Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Rakha E, Tozbikian G. Invasive breast cancer of no special type (NST). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantductalNOS.html. Accessed May 12th, 2024.
Definition / general
- Most common type of invasive breast carcinoma (75 - 80%)
- Invasive carcinoma with evidence of mammary epithelial origin either by morphology or immunohistochemistry
- Diagnosis of exclusion, lacks the histologic features to classify morphologically as a special subtype of breast cancer
- Arises from epithelial (progenitor / stem) cells at the terminal duct lobular unit (as do lobular and other types of breast carcinoma)
- Although the ductal and lobular nomenclature does not reflect the histogenesis of these tumor types, this terminology remains in common use
- Patient age, tumor stage, lymph node status, histologic grade, clinical biomarker profile and gene expression signature are important prognostic factors
- Treated with surgical excision, with or without radiotherapy, systemic chemotherapy or targeted therapies
- ASCO / CAP guidelines require assessment by standard breast biomarkers (estrogen and progesterone receptors and HER2)
Essential features
- Large and heterogenous group of tumors that lack features diagnostic of special types
- Range in morphology, grade, biomarker profile, molecular subtype and clinical behavior
Terminology
- Invasive breast carcinoma, not otherwise specified (NOS)
- Invasive ductal carcinoma
- Not recommended: scirrhous carcinoma
ICD coding
Epidemiology
- Breast cancer is the commonly diagnosed cancer in females (24%) (CA Cancer J Clin 2018;68:394)
- Leading cause of female cancer death worldwide (CA Cancer J Clin 2018;68:394)
- 8 - 9% of women diagnosed with invasive breast cancer before age 75 in North America and West Europe (NIH: SEER Cancer Statistics Review (CSR) 1975-2018 [Accessed 23 June 2022])
- Majority are sporadic; 5 - 10% of all breast cancers are associated with hereditary cancer susceptibility genes (Am J Hum Genet 1991;48:232, Semin Oncol 2016;43:528, Eur J Hum Genet 2009;17:722)
Sites
- Breast
- Can occur in axilla accessory breast tissue
Etiology
- Origin of breast cancer is multifactorial and complex but most studies point to hormones, reproductive factors, diet, environment and genetics as general factors
- After menopause, about 40% of risk is modifiable (Am J Epidemiol 2008;168:404)
- Hormone related risk factors:
- Prolonged exposure to estrogen: early menarche, late menopause, nulliparity, having first child after age 30 and lack of breast feeding (Br J Cancer 2009;100:538)
- Postmenopausal women with obesity and estrogen producing ovarian tumors (BJOG 2006;113:1160)
- Women using combined hormone replacement therapy with progestins or estrogens alone (Int J Cancer 2007;121:645)
- Risk with oral contraceptives is controversial (Mayo Clin Proc 2006;81:1290)
- Associated with increased risk of hormone receptor positive breast cancer (Breast Cancer Res Treat 2014;144:1)
- Parity may be associated with an increased risk of triple negative breast cancers
- Hormone factors associated with reduced risk of breast cancer:
- Oophorectomy before age 35 or first child before age 18
- Obesity prior to age 40 - due to anovulatory cycles and lower progesterone levels in late cycle (J Natl Cancer Inst 2011;103:250)
- Genetic risk factors:
- Breast cancer shows familial clustering
- First degree relatives with breast cancer; having 1 first degree relative (mother, sister, daughter) creates a relative risk of 2 - 3x, higher if relative is affected before age 50 or had bilateral disease; relative risk with 2 first degree relatives is 4 - 6x (Int J Cancer 1997;71:800)
- Li-Fraumeni syndrome (germline p53 mutations) - 25% of patients develop breast cancer
- Mutations of BRCA1 and BRCA2 genes are associated with familial breast cancer at an early age, account for 20 - 60% of familial breast cancer but only 5% of all cases
- Germline BRCA1 mutations are associated with risk for triple negative breast cancers, whereas germline BRCA2 mutations are associated with hormone receptor positive breast cancers (J Clin Oncol 2015;33:304)
- Cowden disease (multiple hamartoma syndrome): autosomal dominant, due to 10q mutation: 30 - 50% risk of breast cancer (ductal carcinoma in situ or invasive ductal carcinoma) by age 50; also benign skin tumors (Hum Pathol 1998;29:47)
- Heterozygous carriers for ataxia telangiectasia have an 11% risk of breast cancer by age 50
- Black women (compared to white women) have more frequent breast cancers in women < age 40; present with higher stage tumors with higher nuclear grade that are more likely ER / PR negative, have higher mortality rate (J Natl Cancer Inst 200;100:1804, Cancer 1998;83:2509)
- Women have 100x risk of breast cancer compared to men (J Clin Oncol 2011;29:4381)
- Environmental risk factors:
- Rates in U.S. > Japan / Taiwan (5:1), also high in Northern Europe, low in Asia / Africa; may be due to known risks of obesity / high fat diet and heavy alcohol use; however, differences diminish with immigration (Nutr Cancer 2008;60:492, Am J Epidemiol 2000;152:950)
- Alcohol consumption has been consistently associated with a moderate increase in risk, particularly hormone receptor positive breast cancers (JAMA 1998;279:535)
- Adult dietary soy foods and carotenoids have protective effect (Am J Clin Nutr 2009;89:1920, Int J Cancer 2009;124:2929)
- Physical activity has a protective effect (J Natl Cancer Inst 2008;100:728, Breast Cancer Res Treat 2010;120:235)
- Other risk factors:
- Female sex and older age are probably the most common risk factors of breast cancer
- Proliferative breast disease (see individual topics in Breast chapter), particularly in situ carcinoma (Clin Cancer Res 2007;13:5474)
- Carcinoma of opposite breast or endometrium
- Radiation exposure in young women, including women < age 30 with supradiaphragmatic radiation for Hodgkin lymphoma; reduced risk if also have irradiation of ovaries > 5 Gy (Int J Radiat Oncol Biol Phys 2009;73:69, J Clin Oncol 2009;27:3901)
- Mammographic density is a highly heritable risk (J Br Menopause Soc 2006;12:186, Breast Cancer Res 2011;13:R132)
Clinical features
- In patients > 65 years, 87% of patients with invasive breast carcinoma have NST (Crit Rev Oncol Hematol 2008;67:263)
- Most (~90%) are unifocal and with higher frequency in the upper outer quadrant (Cancer Epidemiol 2016;44:186)
- In nonscreened populations, NST carcinoma commonly presents as a palpable mass
- Less commonly, it presents with skin retraction, nipple discharge, nipple inversion, a change in the size or shape of the breast or a change in the color or texture of the skin
- In screened populations, it commonly presents as a spiculated mass with or without calcifications but may present as well circumscribed masses, architectural distortion or calcifications alone
- Clinically, IBC NST is further divided into the following biomarker defined subtypes for treatment purposes:
- ER positive, HER2 negative
- ER positive, HER2 positive cancers
- ER negative, HER2 positive cancers
- ER negative, HER2 negative cancers
- Frequency of these biomarker / clinical subtypes varies by population characteristics but the ER positive subtype is the most common
- HER2 positive subtype comprises 10 - 15%, whereas ER negative / HER2 negative subtype comprises approximately 15 - 30% (Crit Rev Oncol Hematol 2010;76:44, JAMA 2006;295:2492)
Diagnosis
- Histologic examination of involved tissue
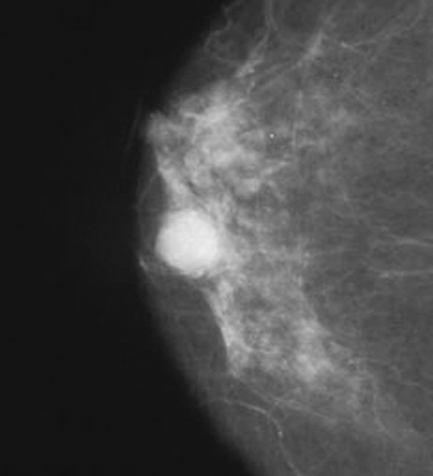
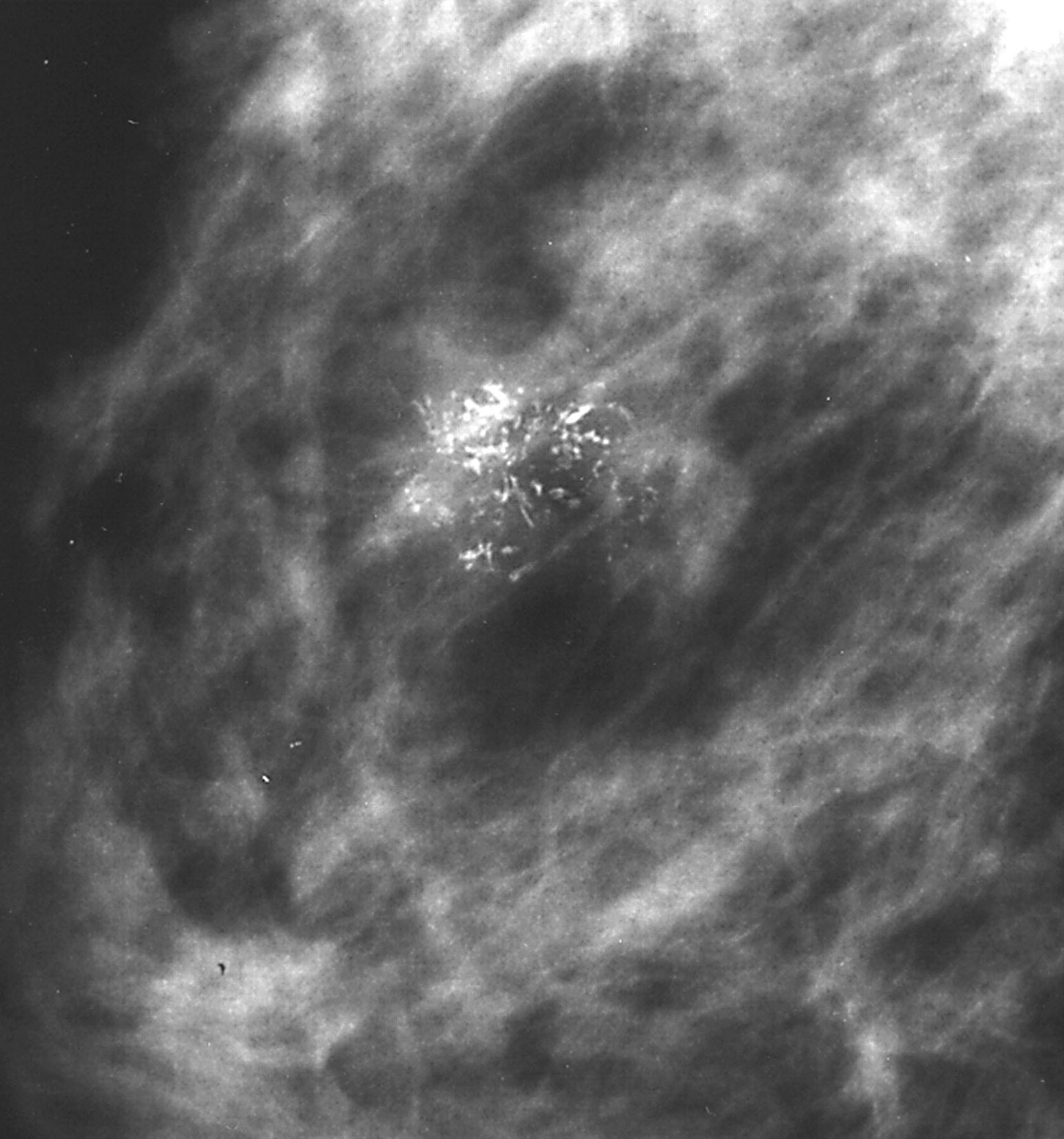
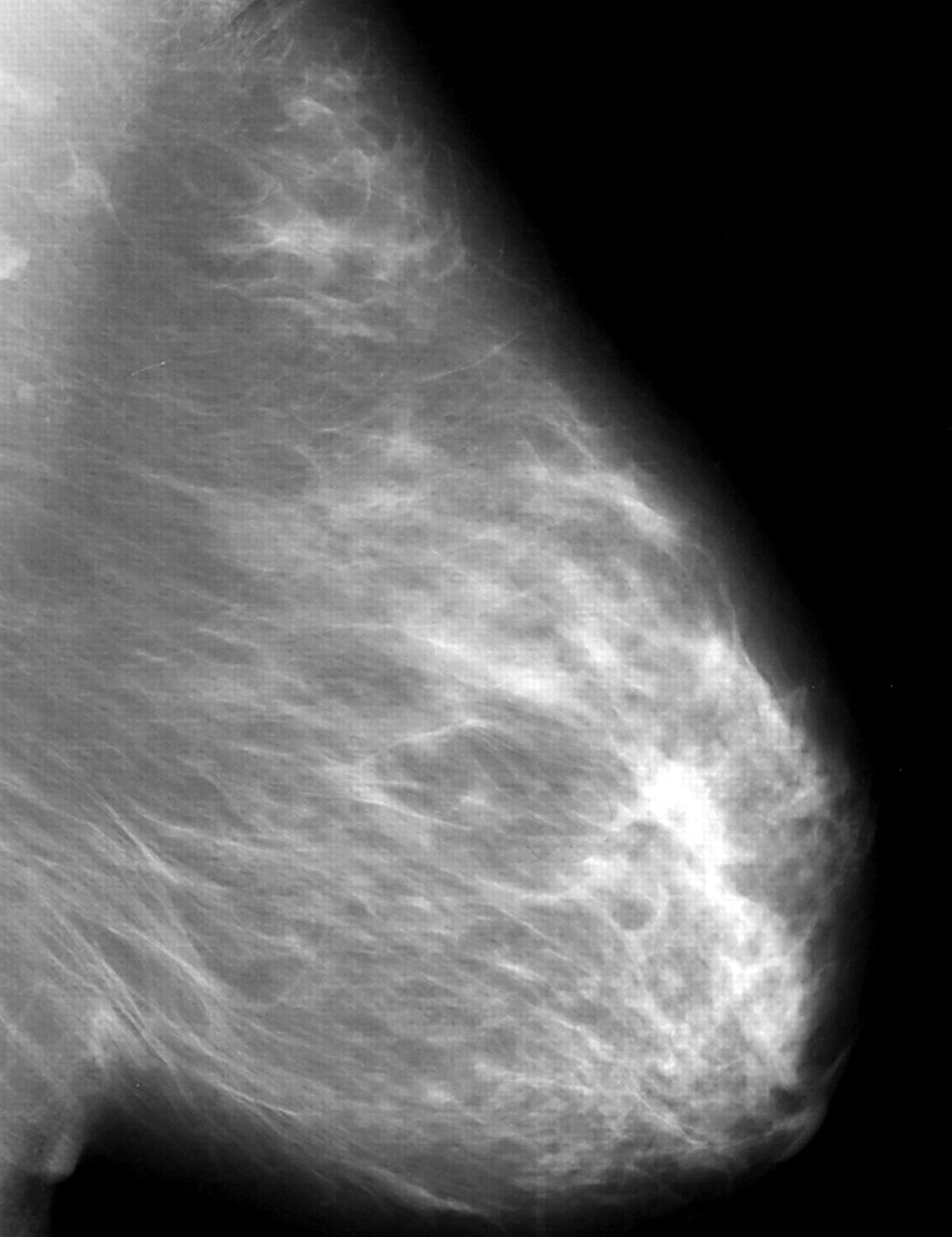
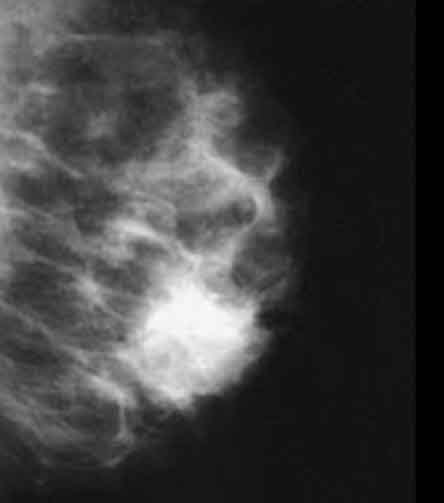
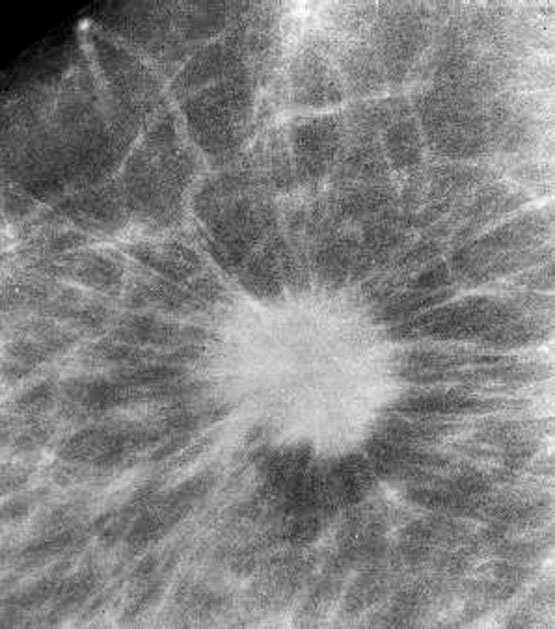
Radiology description
- Mammographically often presents as a spiculated mass with or without calcifications but may present as an architectural distortion or calcifications alone
- Targeted ultrasonography often used to enhance visualization
- MRI often shows enhancing mass lesion with variable washout kinetic patterns (Breast J 2010;16:394)
- MRI is sensitive but nonspecific technique is reserved for screening patients at high risk (e.g., BRCA mutation carriers), patients with dense breast tissue, estimating extent of disease in patients with lobular carcinoma, evaluating response to neoadjuvant chemotherapy or evaluating indeterminate abnormalities on mammography / ultrasound (Breast 2013;22:S77)
Prognostic factors
- Patient age (Cancer Epidemiol Biomarkers Prev 2019;28:303)
- Stage as determined by pT size and lymph node status (Cancer 2003;98:2133)
- Histologic grade is an independent prognostic factor (Arch Pathol Lab Med 2014;138:1048)
- Lymphovascular invasion associated with higher risk of local recurrence and distant recurrence (Cancer 2012;118:3670)
- Biomarker profile:
- Hormone receptor expression associated with better short term survival
- HER2 expression associated with improved survival when anti-HER2 therapy is received
- Gene expression signature, observed survival is highest in luminal A, lowest in basal-like (Cancer Treat Rev 2012;38:698)
Case reports
- 47 year old woman with a subareolar breast mass (Int J Clin Exp Pathol 2014;7:7020)
- 53 year old woman with a breast mass (Medicine (Baltimore) 2022;101:e28433)
- 56 year old woman with cystic breast lesion (Medicine (Baltimore) 2018;97:e13740)
- 70 year old woman with a benign appearing breast mass (Am J Case Rep 2017;18:813)
Treatment
- Surgical excision by breast conserving surgery (lumpectomy) or mastectomy, with or without axillary lymph node dissection
- May be offered local radiotherapy to the breast after lumpectomy or to the chest wall after mastectomy to reduce local recurrence risk
- May be offered systemic chemotherapy either neoadjuvantly or adjuvantly
- May be offered targeted therapies (e.g., antiendocrine or anti-HER2 directed)
- Selection of treatment (type of surgery, inclusion of radiotherapy and selection of systemic therapies) is based on multifactorial consideration of several factors including but not limited to tumor size, histologic grade, stage, biomarker status, results of genomic risk assessment (e.g., Oncotype DX Recurrence Score ®), anatomic location, patient age and comorbidities, heritable breast cancer risk (e.g., BRCA status), prior exposure to chemotherapy or radiation therapy, cosmetic outcome and patient's preference
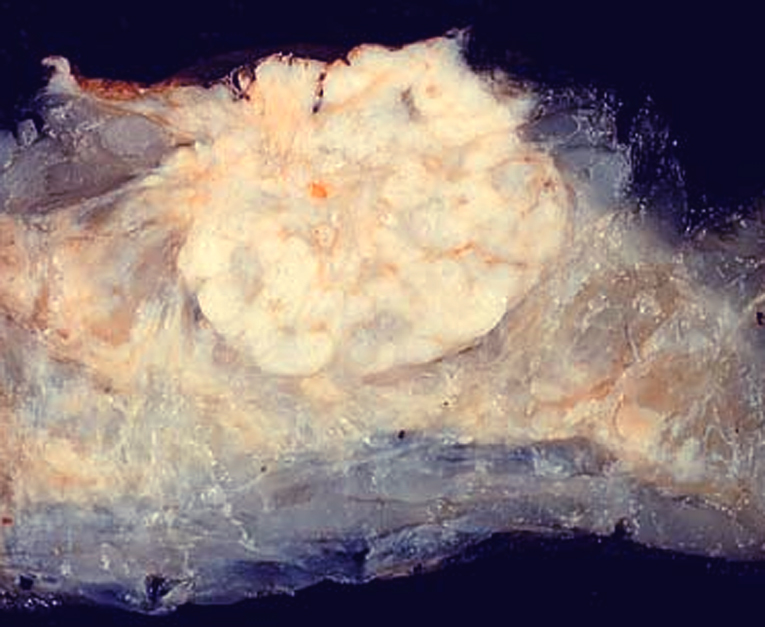
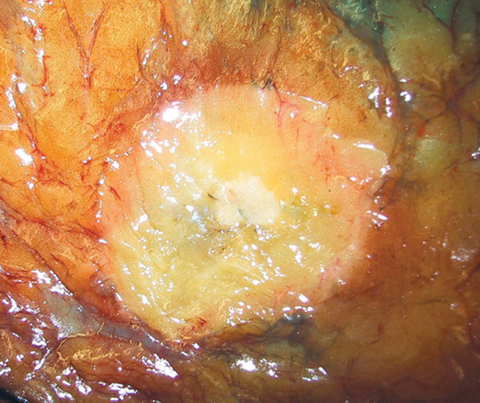
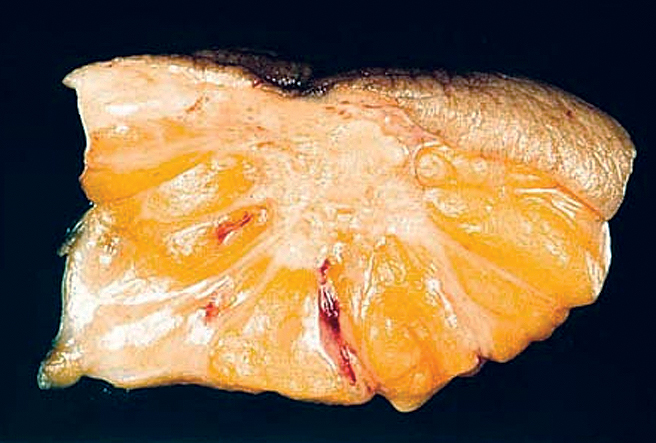
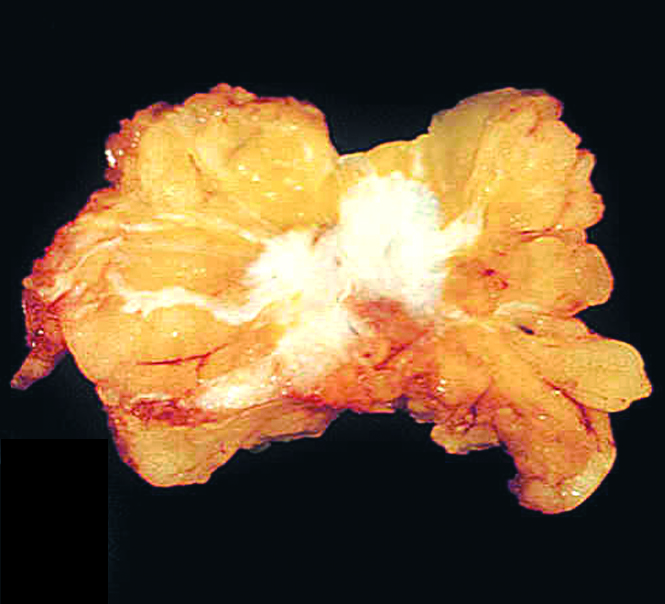
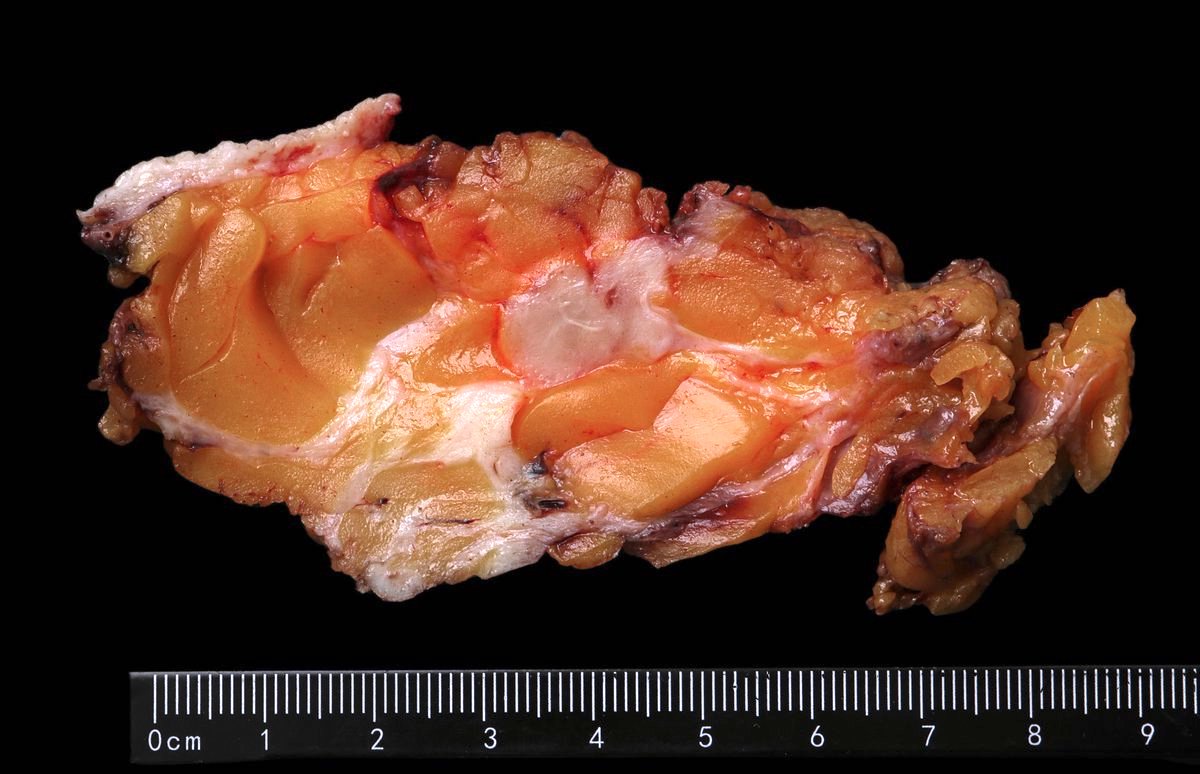
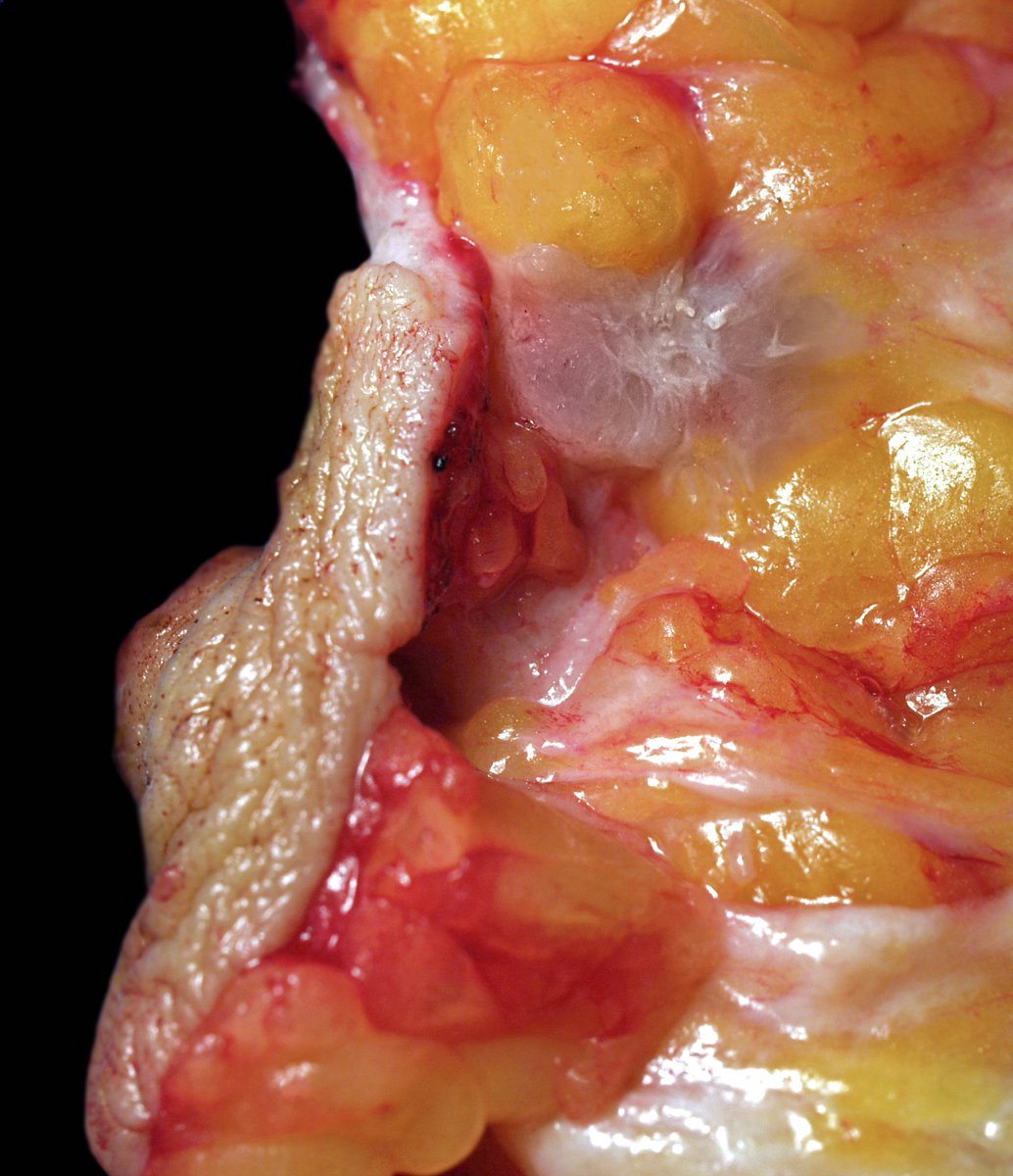
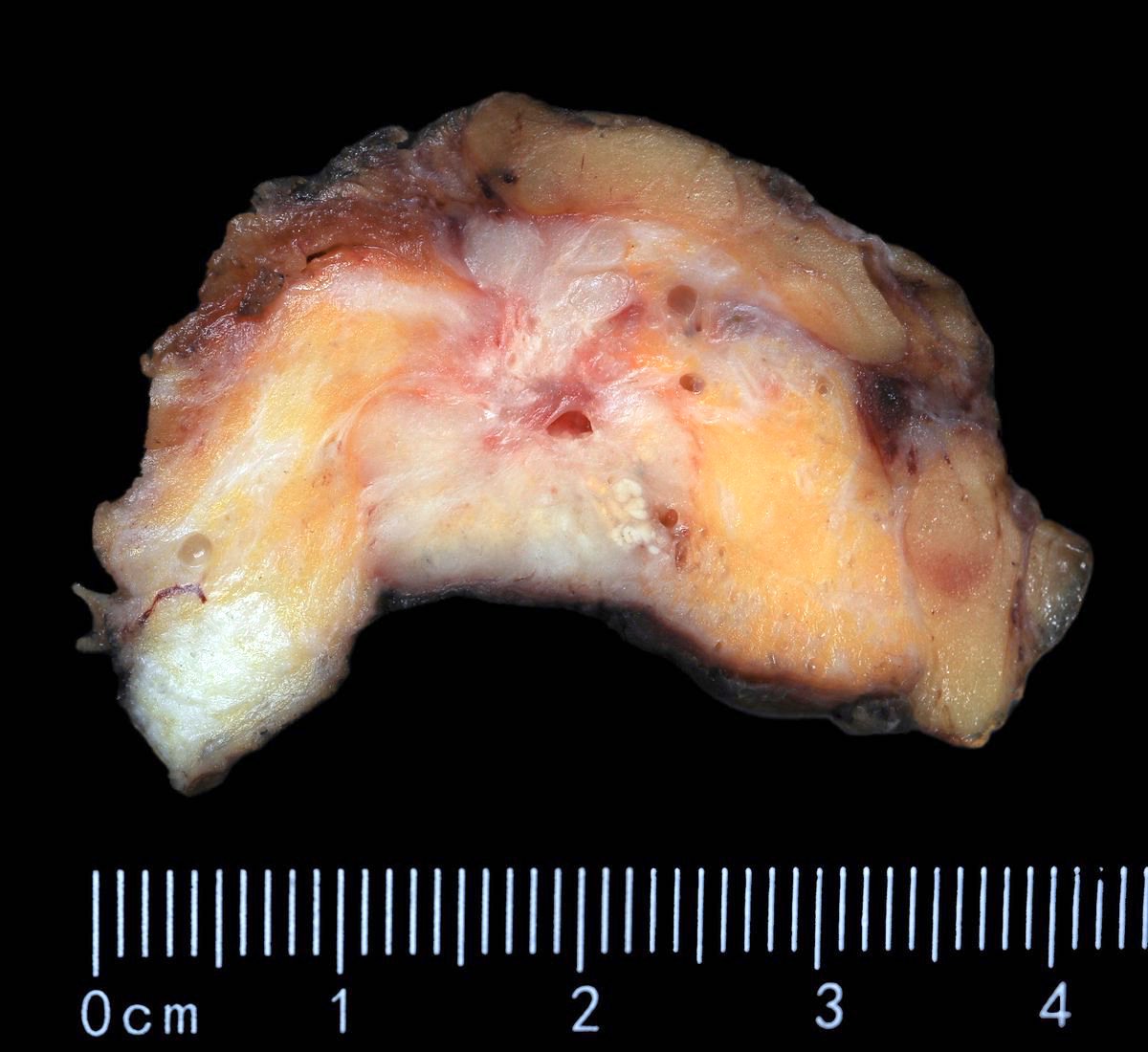
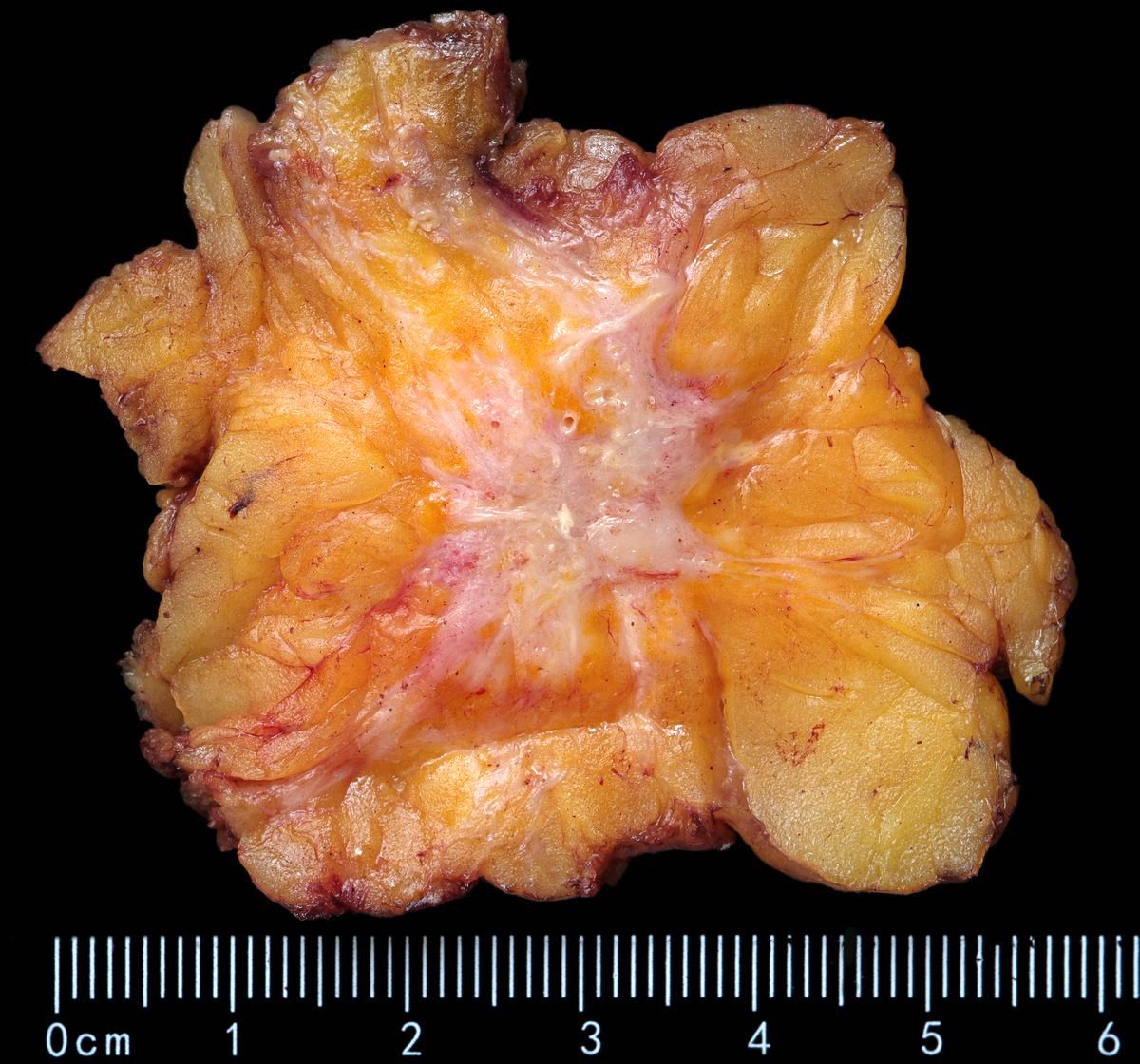
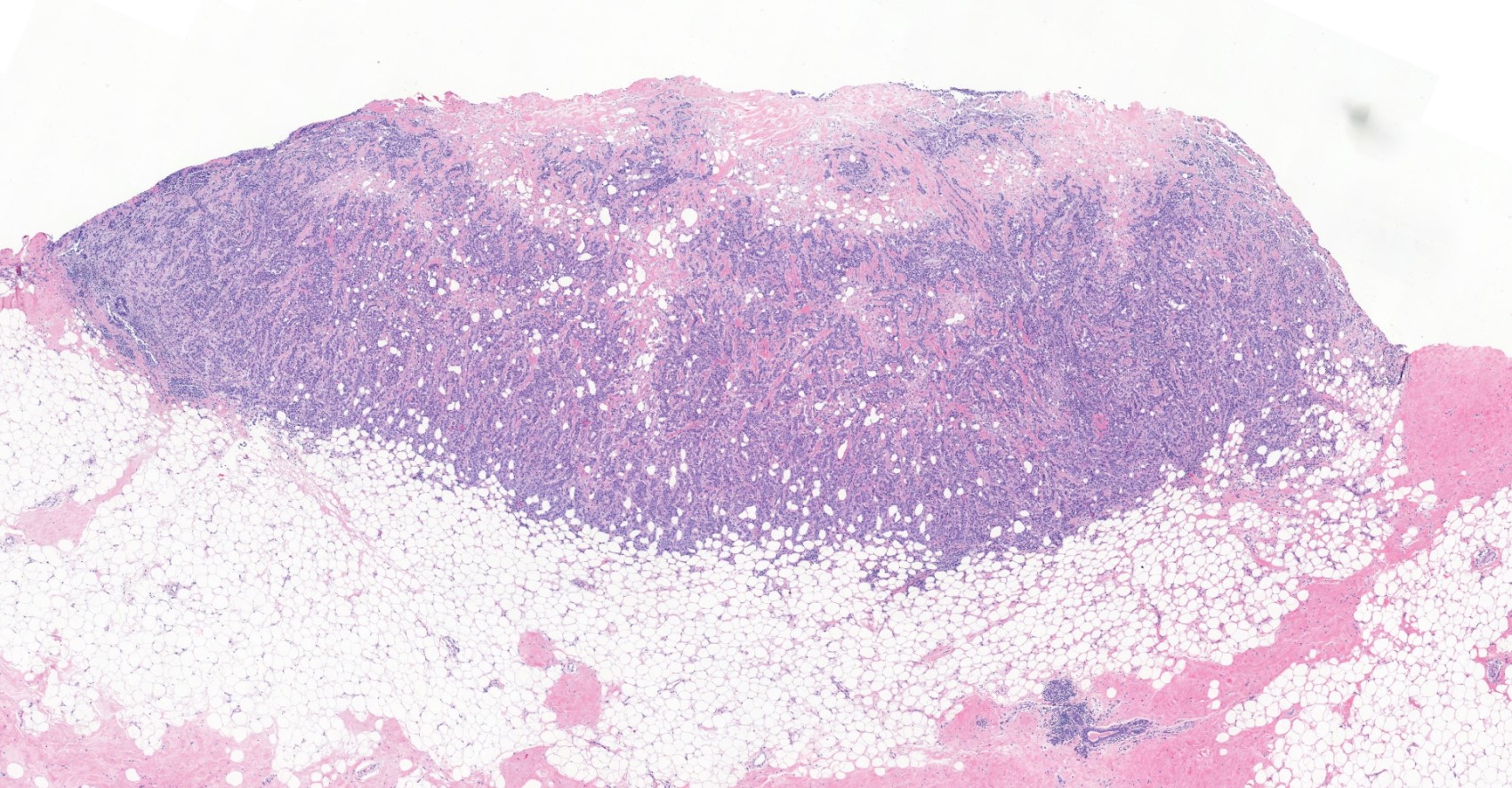
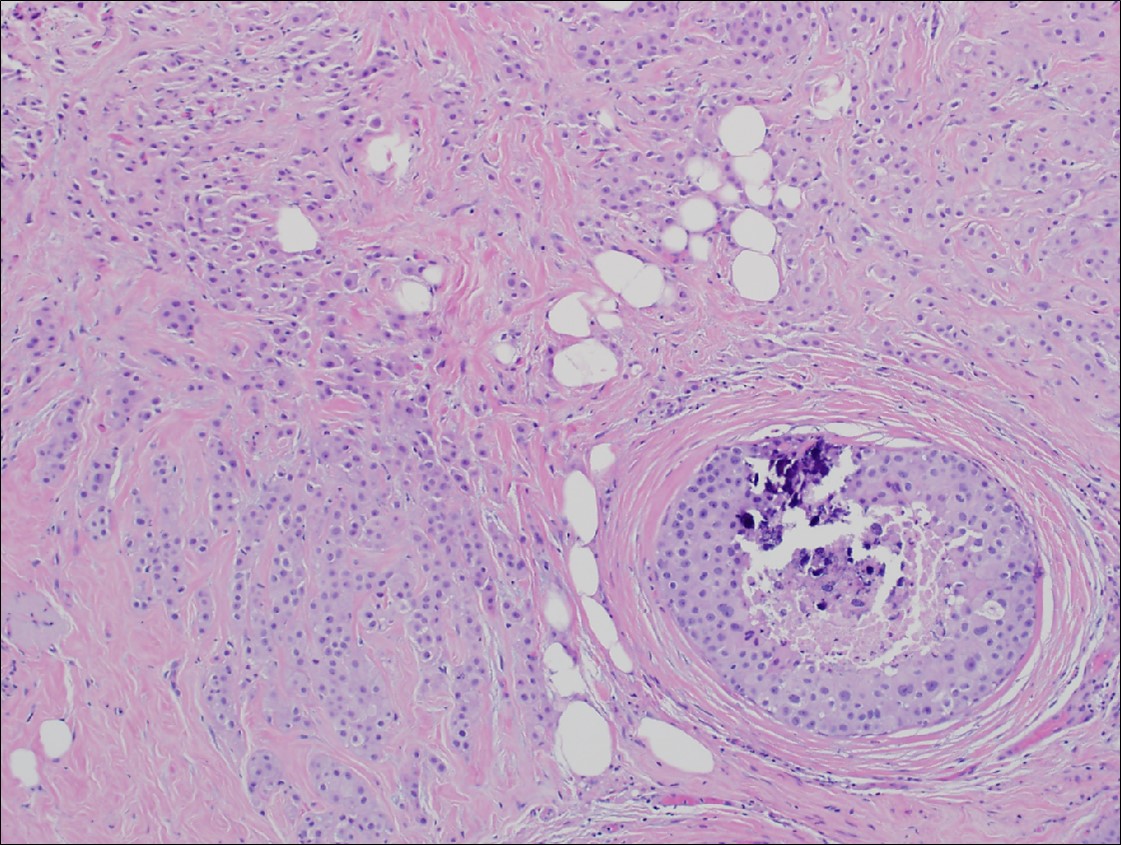
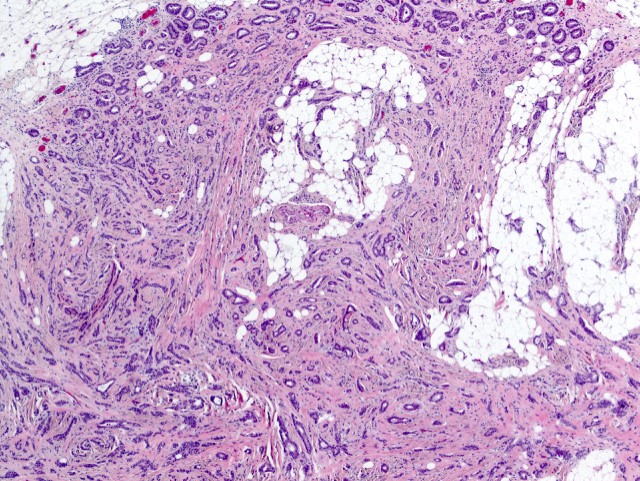
Gross description
- Grossly evident mass, with an irregular, stellate outline or nodular configuration
- Mass is usually poorly circumscribed and contracts from surrounding tissue
- Firm or even hard on palpation and may have a gritty feel when cut with a knife, grating sound when scraped
- May show streaks of chalky white elastotic stroma penetrating surrounding stroma (crab-like), calcification
- Large tumors have hemorrhage, necrosis and cystic degeneration
- May be fixed to chest wall and cause skin dimpling or nipple retraction
- Some tumors, including neoadjuvant treated cancers, may be grossly inapparent and require careful correlation with the imaging at the time of gross examination and tissue sampling
Gross images
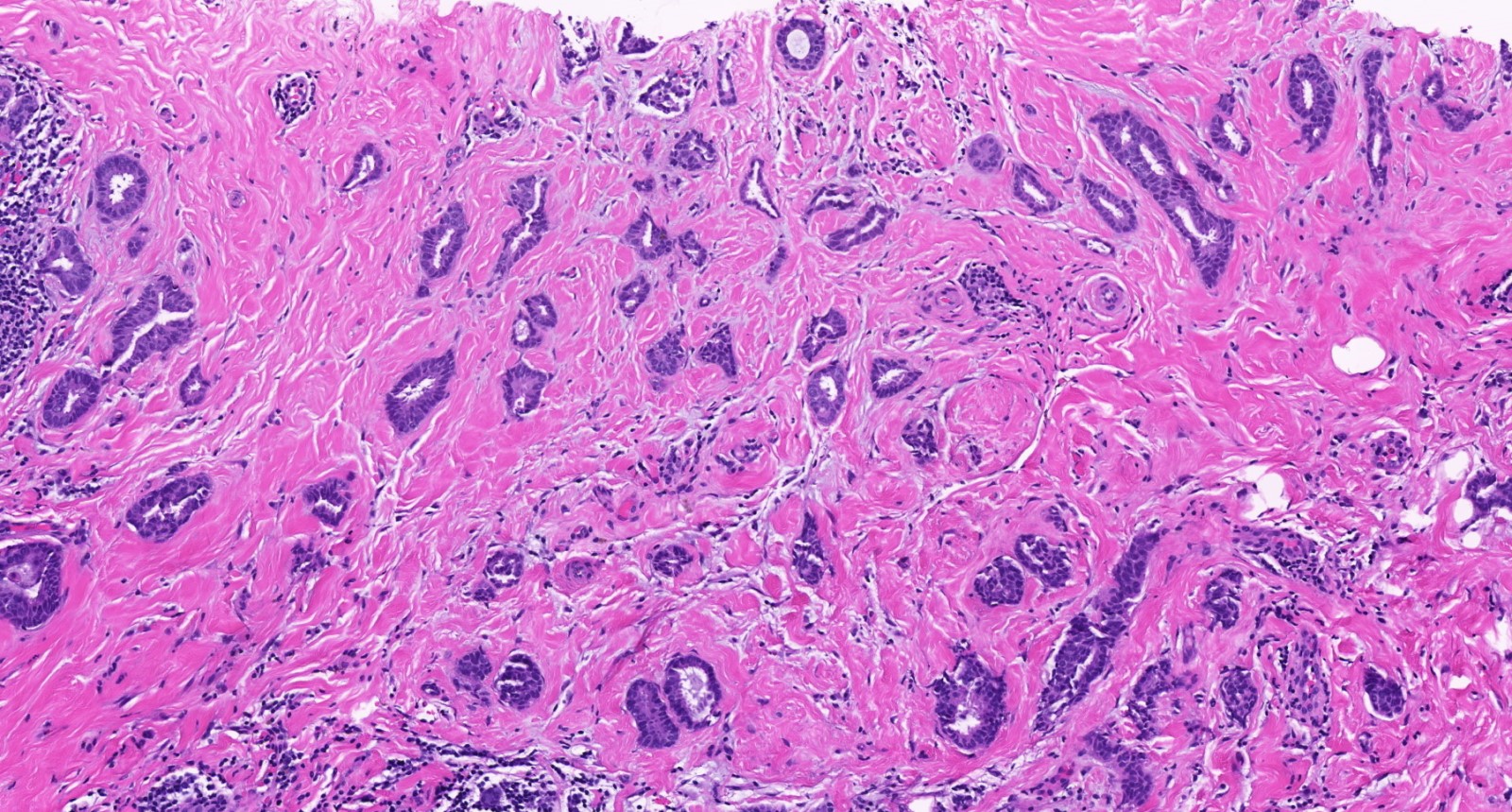
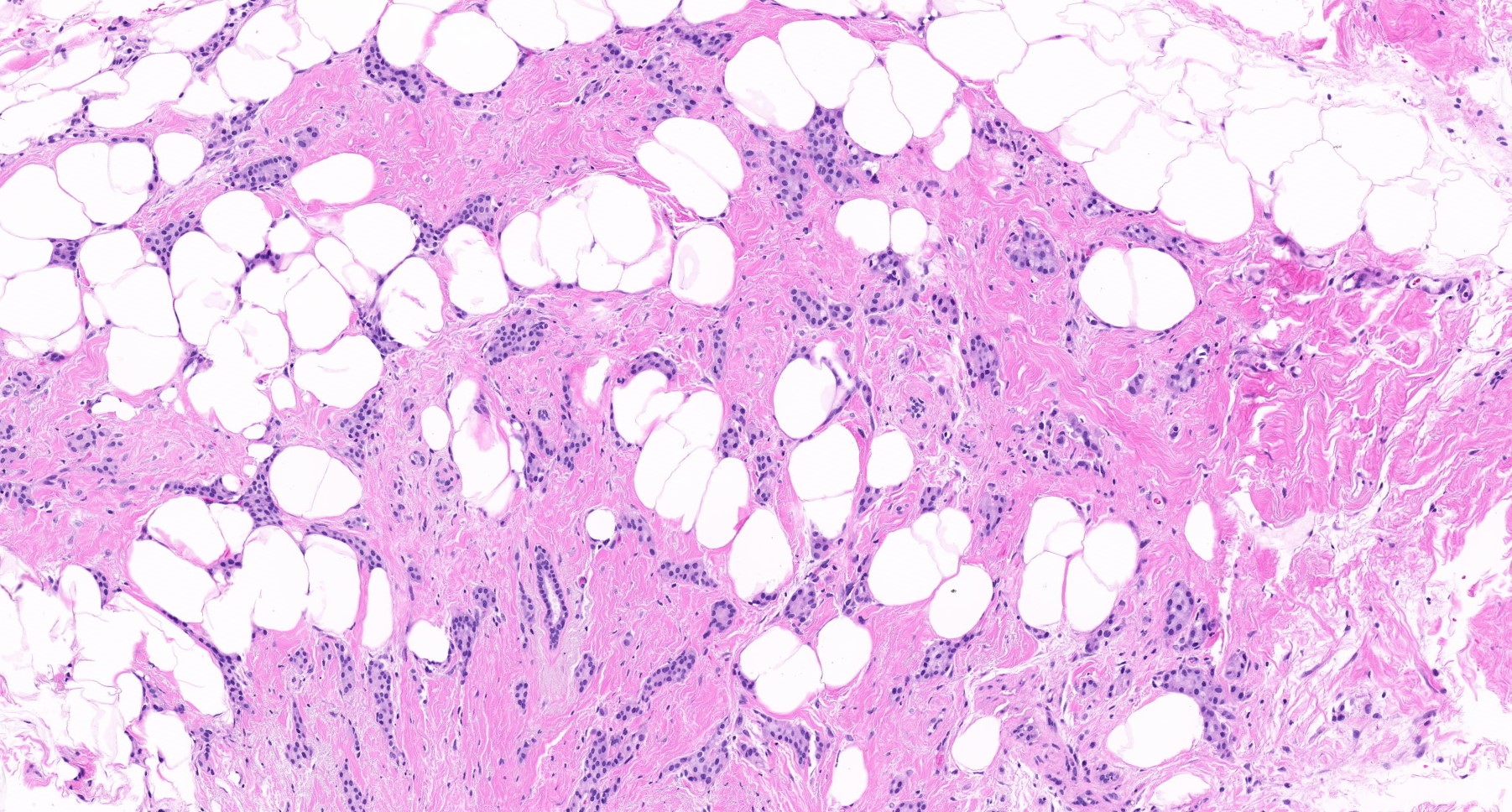
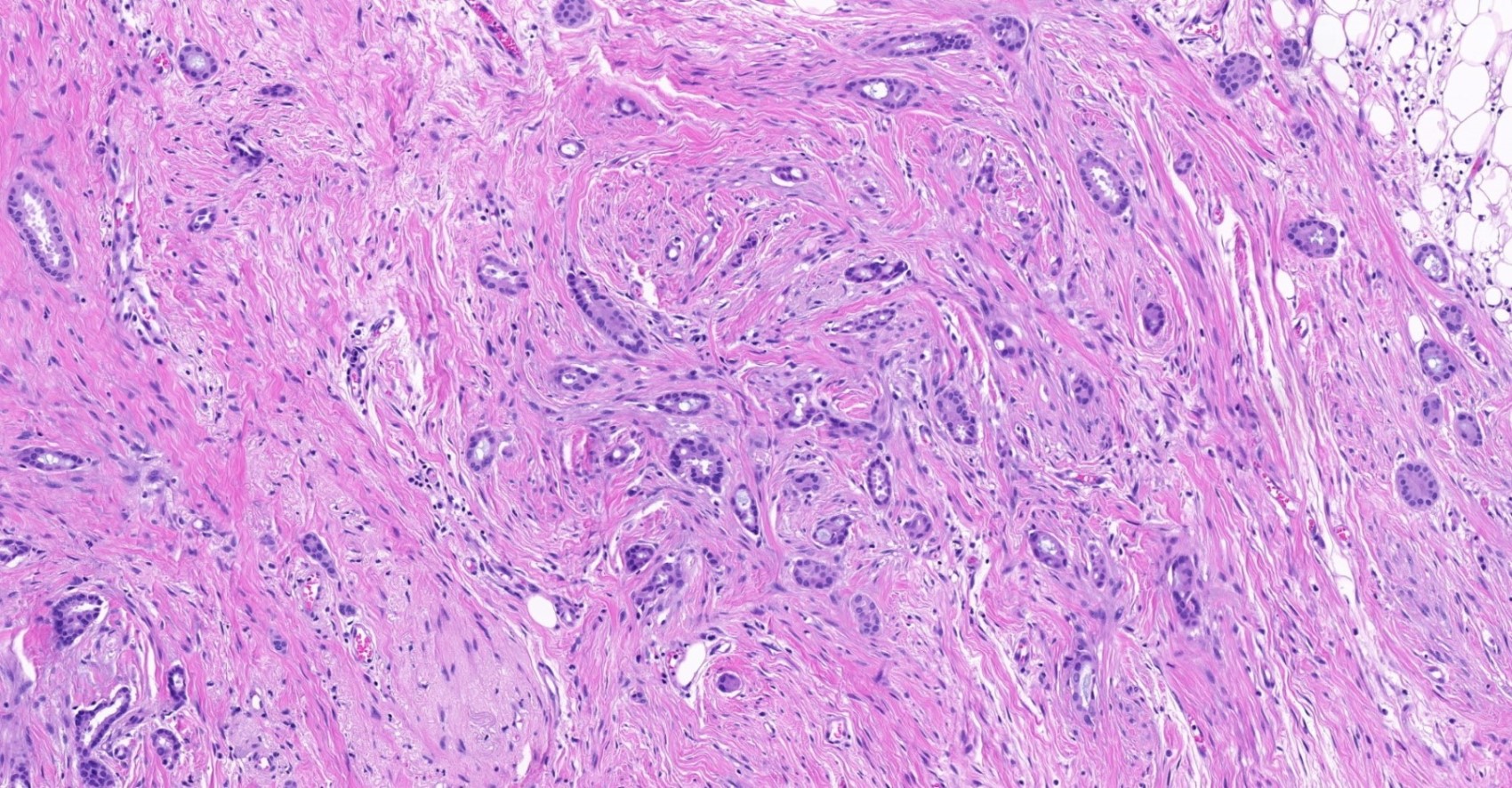
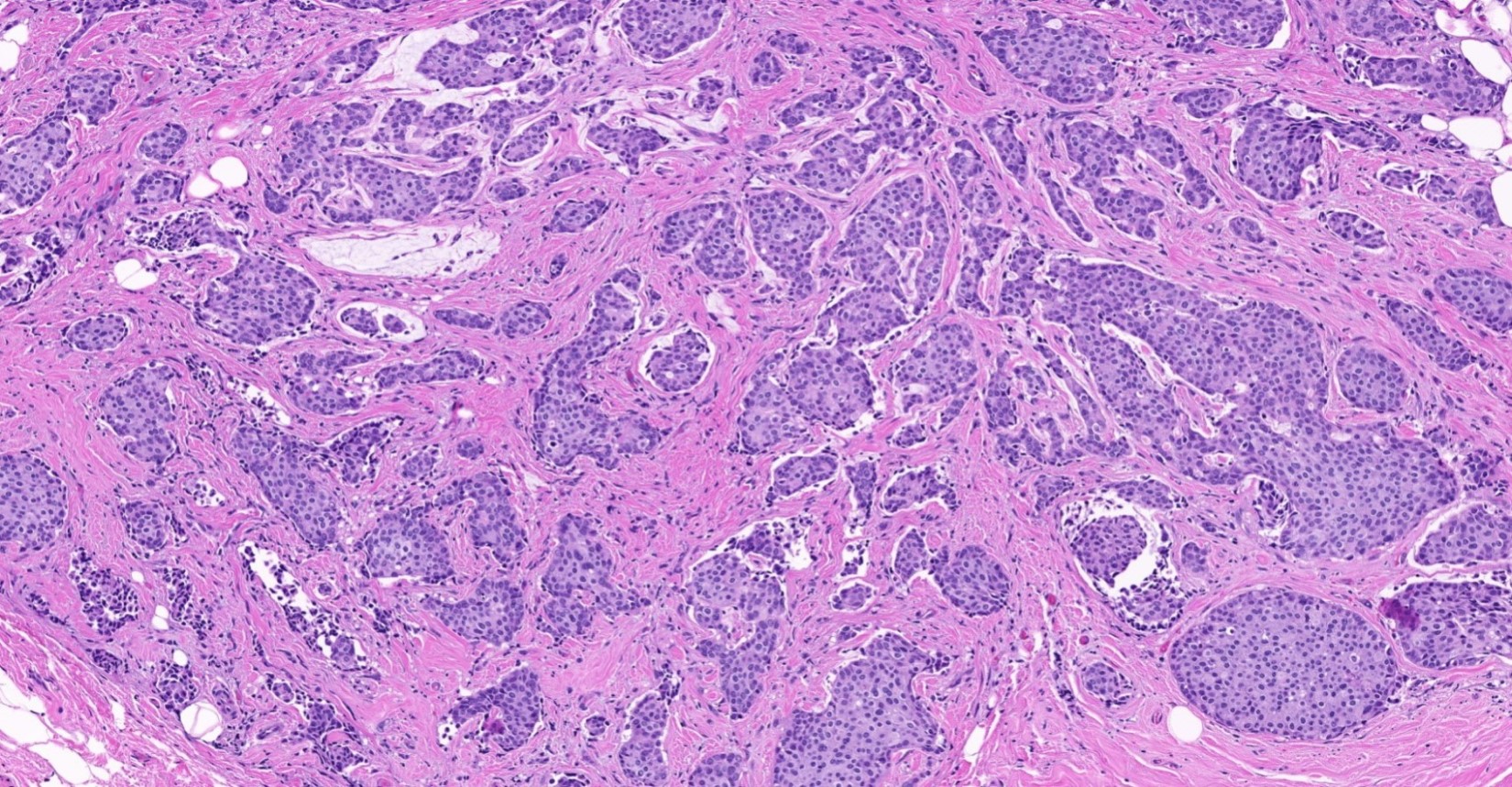
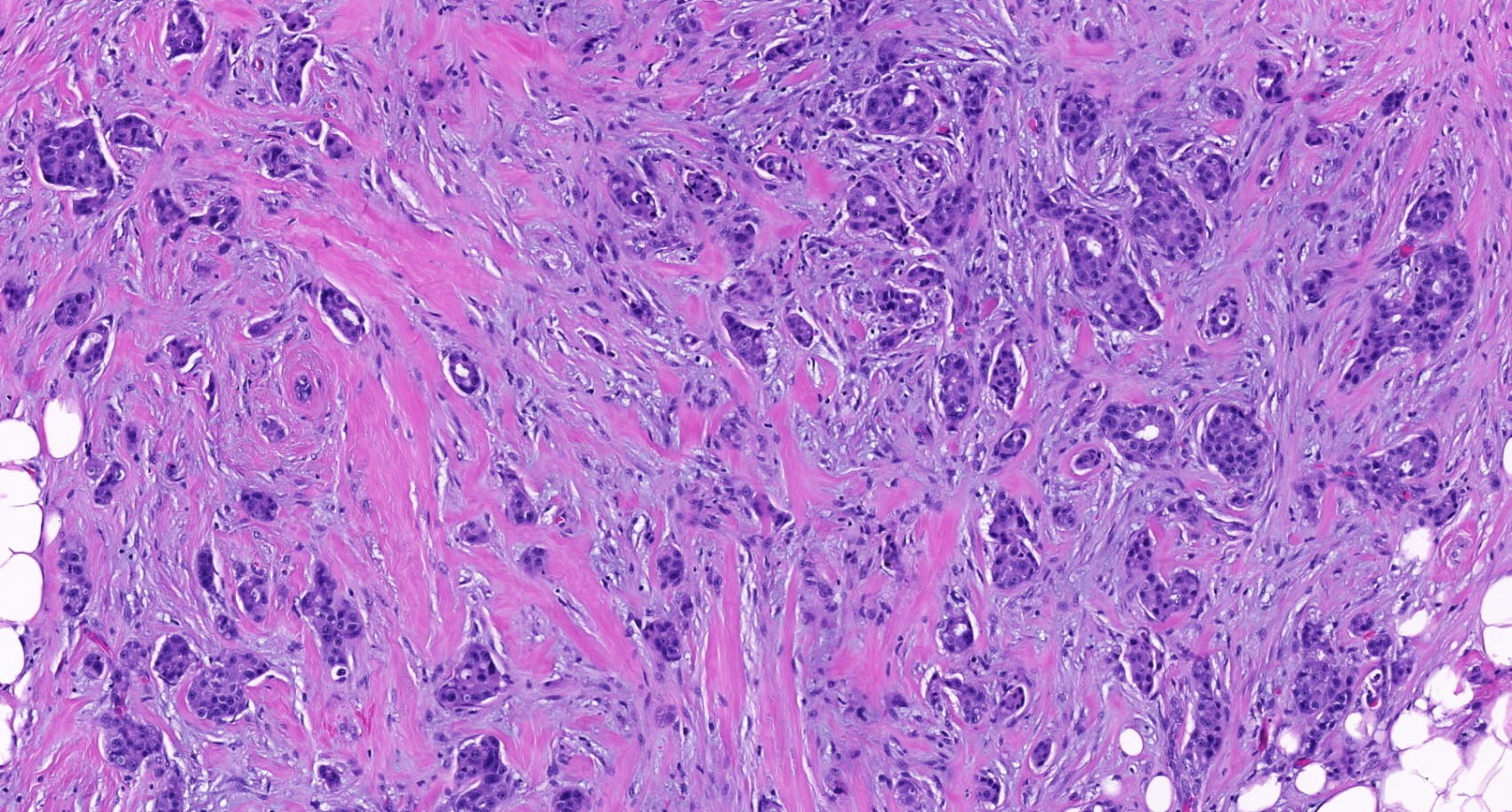
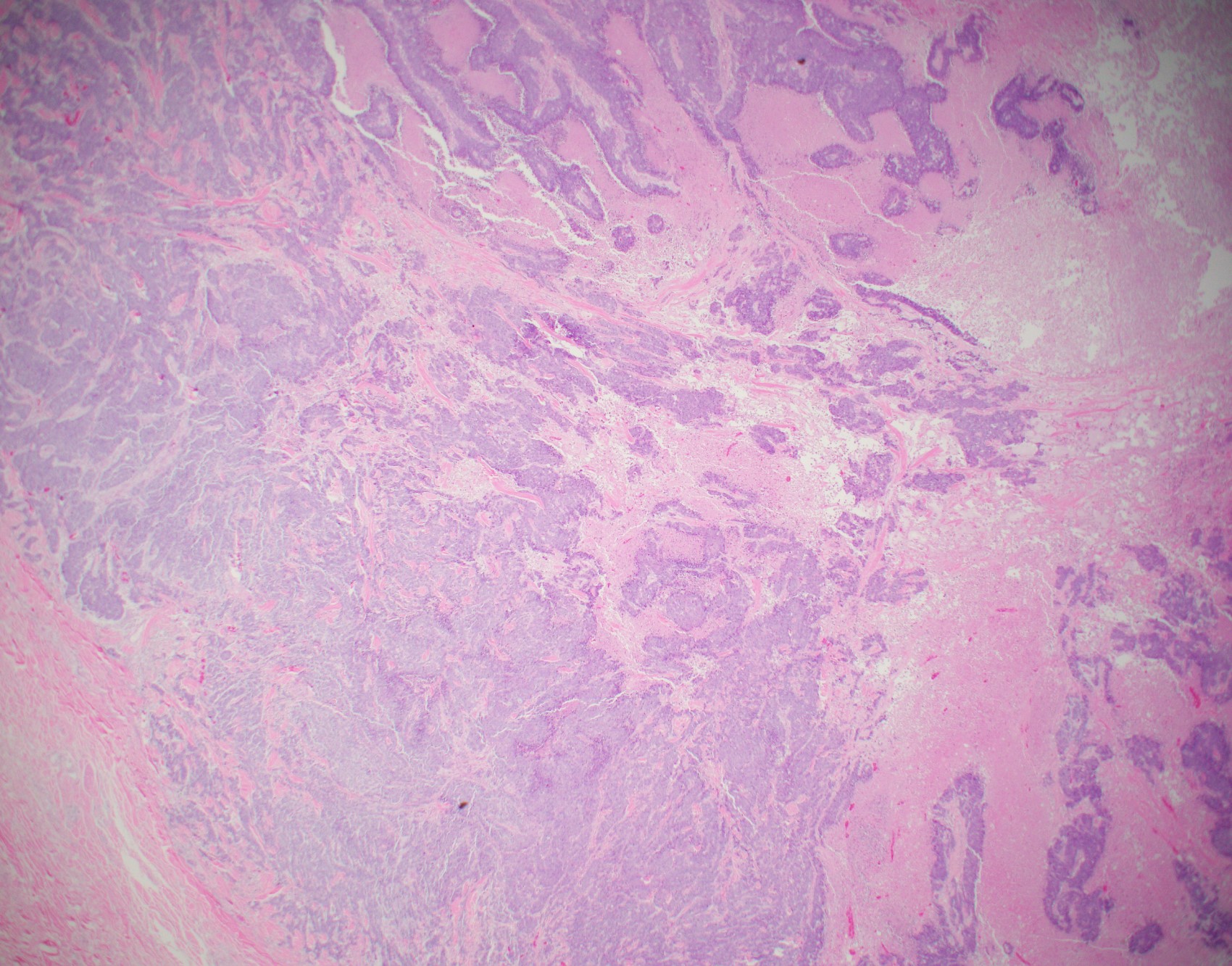
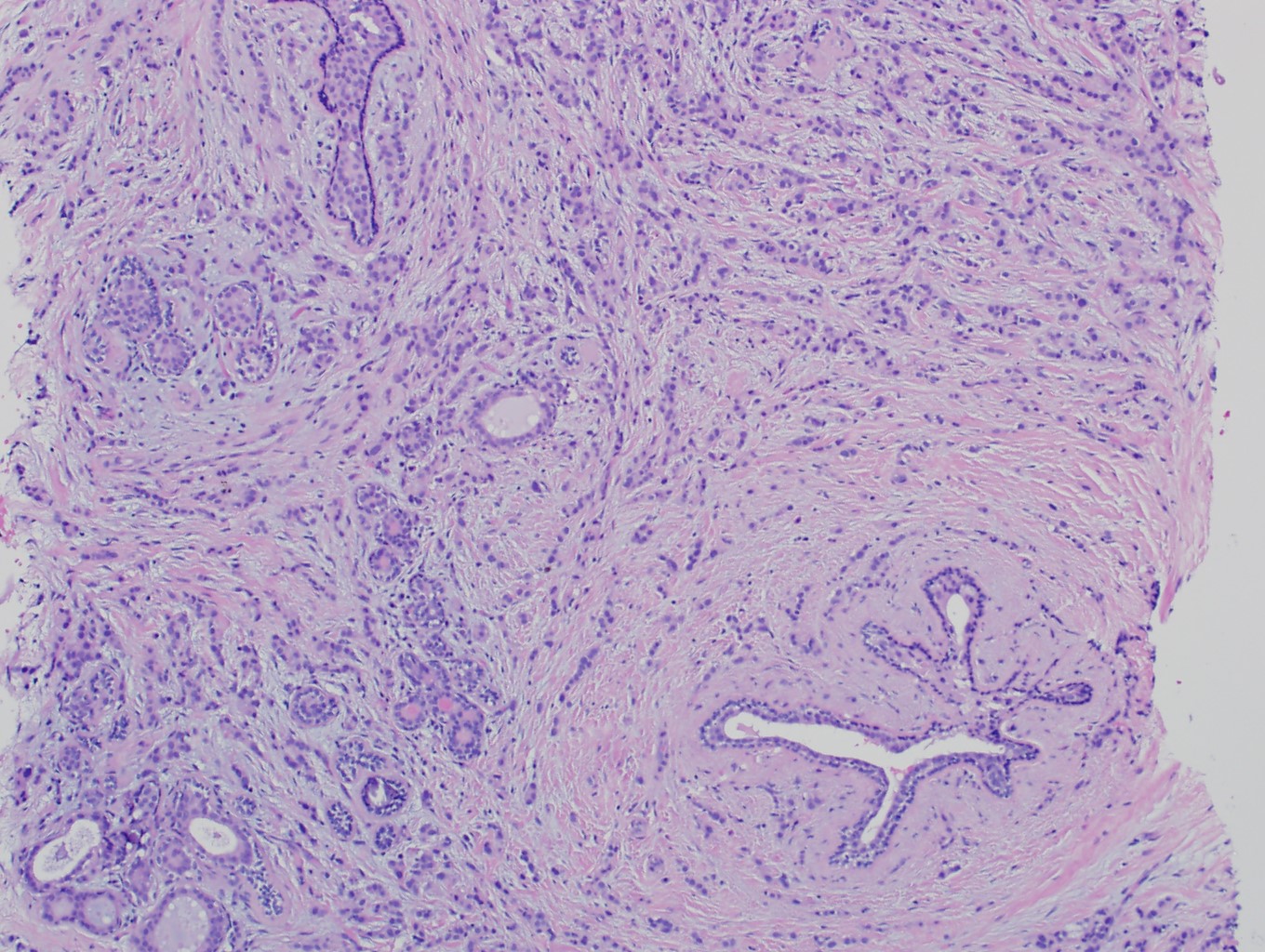
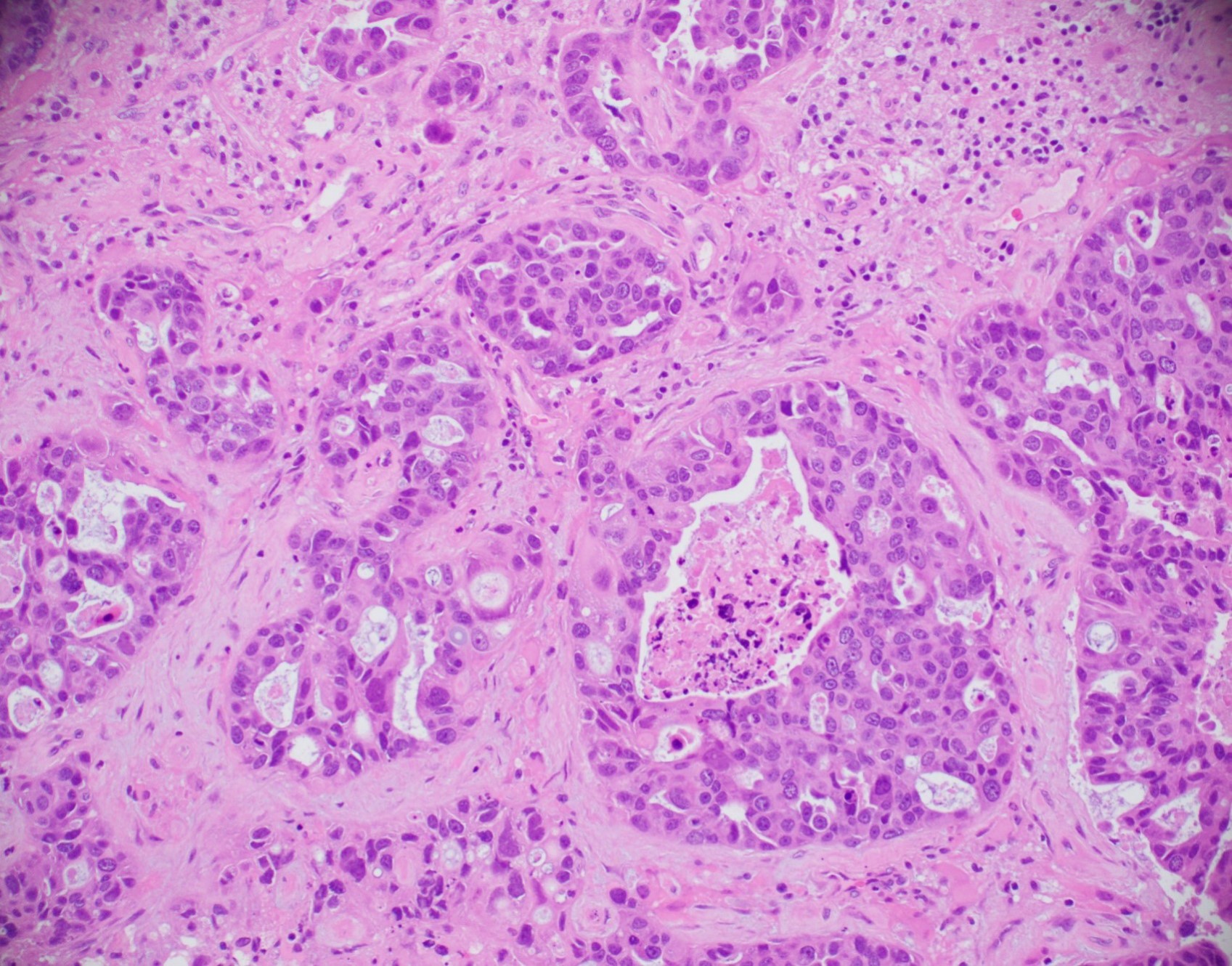
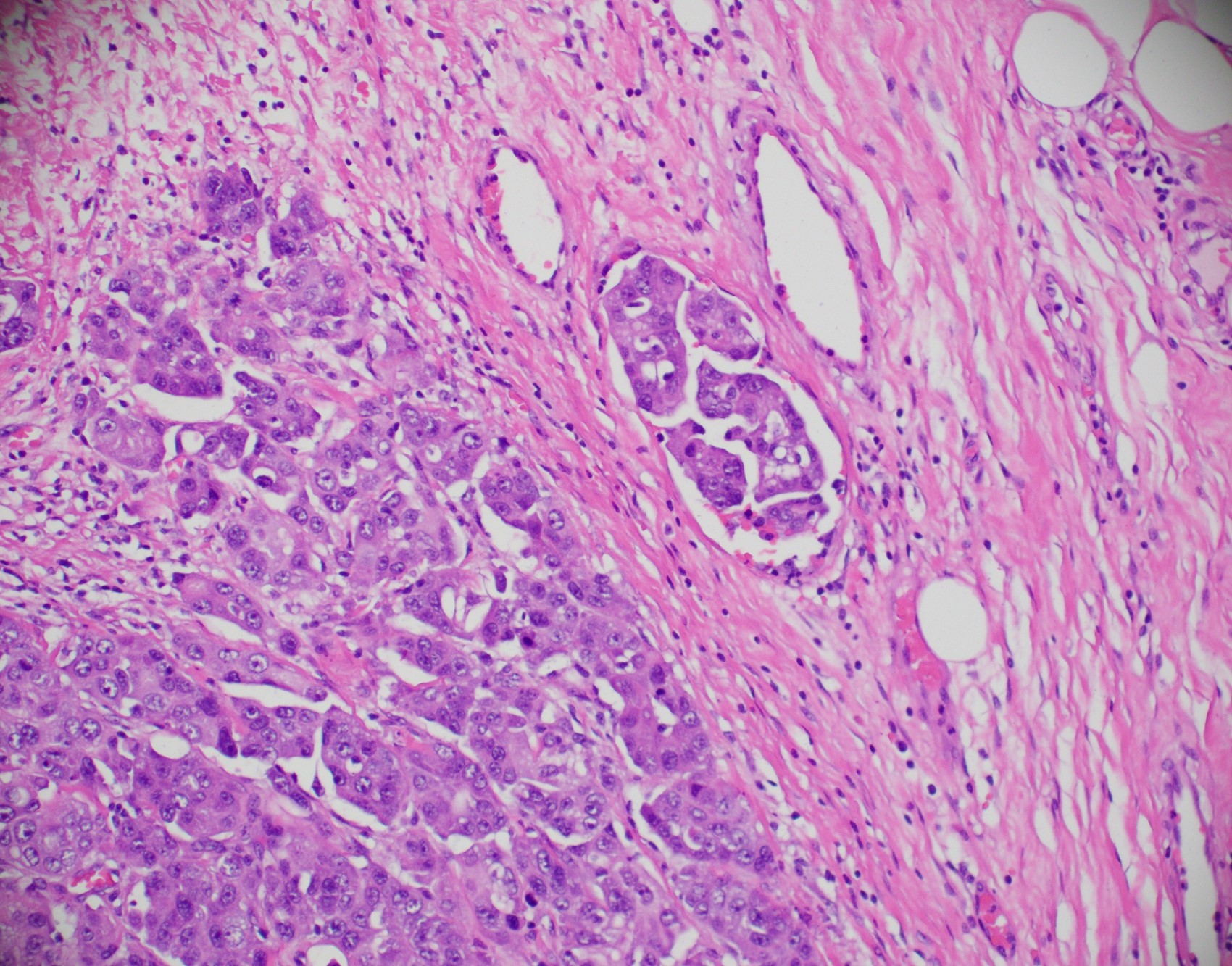
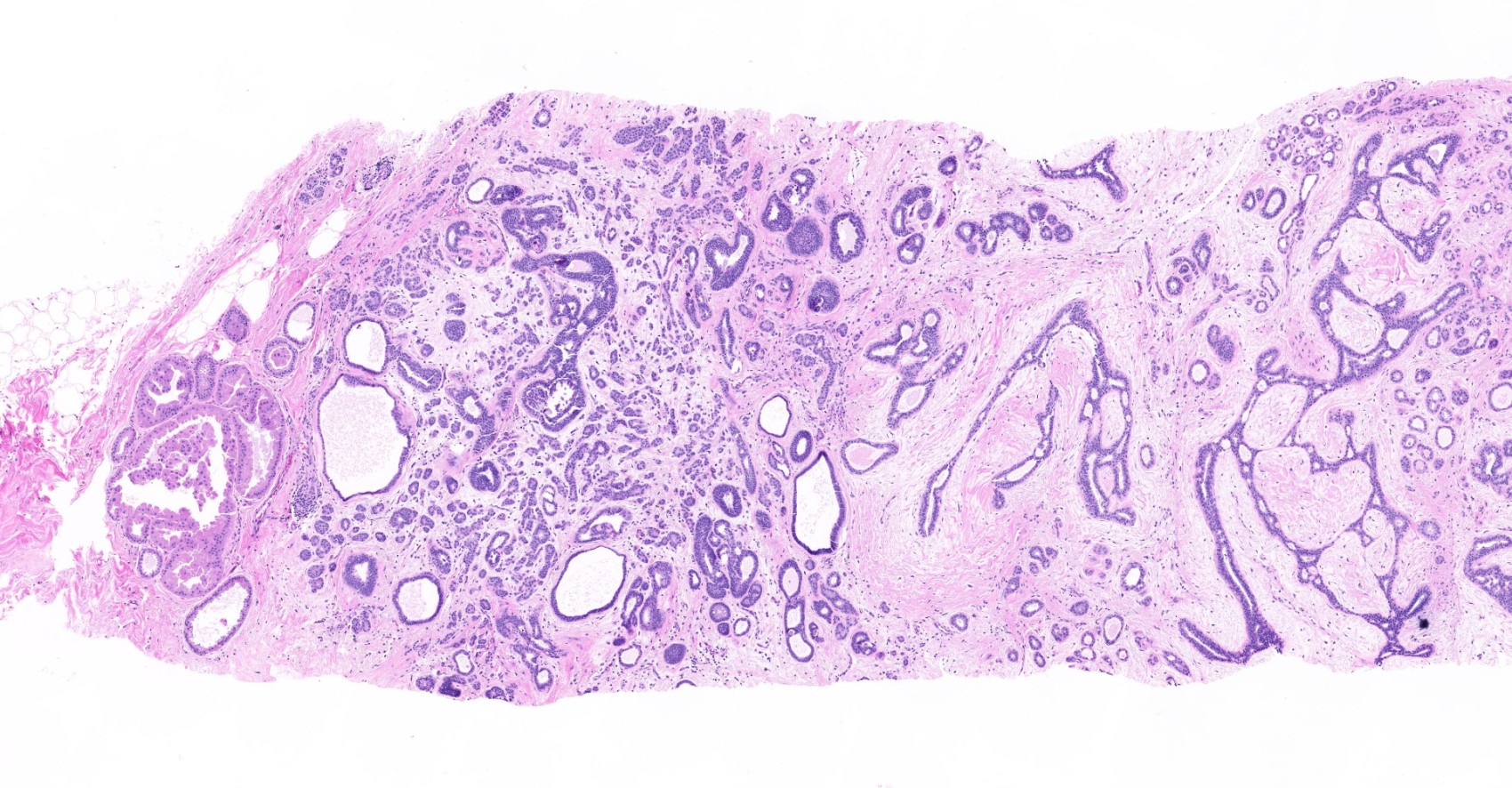
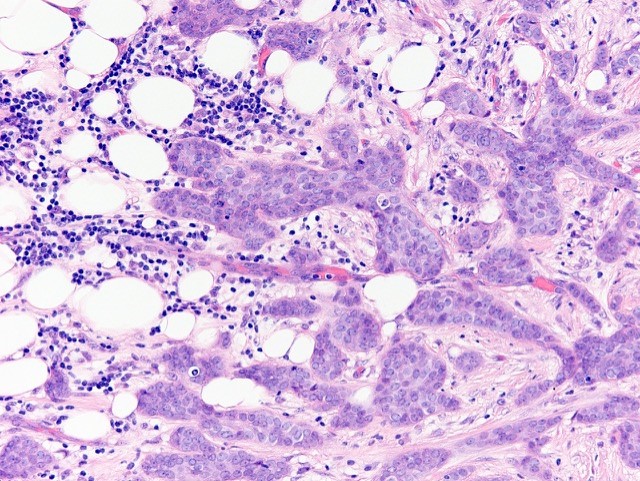
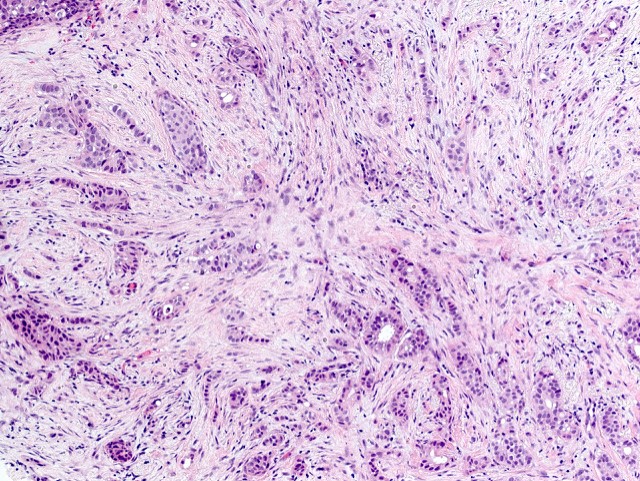
Microscopic (histologic) description
- Histologic grading is based on the Nottingham / modified Bloom & Richardson Score (Histopathology 1991;19:403):
- Tubule formation (1 - 3 points):
- > 75% (1 point)
- 10 - 75% (2 points)
- < 10% (3 points)
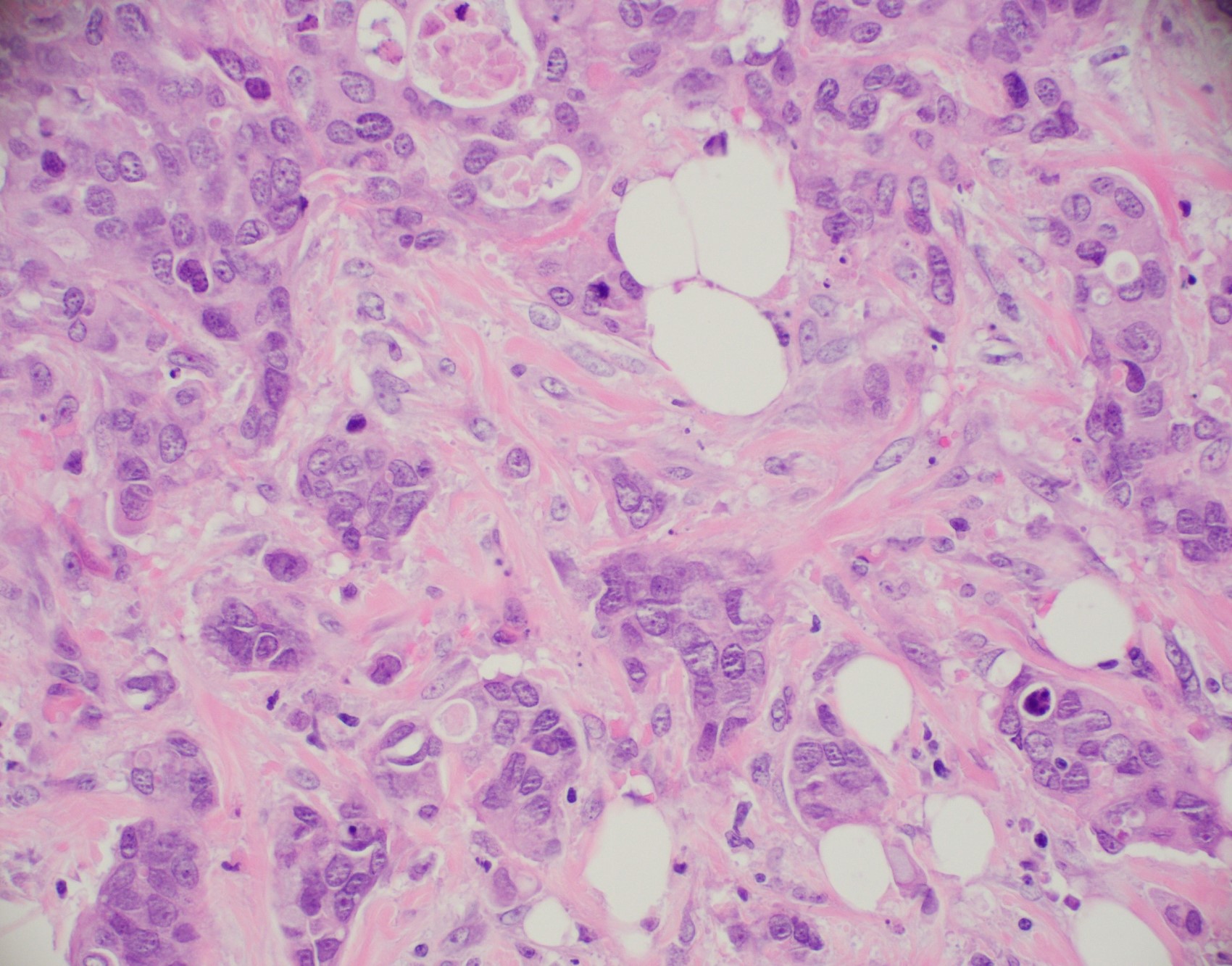
- Nuclear pleomorphism (1 - 3 points):
- Small, regular, uniform, similar to normal ductal epithelial cells, 2 - 3x RBC (1 point)
- Moderate increase in size / variability (2 points)
- Large nuclei, marked variation, often vesicular chromatin with prominent nucleoli (3 points)
- Mitotic count (1 - 3 points), dependent on microscopic field area
- Total score (add points for tubule formation, nuclear pleomorphism and mitotic count):
- 3 - 5 points: grade 1
- 6 - 7 points: grade 2
- 8 - 9 points: grade 3
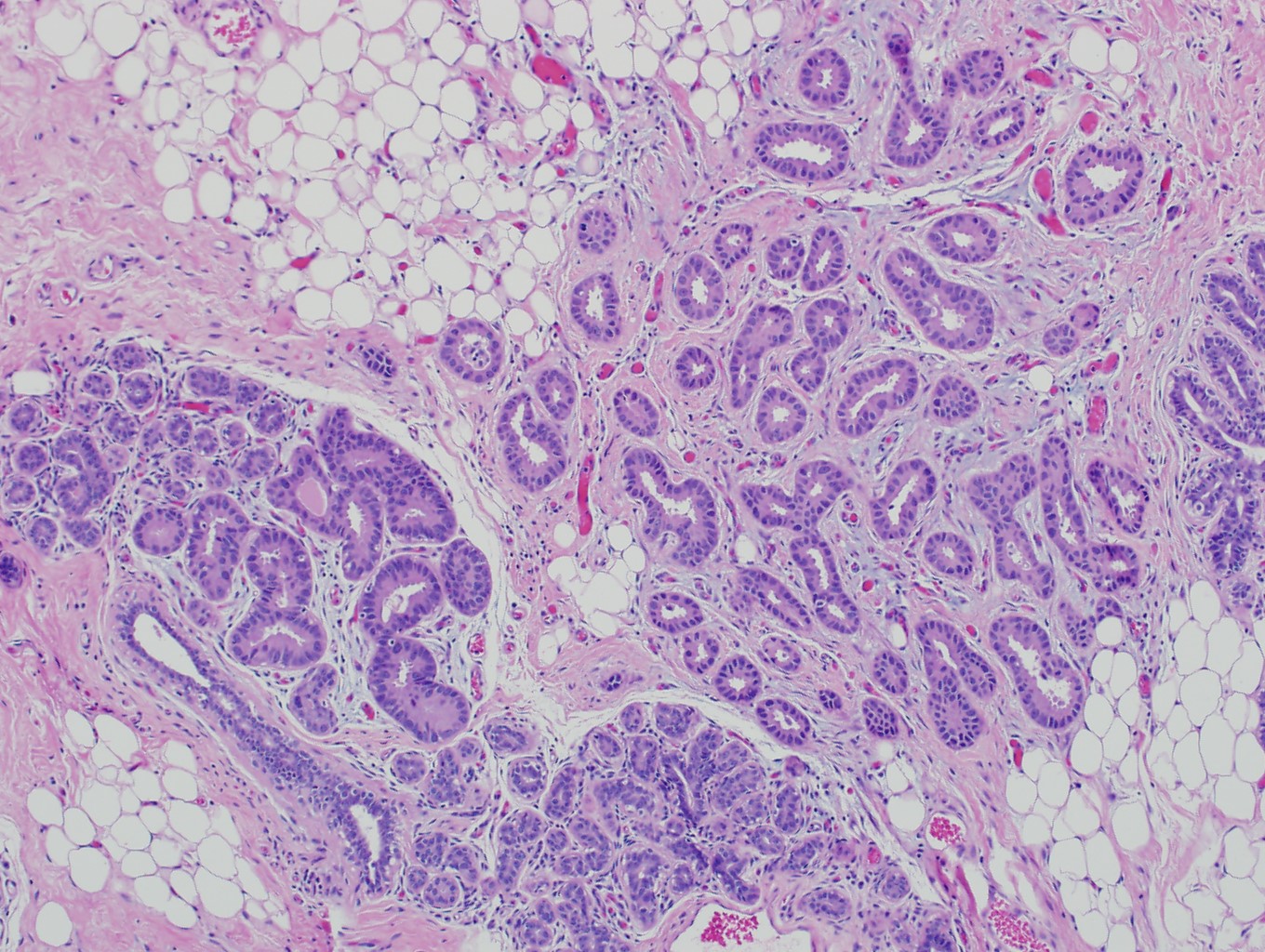
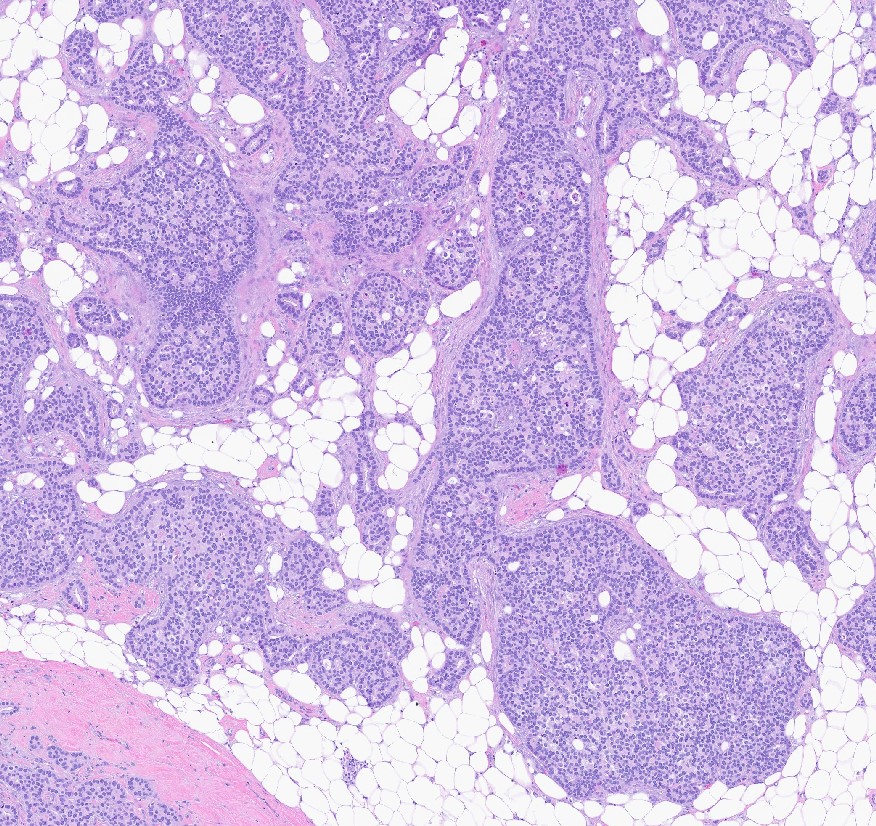
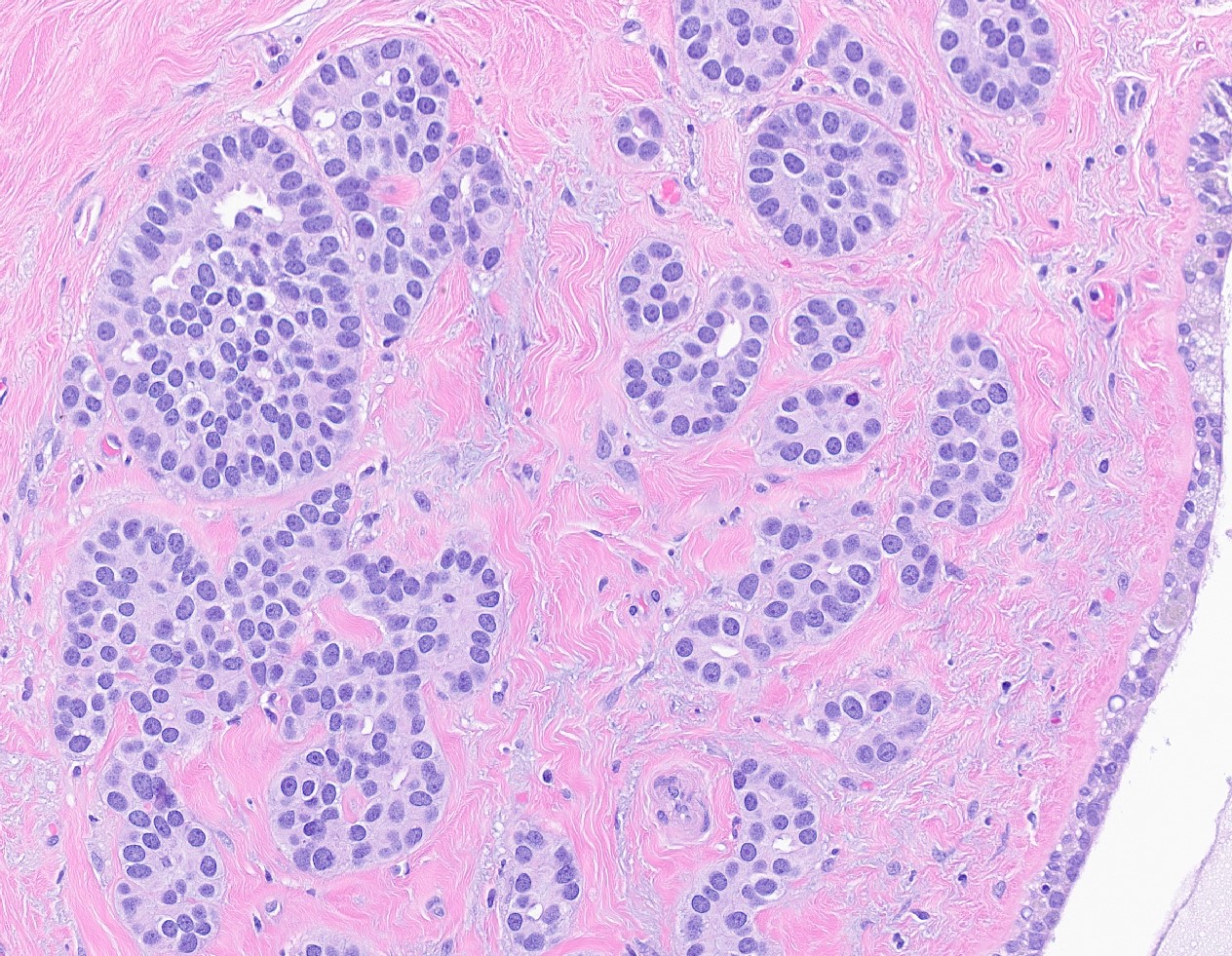
- Histological features of IBC NST vary considerably from case to case and even within the same case
- Margins vary from highly infiltrative, permeating the surrounding tissue, to continuous pushing margins
- Architecture varies from sheets, nests, clusters, cords or individual cells (but lacks the cytomorphological characteristics of invasive lobular carcinoma)
- Tubular formations are prominent in well differentiated tumors but absent in poorly differentiated tumors
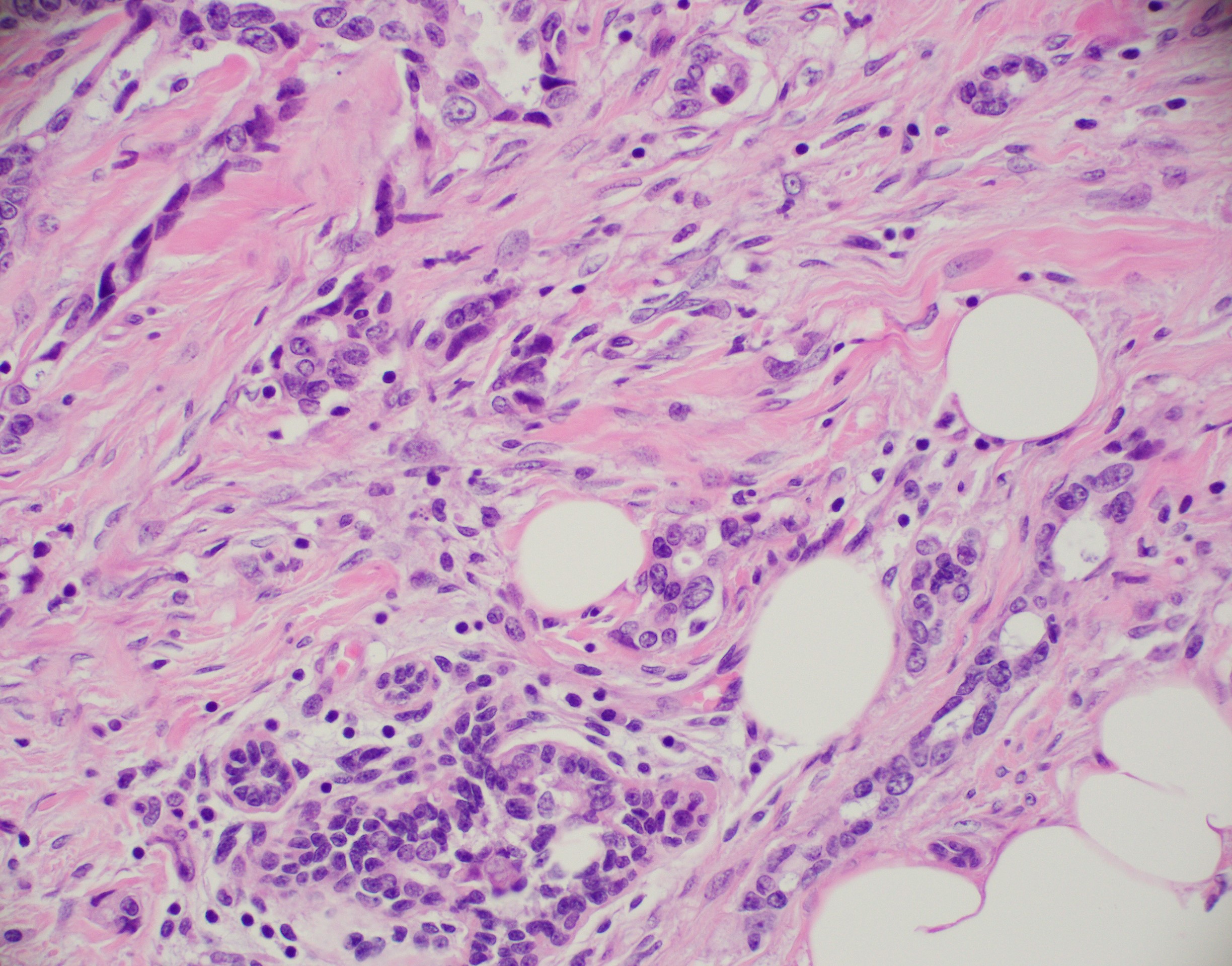
- Variable cytological features:
- Cytoplasm may be abundant and eosinophilic but it can show other features in some tumors, including as clear, foamy or granular
- Nuclei may be regular and uniform or highly pleomorphic with prominent or multiple nucleoli
- Mitotic figures are variable from virtually absent to extensive
- 2 distinct growth patterns exist:
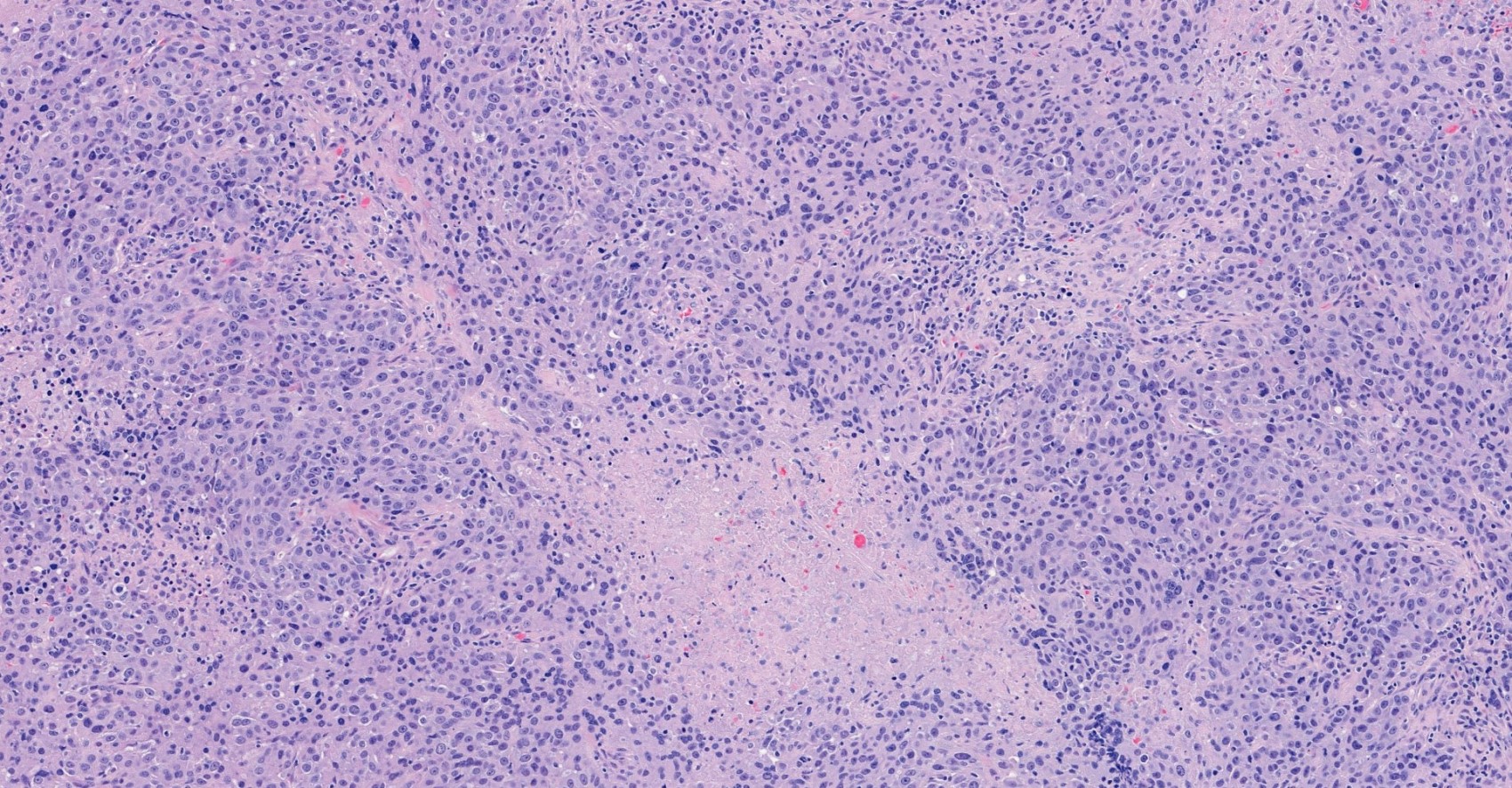
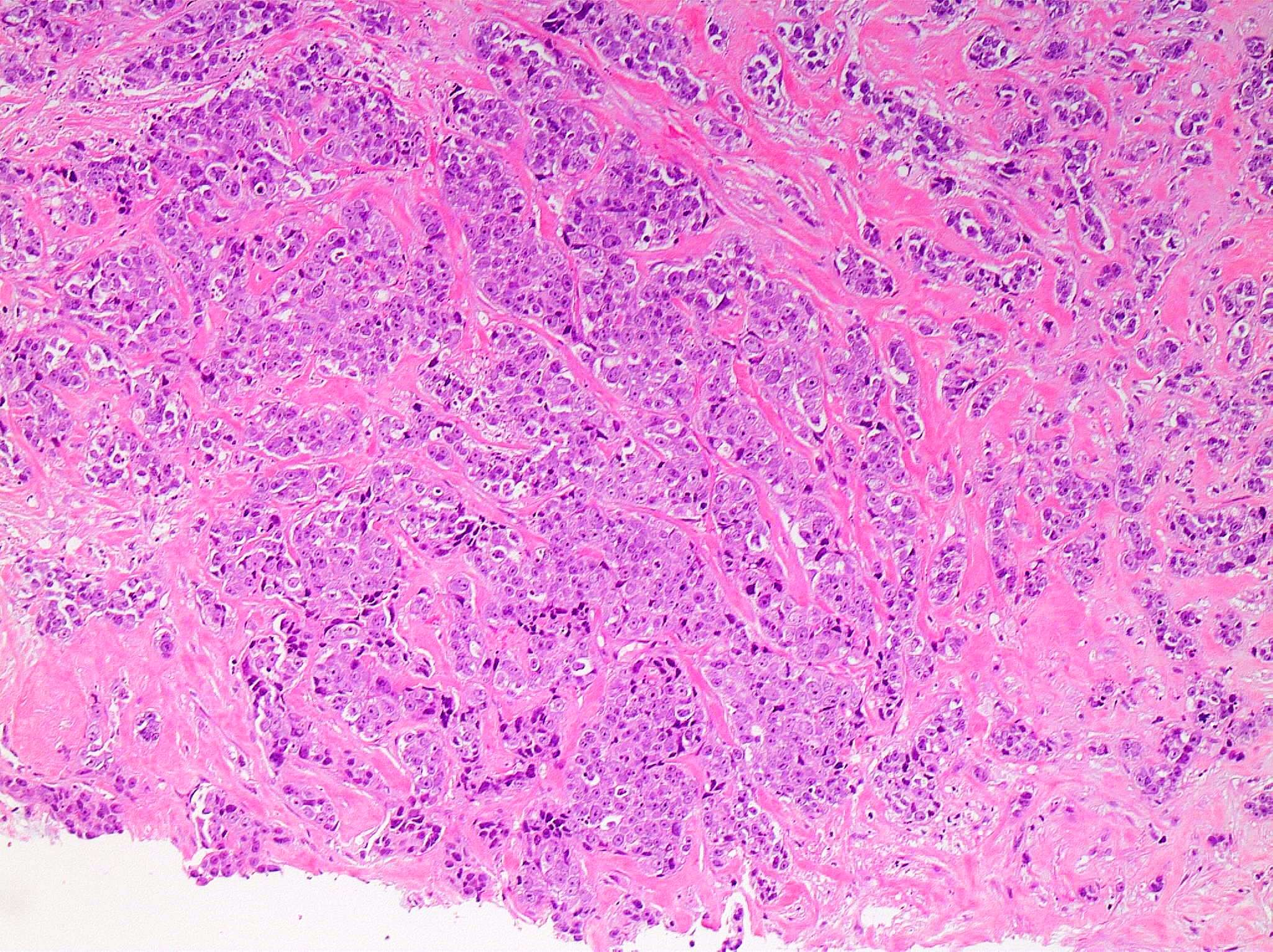
- Large and solid nests or syncytial infiltrative growth pattern with little associated stroma and an expansive growth that compresses the surrounding stroma (e.g., most basal-like breast cancers)
- Tumors characterized by small cancer nests accompanied by marked fibrosis (desmoplastic / scirrhous); this type diffusely infiltrates the surrounding tissue as an irregular shaped spiculated mass
- Calcification in 60% of cases, variable necrosis
- Elastosis involves stroma, wall of vessels and ducts and causes grossly noted chalky streaks
- Often ductal carcinoma in situ (DCIS) (up to 80%)
- In some cases, DCIS is extensive
- Associated DCIS is usually of same nuclear grade as the invasive carcinoma
- Perineural invasion (28%)
- Mast cells are associated with low grade tumors
- Uncommon features: eosinophils intraluminal crystalloids (BMC Cancer 2007;7:165, Arch Pathol Lab Med 1997;121:593)
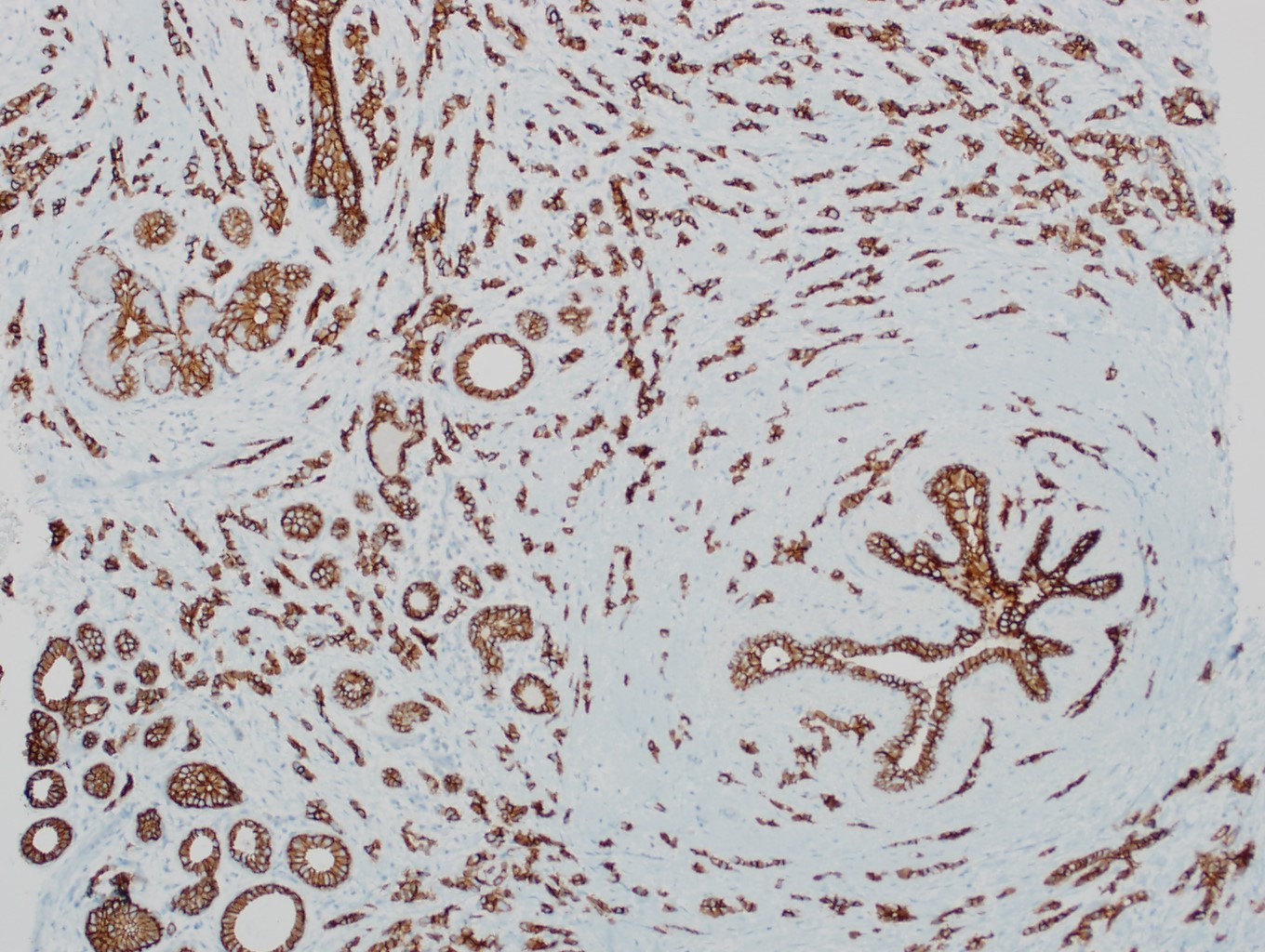
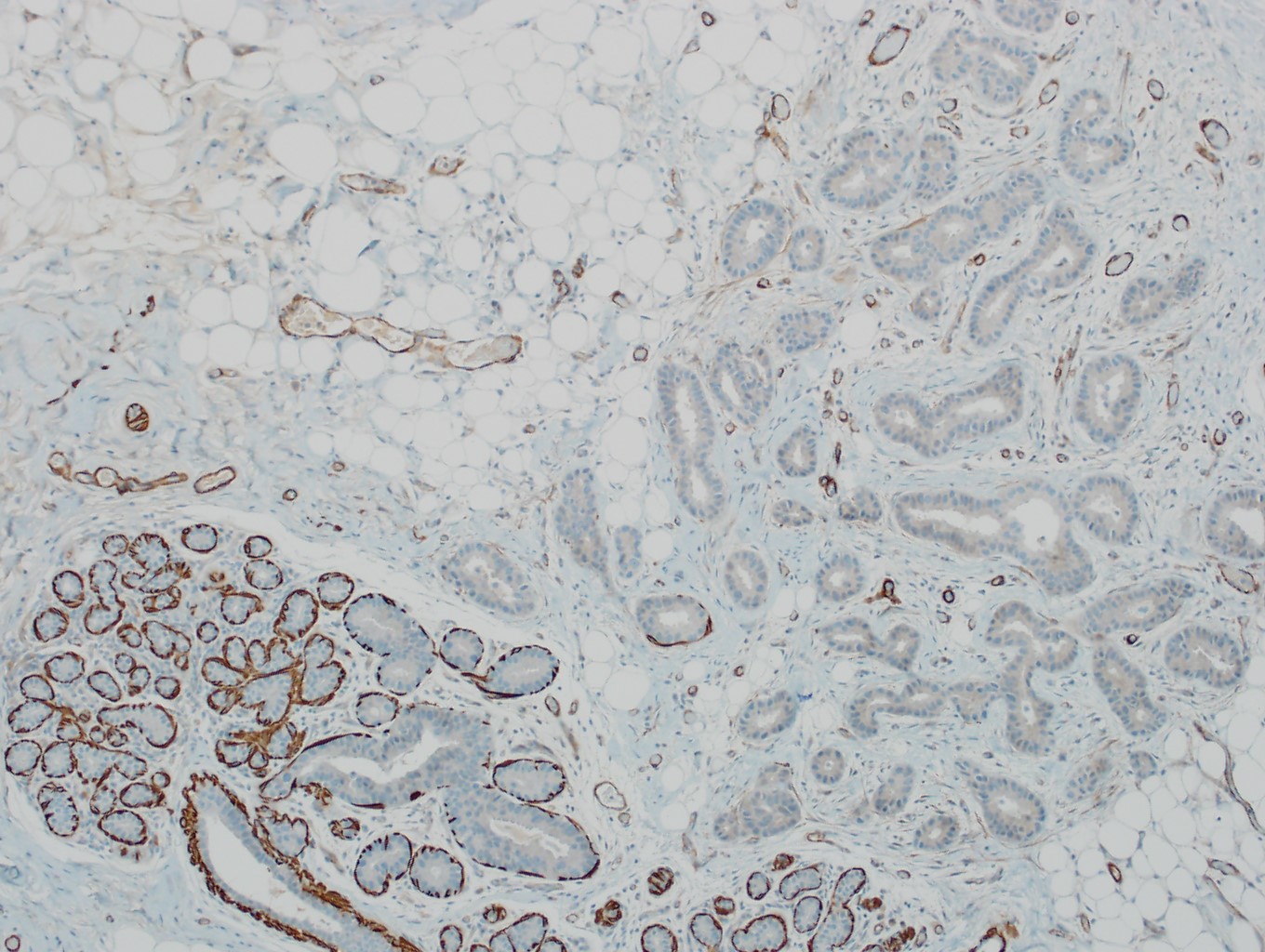
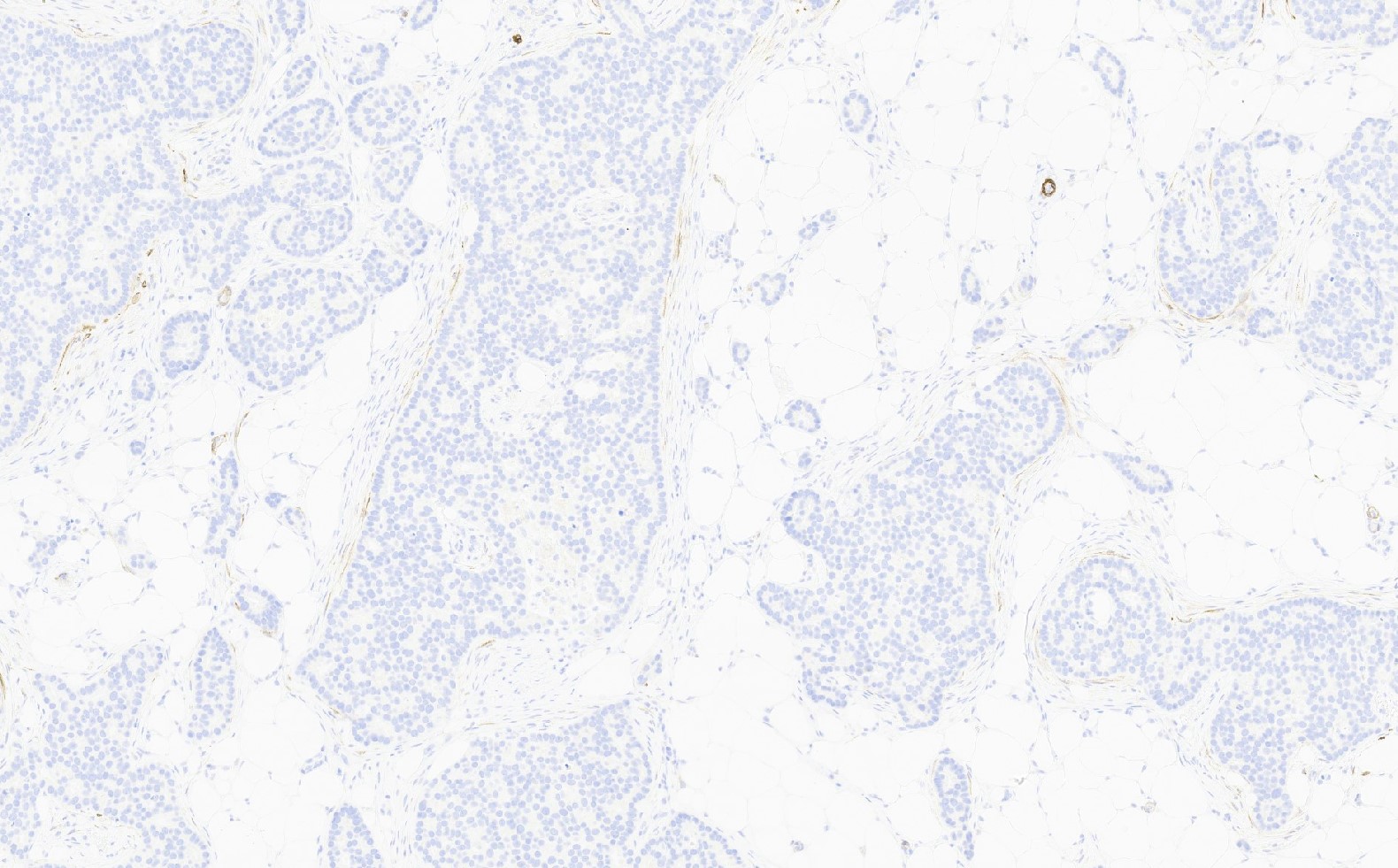
- No myoepithelial cell lining (as seen in DCIS or benign lesions)
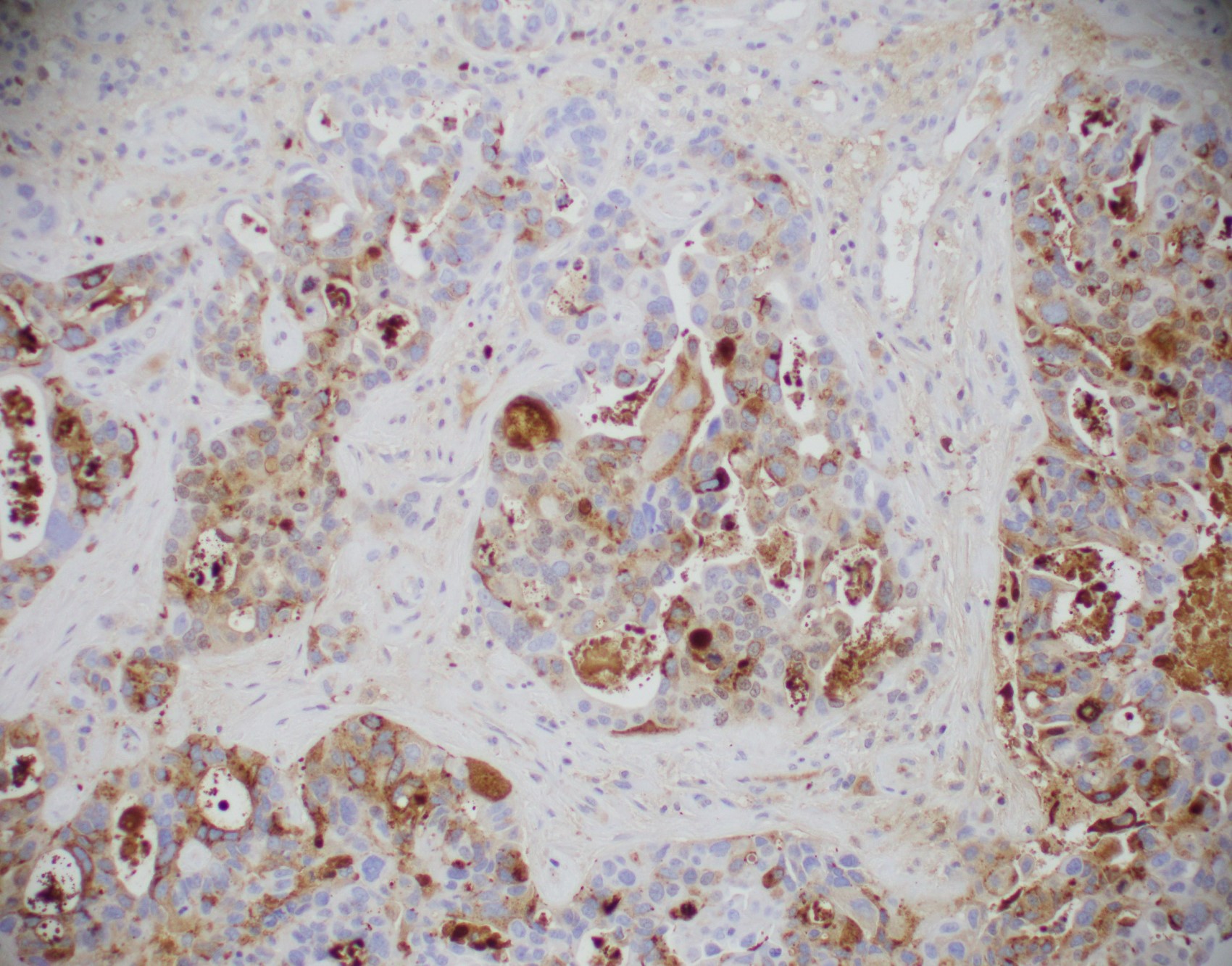
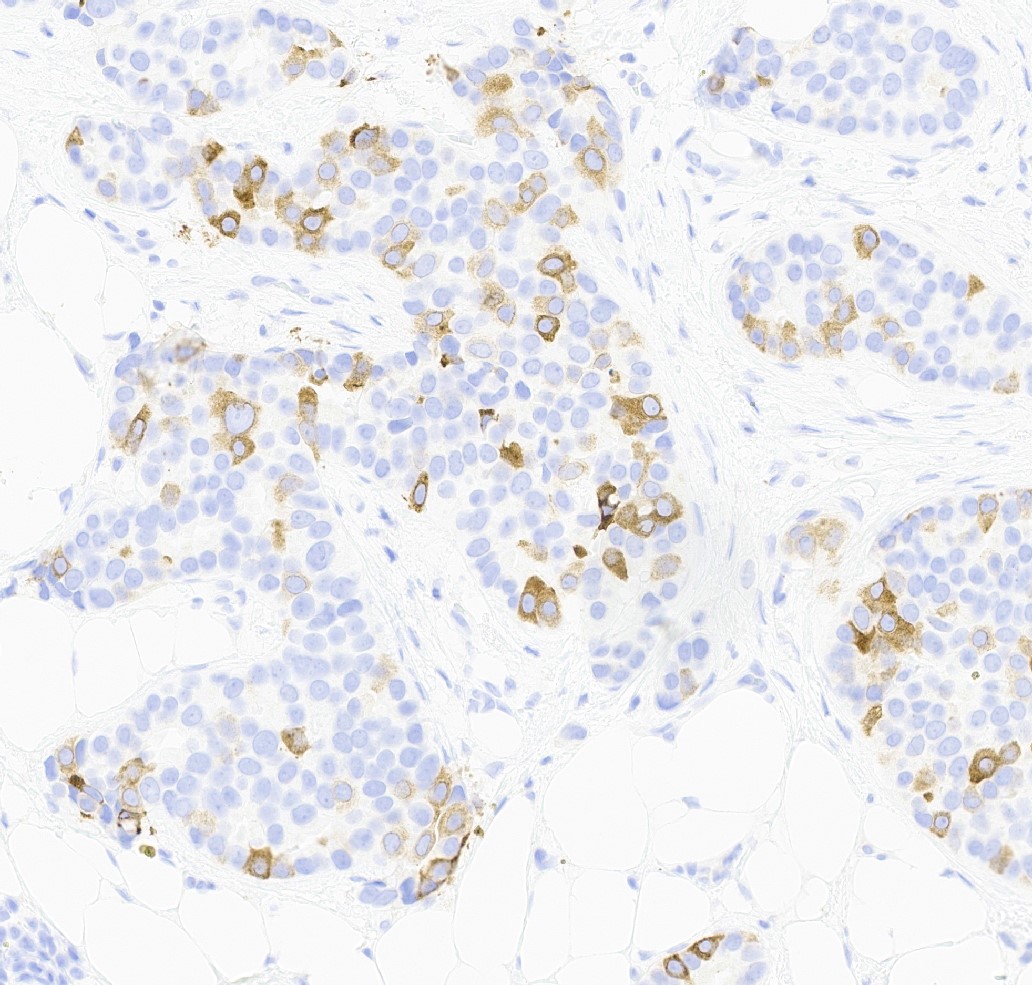
- Angiolymphatic invasion in 35%; differs from tissue retraction because:
- Occurs outside margin of carcinoma
- Does not conform precisely to space it is in
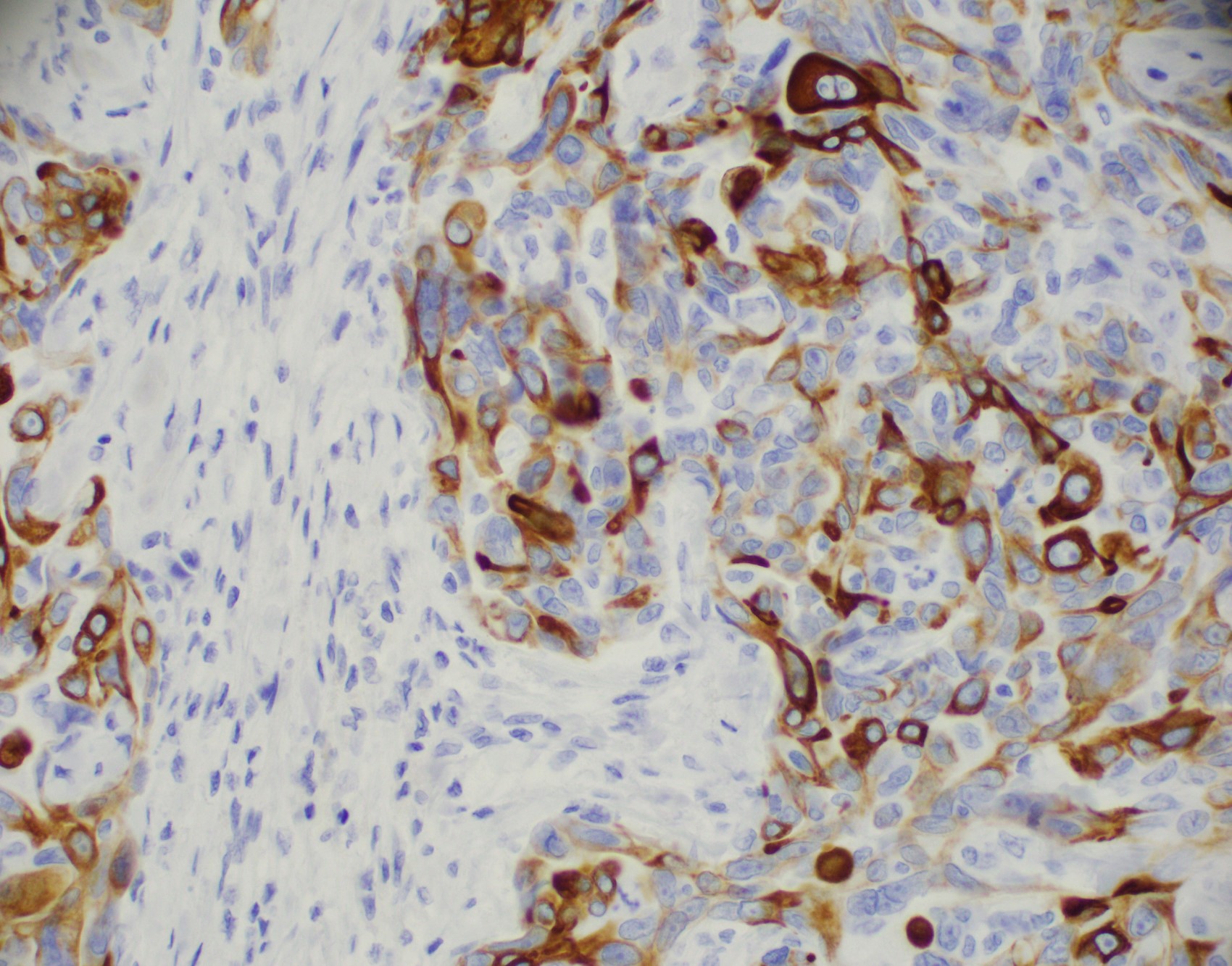
- Endothelial lining is present and is CD31+, ERG, D2-40+, CD34+ and factor VIII+
- Mixed IBC NST and special subtype pattern:
- IBC NST with < 10% special type should be classified as IBC NST with an option to describe the focal specialized pattern in the report comment
- Invasive breast carcinoma with > 90% specialized type patterns should be classified as the special type
- When IBC NST contains a component of specialized tumor type that comprises > 10% but < 90%, the tumor is classified as mixed tumor
- It is recommended to report both the specific special type present and the overall percentage of the cancer with the special pattern (e.g., mixed IBC NST and mucinous carcinoma [40% mucinous])
- Biomarker status of both areas should be reported since they can be distinct
- Grade of the overall tumor should be considered
- Special morphologic patterns of NST:
- IBC NST shows a wide spectrum of differentiation with variable extent of these differentiation lineages
- Some tumors comprises the end of the differentiation spectrum but the prognostic value and the morphological features are not sufficient to classify them as special types, even if such differentiation features comprise > 90%
- Current opinion is to consider such tumors as special morphologic patterns of IBC NST to improve the diagnostic concordance of the classification system and to highlight the lack of prognostic and management implications
- These patterns include:
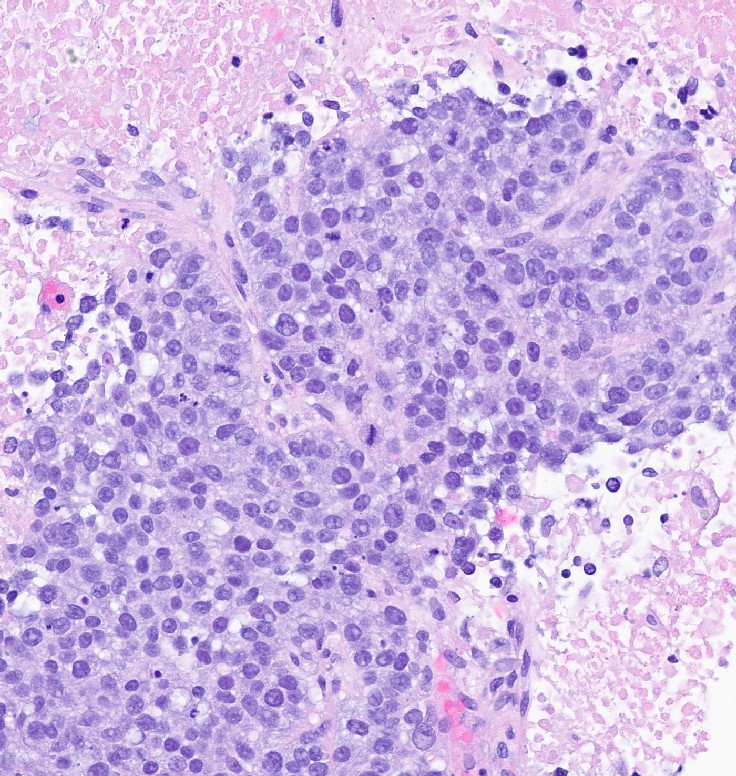
- Basal-like or medullary-like patterns:
- These include breast carcinomas previous described as medullary-like carcinoma or carcinoma with medullary features, as well as basal-like carcinoma
- These tumors typically show high histological grade, a prominent tumor associated lymphocytic (TIL) infiltrate, a triple negative phenotype and often have basal-like molecular profiles
- Oncocytic, sebaceous, melanocytic, glycogen rich, lipid rich, carcinomas with osteoclast-like stromal giant cells, pleomorphic and choriocarcinomatous carcinoma patterns
- Invasive carcinoma with neuroendocrine differentiation:
- A proportion of IBC NST shows neuroendocrine differentiation of variable extent as determined by histological, histochemical and immunohistochemical analysis occurs but lack histologic features to classify as large cell or small cell neuroendocrine carcinoma or neuroendocrine tumor of the breast
- These tumors do not carry prognostic or clinical significance and they can be considered as variant (morphological pattern) of IBC NST (Hum Pathol 2003;34:1001)
- Basal-like or medullary-like patterns:
- Tubule formation (1 - 3 points):
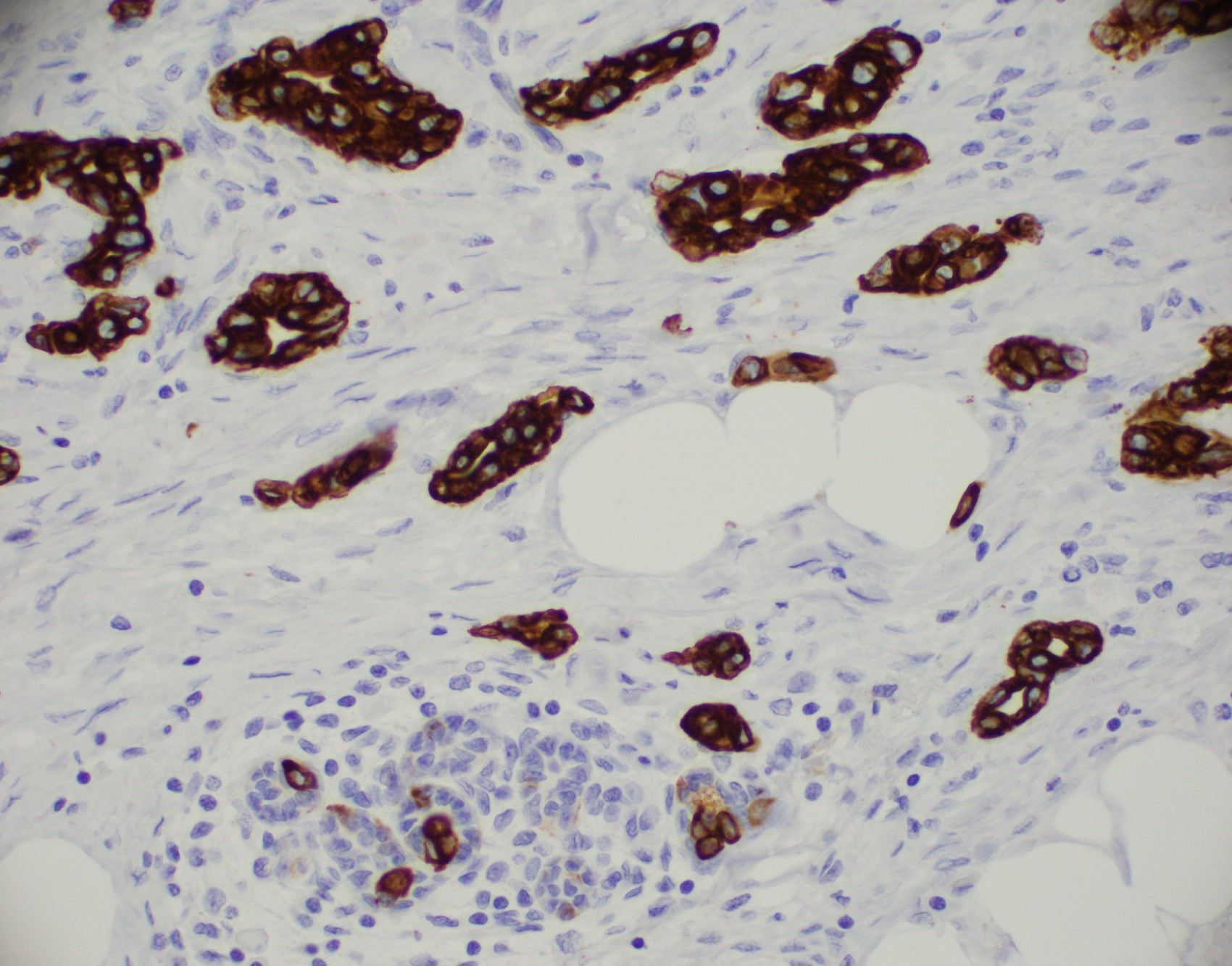
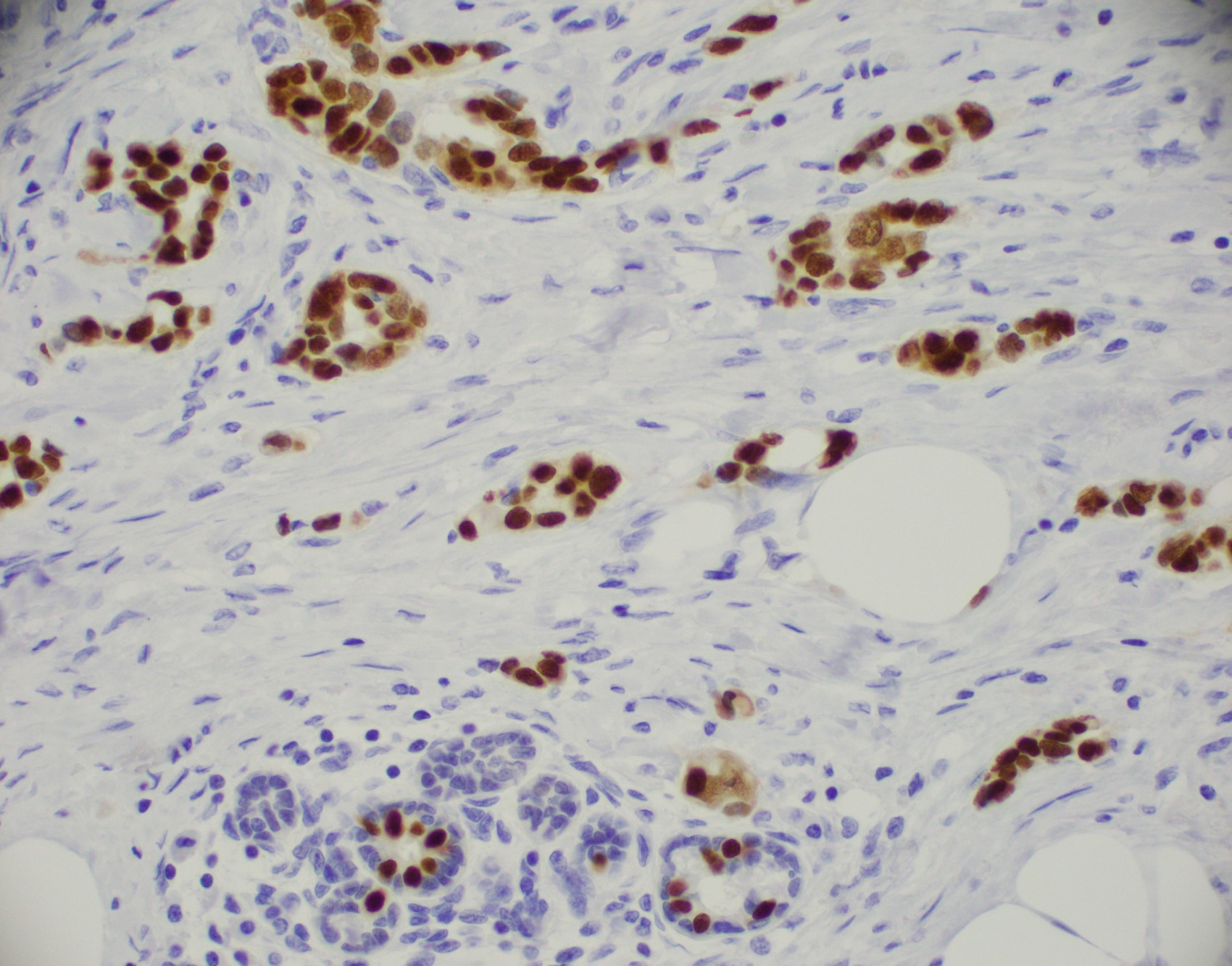
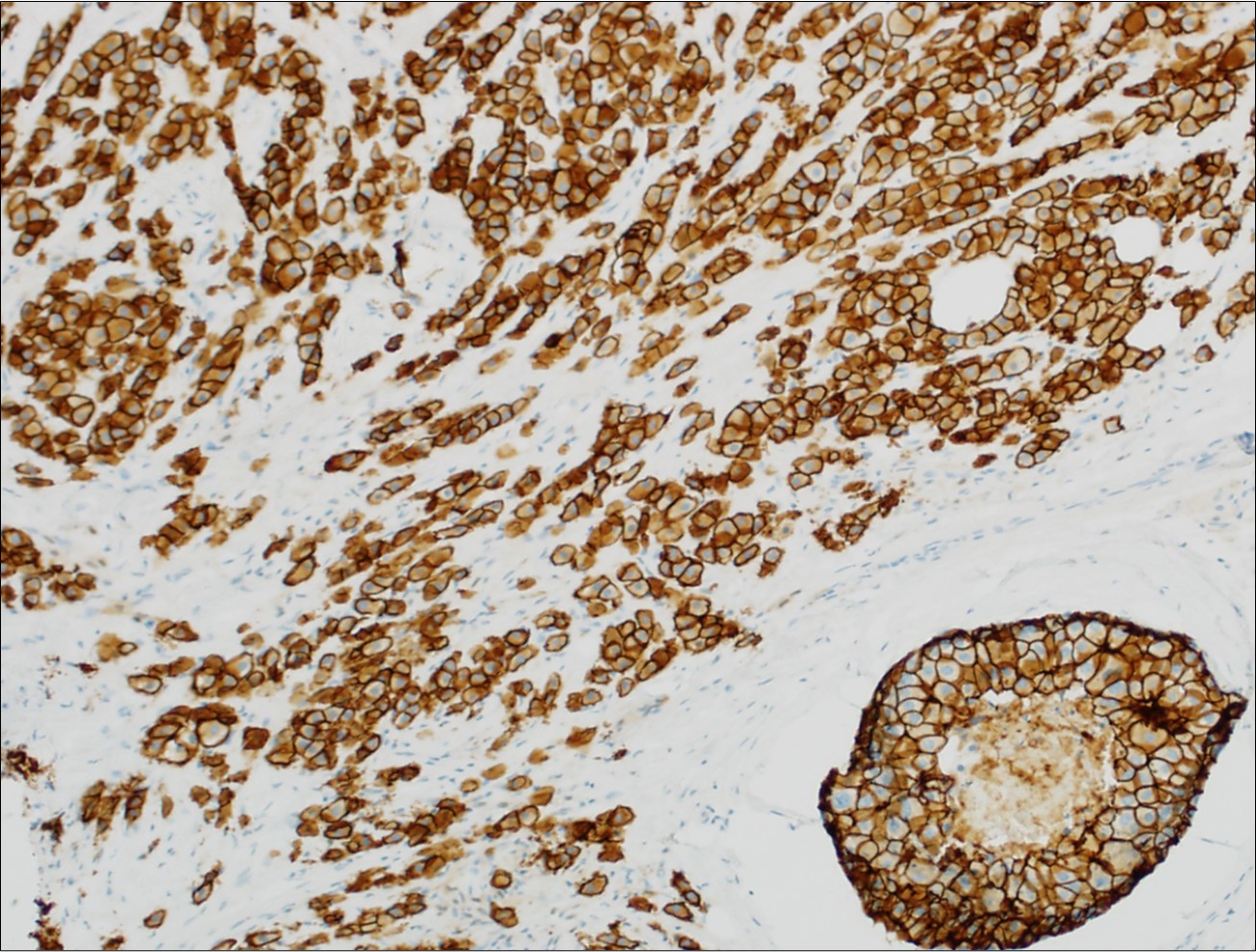
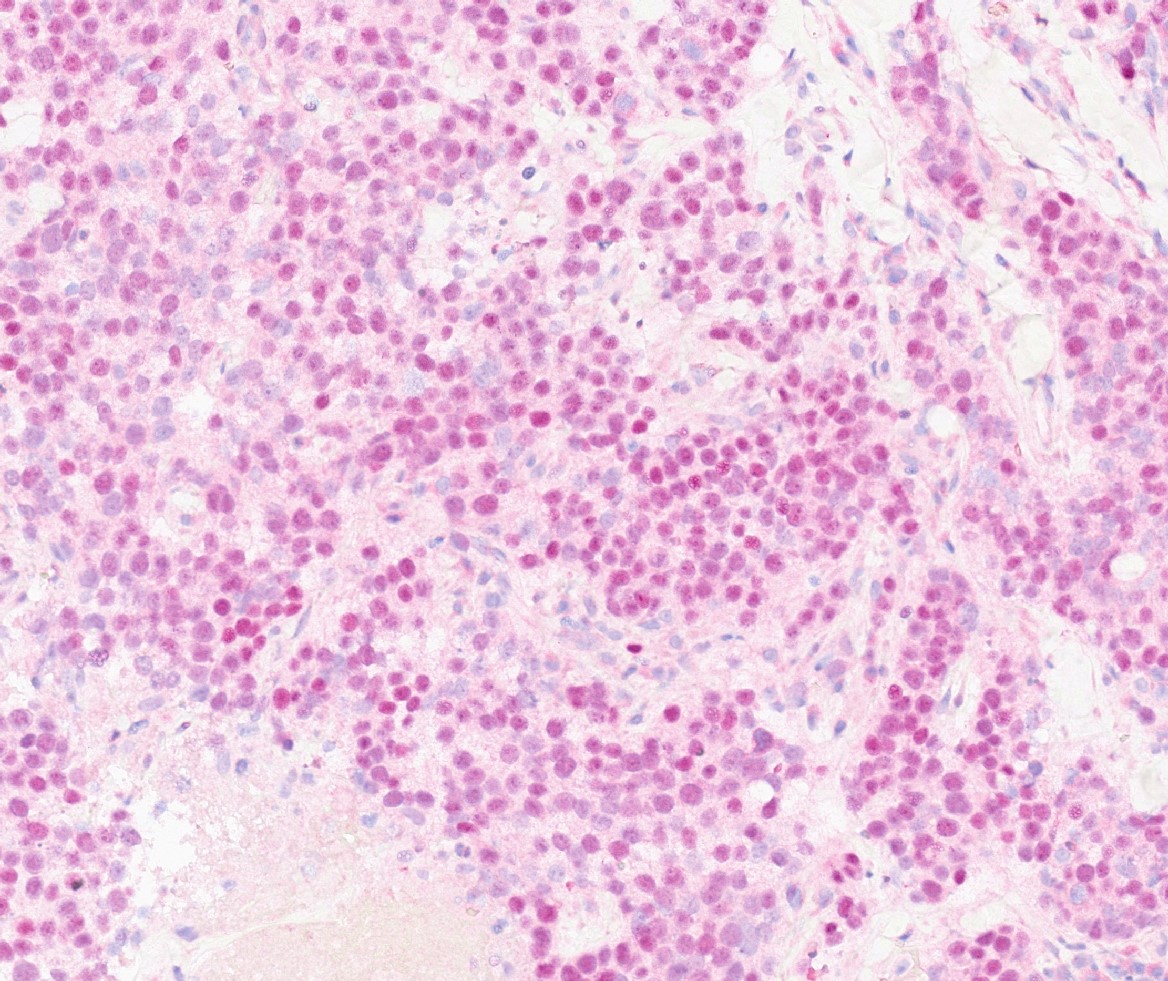
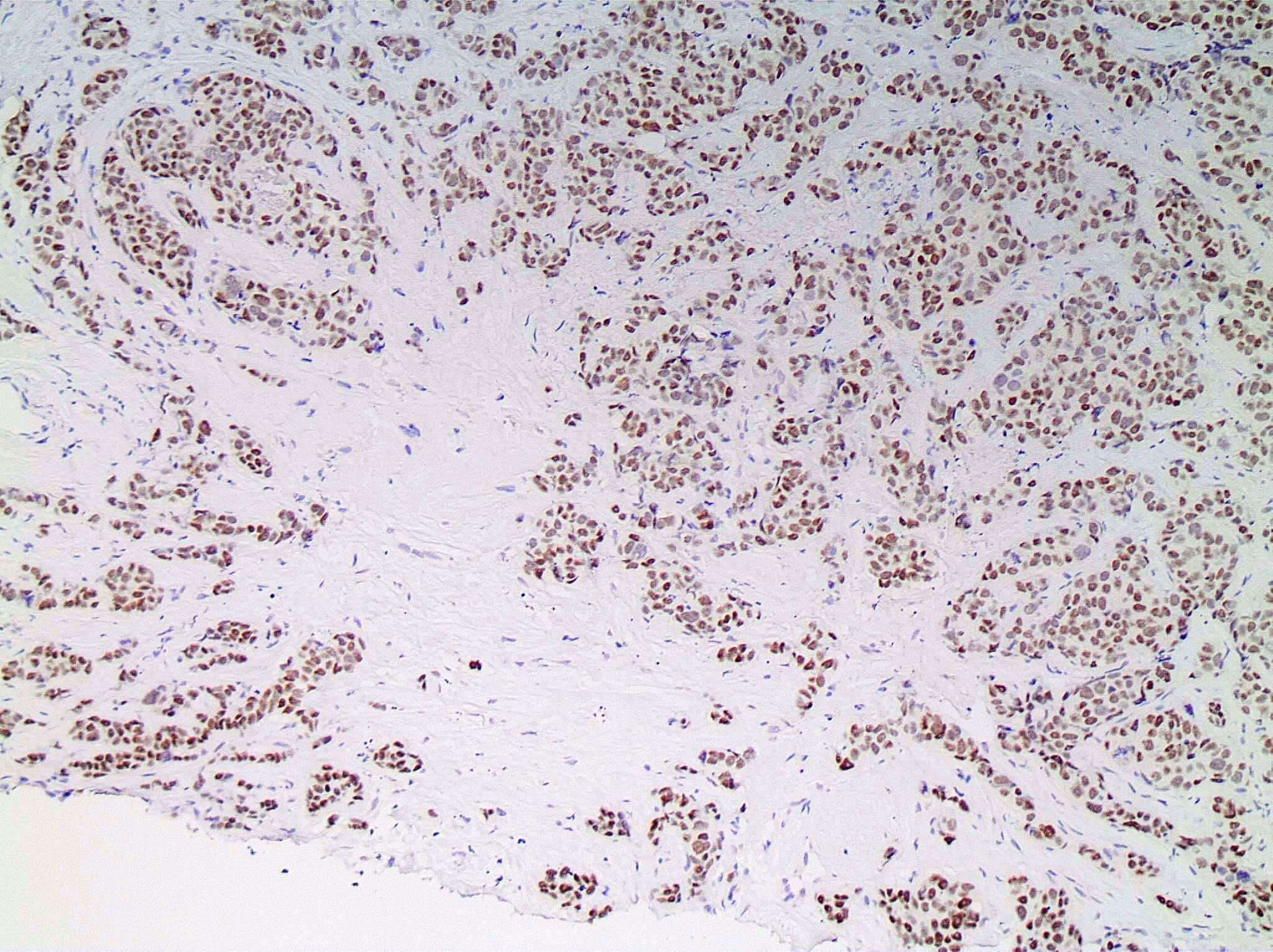
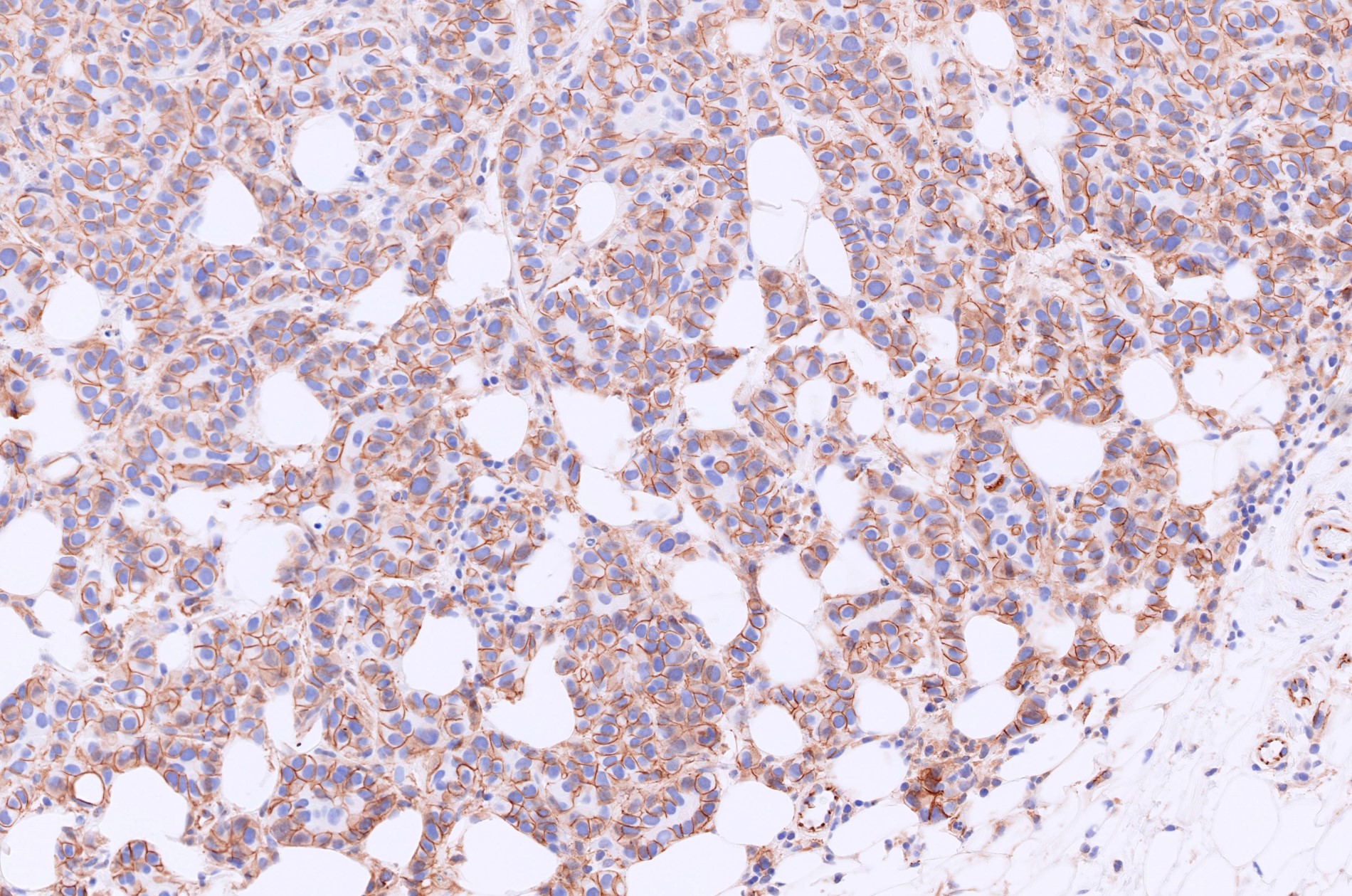
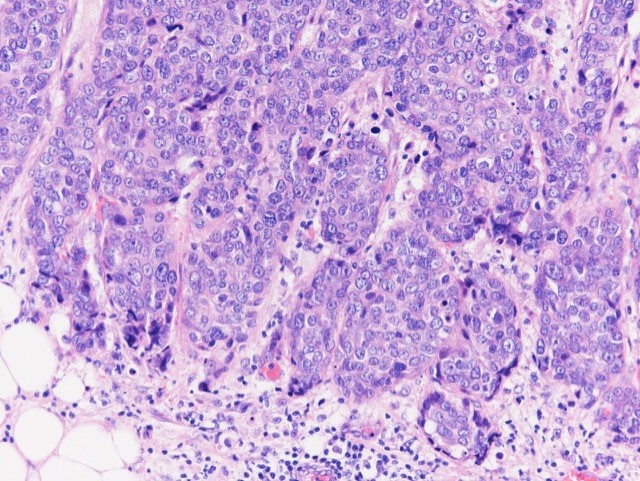
Microscopic (histologic) images
Contributed by Julie M. Jorns, M.D., Kristen E. Muller, D.O., Gary Tozbikian, M.D. and Emad Rakha, M.D.
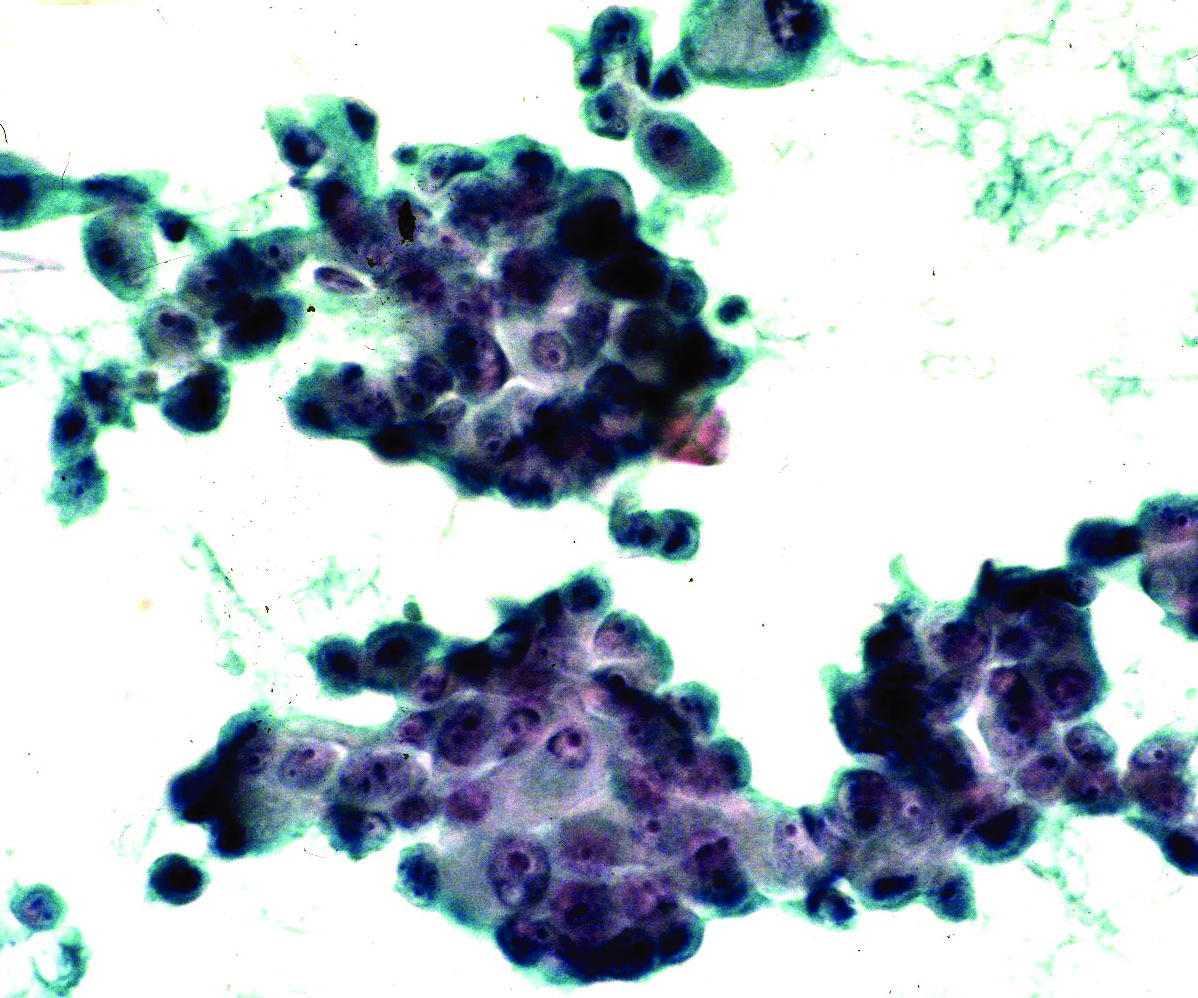
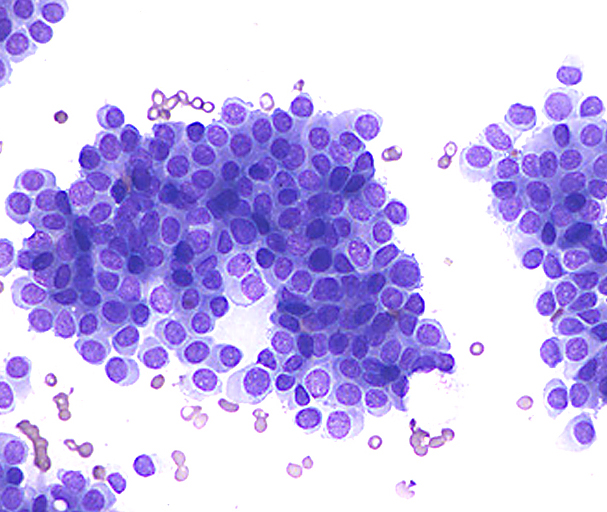
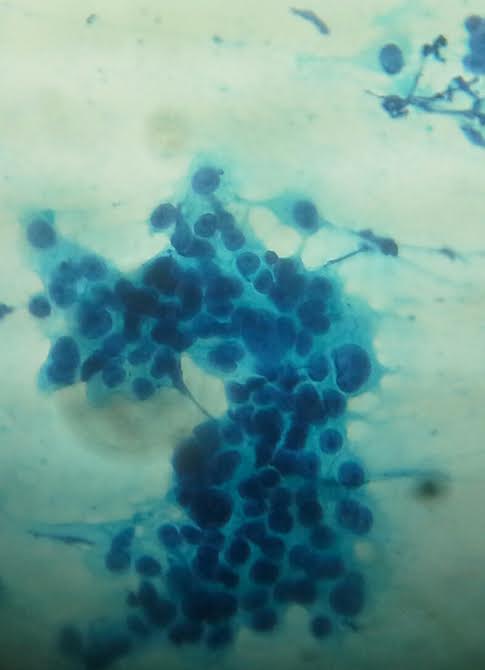
Cytology description
- Can use cellular pleomorphism, nuclear size, nuclear margin, nucleoli, lack of naked nuclei, cellular dyscohesion and mitoses in addition to necrosis to assess cytologic tumor grade, which correlates with histologic grade (Diagn Cytopathol 2003;29:185)
Cytology images
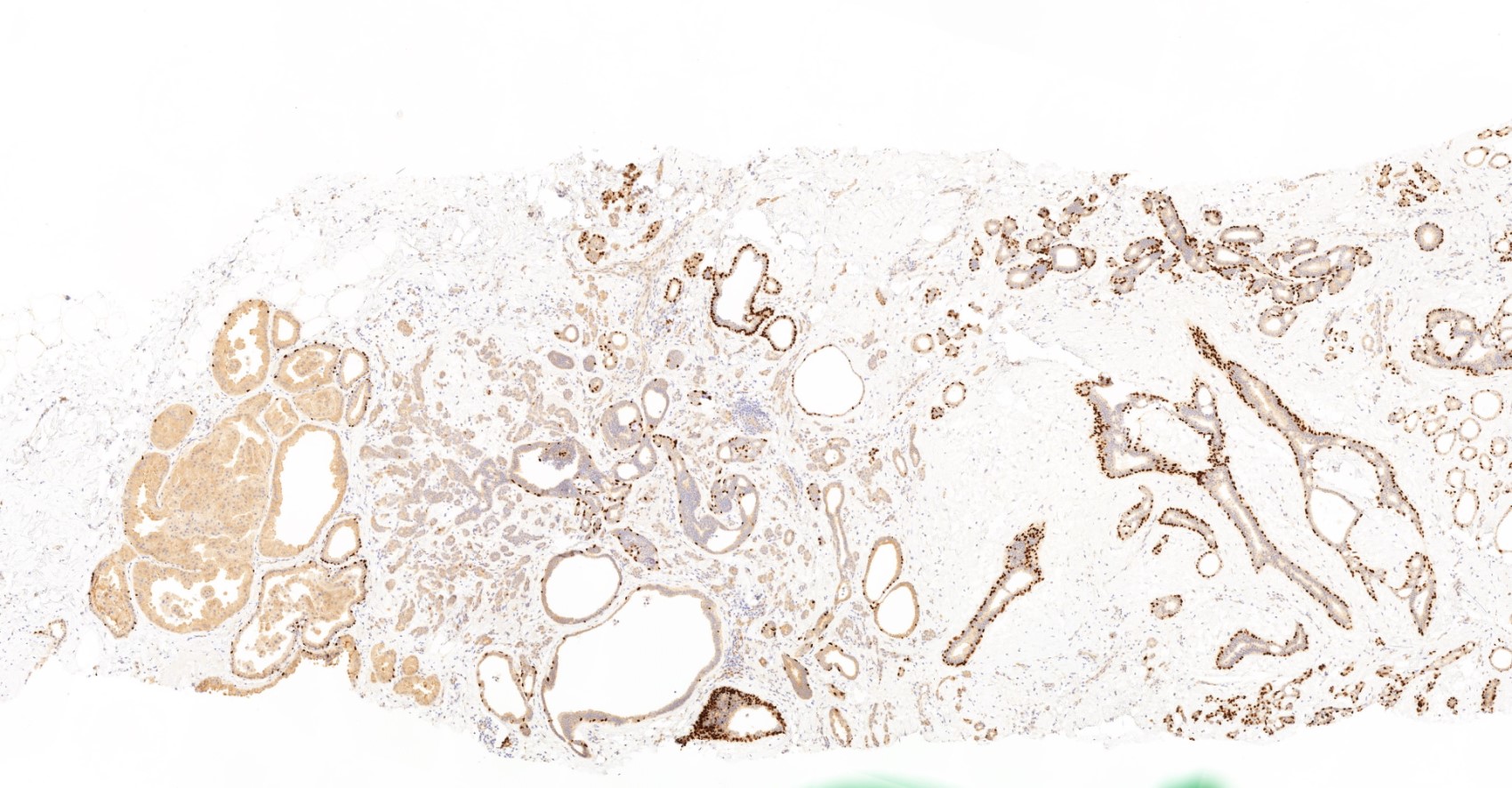
Positive stains
- Luminal low molecular weight cytokeratins (CK8 / 18, CK19 and CK7 and pancytokeratins such as AE1 / AE3, CAM 5.2, MNF-116), EMA, E-cadherin, p120, ER (60 - 80%), PR (50 - 70%), HER2 (15 - 20%) (Am J Clin Pathol 2006;125:377)
- GCDFP-15 (30 - 40%)
- GATA3 (~91 - 100% of hormone receptor positive breast cancers, ~43 - 66% in triple negative breast cancer) (Hum Pathol 2014;45:2225, Hum Pathol 2015;46:1829)
- Also mammaglobin, milk fat globule, lactalbumin, CEA, B72.3, BCA225
- High molecular weight cytokeratins (CK5/6, CK14 and CK17) (10 - 30%)
- Glycogen (60%), mucin (moderate / marked in 20%)
- S100 (10 - 45%), RCC Ma (renal cell carcinoma marker)
- CD5 clone 4C7 (Arch Pathol Lab Med 2001;125:781)
- TRPS1 (Mod Pathol 2021;34:710)
- SOX10 (57 - 58% of triple negative breast cancers) (Hum Pathol 2019;85:221, Hum Pathol 2017;67:205)
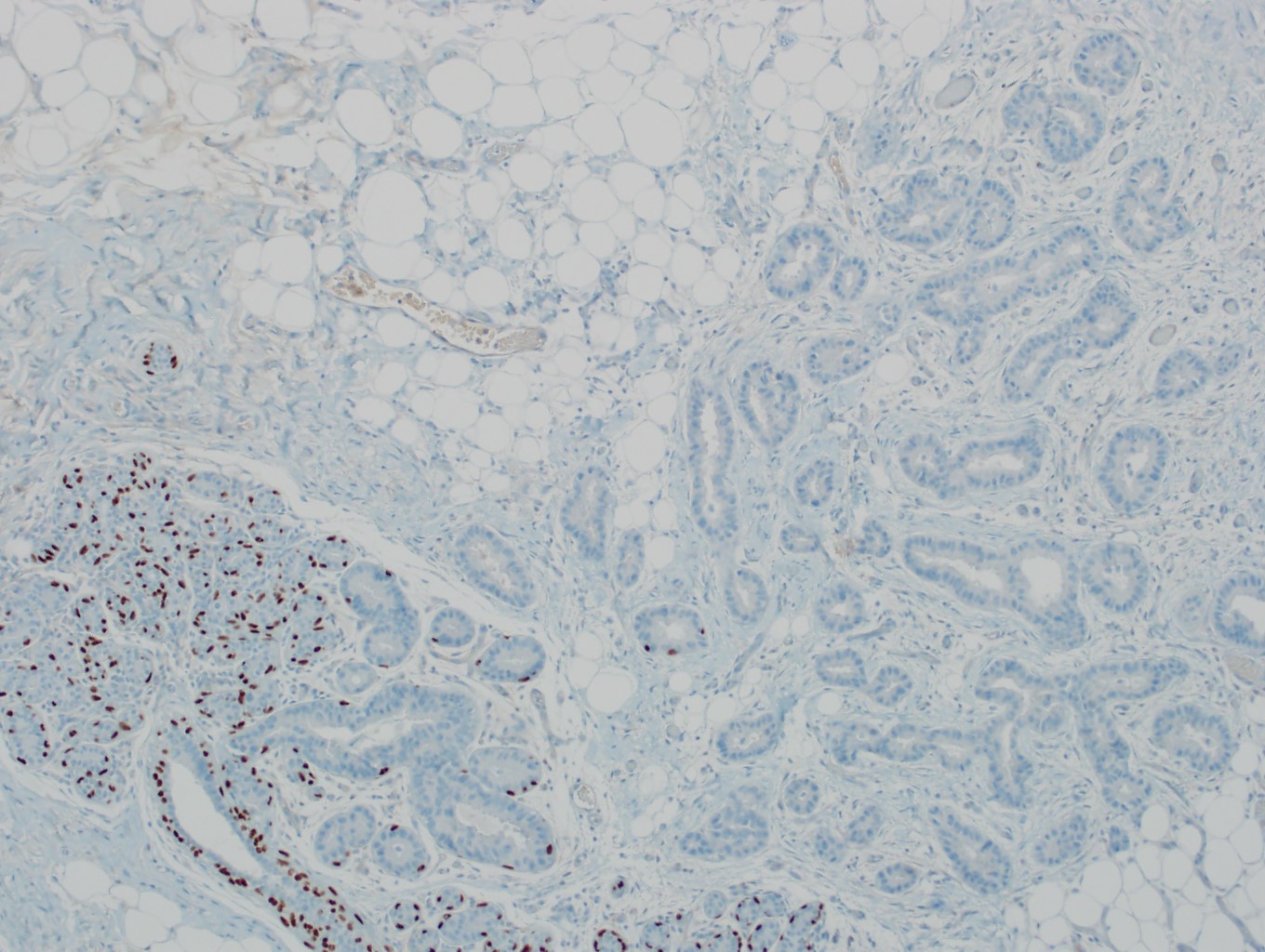
Negative stains
- CK20, CD34
- Myoepithelial markers: p63 (positive in benign lesions), CD10, calponin (Am J Surg Pathol 2001;25:1054, Mod Pathol 2002;15:397)
- Other tissue specific transcription factors: TTF1, CDX2, PAX8 and WT1 (nonspecific weak to moderate or strong focal staining can be in up to 10% of cases but typically weaker than its expression in the relevant tumors)
Electron microscopy description
- Glandular differentiation (microvilli and terminal bars on luminal side)
Molecular / cytogenetics description
- ER+ pathway: characterized by gains of 1q, loss of 16q, sometimes amplification of 17q12
- ER- pathway: characterized by loss of 13q, gain of 11q13, amplification of 17q12, p53 mutations common, increased expression of genes associated with cell proliferation
- PI3KCA mutations associated with endocrine therapy resistance (Virchows Arch 2016;469:35, Int J Breast Cancer 2020;2020:3759179)
- p53 mutations in many basal-like tumors (Nature 2012;490:61)
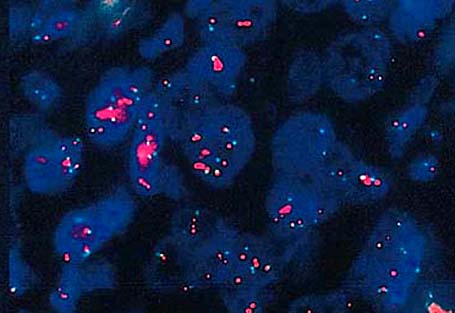
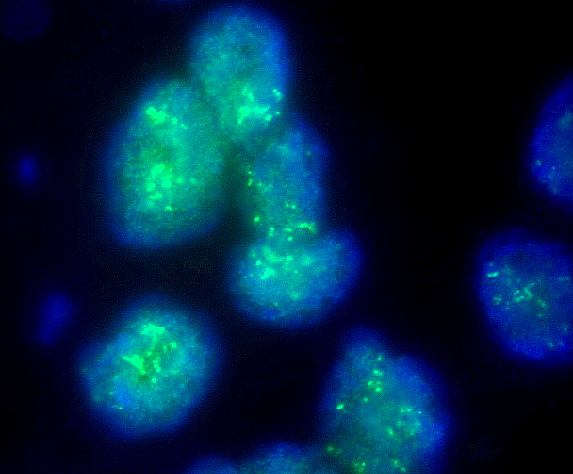
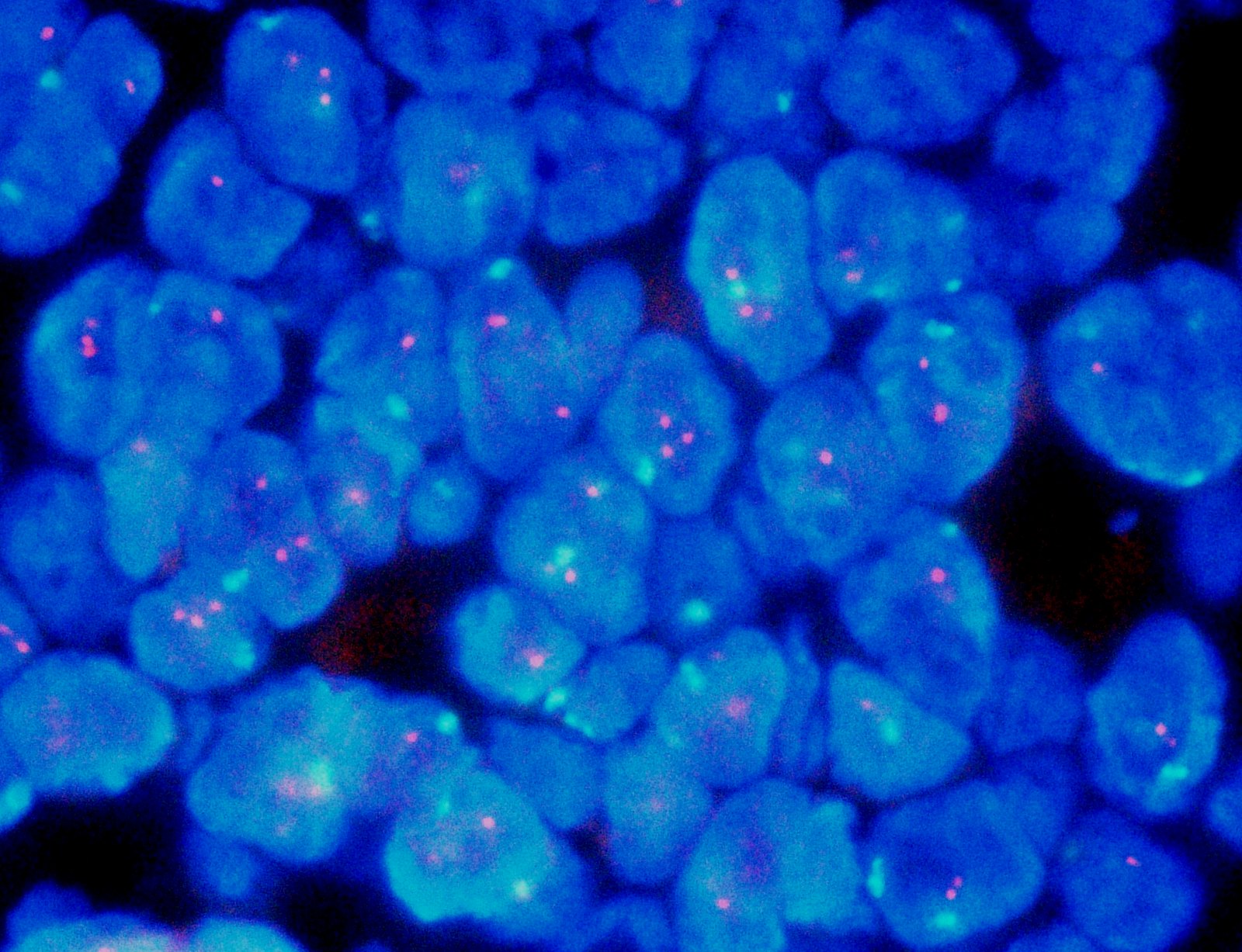
- HER2 gene amplification in approximately 15% (Cancer Res 1993;53:4960, J Clin Oncol 1989;7:1120)
- Gene expression profiling stratifies breast cancers into several intrinsic molecular subtypes (e.g., luminal A, luminal B, HER2 enriched, basal-like, etc.) (Nature 2012;490:61)
- 5 - 10% of breast cancers are hereditary as result of mutations in various genes, such as BRCA1, BRCA2, TP53, STK11, CD1, PTEN, MDM2, RB, CHEK2 (Acta Oncol 2019;58:135)
- ESR1 mutations associated with resistance to endocrine therapy (JAMA Oncol 2016;2:1310)
Molecular / cytogenetics images
Videos
Ductal carcinoma - breast
Breast ductal carcinoma (breast cancer)
Breast carcinoma: invasive - pathology mini tutorial
Sample pathology report
- Left breast, mass, ultrasound guided core needle biopsy:
- Invasive breast carcinoma, NST (invasive ductal carcinoma), grade 2, measuring 1.5 cm
- Estrogen receptor: positive (95%, strong intensity)
- Progesterone receptor: negative (0%)
- HER2: negative (score 1+)
Differential diagnosis
- IBC NST should be differentiated from special types of breast carcinoma:
- > 90% special tumor characteristics
- IBC NST admixed with 10 - 90% of other special subtype(s) is designated as mixed IBC NST and special subtype with description of the percentage of special subtype components provided
- Metastatic adenocarcinoma with overlapping features, including lung adenocarcinoma, endometrioid carcinoma, serous carcinoma and renal cell carcinoma:
- Correlation with clinical and radiologic history
- Appropriate use of immunohistochemical panel of stains (e.g., estrogen receptor, GATA3, TRPS1, etc.)
- Rarely, examples of skin adnexal (sweat gland) carcinoma of the breast skin:
- Morphology and immunohistochemical profile may be indistinguishable from breast cancer, superficial location centered in the skin versus within the breast (adjacent ducts / lobules)
- Melanoma:
- Can mimic high grade breast cancer
- Melanin pigment, lack of cytokeratin expression, positive for melanoma melanocytic markers
- Ductal carcinoma in situ involving sclerosing lesions (e.g., sclerosing adenosis):
- IHC using myoepithelial markers shows retained myoepithelial cell layer
Additional references
Board review style question #1
Board review style answer #1
A. Calponin and p63. The use of myoepithelial markers is most useful for distinguishing in situ from invasive carcinoma. Utilization of 2 myoepithelial markers, including both a cytoplasmic marker (e.g., calponin or smooth muscle myosin) and a nuclear marker (p63 or p40) is recommended, as myoepithelial expression can be patchy or attenuated (p63 / p40) and show nonspecific expression in endothelial cells (SMMS); the combination of 2 markers provides increased sensitivity and specificity. Both DCIS and invasive breast carcinoma (NST) may show expression of hormone receptors, HER2 and cytokeratins.
Comment Here
Reference: Invasive breast cancer of no special type (NST)
Comment Here
Reference: Invasive breast cancer of no special type (NST)
Board review style question #2
Which of the following is true regarding the diagnosis of invasive breast carcinoma of no special type (NST)?
- Always shows extensive (> 90%) tubular / acinar formation
- Generally negative for E-cadherin and p120 (cytoplasmic)
- Low grade tumors are generally positive for HER2 overexpression by immunohistochemistry
- Majority of cases are hereditary due to mutations in either BRCA1 or BRCA2
- Requires assessment of standard biomarkers estrogen and progesterone receptors and HER2
Board review style answer #2
E. Per ASCO / CAP guideline recommendations, all new primary invasive breast cancers require assessment of standard breast biomarkers: estrogen receptor, progesterone receptor and HER2. IBC NST show positive membranous reactivity of E-cadherin and p120. Degree of tubular formation can range between tumors and within tumors. Generally, low grade tumors show greater tubule formation, are positive for hormone receptor expression and are negative for HER2. The majority of breast cancer is sporadic; only 5 - 10% of breast cancer is considered familial.
Comment Here
Reference: Invasive breast cancer of no special type (NST)
Comment Here
Reference: Invasive breast cancer of no special type (NST)
Board review style question #3
Which of the following findings would you expect from a low grade invasive breast carcinoma of no special type?
- Basal-like intrinsic molecular subtype
- Lack of desmoplastic stromal response, positive for S100, positive for laminin and collagen IV around glands, estrogen receptor negative
- Positive for HER2 overexpression
- Positive for hormone receptors (estrogen and progesterone receptors)
- Positive for p63 and calponin
Board review style answer #3
D. The majority of low grade invasive breast carcinomas of no special type belong to the luminal A intrinsic molecular subtype, are positive for hormone receptors (estrogen and progesterone receptors) and are negative for HER2. The invasive component will lack expression of myoepithelial markers (e.g., p63 and calponin). Lack of desmoplasia, expression of S100, negativity for estrogen receptor and expression of laminin and collagen IV around infiltrative glands would be consistent with microglandular adenosis.
Comment Here
Reference: Invasive breast cancer of no special type (NST)
Comment Here
Reference: Invasive breast cancer of no special type (NST)