Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2 | Practice question #3 | Practice answer #3Cite this page: Rytych J, Krogh K, Yang GY, Escobar DJ. Well differentiated neuroendocrine tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreaswdnet.html. Accessed September 14th, 2025.
Definition / general
- Low, intermediate or high grade subset of neuroendocrine tumors (NET) that lack necrosis and express neuroendocrine markers (synaptophysin or chromogranin A)
Essential features
- WHO Classification of Tumours of Endocrine Organs (2017) and WHO Classification of Tumours of the Digestive System (2019) classifies well differentiated pancreatic neuroendocrine tumors (PanNETs) as grade 1, grade 2 or grade 3 (see Diagnosis)
- Classification uses criteria similar to other gastrointestinal neuroendocrine neoplasms
- May be nonfunctioning or secrete 1 or more peptides; multiple tumors in the same patient may secrete the same or different peptides
Terminology
- Synonyms: well differentiated neuroendocrine tumor (WD NET), pancreatic neuroendocrine tumor (PanNET), pancreatic endocrine tumor, islet cell tumor
- Specific functional terms (glucagonoma, insulinoma, gastrinoma) not recommended for use unless hormonal syndrome exists clinically
- Microscopic lesions < 0.5 cm are termed microadenomas
- Carcinoid is a term that should be avoided if possible
ICD coding
- ICD-10: C25 - malignant neoplasm of pancreas
Epidemiology
- M = F
- Peak incidence: 30 - 60 years
- Comprise ~2 - 5% of pancreatic neoplasms (J Gastrointest Surg 2010;14:541)
- Most are sporadic; risk factors include (Ann Oncol 2016;27:68):
- Family history of cancer (not site dependent; lung, colon, among others)
- Elevated BMI
- Diabetes
- Cigarette smoking
- Elevated alcohol consumption
- 10 - 20% are associated with syndromes:
- Multiple endocrine neoplasia type 1 (MEN1): nearly 100% pancreatic involvement at autopsy (Surgery 1979;86:475)
- Von Hippel-Lindau (VHL): 12.3 - 17% of patients (Surgery 2007;142:814, Gastroenterology 2000;119:1087)
- Neurofibromatosis type 1: 10% of patients (Crit Rev Oncol Hematol 2022;172:103648)
- Tuberous sclerosis: 1 - 9% of patients (Crit Rev Oncol Hematol 2022;172:103648)
- Nonfunctional tumors represent 60 - 90% of all PanNETs (Neuroendocrinology 2016;103:153, Biomed Res Int 2017;2017:9856140)
- Functional tumors (10 - 40%):
- Insulinoma (most common, account for 4 - 20% of PanNETs) (J Gastroenterol 2015;50:58)
- Gastrinoma (second most common, account for 4 - 8% of PanNETs) (J Gastroenterol 2015;50:58)
- VIPoma (< 2% of PanNETs) (Endocr J 2022;69:1201)
- Glucagnoma (1 - 2% of PanNETs) (Ann Surg Oncol 2007;14:3492)
- Somatostatinoma (< 1% of PanNETs) (J Gastroenterol Hepatol 2008;23:521)
- ACTH producing neuroendocrine tumor (< 1% of PanNETs) (Am J Surg Pathol 2015;39:374)
- Serotonin producing neuroendocrine tumor (up to 3.9%) (Am J Surg Pathol 2015;39:592)
Sites
- Found throughout the pancreas
- Nonfunctional, gastrinomas or serotonin producing tumors are more likely to be found in the head of the pancreas
- Insulinomas, glucagonomas and VIPomas are more likely to be found in the tail (Ann Surg Oncol 2007;14:3492)
Pathophysiology
- Theorized to arise from totipotent stem cells in the pancreatic ductal system and islets of Langerhans (Endotext: Pathophysiology and Treatment of Pancreatic Neuroendocrine Tumors [Accessed 30 September 2022])
Etiology
- Majority are sporadic and nonsyndromic
- Minority are associated with multiple endocrine neoplasia syndrome (80 - 100% incidence), tuberous sclerosis (rare), von Hippel-Lindau (VHL, 10 - 17% incidence), among others (Cancer 2008;113:1807)
- Hyperplastic and preneoplastic lesions were removed from the WHO 2017; PanNEN precursor lesions have not been clearly associated with sporadic neoplasms (but are described in MEN1 and VHL)
Clinical features
- Demonstrate a spectrum of clinical behavior dependent on hormones produced
- Commonly causes abdominal pain, jaundice
- Asymptomatic pancreatic neuroendocrine tumors are increasingly common, likely because of an increase in detection due to increased imaging frequency and improvement in imaging modalities (Cancer 2015;121:589)
- Functional tumors may have elevated serum peptides corresponding to tumor cell type
- May be associated with carcinoid syndrome (flushing, diarrhea) if metastatic to liver
- Most nonfunctional tumors are large and detected at an advanced stage at diagnosis, with 60 - 85% having liver metastases (Cancer 2008;113:1807)
- Liver is the most common site of metastasis
- Insulinoma:
- Composed of insulin producing cells with uncontrolled secretion of insulin
- Clinical: Whipple triad (hypoglycemic symptoms, plasma glucose < 40 mg/dL and symptom relief after glucose administration)
- Typically, small (< 2 cm diameter) and solitary (~10% multiple, usually in MEN1 patients) (Neoplasma 2015;62:484)
- Gastrinoma:
- Composed of gastrin producing cells with uncontrolled secretion of gastrin
- Clinical: Zollinger-Ellison syndrome (reflux symptoms and duodenal ulcers)
- Typically, large size (average 3.8 cm diameter), more common in the duodenum than in the pancreas (Wien Klin Wochenschr 2007;119:579)
- VIPoma:
- Composed of uncontrolled vasoactive intestinal peptide (VIP) secreting cells
- Clinical: WDHA syndrome (watery diarrhea [persists when fasting], hypokalemia and achlorhydria)
- Typically, large (average ~5 cm diameter) and solitary (Ann N Y Acad Sci 1988;527:508)
- Glucagonoma:
- Composed of cells with uncontrolled secretion of glucagon and preglucagon derived peptide
- Clinical: triad of skin rash (necrolytic migratory erythema), diabetes mellitus and weight loss
- Typically, variable in size (average 3 - 7 cm diameter) and solitary (Medicine (Baltimore) 1996;75:53)
- Somatostatinoma:
- Composed of cells that demonstrate D cell differentiation with uncontrolled secretion of somatostatin
- Clinical: less distinctive than other functioning PanNETs (glucose intolerance, cholelithiasis and diarrhea)
- Typically, large (average 5 - 6 cm diameter) and multinodular (J Exp Clin Cancer Res 1999;18:13)
- ACTH producing neuroendocrine tumor:
- Composed of cells secreting uncontrolled adrenocorticotropic hormone (ACTH)
- Clinical: features of Cushing syndrome (central obesity, weight gain, moon face, hypertension, hyperglycemia, osteoporosis, hypokalemia and cutaneous hyperpigmentation)
- Typically, large (average 4.8 cm diameter) and solitary (Am J Surg Pathol 2015;39:374)
- Composed of cells secreting uncontrolled adrenocorticotropic hormone (ACTH)
- Serotonin producing neuroendocrine tumor:
- Composed of cells secreting uncontrolled serotonin
- Clinical (usually only after metastasizing to the liver): carcinoid syndrome (abdominal pain, flushing, diarrhea and weight loss)
- Typically large (average ~5 cm diameter) and solitary (Pancreas 2011;40:883)
Diagnosis
- Requires neuroendocrine histology and positive staining for neuroendocrine and cytokeratin makers (and the presence of clinical symptoms if functional)
- Contains < 30% of the neoplasm volume of a second tumor type (ductal, acinar etc.) (otherwise, see mixed neuroendocrine nonneuroendocrine neoplasms [MiNEN])
- Does not contain necrosis or severe atypia (otherwise, see neuroendocrine carcinoma [PanNEC])
- Well differentiated tumors are classified by mitotic count and proliferative index (Ki67):
- G1 (NET G1): mitotic count < 2/2 mm2 and < 3% Ki67 index
- G2 (NET G2): mitotic count 2 - 20/2 mm2 or 3 - 20% Ki67 index
- G3 (NET G3): mitoses > 20/2 mm2 or > 20% Ki67 index
Laboratory
- Elevated serum chromogranin A levels (> 15 ng/mL) show a sensitivity of 66% and a specificity of 95% for the diagnosis pancreatic neuroendocrine tumors (World J Gastroenterol 2020;26:2305)
- Elevated serum neuron specific enolase (NSE) and pancreatic polypeptide (PP) are respectively seen in up to 30 - 50% and 50% of PNET patients (Front Oncol 2020;10:831)
- Specific biomarkers for functional PNET are not currently recommended due to their poor sensitivity, specificity and lack of standardization between laboratories (Surg Oncol Clin N Am 2020;29:165)
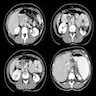
Radiology description
- General: highly vascular, well circumscribed enhancing lesion that can be solid or cystic
- Ultrasound: circumscribed masses with smooth margins, suboptimal visualization of the body and tail due to bowel gas obstruction, sensitivity of 20 - 80%
- CT: well defined, uniformly hypervascular masses on arterial phase CT, most widely available technique
- MRI: hyperintense on T1 weighted imaging due to an abundance of proteinaceous material and variable signal on T2 weighted images; better resolution than CT (Semin Ultrasound CT MR 2019;40:469)
- 68Ga SRS (stimulated Raman scattering) is a type of PET imaging of somatostatin receptor, current gold standard for functional imaging of NETs (Nat Rev Endocrinol 2018;14:656)
Prognostic factors
- Relatively indolent but with variable outcome
- Features associated with adverse outcome (Am J Surg Pathol 2007;31:1677, HPB (Oxford) 2009;11:422, Sci Rep 2018;8:7271):
- Size > 2 cm
- Tumor necrosis
- Lymph node positivity
- Mitoses > 2/10 high powered fields
- Vascular invasion
- Perineural invasion
- High Ki67 index
- CK19 positivity
- Loss of DAXX / ATRX expression and alternative lengthening of telomeres suggest poor outcome (Clin Cancer Res 2017;23:600, Front Endocrinol (Lausanne) 2021;12:691557)
Case reports
- 23 year old woman with VHL and SDHA pathogenic variant co-occurrence in PanNET (Front Oncol 2022;12:925582)
- 43 year old man with MEN1 and metastatic grade 3 PanNET (J Endocr Soc 2022;6:bvac122)
- 44 year old woman with recurrent pancreatic glucagonoma (Ann Med Surg (Lond) 2022;77:103604)
- 62 year old man with metastases (J Med Life 2018;11:57)
- 69 year old man with pancreatic insulinoma presenting with postprandial hypoglycemia (AACE Clin Case Rep 2022;8:154)
Treatment
- Primarily surgical (benefits shown for primary and metastatic disease)
- Enucleation can be used for small and localized tumors
- First line treatments for metastatic disease are somatostatin analogs (octreotide and lanreotide)
- Second line treatments include tyrosine kinase inhibitors (sunitinib) and mTOR inhibitors (everolimus)
- Novel chemotherapy regime of capeciabine and temozolomide (CAPTEM) and peptide receptor radionuclide therapy (PRRT) has been approved
- Reference: Surg Clin North Am 2019;99:793
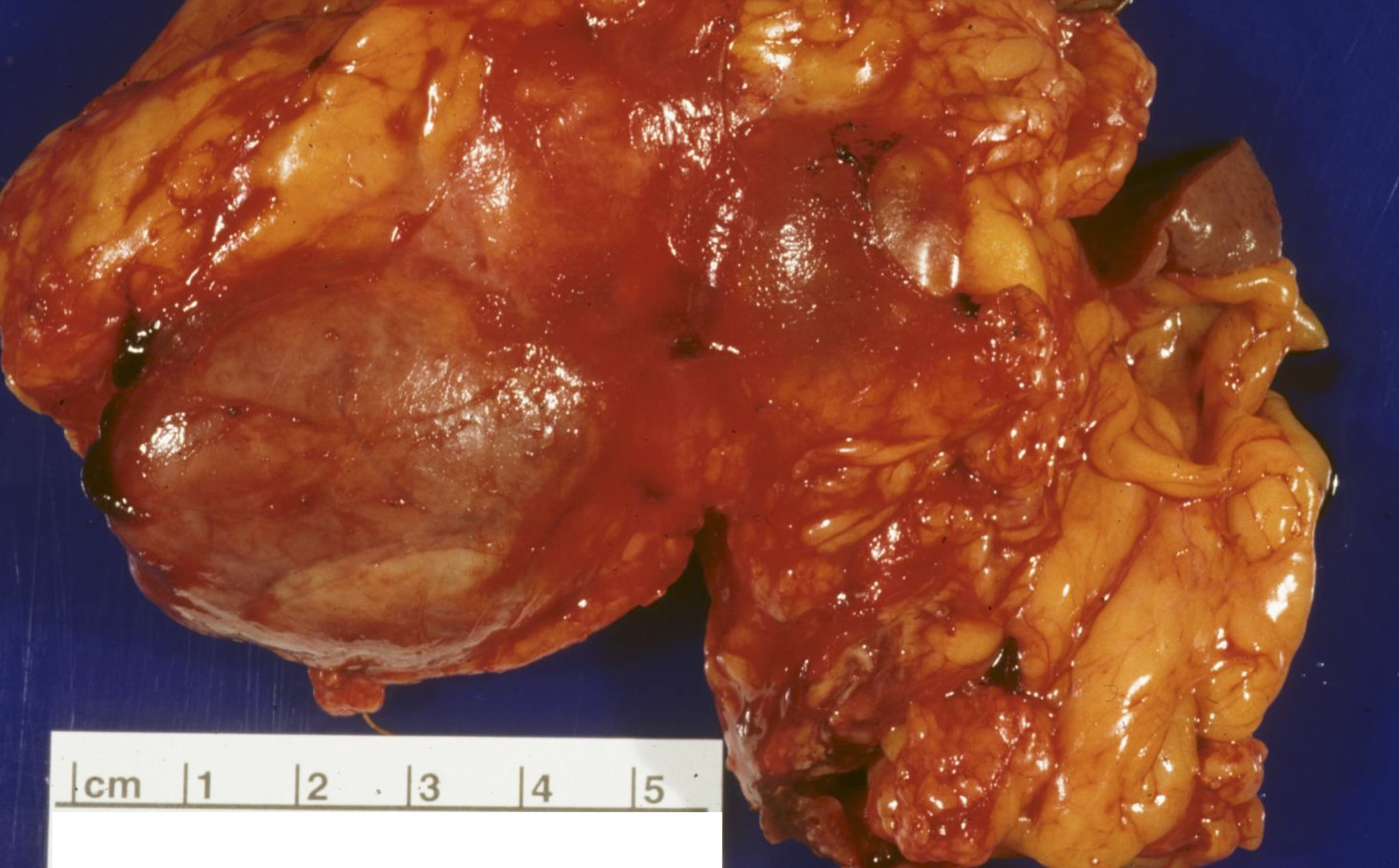
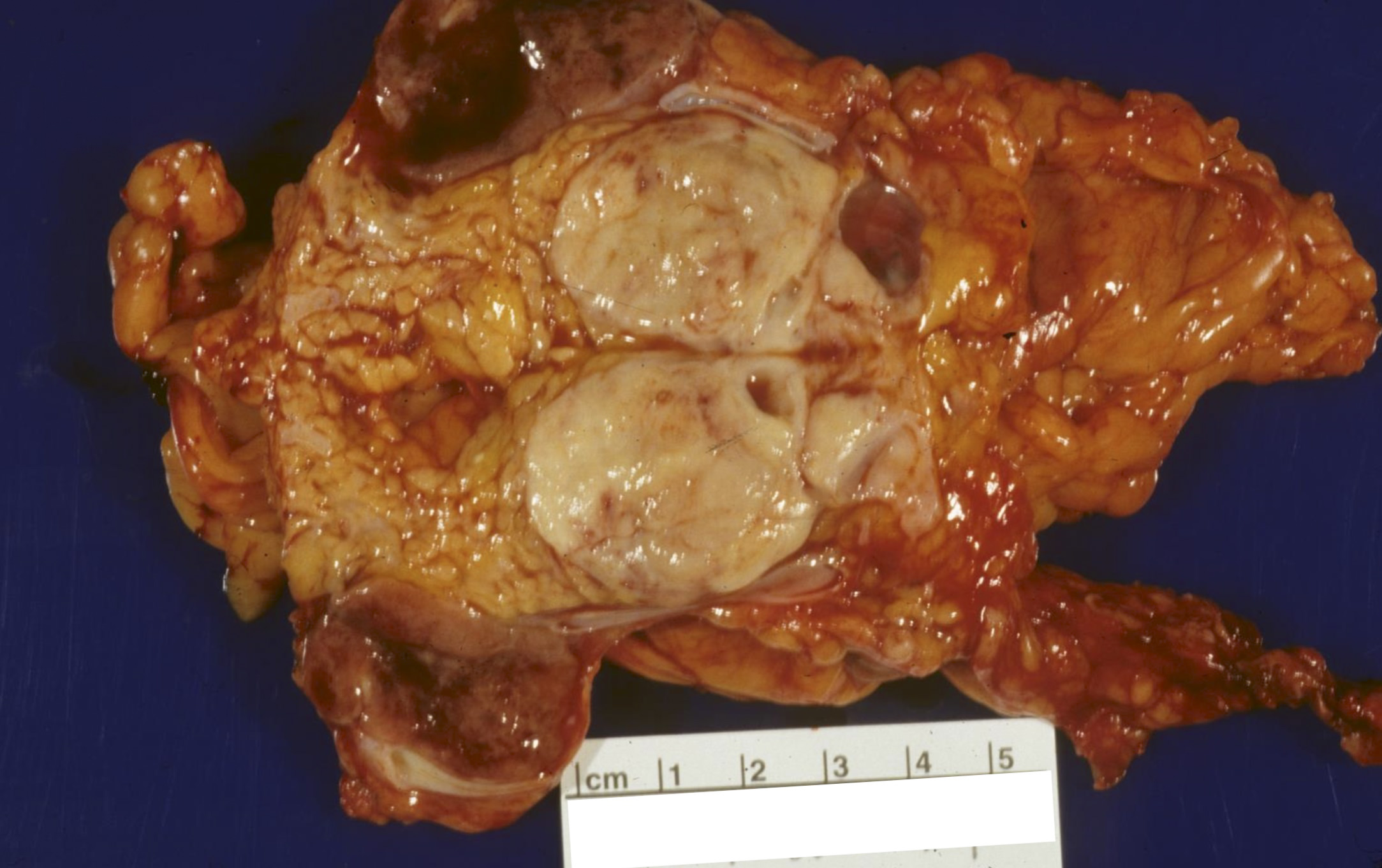
Gross description
- Solid, well circumscribed mass, typically 2 - 5 cm in diameter
- Larger tumors often show heterogenous and lobulated cut surfaces
- Color can range from red-tan to brown-yellow
- May show extensive fibrosis, calcification or ossification
- 5% are cystic (unilocular, less commonly multilocular) but necrosis is uncommon in well differentiated PanNETs
- Reference: Arch Pathol Lab Med 2019;143:1317
Gross images
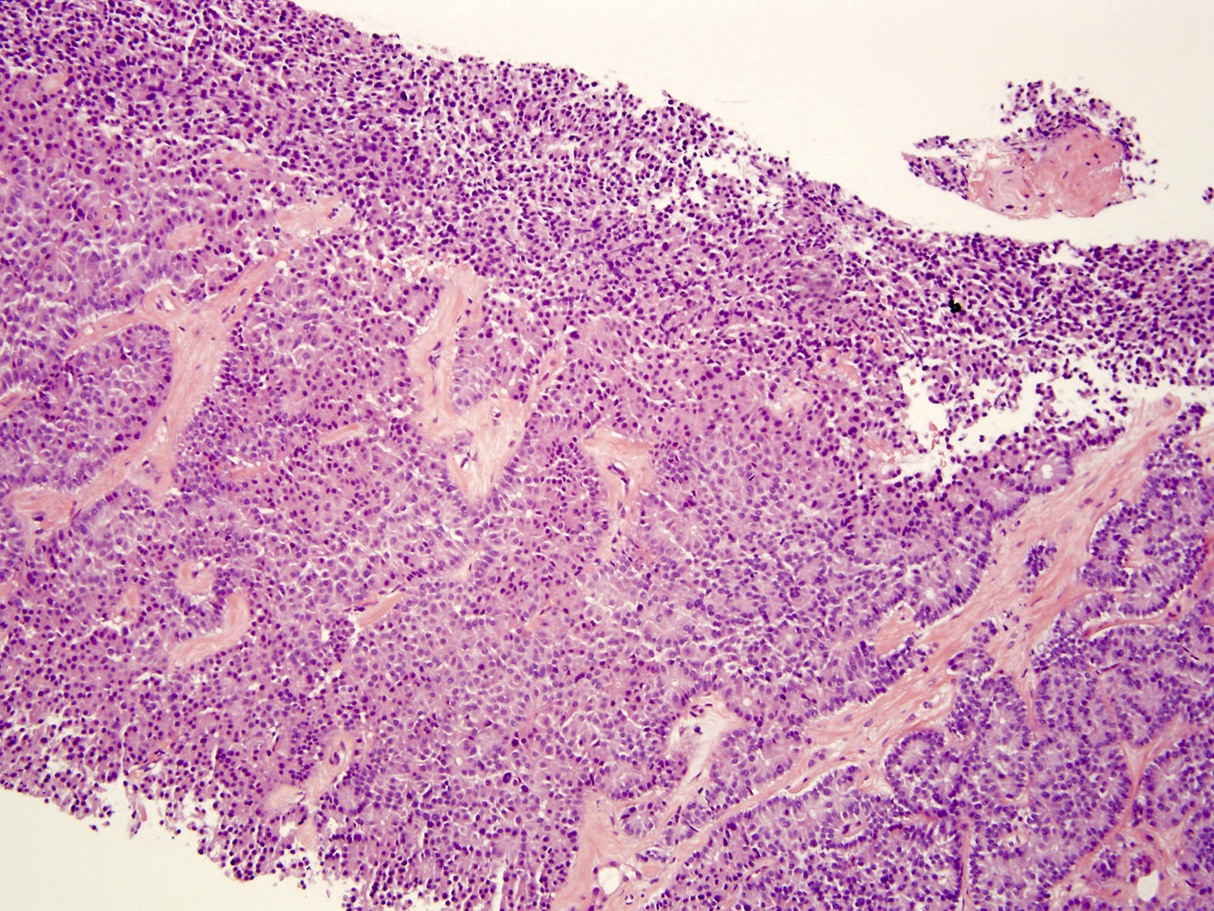
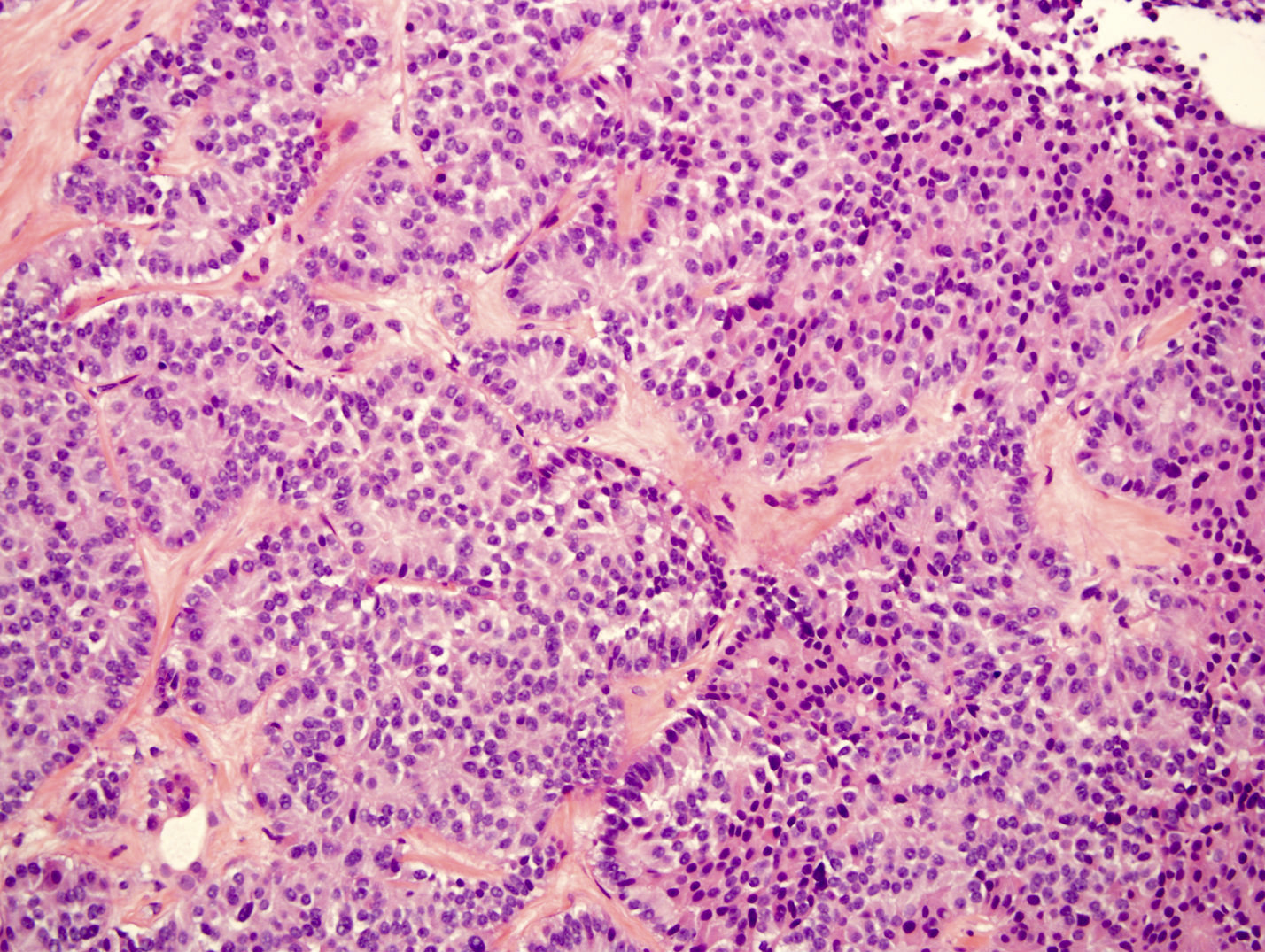
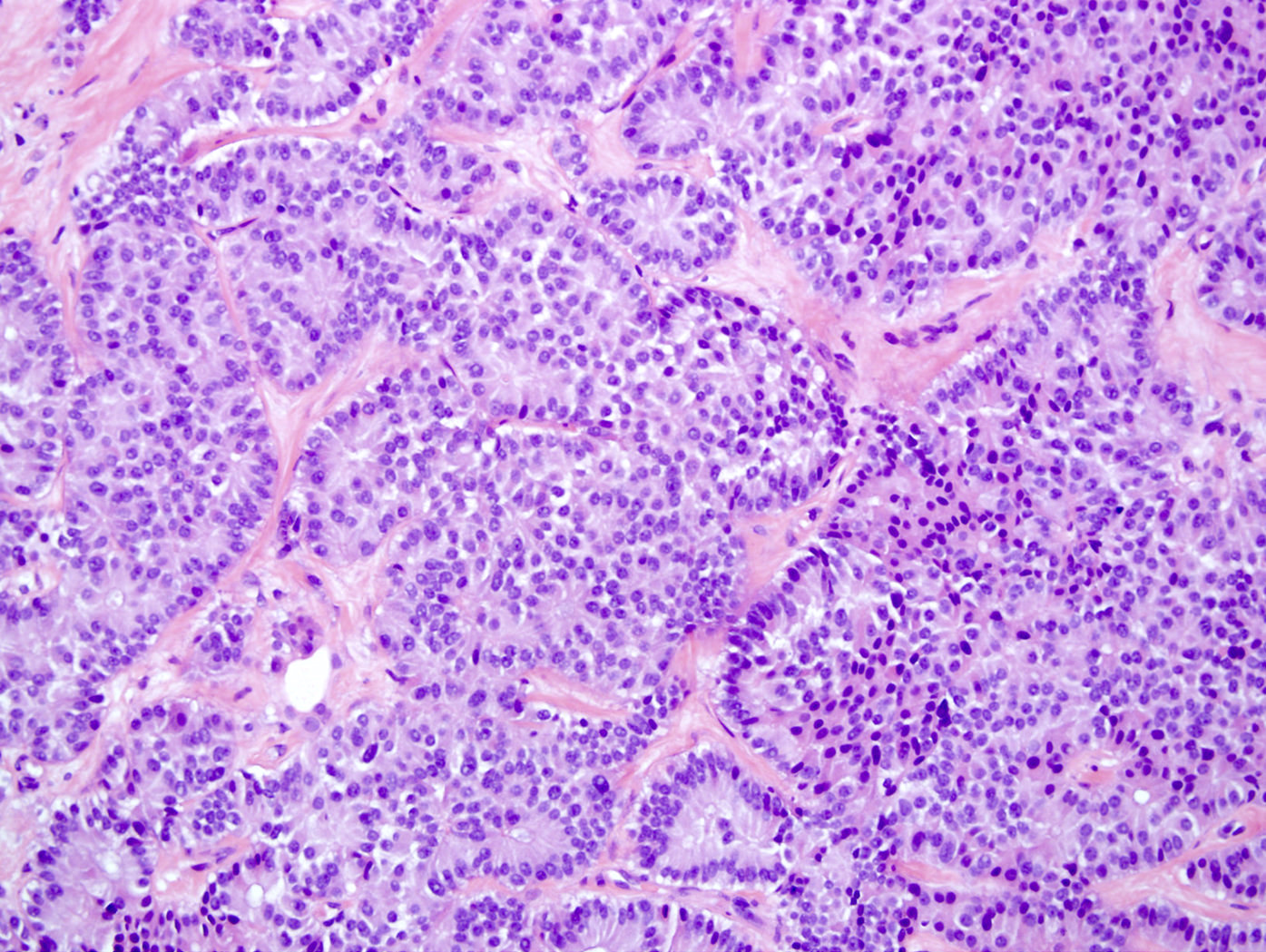
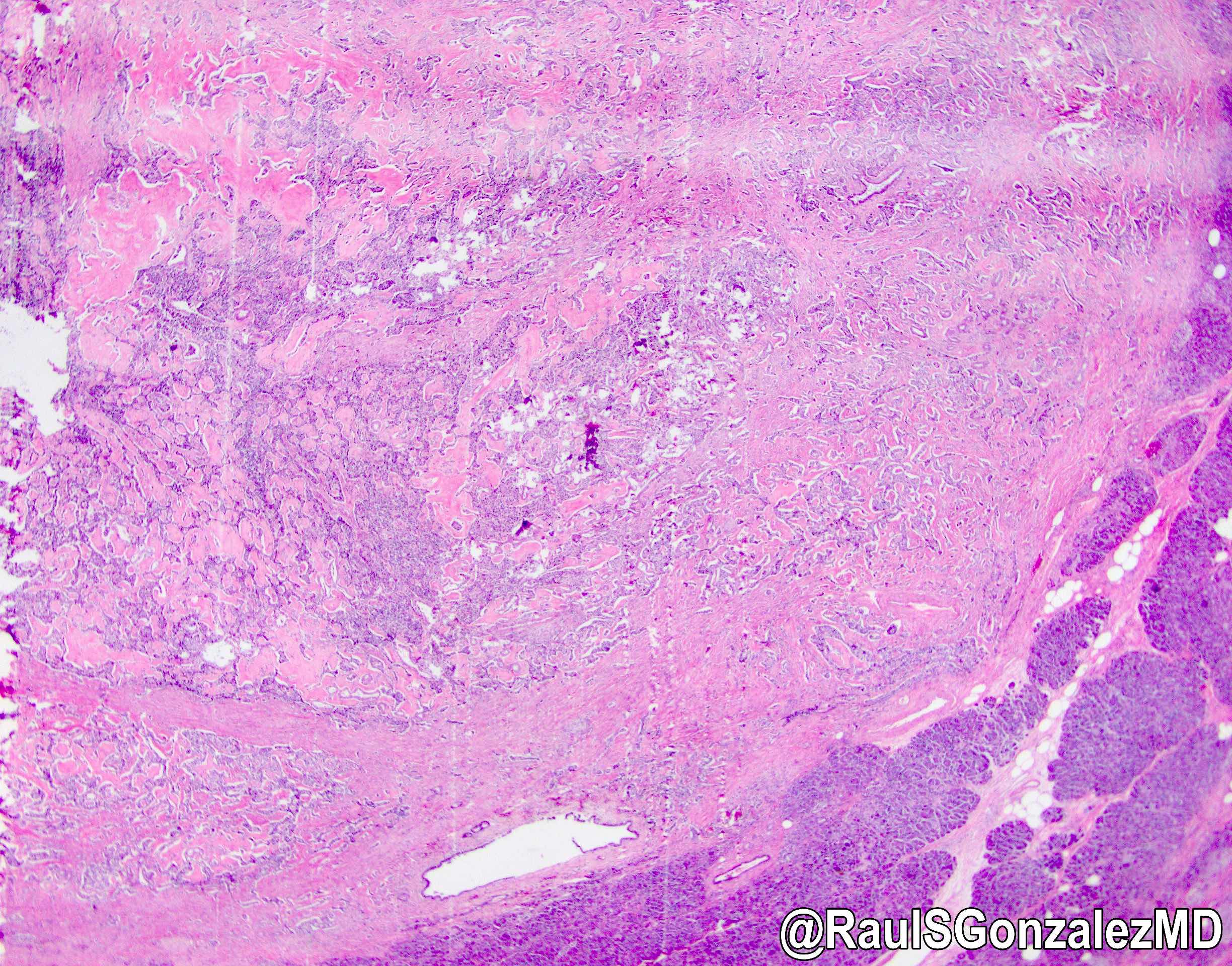
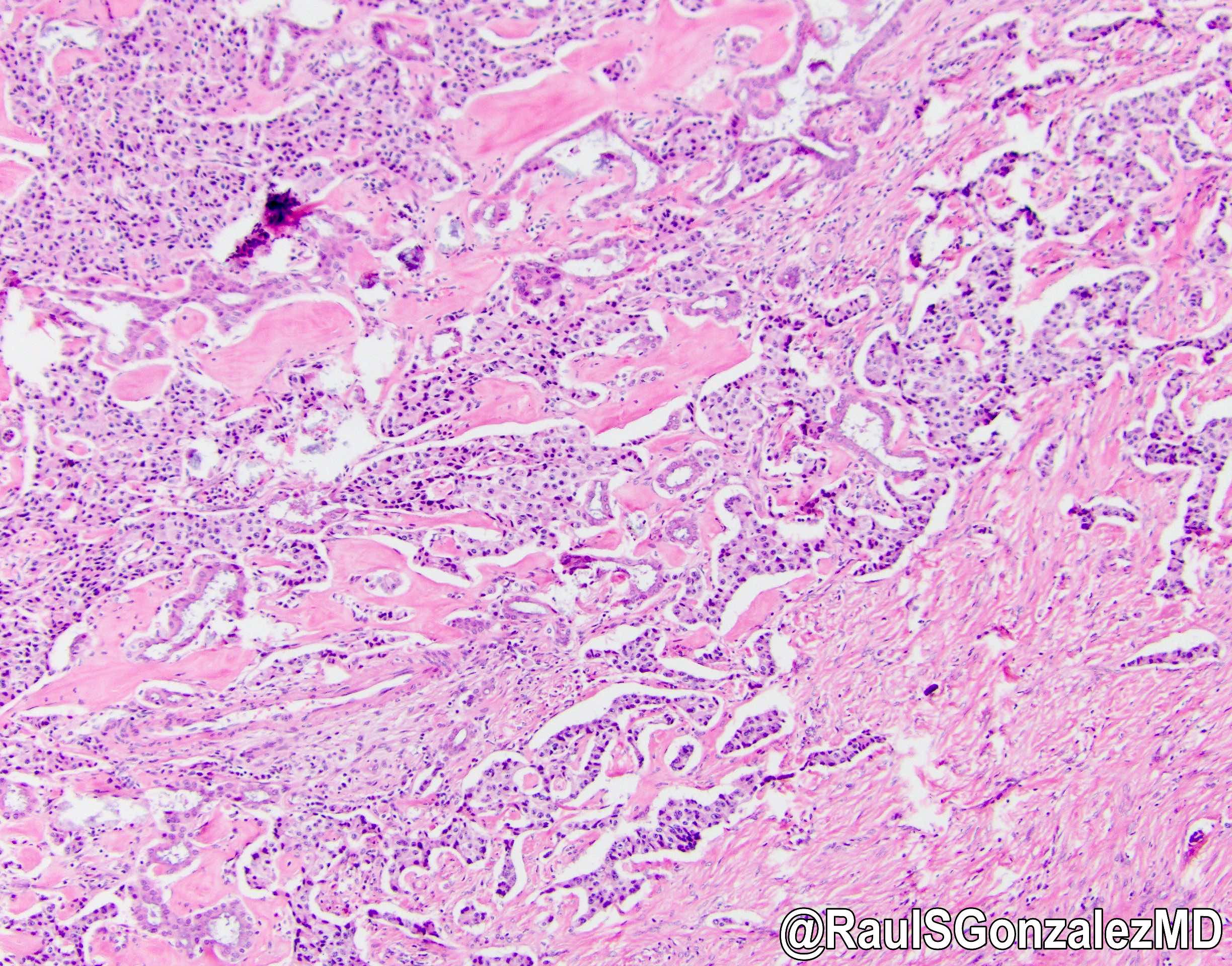
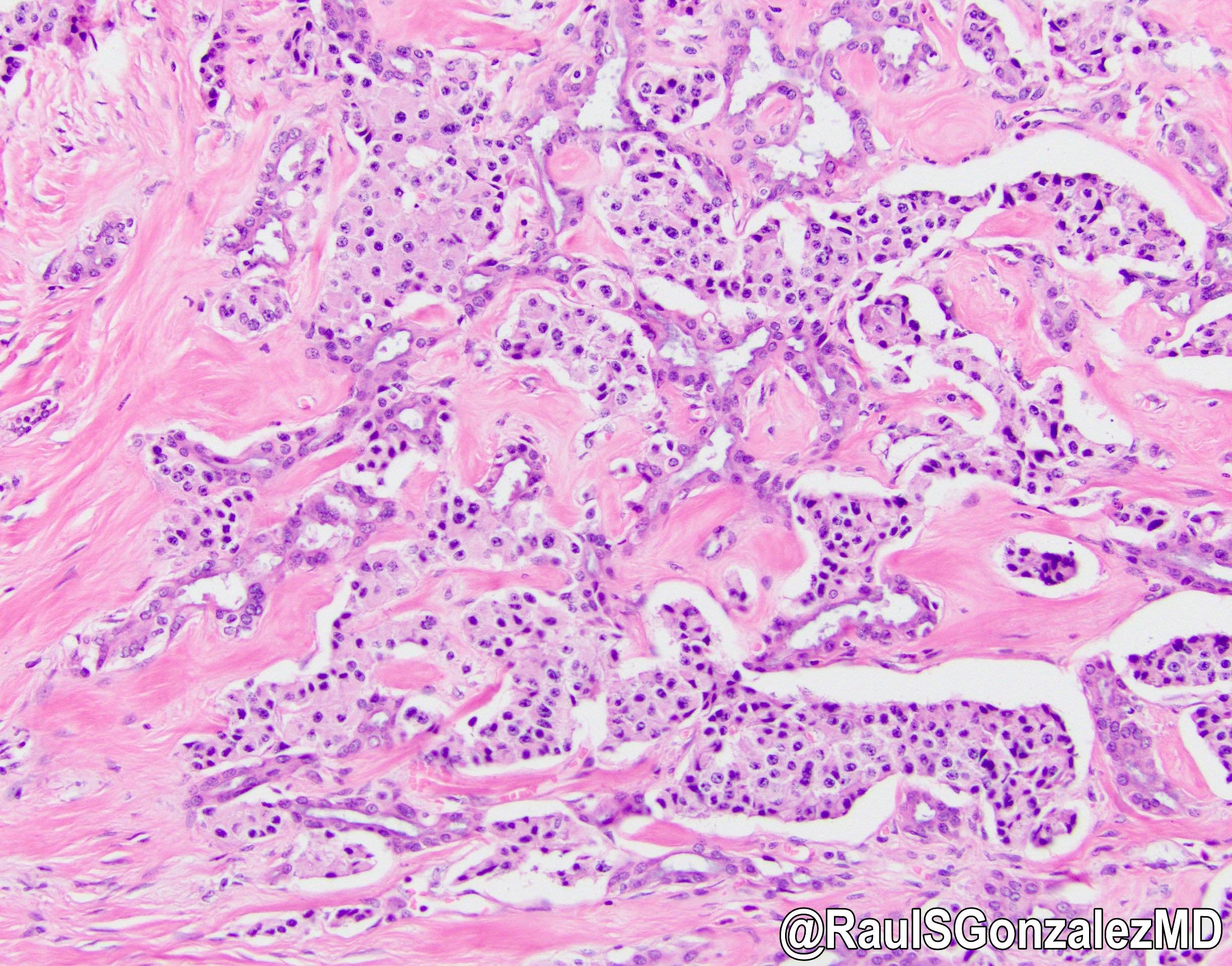
Microscopic (histologic) description
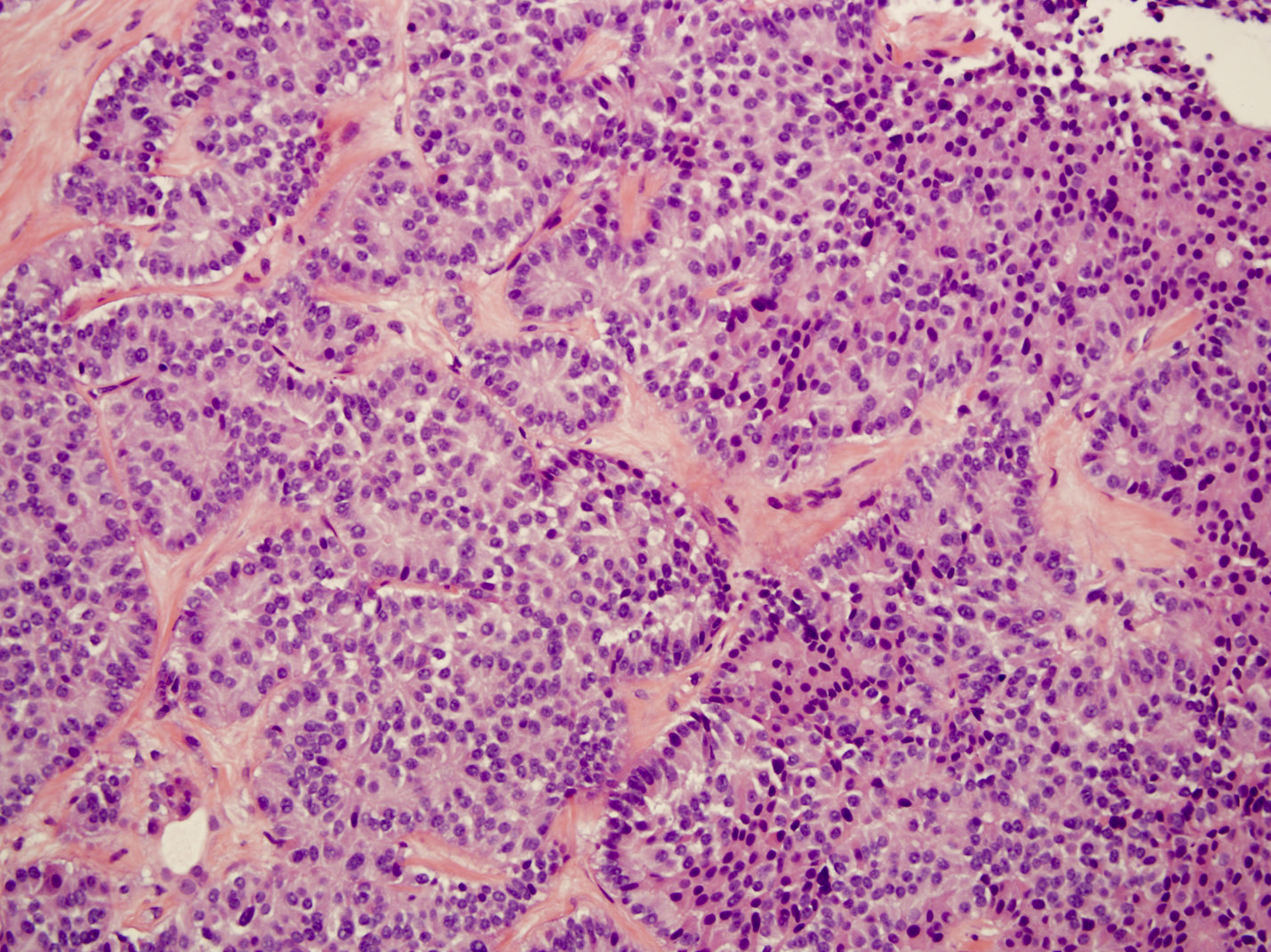
- Architecture can be solid, organoid, nesting, gyriform, trabecular or glandular
- Small, round monotonous cells with coarse, salt and pepper nuclear chromatin and minimal atypia
- Occasional small nucleoli are most common; large nucleoli can be seen
- Cytoplasm can be pale pink, oncocytic, granular or lipid rich / vacuolated
- Rarely, nuclei can be eccentrically located (rhabdoid cell appearance) (Am J Surg Pathol 2003;27:642)
- Islet amyloid polypeptide (amylin) deposition is specific for insulinoma but is only seen in ~5% of cases
- Psammoma bodies can be seen in somatostatinomas
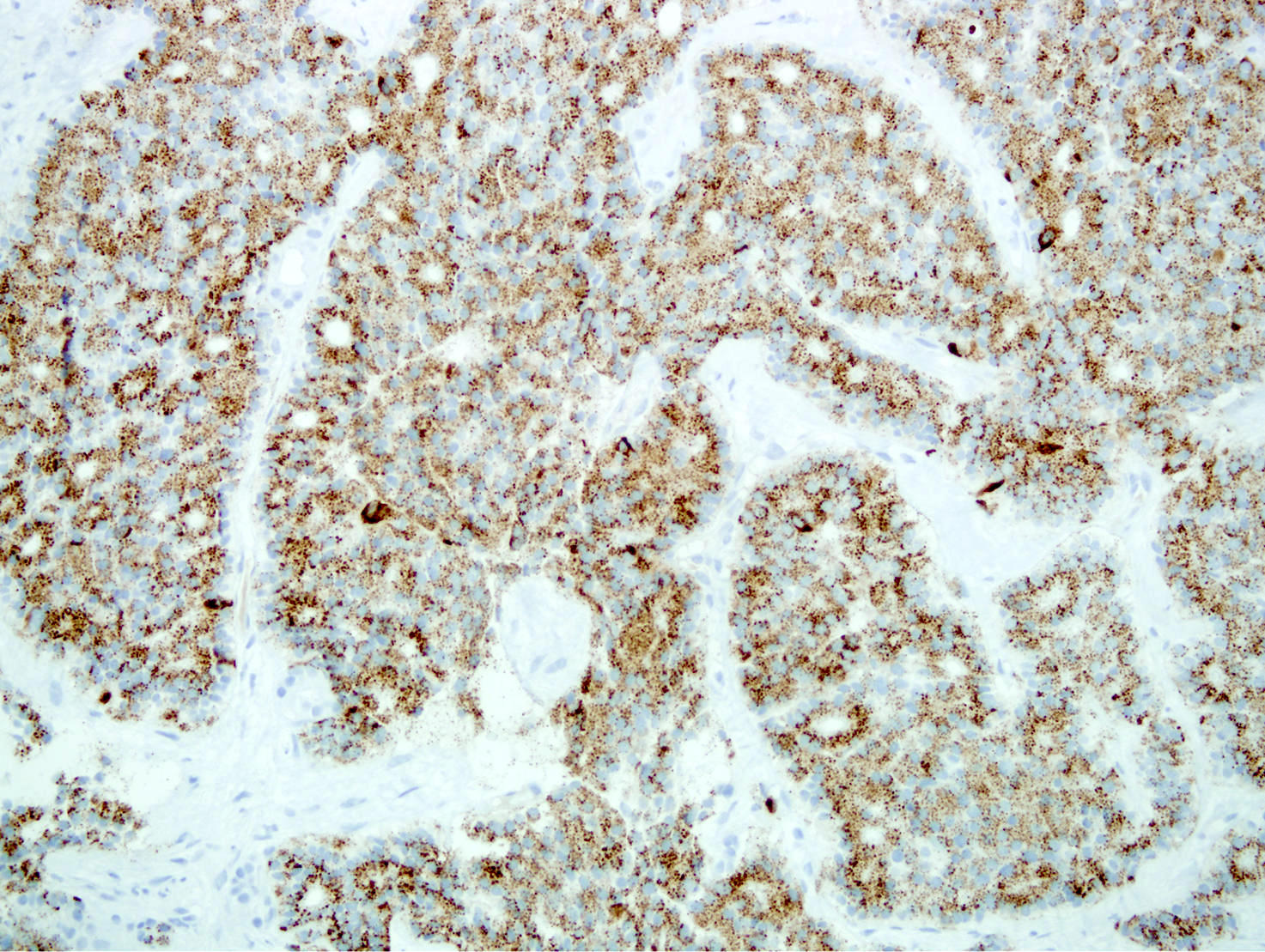
Microscopic (histologic) images
Cytology description
- Single cell type; monotonous plasmacytoid cells with moderate amount of cytoplasm and distinctive neuroendocrine chromatin (Clin Cancer Res 2017;23:600)
- Can have rosette-like aggregates and eccentric nuclei
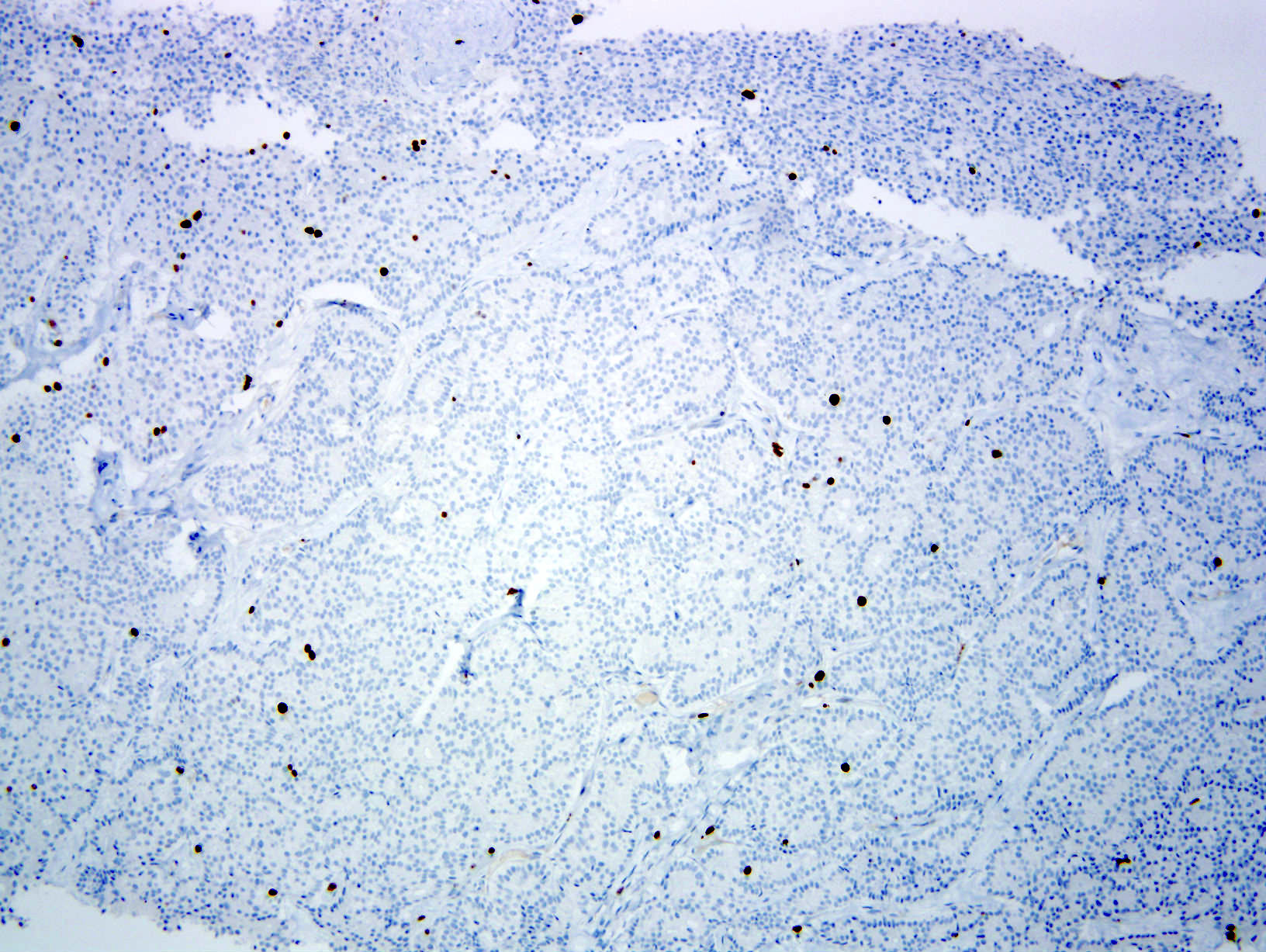
Positive stains
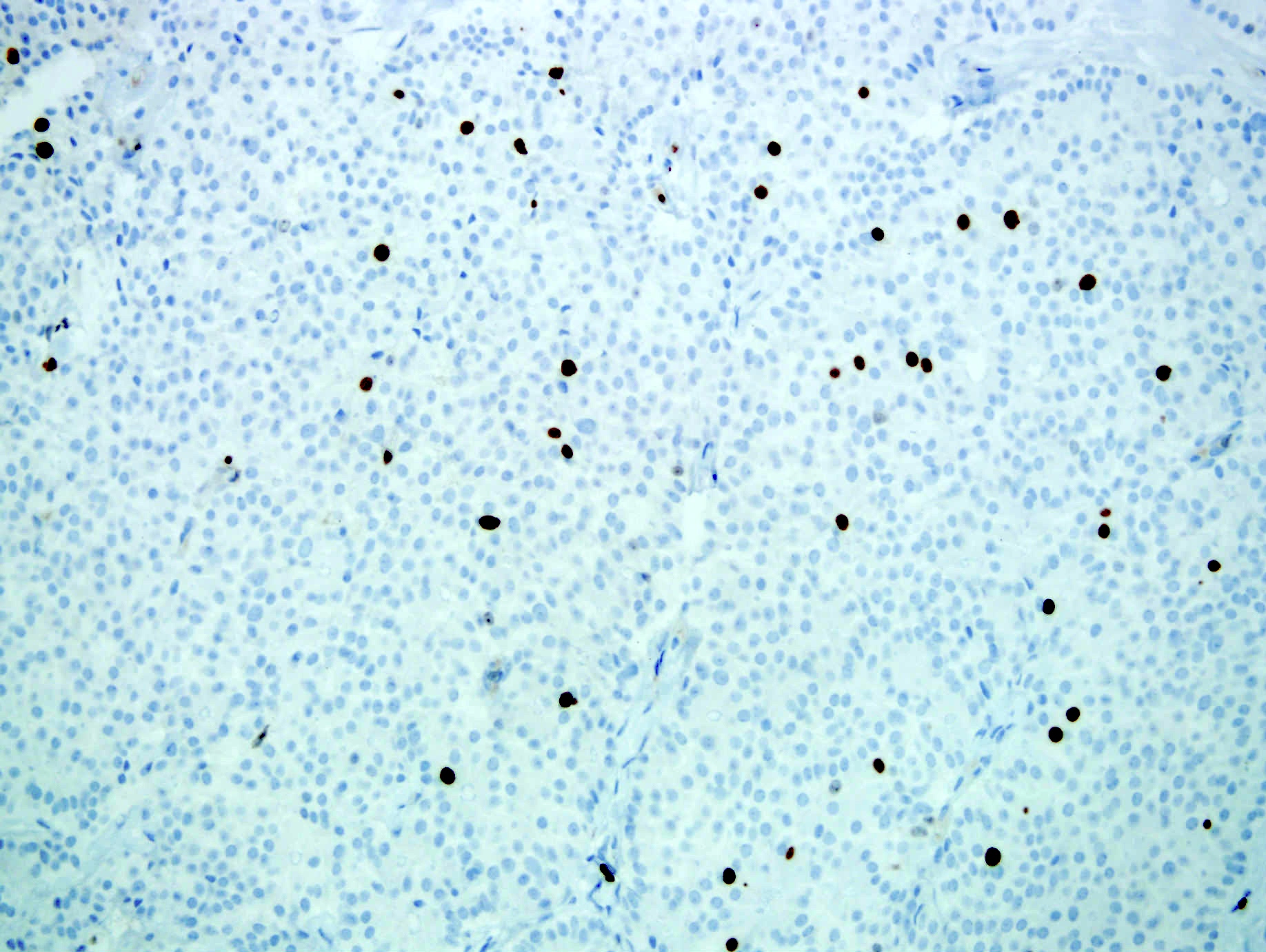
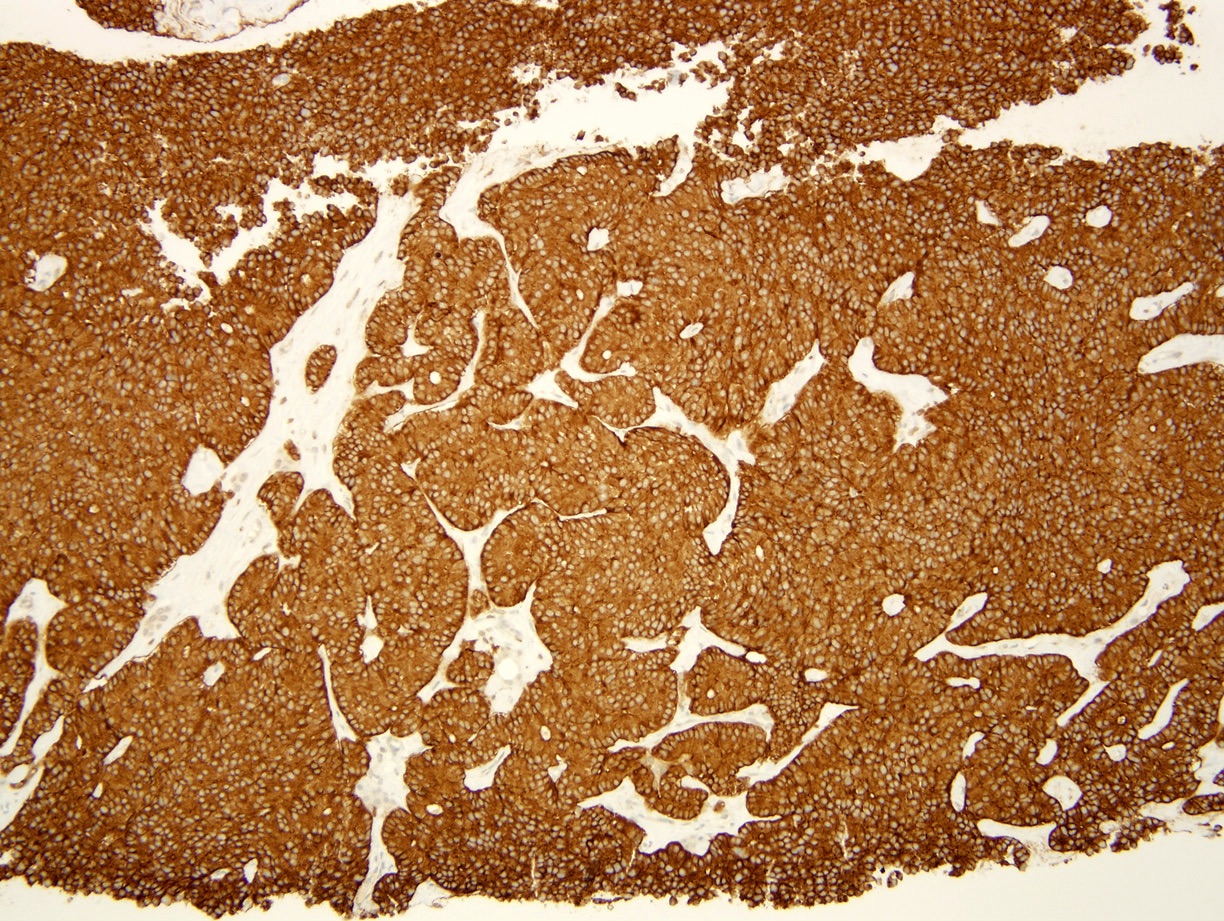
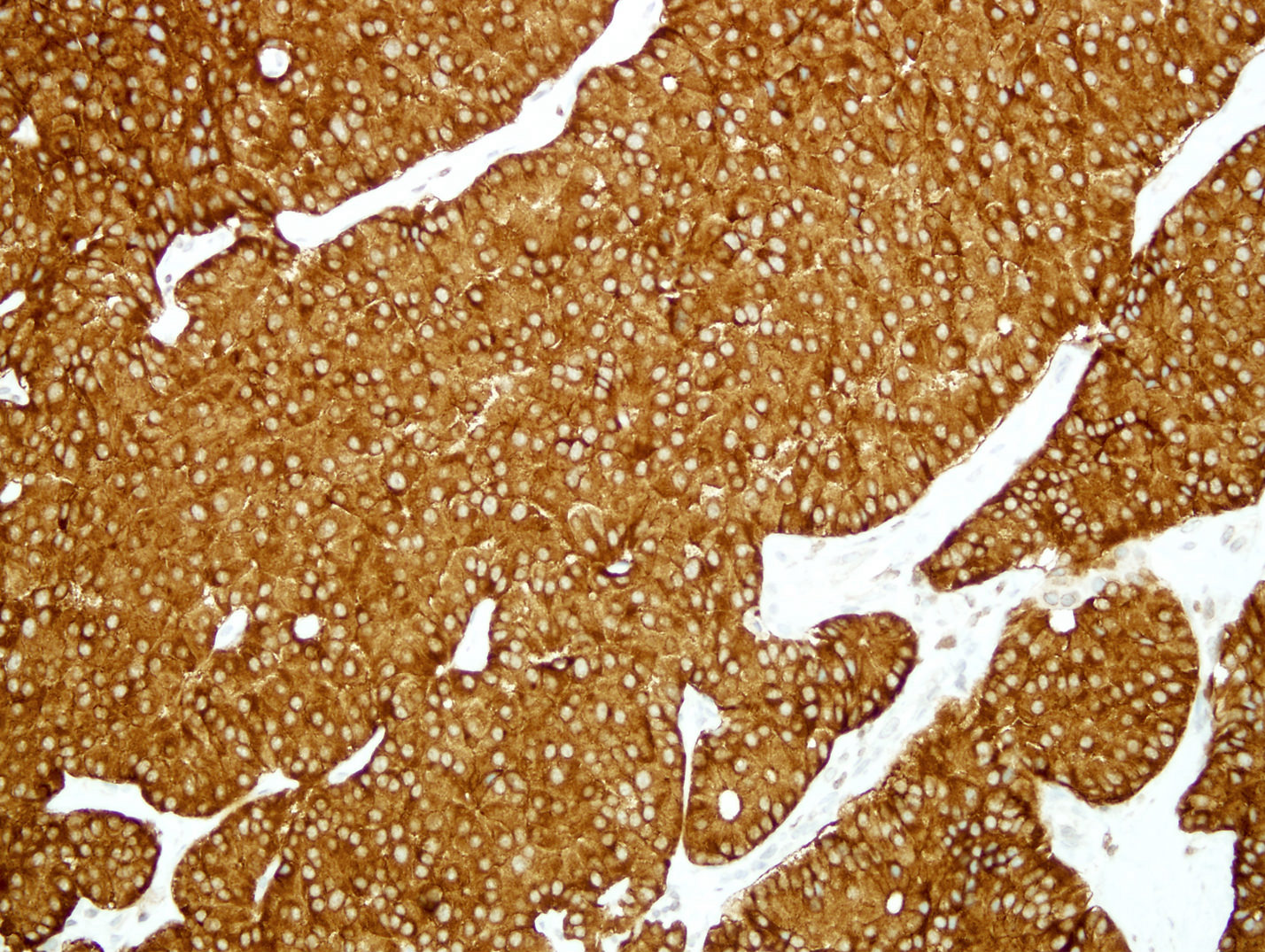
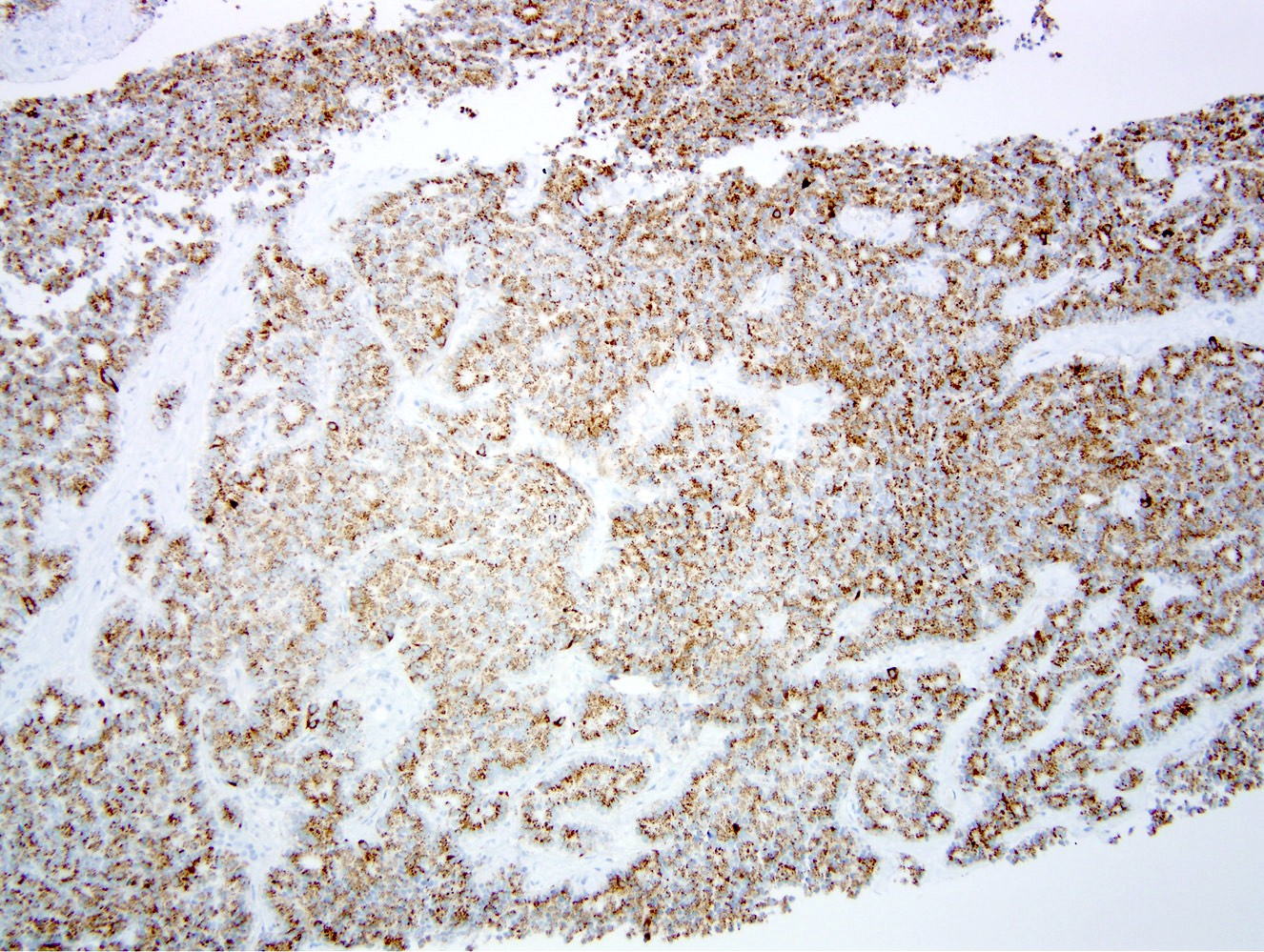
- Chromogranin A (more specific) and synaptophysin (more sensitive): diffuse and strong
- NSE, CD56, CD57
- AE1 / AE3, CAM5.2 and CK8/18
- Somatostatin receptor is positive in 80 - 90% and PR is positive in 58% of PanNETs (Hum Pathol 2020;96:8)
- Immunohistochemistry for peptide hormones (insulin, glucagon, VIP, etc.) is rarely required and nonfunctional tumors may be positive
- SMAD4 and Rb retained
- In metastatic setting: Isl1 (most sensitive) and PAX6 positivity is supportive
- PAX8 (polyclonal) is positive in 50 - 60% of cases metastatic to the liver (monoclonal PAX8 is negative) (Hum Pathol 2020;96:8)
- NESP55+ and PDX1+, in the presence of negative CDX2 and TTF1, is 97% specific for pancreatic origin (Clin Cancer Res 2017;23:600)
Negative stains
- CDX2, SATB2 and TTF1 (Surg Oncol Clin N Am 2020;29:185)
- Nuclear beta catenin
- Trypsin / chymotrypsin
- BCL10
- SMA
Molecular / cytogenetics description
- Frequently mutated genes (Science 2011;331:1199, Endocr Relat Cancer 2009;16:1219, Expert Rev Gastroenterol Hepatol 2015;9:1407):
- MEN1 (44.1%)
- DAXX (death domain associated protein, 25%)
- ATDX ([alpha] thalassemia / intellectual disability syndrome X linked, 17.6%)
- TSC2 (8.8%)
- PTEN (7.3%)
- PI3KCA (1%)
- VHL (up to 25% including deletion by promoter methylation)
Sample pathology report
- Distal pancreas and spleen, distal pancreatectomy and splenectomy:
- Well differentiated neuroendocrine tumor, WHO grade 2, measuring 4.1 cm in greatest dimension (see comment)
- Lymphovascular and perineural invasion identified
- Pancreatic resection margin, negative for tumor
- 2 of 10 lymph nodes, positive for metastatic tumor (2/10)
- Spleen with no significant pathologic change
- Pathologic stage: pT3, pN1
- Comment: Immunohistochemical stains are performed and show the tumor cells are positive for cytokeratin AE1 / AE3, synaptophysin and chromogranin (patchy). The Ki67 proliferative index is 15%. Beta catenin shows membranous staining (negative result). Overall findings are consistent with 2019 WHO grade 2 (out of 3).
Differential diagnosis
- Solid pseudopapillary neoplasm:
- Positive stains: nuclear beta catenin, cytoplasmic / perinuclear CD10, nuclear PR, synaptophysin (sometimes), pankeratins (variable)
- Negative stains: membranous E-cadherin, ER, chromogranin (usually)
- Contains pseudopapillary architecture, which is rare in neuroendocrine tumors
- Mixed neuroendocrine nonneuroendocrine neoplasms (MiNEN):
- Typically, neuroendocrine component is mixed with a ductal adenocarcinoma, occasionally with acinar cell carcinoma
- Each component must account for > 30% of the tumor
- Metastatic neuroendocrine tumors of other origin:
- Negative for islet 1, PAX6, PDX1
- Positive for TTF1, SATB2, CDX2 (Surg Oncol Clin N Am 2020;29:185)
- Neuroendocrine carcinoma (NEC):
- High mitotic rate (> 20/2 mm2 or Ki67 > 20%)
- Large or small cell with corresponding high grade morphology
- Acinar cell carcinoma (Am J Surg Pathol 1992;16:815, Cancer Cytopathol 2013;121:459):
- PAS+ diastase resistant granules sometimes detected
- Positive stains: trypsin, chymotrypsin, lipase, CK8/18
- Occasionally positive for synaptophysin or chromogranin
- Pancreatoblastoma (Cancer Cytopathol 2013;121:459):
- Solid and acinar patterns, squamoid nests are present
- Trypsin, chymotrypsin, lipase, BCL10, synaptophysin and chromogranin can be focally positive
- Glomus tumor:
- Composed of 3 cell types: glomus, vasculature and smooth muscle cells
- Positive SMA, negative synaptophysin, chromogranin
Additional references
Practice question #1
Which of the following statements is true regarding the pancreatic lesion seen above?
- Commonly associated with familial adenomatous polyposis (FAP)
- Loss of DAXX / ATRX expression are associated with better outcomes
- More common than pancreatic adenocarcinoma
- More likely to be nonsecreting than secreting
Practice answer #1
D. More likely to be nonsecreting than secreting
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor
Practice question #2
Practice answer #2
D. Treatment depends on Ki67 interpretation
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor
Practice question #3
A patient presents to his primary care doctor with a 1 month history of constant watery diarrhea that wakes him up several times each night. Laboratory values were remarkable for a potassium of 3.2 mmol/L (normal: 3.6 - 5.2 mmol/L). An abdominal CT showed a solitary, solid, well demarcated and vascular 5 cm lesion in the tail of the pancreas that is consistent with a neoplasm. What is the most likely gene mutation in this lesion?
- APC
- CTNNB1
- MEN1
- SMAD4
- STK11
Practice answer #3
C. MEN1. The patient is presenting with WDHA syndrome (watery diarrhea that persists when fasting, hypokalemia and achlorhydria) and a pancreatic mass. This is likely due to a VIPoma, which is a type of pancreatic neuroendocrine tumor (PanNET). The most common mutation in these tumors is MEN1.
Comment Here
Reference: Well differentiated neuroendocrine tumor
Comment Here
Reference: Well differentiated neuroendocrine tumor