Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Chatzopoulos K. Large cell neuroendocrine carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumorlargecellNE.html. Accessed April 27th, 2024.
Definition / general
- Aggressive, poorly differentiated carcinoma composed of large cells with neuroendocrine architecture, prominent nucleoli, high mitotic activity and necrosis
- Grouped with other neuroendocrine tumors in the 2021 WHO classification of thoracic tumors (J Thorac Oncol 2022;17:362)
- More likely to recur and has shorter patient survival than other types of non-small cell lung carcinoma, even in stage I disease (Cancer Control 2006;13:270)
- May coexist with other lung cancers such as adenocarcinoma and squamous cell carcinoma
Essential features
- High grade non-small cell carcinoma with neuroendocrine architecture and immunohistochemical markers, characterized by > 10 mitoses/2 mm2, prominent nucleoli and extensive necrosis (J Thorac Oncol 2022;17:362)
- Poor overall prognosis
- Should be distinguished from atypical carcinoid, basaloid squamous cell carcinoma and poorly differentiated adenocarcinoma, although diagnosis can be difficult on small biopsies or cytology specimens
Terminology
- Large cell neuroendocrine carcinoma (LCNEC): proposed by Travis et al. in 1991 as distinct from small cell carcinoma (Am J Surg Pathol 1991;15:529)
- Combined LCNEC: also has components of adenocarcinoma (most common), squamous cell carcinoma, giant cell carcinoma or spindle cell carcinoma (Thorac Surg Clin 2014;24:257)
ICD coding
Epidemiology
- Incidence varies between 0.3% and 3% of all lung tumors (Pathol Oncol Res 2022;28:1610730, J Clin Med 2022;11:1461)
- Older age and male sex predominance (Pathol Oncol Res 2022;28:1610730)
- Strong association with heavy smoking (Cancers (Basel) 2012;4:777)
Sites
- Thorax: lung, anterior mediastinum, thymus (Mod Pathol 2022;35:36, J Clin Med 2022;11:1461, Mol Clin Oncol 2021;14:34, BMJ Case Rep 2021;14:e240453, Int J Surg Case Rep 2019;60:53, J Med Case Rep 2017;11:155, Surg Case Rep 2018;4:133)
- Head and neck: parotid and submandibular gland, nasopharynx, hypopharynx, oral cavity, larynx (Auris Nasus Larynx 2005;32:89, Auris Nasus Larynx 2014;41:105, J Pediatr Hematol Oncol 2015;37:474, BMJ Case Rep 2015;2015:bcr2015211908, Int J Oral Maxillofac Surg 2022;51:1520, Head Neck Pathol 2010;4:198)
- Gastrointestinal tract: esophagus, esophagogastric junction, colon and rectum, ampulla (Int J Surg Case Rep 2019;60:291, Pathol Int 2019;69:481, Clin Colorectal Cancer 2020;19:61, Cureus 2022;14:e20949, Med Oncol 2010;27:1144)
- Pancreatobiliary: liver, extrahepatic biliary tract, gallbladder, pancreas (Int J Surg Pathol 2019;27:893, Surg Case Rep 2016;2:141, Jpn J Clin Oncol 2013;43:571, J Laparoendosc Adv Surg Tech A 2022;32:639)
- Genitourinary tract: kidney, urinary bladder, prostate (J Med Case Rep 2017;11:297, Cureus 2022;14:e20949, IJU Case Rep 2022;5:505)
- Gynecologic: ovary, uterus and uterine cervix (Gulf J Oncolog 2021;1:82, Eur J Gynaecol Oncol 2004;25:109, Case Rep Oncol 2018;11:665)
- Skin (J Cutan Pathol 2016;43:1067)
- Breast (Mod Pathol 2022;35:1494)
Pathophysiology
- Proposed model of histogenesis of lung neuroendocrine carcinomas, in view of similarities in the expression of primitive neural / neuroendocrine cell specific transcription factors (Pathol Int 2015;65:277)
Etiology
- Strong association with heavy smoking (Cancers (Basel) 2012;4:777)
- Unclear association with preneoplastic lesions, such as neuroendocrine cell hyperplasia and carcinoid tumorlets (Endocrine 2015;50:315)
Clinical features
- Symptoms vary according to anatomic localization
- Centrally located tumors present with cough, hemoptysis, bronchial obstruction or recurrent pneumonia (J Thorac Dis 2020;12:466)
- Peripherally located tumors can be asymptomatic and identified incidentally (Ann Thorac Surg 1998;65:1410)
- Paraneoplastic syndromes are uncommon but may include Cushing syndrome, carcinoid syndrome or Lambert-Eaton myasthenic syndrome (J Bronchology Interv Pulmonol 2015;22:267, Clin Pathol 2021;14:2632010X211051741)
Diagnosis
- Difficult to make specific diagnosis on small biopsy and cytology specimens
- Frequently recognized in cytology as non-small cell lung cancer (NSCLC), not otherwise specified or as adenocarcinoma
- Neuroendocrine features by light microscopy and confirmation by immunohistochemical staining for neuroendocrine markers
Laboratory
- Serum AFP levels can be elevated (Mol Clin Oncol 2021;14:34)
Radiology description
- Nonspecific findings, indistinguishable from other NSCLC
- CT findings: peripherally located tumors, expansively growing with irregular margins, with or without calcification and infrequent cavitation (Clin Imaging 2007;31:379)
Prognostic factors
- Poor prognosis with median overall survival 17.9 months and 3 year survival rate of 1.8% (Bull Cancer 2021;108:981)
- Worse prognosis than stage equivalent non-small cell lung carcinoma (Semin Thorac Cardiovasc Surg 2006;18:206)
- Adverse prognosticators (Bull Cancer 2021;108:981, Ann Thorac Surg 2015;99:983)
- Male sex
- History of smoking
- Obstructive atelectasis
- Advanced nodal stage
- Coexpression of chromogranin A and CD56
Case reports
- 38 year old pregnant woman with a 5 cm left lung lower lobe mass with brain and adrenal metastases (Front Oncol 2022;12:823813)
- 64 year old man with hemoptysis and a 3 cm left lung upper lobe mass (Ann Palliat Med 2020;9:3705)
- 69 year old man with progressive weakness and a 2.5 cm right lung lower lobe mass (Clin Pathol 2021;14:2632010X211051741)
- 76 year old woman with a 2.4 cm left lung upper lobe mass, multiple liver masses and carcinoid syndrome (Intern Med 2022 Oct 5 [Epub ahead of print])
Treatment
- Surgical resection in patients with stage I - IIIA disease with adjuvant chemotherapy (J Thorac Oncol 2019;14:2143, Br J Cancer 2021;125:1210)
- Chemotherapy in patients with advanced stage disease (Eur Respir J 2017;50:1701658)
- Immunotherapy is currently in clinical trials (Front Oncol 2021;11:655752)
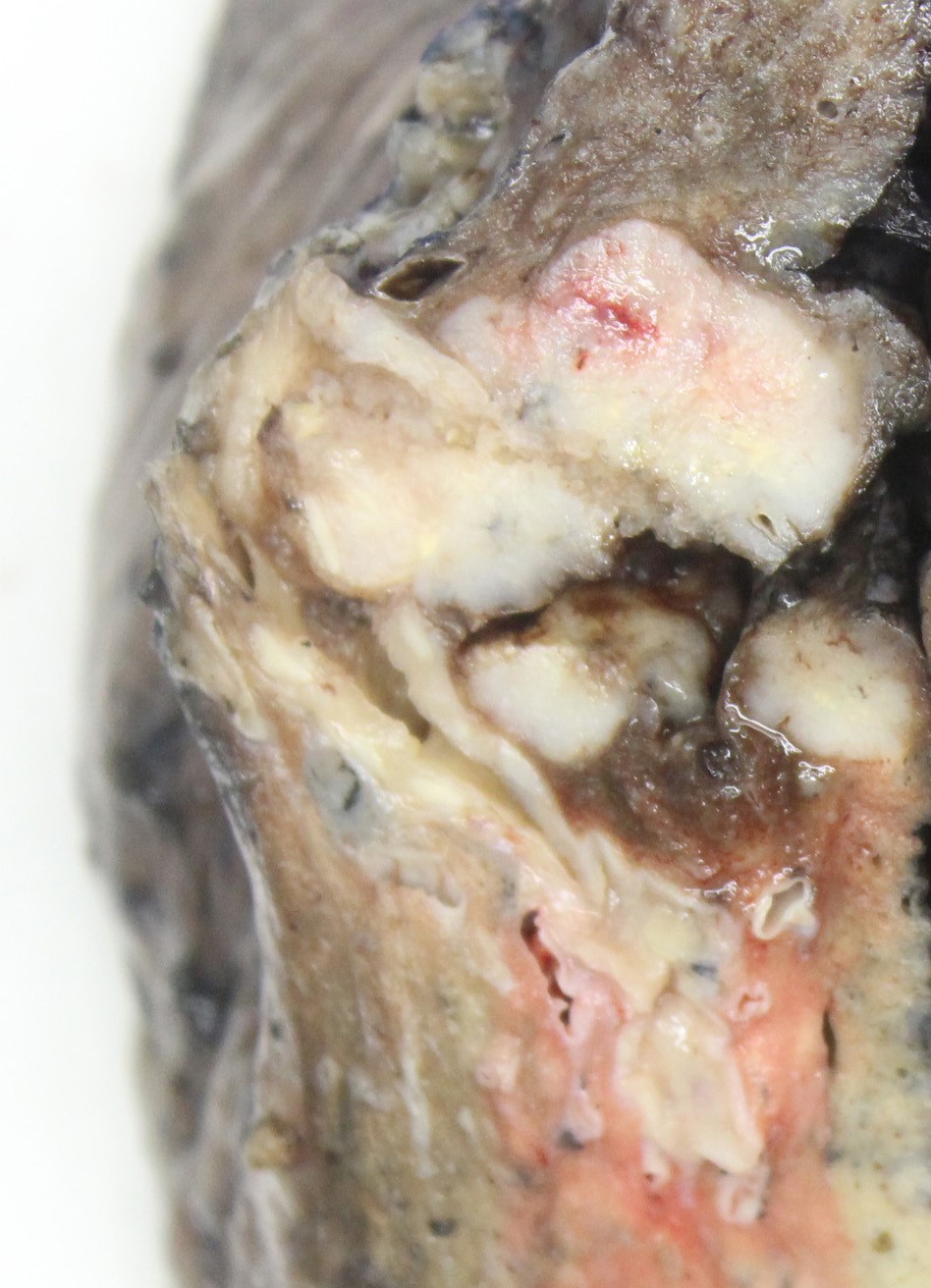
Gross description
- Gross appearance is indistinguishable from other lung malignancies
- Most tumors tumors are located peripherally and are usually nodular and circumscribed with tan-red, necrotic cut surface (Am J Clin Pathol 2004;122:884, AJR Am J Roentgenol 2004;182:87)
- Size range: 0.9 - 12 cm, with average size 3 - 4 cm (Cancer 2001;91:1992)
Frozen section description
- Neuroendocrine organoid architecture, prominent nucleoli, abundant necrosis
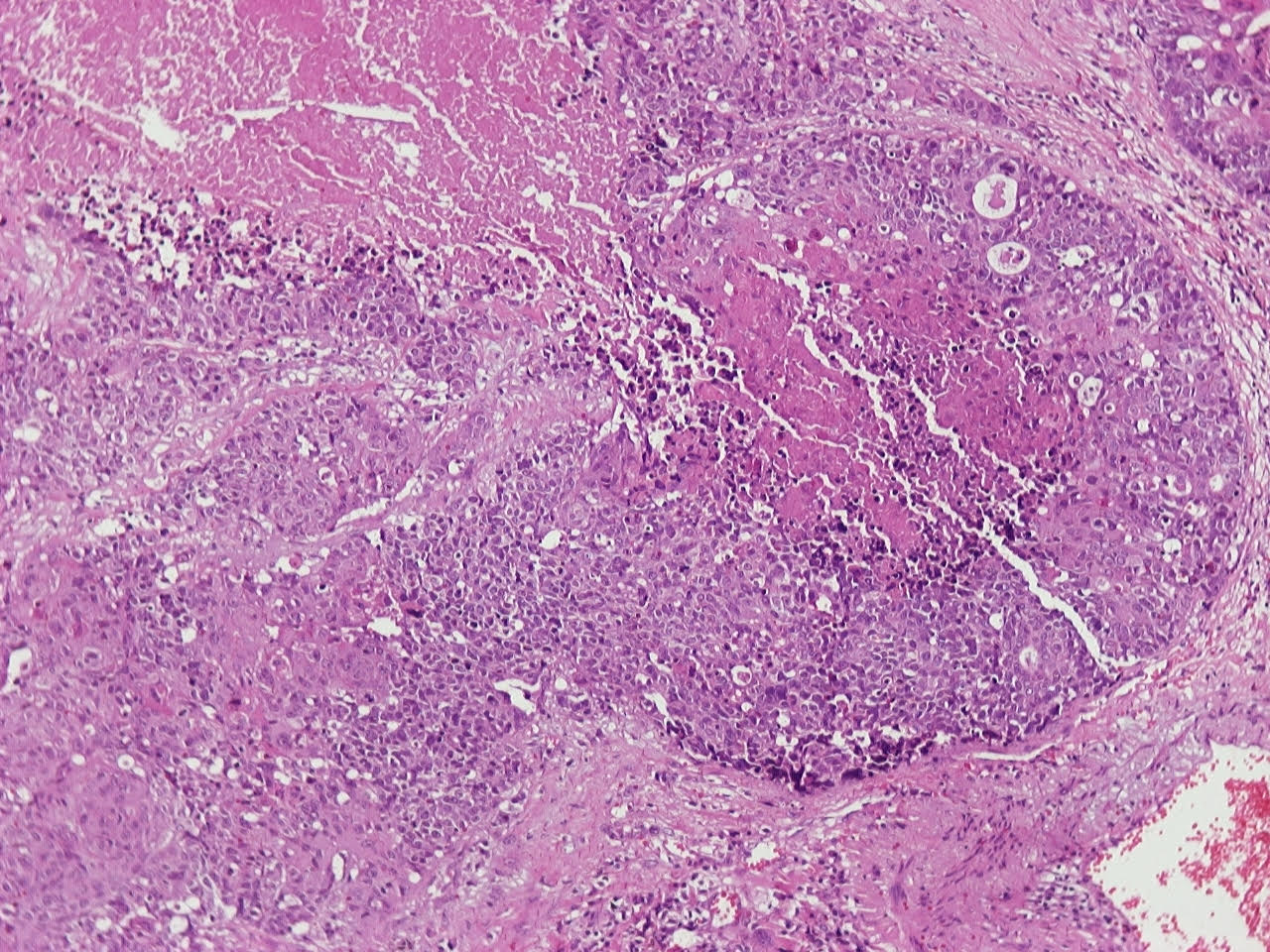
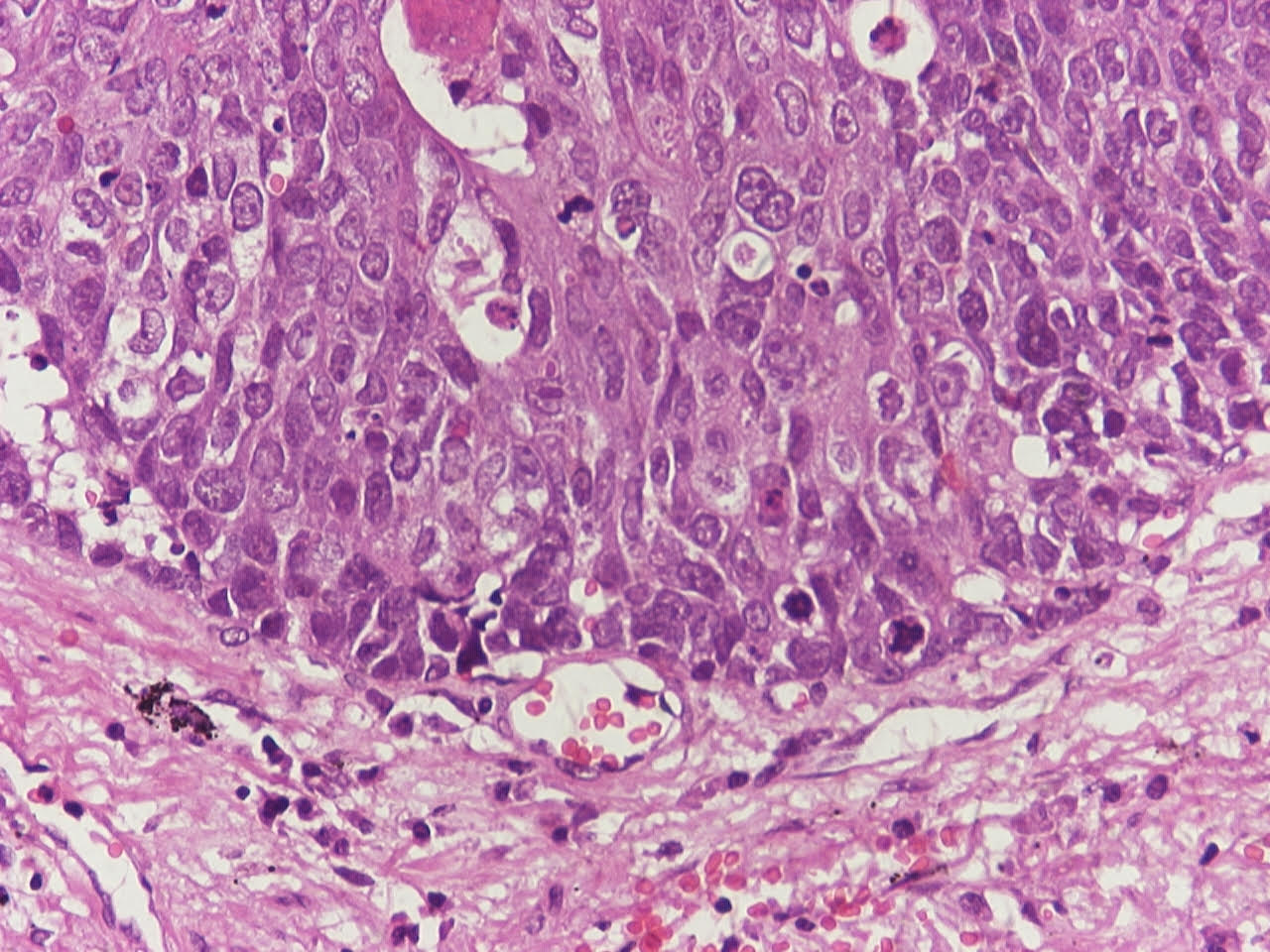
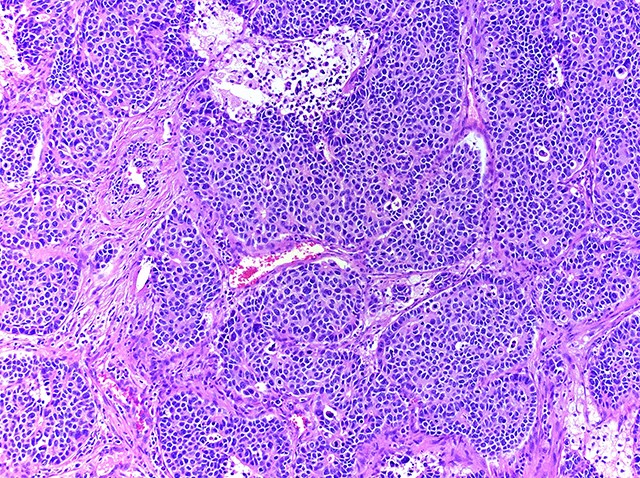
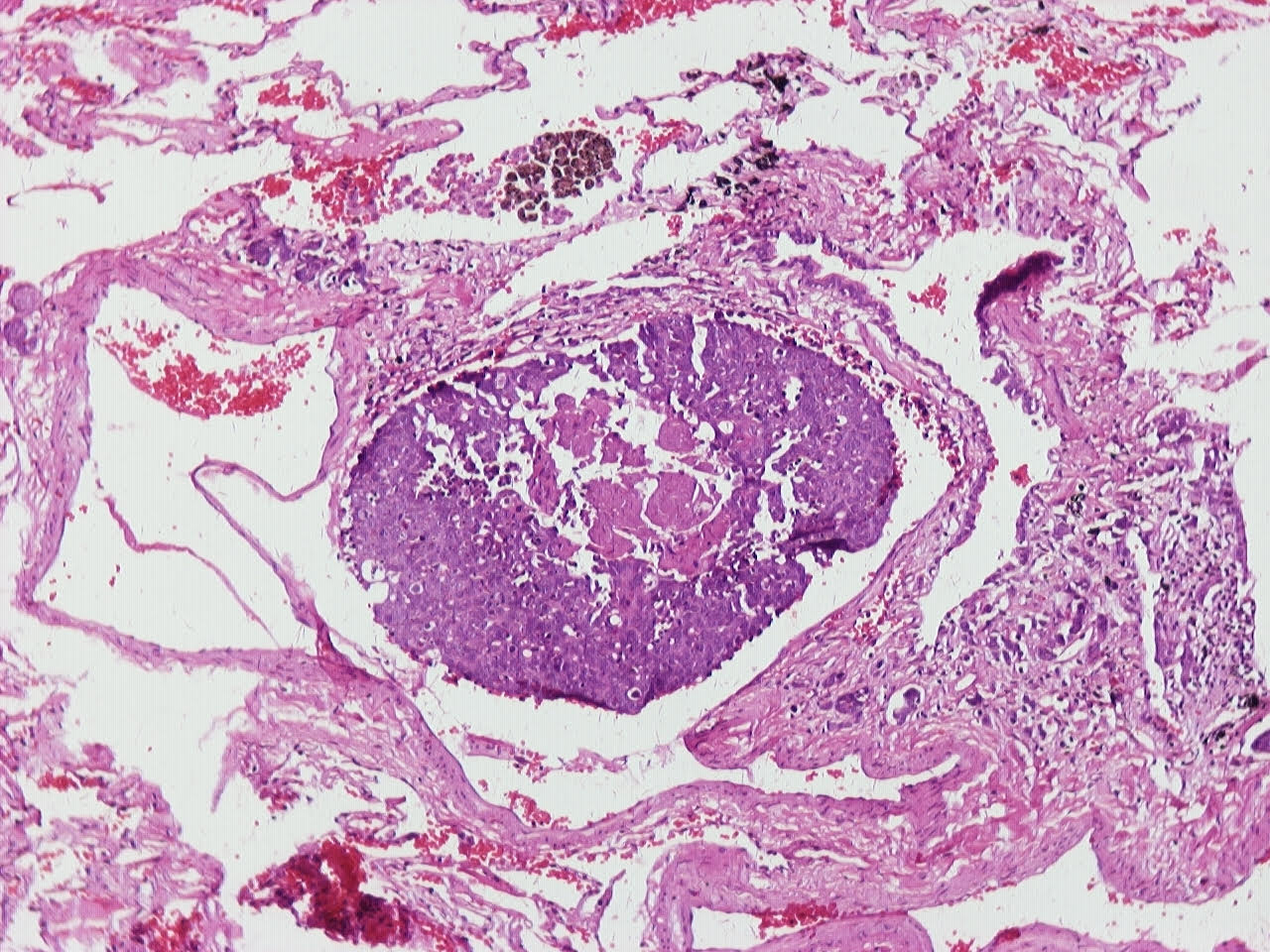
Microscopic (histologic) description
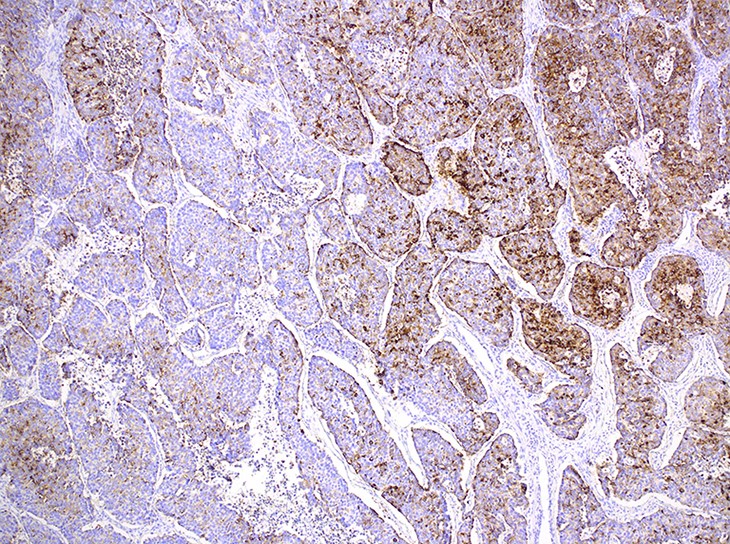
- Neuroendocrine organoid architecture may include nesting with peripheral palisading, anastomosing trabeculae and rosette-like structures (Mod Pathol 2022;35:36)
- > 10 mitoses/2 mm2, extensive / geographic necrosis
- Large cells (~3x size of small cell carcinoma) with abundant amphophilic cytoplasm, intercellular membranes, nuclear pleomorphism, variably coarse, granular or vesicular chromatin with prominent nucleoli (Transl Lung Cancer Res 2020;9:860, Mod Pathol 2022;35:36)
- Occasional rhabdoid features (Pathol Int 2019;69:481, J Cancer Res Ther 2015;11:657)
- Larger tumor cells than atypical carcinoid, high nuclear grade, increased mitotic activity and necrosis (Arch Pathol Lab Med 2010;134:1628)
Microscopic (histologic) images
Contributed by Ioanna Abba Nteka, M.D., Aggeliki Cheva, M.D., Ph.D., Antonia Loukousia, M.D., Roseann Wu, M.D., M.P.H. and Kyriakos Chatzopoulos, M.D., Ph.D.
Cytology description
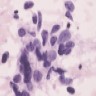
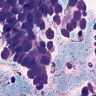
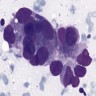
- Hypercellular, with numerous, single medium to large cells
- 3 dimensional and variably sized groups
- Large, pleomorphic cells with irregular vesicular chromatin, prominent nucleoli, abundant cytoplasm, sharp cellular borders
- Naked nuclei are abundant but with a variable subset of cells showing evident cytoplasm
- Molding, mitoses, necrotic background
- Peripheral nuclear palisading; rosette-like structures (Cancer 2008;114:180, Diagn Cytopathol 2001;24:58)
Cytology images
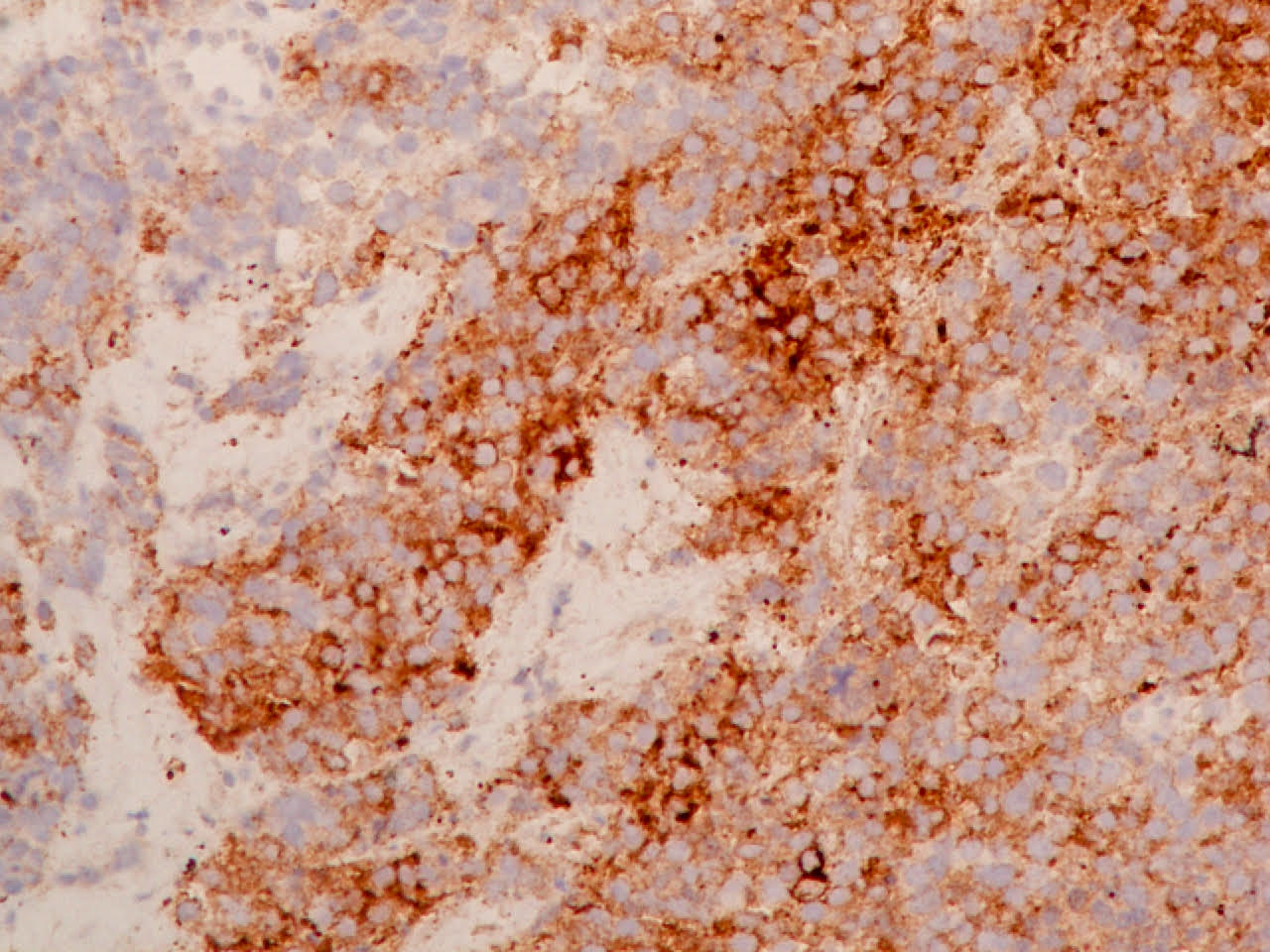
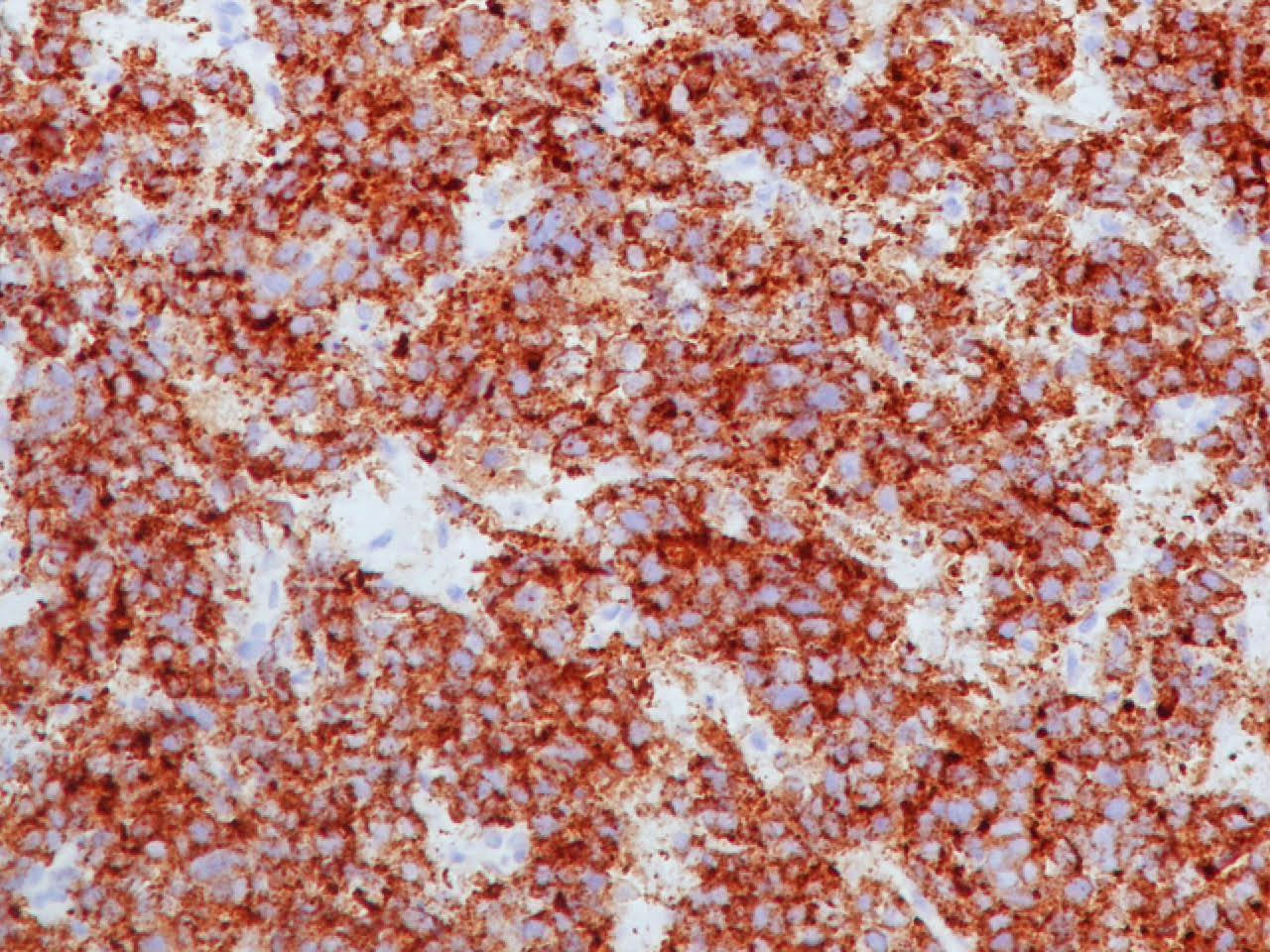
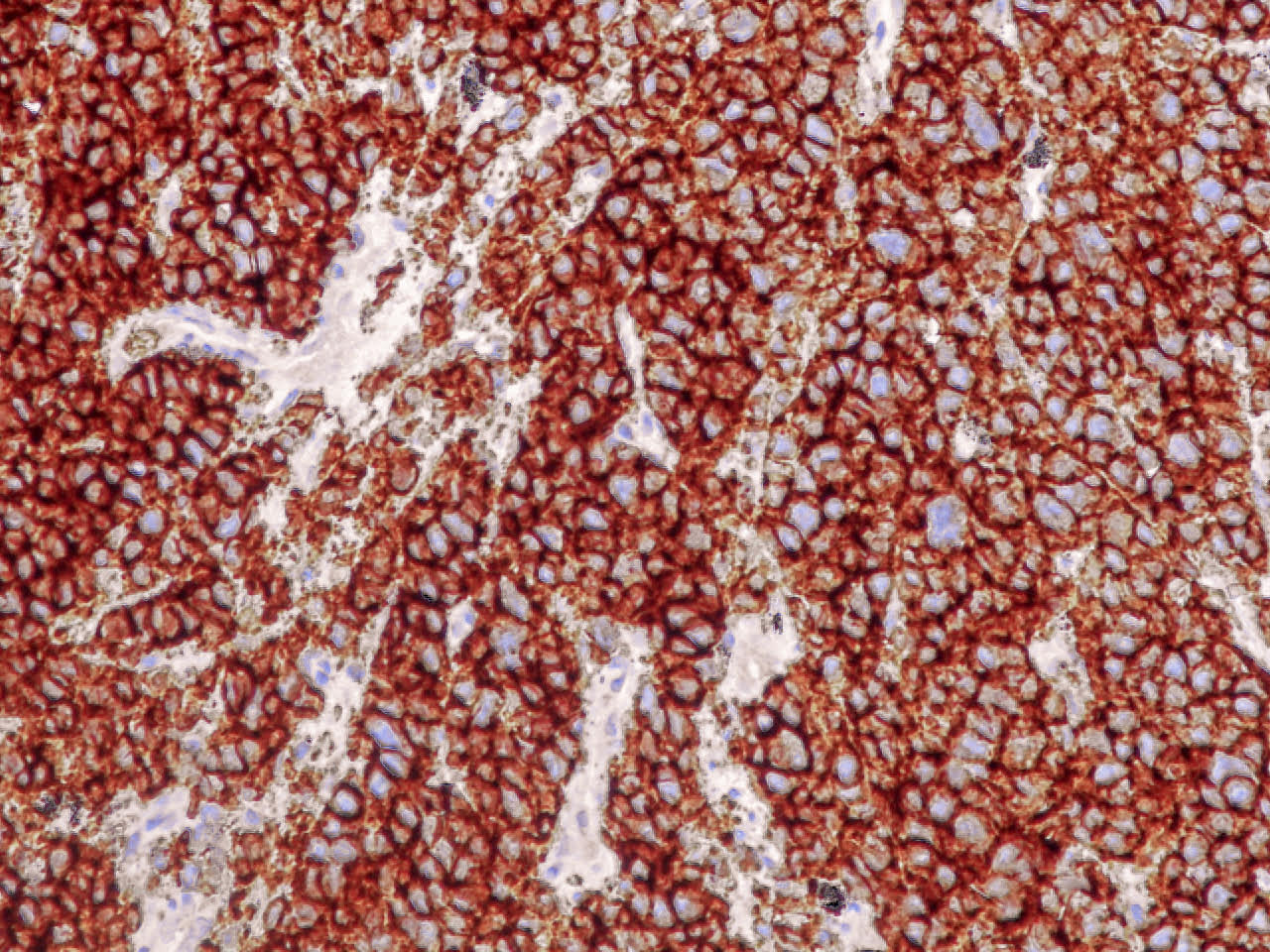
Positive stains
- Neuroendocrine markers, usually at least 2 focal to diffuse (chromogranin, synaptophysin, CD56), caution if CD56 is the only neuroendocrine marker expressed
- ISNM1: panneuroendocrine marker; nuclear positivity in 91.3% of large cell neuroendocrine carcinomas (Am J Surg Pathol 2017;41:1561)
- ASCL1: nuclear expression in 72.7%; neurogenic / neuroendocrine transcription factor (Hum Pathol 2016;48:142)
- Cytokeratins AE1 / AE3, CAM5.2
- Variable CK7 and 34 beta E12 (Hum Pathol 2000;31:980)
- pRb and cyclin D1 (Histopathology 2022;81:205)
- High Ki67 (40 - 80%) helpful to differentiate from carcinoid tumors, especially in small biopsies (Semin Diagn Pathol 2015;32:469)
- NKX6.1 in 80% (Diagn Cytopathol 2018;46:1010)
- CD117 in 77% (Virchows Arch 2004;445:449)
- GLUT1 in 74% (Mod Pathol 2009;22:633)
- SATB2 in 70% (Int J Surg Pathol 2022;30:151)
- TTF1 in 50% (Mod Pathol 2018;31:111)\
Negative stains
- p63 and p40 (Am J Clin Pathol 2014;142:320)
- Napsin A (can be focally positive in 15%) (Mod Pathol 2018;31:111)
- Loss of YAP1 in 60% (Cancer Sci 2016;107:1527)
- PDL1 in 10% (Lung Cancer 2017;108:115)
Electron microscopy description
- Neurosecretory granules, occasional evidence of granular differentiation and intercellular junctions suggestive of squamous differentiation (Am J Surg Pathol 1991;15:529)
Molecular / cytogenetics description
- 3 molecular subsets identified by next generation sequencing; no statistically significant differences in clinicopathologic parameters or survival among subsets:
- Small cell carcinoma-like: TP53 and RB1 comutation and MYCL amplification
- Non-small cell carcinoma-like: STK11, KRAS, KEAP1 mutations, no TP53 / RB1 comutation, molecular profile resembling adenocarcinoma, with additional NOTCH family mutations
- Carcinoid-like: MEN1 mutations, low mutation burden (Clin Cancer Res 2016;22:3618)
- Other less commonly mutated genes: PIK3CA, PTEN, AKT2, FGFR1, KIT, ERBB2, HRAS, EGFR, NTRK2 and NTRK3 (Clin Cancer Res 2017;23:757, Hum Mutat 2008;29:609)
- Case reports of tumors harboring an EML4::ALK fusion (Cold Spring Harb Mol Case Stud 2022;8:a00623, Front Oncol 2022;12:823813)
- BRAF alterations including mutations, amplifications reported in small case series (JCO Precis Oncol 2018;2:1)
Sample pathology report
- Lung, left upper lobe, CT guided needle core biopsy:
- Large cell neuroendocrine carcinoma (see comment)
- Comment: Multiple sections from the biopsy material reveal an infiltrative malignant tumor consisting of large cells with abundant amphophilic cytoplasm and large, ovoid or round nuclei with prominent nucleoli and increased mitotic activity. Tumor cells form solid nests with peripheral palisading and central necrosis. Immunostains performed on paraffin sections show diffuse and strong expression of keratins AE1 / AE3, synaptophysin, chromogranin, CD56 and nuclear expression of TTF1 and ISNM1. No expression of p40 or napsin A is seen. These findings support the diagnosis.
Differential diagnosis
- Small cell carcinoma:
- Sheet-like growth, nuclear molding, round oval or spindle cells, crushing artifact, Azzopardi effect, scant cytoplasm, finely granular chromatin, no nucleoli, extensive necrosis, high mitotic count (Am J Surg Pathol 2002;26:1184, Hum Pathol 1998;29:272)
- Atypical carcinoid:
- Lack of prominent nucleoli, monotonous cells with nesting and salt and pepper chromatin distribution, mitotic count between 2 - 10/2 mm2 (Transl Lung Cancer Res 2020;9:860)
- Basaloid squamous cell carcinoma:
- Immunopositivity for p40 and CK5/6, lack of neuroendocrine granules in electron microscopy (Transl Lung Cancer Res 2017;6:530)
- Adenocarcinoma, solid pattern:
- Absence of neuroendocrine markers by immunohistochemistry, lack of peripheral palisading (Transl Lung Cancer Res 2017;6:530)
- NUT carcinoma:
- Can occasionally be positive for synaptophysin (pitfall), presence of abrupt keratinization, NUT immunopositivity (Virchows Arch 2021;478:21)
- SMARCA4 deficient undifferentiated tumor:
- Discohesive cells with occasional rhabdoid features in an otherwise diffuse and monotonous cellular population, BRG1 loss by immunohistochemistry (Virchows Arch 2021;478:21)
Board review style question #1
A 76 year old man with a history of heavy smoking is diagnosed with a large pulmonary mass and undergoes lobectomy. The histologic features of the mass can be seen in the photograph above. Which initial panel of immunostains is more likely to be helpful in the differential diagnosis of this pulmonary malignancy from its most frequent counterparts?
- BRG1, INI1 and NUT
- Chromogranin, synaptophysin and cytokeratin 8/18
- ISNM1, p40 and napsin A
- p63, TTF1 and CD56
Board review style answer #1
C. ISNM1, p40 and napsin A. The differential diagnosis of this malignancy includes large cell neuroendocrine carcinoma, poorly differentiated adenocarcinoma and squamous cell carcinoma. In any case, additional immunostains will be needed to confirm the diagnosis.
Answer D is incorrect because although CD56 can be expressed in LCNEC, it should be used in caution when alone, as it is not as specific as other neuroendocrine markers. TTF1 can be expressed in half of LCNECs. Although p63 is a marker of squamous differentiation, it is not as specific as its p40 isoform. Answer B is incorrect because although LCNEC is likely to express all these 3 markers, the panel will not help rule out poorly differentiated adenocarcinoma. Answer A is incorrect because BRG1 expression is lost in undifferentiated SMARCA4 deficient thoracic tumors. INI1 can be partially lost in poorly differentiated non-small cell carcinomas with rhabdoid features or totally lost in SMARCB1 deficient carcinomas. NUT immunopositivity would raise the possibility of NUT carcinoma. None of these markers will be helpful in differentiating LCNEC from its more frequent mimics. Answer C is correct because ISNM1 is a highly sensitive and specific marker of neuroendocrine differentiation . Similarly, p40 isoform of p63 is highly specific for squamous differentiation. Napsin A is most frequently expressed in lung adenocarcinomas. This panel of immunostains will be the most helpful, since ISNM1 positivity will point towards the diagnosis of LCNEC, while p40 and napsin A negativity will make the possibilities of squamous cell carcinoma and adenocarcinoma remote.
Comment Here
Reference: Large cell neuroendocrine carcinoma
Comment Here
Reference: Large cell neuroendocrine carcinoma
Board review style question #2
Which of the following histologic features is the most helpful in the differential diagnosis of large cell neuroendocrine carcinoma from small cell carcinoma?
- Extensive necrosis
- Mitotic count > 10/2 mm2
- Organoid nesting
- Prominent nucleoli
Board review style answer #2
D. Prominent nucleoli are a characteristic feature of LCNEC. In contrast, small cell carcinoma cells usually have hyperchromatic nuclei with salt and pepper chromatin distribution and indiscrete nucleoli. Answer B is incorrect because mitotic count is elevated both in LCNEC and small cell carcinoma. Answer A is incorrect because extensive geographic necrosis is a feature of both LCNEC and small cell carcinoma. Answer C is incorrect because although organoid nesting is very characteristic of LCNEC, in can be frequently seen in areas of small cell carcinoma as well.
Comment Here
Reference: Large cell neuroendocrine carcinoma
Comment Here
Reference: Large cell neuroendocrine carcinoma