Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Sharma A, Bennett JA. Signet ring stromal tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ovarytumorsignetringstromal.html. Accessed April 26th, 2024.
Definition / general
- Rare, benign ovarian stromal tumor with signet ring cells
Essential features
- Primary ovarian stromal neoplasm comprised of various proportions of signet ring and spindle cells
- Cytoplasmic vacuoles in signet ring cells are negative for lipid, mucin and glycogen
- Usually positive, at least focally, for 1 or more sex cord markers (SF1, inhibin, calretinin) but typically negative for epithelial membrane antigen (EMA)
Terminology
- Signet ring stromal cell tumor (SRSCT)
- Signet ring stromal tumor (SRST)
ICD coding
Epidemiology
- ~20 cases reported to date
- No age predilection (range: 28 - 70 years; mean: 49 years) (Am J Surg Pathol 2022;46:1599)
Sites
- Ovary
Pathophysiology
- Unknown; hypothesized to arise from ovarian stromal cells or multifocal conversion of a fibroma (Adv Anat Pathol 2014;21:443)
Etiology
- Unknown
Clinical features
- Nonspecific including abdominopelvic pain, abnormal uterine bleeding or incidental finding on imaging
Diagnosis
- Oophorectomy
Laboratory
- CA-125 typically within normal limits
- Mildly elevated (76.1 U/mL; normal < 46 U/mL) in one report (Int J Gynecol Pathol 2020;39:193)
Radiology description
- No defining features to distinguish from other ovarian neoplasms on imaging
- Solid or cystic, may appear complex
Prognostic factors
- No reports of metastasis or recurrence but overall limited follow up data
Case reports
- 44 year old woman with polymenorrhea and 4 cm right adnexal mass (J Turk Ger Gynecol Assoc 2011;12:59)
- 69 year old obese woman with bilateral adnexal masses on imaging (Obstet Gynecol 2010;116:556)
- 70 year old woman with abdominal distention, rectal bleeding and bilateral ovarian masses (Int J Gynecol Pathol 2020;39:193)
- 76 year old woman with incidental complex adnexal mass (Med Mol Morphol 2008;41:165)
Treatment
- Surgical excision (oophorectomy)
Gross description
- Most are unilateral
- Typically well circumscribed and unencapsulated
- Yellow to tan and soft to firm cut surface
- May show white fibromatous areas
- Variable hemorrhage, necrosis and cystic spaces
- Range: 3.0 - 20 cm (mean: 12.8 cm) (Am J Surg Pathol 2022;46:1599)
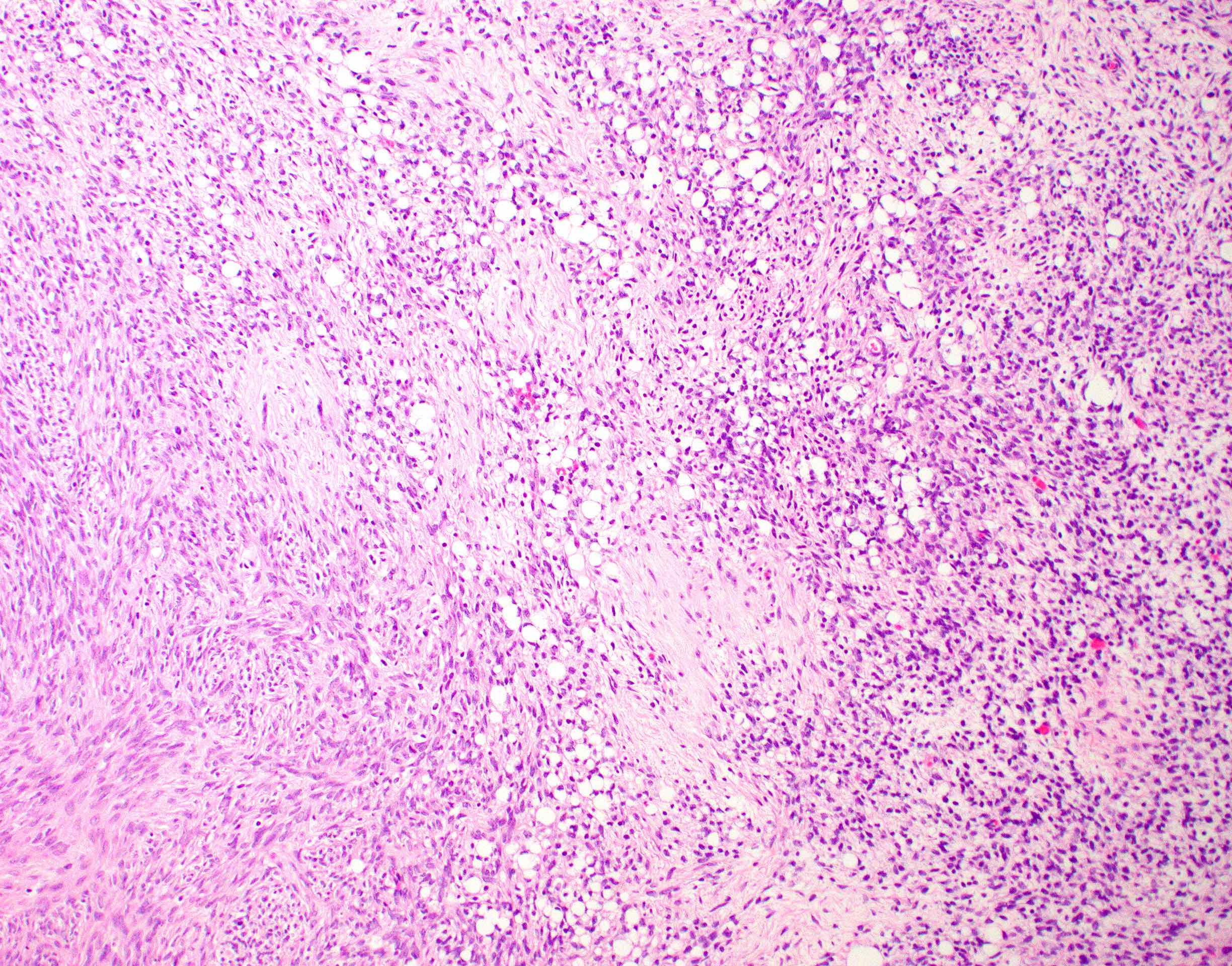
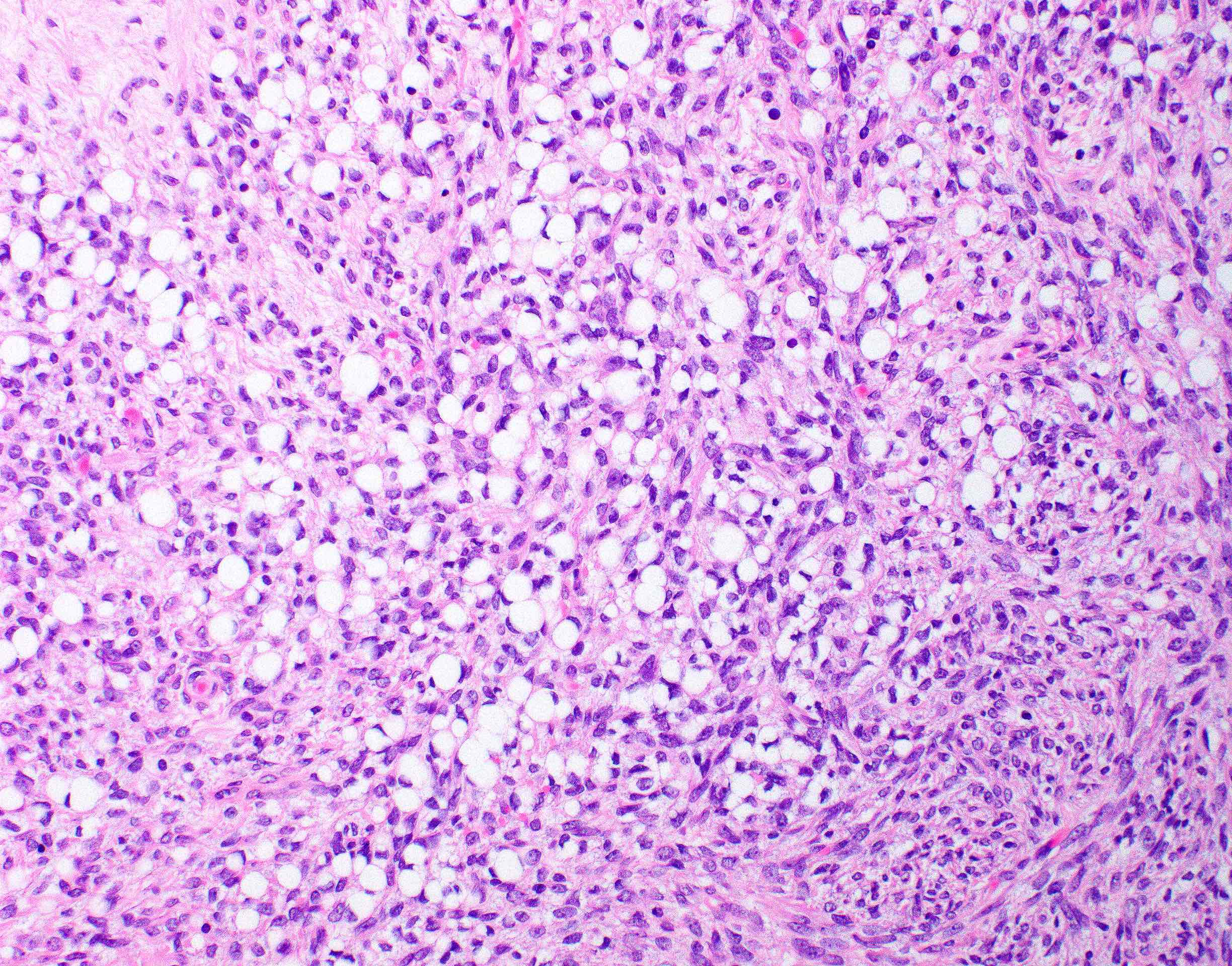
Microscopic (histologic) description
- 2 cell populations in variable proportions
- Diffuse growth of round cells with single to occasionally multiple intracytoplasmic, optically clear vacuoles, which peripherally displace and indent nuclei into a crescent shape (signet ring cells)
- Fusiform cells with eosinophilic cytoplasm and ovoid nucleus
- May have fibroma-like areas that merge with signet ring component
- Absent to minimal cellular atypia, mitotic activity and necrosis
- Rarely show brisk mitoses (13/10 and 16/10 high power fields) (Ultrastruct Pathol 1995;19:401, Int J Gynecol Pathol 2004;23:45)
- PAS+ intracytoplasmic hyaline globules noted in ~50% (Ultrastruct Pathol 1995;19:401, Virchows Arch A Pathol Anat Histopathol 1993;422:333, Int J Gynecol Pathol 2004;23:45, Int J Surg Pathol 2008;16:180, Pathol Oncol Res 2008;14:333, Med Mol Morphol 2008;41:165, J Turk Ger Gynecol Assoc 2011;12:59, Int J Gynecol Pathol 2020;39:193)
Microscopic (histologic) images
Positive stains
- Usually positive, at least focally for 1 or more sex cord markers (SF1, calretinin, inhibin, WT1) (Am J Surg Pathol 2022;46:1599)
- Variable expression for CD10 (usually focal), AE1 / AE3 and CAM5.2
Negative stains
- Hallmark is negativity of intracytoplasmic vacuoles for
- Mucins (mucicarmine, PASD, Alcian blue)
- Glycogen (PAS, Alcian blue)
- Lipid (Sudan III, Oil Red O)
- Usually negative for cyclin D1, with cytoplasmic beta catenin expression (not nuclear)
- EMA, CK7, CK20
- Reference: Am J Surg Pathol 2022;46:1599
Electron microscopy description
- Cytoplasmic vacuoles result from (Ultrastruct Pathol 1995;19:401)
- Generalized edema of cytoplasmic matrix
- Dilatation of mitochondria
- Invagination of cell membranes (pseudoinclusions) by extracellular matrix
- No basement membrane formation and abundant free ribosomes (Cancer 1976;38:166, Gynecol Oncol 2000;77:323)
- Hyaline globules show features of lysosomes or degenerating erythrocytes (Med Mol Morphol 2008;41:165, Ultrastruct Pathol 1995;19:401)
Molecular / cytogenetics description
- Negative for FOXL2 and CTNNB1 mutations (Int J Gynecol Pathol 2020;39:193, Am J Surg Pathol 2022;46:1599)
Sample pathology report
- Right ovary, oophorectomy:
- Signet ring stromal tumor (5.0 cm)
Differential diagnosis
- Krukenberg tumor (Am J Surg Pathol 2006;30:277):
- Bilateral in > 50%, often with extraovarian disease at presentation
- Nodular growth
- Admixed with glands, nests or cords of malignant cells (may be very focal)
- Cytokeratin strongly and diffusely positive
- Vacuoles are positive for PASD and mucicarmine
- Similar to SRST, it might have prominent fibromatous component
- Microcystic stromal tumor (Am J Surg Pathol 2009;33:367, Am J Surg Pathol 2015;39:1420, Am J Surg Pathol 2018;42:137, Am J Surg Pathol 2022;46:1599):
- Often separated into lobules by hyalinized bands
- Admixture of microcysts, solid cellular areas and fibrous stroma
- Up to 60% have bizarre nuclei
- Diffuse cyclin D1 and CD10
- Aberrant (nuclear) beta catenin
- Negative for calretinin and inhibin
- Characterized by CTNNB1 or APC mutations
- Signet ring cell change (Adv Anat Pathol 2014;21:443):
- Recent study suggests that some signet ring stromal tumors are fibromas with signet ring cell change (Am J Surg Pathol 2022;46:1599)
- Described in adult granulosa cell tumor (n=1), Brenner tumor (n=1) and serous cystadenofibroma (n=2)
Board review style question #1
Board review style answer #1
B. It is negative for EMA. This is a signet ring stromal tumor. Answers A and E are incorrect as this is a benign entity for which excision is curative. Answer D is incorrect because the tumor is usually unilateral but may rarely be bilateral. Answer C is incorrect as it is negative for mucicarmine. Given the signet ring cell morphology and cytokeratin expression, there may be concern for a metastatic mucinous carcinoma with signet ring cells (i.e., Krukenberg tumor). However, EMA helps to distinguish between the 2 entities as it is negative in signet ring stromal tumor but positive in Krukenberg tumor.
Comment Here
Reference: Signet ring stromal tumor
Comment Here
Reference: Signet ring stromal tumor
Board review style question #2
A 32 year old woman without significant medical history presents with abdominal pain. Workup reveals a 2 cm right adnexal mass on ultrasound and she undergoes salpingo-oophorectomy. Histologic examination of the ovarian tumor reveals sheets of cells with crescent shaped nuclei and intracytoplasmic vacuoles. The vacuoles are negative for mucicarmine and periodic acid Schiff (PAS). The cells are positive for inhibin and steroidogenic factor 1 (SF1) but negative for cyclin D1 and epithelial membrane antigen (EMA). Beta catenin shows cytoplasmic expression. What is the likely diagnosis?
- Adult granulosa cell tumor
- Brenner tumor
- Krukenberg tumor
- Microcystic stromal tumor
- Signet ring stromal tumor
Board review style answer #2
E. Signet ring stromal tumor. All of the answers above are reasonable considerations for which clinicopathologic and immunohistochemical features should aid towards the diagnosis; however, in contrast to the other options, signet ring stromal tumors should present as a unilateral mass with expression of sex cord markers and negativity for cyclin D1 and beta catenin (membranous positivity only). Answer A is incorrect because adult granulosa cell tumor usually occurs in postmenopausal females who present with estrogenic manifestations. Histologically, multiple growth patterns are common and cells are usually small with coffee bean nuclei, longitudinal nuclear grooves and scant cytoplasm. Answer C is incorrect because Krukenberg tumor also presents in older females, usually as bilateral ovarian masses, with positivity for cytokeratin and negativity for sex cord markers. Answer D is incorrect because microcystic stromal tumor can show signet ring cells along with variably thick collagenous septations and strong nuclear expression of beta catenin and cyclin D1. Answer B is incorrect because Brenner tumor usually shows a nested architecture of transitional type cells embedded in a fibromatous stroma and should be positive for CK7 and EMA.
Comment Here
Reference: Signet ring stromal tumor
Comment Here
Reference: Signet ring stromal tumor