Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Nezami BG, MacLennan G. Clear cell. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html. Accessed September 24th, 2025.
Definition / general
- Most common renal epithelial tumor, accounting for ~2% of all malignancies, typically with clear cytoplasm and a compact nested or acinar growth pattern, intersected by delicate vasculature and with characteristic alterations to chromosome 3p involving VHL (von Hippel-Lindau) gene inactivation
Essential features
- Cortical mass with golden yellow variegated cut surface, with diverse architecture, primarily solid and nested
- Clear or granular eosinophilic cytoplasm and prominent but delicate capillary network
- > 95% sporadic, mostly single mass, in the sixth to seventh decade, commonly harboring VHL gene inactivation located on the short arm of chromosome 3 (3p25)
- Small percentage of tumors are familial, mostly VHL disease with multiple bilateral tumors and earlier onset
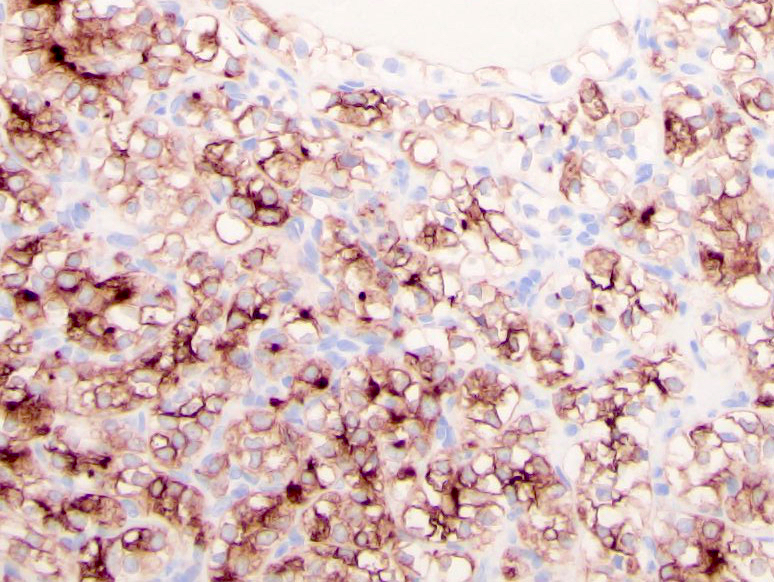
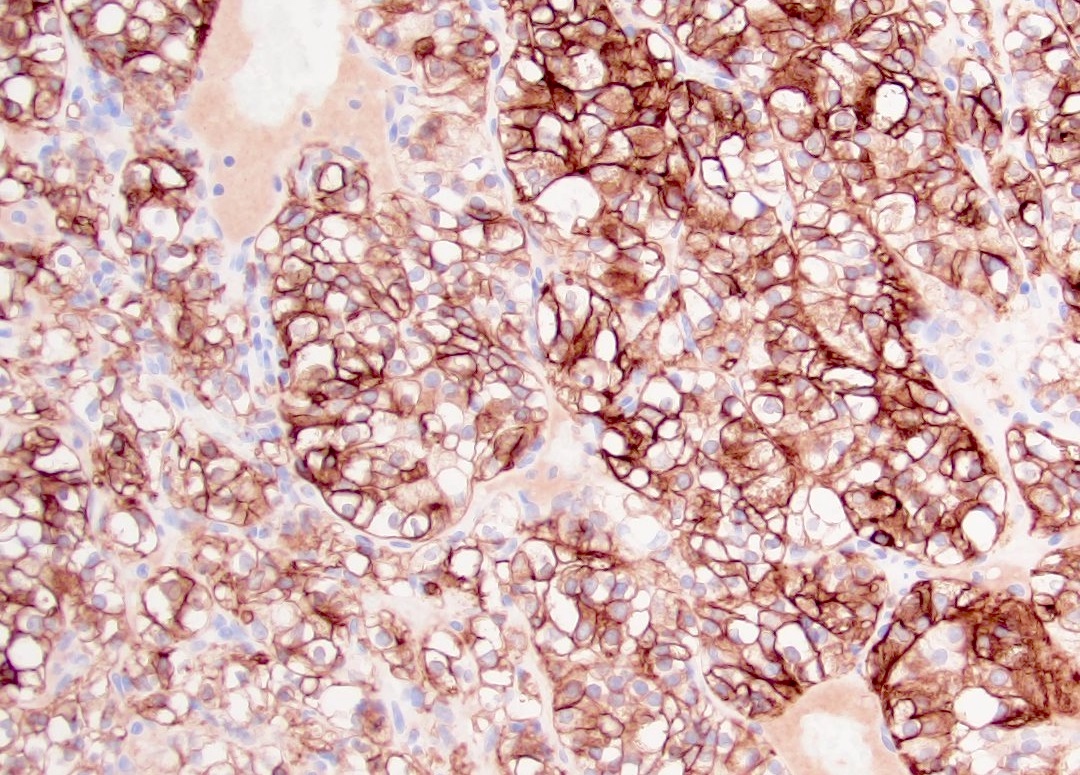
- Characteristic immunohistochemical profile: positive for PAX8, CAIX (box-like) and CD10; generally negative for AMACR (35% positive), CK7 (15% positive) and CD117 (2% positive)
ICD coding
- ICD-O: 8310/3 - clear cell renal cell carcinoma
Epidemiology
- ~2% of all malignancies
- 65 - 70% of all renal cell carcinomas (RCC)
- Most occur after age 40, predominantly in sixth and seventh decades
- M:F = 1.5:1
- References: Eur Urol 2019;75:74, Semin Oncol 2000;27:124, CA Cancer J Clin 2019;69:7
Sites
- Kidney, typically solitary cortical mass in sporadic tumors
- Multiple tumors may represent familial syndromes but retrograde venous extension from the dominant sporadic tumor is also possible
- Renal sinus invasion is the most common pathway of spread, often accompanied by invasion of the renal vein or its segmental branches, leading to a higher risk of distant metastasis
- Metastases
- Hematogenous is more common: lung (most common), bone, liver, retroperitoneum, pleura, central nervous system (CNS), head and neck (J Urol 2008;179:474, Urol Case Rep 2019;27:100989)
- Lymphatic is less common compared to papillary RCC and chromophobe RCC: hilar, aortic, caval and thoracic lymph nodes (BJU Int 2008;102:1381, Cancer 2008;112:1480)
Pathophysiology
- Suggested to arise in epithelial cells lining the proximal convoluted tubule (EBioMedicine 2023;92:104596, Annu Rev Pathol 2015;10:263)
- Loss of the VHL protein function by a 2 step process in 50 - 82% of somatic clear cell renal cell carcinomas (ccRCC) leading to deletion, unbalanced translocation or biallelic alteration in VHL (von Hippel-Lindau) tumor suppressor gene (3p25-26)
- First event of 3p loss, usually by chromothripsis, occurs in childhood or adolescence in a few hundred cells (Cell 2018;173:611)
- Second allele undergoes somatic mutation or epigenetic inactivation through hypermethylation
- VHL protein loss leads to accumulation of hypoxia inducible transcription factor alpha (HIF1α)
- HIF1α accumulation drives transcription of hypoxia associated genes, including VEGF, PDFGβ, GLUT1, TGFα, CAIX, EPO and metalloproteinases (Surg Pathol Clin 2009;2:199)
- Other driver mutations are present in smaller percentages (see Molecular / cytogenetics description)
Etiology
- Risk factors: smoking, obesity, hypertension, long term dialysis (particularly in acquired adult cystic kidney disease) and family history of kidney cancer
- Familial tumors: mostly von Hippel-Lindau disease, less commonly in families segregating constitutional chromosome 3 translocations, BAP1 tumor predisposition syndrome, Cowden syndrome, Birt-Hogg-Dubé syndrome and tuberous sclerosis (Genet Couns 2003;14:149, Am J Surg Pathol 2015;39:e1, Eur Urol 2019;76:754, J Urol 2013;190:1990, Adv Anat Pathol 2013;20:245)
Clinical features
- Most commonly: anemia, gross hematuria, flank pain and mass
- Weight loss and fever in late stages
- Classic triad of flank mass, pain and hematuria present in < 10% of cases (Urol Oncol 2002;7:135)
Diagnosis
- 60 - 80% found incidentally on radiologic imaging
- Nephrectomy or partial nephrectomy; definitive diagnosis may be possible by needle biopsy
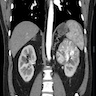
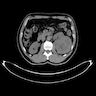
Radiology description
- Computed tomography (CT): exophytic with mixed enhancement and heterogeneous appearance (due to internal necrosis, cystic change or hemorrhage)
- Bosniak classification (category I to IV) for renal cysts guides the management by approximating the risk of malignancy
- Magnetic resonance imaging (MRI): similar accuracy to CT, heterogeneous in T1 and hyperintense (bright) T2 when use of contrast materials is contraindicated
- In metastatic cases, MRI and positron emission tomography (PET / CT) are the preferred methods
- Ultrasonography: useful for incidental detection of renal masses
- Reference: Radiology 2013;267:444
Prognostic factors
- 5 year survival: 50 - 70% after nephrectomy, 10% in metastatic disease
- Survival difference is mostly due to differences in TNM stage and nuclear grade, regardless of the histologic type of the RCC (J Clin Oncol 2005;23:2763, Eur Urol 2005;48:593)
- Worse prognosis within the same stage: higher histologic grade, sarcomatoid and rhabdoid differentiation and > 10% coagulative tumor necrosis (Pathology 2021;53:120, Am J Surg Pathol 2013;37:311, Pathology 2015;47:34)
- Worse prognosis than papillary and chromophobe RCC
- Better prognosis with only VHL loss or PBRM1 mutation, poor prognosis with multiple driver gene mutations or loss of 4p, 9p or 14q
- BAP1 mutated ccRCCs have distinct morphologic and immunophenotypic features associated with more aggressive behavior than typical VHL only mutated ccRCC (Am J Clin Pathol 2021;155:718)
- PBRM1 is mutually exclusive to BAP1 mutation and is associated with improved response to immunotherapy
- TNM staging: the most accurate predictor (see Kidney tumor staging)
- pT1 and pT2: limited to the kidney, classified based on size
- T1a (≤ 4 cm)
- T1b (> 4 cm to 7 cm)
- T2a (> 7 cm to 10 cm)
- T2b (> 10 cm)
- pT3: regional spread beyond kidney parenchyma
- pT3a: regional extrarenal spread into perinephric fat, renal sinus fat, involvement of the renal vein or segmental veins or invasion of the pelvicalyceal system
- pT3b: extension into the inferior vena cava below the diaphragm
- pT3c: tumor extension into the inferior vena cava above the diaphragm or invasion of the vena cava wall
- pT4: distant spread, beyond Gerota fascia, including contiguous extension into the ipsilateral adrenal gland
- pT1 and pT2: limited to the kidney, classified based on size
- Histologic grading
- WHO / ISUP grading system: 4 tiers, uses nucleolar prominence; used for clear cell and papillary renal cell carcinoma (chromophobe RCC not graded)
- G4: extreme nuclear pleomorphism, multinucleated giant cells or rhabdoid or sarcomatoid differentiation
- G3: nucleoli are conspicuous and eosinophilic at 100x magnification
- G2: nucleoli are conspicuous and eosinophilic at 400x magnification (but not prominent at 100x magnification)
- G1: nucleoli are absent or inconspicuous and basophilic at 400x magnification (Urology 2014;83:969)
- WHO / ISUP grading system: 4 tiers, uses nucleolar prominence; used for clear cell and papillary renal cell carcinoma (chromophobe RCC not graded)
- > 50% granular eosinophilic features may predict poor response to IL2 therapy (J Immunother 2005;28:488)
- Clear cell RCCs with > 40% eosinophilic component may have worse clinical outcome (Mod Pathol 2015;28:S217)
Case reports
- 41, 55 and 68 year old men with clear cell RCC metastases that significantly decreased in size or resolved with no surgical or other types of treatment (Case Rep Oncol 2020;13:1285)
- 52 year old man with a metastatic clear cell RCC to the forearm without an identifiable primary renal mass (Urol Case Rep 2019;27:100989)
- 63 year old man with 15 cm clear cell RCC with syncytial giant cells (Arch Pathol Lab Med 2004;128:1435)
- 65 year old man with a 2.4 cm tumor with nonnecrotizing sarcoid-like granulomata (Clin Pathol 2020;13:2632010X20954215)
Treatment
- Choice of treatment depends on the stage, overall patient health and other individualized factors
- Surgical resection of stages 1 - 3 can be curative but up to 33% will recur
- Partial nephrectomy for smaller and localized tumors; radical nephrectomy for tumors larger than 4 cm and centrally located tumors
- Cryotherapy and radiofrequency ablation for unresectable tumors or some small tumors
- Systemic chemotherapy has limited efficacy
- Immunotherapy with checkpoint inhibitors has a 15 - 20% response rate: monoclonal antibodies against PD-1 (nivolumab and pembrolizumab), PDL1 (avelumab and atezolizumab) and CTLA4 (ipilimumab) (J Natl Compr Canc Netw 2019;17:587)
- Inhibitors of mammalian target of rapamycin (mTOR) pathways (such as temsirolimus)
- Tyrosine kinase inhibitors targeting VEGFR (including lenvatinib, axitinib and sunitinib), PDGFR or multitargeted (sorafenib, tivozanib, cabozantinib, lenvatinib and pazopanib) (Surg Pathol Clin 2009;2:199, Semin Oncol 2006;33:588)
- Hypoxia inducible factor 2 alpha (HIF2α) inhibitor belzutifan for certain types of VHL disease associated tumors
- Immune checkpoint inhibitors have shown significant response in ccRCC with sarcomatoid and rhabdoid features
- Combined tyrosine kinase inhibitors and checkpoint inhibitors received Food and Drug Administration (FDA) approval for advanced RCCs (J Natl Compr Canc Netw 2022;20:71)
- IL2 therapy: used less frequently
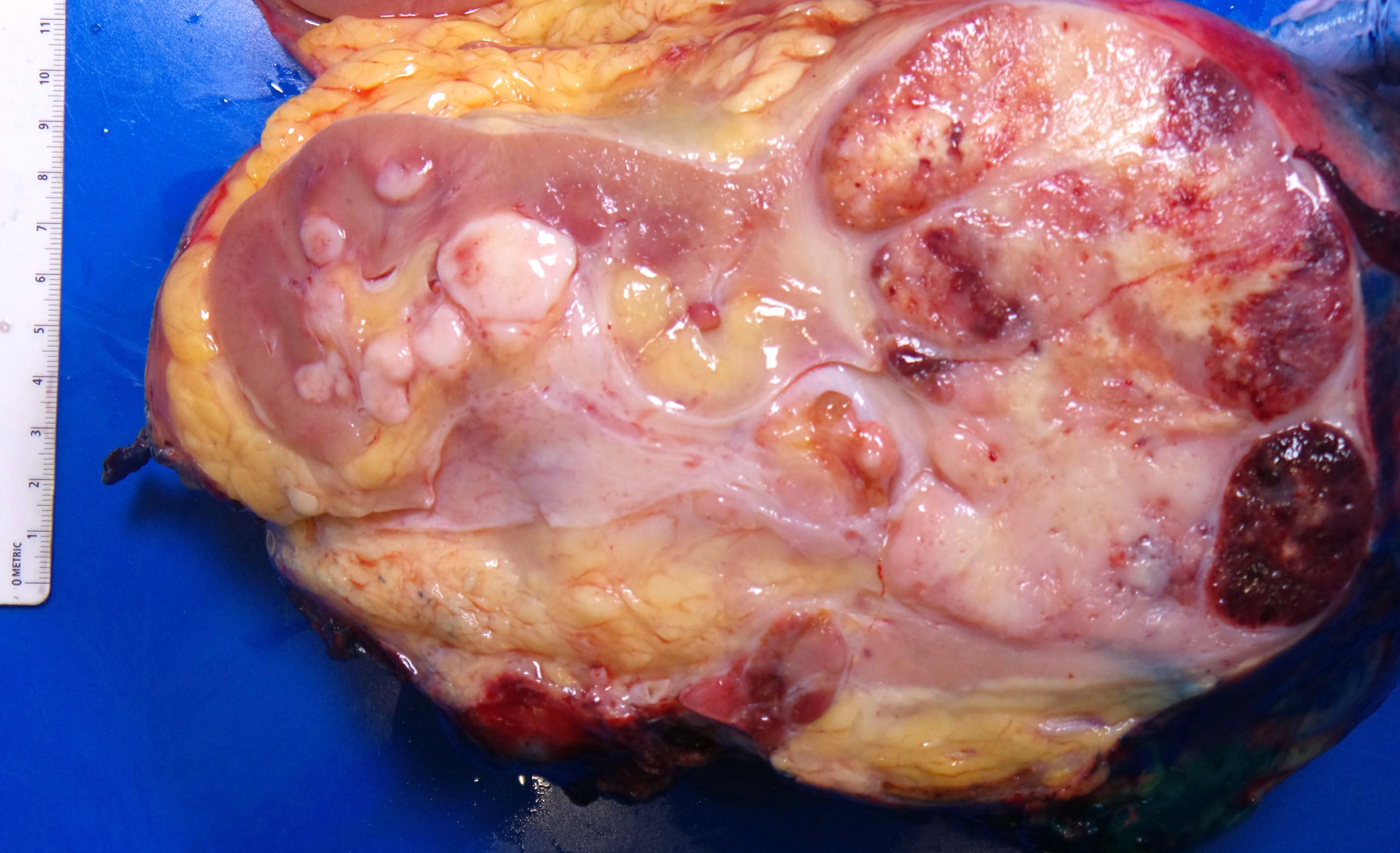
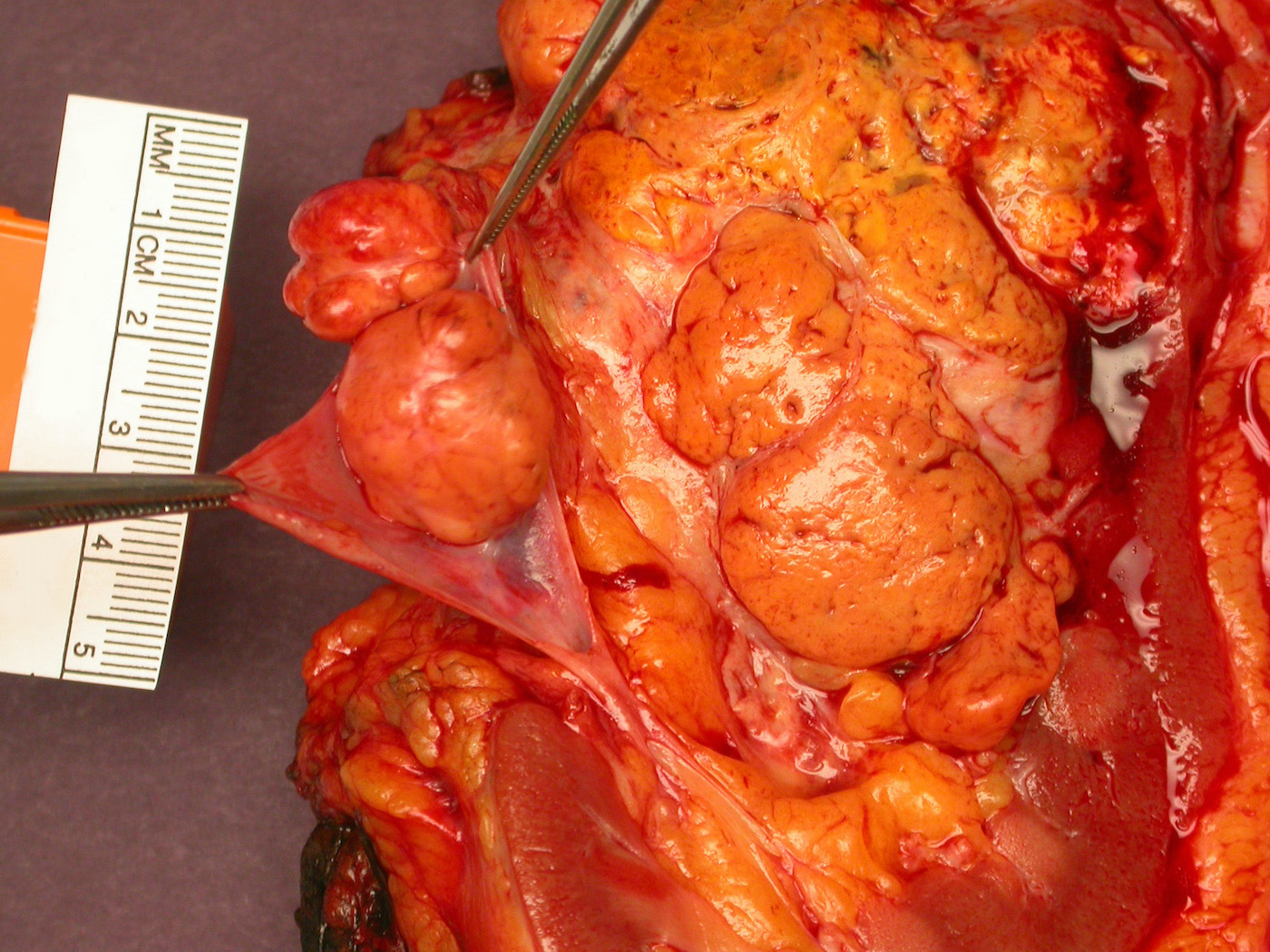
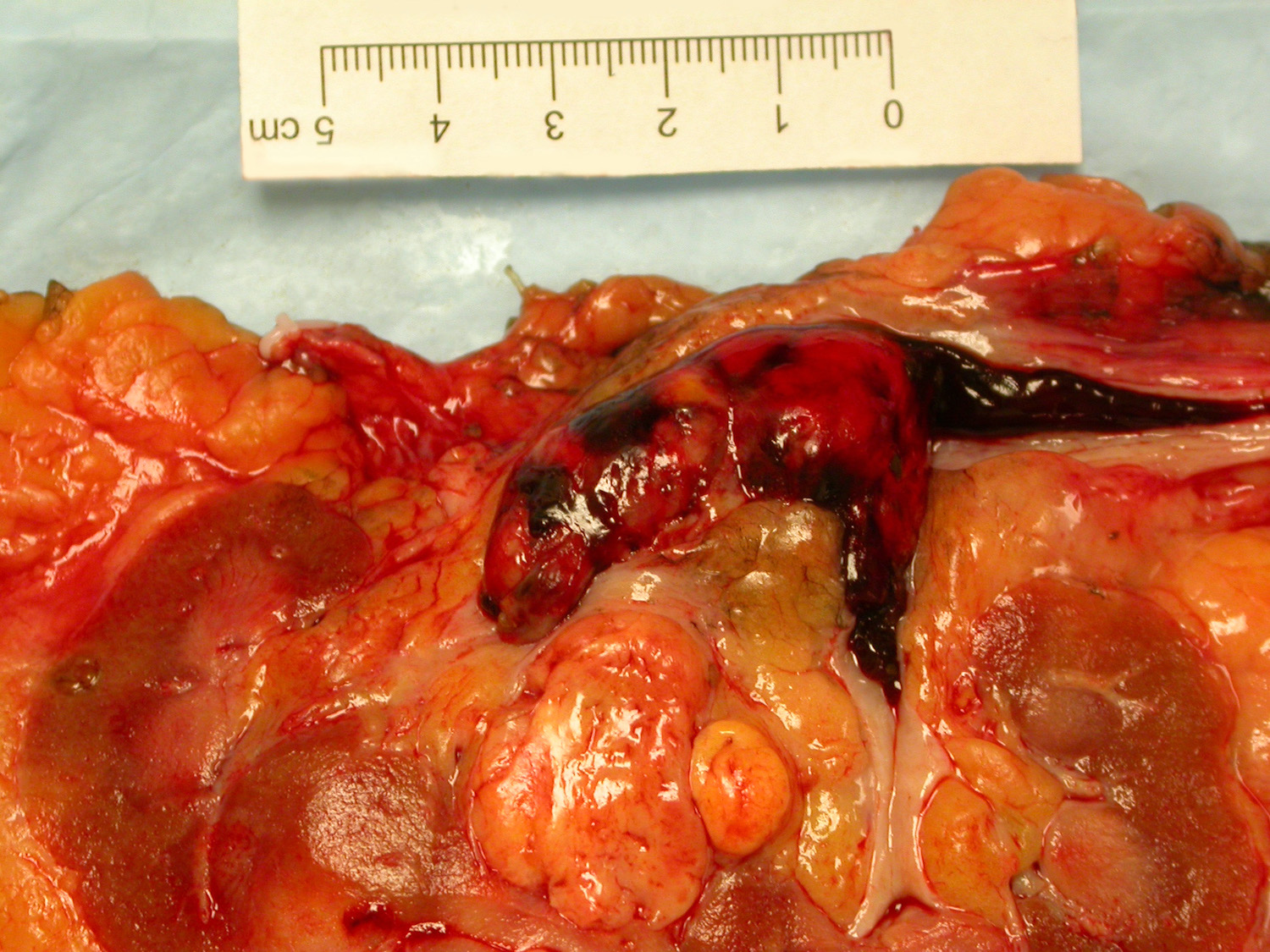
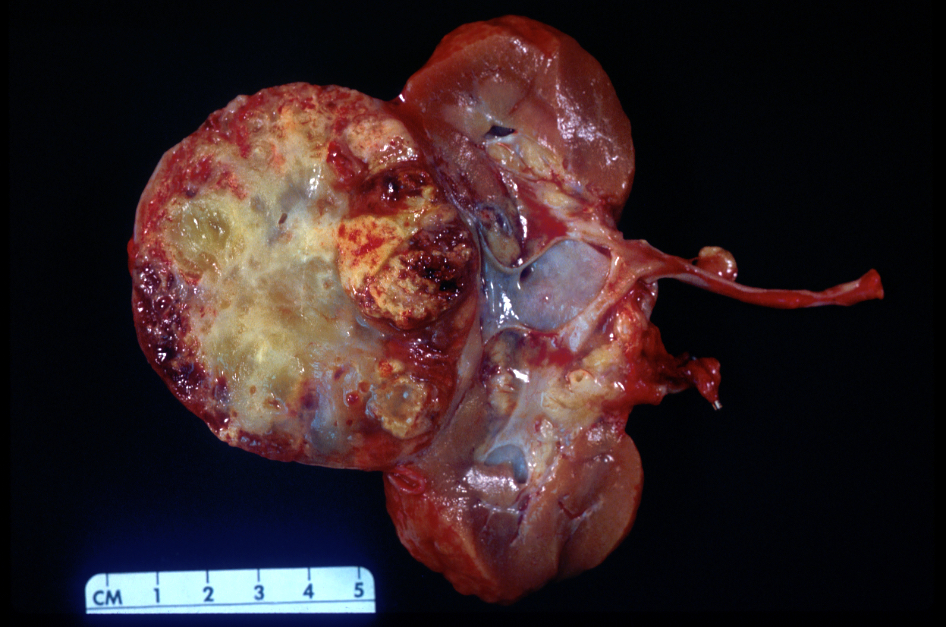
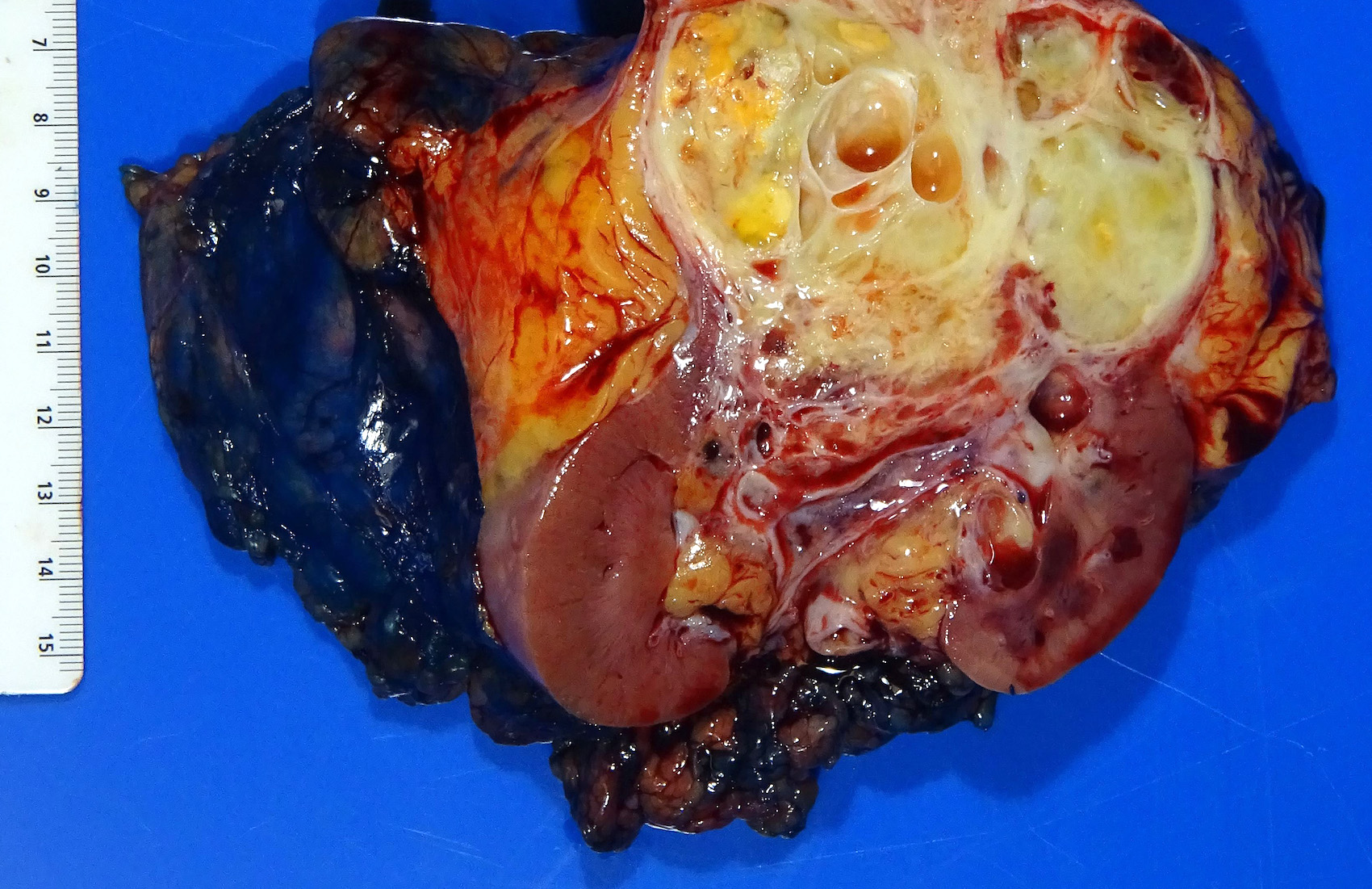
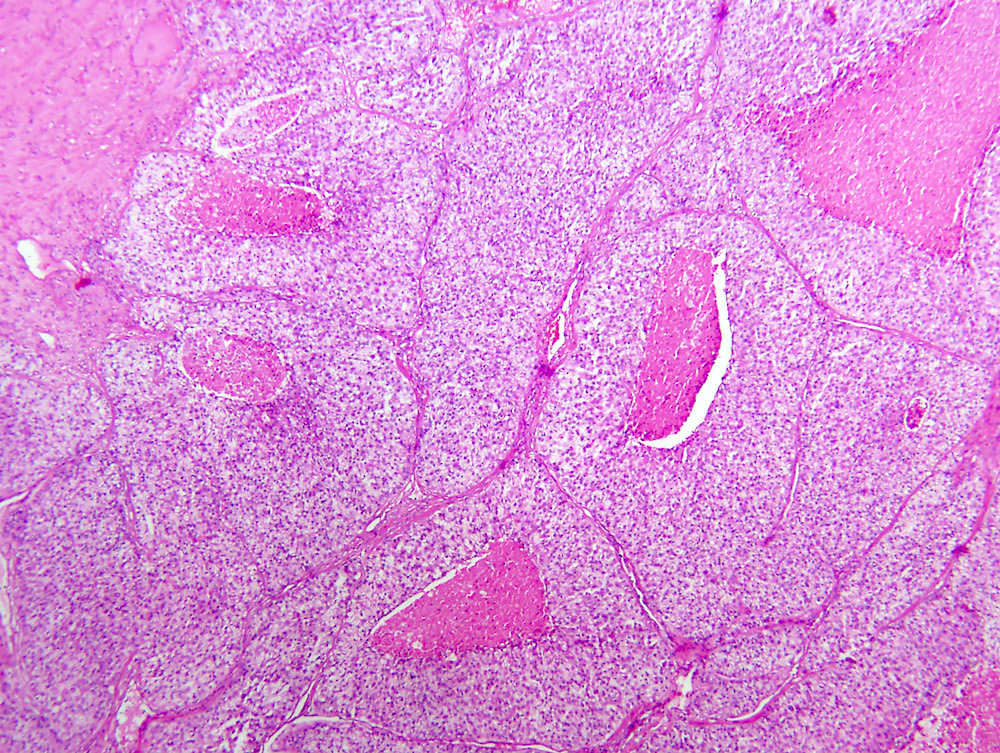
Gross description
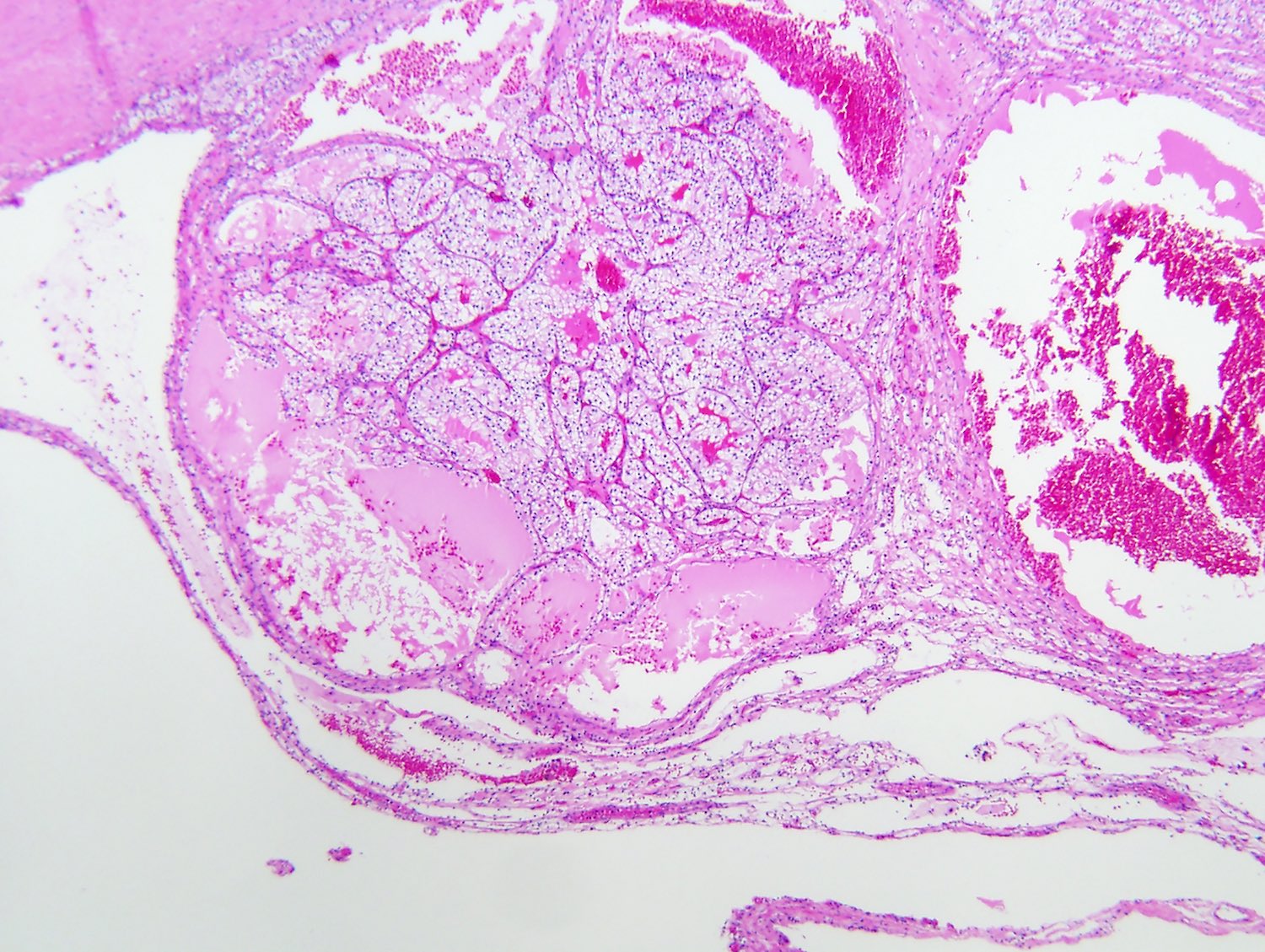
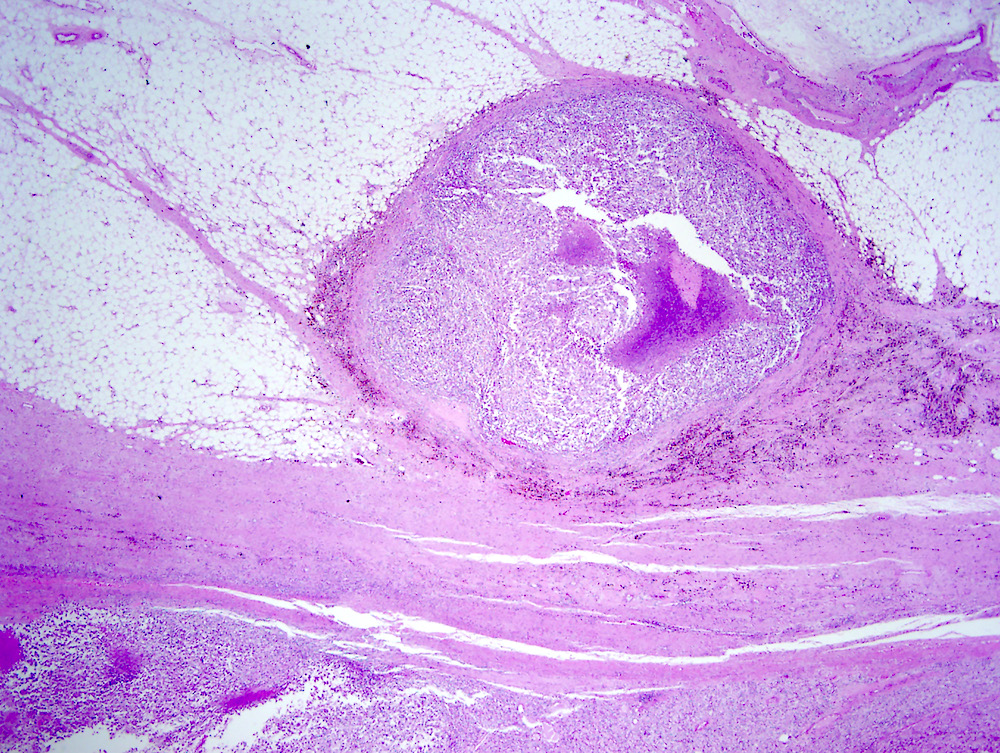
- Typically unilateral and unicentric renal cortical mass (average size: 7 cm)
- Typically well circumscribed by a pseudocapsule and expansile pushing margin protruding from the renal cortex
- Variegated solid and cystic with areas of fibrosis (gray) and recent or old hemorrhage (brown); necrosis and cystic changes are common
- Golden yellow color due to high lipid content
- Higher grade tumors may not be yellow due to less lipid and glycogen content
- Soft fleshy areas may reflect sarcomatoid differentiation
- Frequent involvement of renal vein and renal sinus
- Bilateral and multicentric masses are features of hereditary disease
- Notes on tumor staging
- Tumors larger than 7 cm almost always invade renal sinus fat; if no invasion is seen in larger tumors, an additional gross review is warranted (Pathology 2021;53:120)
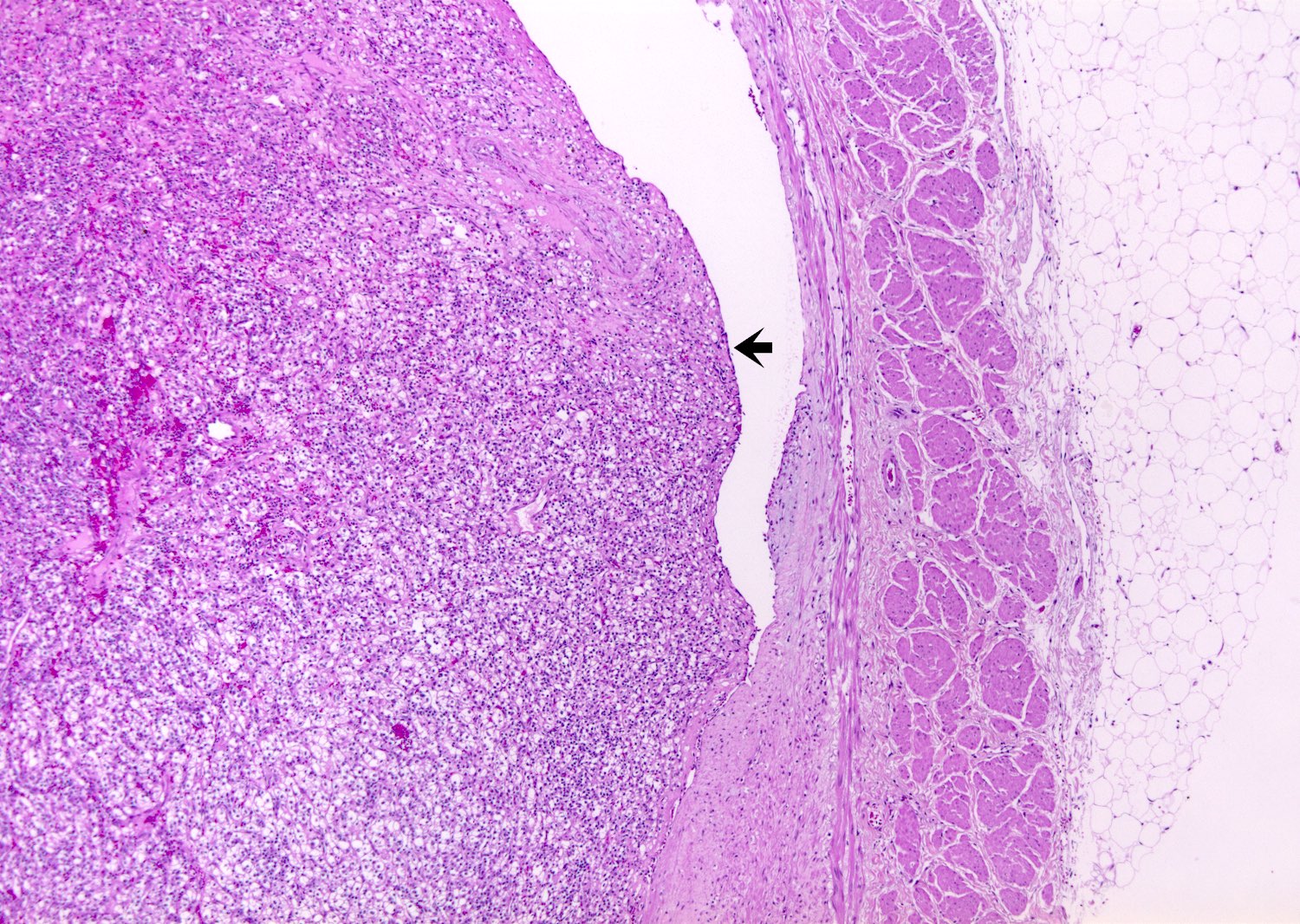
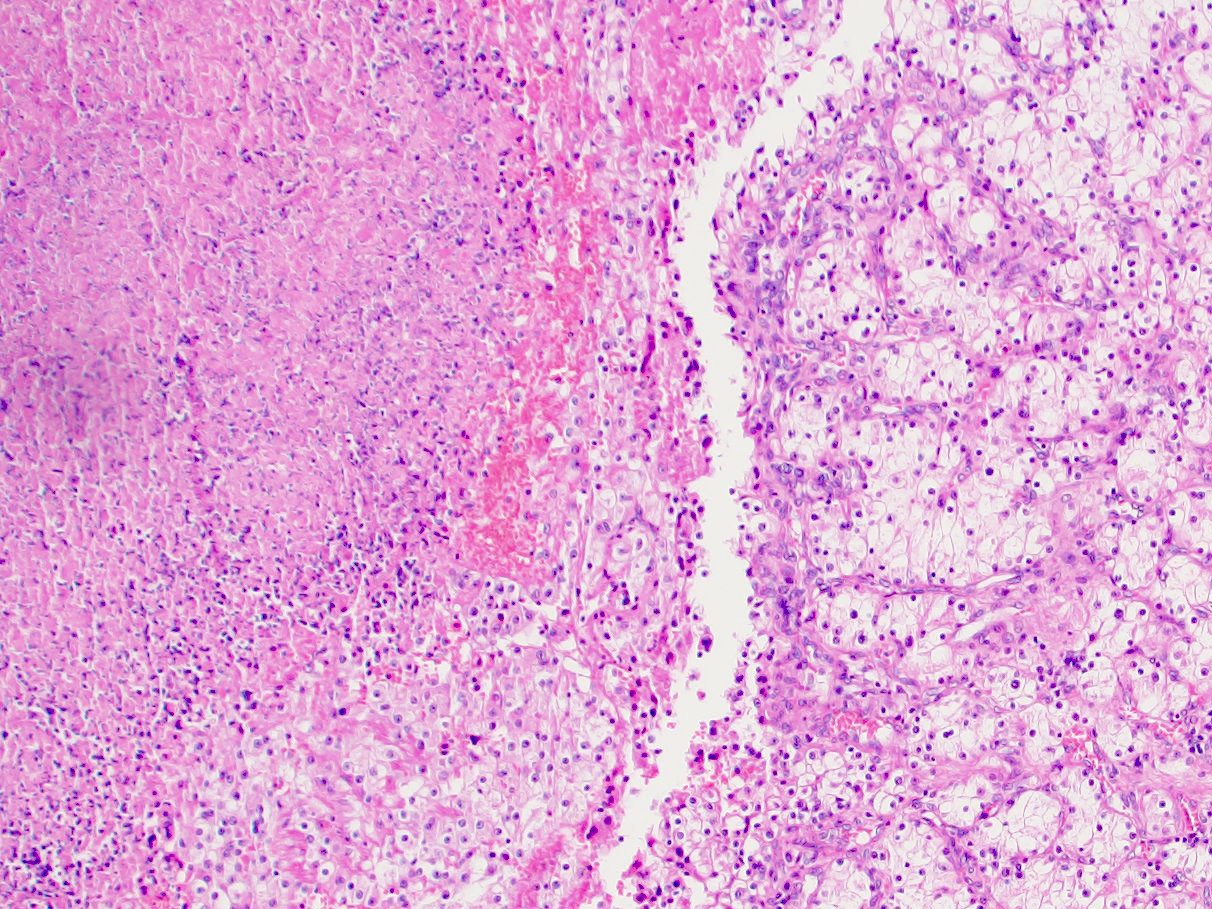
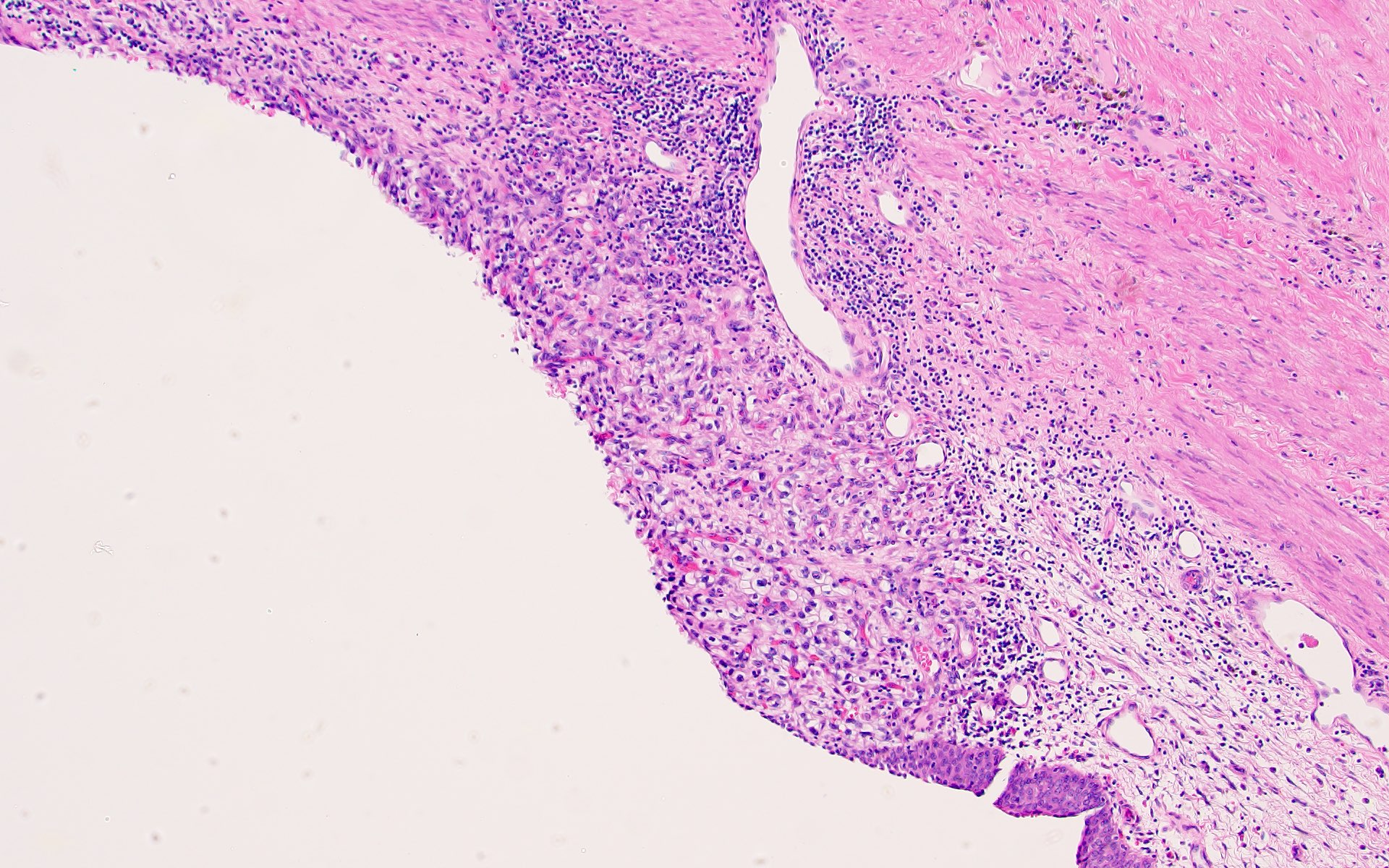
- Capsular invasion
- Disrupted and irregular advancement of tumor into the perirenal adipose tissue and loss of the smooth convex outer contour
- Smooth bulging tumor covered by the cancer pseudocapsule is not considered perirenal fat involvement
- Tumor cell should touch fat or extend irregular tongues into perinephric tissues, with or without desmoplasia (J Kidney Cancer VHL 2014;1:26)
- Renal sinus invasion
- Most common route of extrarenal spread
- Usually happens before capsular invasion
- Unlike renal capsule, the renal sinus is not delineated from the renal parenchyma by a fibrous capsule
- Not regarded as true invasion if tumor is separated from sinus structures by a rim of renal parenchyma
- Regarded as sinus invasion if tumor bulges into the renal sinus fat clearly beyond renal parenchyma, even if covered by loose connective tissue (J Kidney Cancer VHL 2014;1:26)
- Tumor surrounding large lymphovascular structures is a sign of sinus fat invasion (Am J Surg Pathol 2004;28:1594, Am J Surg Pathol 2000;24:451)
- Vascular invasion
- May show as tumor nodules in renal sinus
- In partially obliterate vessels, a single layer of endothelial lining overlying the tumor does not preclude vascular invasion
- Small vein involvement usually implies large vein involvement
- Renal sinus invasion usually implies renal vein invasion, cautious examination is warranted (J Urol 2005;174:1199)
Gross images
Frozen section description
- Limited application in determining the subtype of renal cell carcinoma
- Sometimes important to distinguish urothelial carcinoma from RCC
- Characteristic findings on microscopy include clear cell cytoplasm, highly dense vascularization (chicken wire) and nested growth pattern
- Sometimes only naked abnormal nuclei are present
Frozen section images
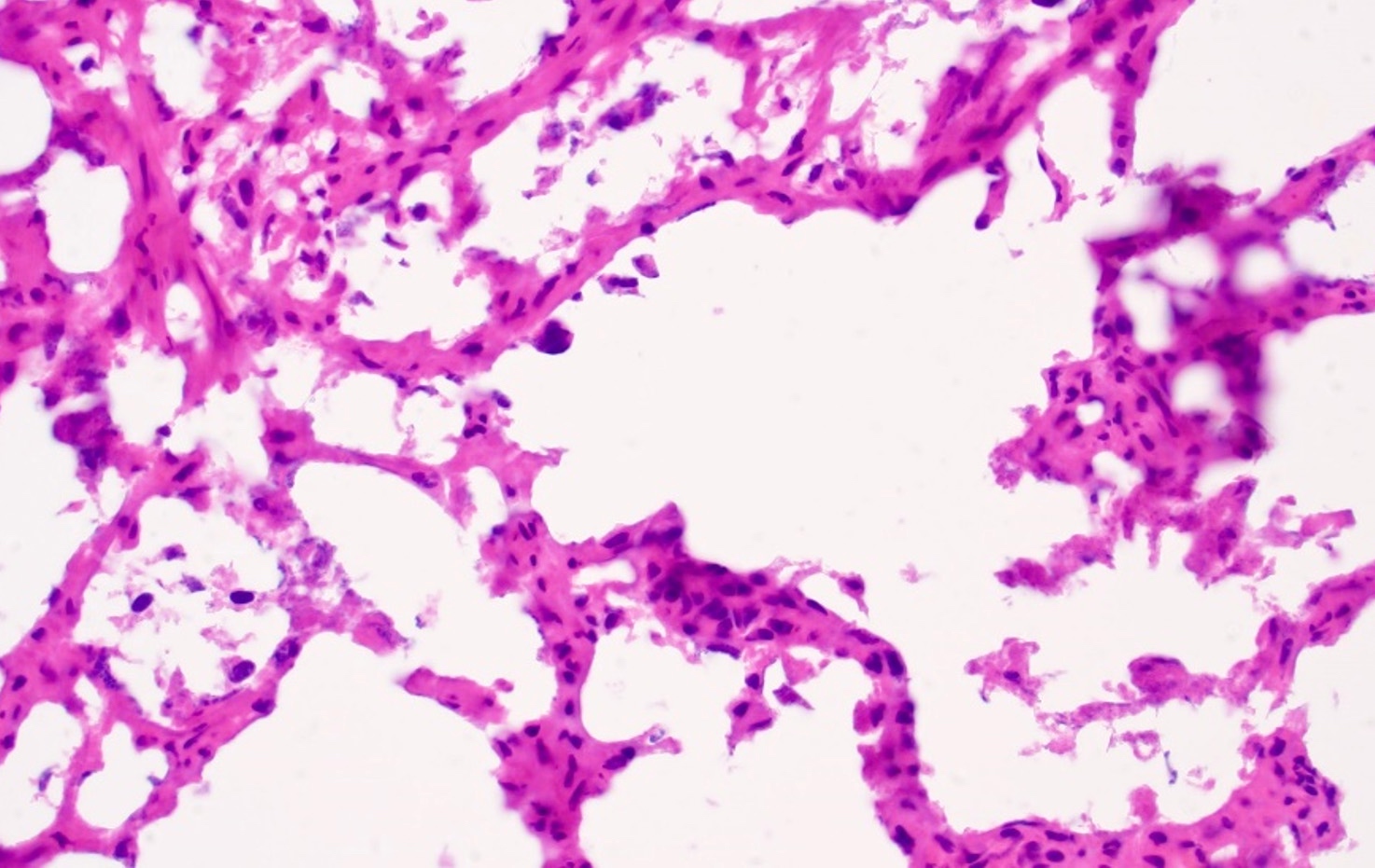
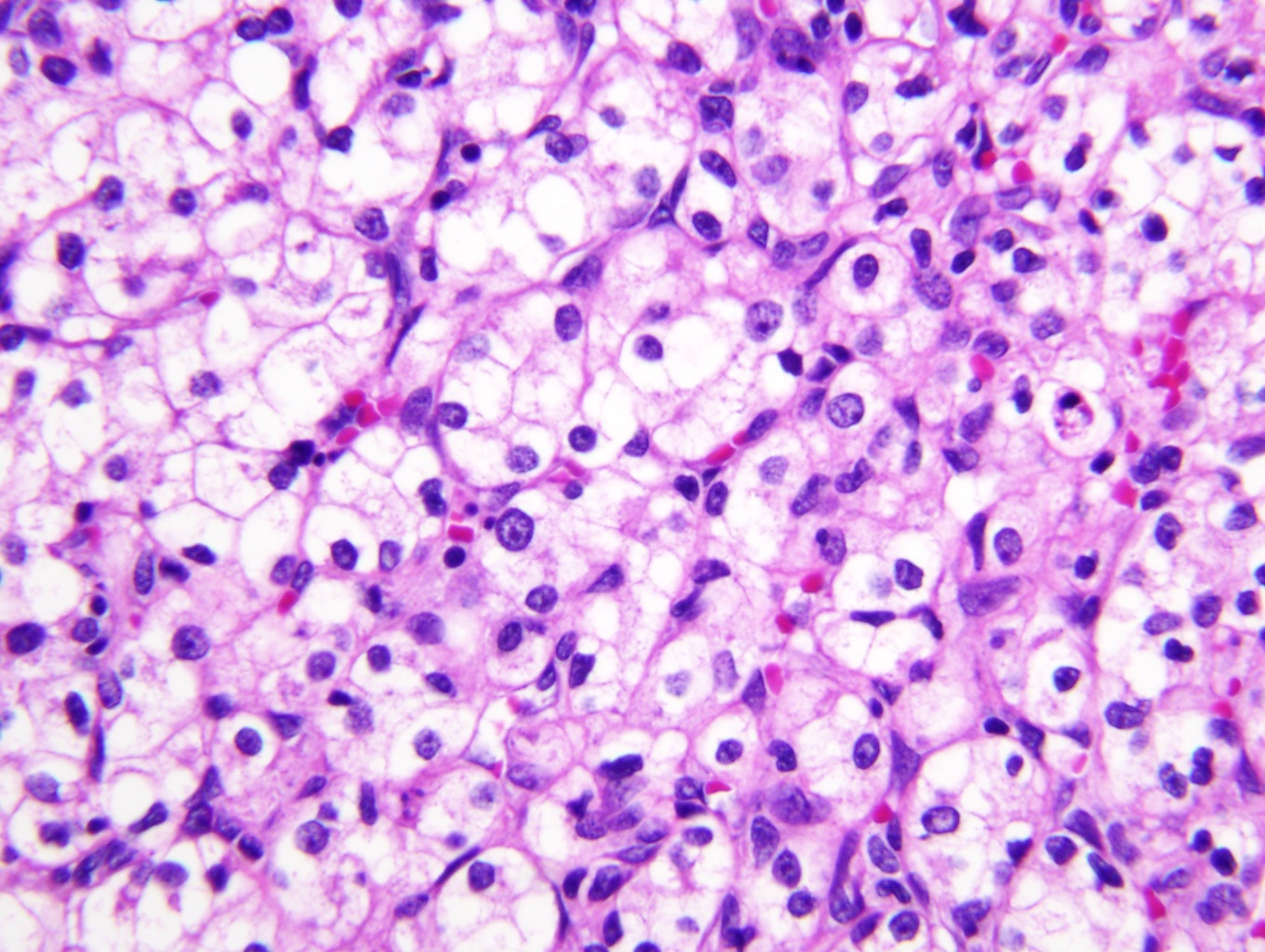
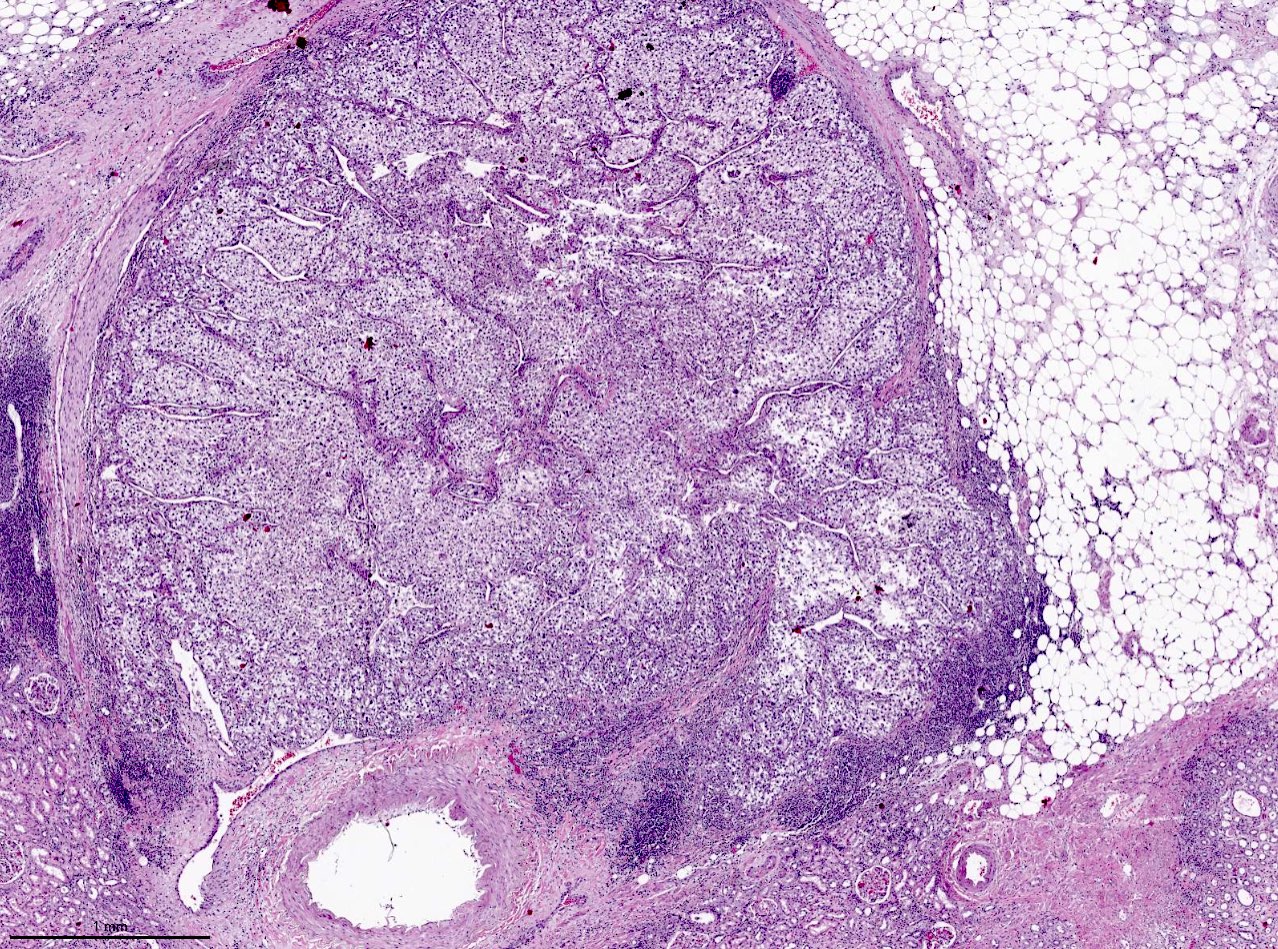
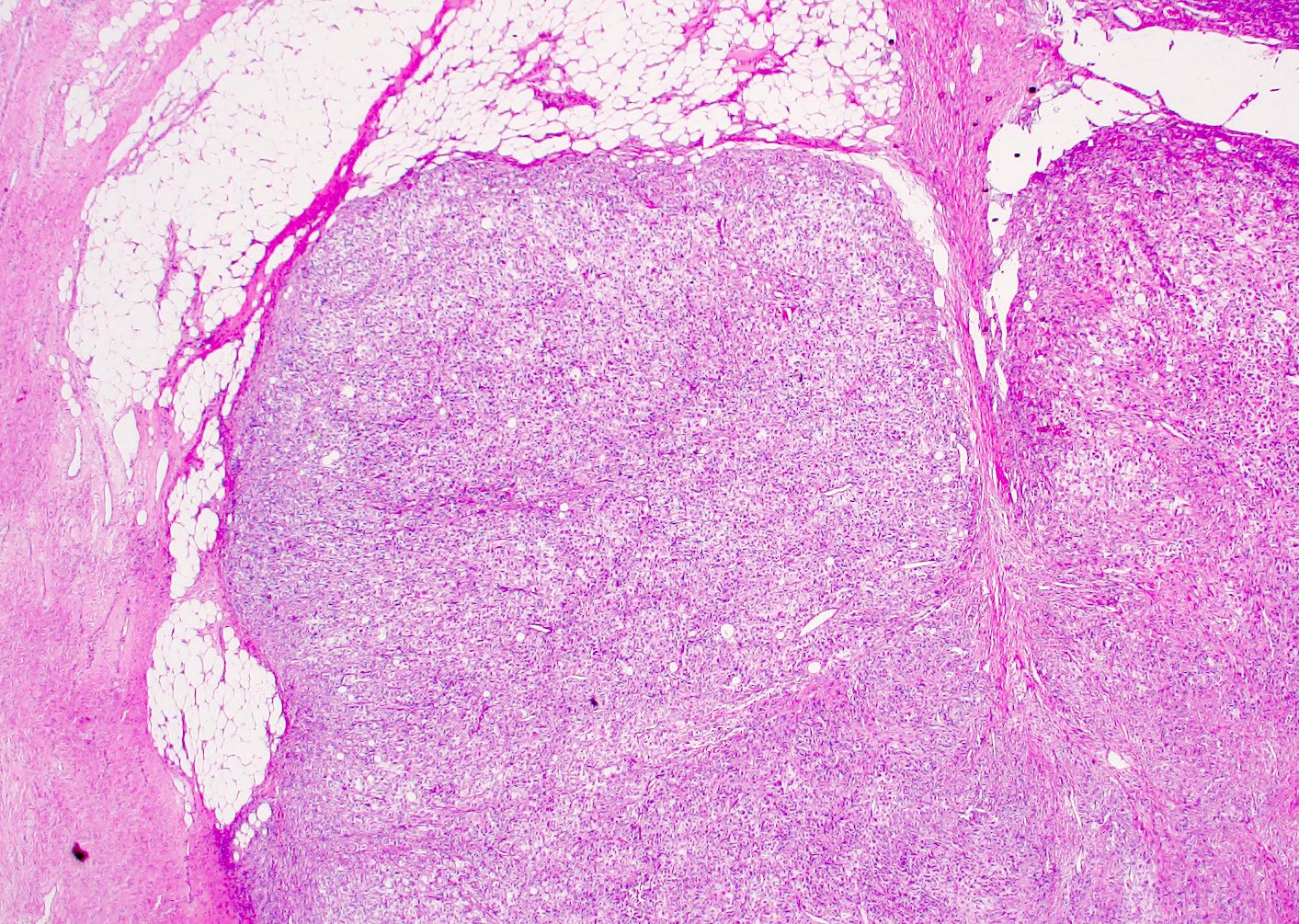
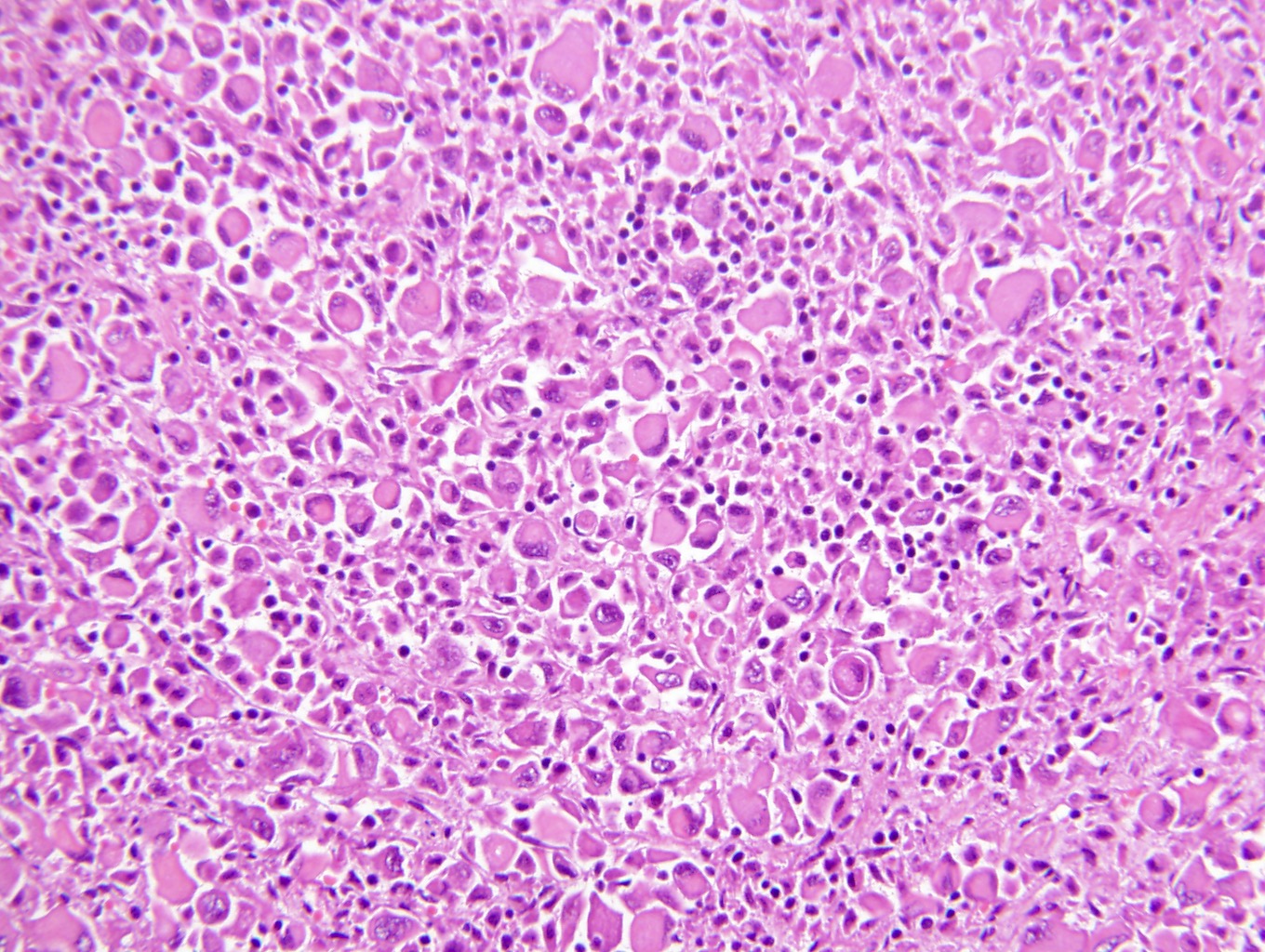
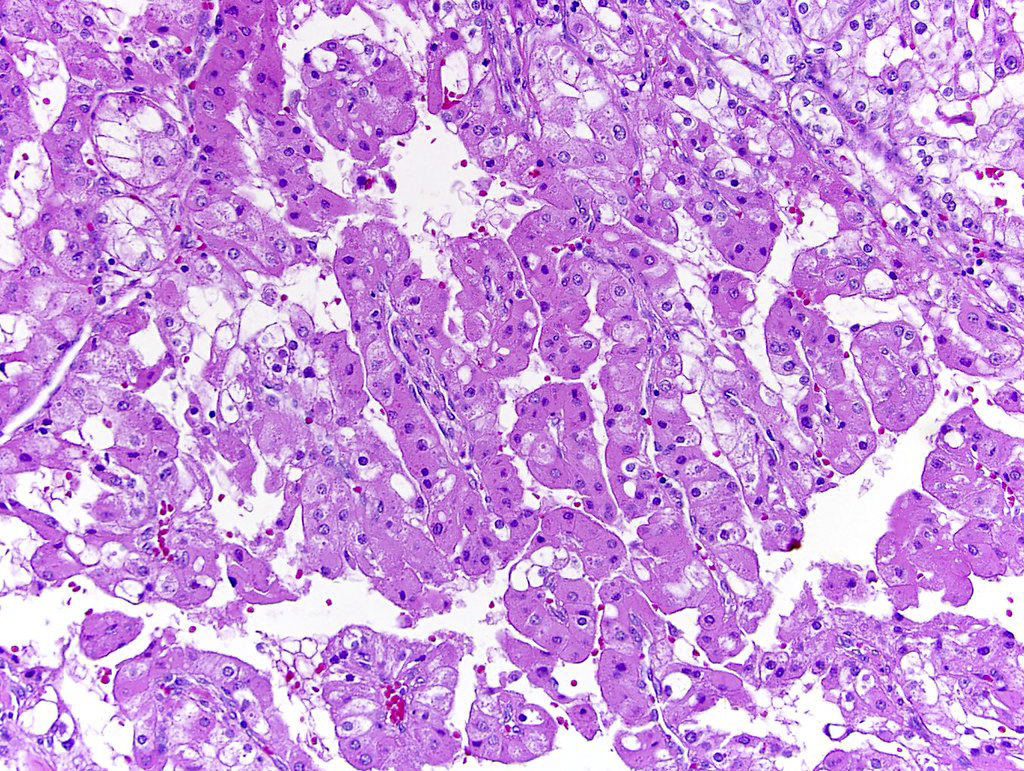
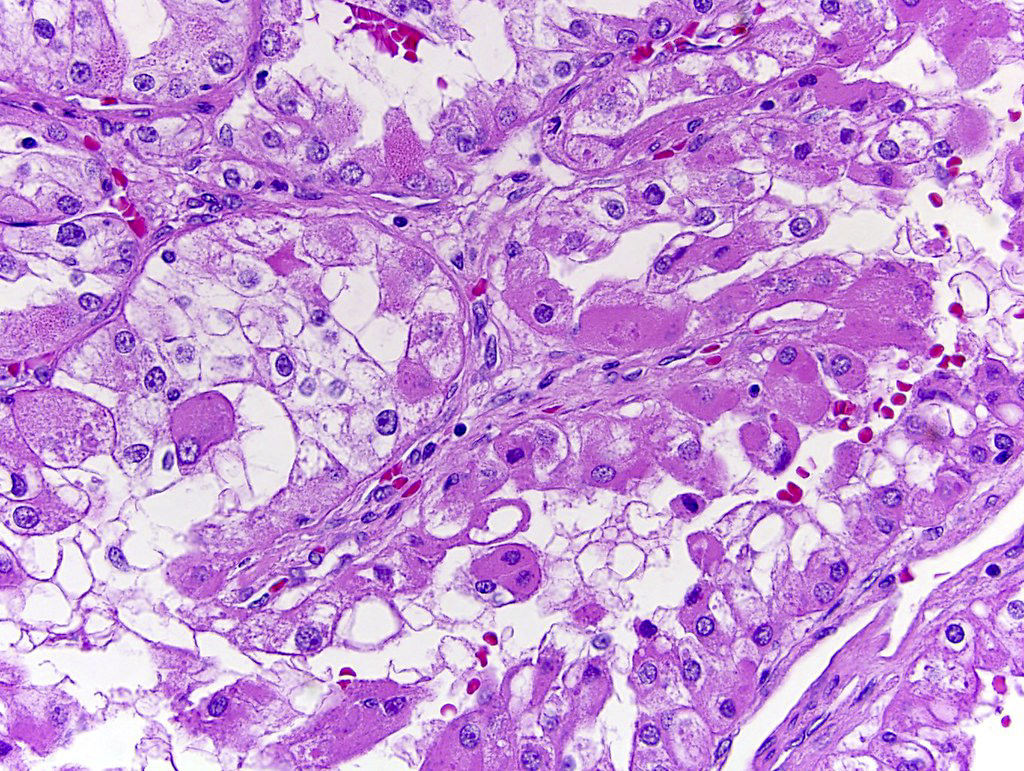
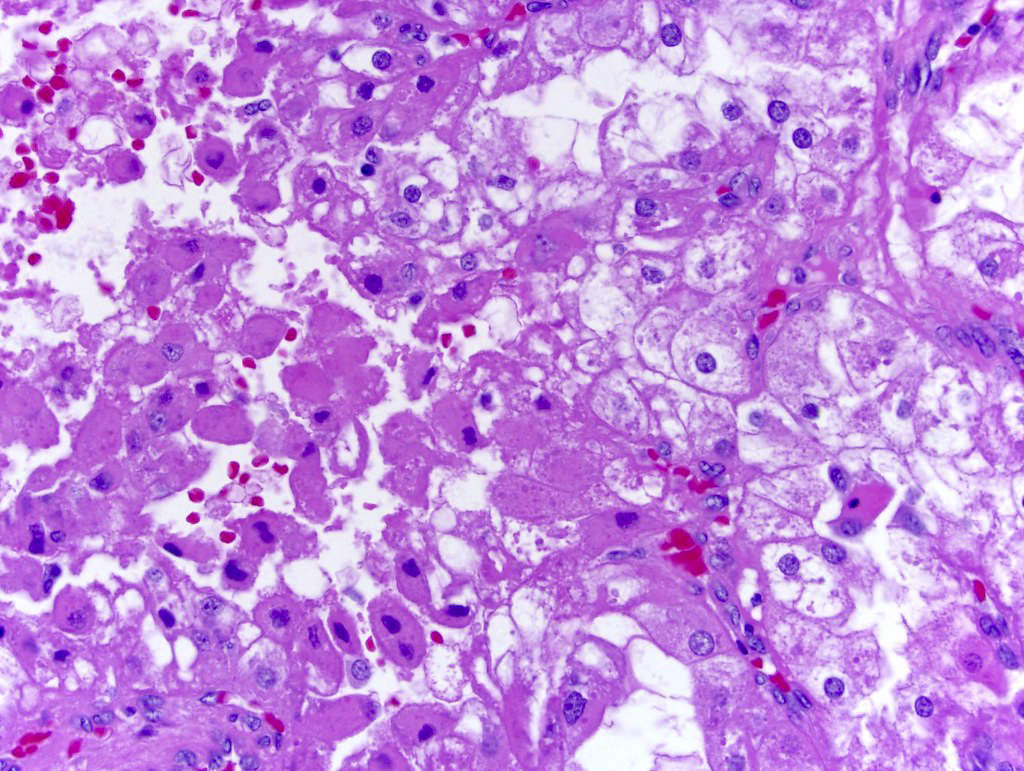
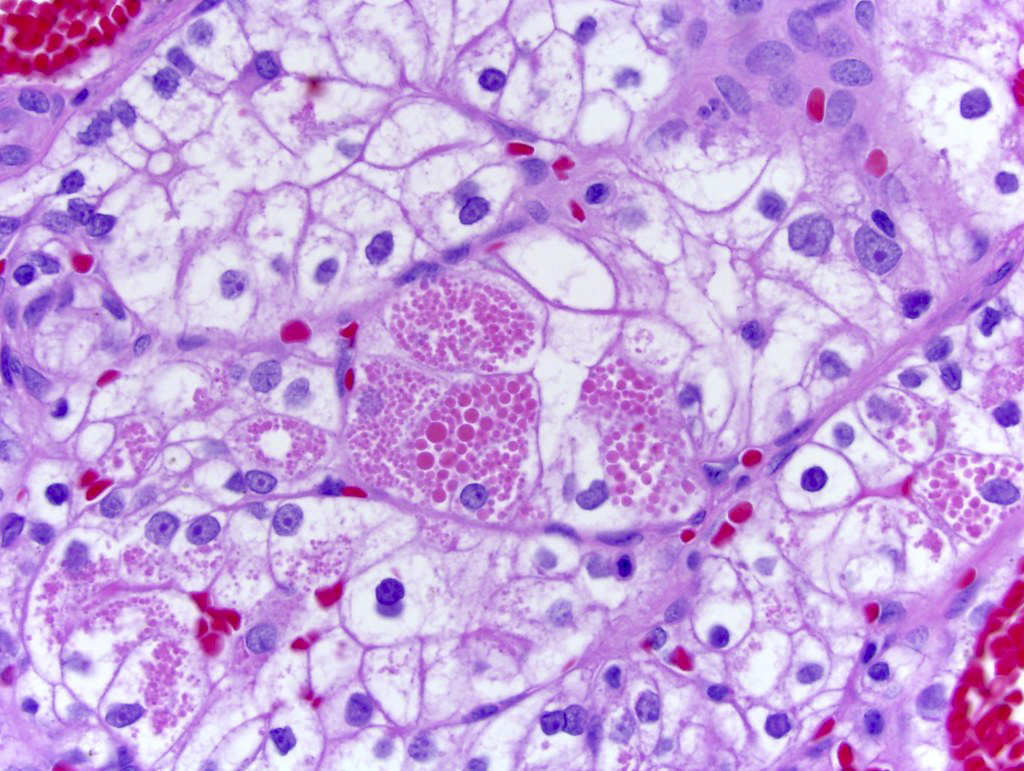
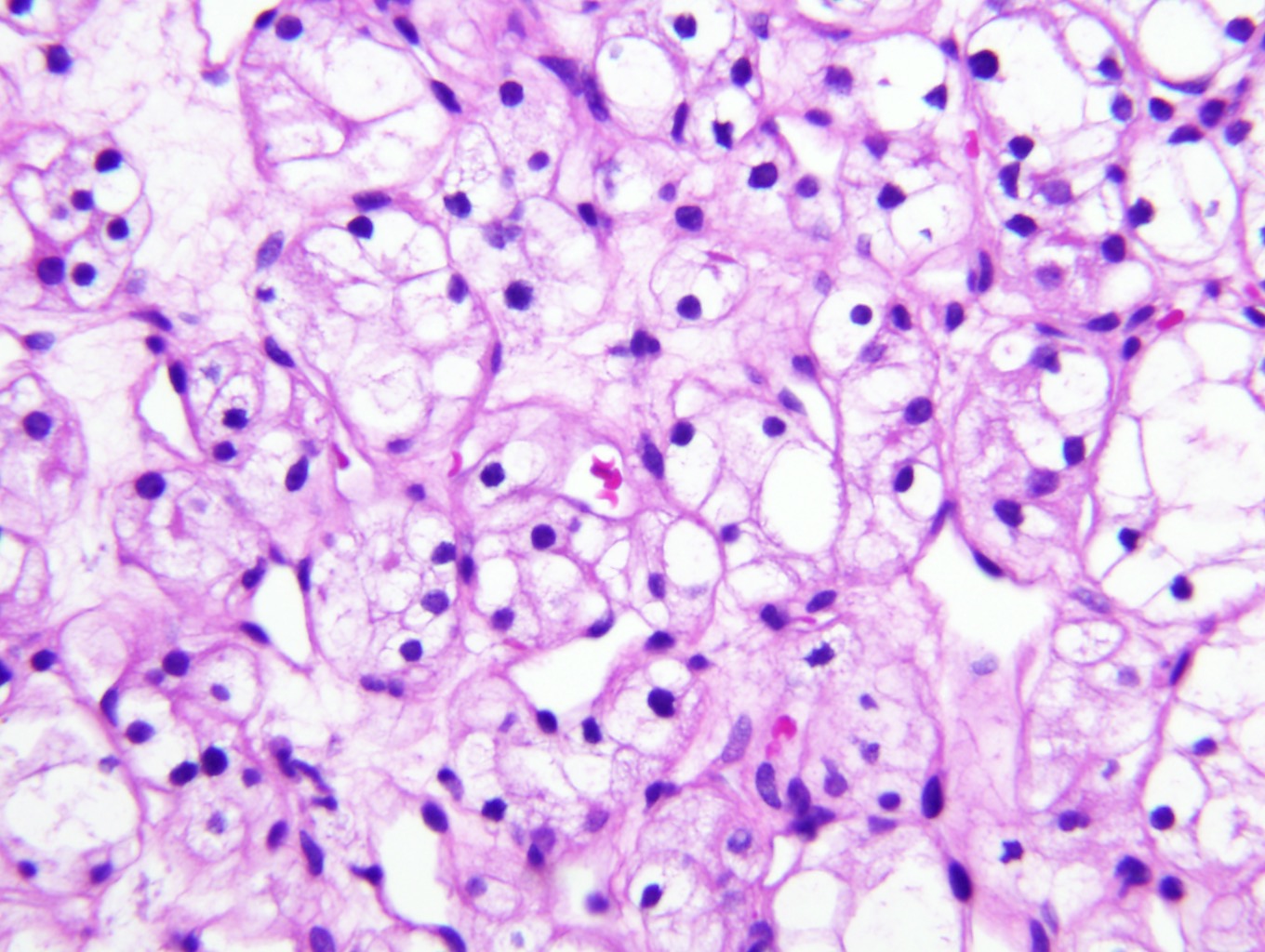
Microscopic (histologic) description
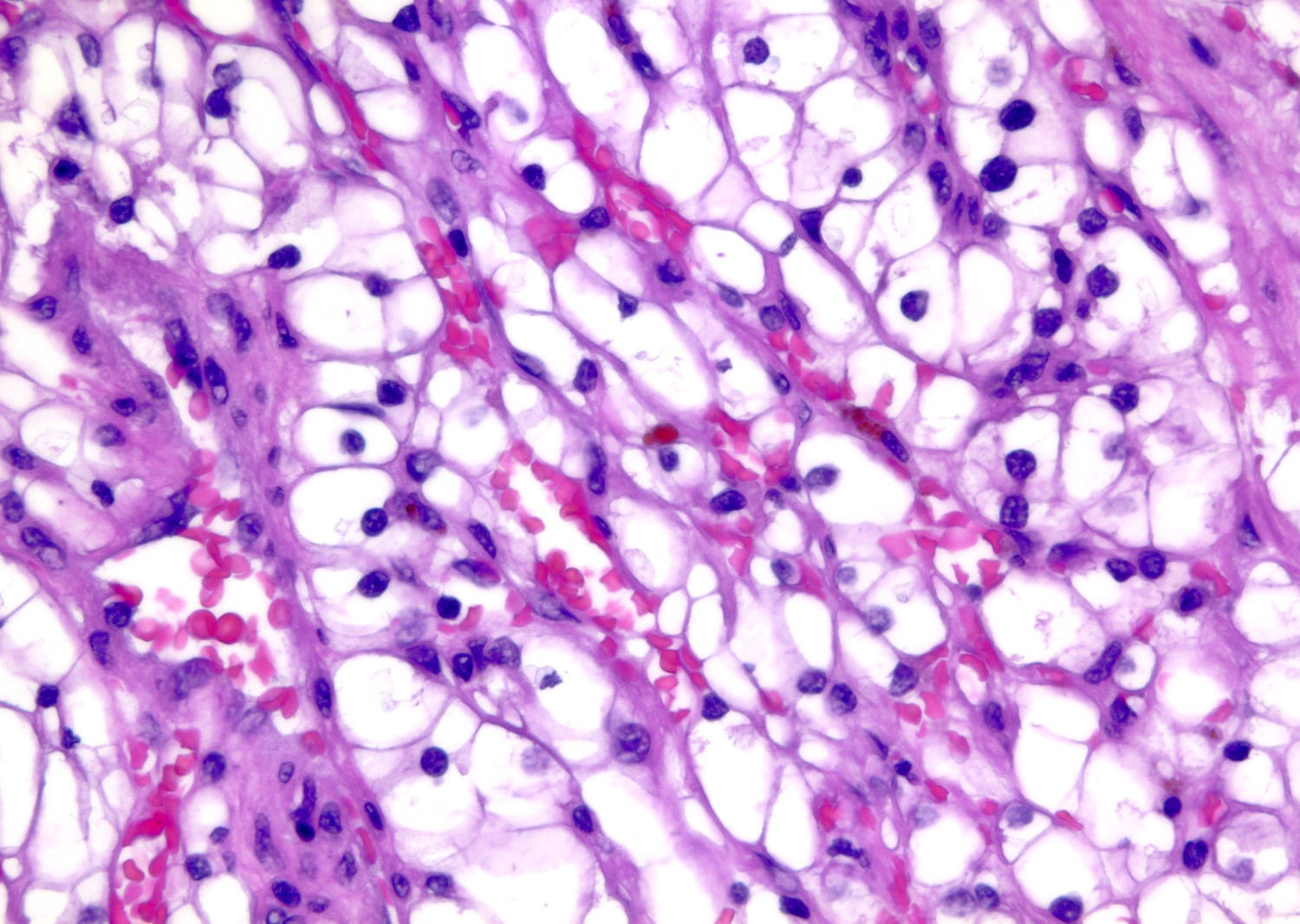
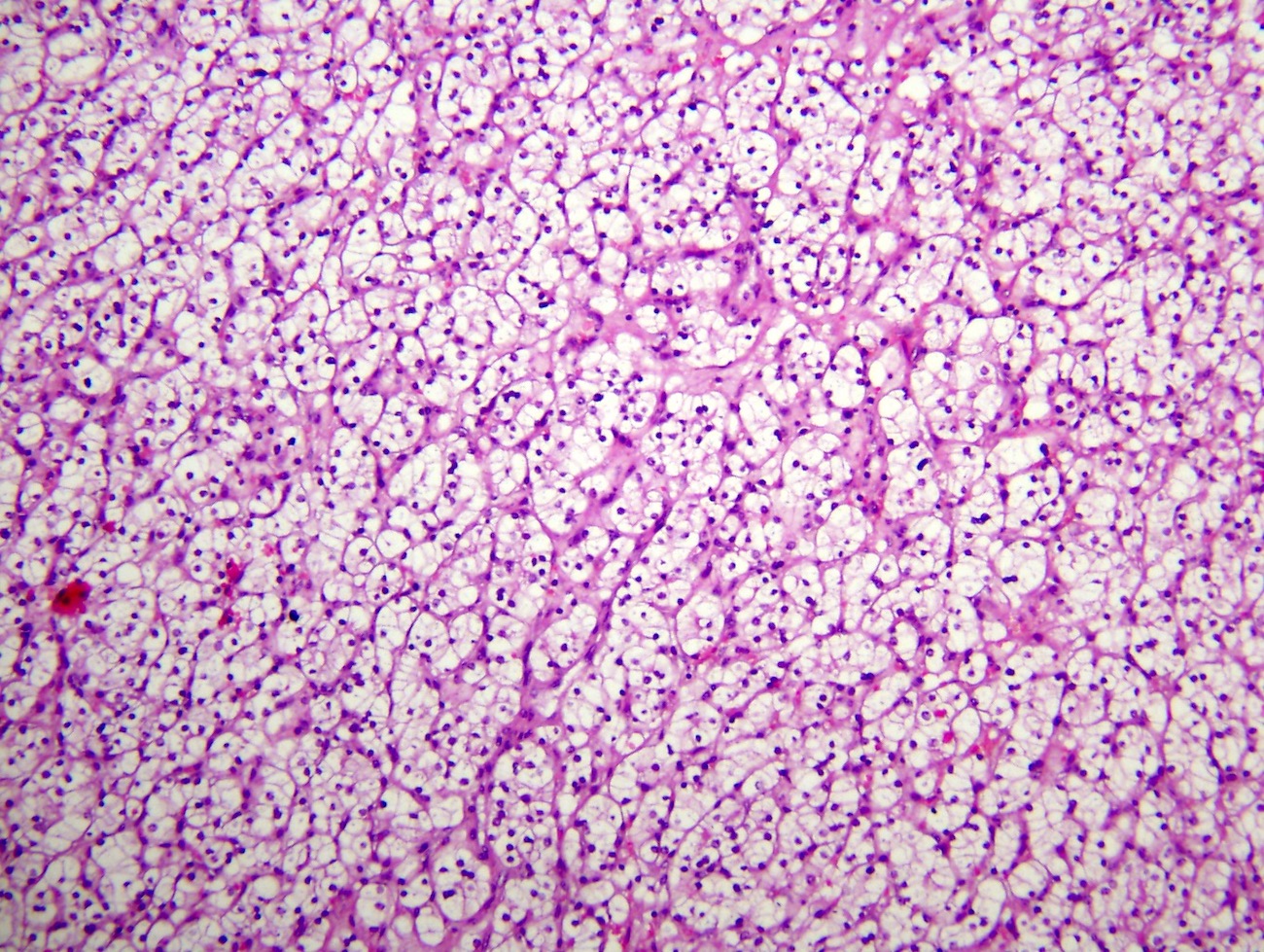
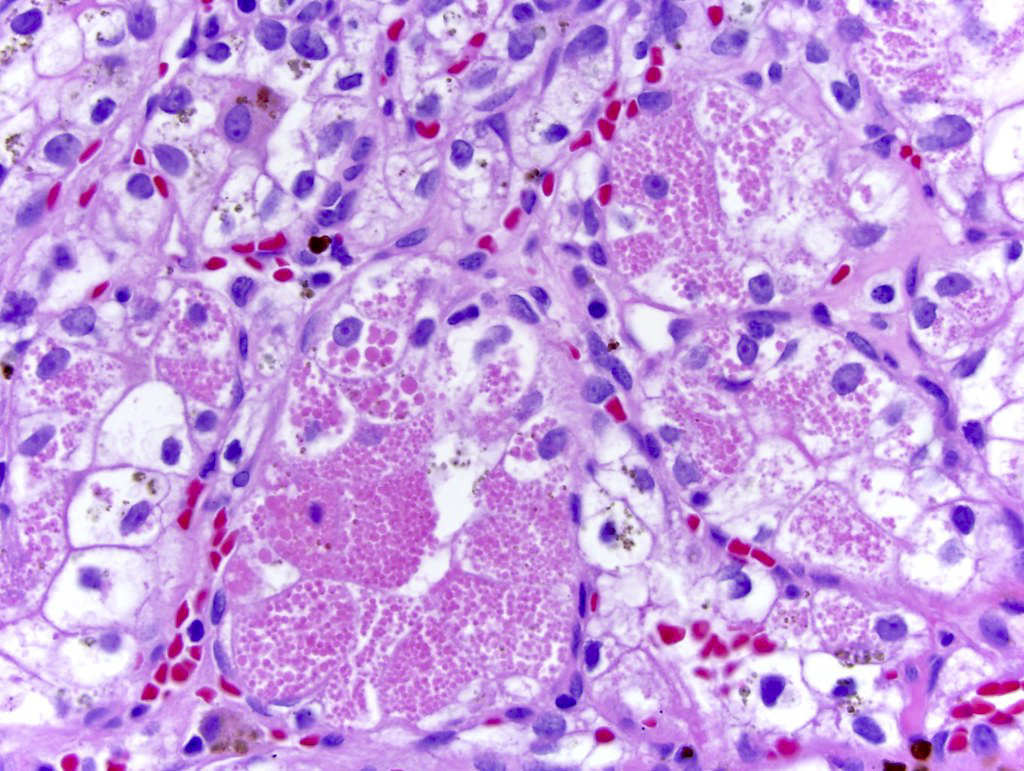
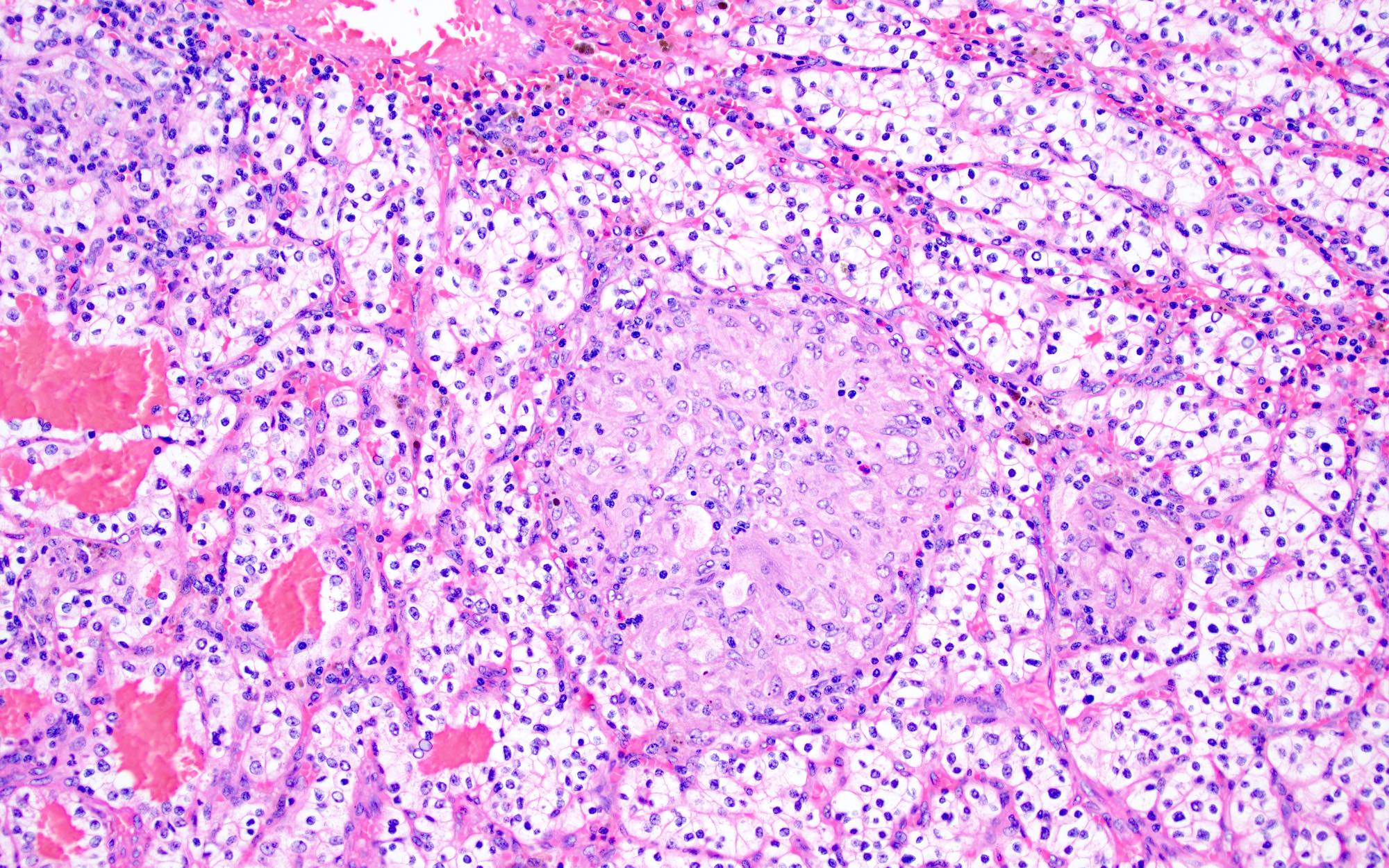
- Typically compact nests and sheets of cells with clear cytoplasm and distinct membrane
- Granular eosinophilic cytoplasm observed in high grade tumors or near areas of hemorrhage or necrosis
- Network of arborizing small, thin walled vessels (important diagnostic feature for cases with granular eosinophilic cytoplasm)
- Architectural patterns: solid, alveolar (nested), acinar (tubular), microcystic (containing extravasated red blood cells or eosinophilic fluid) and occasionally macrocystic
- Focal papillary architecture may be seen but prominent papillary formation raises the possibility of other subtypes (clear cell papillary renal cell tumor, TFE3 rearranged, TFEB altered, ELOC mutated RCC)
- Stroma: nondescript, no desmoplastic reaction (unlike collecting duct carcinoma or urothelial carcinoma) with little inflammatory response
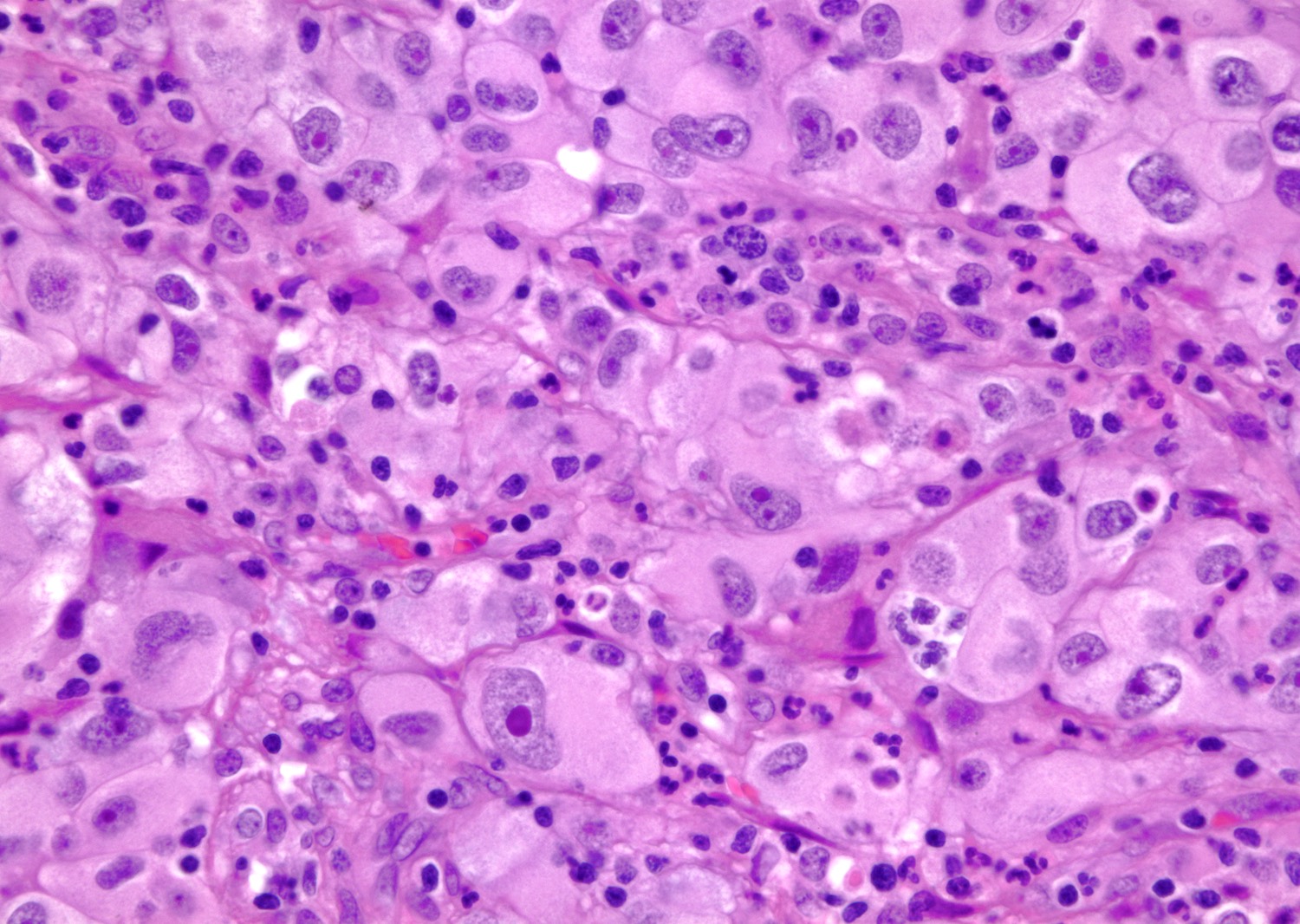
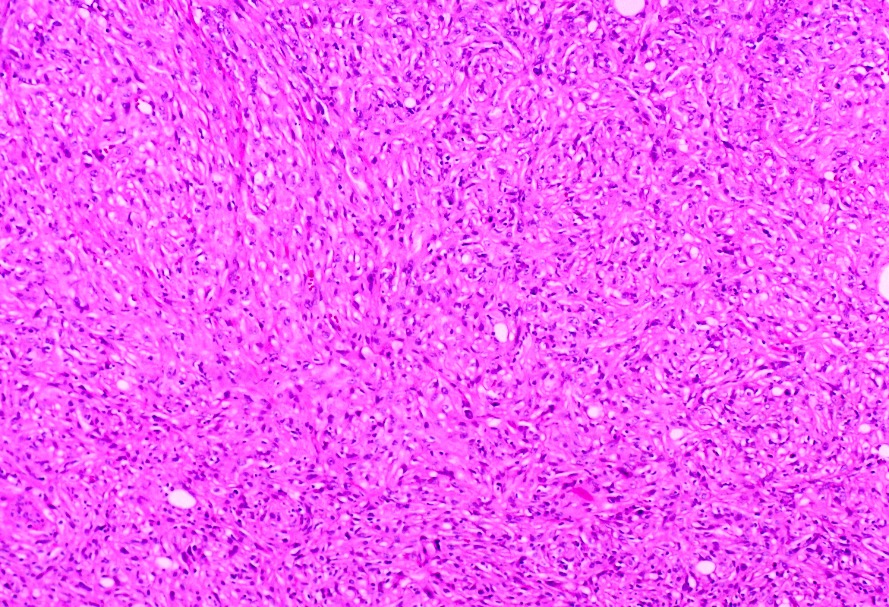
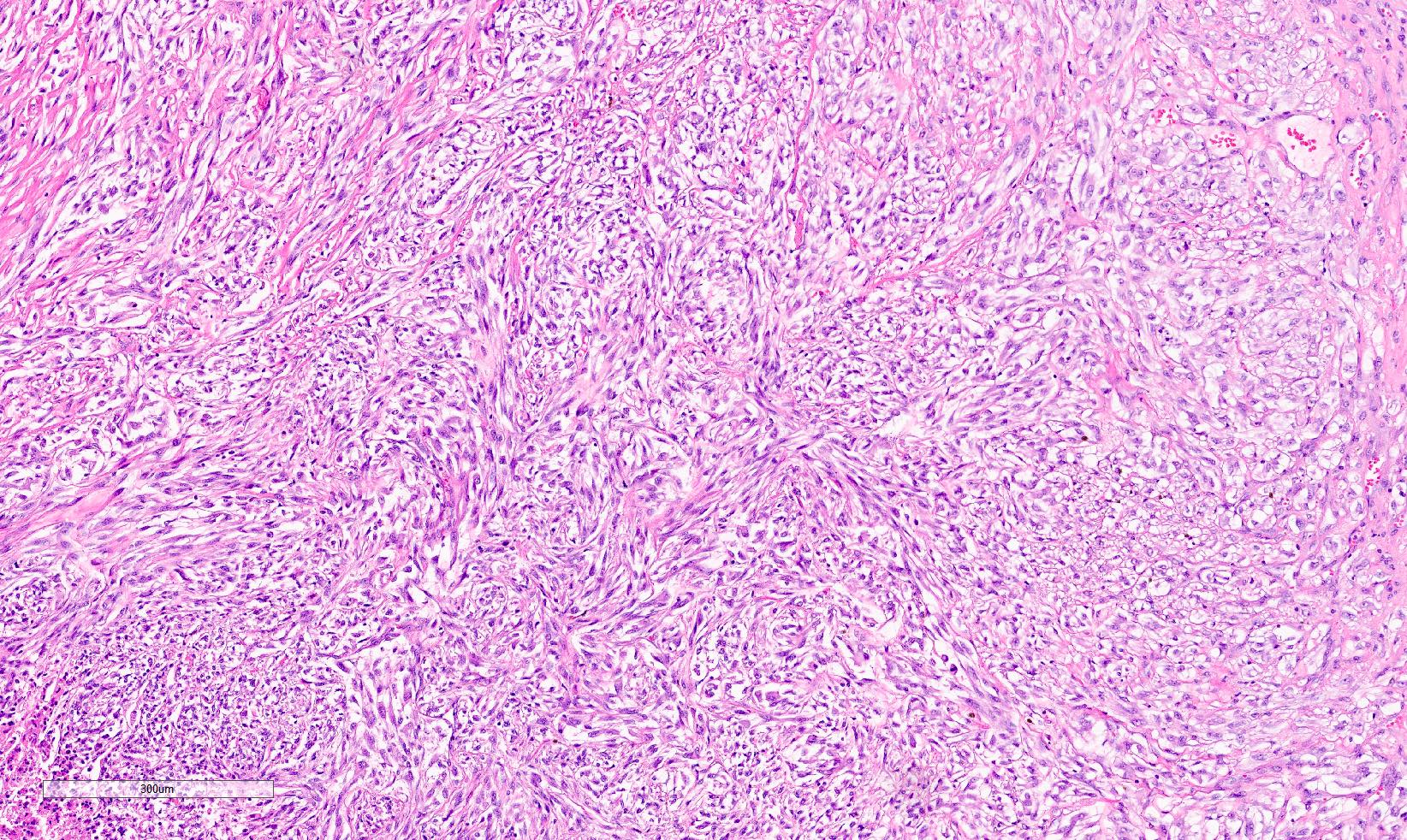
- High grade features
- Rhabdoid differentiation: large, high grade malignant cells with abundant homogeneous eosinophilic cytoplasm, eccentric nuclei and globular eosinophilic intracytoplasmic inclusions
- Sarcomatoid differentiation: may happen in any RCC subtype (Am J Surg Pathol 2004;28:435)
- Tumor necrosis
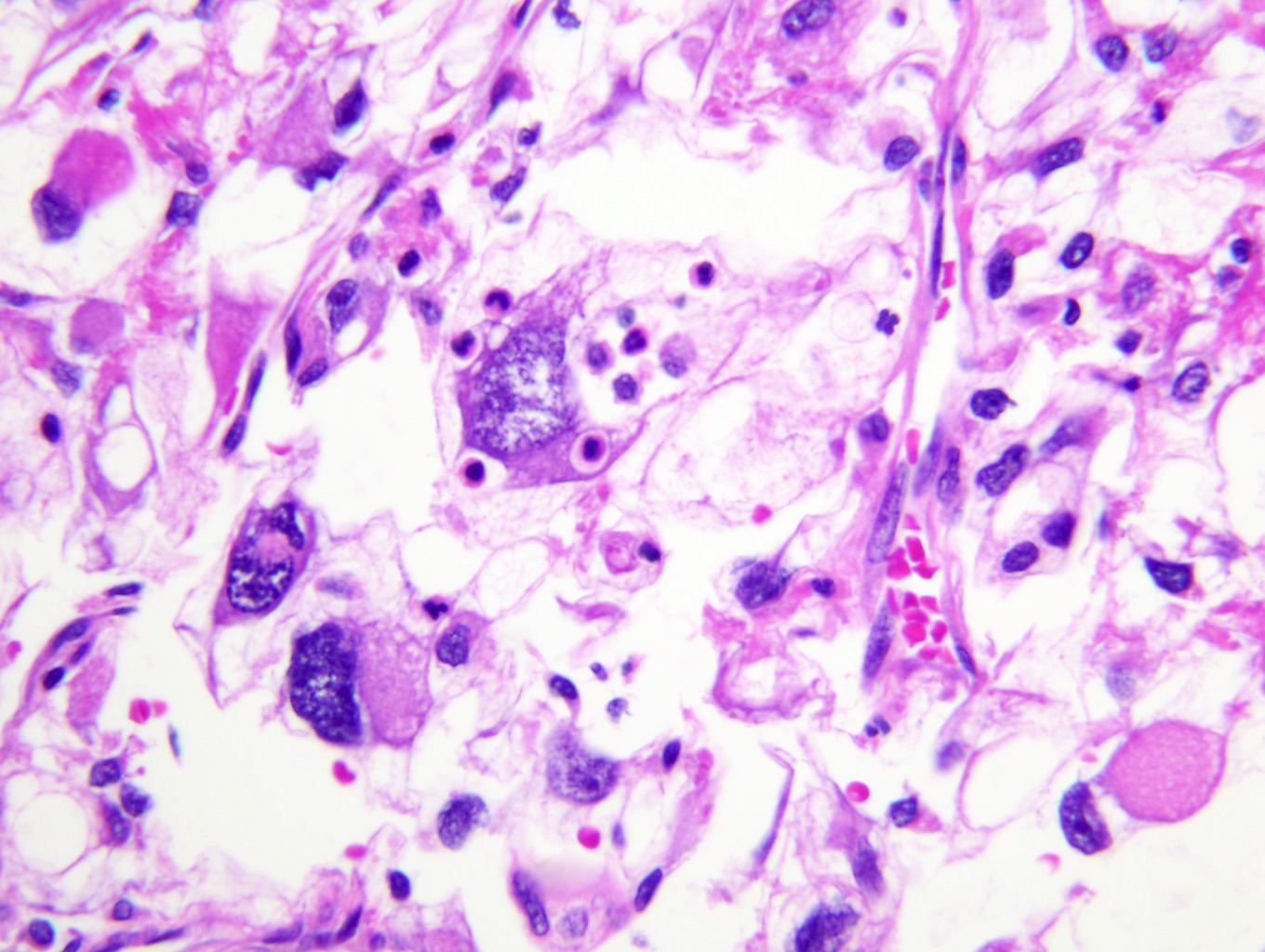
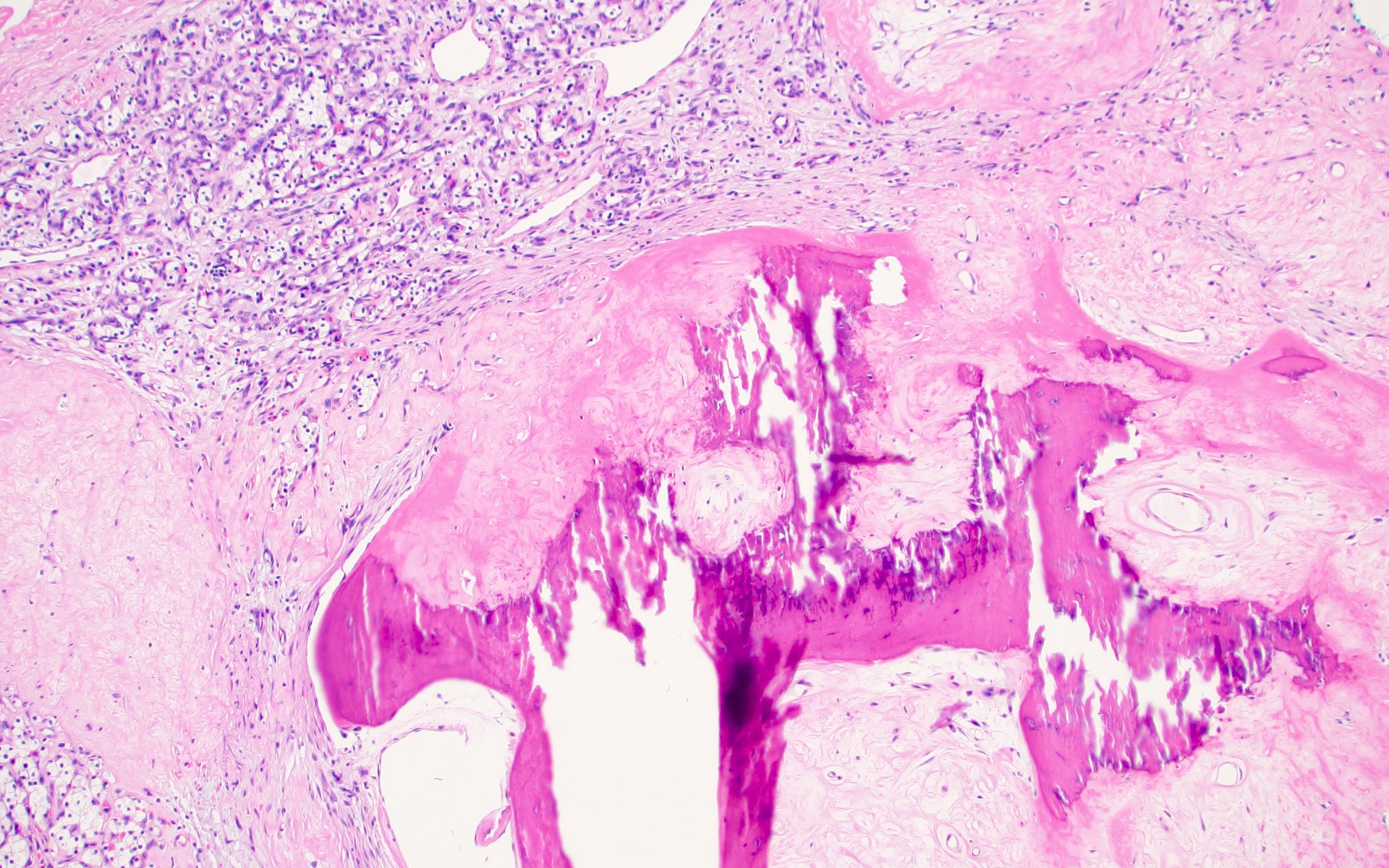
- Uncommon histologic variations (unknown prognostic significance): cystic, pseudopapillary, heterotopic bone formation, intracellular and extracellular hyaline globules, basophilic cytoplasmic inclusions, abundant multinucleated giant cells, sarcoid-like granulomas or myospherulosis features (Hum Pathol 2014;45:735, Am J Clin Pathol 2023;160:603, Histopathology 2022;80:922)
- BAP1 mutated ccRCC frequently shows papillary architecture, eosinophilic cytoplasm and cytoplasmic globules (Am J Clin Pathol 2021;155:718)
- Practically, the lower grade areas of tumor with classic ccRCC histology are most helpful for diagnosis
- Higher grade tumors can demonstrate overlapping features with other RCC types
Microscopic (histologic) images
Contributed by Gregory T. MacLennan, M.D., Behtash Nezami, M.D., Nicole K. Andeen, M.D. and Maria Tretiakova, M.D., Ph.D.
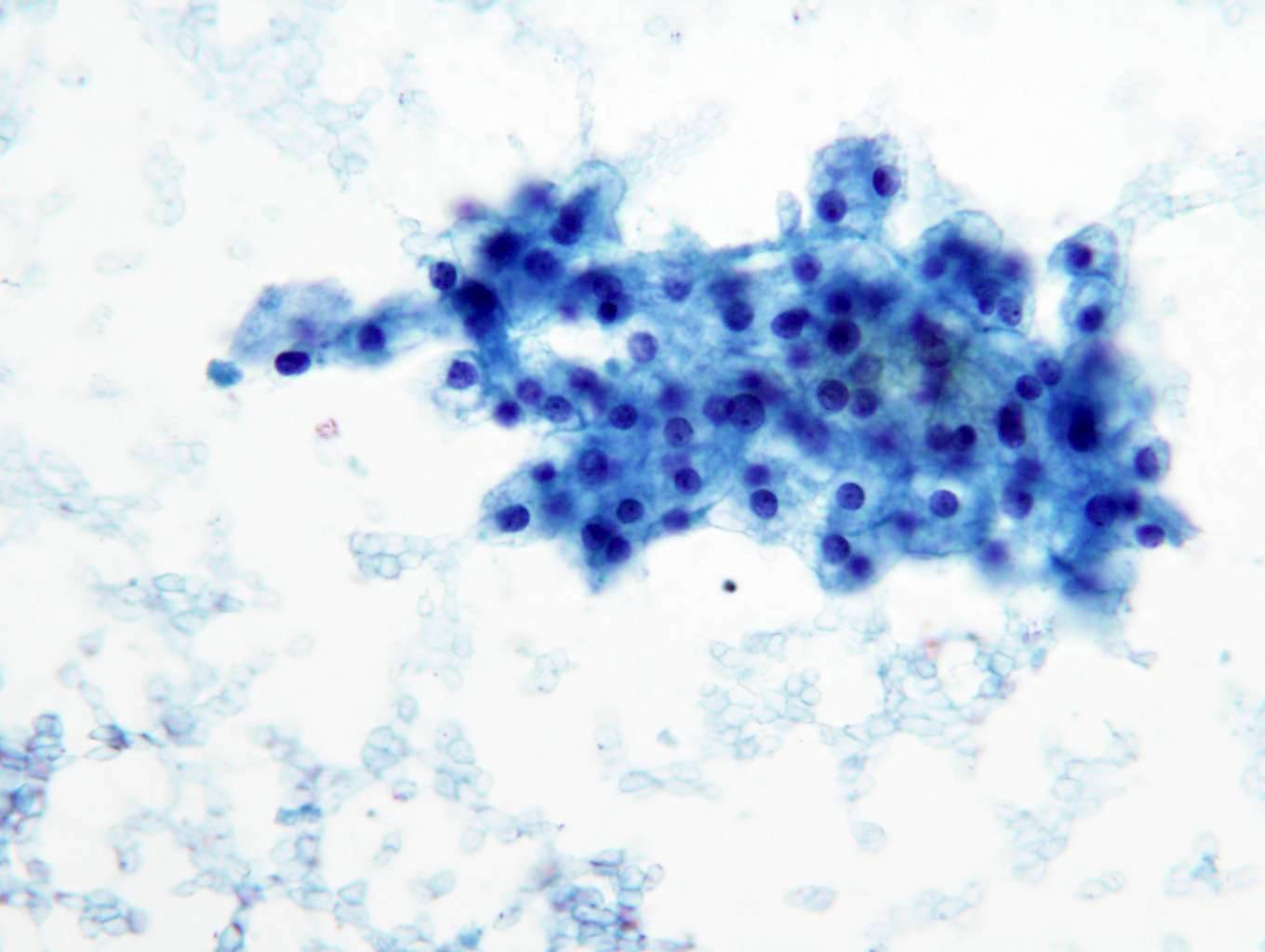
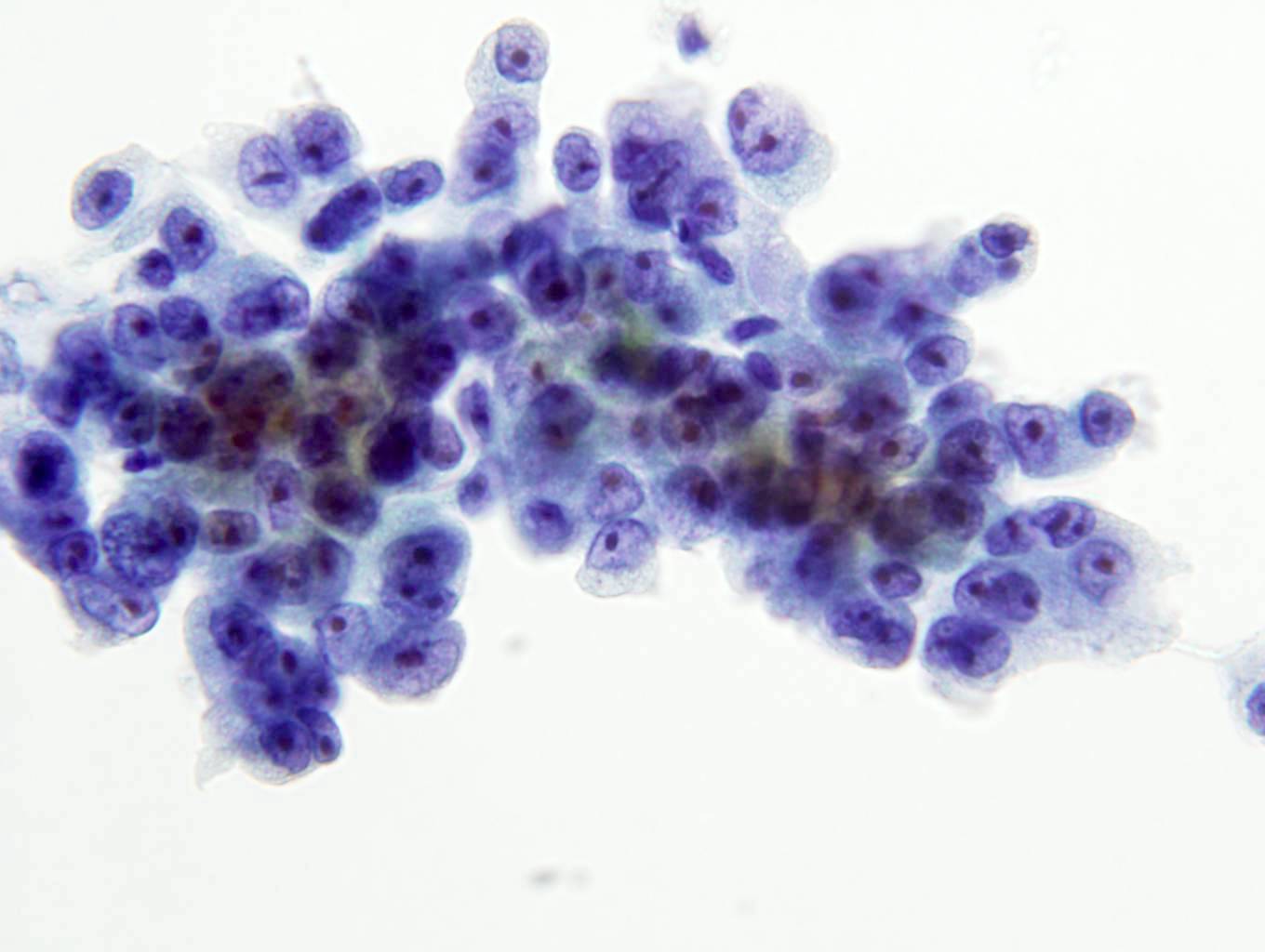
Cytology description
- Cohesive nests of fairly uniform cells with pale cytoplasm, mixed with stromal components and capillaries
- Numerous single cells
- Well defined cell membranes, round central or eccentric nuclei
- Prominent nucleoli in high grade
- Intranuclear vacuoles are common
- Pale, vacuolated or granular cytoplasm
- Background with blood and necrosis is frequent
Positive stains
- Positive and negative stains are less reliable in higher grade tumors
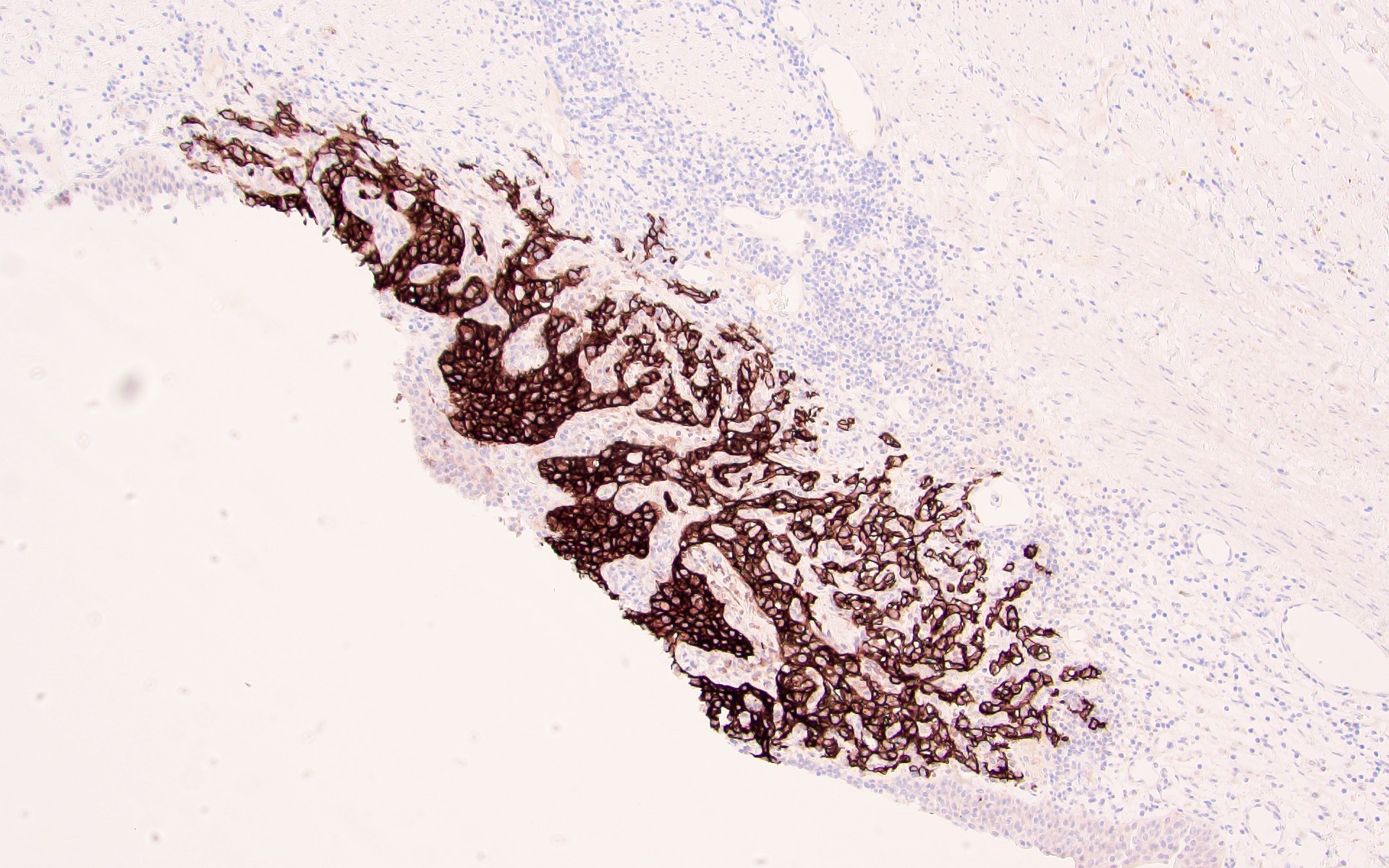
- PAX8: ~95%, nuclear; PAX2 similar but less sensitive (Mod Pathol 2009;22:1218, Am J Surg Pathol 2014;38:e35)
- CAIX: surrogate marker for VHL alterations, 75 - 100%, diffuse membranous (box-like), fairly specific even in tumors with sarcomatoid features (Am J Surg Pathol 2014;38:e35)
- CD10 (82 - 94%, membranous) and RCC (72 - 84%, cytoplasmic and membranous): proximal tubular brush border antigens; also present in normal cells
- Epithelial markers: AE1 / AE3, CAM 5.2, EMA / MUC1 (more sensitive than AE1 / AE3)
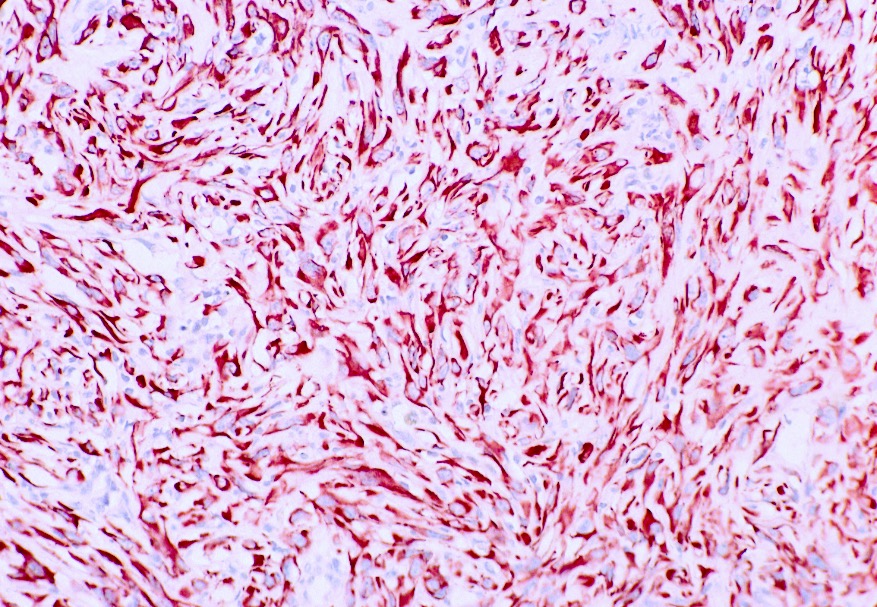
- Vimentin (cytoplasm and membranous)
Negative stains
- CK7: positive in up to 15% of cases; may be focally positive or patchy in high grade areas, cystic components or individual cells in the myxoid and degenerative tumor areas
- 34 beta E12
- CK20 (Am J Surg Pathol 2014;38:e35)
- AMACR: may be focally positive in 25 - 50% of cases (Am J Surg Pathol 2004;28:69, Cancers (Basel) 2020;12:602, Am J Surg Pathol 2015;39:1502)
- CD117
- Cathepsin K
- TFE3
- HMB45
- MelanA
- Inhibin
- BAP1 loss correlates well with BAP1 gene mutation (Nat Genet 2012;44:751)
| PAX8 | CD10 | CAIX | RCC | Melanocytic markers | Vimentin | CK7 | HMWCK | CD117 / KIT | AMACR | GATA3 | |
| Clear cell RCC | + | + | + (box-like) | + | - | + | - | - | - | -/+ | - |
| Papillary RCC | + | + | +/- | + | - | + | + | - | - | + | - |
| Clear cell papillary RCT | + | - | + (cup-like) | +/- | - | + | + | +/- | - | - | +/- |
| Chromophobe RCC | + | -/+ | - | +/- | - | - | + | - | + | - | +/- |
| Epithelioid angiomyolipoma | - | - | -/+ | - | + | -/+ | - | - | - | - | - |
| Tubulocystic carcinoma | + | + | +/- | + | - | +/- | -/+ | - | - | +/- | - |
| TFE3 rearranged / TFEB altered RCC | + | +/- | -/+ | +/- | TFEB: + TFE3: -/+ | + | - | - | - | +/- | - |
| MTSCC | + | -/+ | -/+ | +/- | - | + | + | -/+ | - | +/- | - |
| FH deficient RCC (FH loss and 2SC+) | + | - | - | - | + | - | - | - | - | -/+ | |
| TSC / mTOR / ELOC | + | + | + | - | -/+ | +/- | - | - | + | ||
Legend
| |||||||||||
Electron microscopy description
- Variable cytoplasmic lipid droplets, scant organelles, microvesicles and glycogen
- Evidence of tubular differentiation: well defined long microvilli typical of the brush border seen in normal proximal tubules
- Numerous cell junctions
- Eosinophilic granular cytoplasm due to increased number of large pleomorphic mitochondria
- References: Ultrastruct Pathol 1987;11:483, Am J Surg Pathol 2000;24:1247
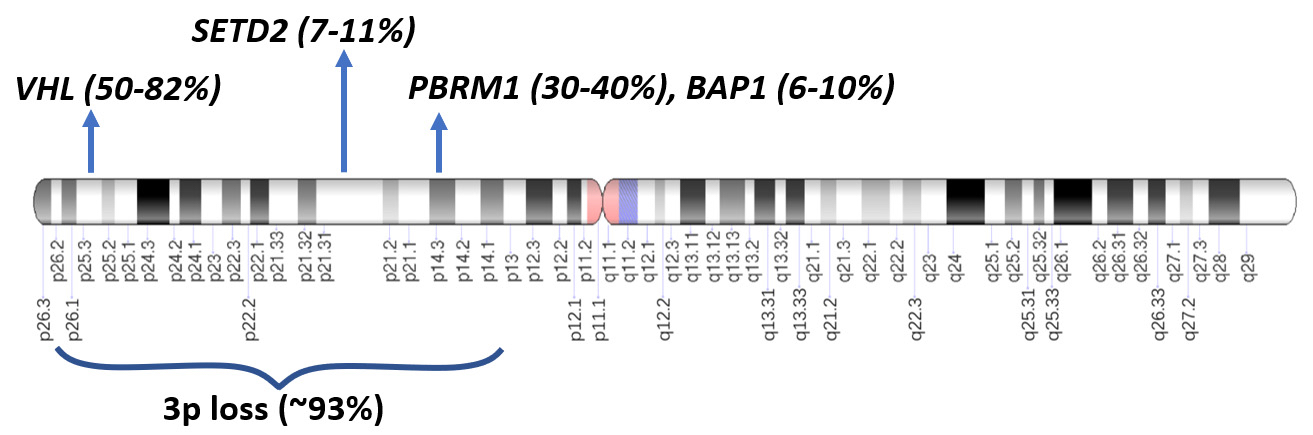
Molecular / cytogenetics description
- VHL gene alteration in the majority of somatic cases
- 3p locus also contains other tumor suppressor chromatin remodeling genes frequently associated with ccRCC: PBRM1 (in 30 - 40% of cases), BAP1 (6 - 10%) and SETD2 (7 - 11%) (Nature 2013;499:43, Clin Cancer Res 2013;19:3259, Nat Genet 2013;45:849, Nat Genet 2013;45:860)
- Other driver alterations: mTOR pathway genes (TSC1, TSC2, MTOR, PIK3CA and PTEN), TP53, loss of 4p, 9p or 14q, mostly occurring in association with VHL alterations as subclonal drivers
- Progression of ccRCC leads to a polyploid karyotype and further gains or losses of genetic material, contributing to disease progression and aggressiveness
- Von Hippel-Lindau familial cancer syndrome
- High risk of developing renal tumors
- Patients born with a germline defect in 1 of the 2 alleles of the VHL gene
- Loss of the second functioning allele (a second hit) leads to the development of clinical disease and tumor formation
- Autosomal dominant inheritance pattern
- Tends to manifest at an early age with a lifetime risk of developing renal tumors of ~70%
- Bilateral multifocal ccRCC in 35 - 45% of patients
- As many as 1,100 cysts and 600 microscopic ccRCC foci have been reported (J Urol 2003;170:2163)
- Other familial syndromes (see Etiology)
Sample pathology report
- Kidney, core needle biopsy:
- Clear cell renal cell carcinoma (see comment)
- Comment: Tumor cells show positive immunostaining for PAX8, CAIX and keratin CAM 5.2. CK7 is negative arguing against clear cell papillary renal cell tumor.
- Kidney, radical nephrectomy:
- Clear cell renal cell carcinoma, WHO / ISUP grade 3, measuring 2.5 cm in greatest dimension (see comment)
- Tumor is confined within the renal capsule.
- All surgical margins are negative for carcinoma.
- See cancer summary report.
- Comment: Immunohistochemical stains are positive for PAX8, CAIX (box-like pattern) and CD10, supportive of this diagnosis.
Differential diagnosis
- Papillary RCC with cytoplasmic clearing:
- Papillary architecture, lacks prominent delicate chicken wire vasculature
- Positive for CK7 and AMACR; negative for CAIX (opposite staining pattern to clear cell RCC) (Am J Surg Pathol 2008;32:1780)
- Clear cell papillary renal cell tumor (CCPRCT):
- Low grade tumor cells with clear cytoplasm (usually G1)
- Well circumscribed, with variable papillary, tubular, acinar and cystic architecture
- Characteristically polarized luminal nuclear arrangement (piano key) away from the basement membrane and cup-like CAIX positivity, rather than diffuse box-like membranous pattern in clear cell renal cell carcinoma
- Lacks high grade areas, tumor necrosis and local invasion
- Lack of chicken wire vasculature and presence of closed nests argue against CCPRCT
- Diffusely positive for CK7 and negative for CD10 (opposite to clear cell RCC)
- Most tumors present as pT1 stage with excellent clinical outcome
- GATA3 positive CCPRCT (Hum Pathol 2017;66:152)
- AMACR (opposite to PRCC) is usually negative
- Fumarate hydratase deficient renal cell carcinoma:
- High grade and aggressive tumor, seen in younger age than ccRCC
- Characteristic inclusion-like nucleoli with perinucleolar clearing
- Positive 2SC IHC (both nuclear and cytoplasmic) and loss of FH expression
- Associated with leiomyomas of skin and uterus in germline cases (HLRCC syndrome)
- Negative for CAIX
- Multilocular cystic renal neoplasm of low malignant potential
(MCRNLMP):
- Clear cells lining the thin fibrous septa of multiple cystic spaces or within the fibrous septa
- Uncommon, usually lower grade associated with an excellent prognosis
- Absence of expansile solid nodules
- Similar IHC staining pattern to clear cell RCC but more commonly CK7 positive and less often CD10 positive (Am J Surg Pathol 2012;36:1425)
- Epithelioid angiomyolipoma (AML):
- Characteristic abnormal AML vessels, smooth muscle and blood pools
- Lacks characteristic nests seen in ccRCC
- May resemble high grade ccRCC
- Look for areas of typical AML with fat and dysmorphic vessels
- Positive for HMB45, cathepsin K
- Negative for PAX8, CAIX, EMA and cytokeratins (Am J Surg Pathol 2001;25:65)
- TFE3 rearranged and TFEB altered renal cell carcinomas:
- TFE3 rearranged (chr Xp11) or TFEB altered (chr 6p21, rearranged or amplified)
- TFE3 and TFEB rearranged RCCs seen in a younger age range (median: 31 years)
- TFEB amplified RCCs occur in older patients (median: 64 years) (Am J Surg Pathol 2002;26:1553, Clin Cancer Res 2009;15:1170, Am J Surg Pathol 2014;38:604, Am J Surg Pathol 2016;40:1484)
- High grade nuclei, mixed clear and eosinophilic (not clear) cytoplasm
- Different architectural patterns: tubulopapillary or trabecular but lacks the classic closed nests of ccRCC
- Positive for TFE3 or TFEB, cathepsin K, CD10, AMACR and variable focal CAIX, EMA and cytokeratins (opposite to clear cell RCC)
- Clear cell RCCs with prominent fibromuscular septation and CK7 positivity, including ELOC mutated renal cell carcinoma:
- Generally exhibit indolent behavior
- Cytologically resemble ccRCC with clear cytology (mostly lower grade tumor, except for ELOC mutated RCC)
- Architecturally prominent fibromuscular stroma with thick fibromuscular bands
- Mutations in mTOR pathway or TSC1 / TSC2 genes; may be germline
- VHL alterations are not observed
- ELOC mutated RCC is mutually exclusive with VHL, PBRM1, TSC1, TSC2 or mTOR
- Positive for CK7 (strong), CD10 and CAIX complete membranous (box-like)
- Chromophobe RCC:
- Circumscribed mass with a homogeneous light tan cut surface
- Large cells with sharply defined hard cell border, finely reticulated cytoplasm, irregular nuclear border (raisinoid) and perinuclear halo
- Lacks chicken wire vasculature
- Positive for CK7, E-cadherin, CD117; negative for CAIX and vimentin (opposite staining pattern to clear cell RCC) (Am J Surg Pathol 2014;38:e35)
- Oncocytoma:
- Adrenal cortical carcinoma:
- Clear cell carcinoma of the ovary:
- Positive for keratin 34 beta E12 and CK7
- PAX8 negative at extrarenal sites but can be positive in kidney (Appl Immunohistochem Mol Morphol 2019;27:477)
- Clear cell carcinoma of the thyroid:
- Positive for thyroglobulin and TTF1
- PAX8 is positive in both
- Mesothelioma:
- Positive for calretinin, mesothelin and CK5/6
- Hemangioblastoma:
- Lack nuclear atypia, prominent nucleoli and necrosis
- PAX8 negative and inhibin positive (opposite to clear cell RCC)
- PAX8 negative at extrarenal sites but can be positive in kidney (Appl Immunohistochem Mol Morphol 2019;27:477)
Additional references
Practice question #1
Practice answer #1
E. Loss of short arm of chromosome 3. Clear cell RCC is associated with losses in short arm of chromosome 3. Answer A is incorrect because trisomy 7 and 17 and deletion of Y is associated with papillary renal cell carcinoma. Answer B is incorrect because germline mutation of c-MET is seen in hereditary papillary RCC. Answer C is incorrect because chromophobe RCC shows loss of 1 copy of chromosomes 1, 2, 6, 10, 13 and 17 in 85% of the tumors. Answer D is incorrect because loss of chromosomes 1 and Y is observed in oncocytoma.
Comment Here
Reference: Clear cell renal cell carcinoma
Comment Here
Reference: Clear cell renal cell carcinoma
Practice question #2
Which of the following statements regarding clear cell renal cell carcinoma is true?
- Associated with trisomies of 7 and 17
- Has a worse prognosis than papillary renal cell carcinoma
- Minimum of 10% of sarcomatoid tumor is required to make a diagnosis of sarcomatoid carcinoma
- PAX8 staining is specific for renal cell carcinomas
- Tends to be CK7 and CAIX positive
Practice answer #2
B. Has a worse prognosis than papillary renal cell carcinoma. ccRCC has a worse prognosis than papillary and chromophobe RCC. Answer A is incorrect because trisomy 7 and 17 along with loss of chromosome Y are features of papillary RCC. Answer C is incorrect because no minimum proportion of sarcomatoid tumor is required to make a diagnosis of sarcomatoid carcinoma. Answer D is incorrect because PAX8 is not specific to renal tissue and carcinomas and is also positive in thyroid tissue. Answer E is incorrect because clear cell RCC is generally CK7 negative, although weak focal staining can be seen in a small percentage.
Comment Here
Reference: Clear cell renal cell carcinoma
Comment Here
Reference: Clear cell renal cell carcinoma