Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Chen IY, Agostini-Vulaj D. Hepatocellular carcinoma overview. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/livertumorHCC.html. Accessed April 28th, 2024.
Definition / general
- Primary malignancy of liver with hepatocellular differentiation
Essential features
- Most common (> 80%) primary liver malignancy worldwide
- ~ 80% of hepatocellular carcinoma cases arise in cirrhosis
- Specific subtypes have associated molecular / cytogenetic abnormalities:
- Scirrhous subtype: TSC1 / TSC2 mutations
- Steatohepatitic subtype: frequent IL6 / JAK / STAT activation
- Macrotrabecular massive subtype: TP53 mutation and FGF19 amplification
- Fibrolamellar subtype: DNAJB1-PRKACA fusion gene
Terminology
- "Hepatoma" is old term for hepatocellular carcinoma
ICD coding
Epidemiology
- Most common (> 80%) primary liver malignancy worldwide (Nat Rev Gastroenterol Hepatol 2019;16:589)
- Sixth most common malignancy and fourth most common cause of cancer mortality worldwide (Global Cancer Observatory: Liver Fact Sheet [Accessed 18 January 2021])
- Incidence and age of onset vary geographically
- Asia (72.5%) has the highest incidence worldwide, followed by Europe (9.8%), Africa (7.7%), North America (5%), Latin America and the Caribbean (4.6%) and Oceania (0.4%) (Global Cancer Observatory: Liver Fact Sheet [Accessed 18 January 2021])
- Median age of onset is older than sixth decade in North America and Europe; median age of onset in Asia and Africa is between third and sixth decades (Nat Rev Gastroenterol Hepatol 2019;16:589)
- Gender
- Risk factors:
- Chronic liver disease leading to cirrhosis; most common etiologies leading to this include chronic viral hepatitides (HBV and HCV), heavy alcohol consumption, nonalcoholic fatty liver disease (Nat Rev Gastroenterol Hepatol 2019;16:589, Hepatology 2018;68:723)
- Chronic viral hepatitis is the leading cause of hepatocellular carcinoma worldwide (Philos Trans R Soc Lond B Biol Sci 2017;372:20160274)
- In the United States, chronic hepatitis C is a critical risk factor for hepatocellular carcinoma (Hepatology 2018;68:723)
Sites
- Primary location: liver
- Most common metastatic sites (in decreasing order of frequency): lung, portal vein, portal lymph node, intra-abdominal lymph node, bone (Case Rep Oncol Med 2020;2020:7526042)
Pathophysiology
- Stepwise process (low grade dysplastic nodule → high grade dysplastic nodule → early hepatocellular carcinoma → progressed hepatocellular carcinoma) accompanied by accumulation of molecular alterations (Hepatology 2014;60:1983)
- Molecular alterations include: telomere shortening, TERT activation, cell cycle checkpoint inhibitor inactivation (Nat Genet 2015;47:505)
- TERT promoter mutation is a salient event to hepatocellular carcinoma progression (Hepatology 2014;60:1983)
Etiology
- Cirrhosis
- ~ 80% of hepatocellular carcinoma cases arise in cirrhosis (Hepatology 2018;68:723)
- Patients with cirrhosis from any etiology are at risk for developing hepatocellular carcinoma
- Infectious
- Hepatitis B virus (HBV)
- Direct oncogenic effect by integration of the HBV DNA into the host genome, which subsequently induces genomic instability and mutagenesis of cancer related genes, including p53 and WNT / β catenin pathway; can cause hepatocellular carcinoma without antecedent cirrhosis (J Hepatol 2016;64:S84, N Engl J Med 2019;380:1450)
- HBV vaccination reduces the incidence of hepatocellular carcinoma
- Hepatitis C virus (HCV)
- Lacks direct oncogenic effect; occurs almost exclusively in HCV patients with advanced cirrhosis (N Engl J Med 2019;380:1450)
- Core HCV proteins (NS5A and NS3) induce oxidative stress, which eventually results in activation of NFκB and MAPK pathway, leading to cell proliferation and alteration of apoptotic pathway (Medicina (Kaunas) 2019;55:526)
- Direct acting antiviral therapy and interferon based therapy lowers the risk of hepatocellular carcinoma (Gastroenterology 2017;153:996, Gastroenterology 2017;152:142)
- Hepatitis B virus (HBV)
- Metabolic
- Nonalcoholic fatty liver disease (NAFLD)
- Increased prevalence due to metabolic syndrome, obesity and type 2 diabetes; insulin resistance leads to increased insulin-like growth factor 1 (IGF1), which eventually activates PI3K and MAPK pathways, leading to cell proliferation and inhibition of apoptosis (Medicina (Kaunas) 2019;55:526, PLoS One 2014;9:e97136)
- Limited treatment options available
- Others: hemochromatosis, alpha-1 antitrypsin deficiency, hypercitrullinemia, fructosemia
- Nonalcoholic fatty liver disease (NAFLD)
- Environmental exposures
- Aflatoxins:
- Mycotoxins produced by Aspergillus flavus (aflatoxin B1) and Aspergillus parasiticus
- Contaminates grain, corns and peanuts in tropical and subtropical regions, particularly in Asia and Africa
- Aflatoxin B1 reacts with DNA to form mutagenic adducts, leading to codon 249 mutation of TP53 (Liver Cancer International 2020;1:41)
- Alcohol: major risk factor in the Western countries (J Hepatol 2018;69:1274)
- Others: anabolic steroids, Thorotrast, oral contraceptives, tobacco
- Aflatoxins:
- Developmental / congenital
- Abernethy malformation: congenital vascular malformation, which causes splanchnic venous blood to bypass the liver and drain directly into the systemic circulation; also known as congenital extrahepatic portosystemic shunt (CEPS) (Hepatology 2020;71:658)
- Alagille syndrome: autosomal dominant disorder caused by loss of function in JAG1 (85%) or NOTCH2 (15%), resulting in bile duct paucity (World J Gastroenterol 2018;24:3980)
- Ataxia telangiectasia: autosomal recessive disorder caused by defect in the ATM gene, resulting in increased oxidative stress (World J Gastroenterol 2018;24:3980, Front Pediatr 2019;7:458)
- Bile salt export protein deficiency: mutation in ABCB11 causes poor excretion of bile salt, resulting in chronic inflammation and carcinogenesis (World J Gastroenterol 2018;24:3980, Hepatology 2006;44:478)
- Tyrosinemia type I: mutation in FAH (15q23-q25) leads to deficiency of fumaryl acetoacetate hydrolase in the tyrosine degradation pathway, resulting in accumulation of toxic metabolites including maleylacetoacetate and fumarylacetoacetate (World J Gastroenterol 2018;24:3980)
- Progression of benign entity
- ~ 5% of hepatocellular adenomas undergo malignant transformation (Hepat Oncol 2014;1:421)
Diagrams / tables
Clinical features
- Signs and symptoms: abdominal pain, weight loss, hepatomegaly and splenomegaly, jaundice, ascites
Diagnosis
- Imaging: ultrasound, contrast enhanced CT / MRI with or without diagnostic tissue biopsy (see also figure 1 under diagrams)
- Tissue confirmation not always necessary to establish diagnosis (unlike for most malignancies)
- See radiology description for more details
Laboratory
- Laboratory tests: α fetoprotein is elevated in 70 - 90% of cases and has sensitivity of 60% and specificity of 90% in hepatocellular carcinoma detection; elevated liver function tests (LFTs) Gastroenterology 2009;137:110
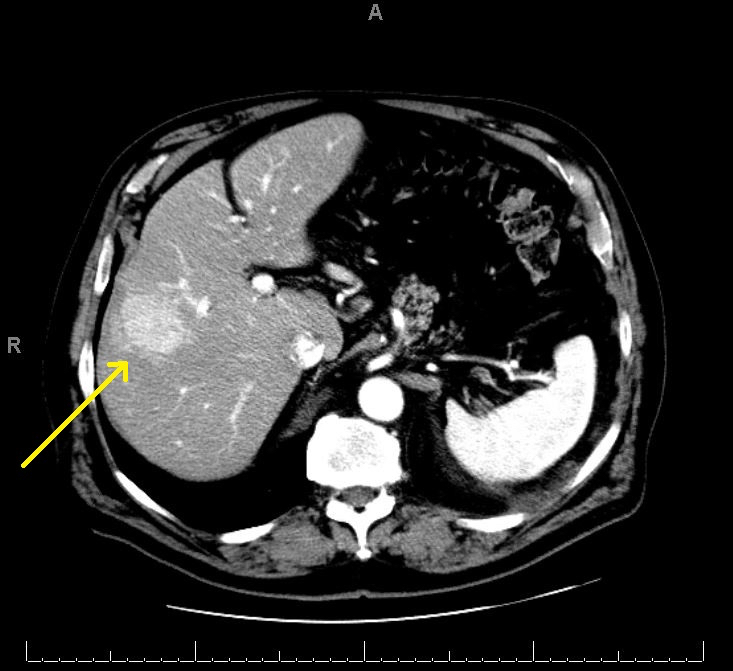
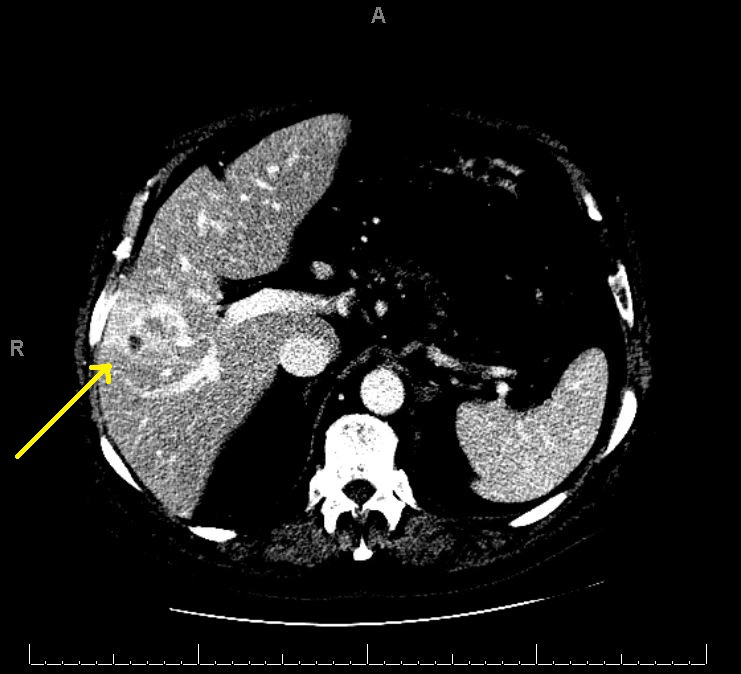
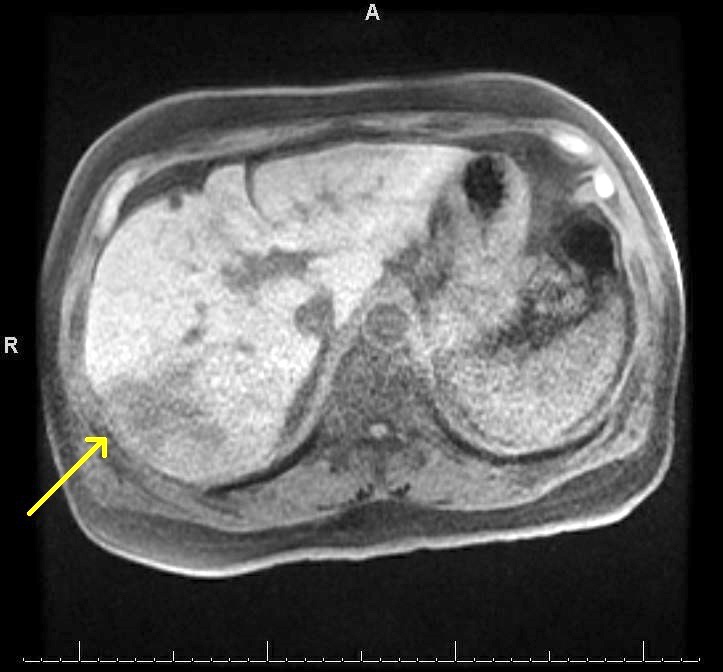
Radiology description
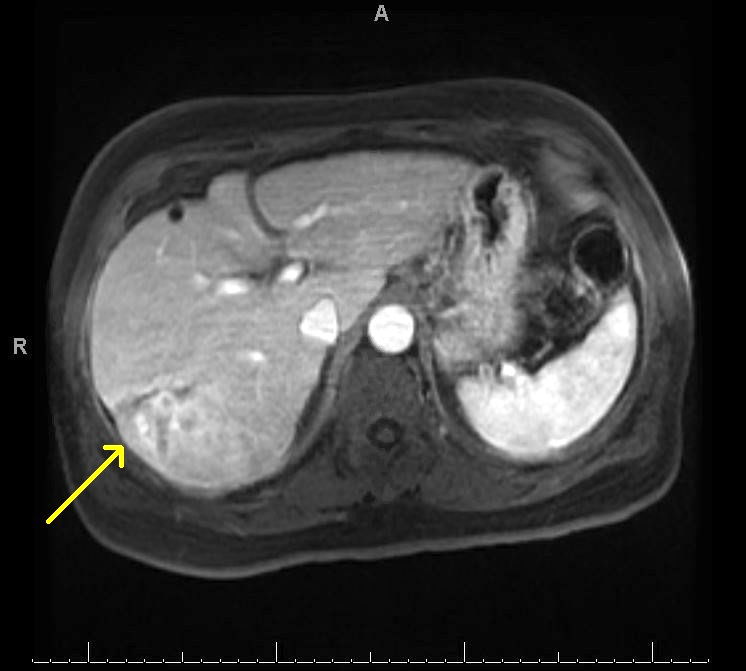
- Liver nodule in cirrhosis (N Engl J Med 2019;380:1450) (see also figure 1 under diagrams)
- If < 1cm: ultrasound surveillance every 3 - 4 months
- If > 1cm: contrast enhanced CT / MRI
- Diagnostic features for hepatocellular carcinoma include hyperenhancement during arterial phase and washout in the venous or delayed phase (due to alteration in blood supply during malignant transformation, as benign hepatocytes receive blood supply from portal vein, whereas malignant hepatocyte receive blood supply from hepatic artery)
- If diagnostic features are not identified, evaluate radiologic features from other imaging techniques and consider liver biopsy for histologic diagnosis
- Liver Imaging Reporting and Data System (LI-RADS) contains 5 diagnostic categories:
- LR-1: definitely benign
- LR-2: probably benign
- LR-3: intermediate probability of malignancy
- LR-4: probably hepatocellular carcinoma
- LR-5: definitely hepatocellular carcinoma
Radiology images
Prognostic factors
- 5 year overall survival is 24 - 70% (Ann Surg 2007;245:909)
- 5 year overall survival after transplant for hepatocellular carcinoma = 64% (J Am Coll Surg 2007;204:1016)
- 5 year recurrence free survival after transplant for hepatocellular carcinoma = 61% (J Am Coll Surg 2007;204:1016)
- Prognosis dependent on TNM stage (Amin: AJCC Cancer Staging Manual, 8th Edition, 2017)
- Presence of lymphovascular invasion and poorly differentiated histology are associated with a worse prognosis in solitary hepatocellular carcinoma > 2 cm (World J Gastroenterol 2018;24:4000, Am J Surg Pathol 2002;26:25, Ann Surg Oncol 2013;20:1223)
- Presence of cirrhosis, background liver damage, multifocality, hepatocellular carcinoma diameter > 2 cm and portal vein thrombosis associated with worse prognosis (Ann Surg 2007;245:909, Liver Int 2009;29:502)
- Immunohistochemical expression of CK19, CD90, EpCAM and CD133 are associated with unfavorable prognosis (World J Gastroenterol 2014;20:2098, Histopathology 2006;49:138, Cancer Sci 2003;94:851)
- Subtypes associated with worse prognosis compared with conventional hepatocellular carcinoma: (Gastroenterol Clin North Am 2017;46:365)
- Cirrhotomimetic
- Sarcomatoid carcinoma: epithelial HCC element and mesenchymal spindle cell element; spindle cells without specific lineage; positive for cytokeratins and vimentin
- Carcinosarcoma: epithelial HCC element and mesenchymal spindle cell element; spindle cells of a specific lineage; positive for vimentin, negative for cytokeratins
- Macrotrabecular massive
- Neutrophil rich
- Subtypes associated with a similar to better prognosis compared with conventional hepatocellular carcinoma: (Gastroenterol Clin North Am 2017;46:365)
- Steatohepatitic
- Clear cell
- Chromophobe
- Fibrolamellar
- Lymphocyte rich
- Subtype with variable/unknown prognosis compared with conventional hepatocellular carcinoma:
- Scirrhous
Case reports
- 30 year old man with unintentional weight loss (BMJ Case Rep 2019;12:e230530)
- 38 year old man with Alagille syndrome presented with acute right upper quadrant pain (Radiol Case Rep 2020;16:90)
- 41 year old otherwise healthy Sri Lankan man with progressive abdominal distension (J Med Case Rep 2017;11:34)
- 53 year old man presented with left forehead nodule associated with ipsilateral vision loss (Case Rep Oncol Med 2020;2020:7526042)
- 62 year old man with 10 year history of hepatitis B presented with fever, fatigue and weight loss (Cancer Res Ther 2015;11:665)
Treatment
- Surgical intervention
- Tumor resection for patients with solitary tumor and patients with preserved liver function
- Liver transplant for patients meeting Milan criteria of solitary tumor < 5 cm or up to 3 tumors < 3 cm (Liver Transpl 2004;10:S115)
- Ablation therapy
- Through radiofrequency, microwave, cryoablation and ethanol injection (Liver Transpl 2004;10:S115)
- Transarterial embolization (TEA) and transarterial chemoembolization (TACE) (Liver Transpl 2004;10:S115)
- Systemic therapy
- Sorafenib is a tyrosine kinase that prolongs median survival (N Engl J Med 2008;359:378)
- Barcelona Clinic Liver Cancer (BCLC) algorithm is the most widely applied hepatocellular carcinoma management system, which classifies patients into five stages (see also figure 2 under diagrams) (Liver Transpl 2004;10:S115)
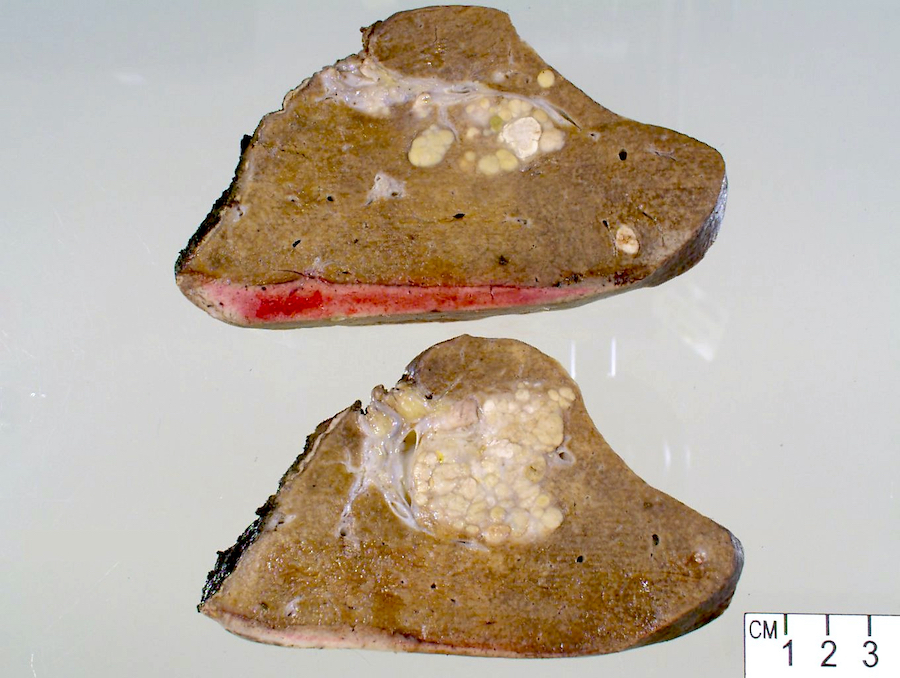
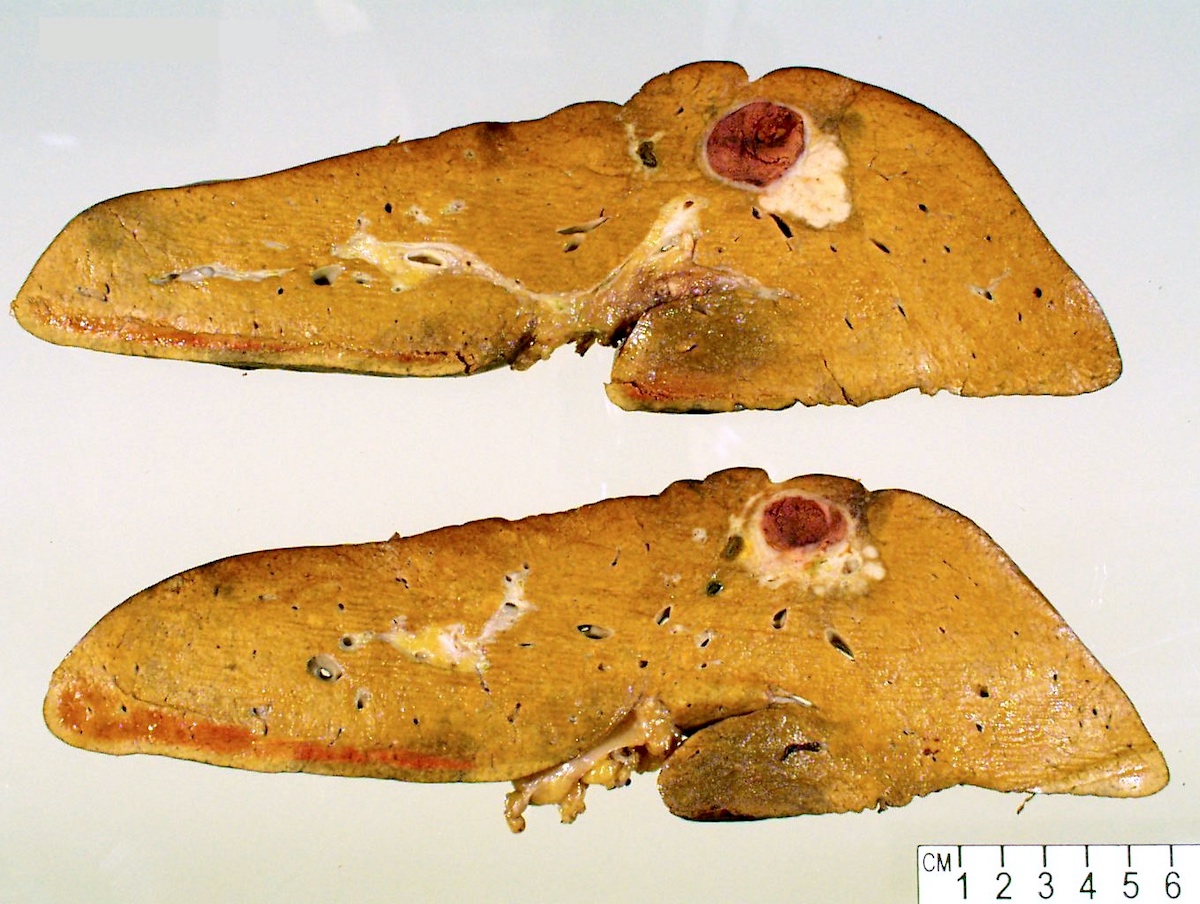
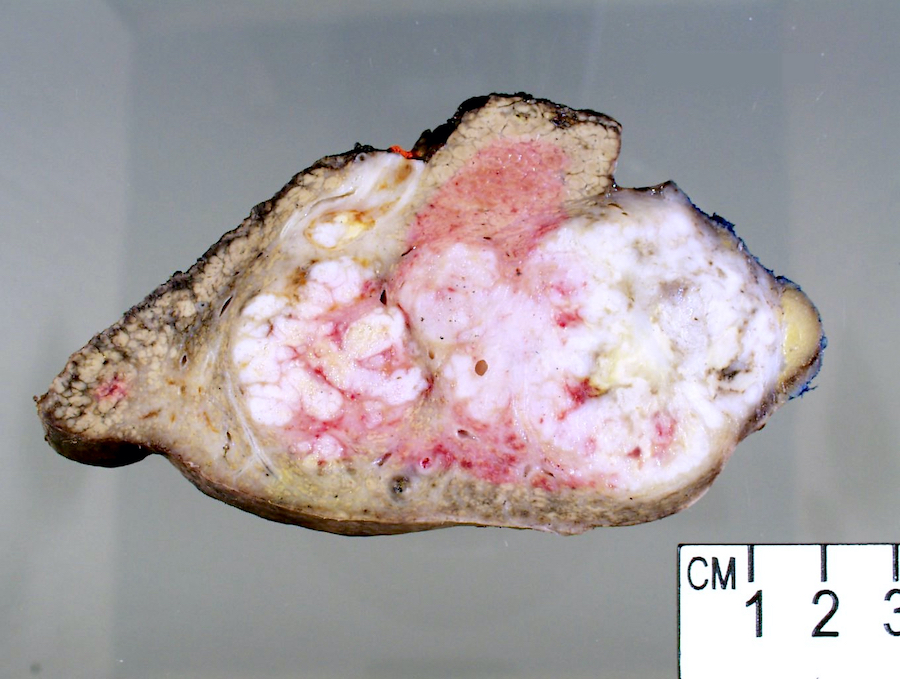
Gross description
- Well circumscribed mass that appears tan-yellow to green (color variation depends on proportion of fat and bile content)
- Areas of hemorrhage and necrosis are common
- Solitary or dominant nodule with multiple satellite nodules or multiple discrete nodules or multiple distinct nodules
- Background liver is usually cirrhotic
- Rarely, hepatocellular carcinoma can have exophytic growth, also known as pedunculated hepatocellular carcinoma (World J Surg 2002;26:1133)
Gross images
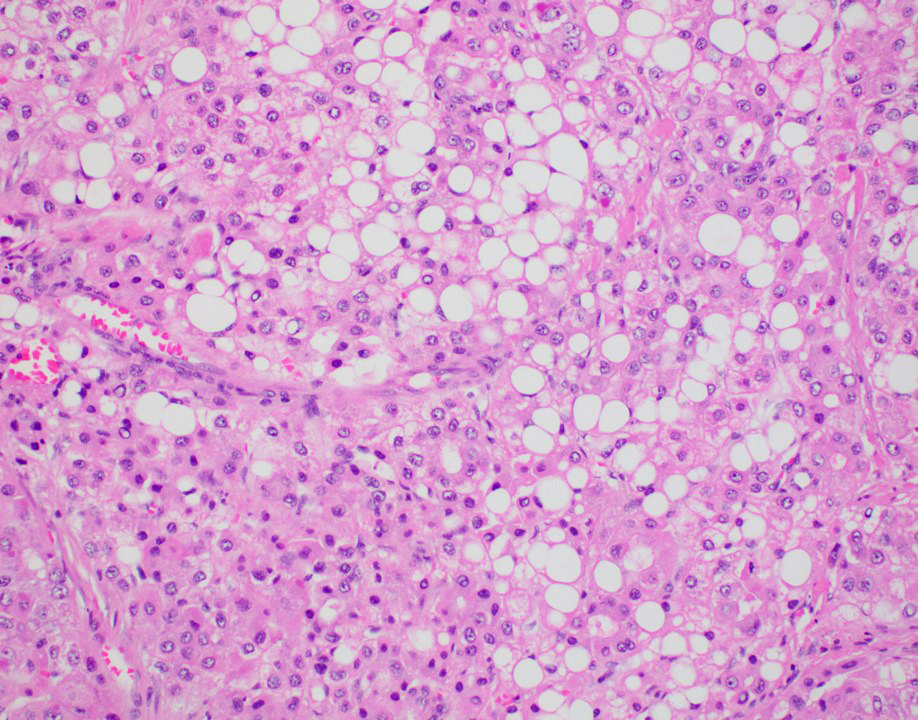
Microscopic (histologic) description
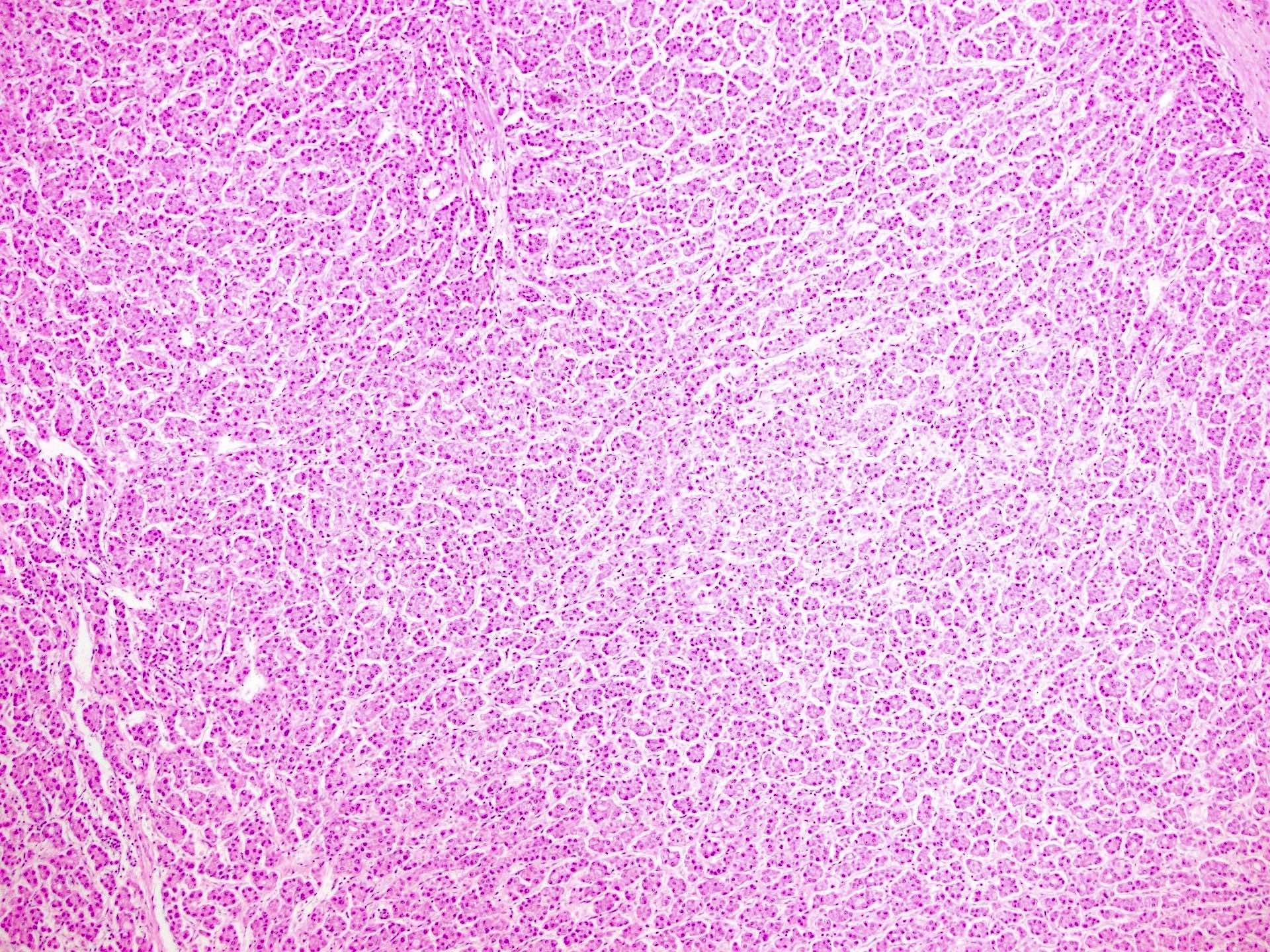
- Architectural patterns:
- 4 principal growth patterns, including trabecular, pseudoglandular, solid and macrotrabecular (in decreasing order of frequency)
- 50% of cases have mixed patterns; macrotrabecular pattern is associated with worse prognosis
- Other features include lack of portal triad in the tumor, reduction of normal reticulin framework, expansion of the hepatocyte plates and increased arterialization with unpaired arteries or arterioles
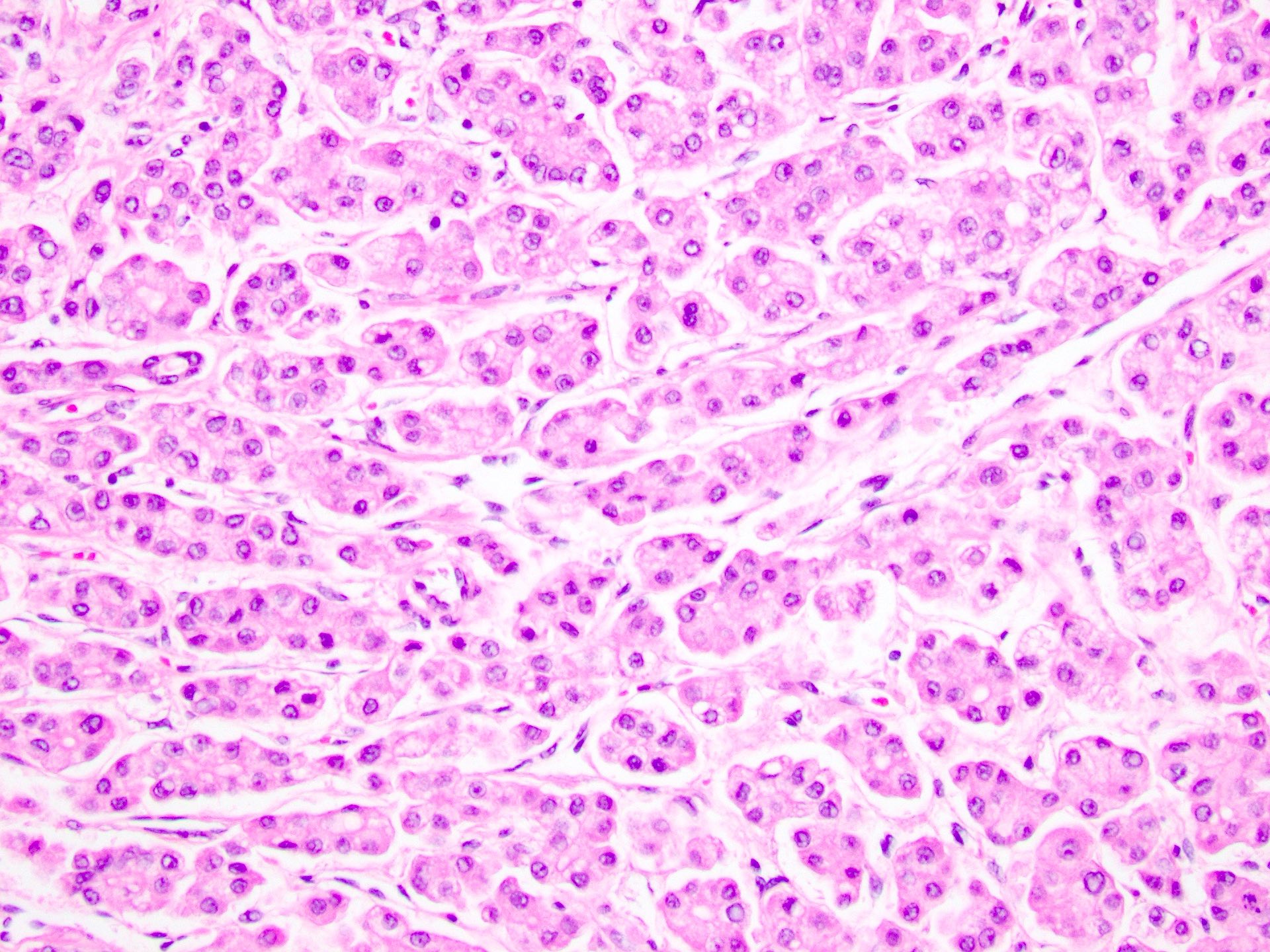
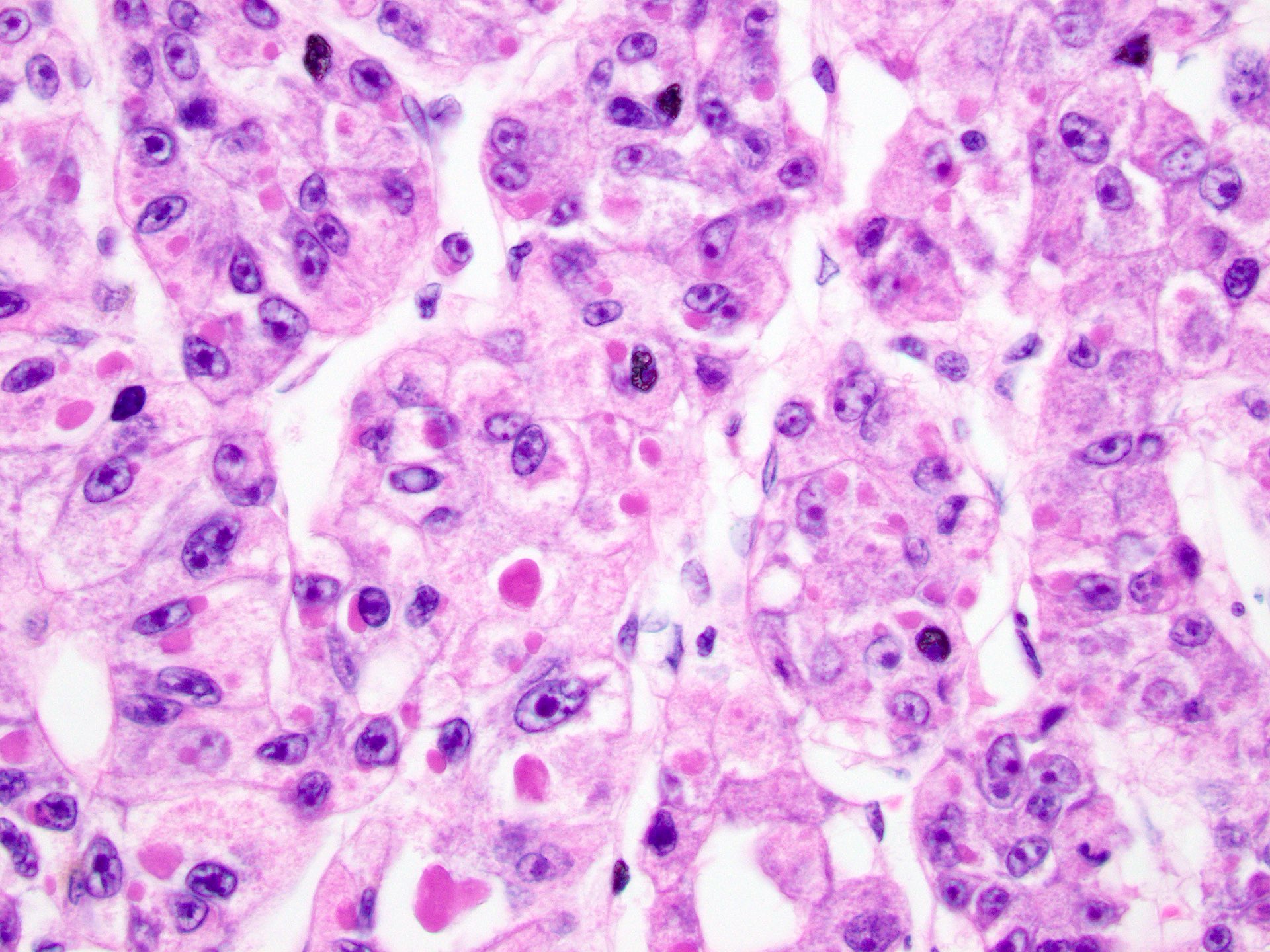
- Cytologic features:
- Polygonal cells with nuclear atypia, including high N/C ratio, irregular nuclear membrane, multinucleation and prominent nuclei
- Cytoplasm varies from clear to eosinophilic, depending on the fat and glycogen content
- Cytoplasmic alterations include Mallory-Denk bodies, hyaline bodies, pale bodies
- Bile production (usually extracellular) may be seen
- Histologic grading (3 tiered system):
- WHO grading system (3 tiered system)
- Well differentiated: tumor cells resemble mature hepatocytes; minimal to mild nuclear atypia
- Moderately differentiated: tumor cells appear malignant on H&E and morphology suggests hepatocellular differentiation; moderate nuclear atypia
- Poorly differentiated: tumor cells appear malignant on H&E and often cannot be distinguished from other poorly differentiated neoplasms; marked nuclear atypia
- Modified Edmondson-Steiner grading system (4 tiered system) (Cancer 1954;7:462)
- Grade I: tumor cells are difficult to differentiated from hyperplastic liver cells
- Grade II: tumor cells resemble mature hepatocytes with slightly larger and more hyperchromatic nuclei; sharp and clear cut cell borders; frequent acini formation
- Grade III: tumor cells are larger and have more hyperchromatic nuclei with less acidophilic cytoplasms; trabecular distortion; numerous tumor giant cells
- Grade IV: tumor cells are intensely hyperchromatic, with scant and less granular cytoplasm; tumor cells appear less cohesive and can appear giant, spindled or short and plump; medullary growth pattern with loss of trabeculation; less acini
- WHO grading system (3 tiered system)
Microscopic (histologic) images
Cytology description
- See also separate cytology topic at HCC-Cytology
- Hypercellular broad clusters of malignant hepatocytes with peripheral endothelial wrapping (Patholog Res Int 2011;2011:587936)
Cytology images
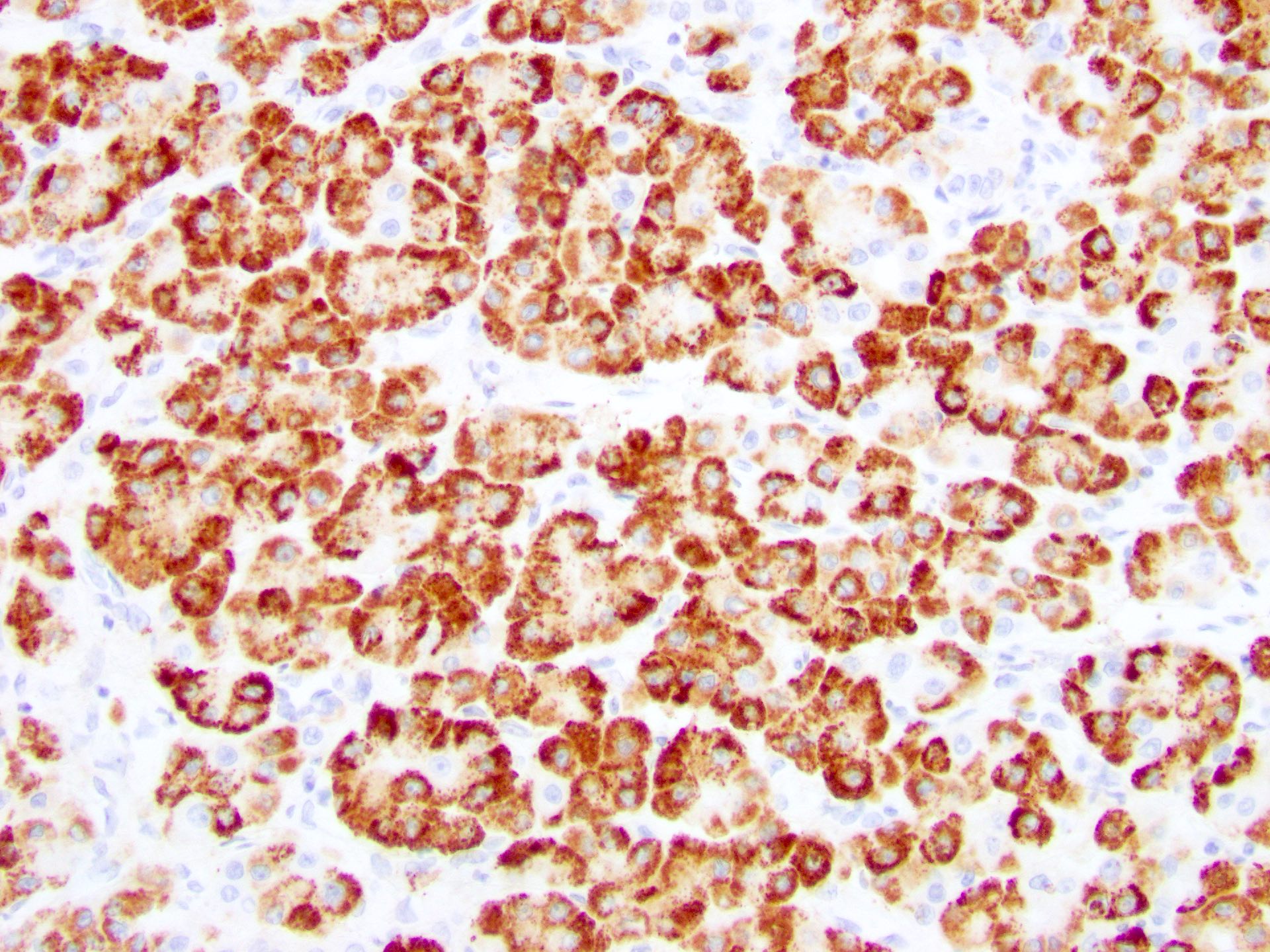
Positive stains
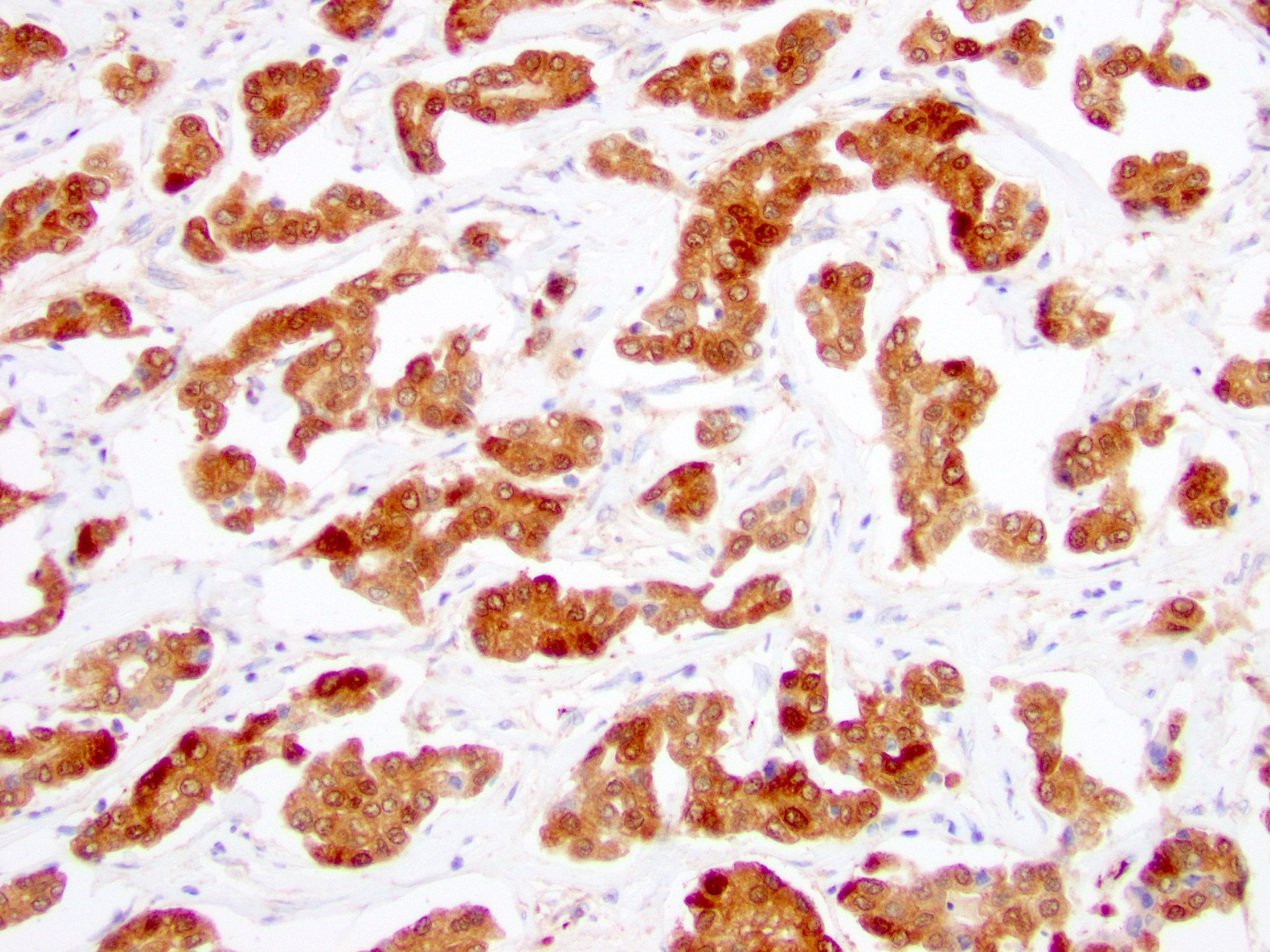
- Arginase1: cytoplasmic or nuclear; useful in confirming hepatocellular differentiation; highly sensitive and specific, thus more useful than HepPar1 for poorly differentiated hepatocellular carcinoma (Am J Surg Pathol 2010;34:1147)
- HepPar1: cytoplasmic and granular; overall highly sensitive but 50% of poorly differentiated hepatocellular carcinoma lose expression (Am J Surg Pathol 2002;26:978)
- Glypican 3: cytoplasmic; high sensitivity in poorly differentiated and scirrhous hepatocellular carcinoma but low sensitivity in well differentiated hepatocellular carcinoma (nonneoplastic liver is negative)
- AFP: cytoplasmic; highly specific but low sensitivity; frequently negative in well differentiated hepatocellular carcinoma
- Polyclonal CEA, villin and CD10 reveal canalicular pattern; limited sensitivity in poorly differentiated hepatocellular carcinoma
- Albumin ISH: high sensitivity for primary liver carcinoma, although this can also be positive in other adenocarcinomas not of biliary origin (Am J Clin Pathol 2019;152:190)
- Pancytokeratins (MNF116 or OSCAR) and CAM5.2 (CK8 / CK18) are positive
- Reticulin: highlights the thickened hepatocyte plates (> 3 cell thick); see also negative stains below
Negative stains
Molecular / cytogenetics description
- Diagnosis of hepatocellular carcinoma often does not require molecular or cytogenetic testing but these ancillary studies can aid in diagnosing difficult cases and identifying a specific subtype of hepatocellular carcinoma (J Hepatol 2017;67:727):
- Scirrhous subtype: TSC1 / TSC2 mutations
- Steatohepatitic subtype: frequent IL6 / JAK / STAT activation
- Macrotrabecular massive subtype: TP53 mutation and FGF19 amplification
- Fibrolamellar subtype: DNAJB1-PRKACA fusion gene
Sample pathology report
- Liver and gallbladder, native, hepatectomy for transplantation:
- Hepatocellular carcinoma, multiple (see comment)
- Comment: Tumor 1 is located in the right lobe and 3 cm in size with no necrosis. Tumor 2 is located in the right lobe and 2.5 cm in size with incomplete necrosis (viable tumor present). Percentage of tumor necrosis for tumor 2 is 75%. Tumor 3 is located in the left lobe and 1.5 cm in size with no necrosis. Tumor 4 is located in the right lobe and 2.5 cm in size with incomplete necrosis (viable tumor present). Percentage of tumor necrosis for tumor 4 is 10%. Tumor 5 is located in the left lobe and 1.5 cm in size with no necrosis. Histologic type is hepatocellular carcinoma with grade G2 - G3: moderately to poorly differentiated. The tumor is confined to the liver and biliary and vascular margins are free of tumor. Vascular invasion present. No lymph nodes submitted or found. AJCC 8th edition pathologic stage is ypT2NX (combined stage II) and UNOS stage is T4a. The background liver shows cirrhosis.
Differential diagnosis
- Hepatocellular adenoma:
- Neoplastic hepatocytes are not expanded on reticulin stain (no loss)
- Noncirrhotic background
- Intrahepatic cholangiocarcinoma:
- Discrete gland formation surrounded by desmoplastic stroma
- Negative for hepatocellular markers; positive for CK7
- Dysplastic nodule in cirrhosis:
- ~ 1 cm lesion in cirrhotic livers seen macroscopically or on imaging
- Intact reticulin network (focal loss may be seen) with preserved portal tracts (although typically reduced in number)
- Cytologic alterations seen may include small cell changes, large cell changes and iron free foci
- Metastatic neuroendocrine neoplasms:
- Positive staining for neuroendocrine markers while negative for hepatocellular markers
- Metastatic carcinomas, such as clear cell renal cell carcinoma (CCRCC):
- Possible clinical history of prior malignancy
- Differing immunohistochemical profile (e.g. clear cell renal cell carcinoma positive for PAX8 while negative for hepatocellular markers)
Additional references
Board review style question #1
Which of the following is true about this liver neoplasm?
- Diffuse CK7 immunohistochemical expression would be expected in this neoplasm
- Hepatitis B is not a risk factor to develop this neoplasm
- Molecular analyses are required to define this neoplasm
- This neoplasm commonly arises in the setting of cirrhosis
Board review style answer #1
D. This neoplasm commonly arises in the setting of cirrhosis. The image demonstrates a hepatocellular carcinoma.
Comment Here
Reference: Hepatocellular carcinoma overview
Comment Here
Reference: Hepatocellular carcinoma overview
Board review style question #2
Which of the following subtypes of hepatocellular carcinoma is associated with a worse prognosis compared with conventional hepatocellular carcinoma?
- Chromophobe hepatocellular carcinoma
- Clear cell hepatocellular carcinoma
- Lymphocyte rich hepatocellular carcinoma
- Sarcomatoid hepatocellular carcinoma
Board review style answer #2
D. Sarcomatoid hepatocellular carcinoma. All of the other answer choices are associated with a similar or better prognosis than conventional hepatocellular carcinoma.
Comment Here
Reference: Hepatocellular carcinoma overview
Comment Here
Reference: Hepatocellular carcinoma overview