Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2 | Practice question #3 | Practice answer #3 | Practice question #4 | Practice answer #4Cite this page: Popescu MC, Tretiakova M. Chromophobe. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccchromo.html. Accessed October 5th, 2025.
Definition / general
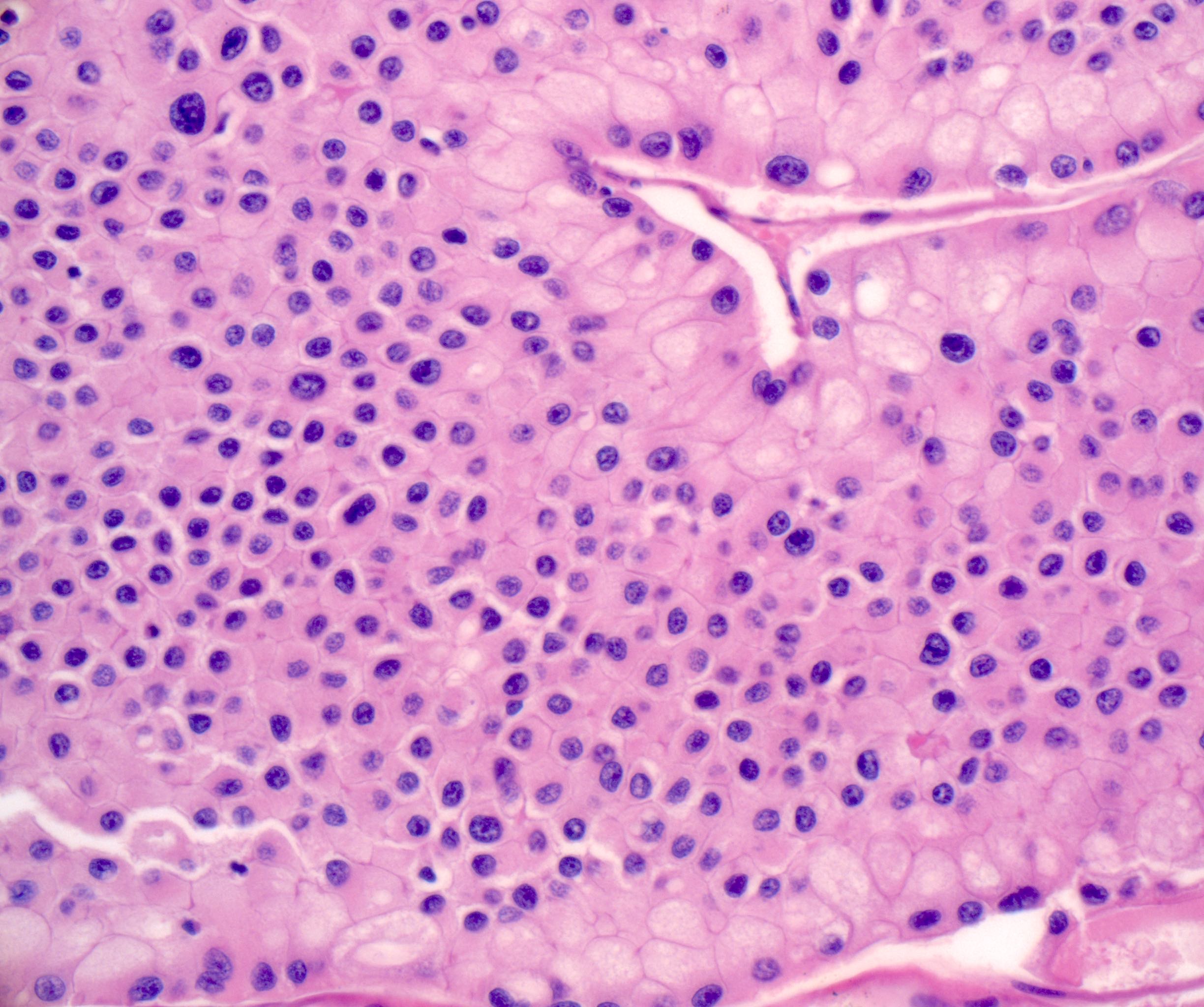
- Third most common renal cell carcinoma arising from the intercalated tubular cells, classically composed of solid sheets and broad trabeculae of alternating larger pale and smaller eosinophilic cells with prominent cell borders, perinuclear halos and wrinkled hyperchromatic nuclei
- Morphologically diverse with several histological subtypes and patterns described to date (Adv Anat Pathol 2022;29:194)
Essential features
- Typically composed of solid sheets of pale polygonal cells traversed by incomplete fibrovascular septa
- Triad of most common cytologic features includes
- Sharply defined cell membranes (plant cell-like)
- Hyperchromatic nuclei with irregular, wrinkled nuclear membrane (raisinoid)
- Perinuclear halos (koilocytic-like)
- Coexpression of CK7 and CD117 / KIT while negative for vimentin
Terminology
- Chromophobe renal cell carcinoma (ChRCC) histologic subtypes
- Classic
- Eosinophilic (≥ 80% eosinophilic cells)
- Sarcomatoid
- Other rare morphologic patterns as described below
ICD coding
- ICD-O: 8317/3 - renal cell carcinoma, chromophobe type
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- ICD-11: 2C90.0 & XH6153 - renal cell carcinoma of kidney, except renal pelvis & renal cell carcinoma, chromophobe type
Epidemiology
- Third most common renal cell carcinoma (RCC), accounting for 5 - 7% of cases (Asian J Urol 2022;9:1)
- In the United States, ~ 3,200 new cases were recorded in 2020 (CA Cancer J Clin 2020;70:7)
- Slight M > F; median age of 59 years (range: 17 - 92) (Virchows Arch 2020;476:409)
- Compared to clear cell RCC, ChRCC is more frequently diagnosed in younger females (Clin Genitourin Cancer 2019;17:373)
Sites
- Most commonly a solitary, unilateral renal mass based in the cortex
- Multiple tumors are more commonly seen in syndromic settings (e.g., Birt-Hogg-Dubé)
- Isolated case reports of multiple, bilateral sporadic tumors (World J Clin Cases 2020;8:3064)
Pathophysiology
Etiology
- Proposed cell of origin: intercalated cells of the distal convoluted tubules (Int J Surg Pathol 2023;31:1027)
- Most occur in a sporadic setting
- 3 main cancer predisposition syndromes
- Birt-Hogg-Dubé (BHD) syndrome
- Autosomal dominant with incomplete penetrance
- Due to germline mutations in FLCN (BHD) gene on chromosome 17p12, which codes for folliculin protein
- Skin lesions: fibrofolliculomas, trichodiscomas, acrochordons
- Lung: cysts, spontaneous pneumothorax
- Renal tumors are multiple and bilateral in 15 - 30%, most commonly hybrid oncocytic / chromophobe tumor, followed by ChRCC and oncocytoma as well as renal oncocytosis (Am J Surg Pathol 2002;26:1542)
- Clear cell RCC, papillary RCC and RCC, NOS can also be seen
- Renal tumors of BHD syndrome are considered low risk tumors suitable for conservative therapy; however, patients develop chronic renal insufficiency due to tumor multifocality
- Tuberous sclerosis complex
- Autosomal dominant syndrome with variable penetrance, due to mutations in TSC1 on chromosome 9q or TSC2 on chromosome 16p (Annu Rev Genomics Hum Genet 2019;20:217)
- Multisystem disorder with numerous manifestations, usually affecting the CNS
- Renal lesions: multiple, bilateral angiomyolipomas or epithelioid angiomyolipoma, renal cysts and variable, typically indolent renal tumors, including eosinophilic solid and cystic RCC, RCC with fibromyomatous stroma, hybrid oncocytic / chromophobe tumors and RCC, NOS (Am J Surg Pathol 2014;38:1457, Am J Surg Pathol 2014;38:895)
- Cowden syndrome
- Part of the PTEN hamartoma tumor syndrome (PHTS), which also includes Bannayan-Riley-Ruvalcaba syndrome
- Autosomal dominant, due to germline mutations in PTEN on chromosome 10q22-23 (Am J Dermatopathol 2022;44:705)
- Multisystem disorder with numerous manifestations
- Affected organs / organ systems include skin, thyroid, breast, endometrium, gastrointestinal tract, genitourinary system (StatPearls: Cowden Disease [Accessed 4 August 2025])
- Renal lesions: most commonly ChRCC and papillary RCC, with no particular morphologic characteristics compared to sporadic examples; presence of concurrent intrarenal microscopic lipomas may be indicative of PHTS associated renal neoplasia (Am J Surg Pathol 2023;47:1001)
- Birt-Hogg-Dubé (BHD) syndrome
Clinical features
N/A
Diagnosis
- Most are incidentally discovered on imaging performed for other reasons
- Computed tomography (CT) scan is the most common imaging modality for diagnosis and staging (Crit Rev Oncol Hematol 2021;160:103287)
- According to the 5th edition of WHO, the following diagnostic criteria are listed as
- Essential: large pale cells with prominent cell membranes and perinuclear halos; groups of CK7 positive cells
- Desirable: absence of vimentin expression (except in sarcomatoid differentiation)
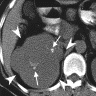
Radiology description
- Usually large, well circumscribed, hypovascular mass with relatively homogeneous contrast enhancement; may show central scar (Radiographics 2021;41:1408)
Prognostic factors
- Commonly organ confined, more favorable clinical outcome and lower metastatic risk than clear cell RCC (Clin Genitourin Cancer 2019;17:373)
- Poor prognostic factors
- Large tumor size (> 7 cm)
- Higher clinical T category and pathologic TNM staging
- Sarcomatoid transformation, tumor necrosis, vascular invasion
- Male sex (Clin Genitourin Cancer 2019;17:373)
- Tumor grading is not prognostically relevant and not recommended
- Histologic diversity of ChRCC does not impact survival outcome (Ann Diagn Pathol 2022;60:151978)
- Recurrence free survival at 5 years is 94.9% (91.7% at 10 years) (Clin Genitourin Cancer 2019;17:373)
- Overall cancer survival at 5 years is 92.3% (82.1% at 10 years) (Clin Genitourin Cancer 2019;17:373)
- Metastatic disease in 5 - 10% of cases (Clin Genitourin Cancer 2019;17:373)
Case reports
- 46 year old man with de novo ChRCC in the graft 3 decades after renal transplantation (Saudi J Kidney Dis Transpl 2020;31:271)
- 47 year old man with massive sarcomatoid differentiation (Indian J Pathol Microbiol 2023;66:839)
- 54 year old woman with isolated metastasis to the brain, 2 years after radical nephrectomy (Indian J Pathol Microbiol 2023;66:363)
- 58 year old woman with osteosarcomatous and chondroblastic sarcomatoid differentiation (J Kidney Cancer VHL 2024;11:59)
- 71 year old man with sarcomatoid and heterologous osteosarcoma-like differentiation (J Surg Case Rep 2023;2023:rjad476)
Treatment
- Surgery, cryoablation and targeted systemic chemotherapy for metastatic disease with antiangiogenic, tyrosine kinase (TK) and mTOR inhibitors (Urol Oncol 2020;38:137, Crit Rev Oncol Hematol 2021;160:103287)
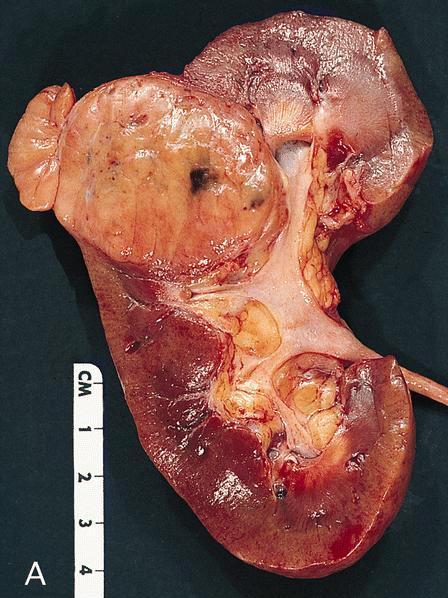
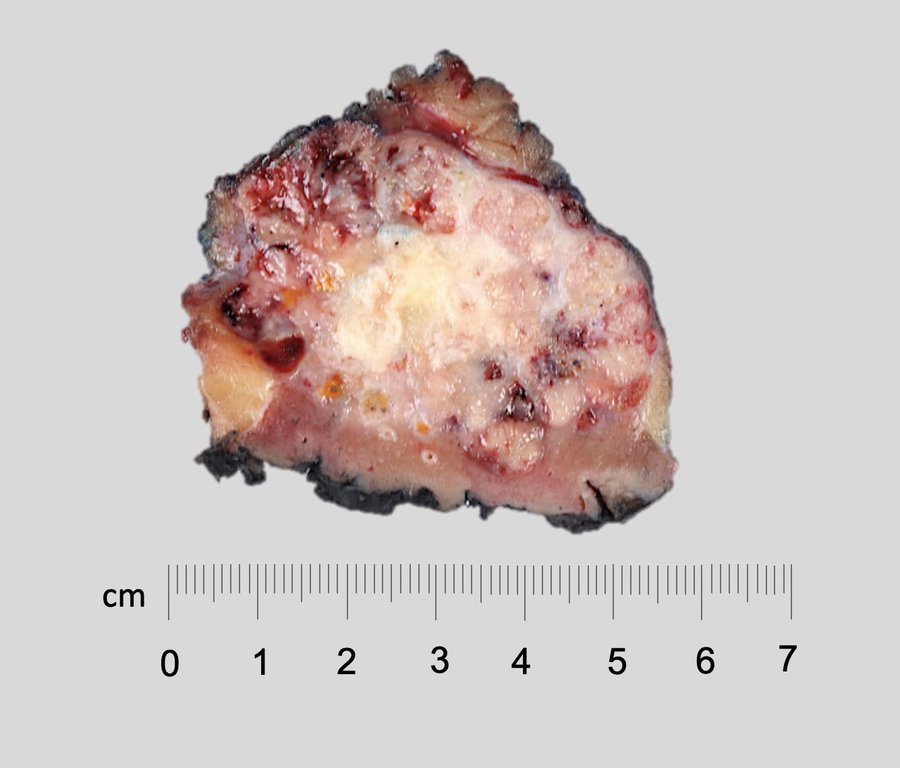
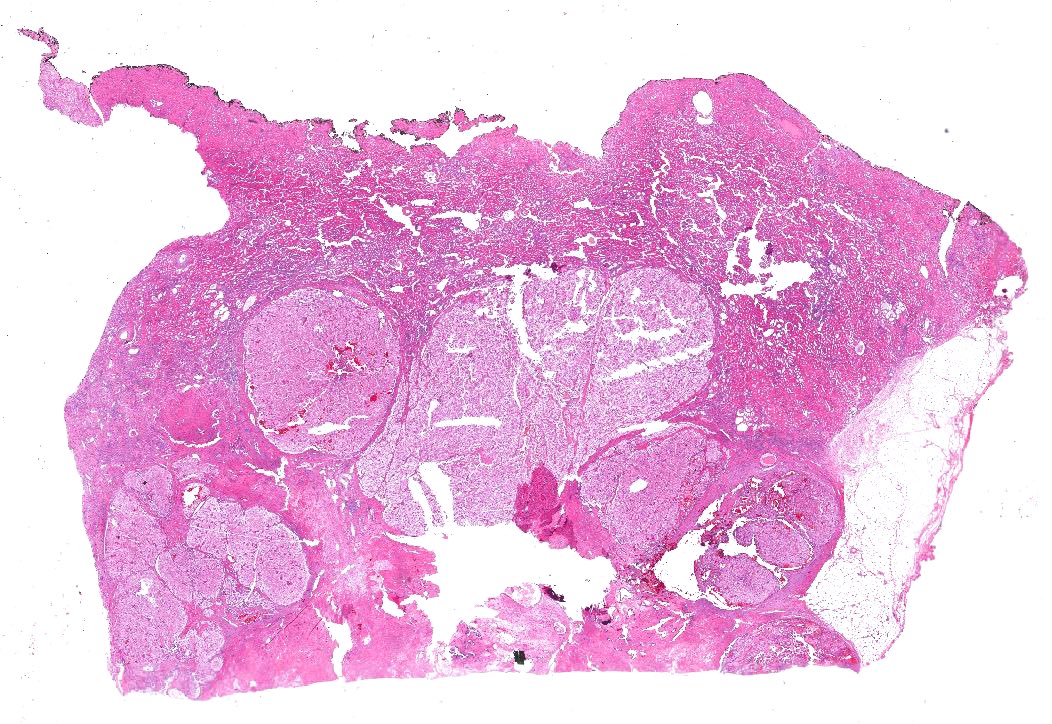
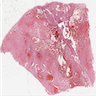
Gross description
- Typically large (median size is 8 cm; range: 1 - 30 cm) and solid (rare cystic examples reported) (Virchows Arch 2016;469:669, Am J Case Rep 2019;20:631)
- Well circumscribed but not encapsulated
- Light tan to gray cut surface, variable intensity of mahogany brown color correlates with amount of eosinophilic cells
- Tan-white, fleshy areas indicate sarcomatoid transformation
- Hemorrhage and necrosis seen in 25% of cases
- Renal vein invasion is rare
- Central scar present in 15% of cases
Gross images
Frozen section description
- Eosinophilic histologic subtype may closely mimic oncocytoma on frozen section (Biomedicine (Taipei) 2019;9:6)
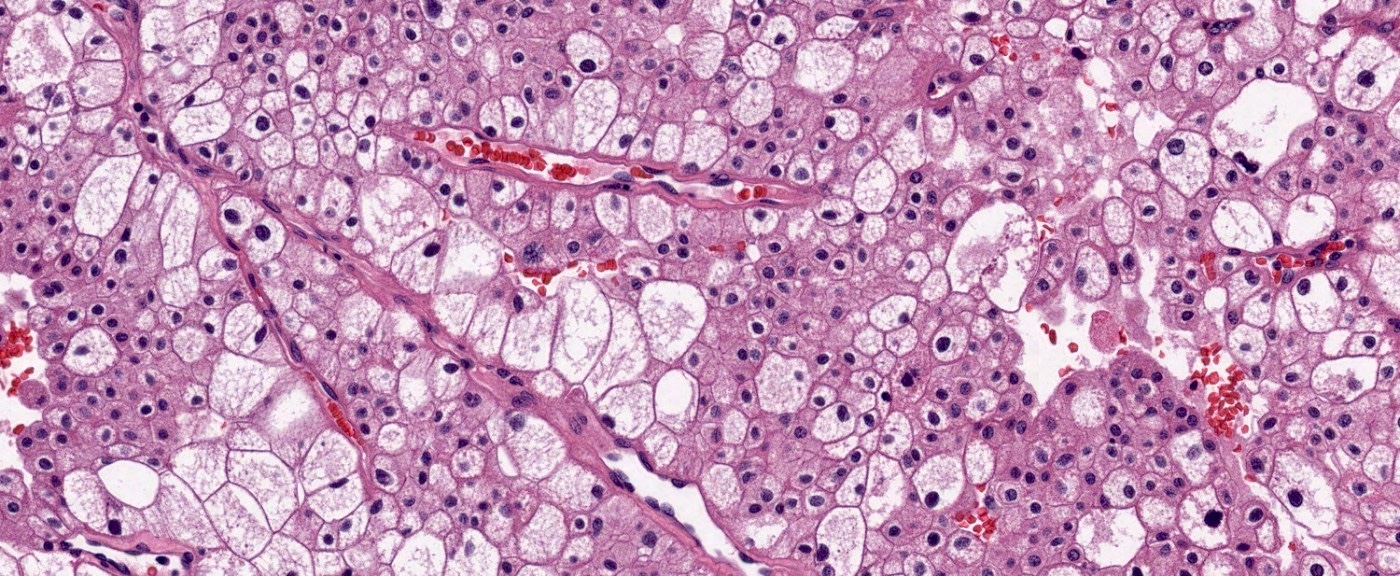
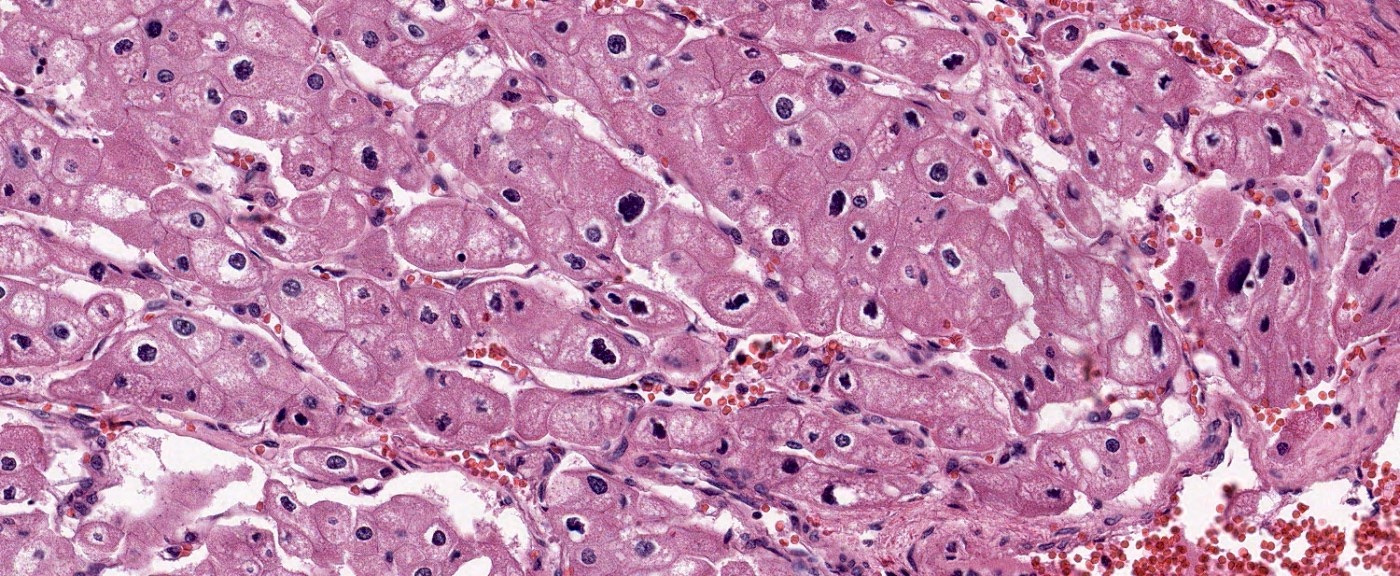
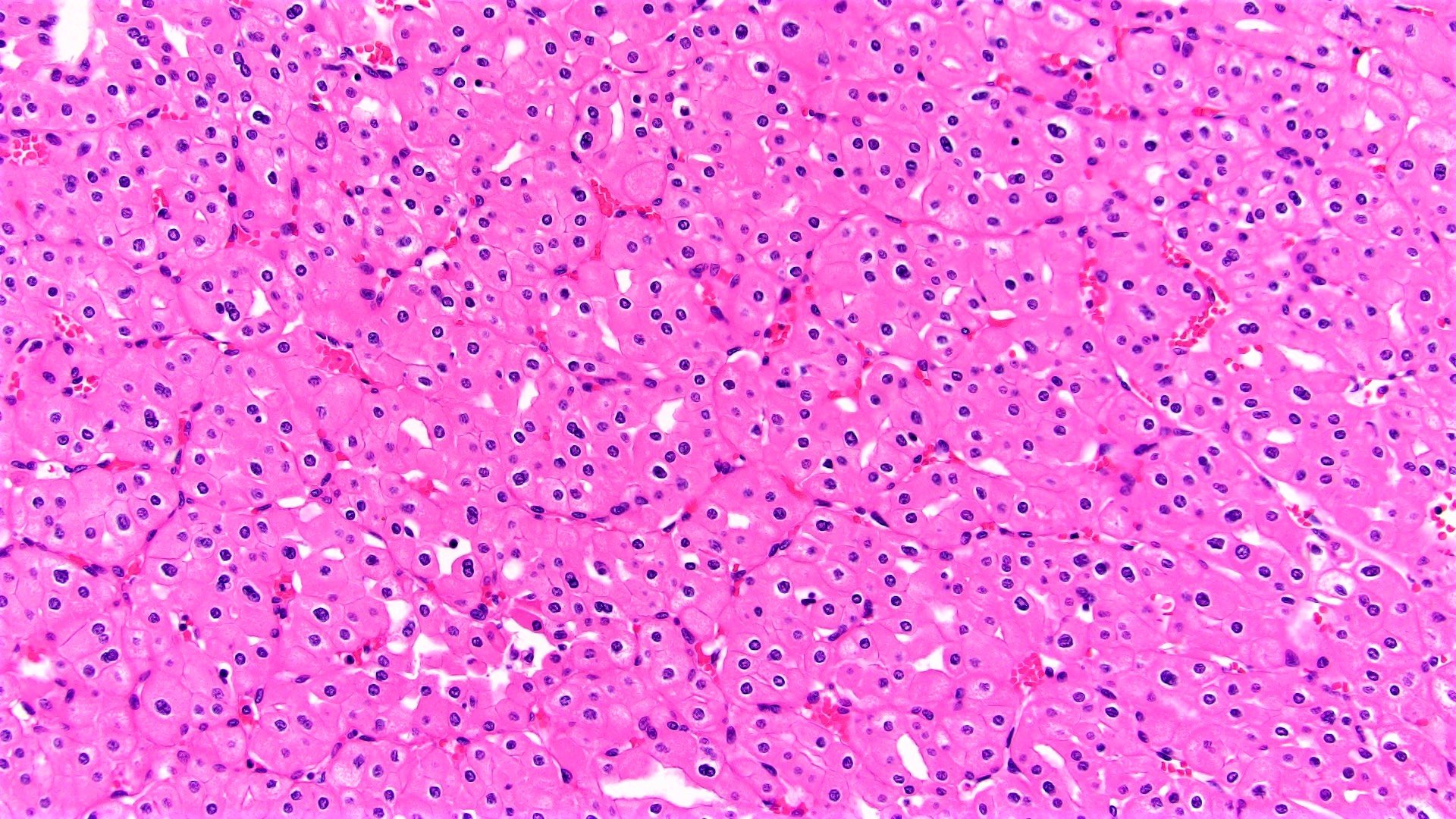
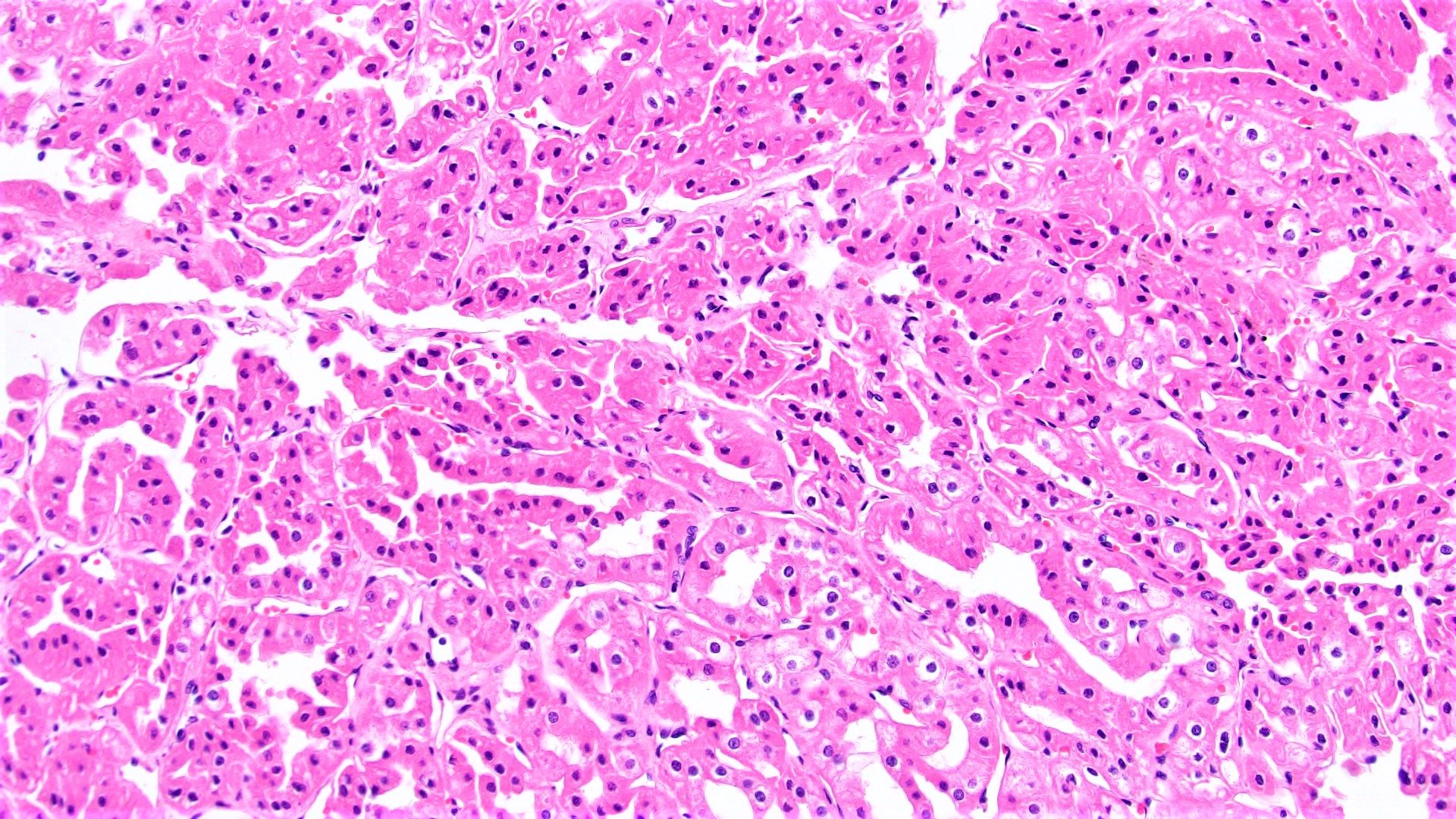
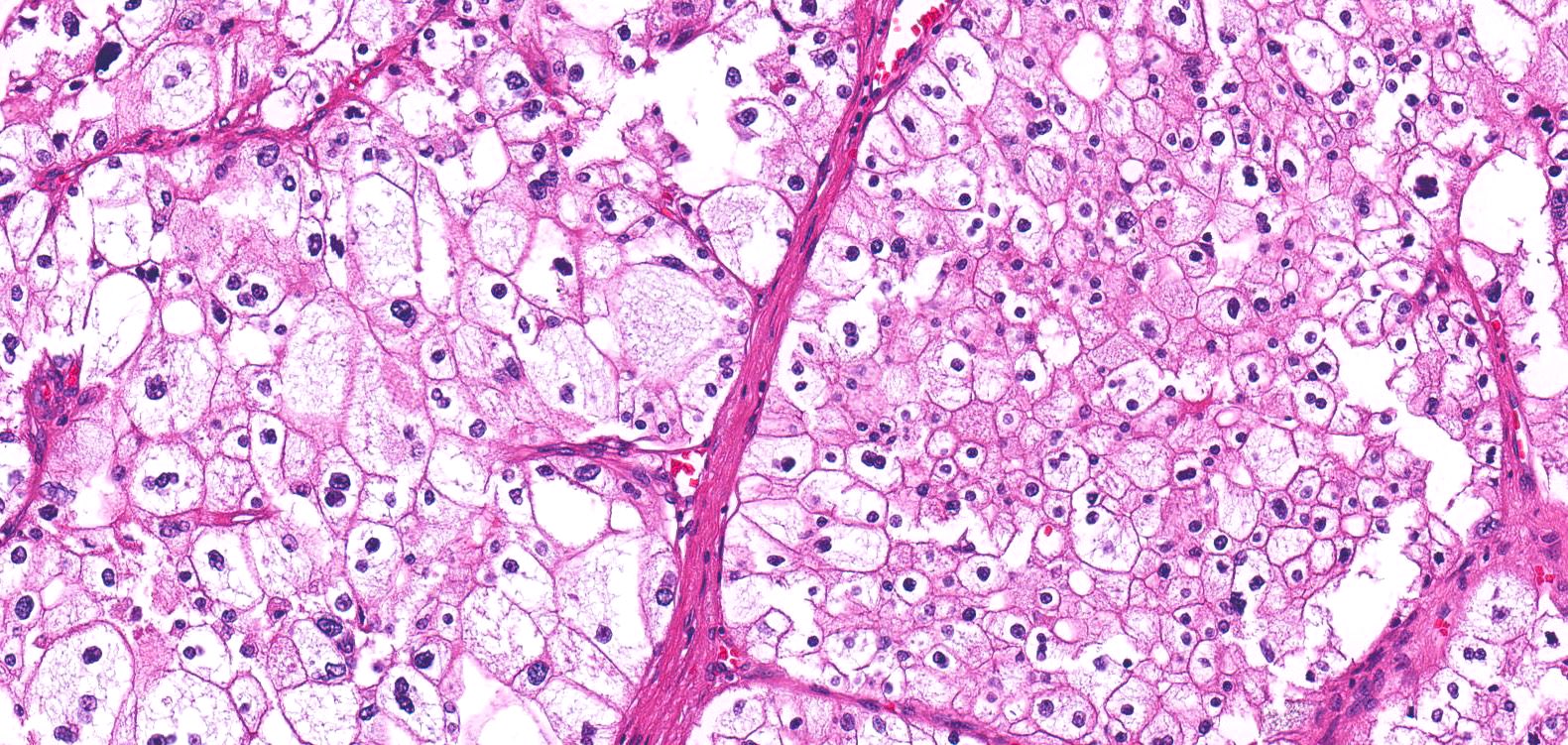
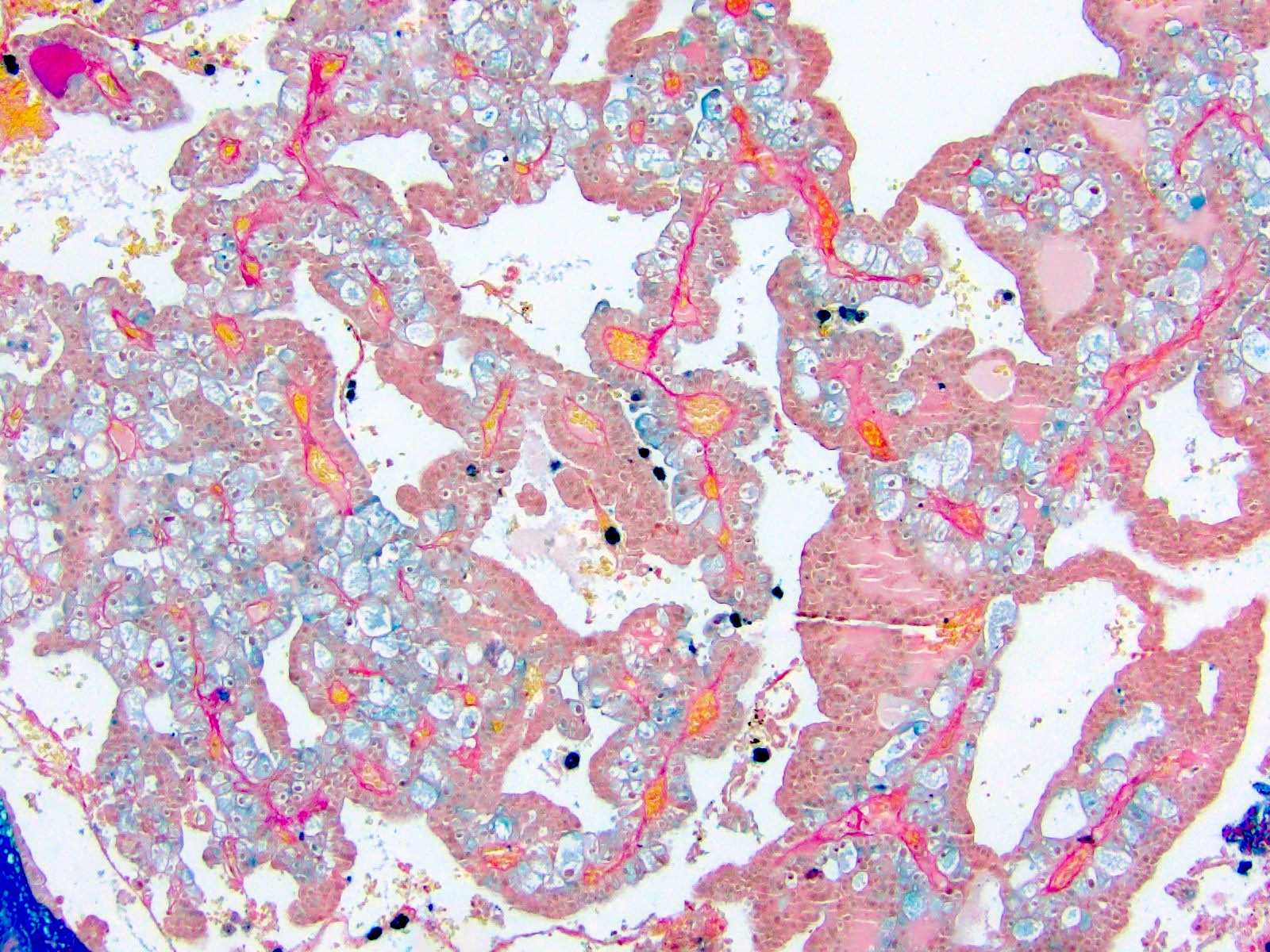
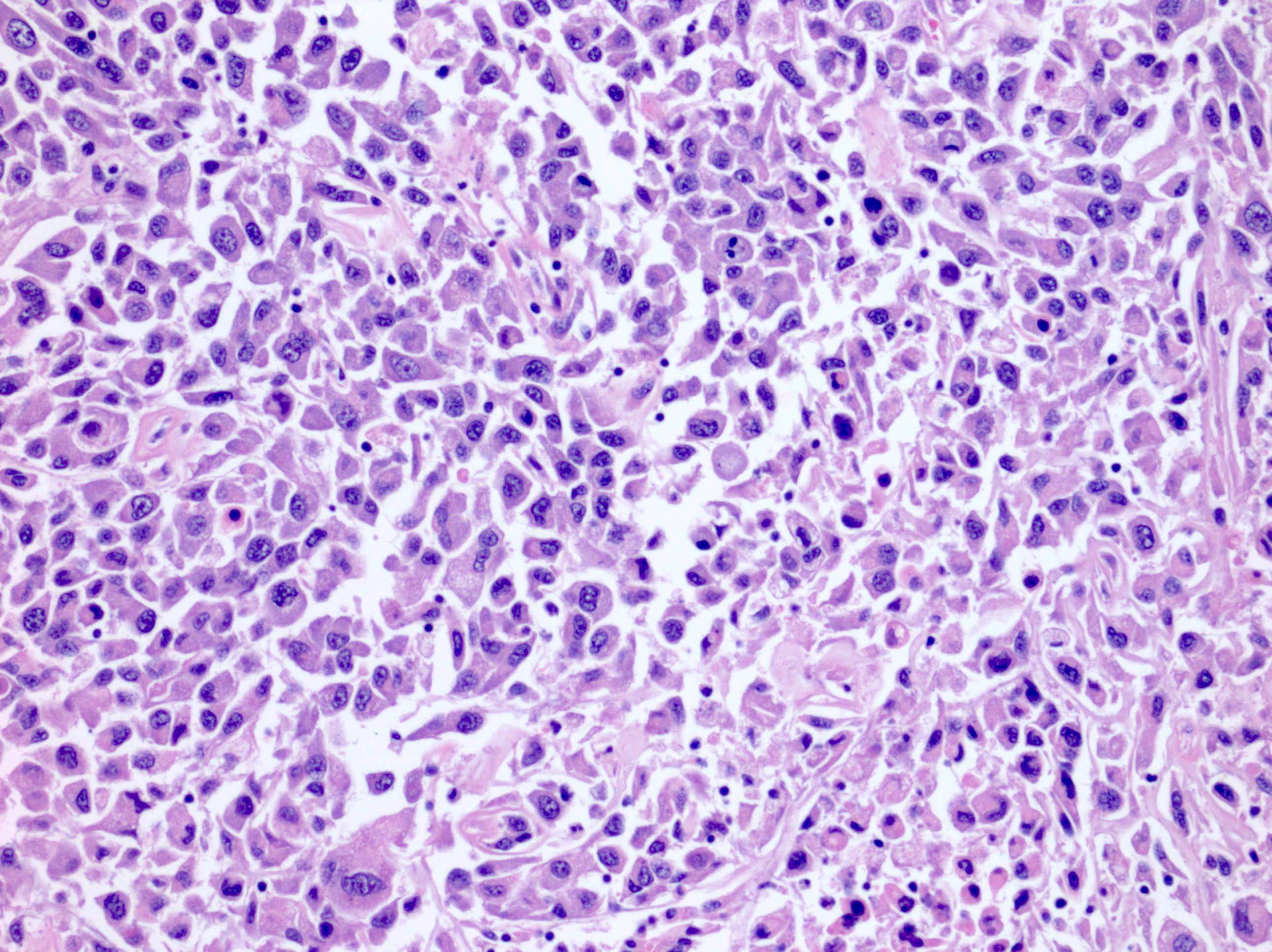
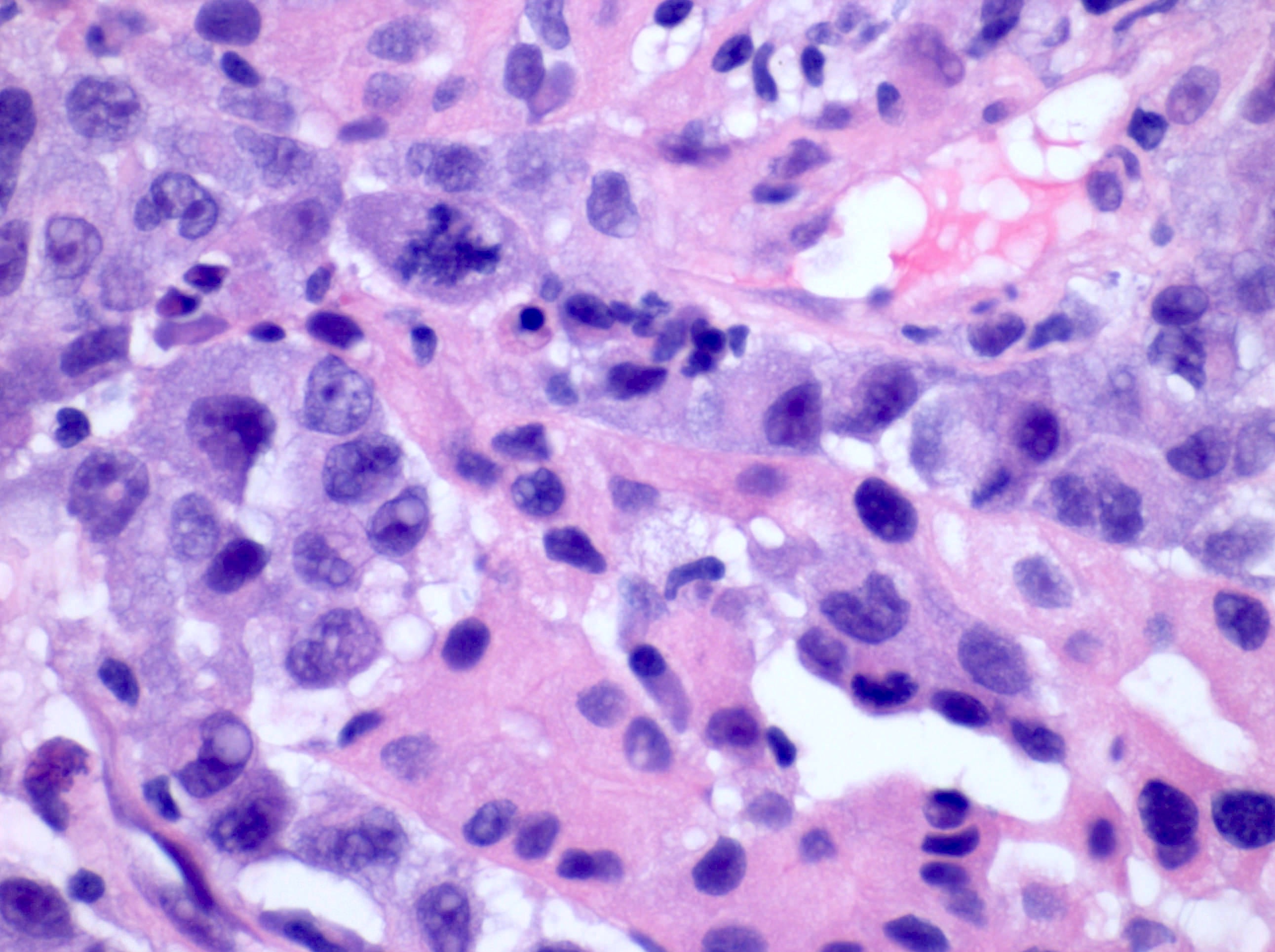
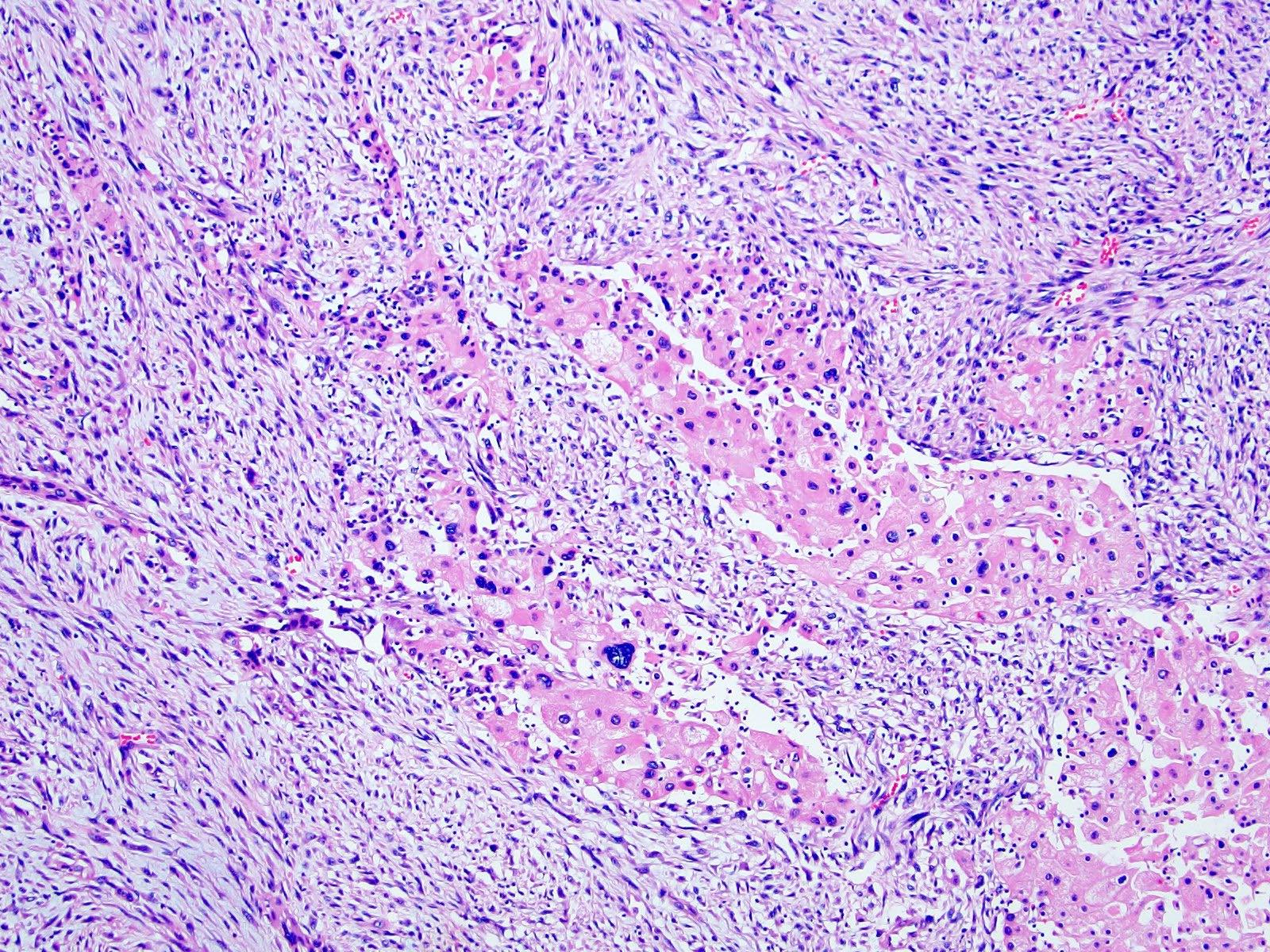
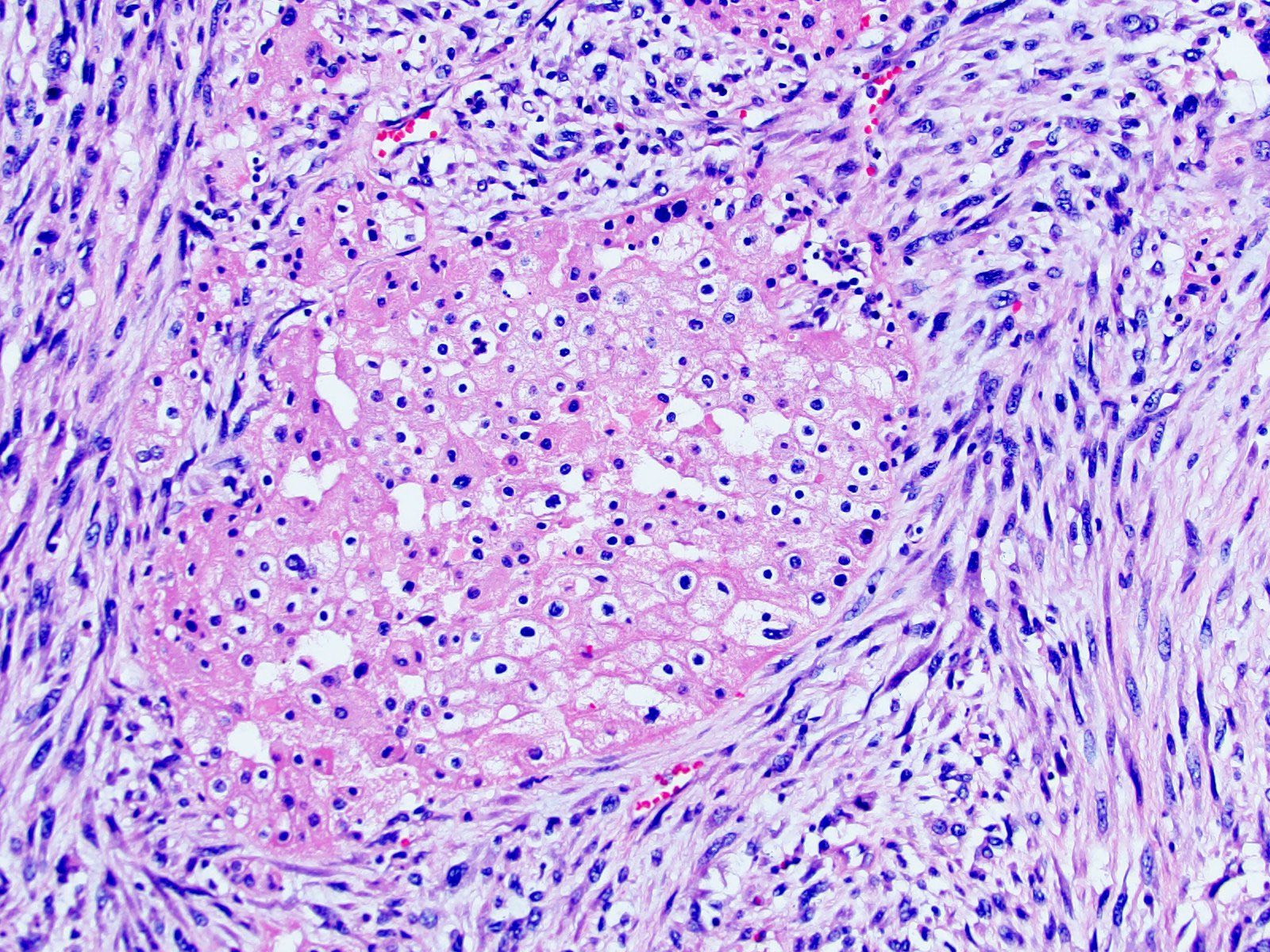
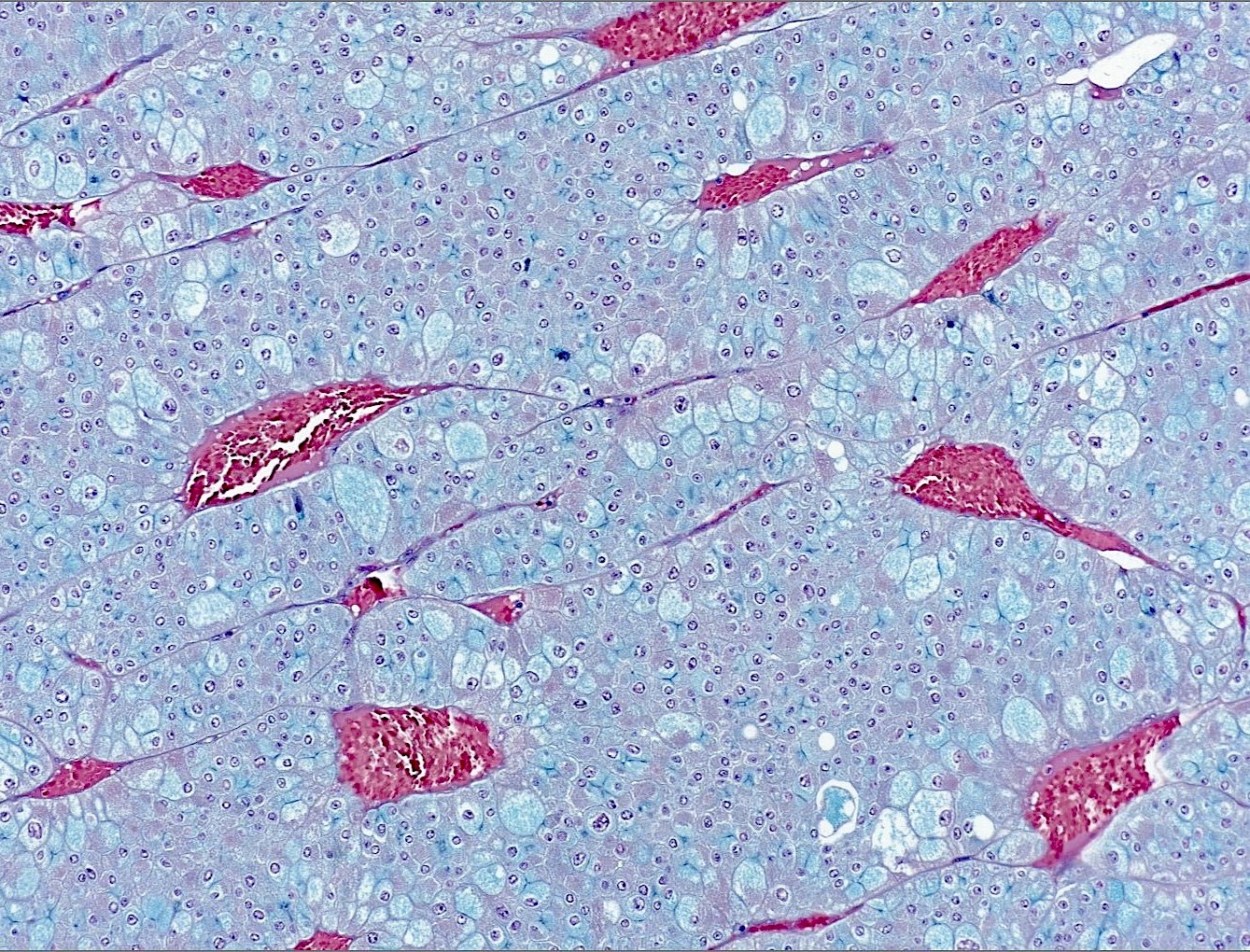
Microscopic (histologic) description
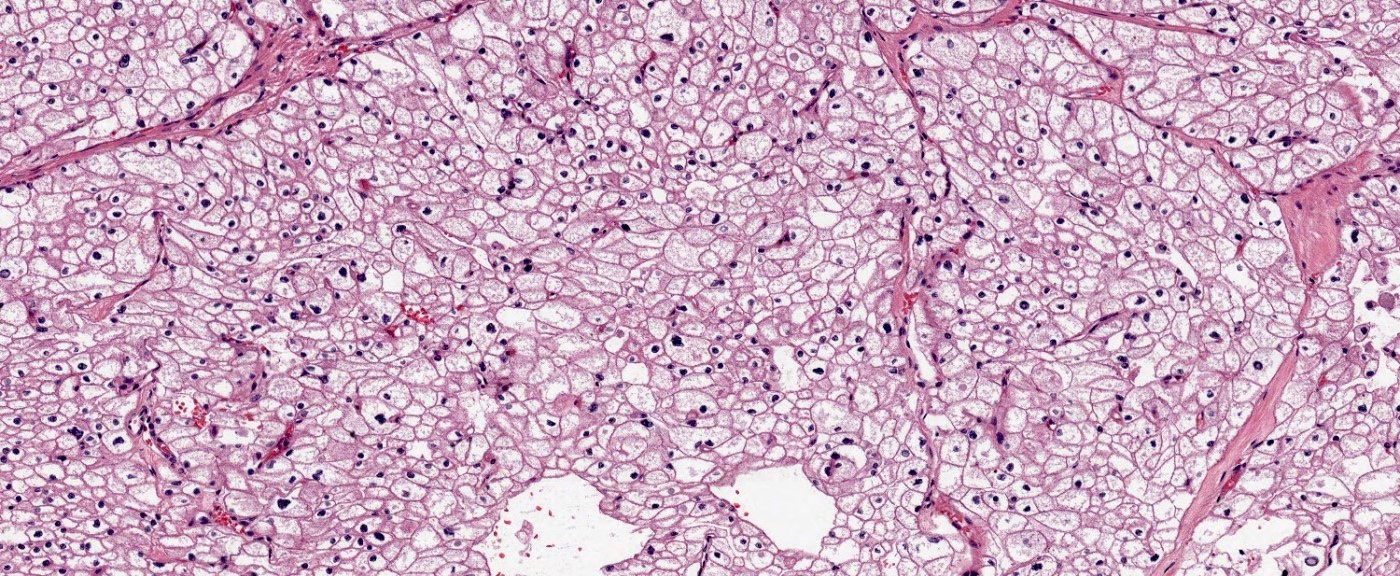
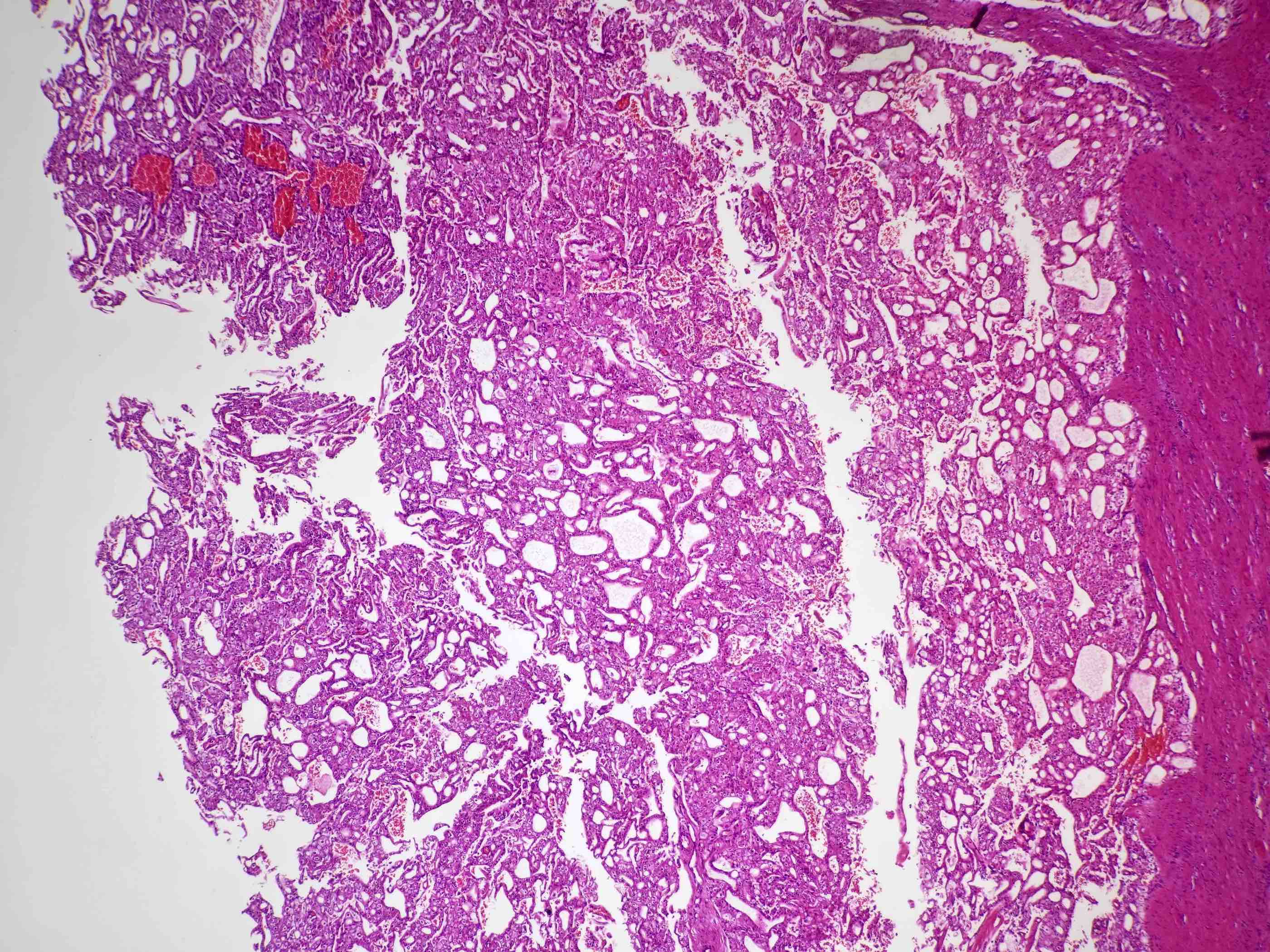
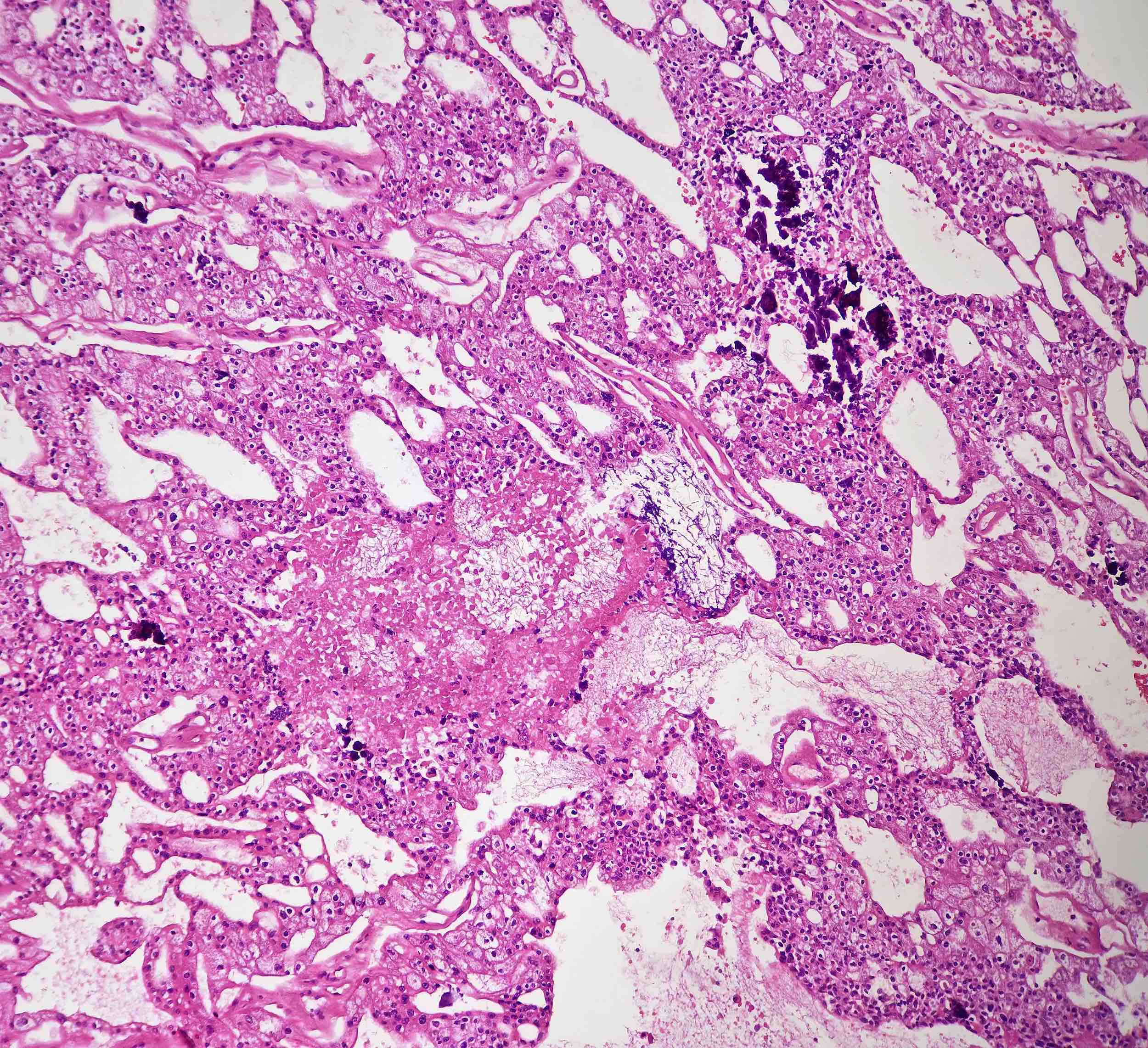
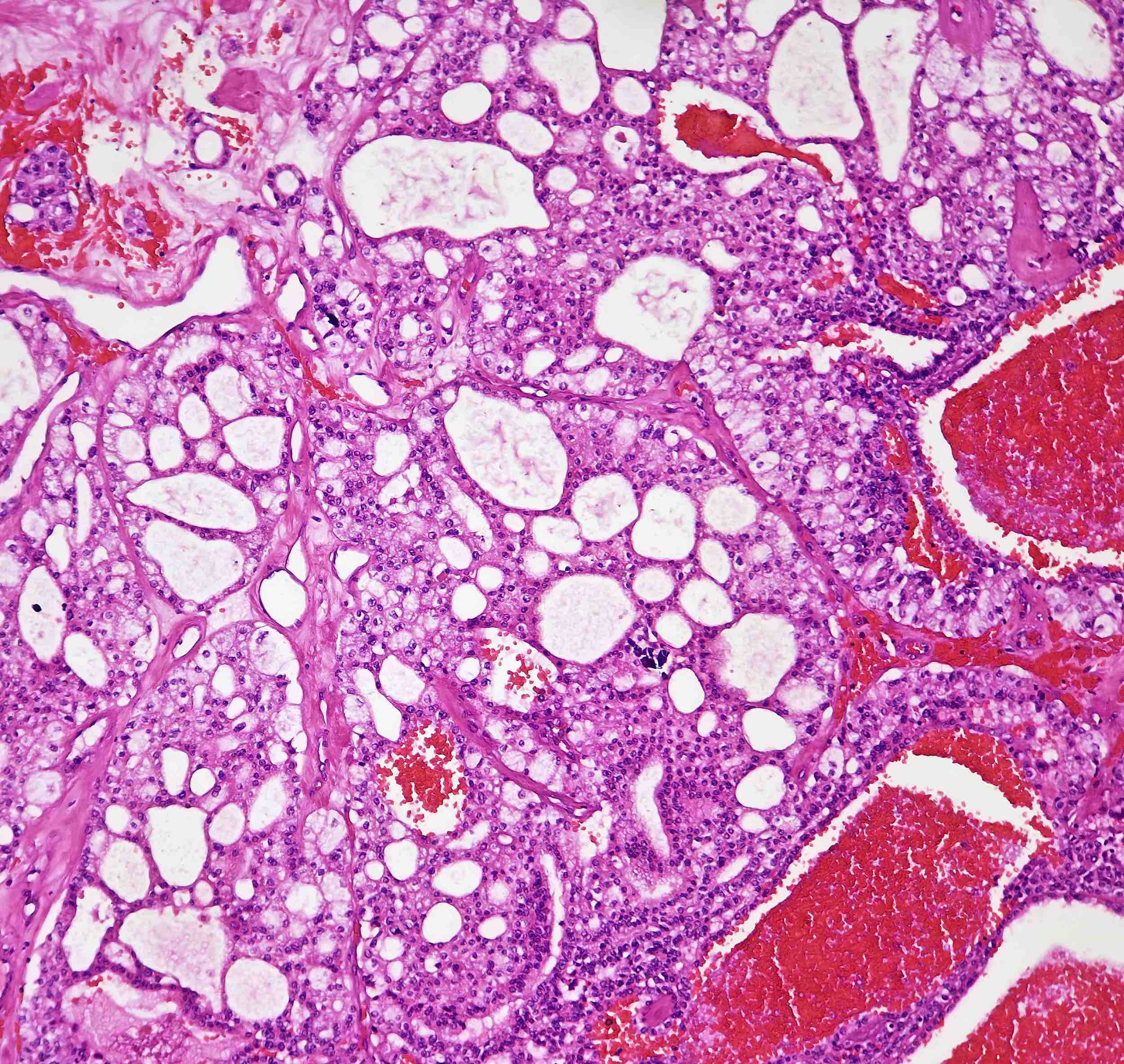
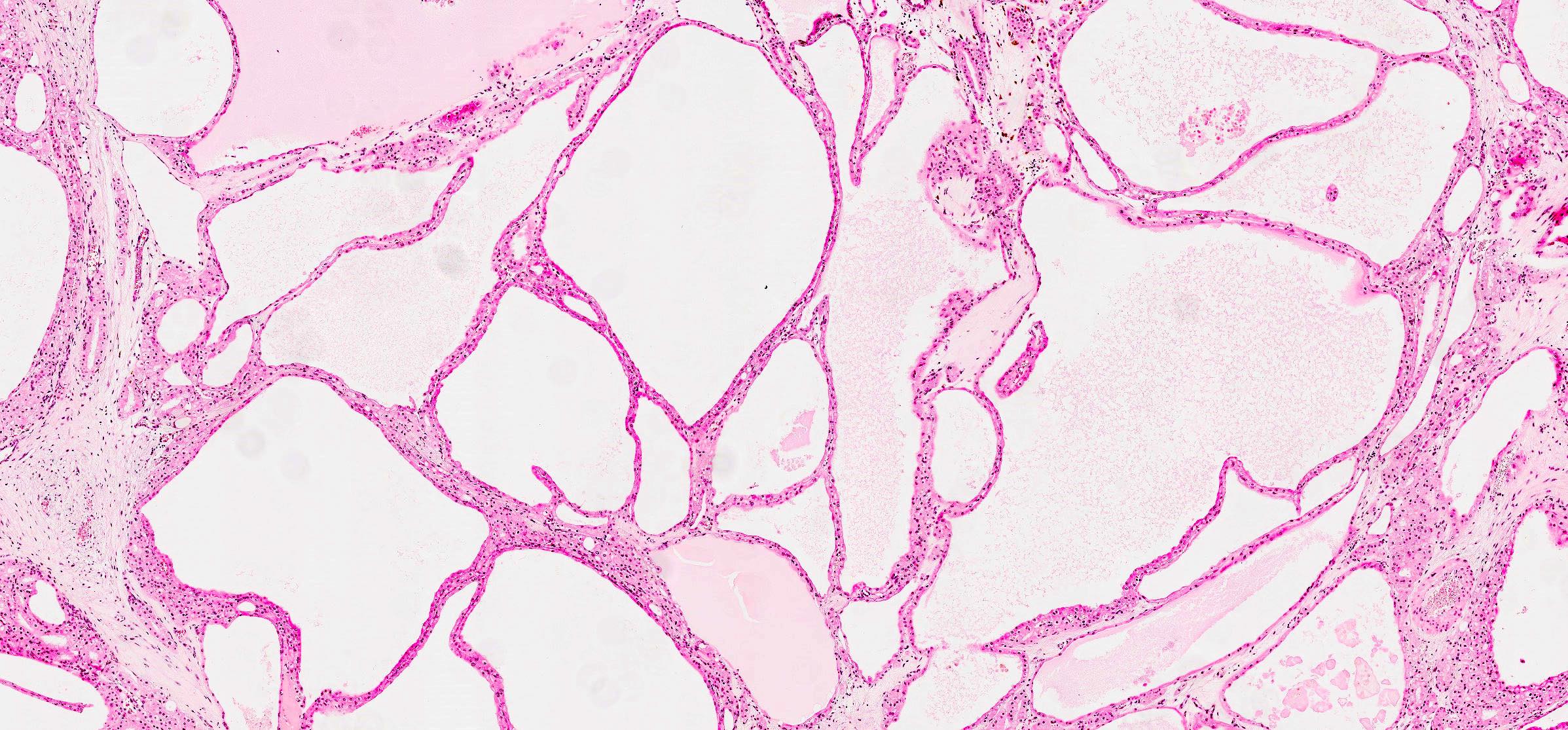
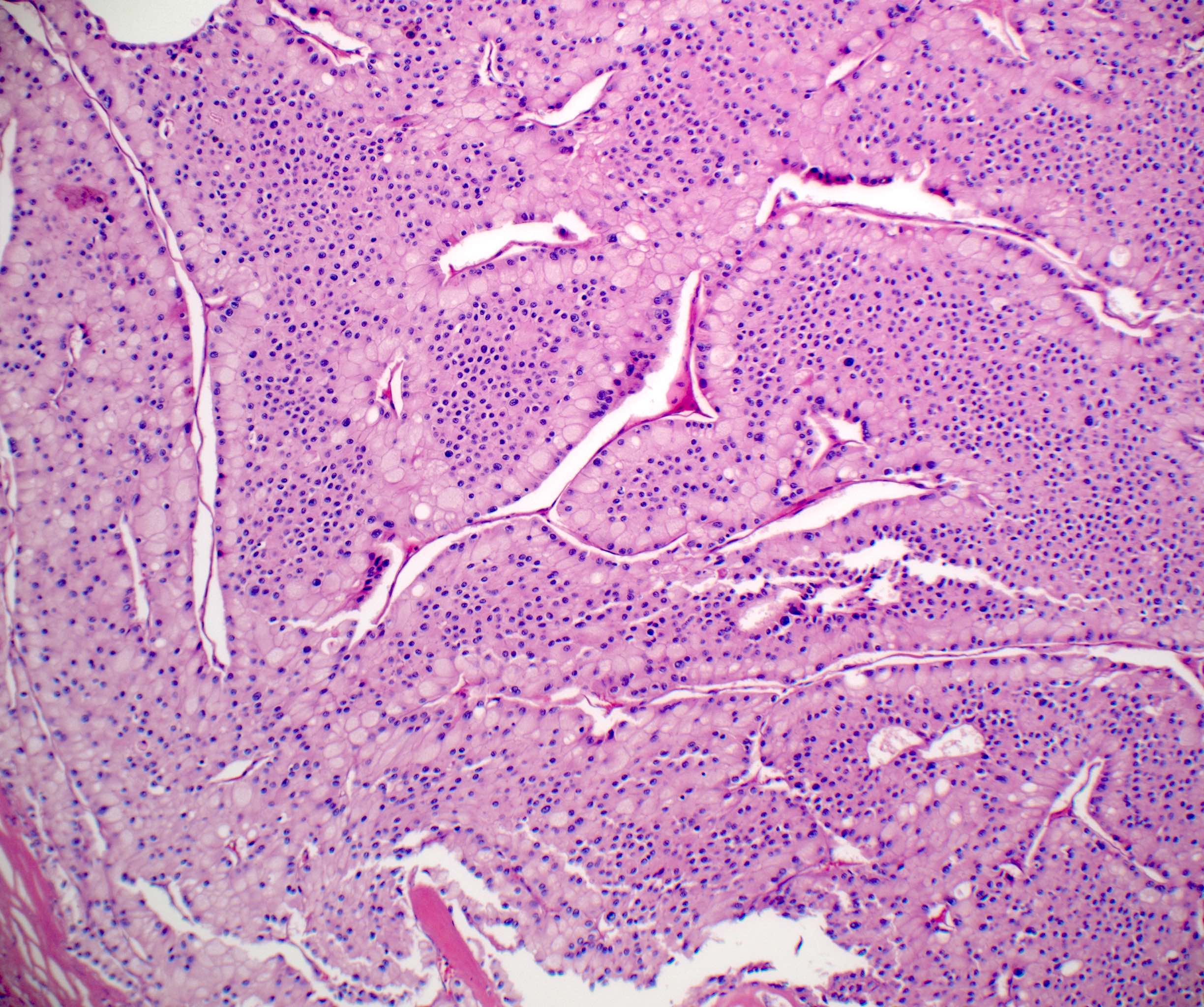
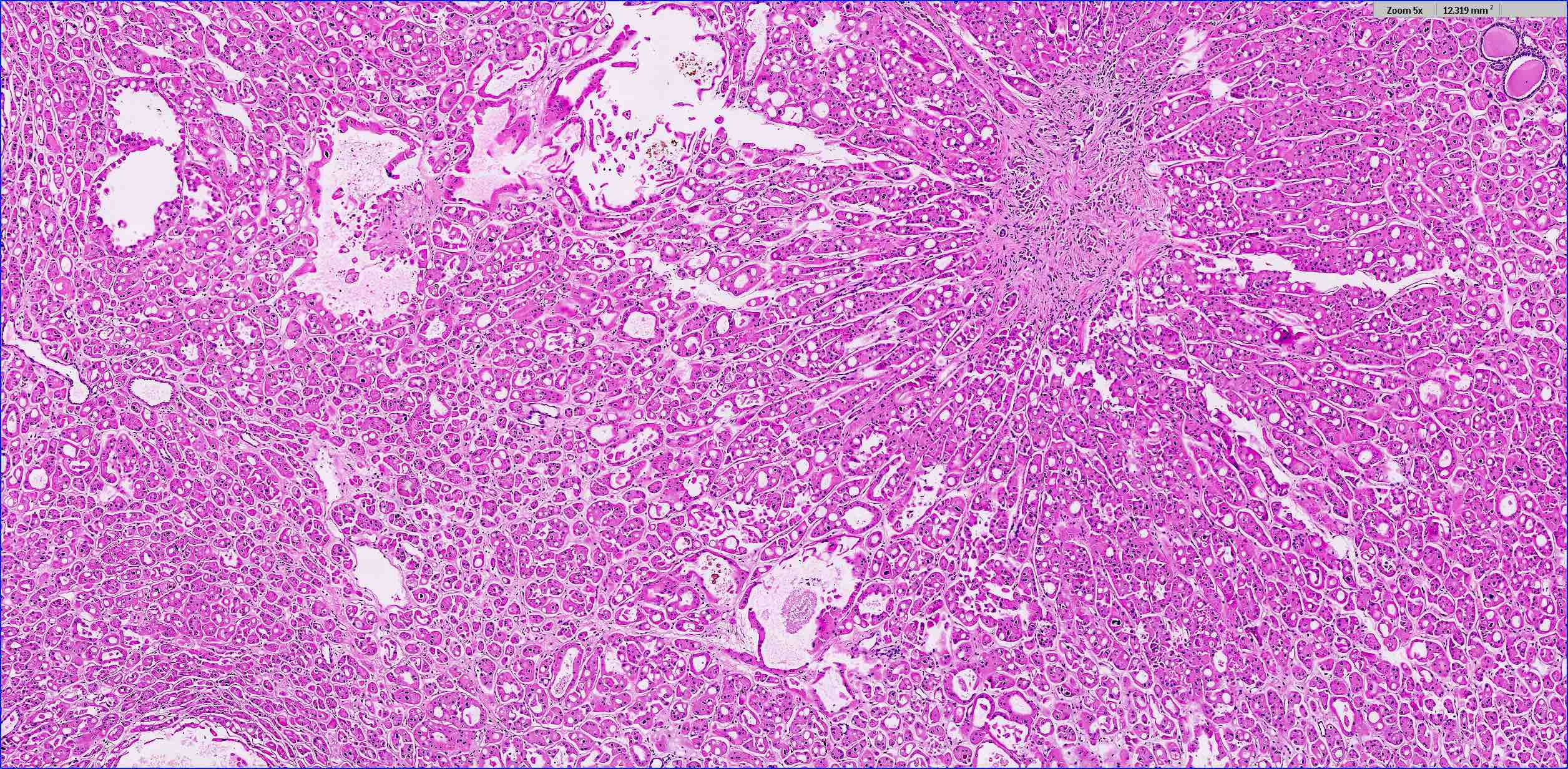
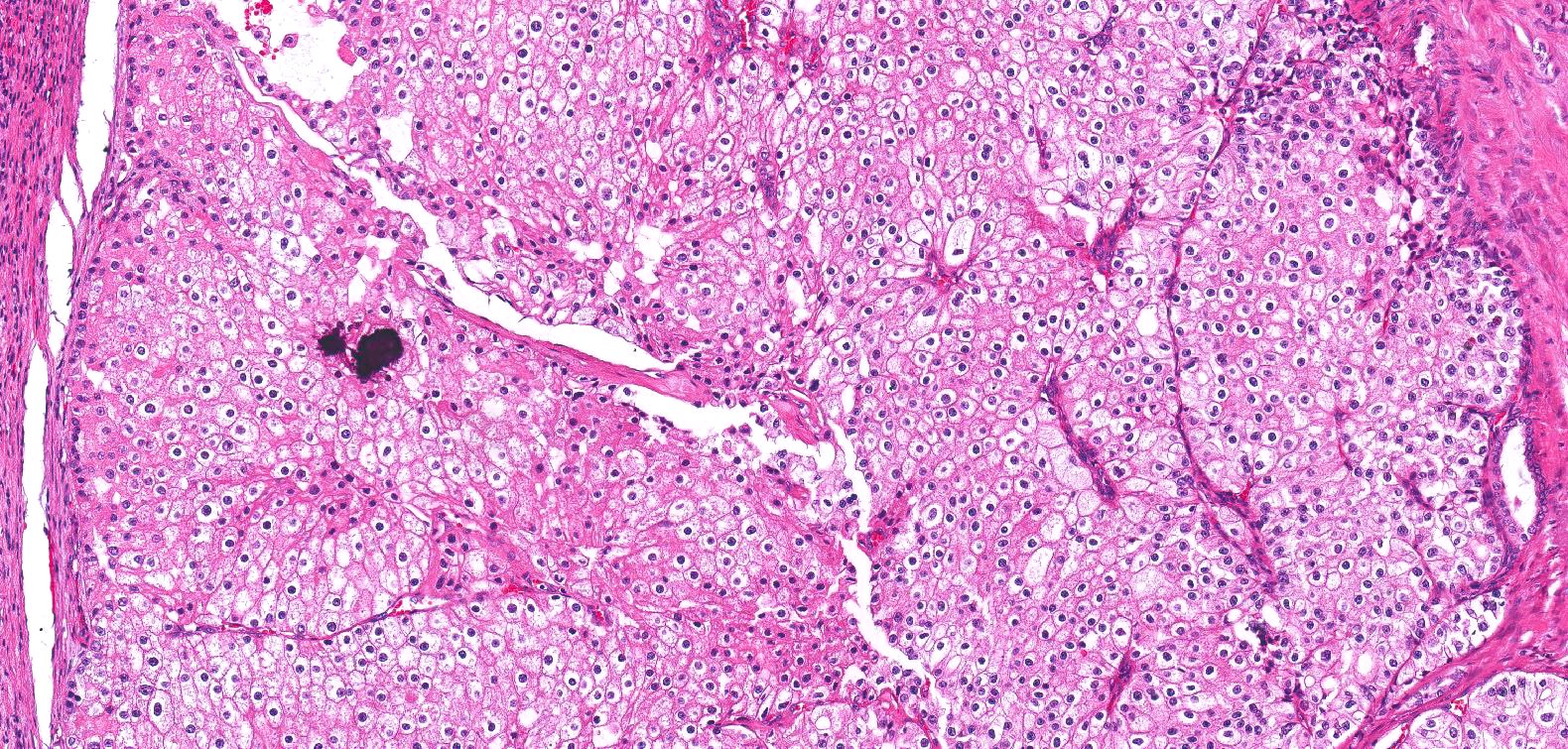
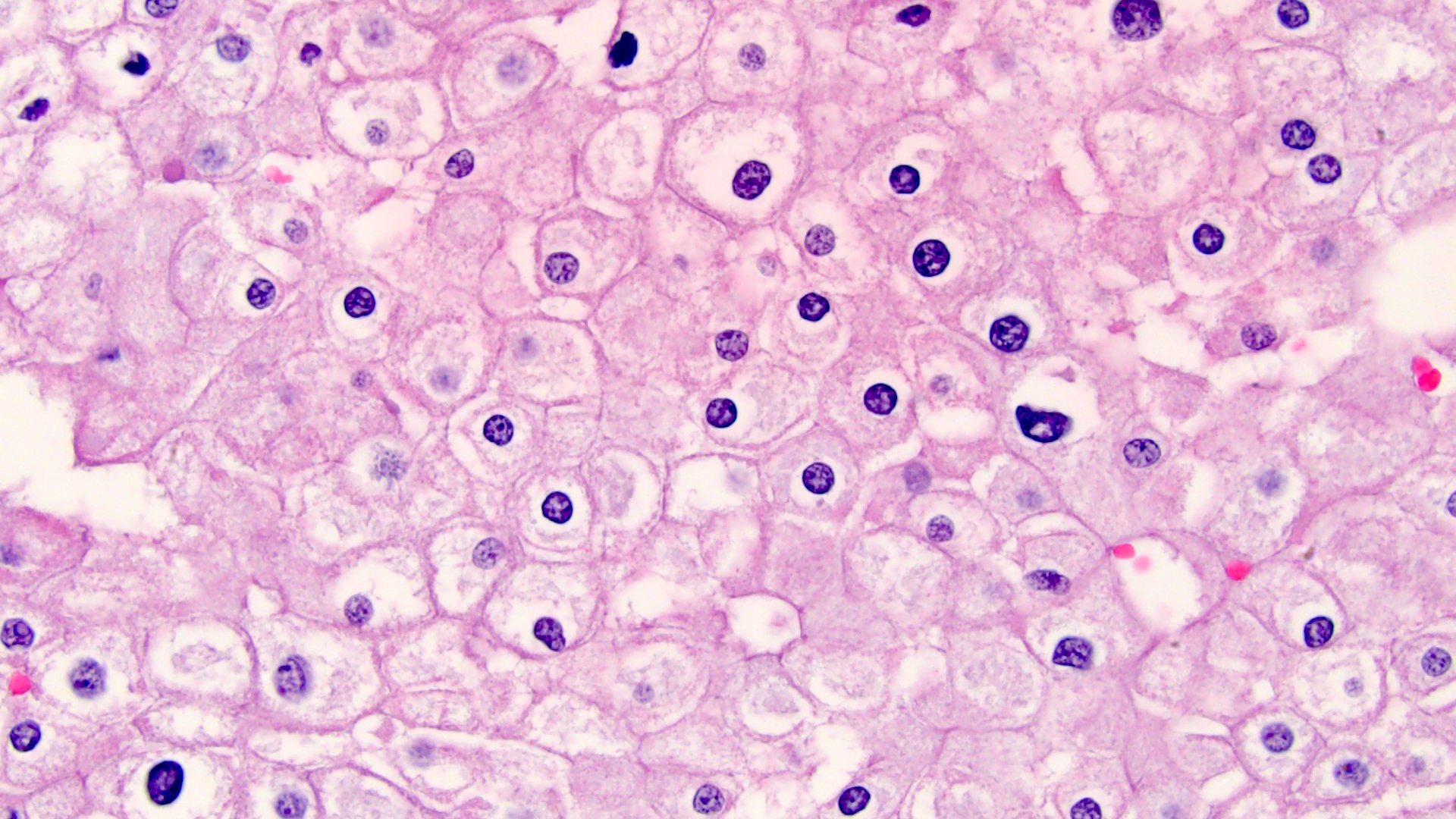
- Classic
- Commonly solid sheets traversed by incomplete fibrovascular septa creating a broad trabecular growth pattern; less common nested or alveolar growth
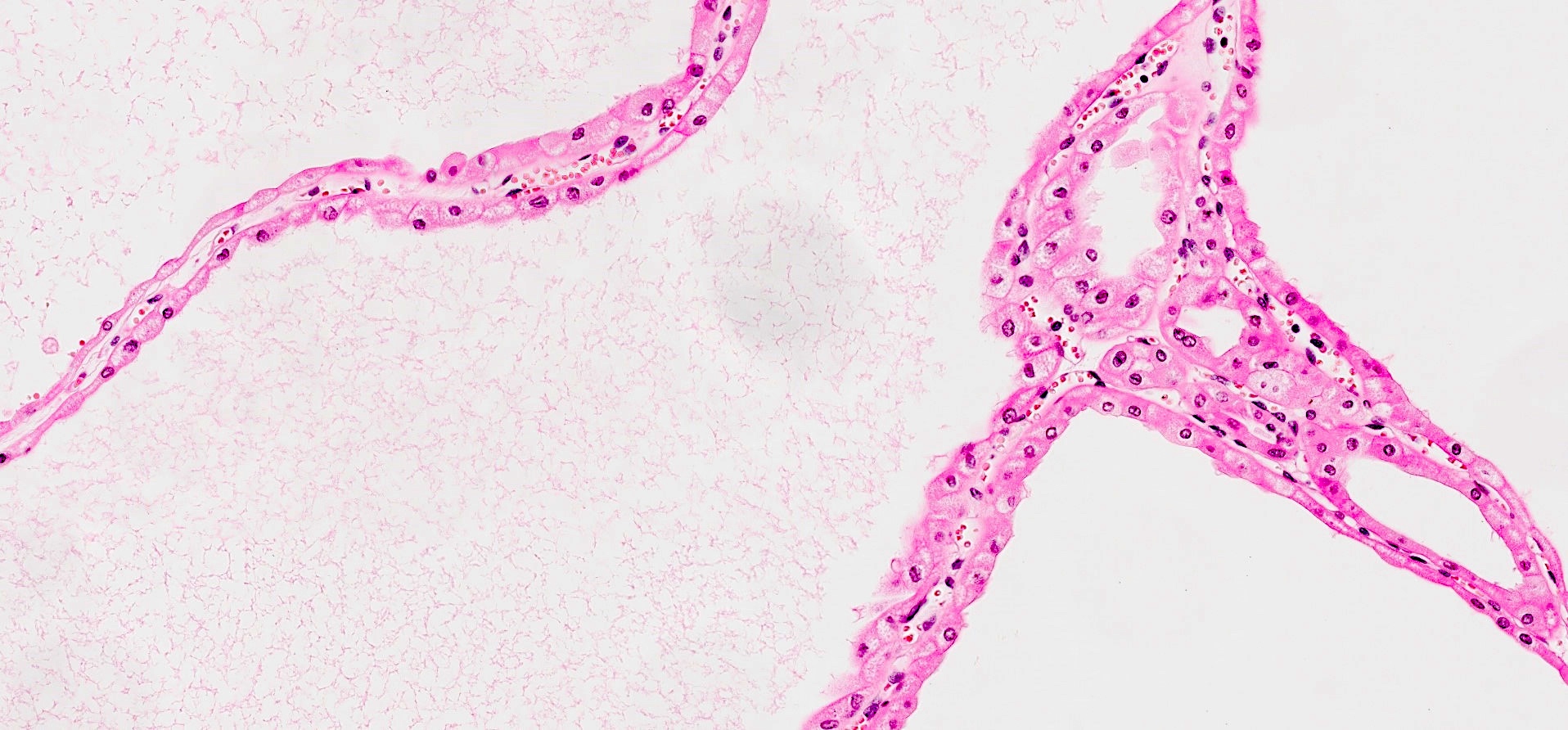
- Larger cells with abundant pale and finely reticulated cytoplasm are most characteristic and define tumor entity (chromo = color, phobe = lacking affinity)
- Alternating smaller cells have eosinophilic cytoplasm
- Larger cells have voluminous cytoplasm, such that the nucleus sometimes is not caught in the plane of section, thus the cells appear anucleate (soft histologic diagnostic clue)
- Prominent cell borders (plant cell-like)
- Perinuclear clear halos (koilocytic-like)
- Hyperchromatic nuclei with irregular, wrinkled nuclear membranes (raisinoid)
- Frequent binucleation
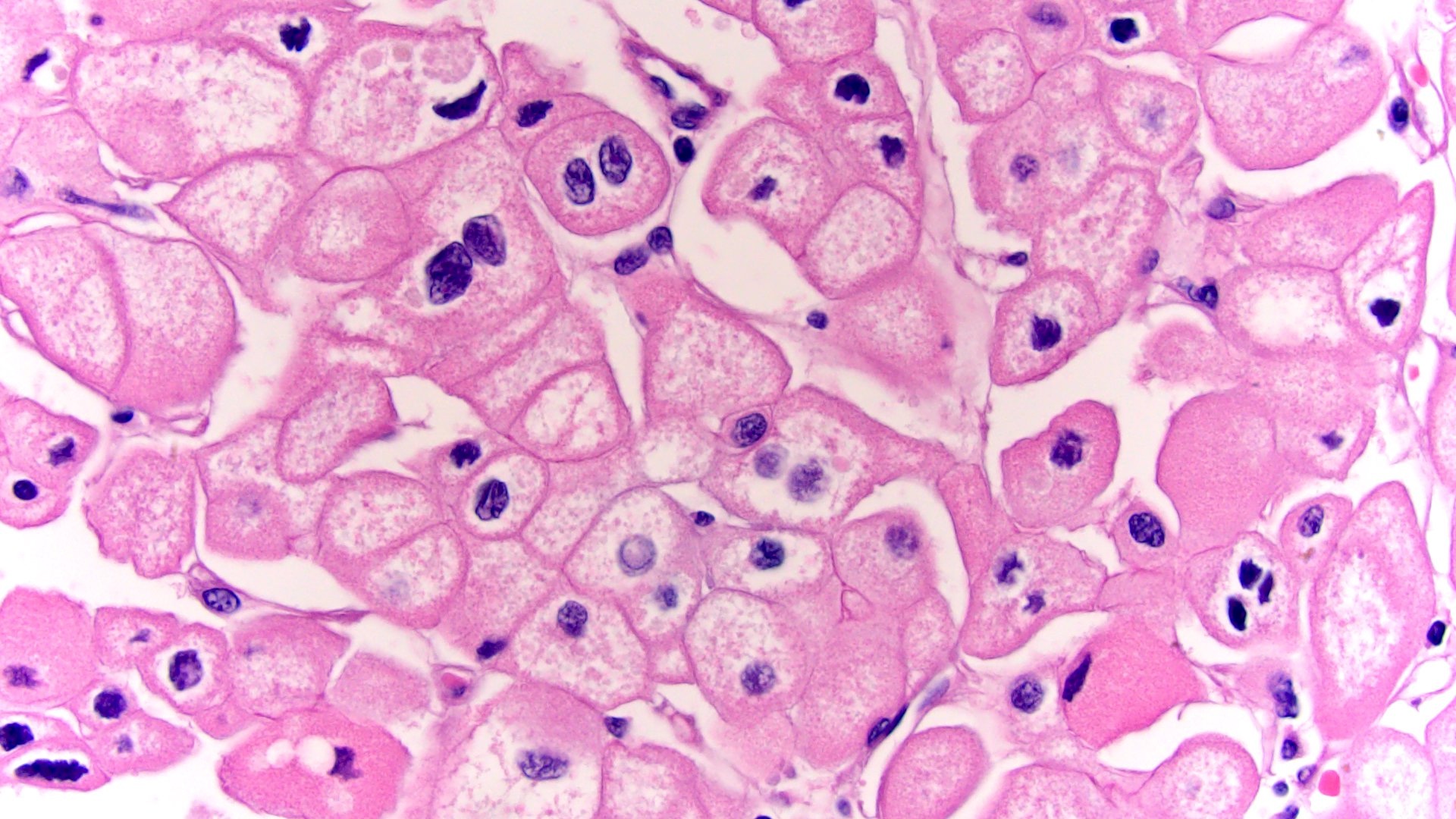
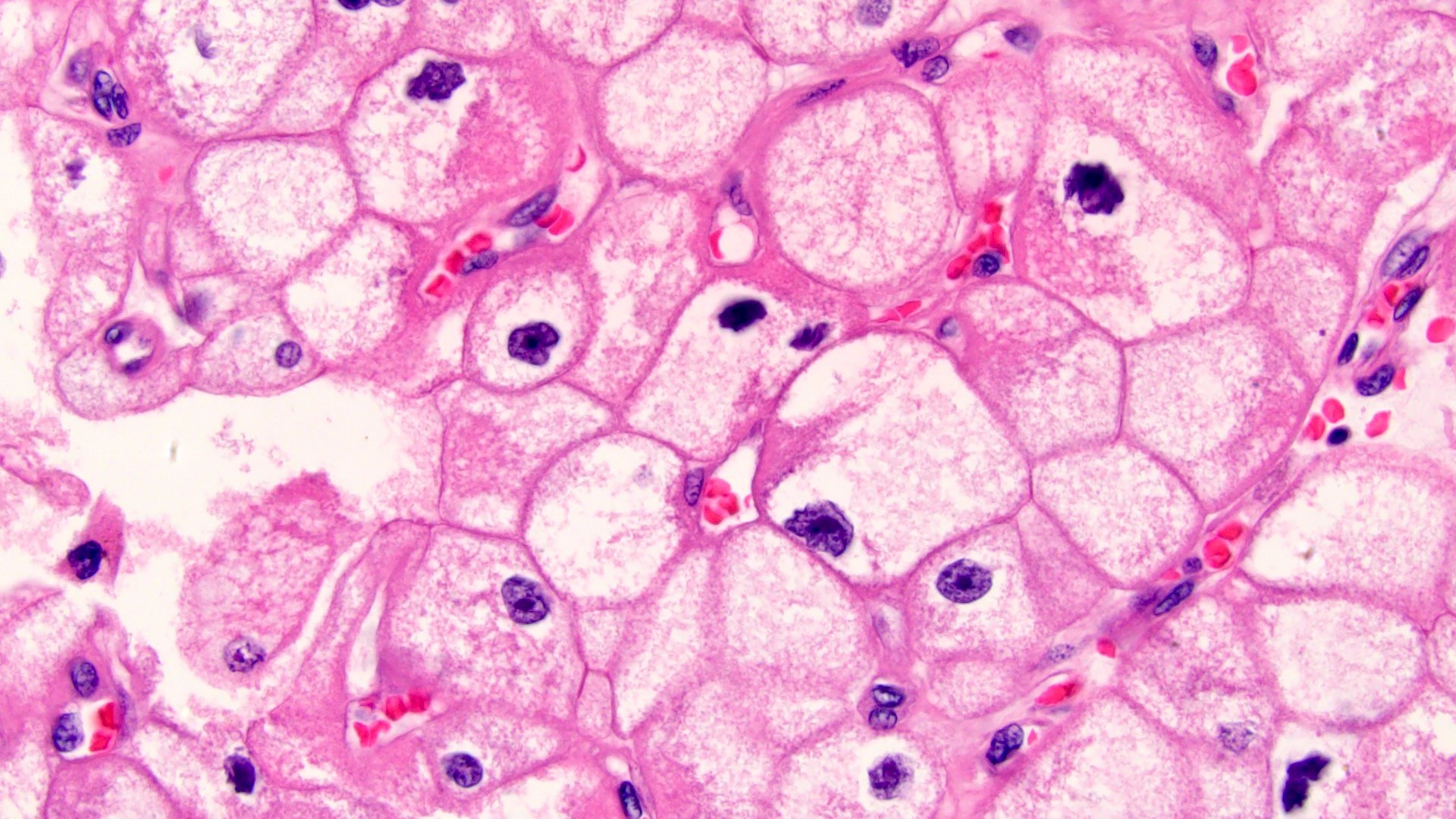
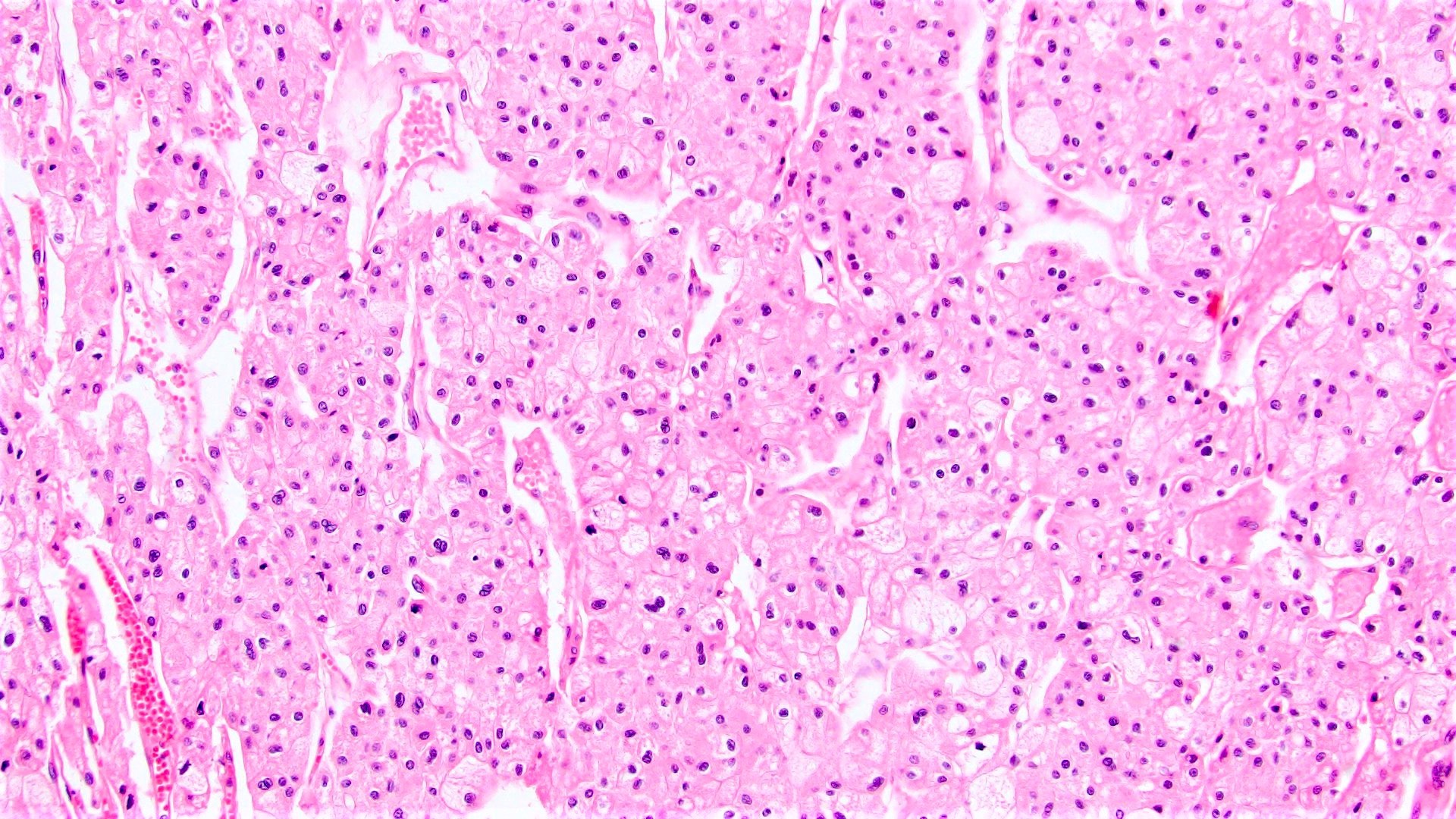
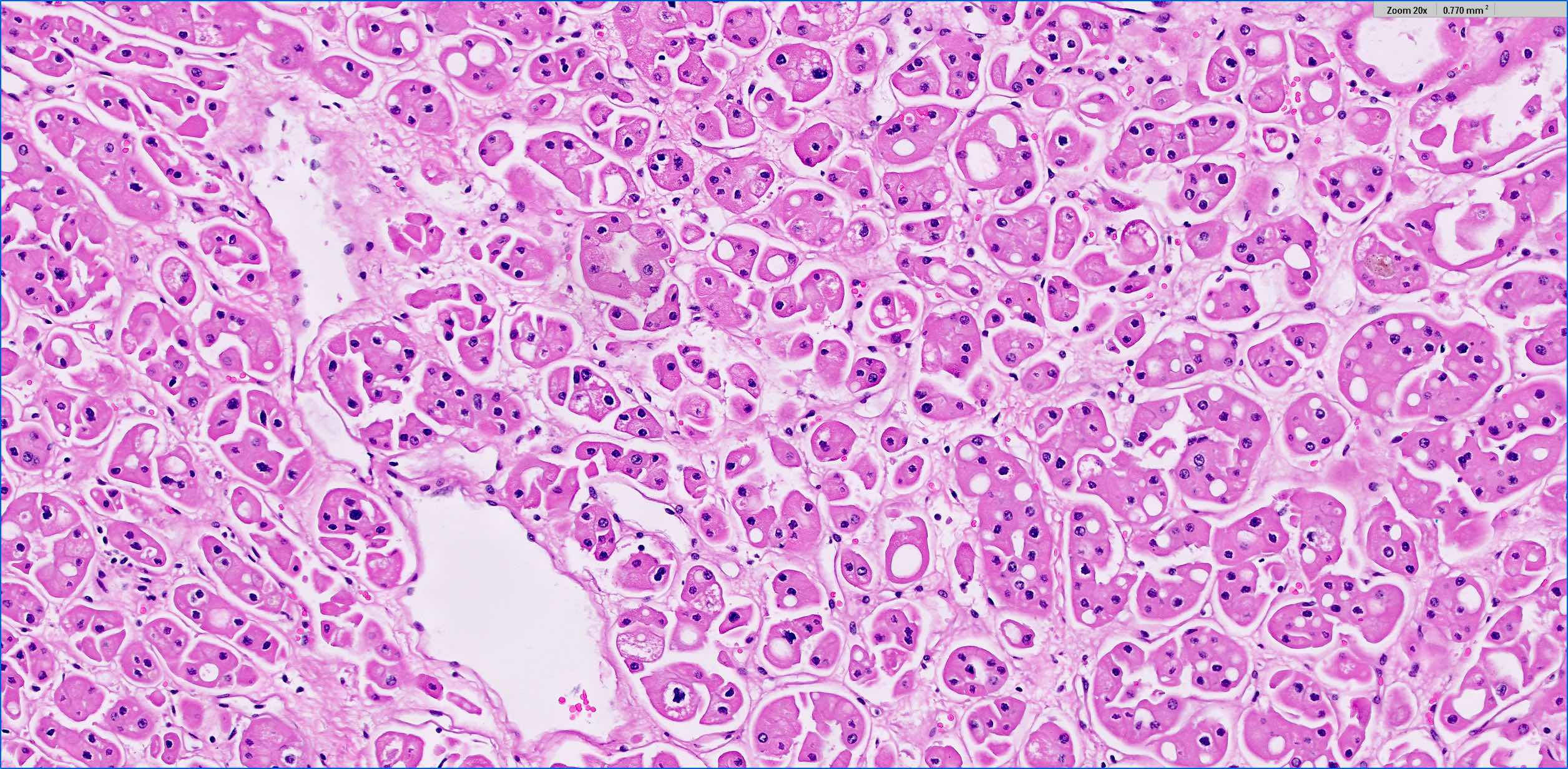
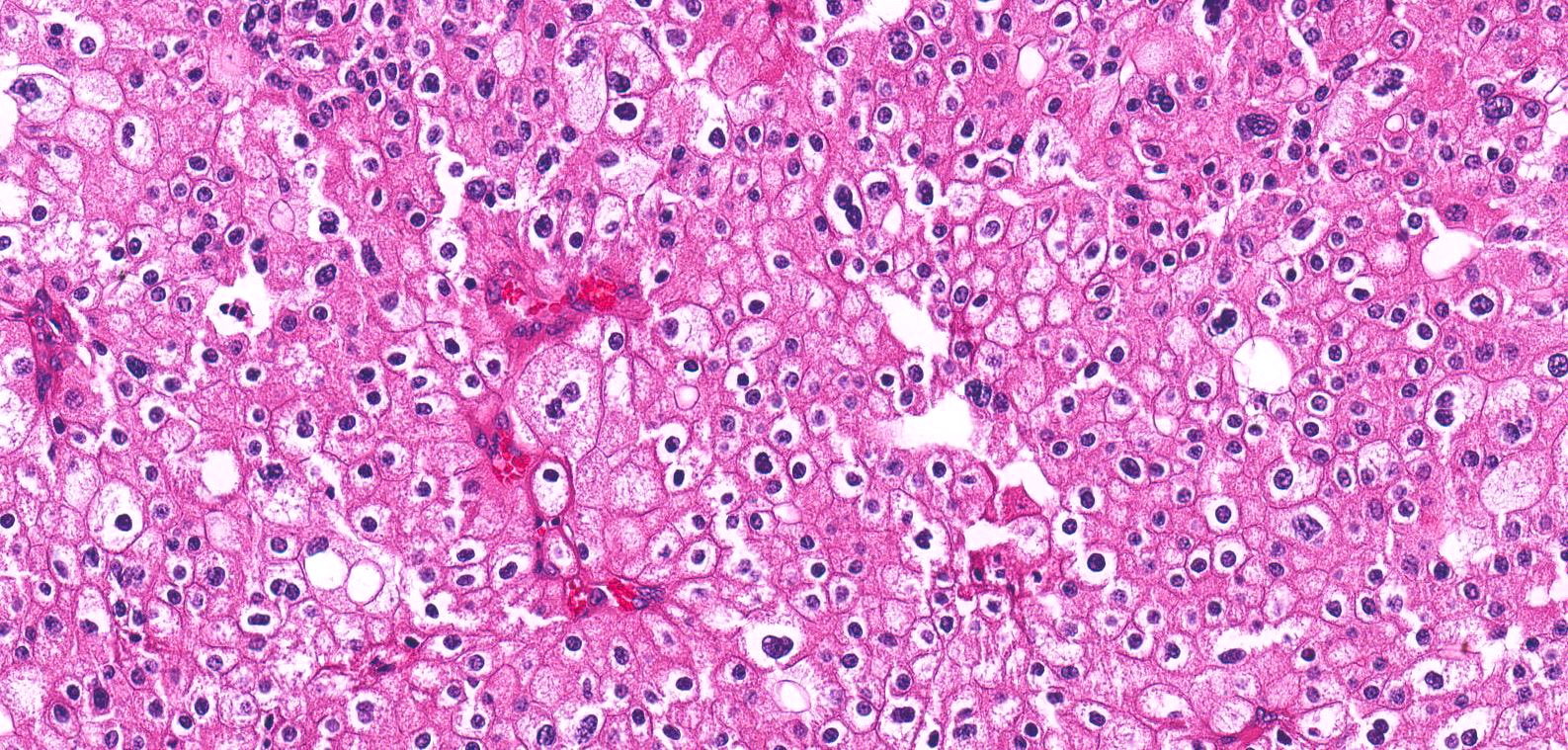
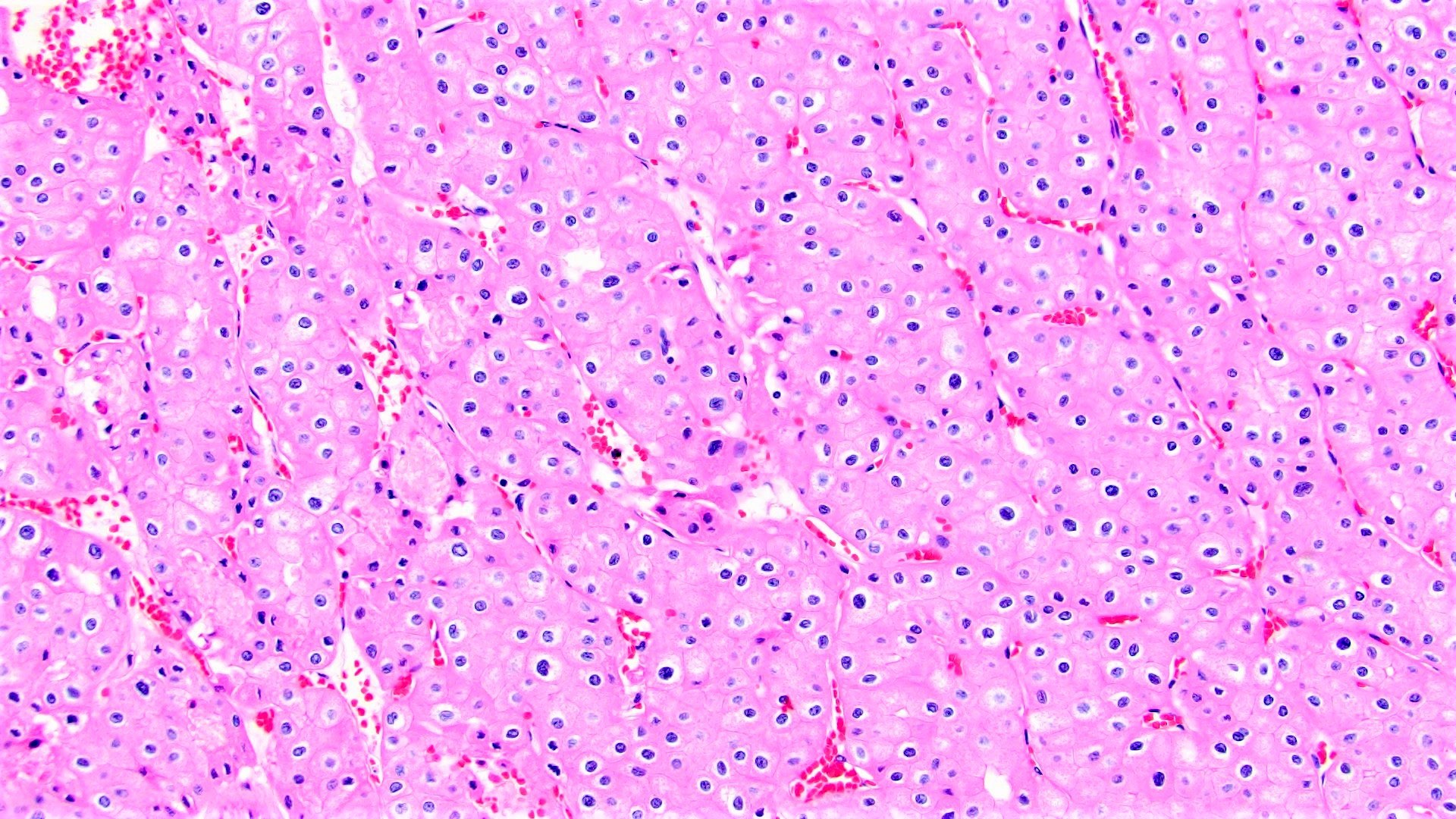
- Eosinophilic
- At least 80% of the tumor must be composed of eosinophilic cells (Adv Anat Pathol 2022;29:194)
- Cells are often smaller than classic chromophobe cells
- Nested, alveolar or sheet-like architecture, whereby nested architecture is more common in eosinophilic variant than in classic subtype
- Otherwise has similar histologic features to classic subtype
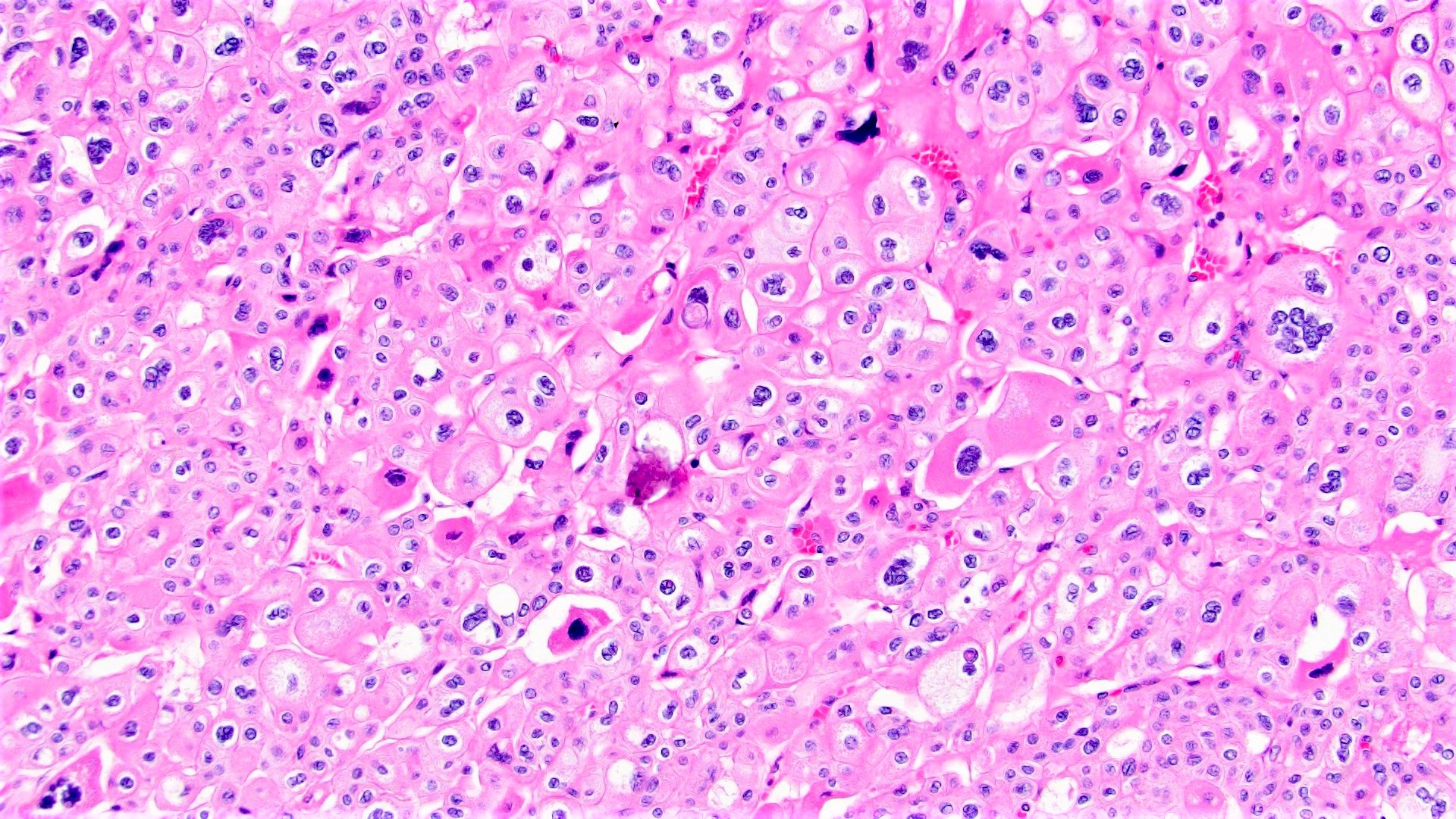
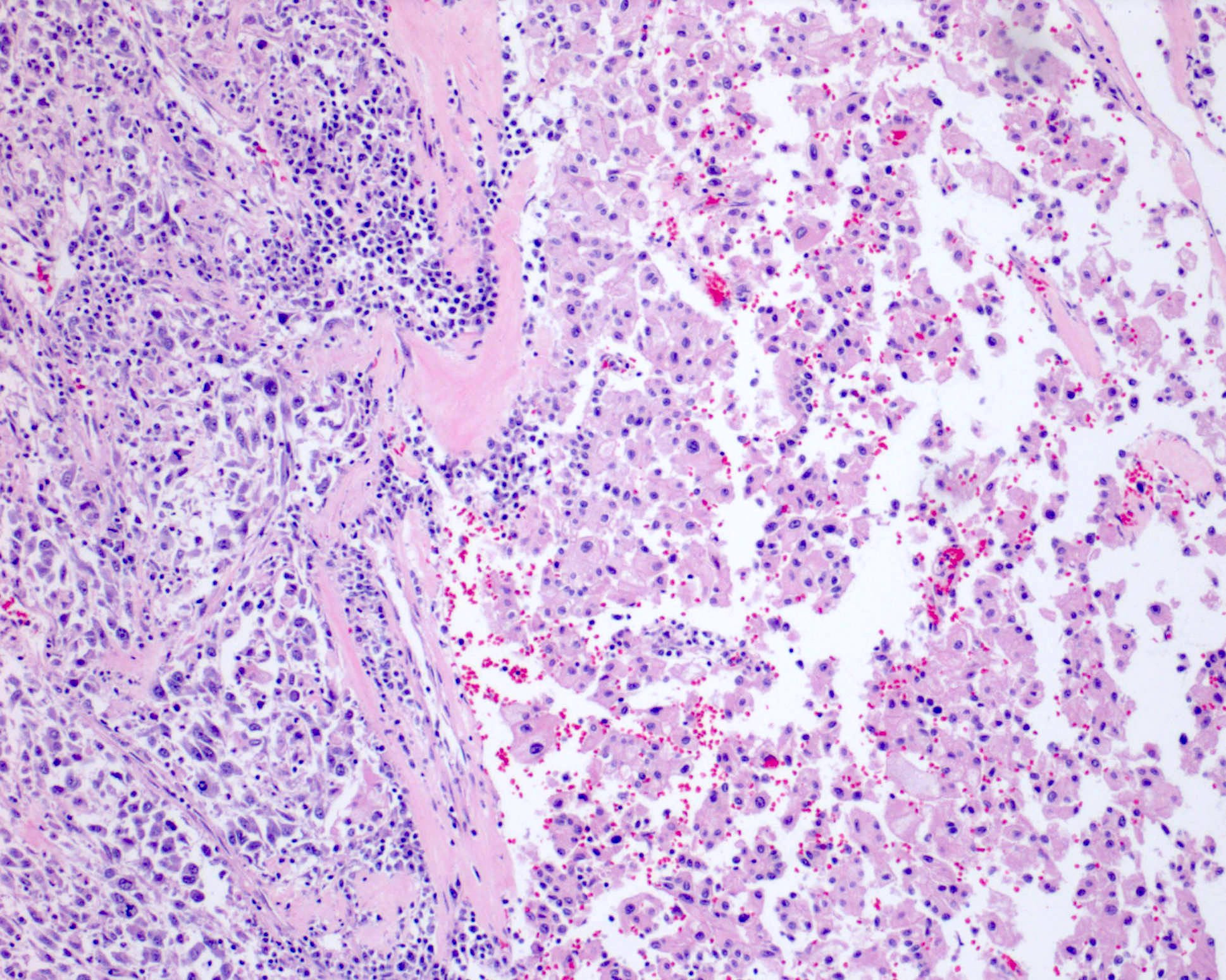
- Sarcomatoid
- Dedifferentiation to high grade, pleomorphic spindle cells with increased mitotic activity, including atypical forms and tumor necrosis
- Other patterns: pleomorphic giant cells, rhabdoid cells, undifferentiated / anaplastic monomorphic cells (Semin Diagn Pathol 2021;38:152)
- All may be associated with heterologous elements (osteoblastic / osteoclastic, chondroblastic, rhabdomyoblastic, liposarcomatous)
- No minimum amount of sarcomatoid histology required for the diagnosis but some authors reserve the diagnosis for when entire fields, examined in isolation, could be mistaken for a sarcoma (Adv Anat Pathol 2022;29:194)
- Other rare histologic patterns (Adv Anat Pathol 2022;29:194)
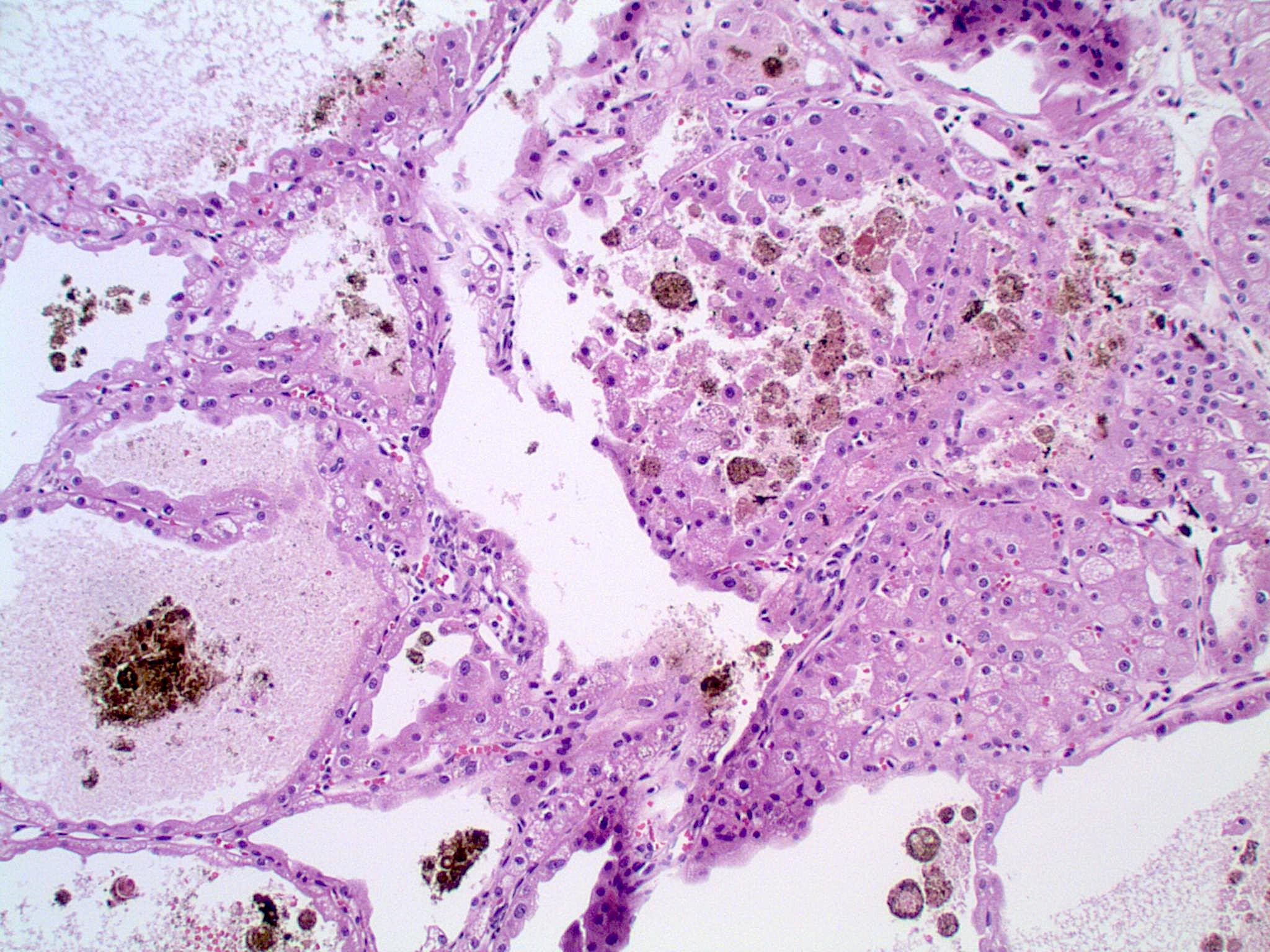
- Adenomatoid / microcystic / pigmented
- Gland-like structures (single tubules or cribriform formations) with a columnar cell lining, basally located nuclei, apical eosinophilic cytoplasm often with a luminal brush border
- Microcysts lined by typical alternating larger pale and smaller eosinophilic chromophobe cells
- Brown or black, extracellular pigment
- Frequent psammomatous or dystrophic calcifications
- Tumor necrosis may be seen
- Frequently admixed with classic component
- Multicystic
- Either variable sized cysts or compressed cystic and tubular structures with slit-like lumina
- Epithelial lining consists of typical 2 cell types of classic component
- Brown extracellular pigment may be seen
- Occasional psammomatous or dystrophic calcifications
- No conventional component associated
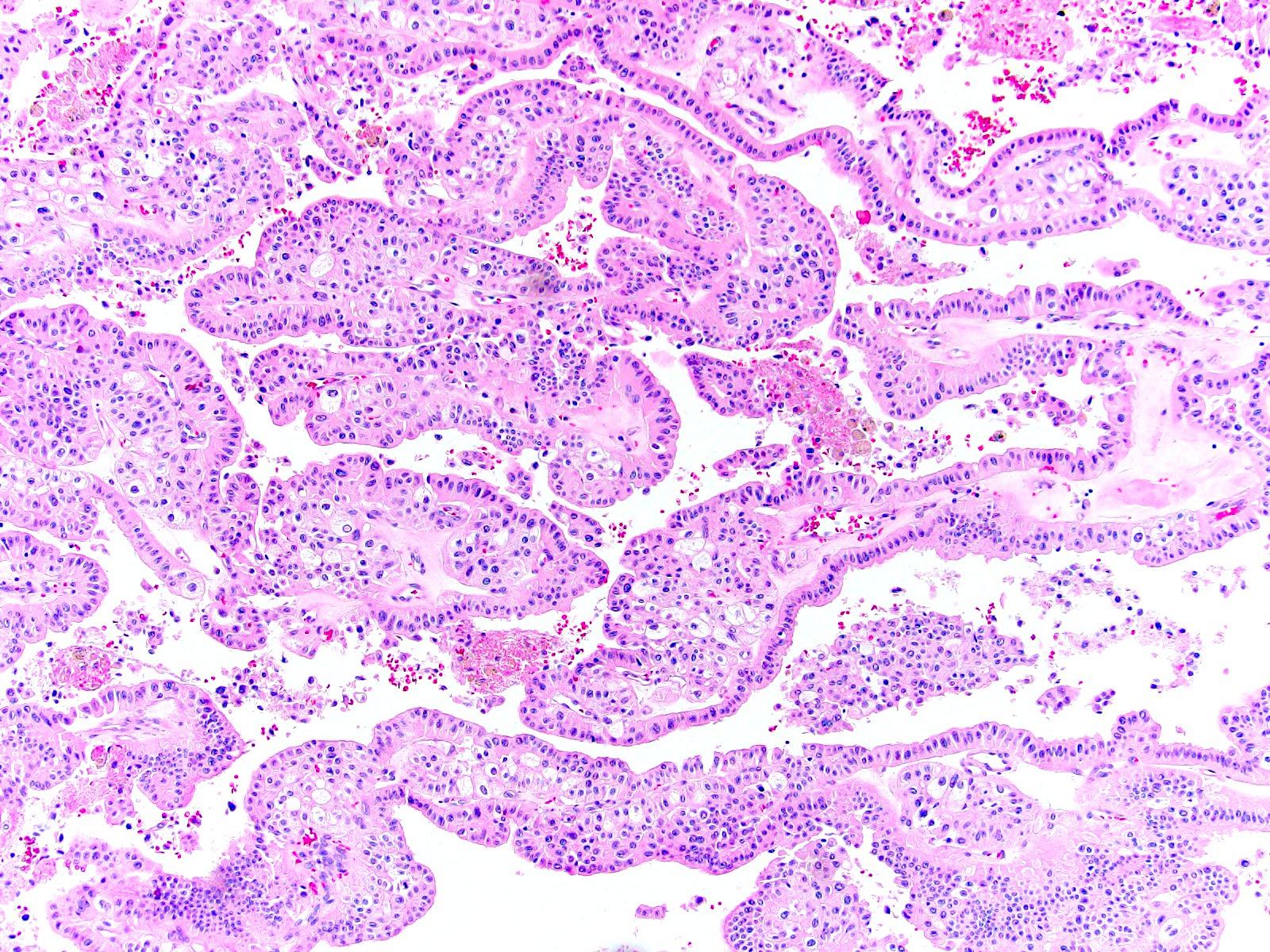
- Papillary
- Variable amount of papillary architecture (can be purely papillary)
- Papillae have slender fibrovascular cores lined by typical larger pale and smaller eosinophilic chromophobe cells
- Psammomatous or dystrophic calcifications as well as pigment may be seen
- Admixed classic areas are useful for the diagnosis
- Neuroendocrine / neuroendocrine-like / small cell-like (Bosn J Basic Med Sci 2022;22:531)
- Admixed classic with tubular, insular, adenomatoid, trabecular and palisaded growth patterns with rosette formation
- Uniform polygonal eosinophilic cells (with scant cytoplasm in small cell-like variant)
- Round to oval nuclei with salt and pepper chromatin and inconspicuous nucleoli
- No significant mitotic activity and usually no tumor necrosis
- Called neuroendocrine when it expresses neuroendocrine markers and neuroendocrine-like when neuroendocrine marker expression is absent
- Micropapillary carcinoma-like (Int J Surg Pathol 2025;33:5)
- Small nests with marked stromal retraction artifact
- Occasional ring forms
- Polygonal cells with bright eosinophilic cytoplasm
- Otherwise, typical cytological features
- Adenomatoid / microcystic / pigmented
- Grading is not recommended
- WHO / ISUP grading system has not been validated (Am J Surg Pathol 2013;37:1490)
- Several grading schemes proposed but no consensus to date (Adv Anat Pathol 2022;29:117)
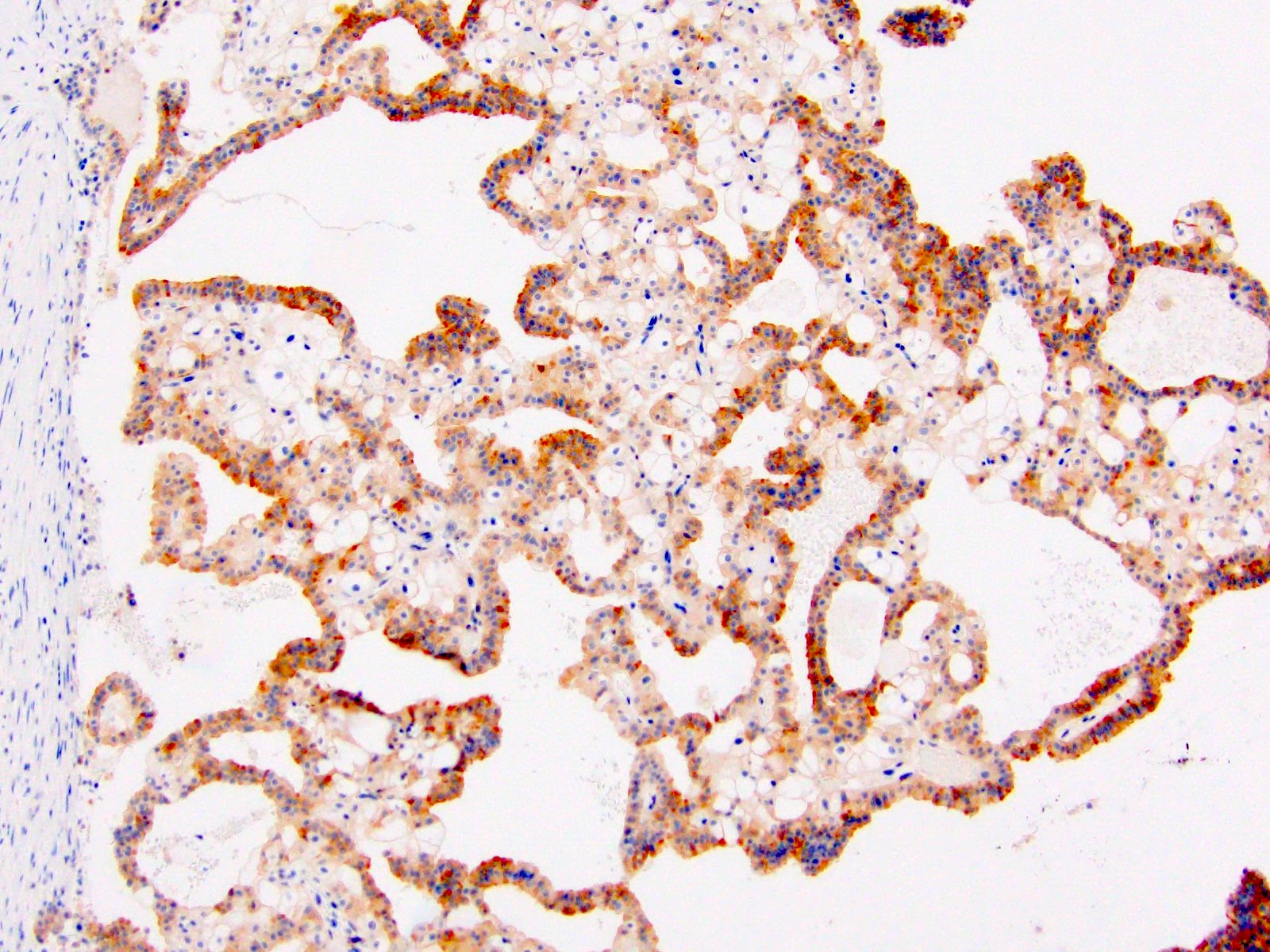
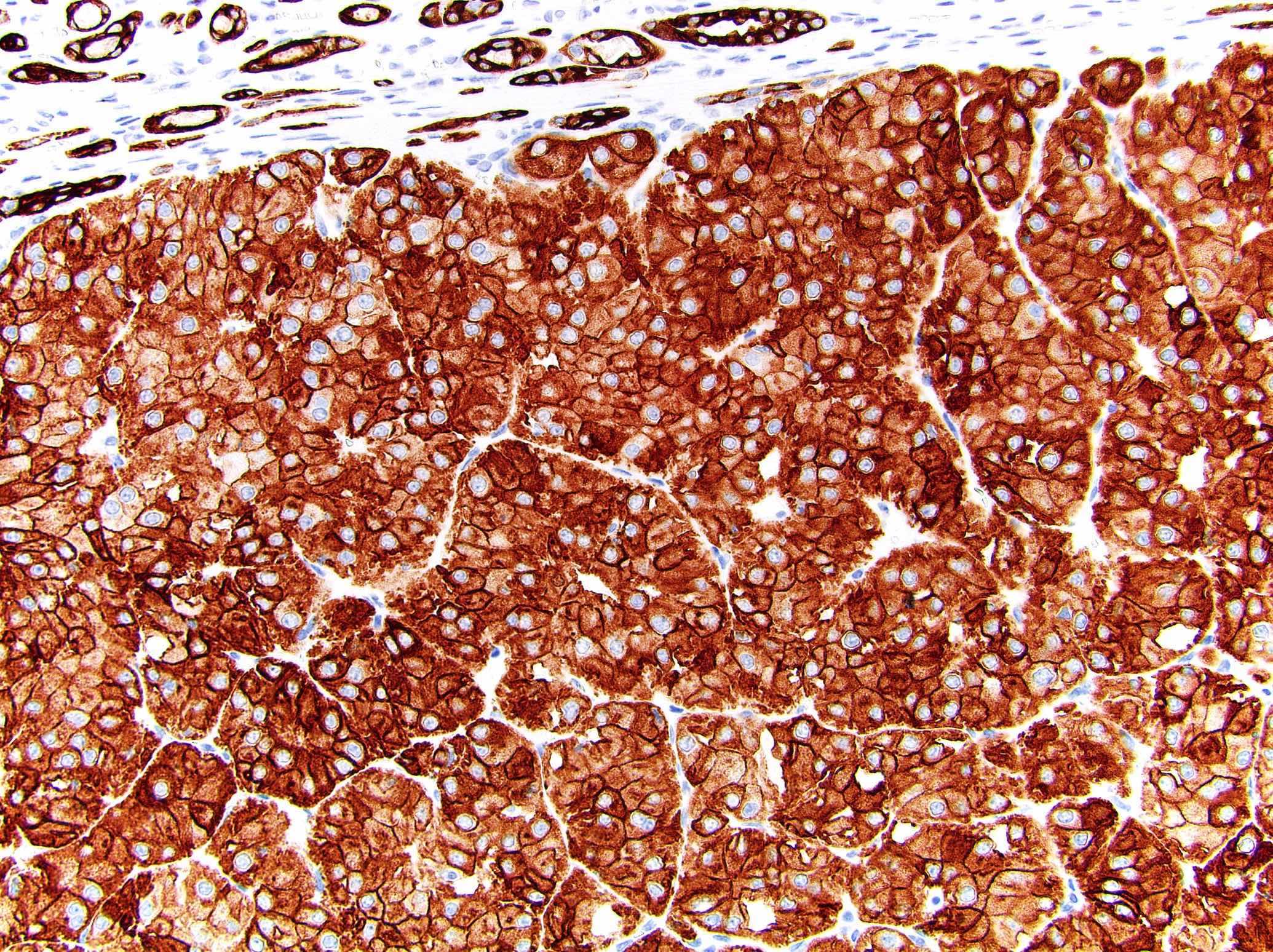
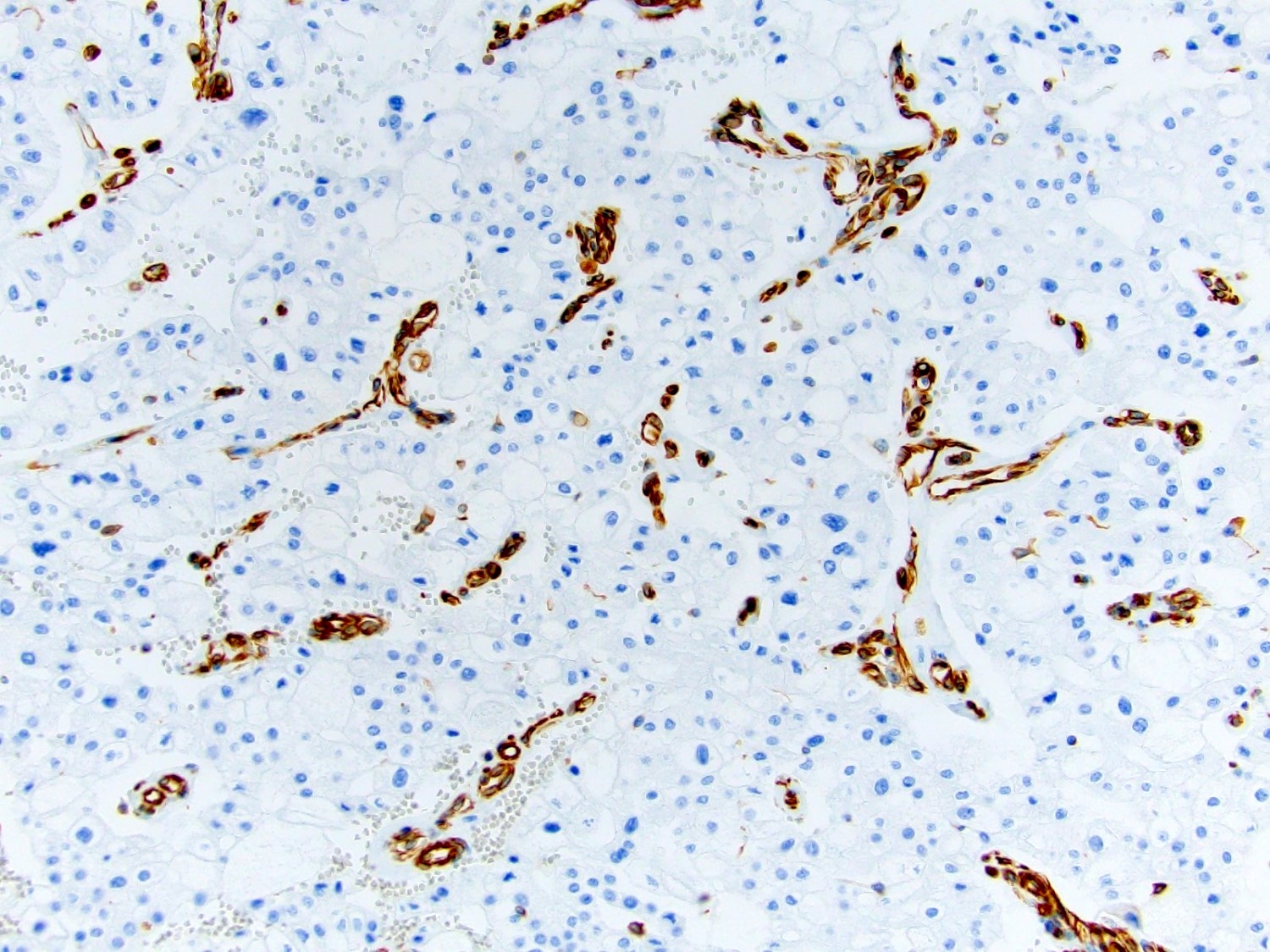
Microscopic (histologic) images
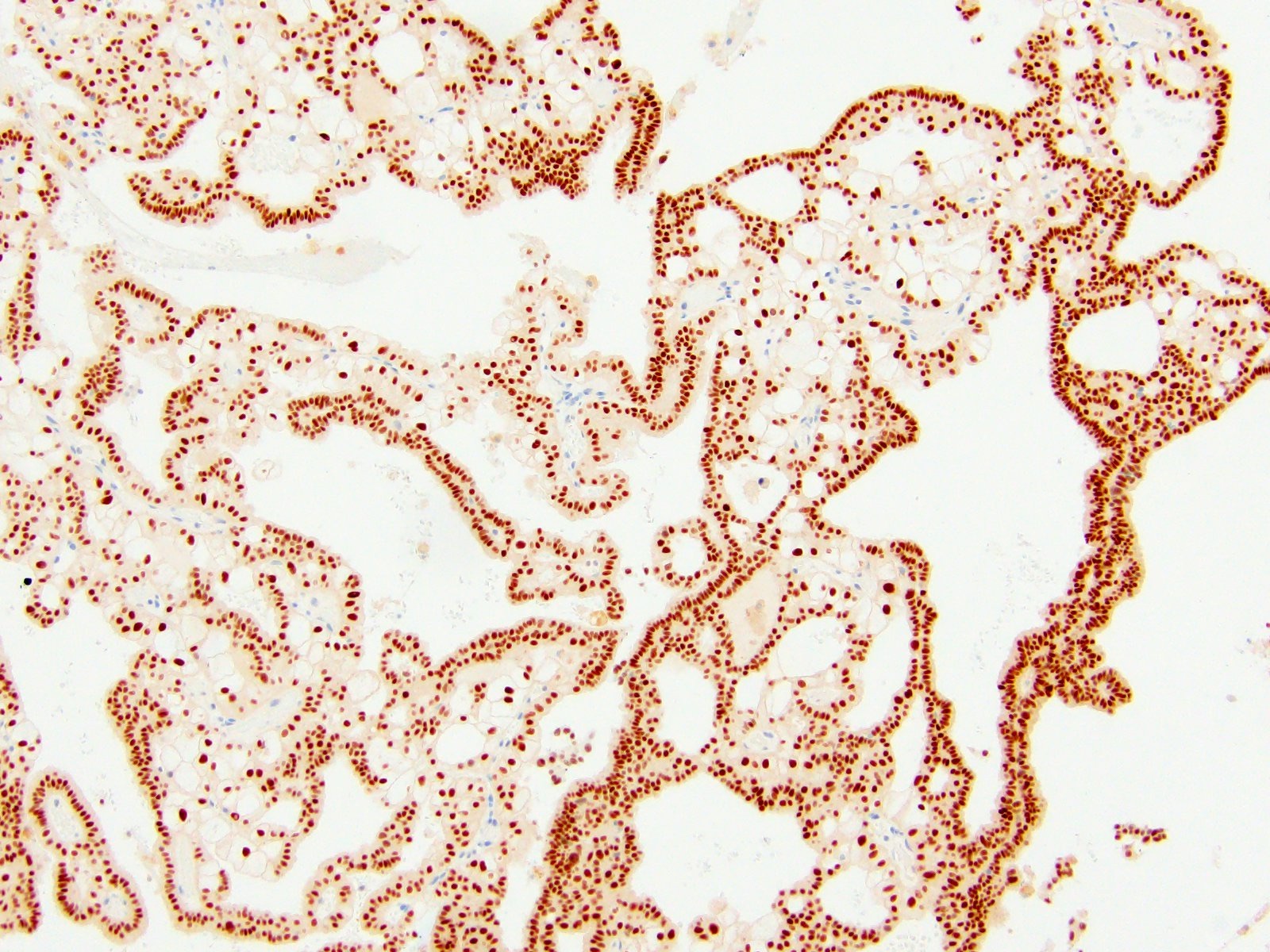
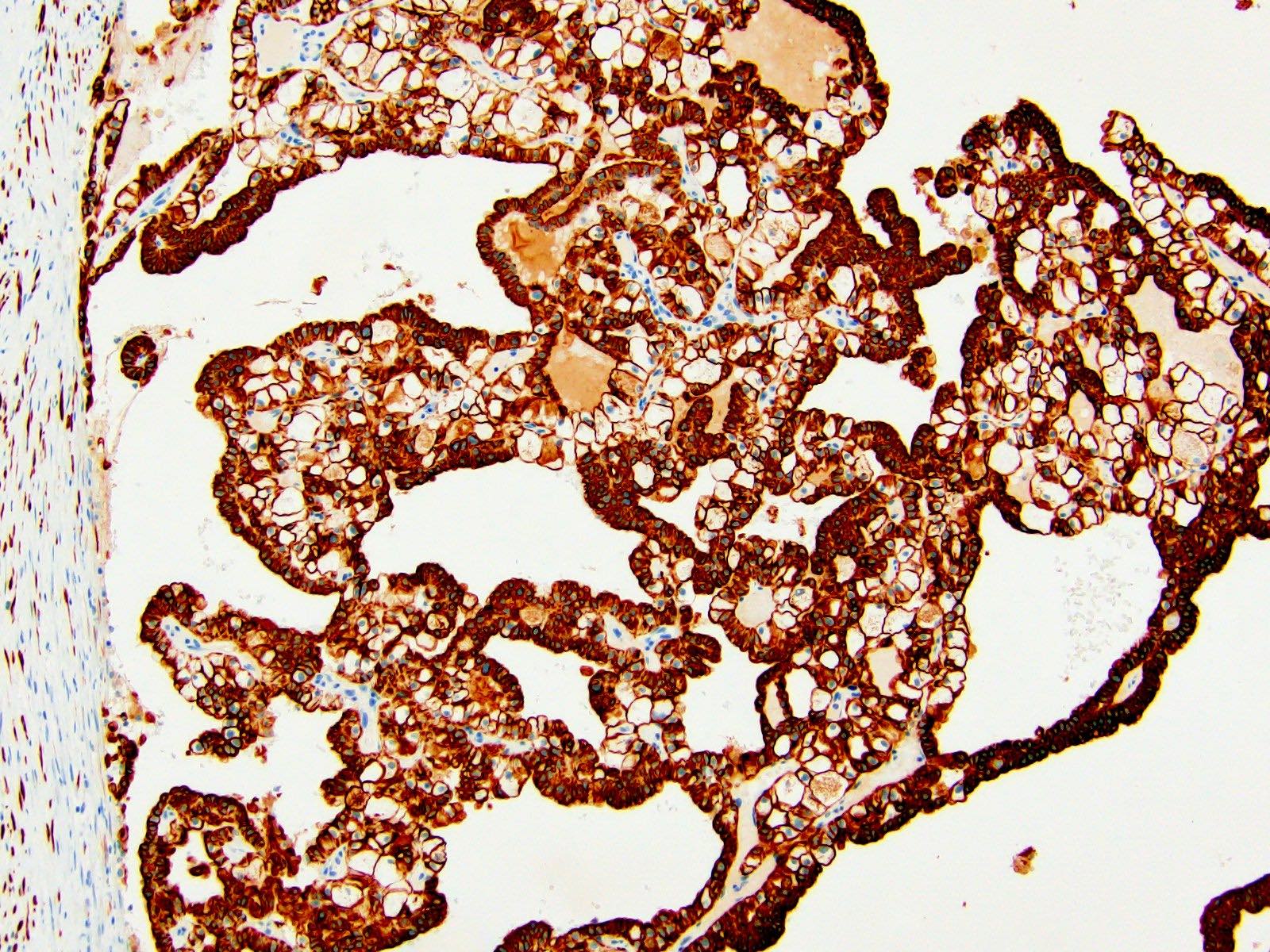
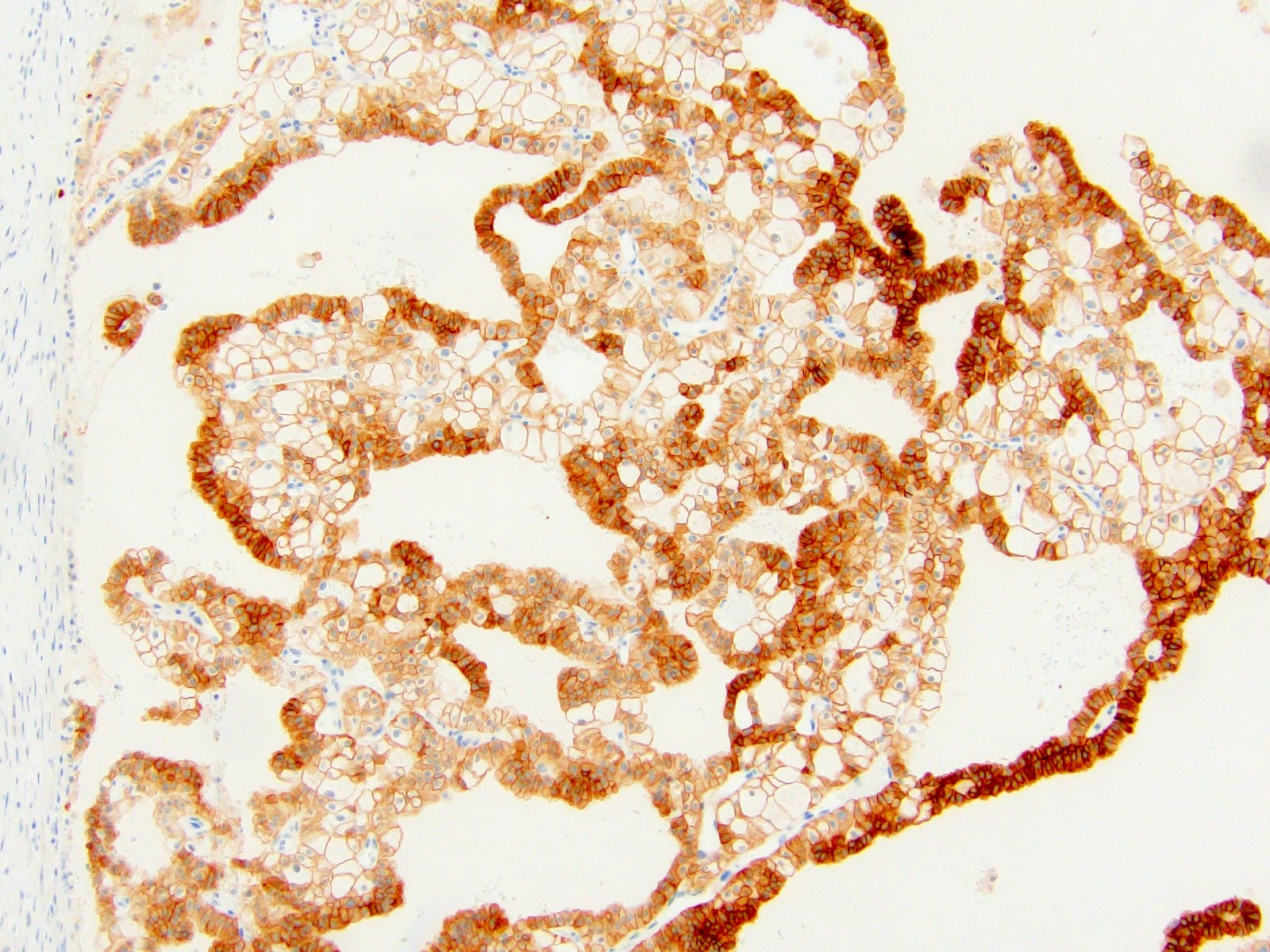
Contributed by Maria Tretiakova, M.D., Ph.D., Timothy Isaac Miller, M.D., M.A., Miruna C. Popescu, M.D., Ankur Sangoi, M.D., Daniel Anderson, M.D., M.B.A. and @katcollmd on Twitter
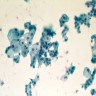
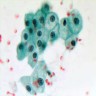
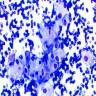
Cytology description
- Single cells and small, discohesive, monolayered groups; cells vary in size from small to large
- Large cells show clear, flocculent cytoplasm with small, eccentric nuclei and frequent binucleation and occasional nuclear pseudoinclusions
- Small cells usually have dense, homogeneous cytoplasm, clear cytoplasmic spaces resembling perinuclear halos, binucleation and marginal nuclear location
- No necrosis, no basement membrane or other stromal material
- Reference: Cancer Cytopathol 2018;126:711
Cytology images
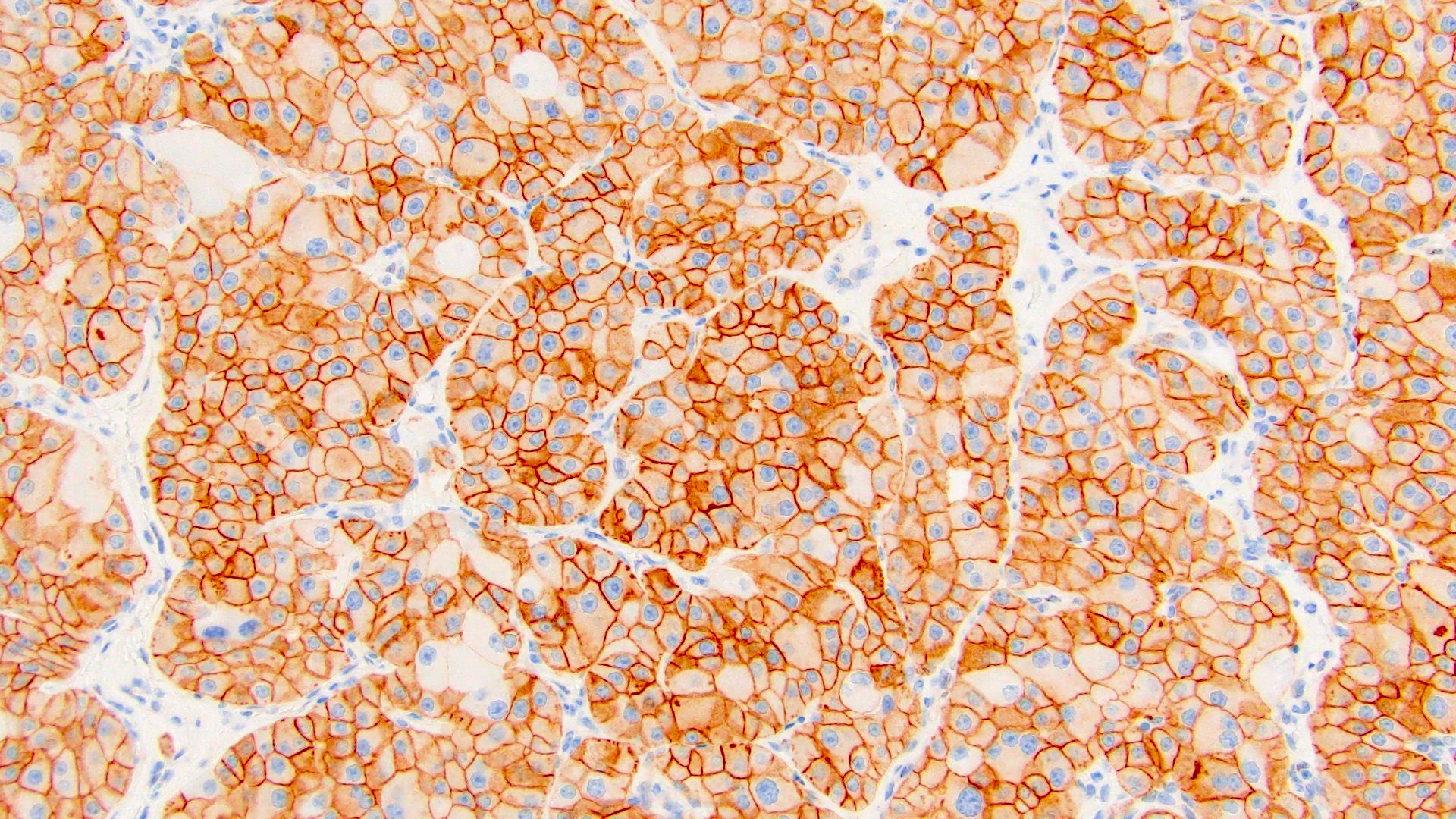
Positive stains
- Most commonly used
- CD117 / KIT: diffuse membranous; negative in 88% of sarcomatoid ChRCC (Am J Surg Pathol 2022;46:1171)
- CK7: diffuse and strong in classic ChRCC, reduced (patchy / isolated cells) in eosinophilic ChRCC; may be negative in 63% of sarcomatoid ChRCC (Adv Anat Pathol 2022;29:194, Am J Surg Pathol 2022;46:1171)
- PAX8 and PAX2: useful to prove renal cell origin; may be negative in sarcomatoid ChRCC (Am J Surg Pathol 2022;46:1171)
- AE1 / AE3, CAM 5.2, EMA: useful to prove epithelial origin; reduced in sarcomatoid ChRCC (Int J Surg Pathol 2024;32:11)
- Hale colloidal iron: diffuse and strong cytoplasmic staining (stains acid mucopolysaccharides in microvesicles)
- FH retained
- SDHB retained
- Other positive stains
- DOG1: helps to distinguish from oncocytoma from clear cell RCC; negative in sarcomatoid ChRCC (Pathol Res Pract 2015;211:303, Am J Surg Pathol 2022;46:1171)
- Neuroendocrine markers (synaptophysin, chromogranin A, CD56, NSE) in the extremely rare neuroendocrine histological variant
Negative stains
- CAIX: significant positive staining may be noted in sarcomatoid component of ChRCC (Hum Pathol 2022;119:85)
- Vimentin: except scar area and sarcomatoid component (Int J Surg Pathol 2024;32:83, Int J Surg Pathol 2024;32:11)
- AMACR
- CD10: rarely positive (focally), more so in sarcomatoid component of ChRCC (Int J Surg Pathol 2024;32:11)
- Cathepsin K: helpful in the distinction from epithelioid angiomyolipoma and MiT family RCC; diffuse positive staining in both ChRCC and oncocytoma, with lesser intensity in ChRCC, has been reported with the EPR19992 clone (Mod Pathol 2012;25:100, Mod Pathol 2009;22:1016, Int J Surg Pathol 2021;29:600)
- GATA3: 30% positive in conventional epithelial component, 50% positive in sarcomatoid component of ChRCC (Am J Surg Pathol 2022;46:1171)
- HMWCK
- Cyclin D1: helpful to distinguish from oncocytoma (81% positive) (Pathol Res Pract 2015;211:303)
Electron microscopy description
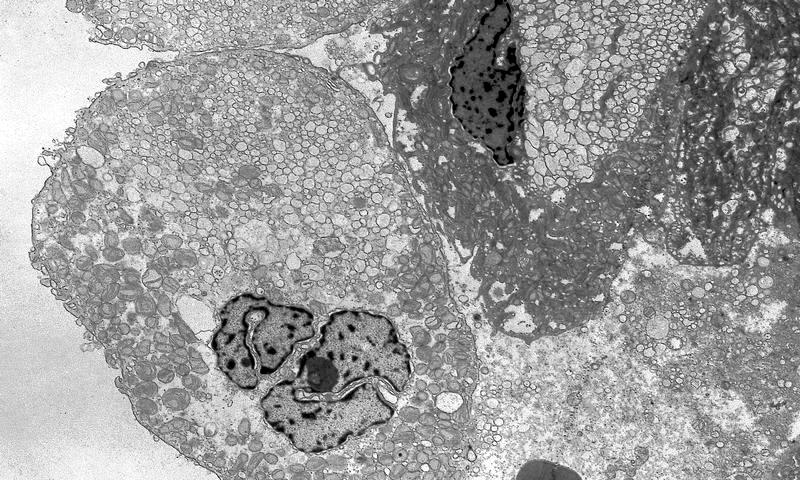
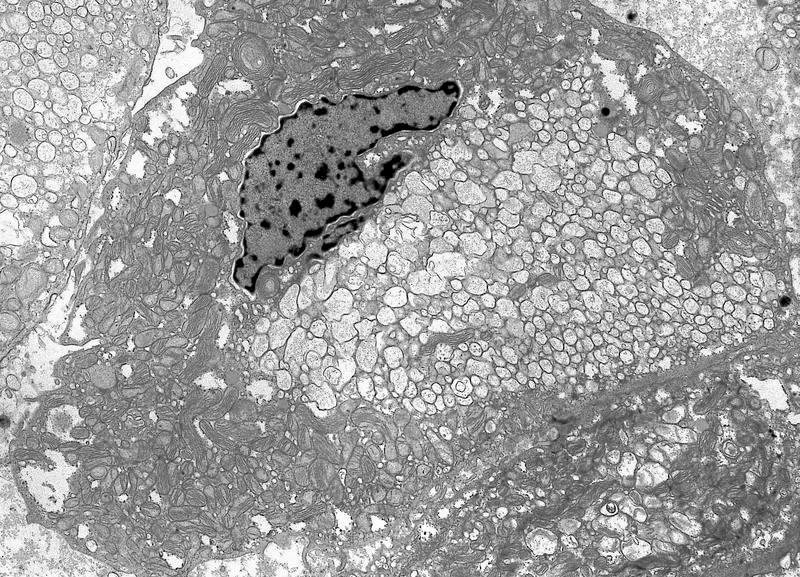
- The larger pale cells of classic ChRCC contain cytoplasmic microvesicles, which are considered a unique and consistent ultrastructural feature (Pathology 2021;53:101)
- These 150 - 350 nm membrane bound microvesicles do not stain with H&E stain, often concentrated in perinuclear location corresponding to perinuclear halos on light microscopy and reticular pale cytoplasm
- Microvesicle origin is uncertain but possibly from mitochondrial outpouchings or defective mitochondriogenesis
- Eosinophilic cells contain abundant mitochondria; mitochondria often show tubulocystic cristae as well as rare, short and stubby microvilli
Electron microscopy images
Molecular / cytogenetics description
- Variable copy number alterations (CNAs), most commonly loss of chromosomes 1, 2, 6, 7, 10, 13, 17 and 21 (86%, mainly classic ChRCC) (Asian J Urol 2022;9:1)
- Additional reported losses of chromosomes 3, 5, 8, 9, 11 and 18 (12 - 58%) (Asian J Urol 2022;9:1)
- Chromosomal gains of 4, 7, 15, 19 and 20 also reported (mainly in classic and sarcomatoid ChRCC) (Adv Anat Pathol 2021;28:8)
- Eosinophilic ChRCC less frequently shows the characteristic chromosomal losses of classic ChRCC and may even have diploid karyotype; no chromosomal gains reported to date (Adv Anat Pathol 2021;28:8)
- Doubled hypodiploidy is a common phenomenon in eosinophilic ChRCC (Hum Pathol 2020;104:18)
- Other rare subtypes of ChRCC (excluding sarcomatoid) show variable CNAs, more often losses than gains (Asian J Urol 2022;9:1)
- Classic ChRCC shows lower tumor mutation burden (TMB) than other RCC subtypes, most commonly involving TP53 (32 - 64%), PTEN (9 - 45%) and TERT promoter (6 - 12%); loss of CDKN2A is also frequently seen (Asian J Urol 2022;9:1)
- Mutations in MTOR, TSC1, TSC2 have been reported in a minority of ChRCC, especially eosinophilic subtypes and associated with a worse prognosis (Mod Pathol 2020;33:2580)
- ChRCC also displays somatic mutations in mitochondrial DNA (Asian J Urol 2022;9:1)
- Recurring RB1 mutations are found in 27% of tumors and suggest that subclonal RB1 mutations can drive the evolution to high grade, nonsarcomatoid ChRCC (Mod Pathol 2024;37:100472)
Videos
Pathology resident led live unknown slide session: pink renal tumors (part 1) by Dr. Miruna C. Popescu
A dummies guide to pink cell (oncocytic) renal tumors by Dr. Rajal B. Shah
Pathology mini tutorials: ChRCC
Pathology mini tutorials: distinguishing eosinophilic ChRCC from oncocytoma
Sample pathology report
- Left kidney, radical nephrectomy:
- Chromophobe renal cell carcinoma, eosinophilic variant (see comment) (see synoptic report)
- Comment: Microscopic sections show solid sheets and broad trabeculae composed predominantly (> 80%) of polygonal cells with eosinophilic cytoplasm, prominent cell borders, perinuclear clear halos and hyperchromatic nuclei with irregular nuclear membranes.
Differential diagnosis
- Clear cell RCC (versus both classic and eosinophilic ChRCC):
- Optically clear cytoplasm instead of finely reticulated
- No raisinoid nuclei, no perinuclear halos, no prominent cell borders
- Negative for CD117 / KIT, CK7, Hale colloidal iron
- Positive for CAIX, vimentin (especially when higher grade and mostly eosinophilic)
- Oncocytoma (versus eosinophilic ChRCC):
- Uniform, round, regular nuclei (no koilocytic-like nuclear atypia)
- Only occasional binucleation
- No perinuclear halos, no prominent cell membranes
- CK7 negative or positive only in scattered, isolated cells (beware of increased staining in central scar area)
- Hale colloidal iron negative or apical bar
- Positive for cyclin D1, S100A1
- Hybrid oncocytic chromophobe tumor:
- Seen in 3 distinct scenarios: renal oncocytosis, Birt-Hogg-Dubé (BHD) syndrome or sporadic (GUPS recommendation to call sporadic hybrid tumors oncocytic renal neoplasm of low malignant potential)
- Presence of multiple, bilateral tumors is a clue
- Tumors with oncocytic cells with mixed morphology between oncocytoma and ChRCC
- Tumors with distinct areas with more typical renal oncocytoma-like and ChRCC-like areas (mosaic alternating pattern)
- Variably positive for CK7
- Positive for CD117 / KIT
- Variable (apical bar to diffuse) Hale colloidal iron
- Sporadic cases exhibit different mutational patterns than in oncocytoma or ChRCC; syndromic cases with FLCN (BHD) gene mutations (Mod Pathol 2019;32:1698)
- Low grade oncocytic tumor (versus eosinophilic ChRCC):
- Solid sheets with abrupt transition to edematous areas with loosely arranged cords and strands of oncocytic cells (boats in a bay pattern)
- Uniform, round / oval, low grade nuclei without nuclear membrane irregularities (no raisinoid nuclei)
- Patchy, usually subtle perinuclear halo
- No prominent, plant cell-like cell borders
- Diffusely positive for CK7 and GATA3
- Negative for CD117 / KIT
- Eosinophilic solid and cystic RCC (versus eosinophilic ChRCC):
- Solid and (micro)cystic components
- Variably high grade, mild pleomorphic nuclei
- Usually abundant granular eosinophilic cytoplasm, sometimes centrally condensed with more flocculent pale periphery
- Fine to coarse basophilic cytoplasmic stippling / inclusions (leishmaniasis-like)
- Cystic areas lined by monolayered, hobnailed, frequently multinucleated cells
- Foamy macrophages and lymphocytic aggregates are common
- Positive for CK20
- Negative for CK7 (may be focally positive but always CK20 > CK7)
- Negative for CD117 / KIT
- High grade papillary RCC (versus eosinophilic ChRCC):
- No raisinoid nuclei, no perinuclear halos, no prominent cell borders
- Negative for CD117 / KIT and CK7 (or focal, as in eosinophilic ChRCC)
- Positive for AMACR, vimentin, CD10
- SDH deficient RCC:
- Characteristic univacuolated cytoplasm with pale eosinophilic, flocculent material
- Uniform round / oval nuclei without nuclear membrane irregularities
- No perinuclear halos, no prominent cell borders
- Loss of SDHB
- Negative for CD117 / KIT, CK7
- MiT family RCC (especially TFEB altered RCC):
- TFEB altered RCC (and especially TFEB amplified RCC) most closely resembles eosinophilic ChRCC
- If present, biphasic pattern with smaller cells clustered around basement membrane hyaline material and larger peripheral epithelioid cells may be helpful
- No prominent cell borders, no perinuclear halos
- Positive for TFEB (must be strong, nuclear and cytoplasmic)
- Variably positive for cathepsin K, MelanA, HMB45
- Negative for CD117 / KIT
- Epithelioid angiomyolipoma:
- Epithelioid to plump spindled cells with prominent nucleoli, intranuclear pseudoinclusions, variable cell discohesion with eosinophilic cytoplasm, sometimes ganglion cell-like appearance
- Negative for PAX8 and CK
- Positive for HMB45, MelanA and cathepsin K (beware of diffusely positive EPR19992 clone in ChRCC) (Int J Surg Pathol 2021;29:600)
- Variably positive for SMA
- Positive for TFE3, GPNMB, TRIM63 RNA ISH
- Oncocytic adrenal cortical carcinoma (versus eosinophilic ChRCC):
- Upper kidney pole localization
- Patchy prominent anisonucleosis (endocrine atypia)
- No raisinoid nuclei, no perinuclear halos, no prominent cell borders
- Nuclei frequently looked pushed to the side of the cell (sometimes even with a rhabdoid appearance)
- Negative for PAX8, CK7, CD117 / KIT, Hale colloidal iron
- Positive for SF1, inhibin, calretinin, MelanA, vimentin, synaptophysin
Additional references
Practice question #1
Which of the following is true about chromophobe renal cell carcinoma (ChRCC)?
- Binucleation is pathognomonic
- Cellulose deposition explains plant-like prominent membranes
- Cytoplasmic clearing is due to high concentration of lipids
- Multinucleation is an unfavorable prognostic feature
- Perinuclear halos are due to accumulation of cytoplasmic microvesicles
Practice answer #1
E. Perinuclear halos are due to accumulation of cytoplasmic microvesicles. The presence of cytoplasmic microvesicles is a unique and consistent ultrastructural feature of ChRCC. Microvesicles are often concentrated in perinuclear location, corresponding to perinuclear halos on light microscopy. These vesicles lack affinity for H&E stain, resulting in cells with clear, reticular or flocculent cytoplasmic appearance on light microscopy (chromophobe). The origin is uncertain but likely related to defective mitochondria. Answer A is incorrect because binucleation may also be seen in other renal cell tumors, including oncocytoma, one of the main differential diagnoses of ChRCC. Answer B is incorrect because the term plant cell-like is only a descriptive term, it does not relate to accumulation of cellulose in the cellular membrane. Answer C is incorrect because the cytoplasmic clearing in ChRCC is due to accumulation of microvesicles, not lipid. Answer D is incorrect because multinucleation, while part of the WHO / ISUP grading system, does not apply to chromophobe RCC. Multinucleated cells are commonly seen in ChRCC and unlike in clear cell or papillary RCC, have no proven prognostic significance.
Comment Here
Reference: Chromophobe
Comment Here
Reference: Chromophobe
Practice question #2
What is the best statement describing chromophobe renal cell carcinoma (ChRCC)?
- Can be reliably distinguished from clear cell RCC by CK7, CD117 / KIT, CAIX and vimentin
- Classic autosomal dominant genetic correlation is Birt-Hogg-Dubé syndrome, which is associated with a mutation of the HOGG gene on chromosome 3p
- Sarcomatoid dedifferentiation is a very common phenomenon
- Typically shows gains of chromosomes 1, 2, 6, 7, 10, 13, 17 and 21
- WHO / ISUP grading system is a useful prognostic indicator
Practice answer #2
A. Can be reliably distinguished from clear cell RCC by CK7, CD117 / KIT, CAIX and vimentin. ChRCC is typically diffusely positive for CD117 / KIT and CK7, while negative for CAIX and vimentin. In contrast, clear cell RCC is typically CAIX positive, may be vimentin positive especially with increasing grade, it is usually negative for CK7 (except predominantly cystic tumors) and is negative for CD117 / KIT. Answer B is incorrect because Birt-Hogg-Dubé is caused by an autosomal dominant mutation of the Folliculin gene (FLCN) at 17p11.2 leading to premature truncation and loss of function of the folliculin protein. Von Hippel-Lindau (VHL) syndrome, also autosomal dominant, is caused by a mutation of the VHL tumor suppressor gene (3p25-26). Depending on the subtype, VHL is associated with clear cell renal cell carcinoma, CNS and retinal hemangioblastomas, visceral cysts in the kidney, pancreas and epididymis, endolymphatic sac tumors, pancreatic neuroendocrine tumors, paragangliomas and pheochromocytomas. Answer E is incorrect because, unlike in clear cell RCC and papillary RCC, the WHO / ISUP grading system has no prognostic value in ChRCC. Answer C is incorrect because sarcomatoid transformation, although being a significant poor prognostic indicator in chromophobe RCC, is observed in ~ 5% of cases. Answer D is incorrect because chromophobe RCC typically shows multiple losses of whole chromosomes, most often 1, 2, 6, 7, 10, 13, 17 and 21.
Comment Here
Reference: Chromophobe
Comment Here
Reference: Chromophobe
Practice question #3
Practice answer #3
A. CK7+, KIT+, vimentin-, CAIX-. The image shown is chromophobe renal cell carcinoma (ChRCC), eosinophilic subtype. Histologic clues to the diagnosis of ChRCC are the raisinoid nuclei, perinuclear clearing / halos and prominent, plant cell-like borders. Furthermore, at least 80% of the cells have an eosinophilic cytoplasm, thus making it the eosinophilic variant of ChRCC. The immunoprofile of ChRCC is typically CK7+, CD117 / KIT+, vimentin- and CAIX-. The other entity to consider is oncocytoma but in this case the degree of nuclear pleomorphism (with enlarged, wrinkled and oval nuclei) favors ChRCC. Answer D is incorrect because CK7-, KIT+, vimentin-, CAIX- would be the immunohistochemical profile most typical of oncocytoma, although vimentin can be focally positive in both ChRCC and oncocytoma in the central scar area. Answer E is incorrect because it is the immunoprofile most typical of clear cell renal cell carcinoma; while high grade clear cell RCC may be predominantly eosinophilic, the perinuclear clearing and raisinoid nuclei are still most characteristic of ChRCC. Answer B is incorrect because CAIX is almost always negative in ChRCC while positivity is more characteristic of clear cell RCC. Answer C is incorrect because ChRCC is typically negative for vimentin. Positive vimentin staining is more characteristic of clear cell RCC, especially when higher grade and mostly eosinophilic.
Comment Here
Reference: Chromophobe
Comment Here
Reference: Chromophobe
Practice question #4
Which of the following is true regarding eosinophilic chromophobe RCC compared to classic ChRCC?
- Classic ChRCC is more likely to have a brown coloration grossly
- Classic ChRCC typically has smaller cells than eosinophilic ChRCC
- Eosinophilic ChRCC must have 100% eosinophilic cells
- No differences in cytogenetics have been found between eosinophilic and classic ChRCC
- The prognosis of eosinophilic ChRCC is the same as classic ChRCC
Practice answer #4
E. The prognosis of eosinophilic ChRCC is the same as classic ChRCC. Studies have shown no difference in prognosis between the different histologic subtypes and morphologic patterns of ChRCC and classic ChRCC. Answer D is incorrect because a recent study showed that eosinophilic ChRCC frequently has less chromosomal instability compared with classic ChRCC and is often characterized by doubled hypodiploidy (Cancers (Basel) 2019;11:1492, Hum Pathol 2020;104:18). Answer A is incorrect because the gross brown coloration of chromophobe RCC correlates with the amount of eosinophilic cells. In this sense, eosinophilic ChRCC is more likely to be brown in coloration grossly. Answer B is incorrect because the eosinophilic cells of eosinophilic ChRCC are typically smaller compared with those of classic ChRCC. Answer C is incorrect because, by definition, eosinophilic ChRCC only needs to have at least 80% eosinophilic cells.
Comment Here
Reference: Chromophobe
Comment Here
Reference: Chromophobe