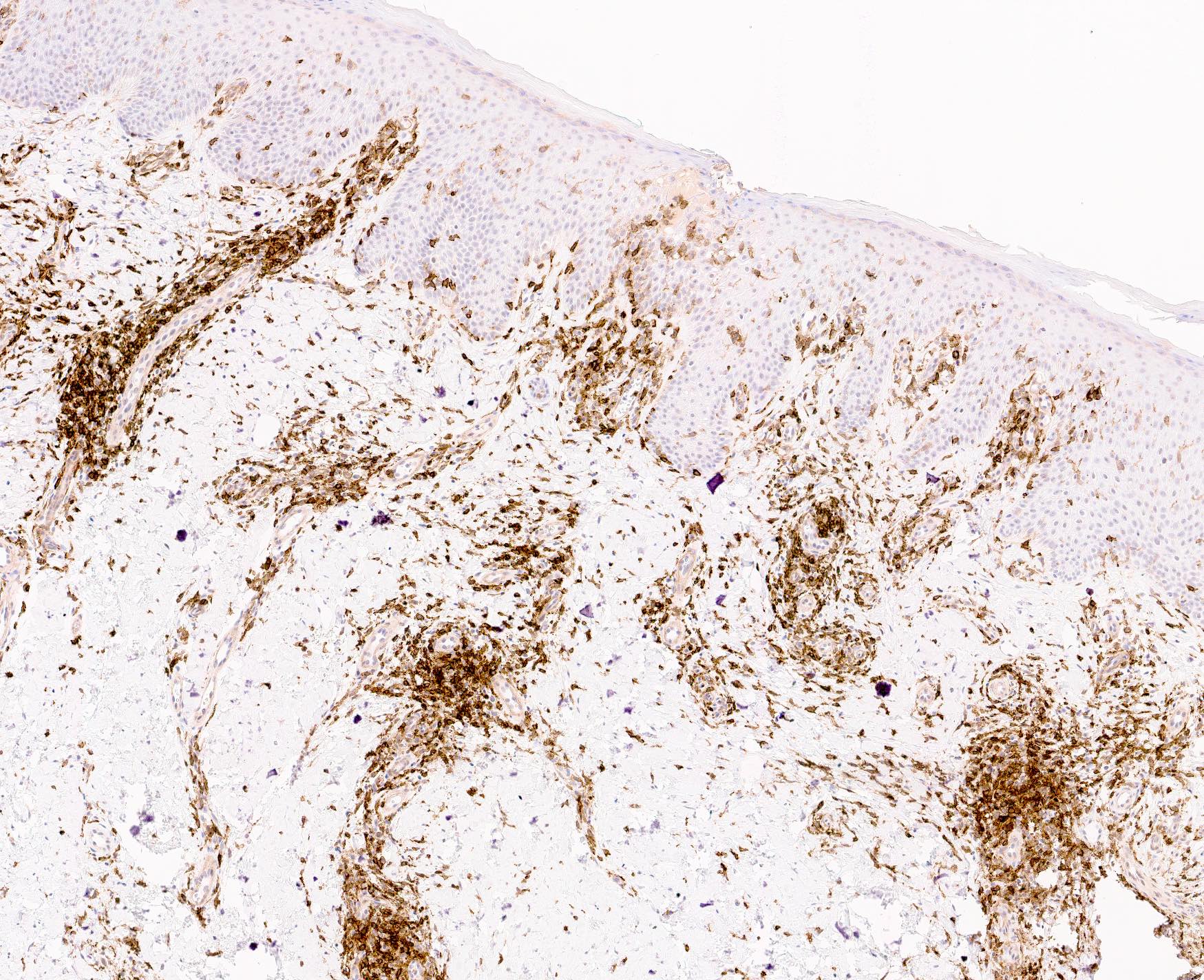
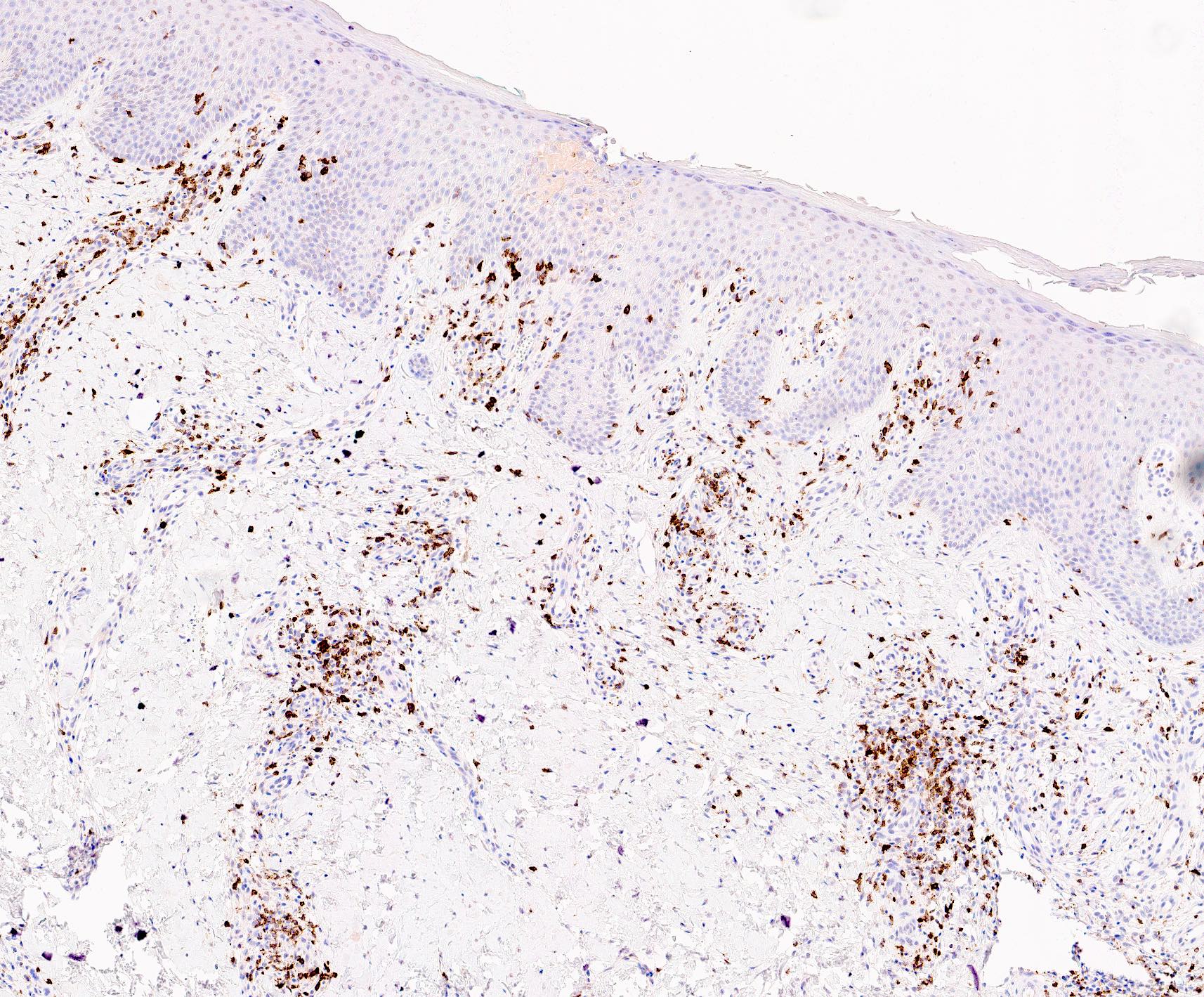
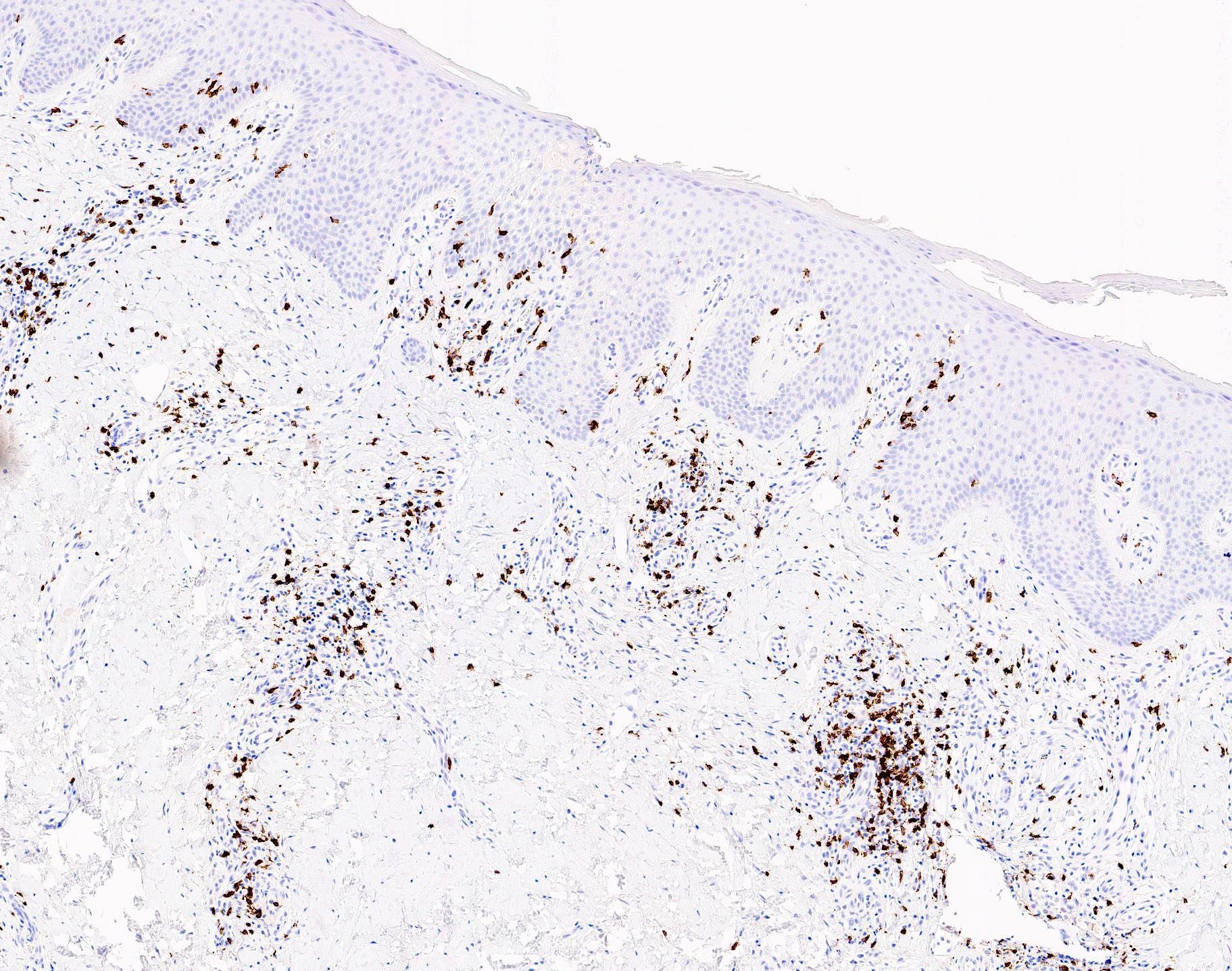
Microscopic (histologic) images
Positive stains
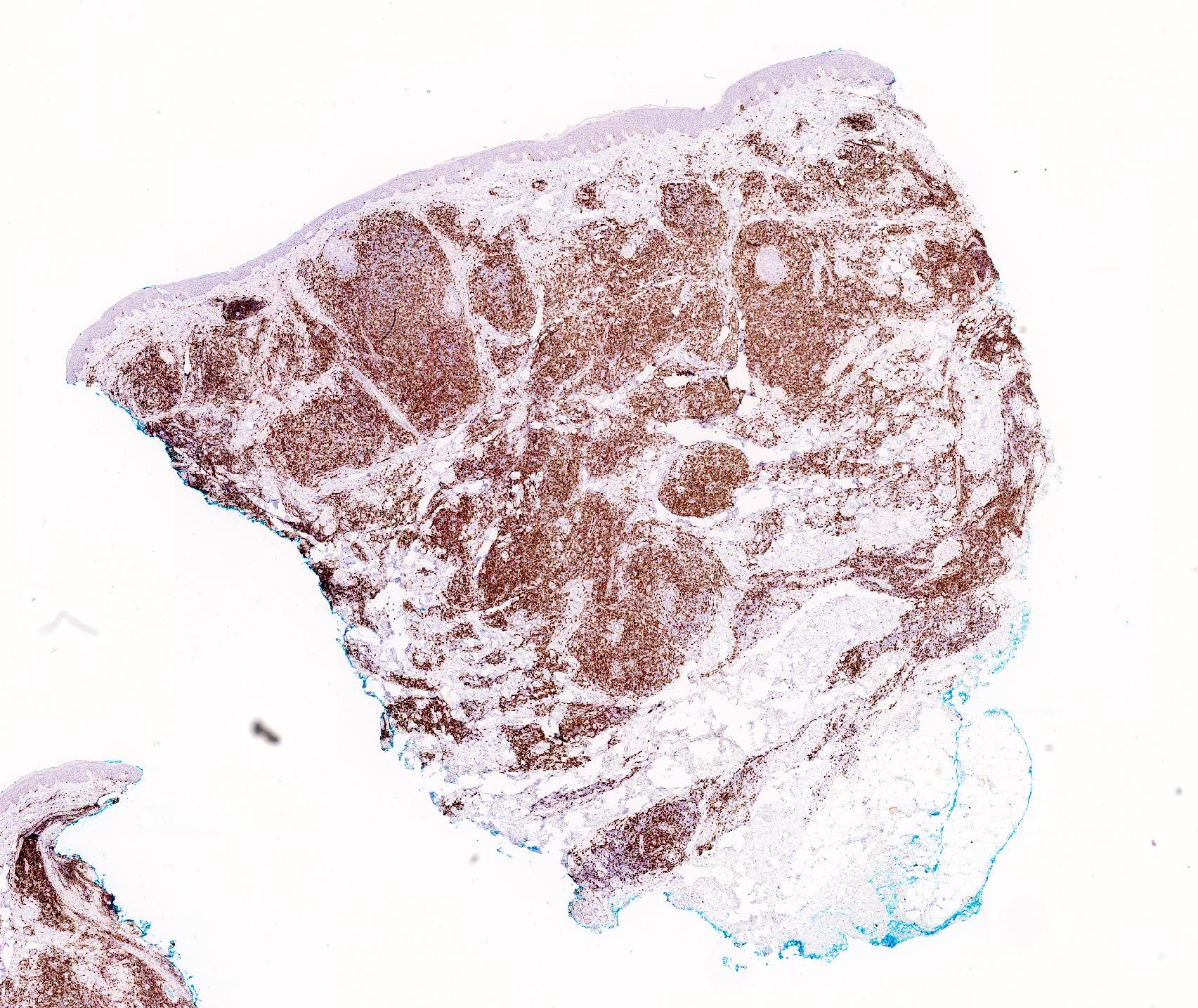
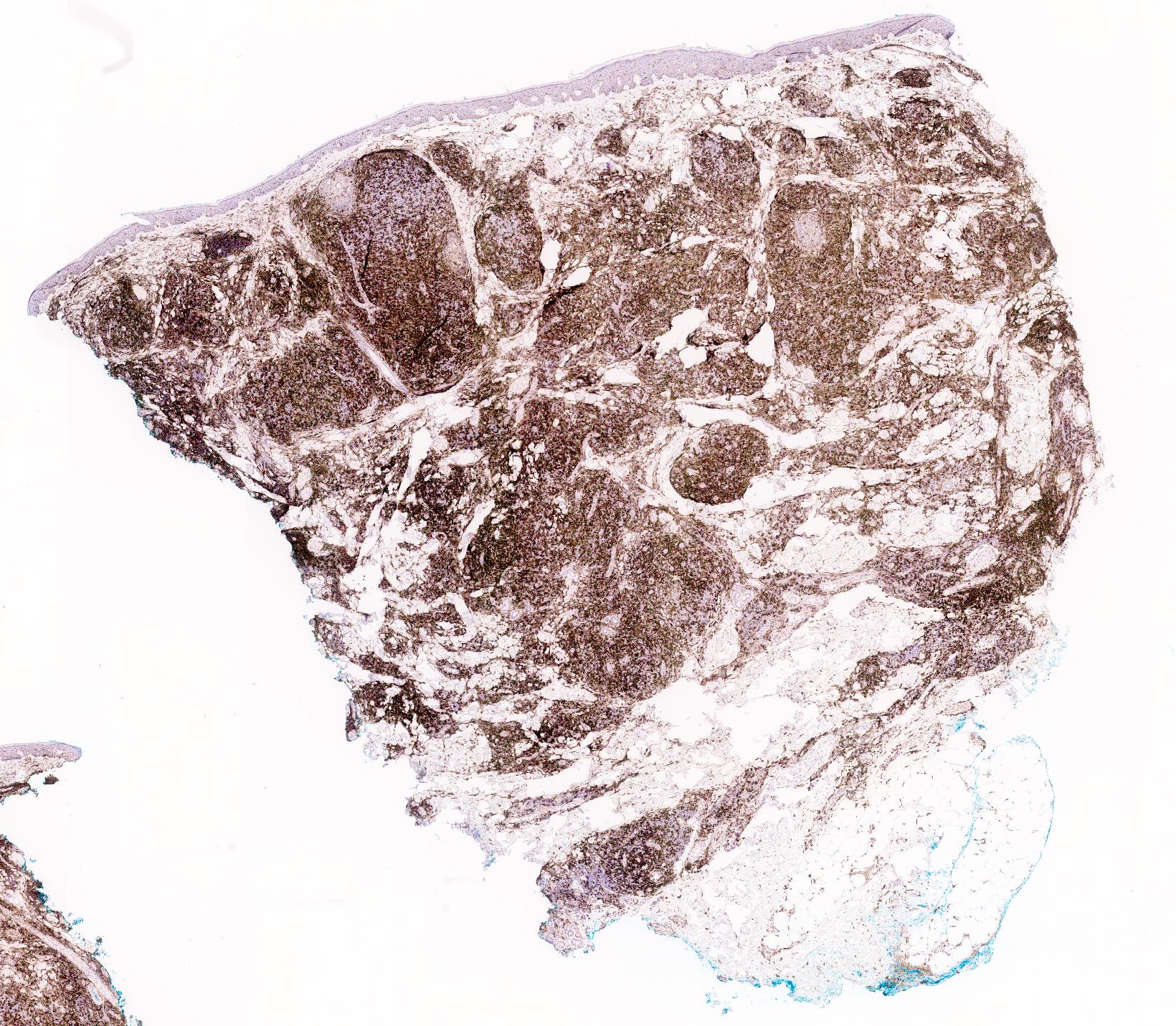
- Immunophenotype is variable depending on the predominant component of the infiltrate (Hum Pathol 2003;34:617)
- In general, the infiltrate is composed of mixed T cells (CD3+) and B cells (CD20+)
- Although variable depending on the subtypes of cutaneous lymphoid hyperplasia, T cell component may show an increased CD4:CD8 ratio
- B cell component expresses CD20, CD79a and BCL6
- Subset of T cells may express follicular helper T cell markers (PD-1, BCL6, CXCL13)
- Plasma cells are usually polytypic (mixed kappa and lambda positive plasma cells)
Negative stains
- T cell component may show partial loss of CD7 and less commonly, CD5 (loss of pan T cell markers, particularly isolated loss of CD7, however, is not pathognomonic for neoplastic processes)
- B cell component lacks BCL2, CD5 or cyclin D1 expression; the mantle zone cells will express BCL2 in cases with well formed germinal centers
- B cell component lacks CD43 coexpression
- CD30, EBER
- CD30 can be positive in T cell variants, particularly with persistent arthropod bite reactions, molluscum contagiosum, HSV infection, etc.
- Scattered cells versus sheets or clusters and weak staining may favor CLH over neoplasia
Molecular / cytogenetics description
- Typically, clonal T or B cell population is absent
- Clonal T cell gene rearrangements or immunoglobulin heavy chain gene rearrangements may occasionally be seen (so called, clonal cutaneous lymphoid hyperplasia; reported frequency varies from 4 - 62% depending on the published series) (Hum Pathol 2003;34:617, Arch Dermatol 1999;135:168, Am J Dermatopathol 2005;27:375, Arch Dermatol 2006;142:1561, Am J Surg Pathol 1995;19:12, Am J Pathol 1989;135:13)
Sample pathology report
- Skin, right arm, punch:
- Nodular dermal lymphohistiocytic infiltrate admixed with scattered eosinophils, consistent with cutaneous nodular hyperplasia (see comment)
- Comment: Sections show a nodular dermal lymphoid infiltrate without significant epidermal involvement. There is a grenz zone between the dermal infiltrate and the overlying epidermis. The infiltrate is forming nodules in the superficial and deep dermis, with focal perivascular and periadnexal distribution patterns and is predominantly composed of lymphocytes and histiocytes. Scattered eosinophils are also noted. Definitive germinal center formation is not identified. Immunohistochemical studies highlight the infiltrate to be composed of mixed CD3+ T cells and CD20+ B cells. The CD3+ T cells show a CD4:CD8 ratio of approximately 4:1. Anti-CD4 also highlights numerous background histiocytes. Anti-CD30 highlights scattered cells admixed within the infiltrate. Anti-PD-1 highlights areas with slightly increased PD-1+ T cells. Kappa and lambda by in situ hybridization fail to reveal increased monotypic plasma cells. Special stains (Fite, Gram and PAS) are negative for definitive fungal and bacterial (including mycobacterial) organisms. If available, correlation with the results of microbiology cultures is necessary. The histologic differential diagnoses include primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder and cutaneous lymphoid hyperplasia. Additional molecular studies by polymerase chain reaction for T cell receptor gene rearrangements fail to reveal monoclonal pattern of amplification of both TCRB and TCRG. Taken together, these morphologic, immunophenotypic and molecular features are consistent with cutaneous lymphoid hyperplasia in the appropriate clinical context. Clinical pathologic correlation and a close follow up are necessary.
Differential diagnosis
- Primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder:
- Distinction from cutaneous lymphoid hyperplasia (particularly nodular T cell and mixed type) is challenging due to frequently overlapping histologic (i.e., dense lymphoid infiltrate composed of small to medium sized lymphocytes involving the dermis or subcutis), immunophenotypic (i.e., CD3+, CD4+, CD8-, CD30- phenotype) and clinical (i.e., solitary plaque or nodule on the head and neck region) features
- Loss of 1 or more pan T cell markers (CD2, CD5 and CD7) more frequently seen in primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder than in cutaneous lymphoid hyperplasia
- Clonal T cell receptor gene rearrangements more commonly seen in primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder than in cutaneous lymphoid hyperplasia
- Presence of monoclonality, in the absence of identifiable triggering factors, favors primary cutaneous + small / medium T cell lymphoproliferative disorder over cutaneous lymphoid hyperplasia
- Primary cutaneous marginal zone B cell lymphoma:
- Simulates Borrelia associated nodular B cell cutaneous lymphoid hyperplasia
- Sheets of plasma cells and Dutcher bodies more frequently seen in primary cutaneous marginal zone B cell lymphoma than in cutaneous lymphoid hyperplasia
- Neoplastic B cells express BCL2 while lacking immunoreactivity for CD5, CD10, BCL6 and cyclin D1
- Neoplastic B cells may coexpress CD43
- Monotypic plasma cells with light chain restriction (either kappa or lambda) more frequently seen in primary cutaneous marginal zone B cell lymphoma than in cutaneous lymphoid hyperplasia
- Clonal immunoglobulin heavy chain gene rearrangements are more commonly seen in primary cutaneous marginal zone B cell lymphoma than in cutaneous lymphoid hyperplasia
- Mycosis fungoides:
- Cutaneous lymphoid hyperplasia may histologically mimic mycosis fungoides, particularly if the infiltrate is T cell predominant and shows prominent lymphocytic exocytosis
- Neoplastic cells of mycosis fungoides, however, typically exhibit cytologic atypia with hyperchromatic nuclei with irregular convoluted nuclear contours
- Both mycosis fungoides and cutaneous lymphoid hyperplasia can show an increased CD4:CD8 ratio in the infiltrate
- Presence of patches / plaques favors mycosis fungoides over cutaneous lymphoid hyperplasia
- Primary cutaneous follicular center lymphoma:
- May simulate nodular B cell cutaneous lymphoid hyperplasia
- Neoplastic follicles lack tingible body macrophages and have attenuated or absent mantle zones
- Neoplastic B cells express BCL6, CD10 (variable; positive in follicular pattern but generally negative in diffuse pattern) while typically lacking immunoreactivity for BCL2
Additional references
Practice question #1
Which of the following features would most likely favor a diagnosis of cutaneous lymphoid hyperplasia over primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder?
- Dermal lymphoid infiltrate predominantly composed of small to medium sized pleomorphic T cells
- Presence of B cells coexpressing CD43
- Presence of T cell receptor beta (TCRB) and gamma (TCRG) gene rearrangements
- Recent history of vaccination to the same site with an erythematous nodule
- Presence of PD-1+ and CXCL13+ cells in the infiltrate
Practice answer #1
D. Recent history of vaccination to the same site with an erythematous nodule. Development of an erythematous nodule at the same site where vaccination was recently administered would favor a reactive process, such as cutaneous lymphoid hyperplasia (CLH). In the absence of identifiable triggering factors, however, distinguishing CLH from primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder (CD4+ small / medium T cell lymphoproliferative disorder) is challenging on histologic grounds alone. Typically, the presence of clonality (choice C) detected by polymerase chain reaction would favor a diagnosis of primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder over CLH, although clonality may also be seen in CLH occasionally. Histologic features of primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder (choice A) are similar to those seen in CLH. Scattered to slightly increased follicular helper T cells (choice E) can be seen in both CLH and primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder. The neoplastic B cells coexpressing CD43 (choice B) may be seen in primary cutaneous marginal zone B cell lymphoma, not in CLH or primary cutaneous CD4+ small / medium T cell lymphoproliferative disorder.
Comment Here
Reference: Cutaneous lymphoid hyperplasia
Comment Here
Reference: Cutaneous lymphoid hyperplasia
Practice question #2
Which of the following statements is true regarding cutaneous lymphoid hyperplasia?
- Clonal T cell receptor beta (TCRB) and gamma (TCRG) gene rearrangements are never found in cutaneous lymphoid hyperplasia
- Cutaneous lymphoid hyperplasia frequently progresses to a lymphoma
- Diagnosis of cutaneous lymphoid hyperplasia requires clinical and pathologic correlation
- Presence of monotypic plasma cells with kappa light chain restriction always favors a diagnosis of primary cutaneous marginal zone B cell lymphoma over cutaneous lymphoid hyperplasia
- Triggering factors or etiologic agents are always identified in cutaneous lymphoid hyperplasia
Practice answer #2
C. Diagnosis of cutaneous lymphoid hyperplasia requires clinical and pathologic correlation. Accurate diagnosis of cutaneous lymphoid hyperplasia (CLH) requires clinical and pathologic correlation, including acquisition of appropriate clinical histories to determine the presence of a possible triggering factor. Albeit rare, monotypic plasma cells with a light chain restriction can also be seen in CLH (choice D). CLH is often idiopathic and definitive etiologic agents may not be found (choice E). Progression of CLH to a lymphoma is not a frequent finding (choice B). The presence of clonality in the infiltrate in the skin is not unique to malignancy (choice A) and can also be seen in various nonneoplastic conditions, including lichen sclerosus, lichen planus, pityriasis lichenoides chronica, pityriasis lichenoides et varioliformis acuta and CLH (J Invest Dermatol 2000;115:254, J Cutan Pathol 2002;29:447, Arch Dermatol Res 2000;292:568, Arch Dermatol 2001;137:305, Arch Dermatol 2000;136:1483).
Comment Here
Reference: Cutaneous lymphoid hyperplasia
Comment Here
Reference: Cutaneous lymphoid hyperplasia