Table of Contents
Definition / general | Erythropoietin and thrombopoietin | Erythrocyte production | Diagrams / tables | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Additional referencesCite this page: Luca DC. Erythroid maturation (erythropoiesis). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowerythroidmaturation.html. Accessed September 15th, 2025.
Definition / general
- Erythropoiesis takes place within the bone marrow in units composed of macrophages surrounded by erythroblasts; either erythroid burst forming units (BFU-E, immature) or erythroid colony forming units (CFU-E, more mature)
- Progresses from myeloid stem cell to pronormoblast, to basophilic normoblast, to polychromatophilic normoblast, to orthochromatic normoblast (then extrusion of nucleus), to reticulocyte (young erythrocyte) and then to erythrocyte (red blood cell)
- Characterized by increasing hemoglobin synthesis, decreasing cell size, decreasing cytoplasmic basophilia and progressive chromatin condensation with extrusion of nucleus at the orthochromatic stage of development
- Extruded nuclei bind on macrophage receptors and are subsequently phagocytized; loss of mitotic capability occurs after the early stage of polychromatophilic normoblast
- Average of 4 cell divisions during maturation, so a proerythroblast can give rise to 16 red cells
- Average time in the bone marrow from proerythroblast to reticulocyte is 7 days; average time for reticulocytes to mature into red cells is 1 - 2 days
- Early erythroid precursors cluster in islands randomly distributed throughout marrow but related to vascular structures
- Erythroblast (pronormoblast) islands may be specialized niches where intercellular interconnections and cytokines regulate erythropoiesis (Curr Opin Hematol 2006;13:137)
Erythropoietin and thrombopoietin
- Erythropoietin (EPO): lineage specific cytokine (glycoprotein) responsible for erythropoiesis (proliferation, differentiation and survival by delaying apoptosis), produced by renal cells in response to hypoxia and by the bone marrow
- EPO receptor present in highest concentrations on erythroid progenitor cells and proerythroblasts but absent on mature erythrocytes
- Thrombopoietin (TPO): may also have a role in erythroid maturation
- Heme protein and tumor necrosis factor (TNF) exert negative feedback on the production of red blood cells
Erythrocyte production
- Steady state: 2 - 4 X 109 erythrocytes/kg/day; 40,000 - 80,000 reticulocytes/µL/day
- In utero: hypoxic environment → high EPO → high erythroid production; after birth: oxygen → drop in EPO → dramatic drop in the number of erythroid elements in the bone marrow in the first few months of life (as low as < 5%)
- After the initial postnatal period: marked decrease in hematocrit → hypoxia → EPO release by kidney → increase in erythroid elements to the normal 20 - 25% of nucleated cells in bone marrow (sustained hypoxia → up to 5 - 7 times increase in number)
- Sideroblast: normoblast containing small iron granules detectable with Prussian blue (approximately 20% of normoblasts)
- Erythropoiesis stimulators: anemia / hypoxia, thyroid hormones, androgens, growth hormone and corticosteroids
Microscopic (histologic) description
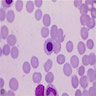
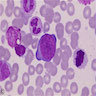
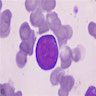
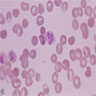
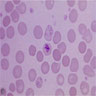
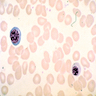
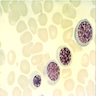
- Pronormoblast: 13 - 18 microns, round / ovoid with thin rim of basophilic cytoplasm, large spherical nucleus with fine chromatin and 1 - 2 nucleoli; usually perinuclear halo; N/C ratio is 90%
- Basophilic normoblast: 12 - 17 microns; increase in deeply basophilic cytoplasm compared to pronormoblast and slightly smaller nucleus with slight chromatin condensation; often perinuclear halo; no granules, no nucleolus; N/C ratio is 75 - 85%
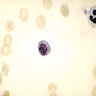
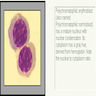
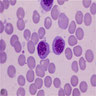
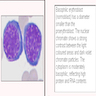
- Polychromatophilic normoblast: 12 - 15 microns; round / ovoid with abundant, dull gray to gray green, variegated cytoplasm due to polyribosomes (basophilic) and hemoglobin (eosinophilic); round, condensed and basophilic nucleus has coarse granules that give it a cartwheel appearance; perinuclear halo present; no nucleolus; N/C ratio is 60 - 80%
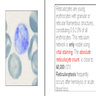
- Orthochromatophilic normoblast: 8 - 12 microns; round / ovoid cells with pink orange uniformly staining cytoplasm, dark and opaque nucleus that may be pyknotic or in the process of being extruded, no nucleolus
- Reticulocyte: 7 - 10 microns; cannot identify without supravital stain (new methylene blue, brilliant cresyl blue) that colors RNA deep blue and granular; must have at least 2 granules to classify as reticulocyte; cytoplasm is red to pale blue due to RNA, no nucleus is present; larger than mature erythrocyte and lacks central pallor, passes through the bone marrow sinus wall and endothelial cells into the circulating blood where it matures
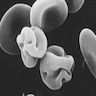
- Erythrocyte: 7 - 8 microns; round / ovoid biconcave disc with orange red cytoplasm, no RNA, no nucleus; survives ~120 days in circulation
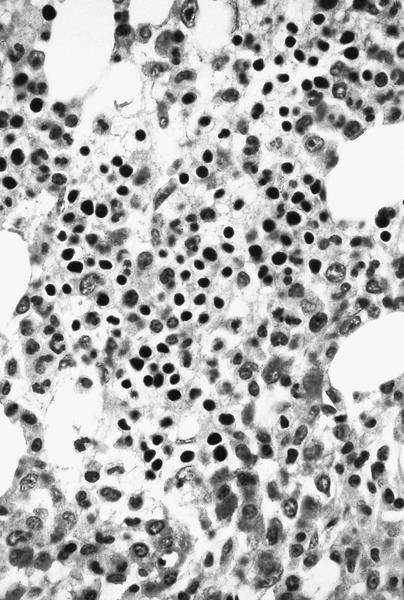
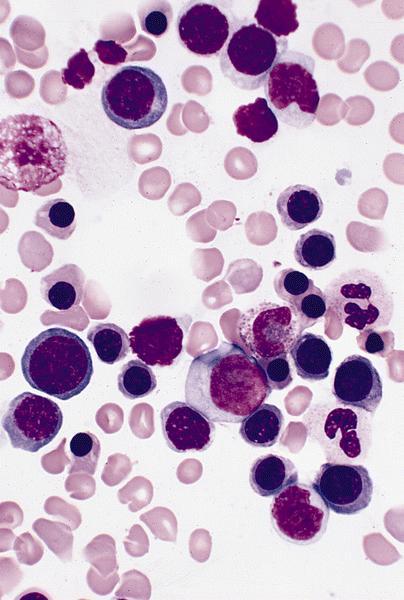
Microscopic (histologic) images
Positive stains
- GLUT1, CD35, CD36 (early precursors), CD38, CD41, CD43, CD44, CD47, CD49d (erythrocyte precursors only), CD58, CD71 (precursors through reticulocytes), CD75, CD105 (erythrocyte precursors only), CD108, CD111, CD139 (weak), CD147, CD233, CD235a (glycophorin A), CD235b (glycophorin B), CD235ab (glycophorin A/B), CD236 (glycophorin C/D), CD236R (glycophorin C), CD238 (Kell), CD239 (Lutheran), CD240 CE (RhC & RhE), CD240 D (RhD), CD240 DCE (RhD/CE) and CD241 (Rh50, RHAG)
- Expression of both common leukocyte and transferrin receptor antigens progressively declines with maturation while glycophorin expression remains high
Negative stains
Electron microscopy description
- Erythroblasts have characteristic surface invaginations developing into small intracytoplasmic vesicles (rhopheocytotic vesicles)
Additional references