Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Novice T, Harms P. Primary cutaneous mucinous carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticmucinouscarcinoma.html. Accessed September 16th, 2025.
Definition / general
- Rare low grade sweat gland carcinoma with nests / strands of atypical epithelial cells floating in lakes of extracellular mucin
- Distinction from metastatic mucinous carcinoma is essential
Essential features
- Very rare tumor, occurring primarily on head / neck (particularly eyelids)
- Characteristic histology of basaloid tumor cell aggregates in pools or lakes of mucin (Am J Surg Pathol 2005;29:764)
- High recurrence rate, low metastatic rate
- Can be difficult to distinguish from metastasis originating from noncutaneous mucinous carcinoma
Terminology
- Mucinous (eccrine) carcinoma
- Primary mucinous carcinoma of the skin
- Colloid carcinoma of the skin
- Cutaneous mucinous adenocarcinoma
- Primary mucinous adenocarcinoma of the skin
- Mucinous sweat gland carcinoma
ICD coding
Epidemiology
- Very rare; 1:150,000 cutaneous lesions (Am J Dermatopathol 2024;46:114)
- Age adjusted incidence of 0.04 per 100,000 person years (Dermatol Surg 2020;46:1141)
- Middle aged to older individuals (mean age: 65.2 years; range: 8 - 89 years old) (Am J Otolaryngol 2018;39:242, Dermatol Surg 2023;49:1091)
- Predominantly reported in White patients (Am J Dermatopathol 2024;46:114)
- In large study, 77% White, 13% Asian and 10% Black (JAMA Dermatol 2014;150:380)
- Freeman et al. reports growing number of cases in patients with skin of color (Dermatol Surg 2023;49:1091)
- No definitive sex predilection (Dermatol Surg 2020;46:1141, Dermatol Surg 2023;49:1091)
Sites
- Head / neck is the most common, especially eyelid (~50% of cases) > nonperiorbital head / neck (20%) > scalp (17%) > trunk / axilla (Am J Otolaryngol 2018;39:242, JAMA Dermatol 2014;150:380, Diagn Cytopathol 2017;45:934)
- Rare reports on hand, vulva, buccal space, scrotum and breast (Urol Ann 2020;12:83, Arch Pathol Lab Med 2002;126:1216)
- Reports of breast and axilla cases could represent misdiagnosed mucinous breast carcinoma, as discussed below (Diagn Cytopathol 2017;45:934, Am J Dermatopathol 2024;46:114)
Pathophysiology
- Recognized as sweat gland derived tumor (Arch Ophthalmol 2010;128:1160)
- Original view: eccrine origin
- Contemporary view: apocrine differentiation (JAMA Dermatol 2014;150:380, Ann Dermatol 2010;22:472)
- Inverse cell polarity (apical secretion by basally located cells) might explain characteristic tumor morphology (Am J Surg Pathol 2023;47:1186)
- Precursor lesions
- Ductal hyperplasia, carcinoma in situ (Am J Surg Pathol 2005;29:764, J Cutan Pathol 1992;19:334)
- Cases with neuroendocrine differentiation, especially on eyelid, might arise from endocrine mucin producing sweat gland carcinoma precursor (Am J Surg Pathol 2005;29:1330, J Cutan Pathol 2014;41:686)
Etiology
- No known environmental causes
Clinical features
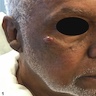
- Skin colored to red, painless and slow growing nodules (Dermatol Surg 2023;49:1091)
- Often delayed presentation due to slow growing nature (Dermatol Surg 2020;46:1141)
- May be ulcerated (Am J Otolaryngol 2018;39:242)
- Can clinically resemble cysts (Arch Craniofac Surg 2024;25:90)
- Color can vary from red to blue to gray or skin colored (Dermatol Surg 2023;49:1091)
- Mean size: 2.1 cm (range: 0.5 - 7 cm) (Am J Otolaryngol 2018;39:242)
Diagnosis
- Required for diagnosis (per the WHO): nests / strands of atypical epithelial cells floating in lakes of extracellular mucin (Am J Surg Pathol 2005;29:764, J Cutan Pathol 1992;19:334)
- In situ component confirms cutaneous origin but is not found in all cases (Am J Surg Pathol 2005;29:764, Am J Dermatopathol 2004;26:353)
- Without in situ component, differentiation from metastatic mucinous carcinoma is essential (i.e., breast, gastrointestinal [GI], lung, ovary, pancreatic biliary, prostate, lacrimal glands, salivary glands) (Am J Surg Pathol 2005;29:764, Am J Dermatopathol 2004;26:353, Dermatol Surg 2020;46:1141)
- Immunohistochemistry
- Review of medical history
- Possibly morphologic comparison with any prior visceral carcinomas (especially of colon or breast)
- Imaging workup for possible visceral primary, if indicated (JAAD Case Rep 2024;45:53)
Prognostic factors
- Overall low grade with favorable prognosis (Dermatol Surg 2020;46:1141, Dermatol Surg 2023;49:1091, JAMA Dermatol 2014;150:380)
- Clinical course
- Recurrences are common (20 - 30%) (J Cutan Pathol 2014;41:686, Dermatol Surg 2023;49:1091)
- Metastases are rare (3 - 7%) (J Cutan Pathol 2014;41:686, Dermatol Surg 2023;49:1091)
- Regional disease: deep extension and lymph node from draining area (Dermatol Surg 2020;46:1141)
- Distant disease: lymph nodes away from draining area or other organs, such as bone and lung (Eur J Plast Surg 2009;32:189, Dermatol Surg 2020;46:1141)
- In a large study, 67% had local disease, 10.5% regional disease and 5.8% distant disease (Dermatol Surg 2020;46:1141)
- Eyelid lesions may present more commonly with distant disease (Dermatol Surg 2020;46:1141)
- At least 1 case of metastatic disease presenting after 10 years of disease free survival has been reported (Dermatol Surg 2023;49:1091, Eur J Plast Surg 2009;32:189)
- Favorable prognostic factors: older age, head / neck location, smaller initial tumor (< 1.5 cm), Asian race (JAMA Dermatol 2014;150:380, Dermatol Surg 2023;49:1091)
- Relationship of neuroendocrine differentiation to prognosis remains unclear due to limited data (J Cutan Pathol 2014;41:686, Dermatol Surg 2023;49:1091)
- Neuroendocrine differentiation might be associated with more favorable prognosis (Am J Surg Pathol 2020;44:1005)
Case reports
- 45 year old man presented with asymptomatic mass on left cheek (Int J Dermatol 2024 Jul 4 [Epub ahead of print])
- 60 year old woman presented with asymptomatic scalp lesion 7 years after positive cervical lymph nodes (J Cutan Pathol 2014;41:686)
- 61 year old man presented with 2 eyelid growths and 76 year old man presented with periorbital mass (Arch Craniofac Surg 2024;25:90)
- 68 year old woman presented with slow growing painless pink papules on eyelids (JAAD Case Rep 2021;9:78)
- 72 year old man presented with 2 year history of growing abdominal nodule (Ann Dermatol 2019;31:339)
- 84 year old man presented with blue mass of buccal space (Am J Otolaryngol 2018;39:242)
Treatment
- No standardized treatment algorithms due to rarity (Dermatol Surg 2023;49:1091)
- Complete excision (e.g., standard excision, wide local excision, Mohs micrographic surgery) is recommended to reduce risk of recurrence (Dermatol Surg 2023;49:1091, JAMA Dermatol 2014;150:380)
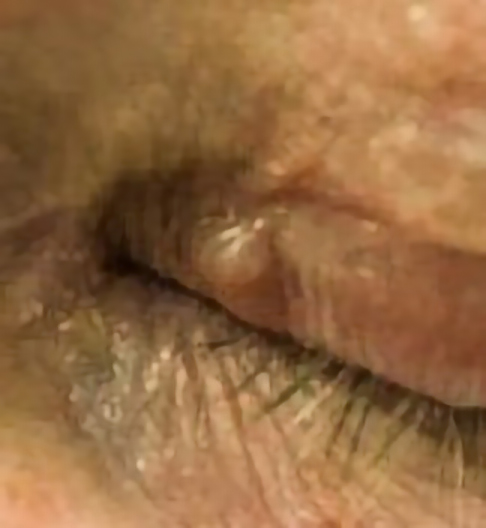
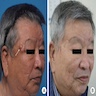
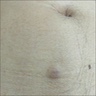
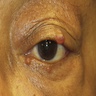
Clinical images
Gross description
- Soft nodule / mass (Dermatol Surg 2020;46:1141)
- May be translucent or cystic (Am J Otolaryngol 2018;39:242, Arch Craniofac Surg 2024;25:90)
- Nonencapsulated and usually poorly circumscribed (Am J Surg Pathol 2005;29:764)
Frozen section description
- Key diagnostic features have not been defined in this context
Microscopic (histologic) description
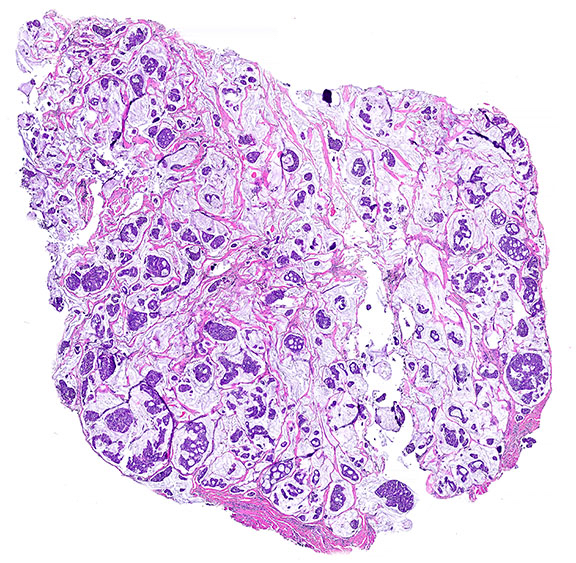
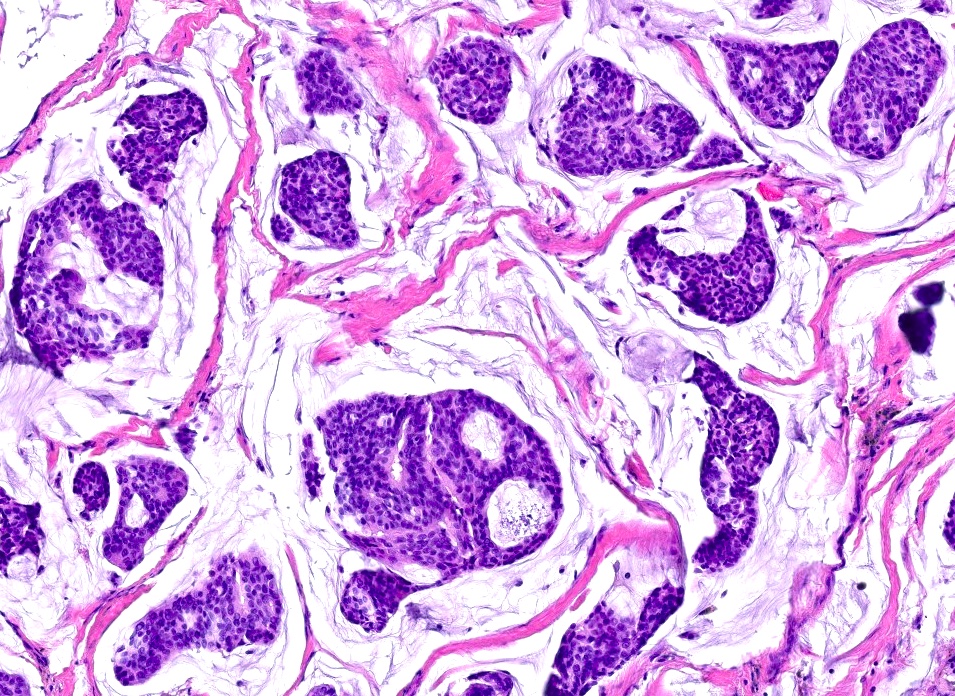
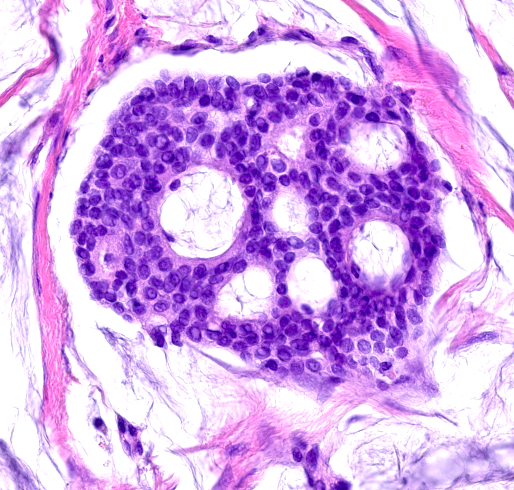
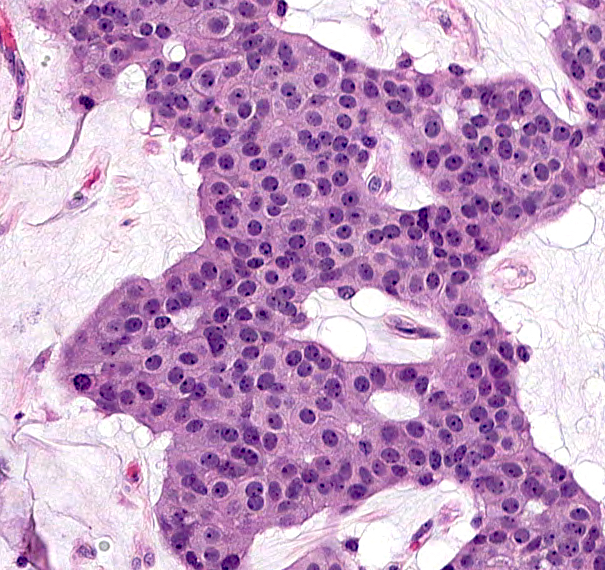
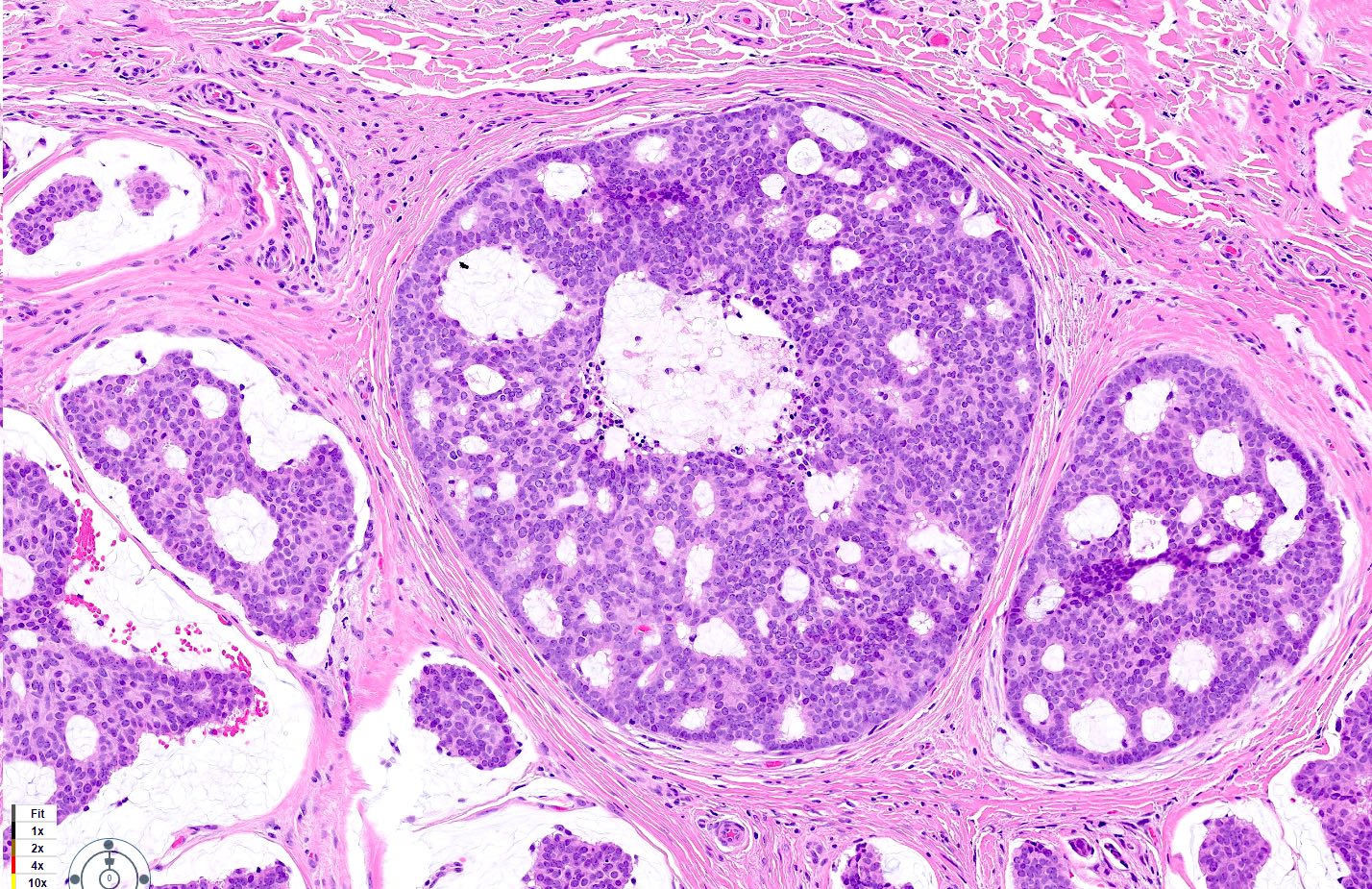
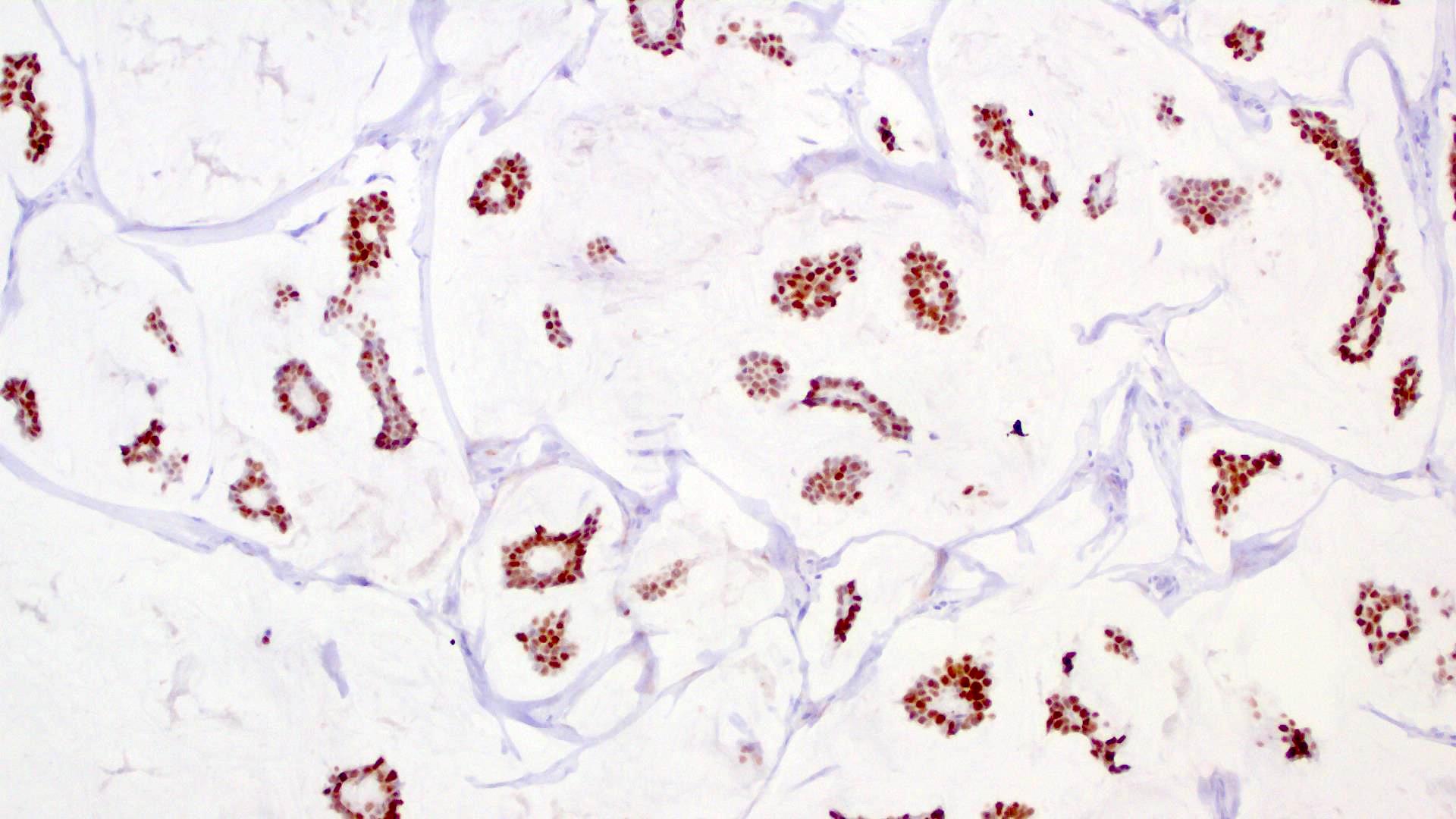
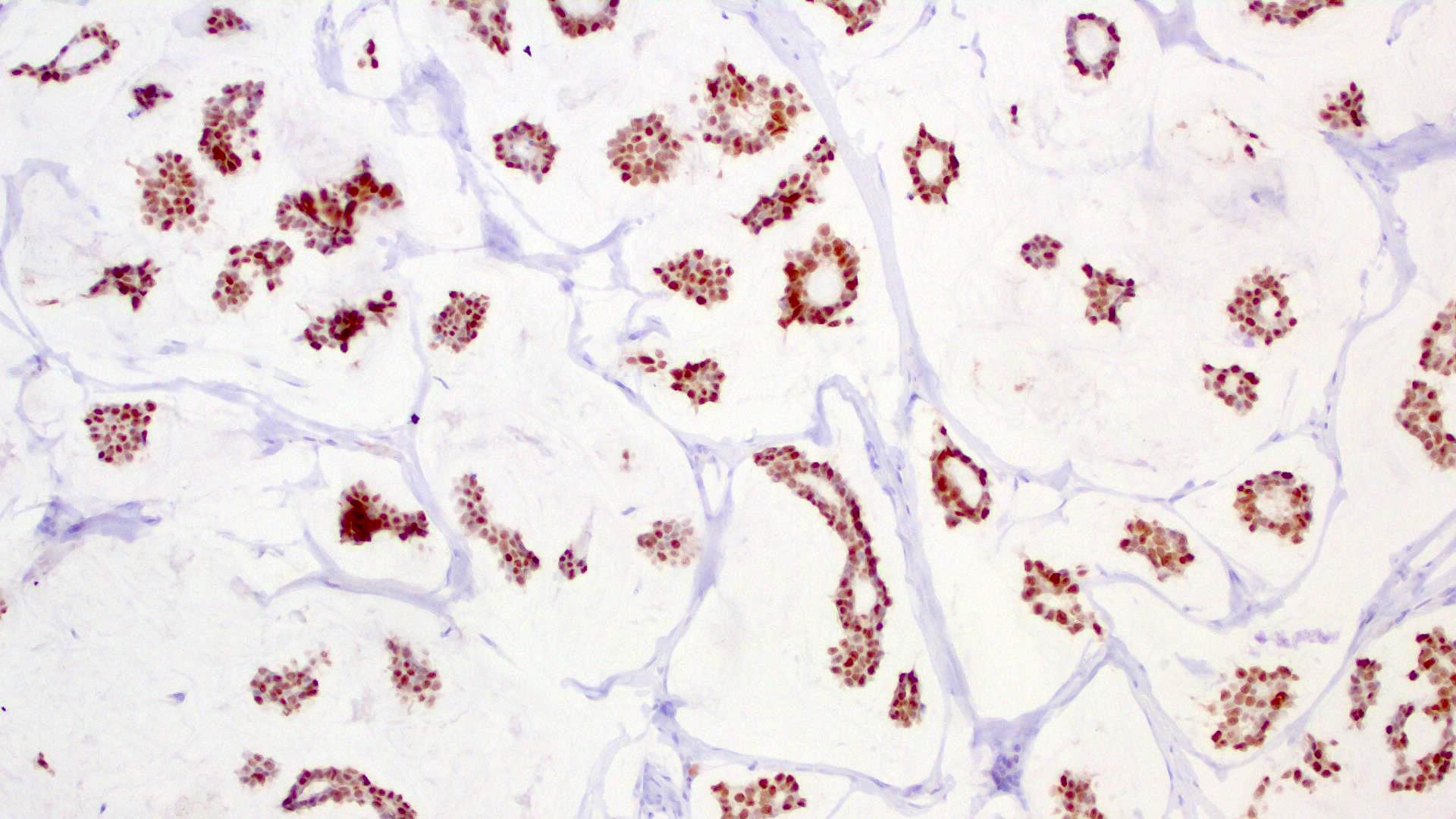
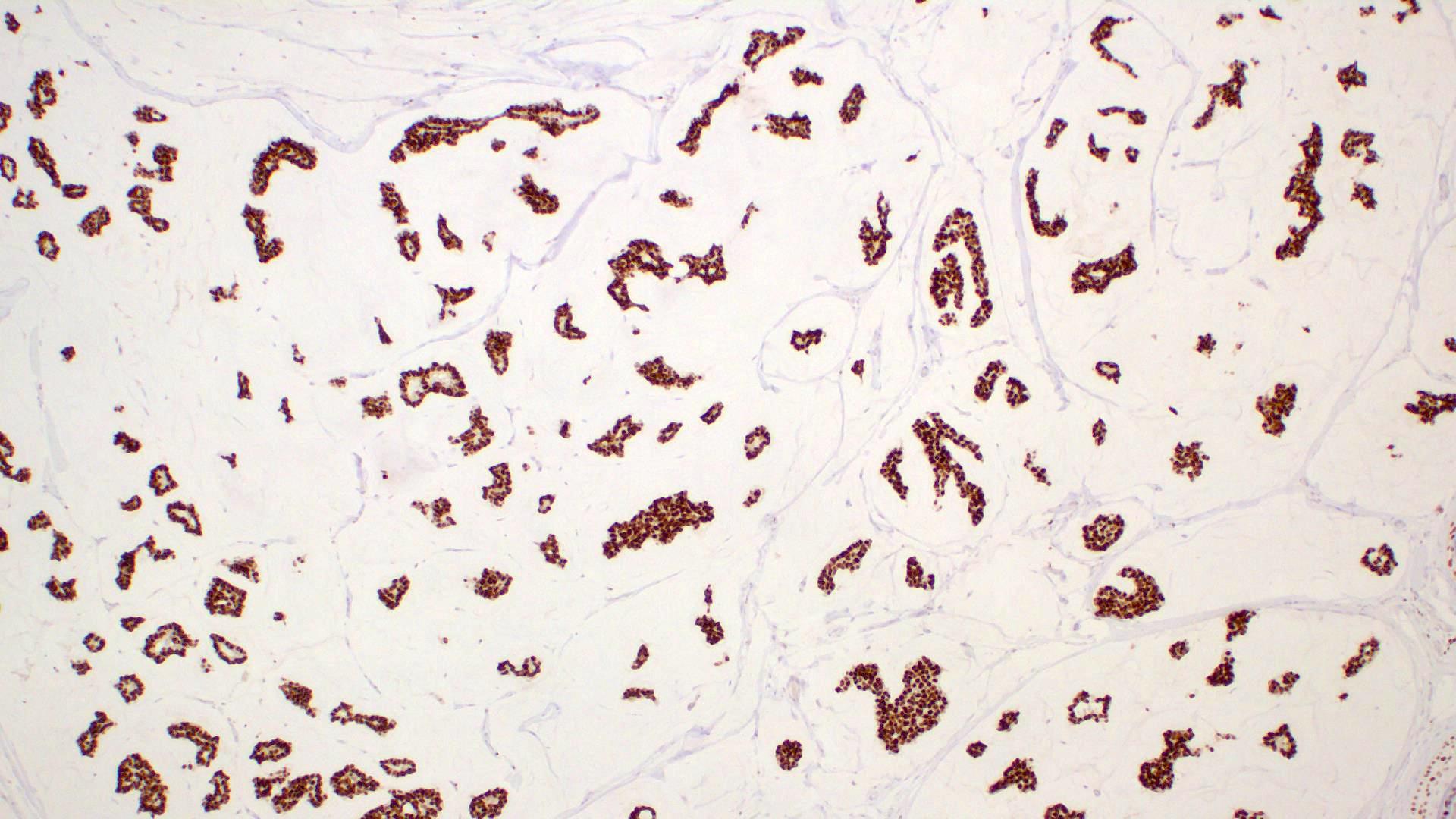
- Islands of basaloid cells surrounded by pools or lakes of mucin (Am J Dermatopathol 2024;46:114)
- Islands can be solid, cystic or cribriform
- With or without tubular / ductal structures between or within epithelial aggregates
- Fibrous septa separate lakes (Am J Dermatopathol 2024;46:114)
- Aggregates of neoplastic cells can extend deep in dermis with or without subcutis (far from main component)
- Polygonal / round / oval cells with small hyperchromatic nuclei and eosinophilic cytoplasm (Dermatol Surg 2023;49:1091)
- Decapitation secretion may be observed
- Low degree of atypia; mitotic figures uncommon (Dermatol Surg 2023;49:1091)
- Minimal inflammation
- In situ component (Am J Surg Pathol 2005;29:764)
- Present in most cases (60 - 70%) but may be focal
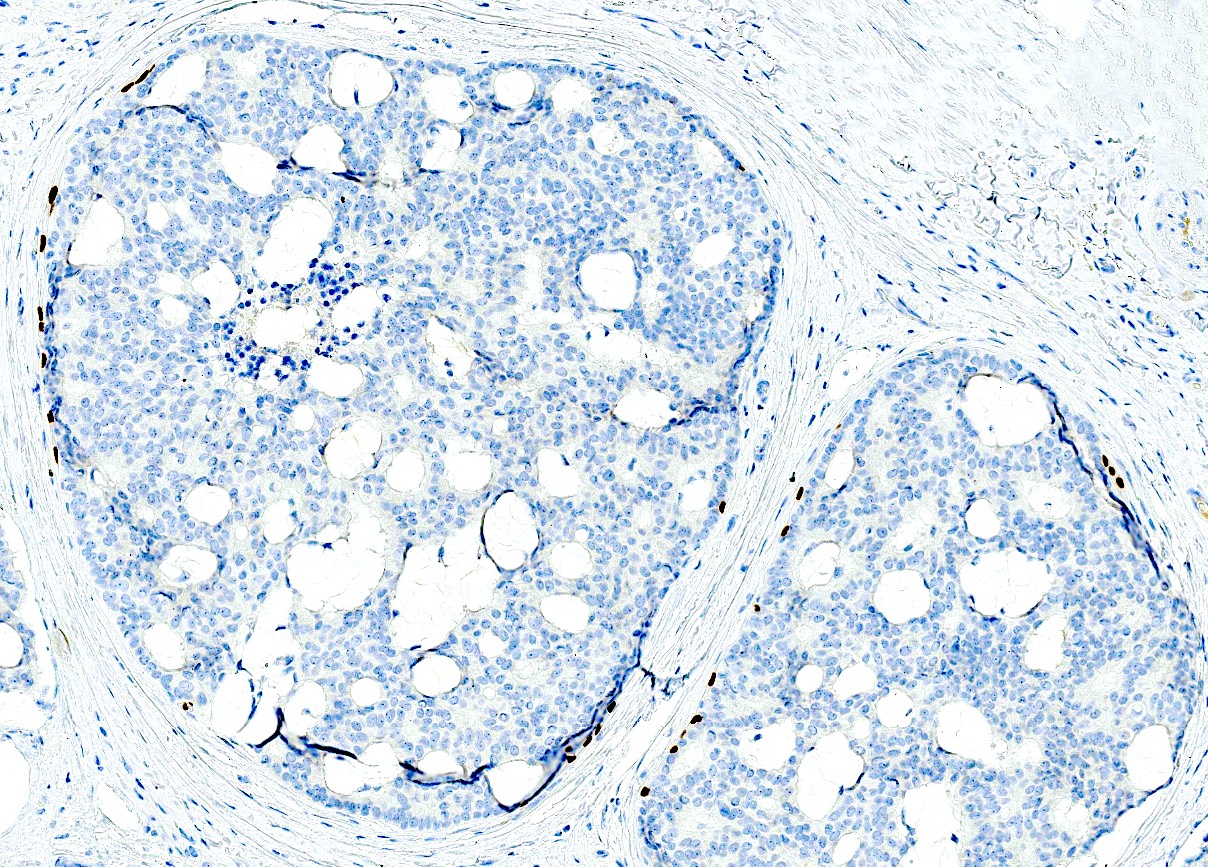
- Myoepithelial layer is highlighted by p63, SMA, calponin
- May resemble (atypical) ductal hyperplasia, ductal carcinoma in situ of the breast
- Potential architectural patterns: cribriform, solid, micropapillary, papillary
- Uncommon features / presentations
- Psammoma bodies (Am J Dermatopathol 2008;30:510)
- Plasmacytoid cells (J Dermatol 2010;37:767)
- Hair follicle induction in epidermis (Am J Surg Pathol 2005;29:764)
- Oxyphilic or columnar metaplasia (Am J Surg Pathol 2005;29:764)
- Mucin with epithelial aggregates dissecting collagen and mimicking lymphatic invasion (Am J Surg Pathol 2005;29:764)
- Peritumoral fibrosis (Am J Surg Pathol 2005;29:764)
- Signet ring cell changes (Am J Surg Pathol 2005;29:764)
- Mixed variant with combination of invasive ductal carcinoma (representing ≥ 10% of total tumor) and classic mucin lakes (representing < 90% total tumor) (Am J Surg Pathol 2005;29:764)
- Perianal lesions: may have pagetoid spread with or without anastomosing cords extending from epidermis into dermis (Am J Surg Pathol 2005;29:764)
Microscopic (histologic) images
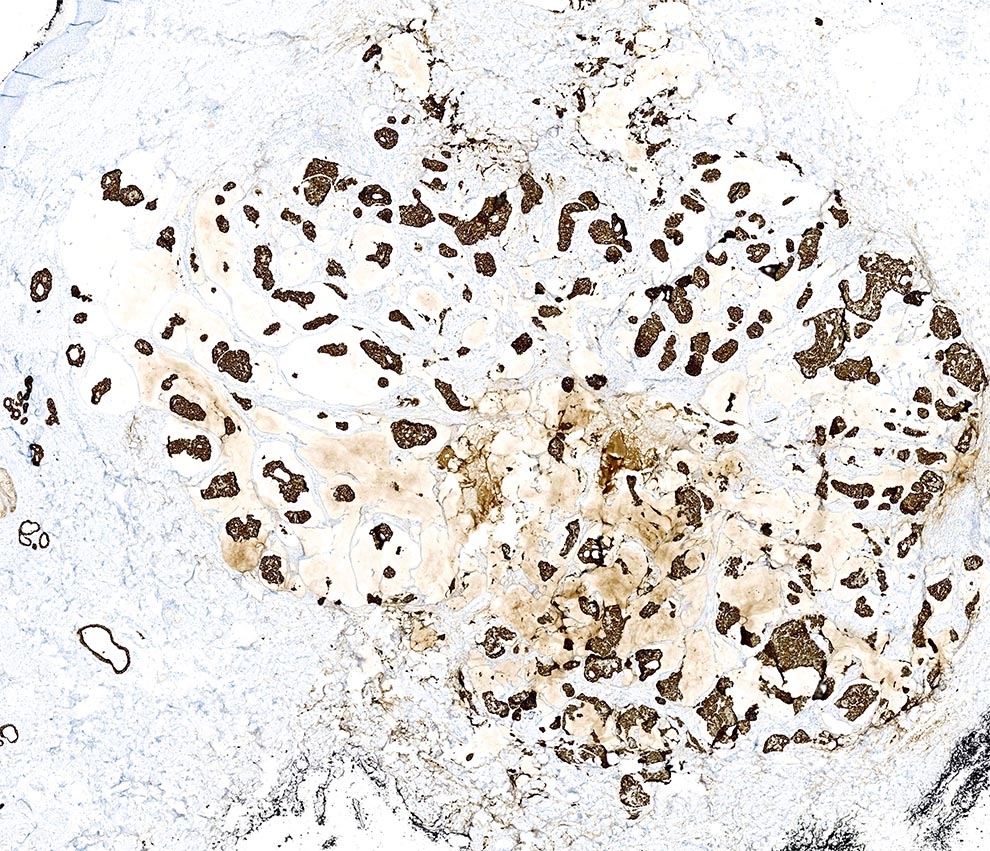
Positive stains
- Immunohistochemistry (Am J Surg Pathol 2020;44:1005, Dermatol Surg 2023;49:1091, Am J Dermatopathol 2024;46:114)
- Cytokeratins: low molecular weight cytokeratins, CK7, AE1 / AE3, CAM 5.2
- EMA, E-cadherin, GATA3, c-MYB (Am J Dermatopathol 2017;39:279, J Cutan Pathol 2017;44:444)
- ER, PR and GCDFP-15 (BRST2) (Am J Surg Pathol 2005;29:764)
- Mucinous carcinoma of breast can have same staining profile
- In situ component (if present): myoepithelial layer positive for p63, p40 or calponin (Am J Surg Pathol 2005;29:764)
- MUC (Am J Dermatopathol 2024;46:114)
- CEA: can be positive (variable reports on sensitivity) (Am J Surg Pathol 2005;29:764)
- Neuroendocrine differentiation in a subset (J Cutan Pathol 2014;41:686)
- Synaptophysin, chromogranin, CD56 and neurospecific enolase can be expressed, including for noneyelid sites but many cases are negative (Ann Dermatol 2010;22:472, J Cutan Pathol 2014;41:686, Am J Surg Pathol 2005;29:764)
- Small study (n = 9) of noneyelid primary cutaneous mucinous carcinoma (PCMC) with the more sensitive neuroendocrine marker INSM1 found that the majority were positive (Am J Surg Pathol 2023;47:1186)
- Endocrine mucin producing sweat gland carcinoma (EMPSGC) associated PCMC proposed to be the neuroendocrine subtype of PCMC (Am J Surg Pathol 2020;44:1005)
- Frequency and significance of neuroendocrine marker expression in PCMC not associated with EMPSGC is unclear (Am J Surg Pathol 2020;44:1005)
- Special stains
- Mucin stains
- Alcian blue (pH 2.5)
- Colloidal iron
- Mucicarmine
- PAS
- Mucin stains
Negative stains
- CK20 (whereas positive in most metastatic GI mucinous carcinomas) (Am J Surg Pathol 2005;29:764)
- CDX2 (whereas positive in most GI mucinous carcinomas) (Am J Surg Pathol 2005;29:764)
- HER2 / neu (Am J Surg Pathol 2005;29:764)
- MUC5AC and MUC6 (Am J Surg Pathol 2005;29:764)
Electron microscopy description
- Electron microscopy is not required for diagnostic confirmation
Molecular / cytogenetics description
- Focused molecular studies have been negative for oncogenic drivers (Surg Pathol Clin 2021;14:251)
- No evidence of HER2 / neu amplification has been demonstrated (Am J Surg Pathol 2005;29:764)
Videos
Primary cutaneous mucinous carcinoma and endocrine mucin producing sweat gland carcinoma
Mucinous carcinoma of skin
Sample pathology report
- Skin of left cheek, excision:
- Mucinous carcinoma, margins negative (see comment)
- Comment: Histology shows islands of basaloid cells surrounded by pools or lakes of mucin. Immunohistochemistry reveals positive expression for CK7 and GCDFP-15. In situ component is present and shows positive expression for p63. Negative staining for CK20, synaptophysin, chromogranin A and CDX2. The findings are most consistent with mucinous carcinoma. Correlation with clinical history and any indicated metastatic workup is recommended for distinction between primary cutaneous mucinous carcinoma and metastatic mucinous carcinoma originating from another site.
Differential diagnosis
- Must rule out cutaneous metastasis or direct extension from visceral mucinous carcinoma (Dermatol Surg 2020;46:1141)
- Presence of in situ component supports cutaneous origin
- No definitive way to rule out metastasis if in situ component is absent (Dermatol Surg 2020;46:1141)
- Morphologic features suggestive of metastasis
- Increased cytologic atypia with or without more mitotic activity (although cutaneous metastasis may also be bland) (Dermatol Surg 2023;49:1091)
- More clusters of cohesive neoplastic cells (J Pathol Transl Med 2018;52:238)
- Less mucin (J Pathol Transl Med 2018;52:238)
- Absence of fibrous septa (J Pathol Transl Med 2018;52:238)
- Intestinal origin: dirty necrosis, glandular components resembling colonic adenocarcinoma (Am J Surg Pathol 2005;29:764)
- Immunohistochemistry (Am J Surg Pathol 2005;29:764, J Pathol Transl Med 2018;52:238)
- In situ component with peripheral myoepithelial cell layer (p63, SMA and calponin): specific but not completely sensitive for cutaneous origin
- Mucinous carcinoma from intestine:
- Mammary mucinous carcinoma:
- Workup for metastatic disease (PET scan, CT, mammography, etc.) (Am J Surg Pathol 2005;29:764, Dermatol Surg 2020;46:1141)
- Endocrine mucin producing sweat gland carcinoma (EMPSGC):
- Proposed precursor lesion for mucinous carcinoma with neuroendocrine differentiation
- Tumors with areas of both mucinous carcinoma and EMPSGC may be encountered (Am J Surg Pathol 2020;44:1005, J Cutan Pathol 2015;42:578)
- These are proposed to exist on a clinical and histopathologic spectrum (Dermatol Surg 2023;49:1091, Am J Surg Pathol 1997;21:1501, Am J Surg Pathol 2005;29:1330, Am J Surg Pathol 2020;44:1005)
- Almost exclusively on eyelids or periorbital skin (rare reports of extrafacial occurrence) (J Cutan Pathol 2014;41:544)
- Association with benign sweat ducts; in situ carcinoma that spreads between luminal ductal epithelium and myoepithelial layer (Am J Surg Pathol 2005;29:1330, J Cutan Pathol 2015;42:578)
- Mucin within cystic spaces and epithelial aggregates but not lakes / pools (Am J Surg Pathol 2005;29:1330)
- Positive for neuroendocrine markers (chromogranin, synaptophysin, neuron specific enolase, INSM1) (Dermatol Surg 2023;49:1091, Am J Surg Pathol 1997;21:1501)
- Can be focal or even absent on small biopsy (Am J Surg Pathol 2020;44:1005)
- If invasive component develops within lakes / pools of mucin, considered to have progressed to cutaneous mucinous carcinoma (Am J Surg Pathol 2020;44:1005)
- Distinction between mucinous carcinoma with more solid areas and EMPSGC can be challenging (Am J Surg Pathol 2020;44:1005, J Cutan Pathol 2015;42:578, Dermatol Surg 2023;49:1091)
- Proposed precursor lesion for mucinous carcinoma with neuroendocrine differentiation
Additional references
Practice question #1
An 81 year old woman presents with a 1.5 x 2.0 cm, slow growing nodule on the right cheek. An excisional biopsy is performed, which shows the findings in the figure above. Immunohistochemistry is positive for CK7 and GCDFP-15. p63, synaptophysin, chromogranin A, CK20 and CDX2 are diffusely negative. What is the most likely diagnosis?
- Basal cell carcinoma
- Cutaneous metastasis of mammary mucinous carcinoma
- Endocrine mucin producing sweat gland carcinoma
- Primary cutaneous mucinous carcinoma
- Unable to distinguish between primary cutaneous mucinous carcinoma and mammary mucinous carcinoma with information provided
Practice answer #1
E. Unable to distinguish between primary cutaneous mucinous carcinoma and mammary mucinous carcinoma with information provided. Both primary cutaneous mucinous carcinoma and mammary mucinous carcinoma have the characteristic histopathology shown in the figure: basaloid islands floating in pools of mucin, separated by fibrous septa. Without the in situ component (positive for p63), it is impossible to distinguish between these 2 entities. CK20 and CDX2 negativity militates against a gastrointestinal visceral metastasis. Further workup for metastatic disease is essential. Answer A is incorrect because the histopathology and staining profile is not characteristic for basal cell carcinoma (BCC). Stromal mucin is seen in BCC but not pools of mucin characteristic of mucinous carcinoma. GCDFP-15 is negative in basal cell carcinoma. Answers B and D are incorrect because histopathology and immunohistochemistry cannot distinguish between these entities. Answer C is incorrect because endocrine mucin producing sweat gland carcinoma has different histopathologic findings (i.e., not characterized by invasive basaloid islands floating in pools of mucin) and it should stain positive for neuroendocrine markers (e.g., synaptophysin, chromogranin A) and p63 in the myoepithelial layer.
Comment Here
Reference: Primary cutaneous mucinous carcinoma
Comment Here
Reference: Primary cutaneous mucinous carcinoma
Practice question #2
Which of the following is true about primary cutaneous mucinous carcinoma?
- Cutaneous mucinous carcinoma is an aggressive tumor with a high metastatic rate
- Neuroendocrine differentiation of primary cutaneous mucinous carcinoma is associated with a worse prognosis
- Primary cutaneous mucinous carcinoma and endocrine mucin producing sweat gland carcinoma are synonymous entities
- Recurrences are common and complete excision with margin assessment is recommended
- The scalp is the most common location for primary cutaneous mucinous carcinoma
Practice answer #2
D. Recurrences are common and complete excision with margin assessment is recommended. Recurrences are common in primary cutaneous mucinous carcinoma (~20 - 30%). This may be a result of small tumor aggregates extending into the deeper dermis / subcutis, far from the main tumor mass. Complete excision with margin assessment is recommended. Answer A is incorrect because primary cutaneous mucinous carcinoma is typically considered a low grade neoplasm with a low metastatic rate. In contrast, metastatic visceral carcinoma to the skin has a worse prognosis. Answer B is incorrect because the association of neuroendocrine differentiation with prognosis is inconclusive. However, reviews by Agni et al. and Au et al. suggest the neuroendocrine subtype of primary cutaneous mucinous carcinoma may have a more indolent behavior and more favorable prognosis (Am J Dermatopathol 2023;45:123). Answer C is incorrect because endocrine mucin producing sweat gland carcinoma is typically considered an in situ precursor to primary cutaneous mucinous carcinoma with neuroendocrine differentiation. Answer E is incorrect because while the head / neck is the most common location for this tumor, ~50% occur on the eyelid. The scalp comprises 17% of cases and nonperiorbital head / neck comprises 20% of cases.
Comment Here
Reference: Primary cutaneous mucinous carcinoma
Comment Here
Reference: Primary cutaneous mucinous carcinoma