- pN: not assigned (no nodes submitted or found)
- pN: not assigned (cannot be determined based on available pathological information)
- pTis: carcinoma in situ
- pT1: tumor limited to one subsite of hypopharynx or ≤ 2 cm in greatest dimension
- pT2: tumor invades > 1 subsite of hypopharynx or an adjacent site, or tumor > 2 cm but ≤ 4 cm in greatest dimension without fixation of hemilarynx
- pT3: tumor > 4 cm in greatest dimension or with fixation of hemilarynx or extension to esophageal mucosa
- pT4a: tumor of any size invading thyroid / cricoid cartilage, hyoid bone, thyroid gland, esophageal muscle or central compartment soft tissue (central compartment soft tissue includes prelaryngeal strap muscles and subcutaneous fat)
- pT4b: tumor of any size that invades prevertebral fascia, encases carotid artery or involves mediastinal structures
Superpage
Superpage Topics
Adenoid cystic carcinoma
Adenosquamous carcinoma (pending)
Amyloidosis-larynx
Amyloidosis-trachea
Anatomy & histology
Basaloid squamous cell carcinoma
Chondrosarcoma
Contact ulcer
Conventional squamous cell carcinoma
Diphtheria
Dysplasia
Grossing & features to report
Laryngeal cysts
Laryngitis / laryngoepiglottitis
Laryngocele (pending)
Neuroendocrine neoplasm
Papillary squamous cell carcinoma
Papilloma
Reactive epithelial hyperplasia
Spindle cell carcinoma
Staging-hypopharynx
Staging-larynx
Verrucous carcinoma
Verrucous hyperplasia
Vocal cord polyp
WHO classification of Laryngeal tumors (pending)Adenoid cystic carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
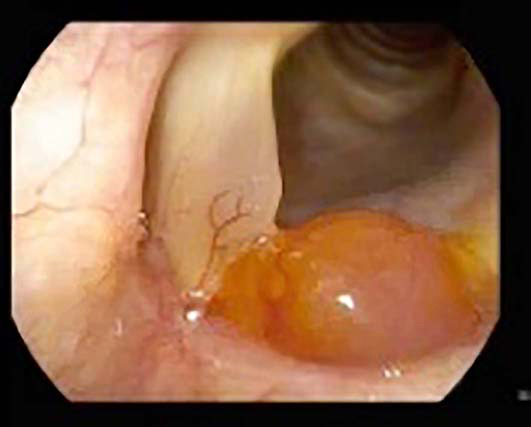
- Most common laryngeal minor salivary gland neoplasm (Laryngoscope 2015;125:2485)
- Only about 142 cases have been reported as of April 2016 (Adv Ther 2016;33:553)
- Same histology as salivary gland tumors (Am J Otolaryngol 2012;33:226)
Essential features
- Slow growing salivary gland tumor that can also be found in the larynx
- Presents at late stage; difficult to achieve clear surgical margins
- Various histologic patterns including tubular, cribriform, solid (similar to the oral cavity)
ICD coding
- ICD-O: 8200/3 - adenoid cystic carcinoma
Epidemiology
- M = F (Otolaryngol Head Neck Surg 2016;154:433)
- Occurs over a wide age range but most commonly presents in the sixth to eighth decade (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
Sites
- Most commonly subglottic (60%) (Adv Ther 2016;33:553)
- Can be supraglottic (30%) or involve the true vocal cords (10%) (Adv Ther 2016;33:553)
- Location is possibly due to the presence of more subepithelial glands in those areas (Head Neck 2008;30:919)
Pathophysiology
- Arises from subepithelial glands (Otolaryngol Head Neck Surg 2016;154:433)
- Associated with t(6;9)(q22-23;p23-24) translocation creating a fusion gene between MYB proto-oncogene and NFIB transcription factor gene (Mod Pathol 2011;24:1169)
- Overall, 56/68 (82%) of adenoid cystic carcinomas expressed MYB-NFIB fusion in a study by Brill et al.; of those 2/5 (40%) were from the larynx (Mod Pathol 2011;24:1169)
- This chromosomal rearrangement juxtaposes super enhancers to the MYB locus to create a positive feedback loop elicited when the MYB protein activates these enhancers (Oncotarget 2016;7:66239)
- Fusion between MYBL1 and NFIB genes without MYB aberration have been identified, demonstrating that tumor development may also be driven by genetic alterations in different members of the same transcription factor family (Oncotarget 2016;7:66239)
Etiology
- Exact cause is currently unknown (Oncol Lett 2015;10:2303)
- Specific risk factors have not yet been identified, smoking does not affect the incidence unlike squamous cell carcinomas (Acta Otorhinolaryngol Ital 2009;29:279)
Clinical features
- Slow growing (Otolaryngol Head Neck Surg 2016;154:433)
- Symptoms include pain (secondary to perineural invasion), dyspnea, hoarseness, dysphagia (Head Neck 2008;30:919)
- Neck metastasis is rare (Adv Ther 2016;33:553)
Prognostic factors
- Often presents at late stage (Otolaryngol Head Neck Surg 2016;154:433)
- Negative margin status is associated with long term survival (Am J Otolaryngol 2012;33:226)
- May be associated with squamous cell carcinoma, particularly if supraglottic and either high grade or solid variants; these cases are extremely aggressive (Ann Thorac Surg 2004;78:1889)
Case reports
- 33 year old woman nonsmoker with subglottic stenosis (Auris Nasus Larynx 2016;43:562)
- 40 year old woman nonsmoker with coexisting bronchoconstriction and asthma (Oncol Lett 2015;10:2303)
- 44 year old woman treated with partial laryngectomy without neck dissection for pT3 adenoid cystic carcinoma (Oncol Lett 2015;10:2303)
- 55 year old man nonsmoker with prelaryngeal pain (Acta Otorhinolaryngol Ital 2009;29:279)
- 70 year old man with a history of smoking and coexisting diabetes mellitus and chronic renal failure (Oncol Lett 2018;16:2783)
Treatment
- Surgery, but clinical margins are difficult due to prevalent perineural invasion (Am J Otolaryngol 2012;33:226)
- Recurrent lesions are associated with unfavorable clinical course (Head Neck 2008;30:919)
- Efficacy of radiotherapy and the radiosensitivity of adenoid cystic carcinoma is not clear (Otolaryngol Head Neck Surg 2016;154:433)
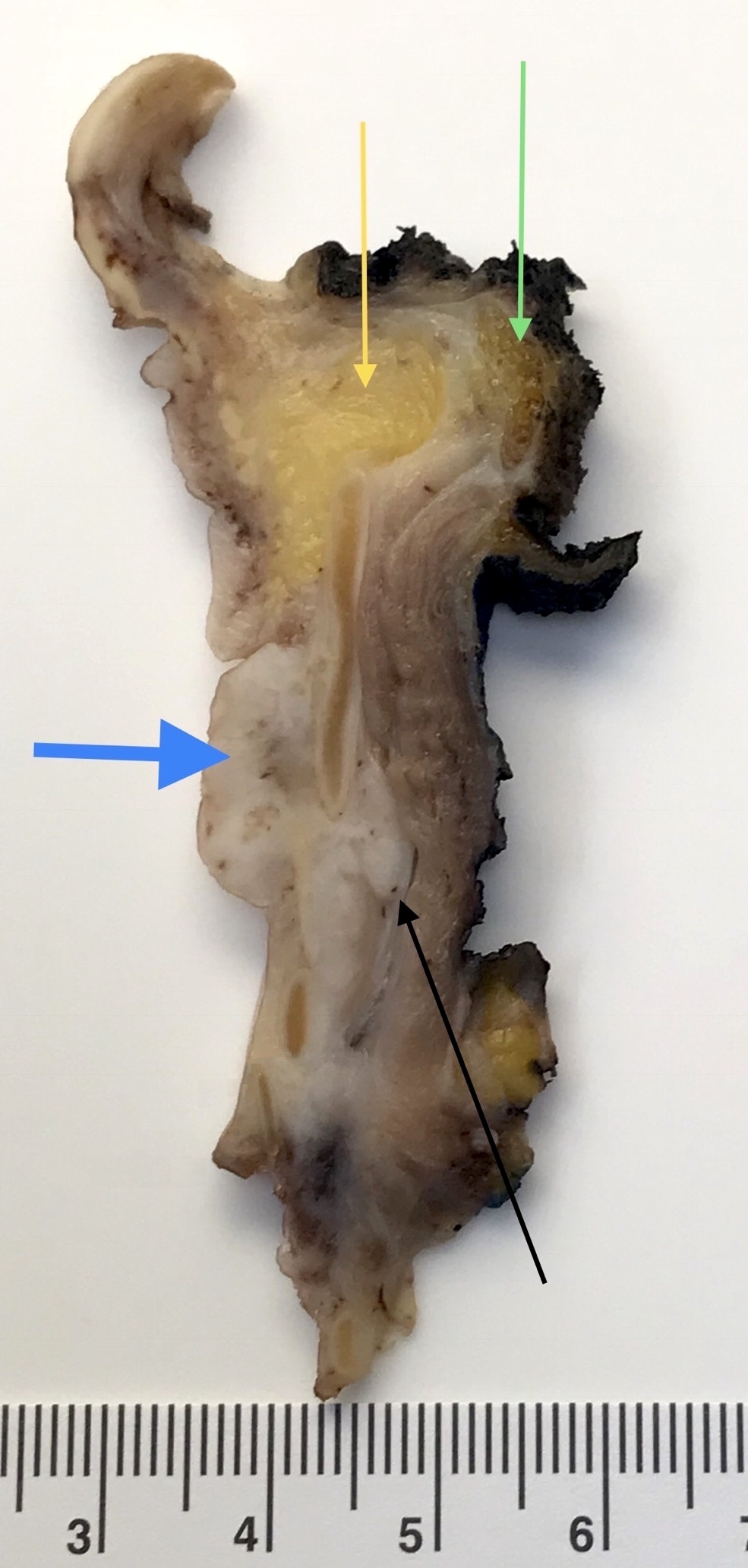
Gross description
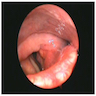
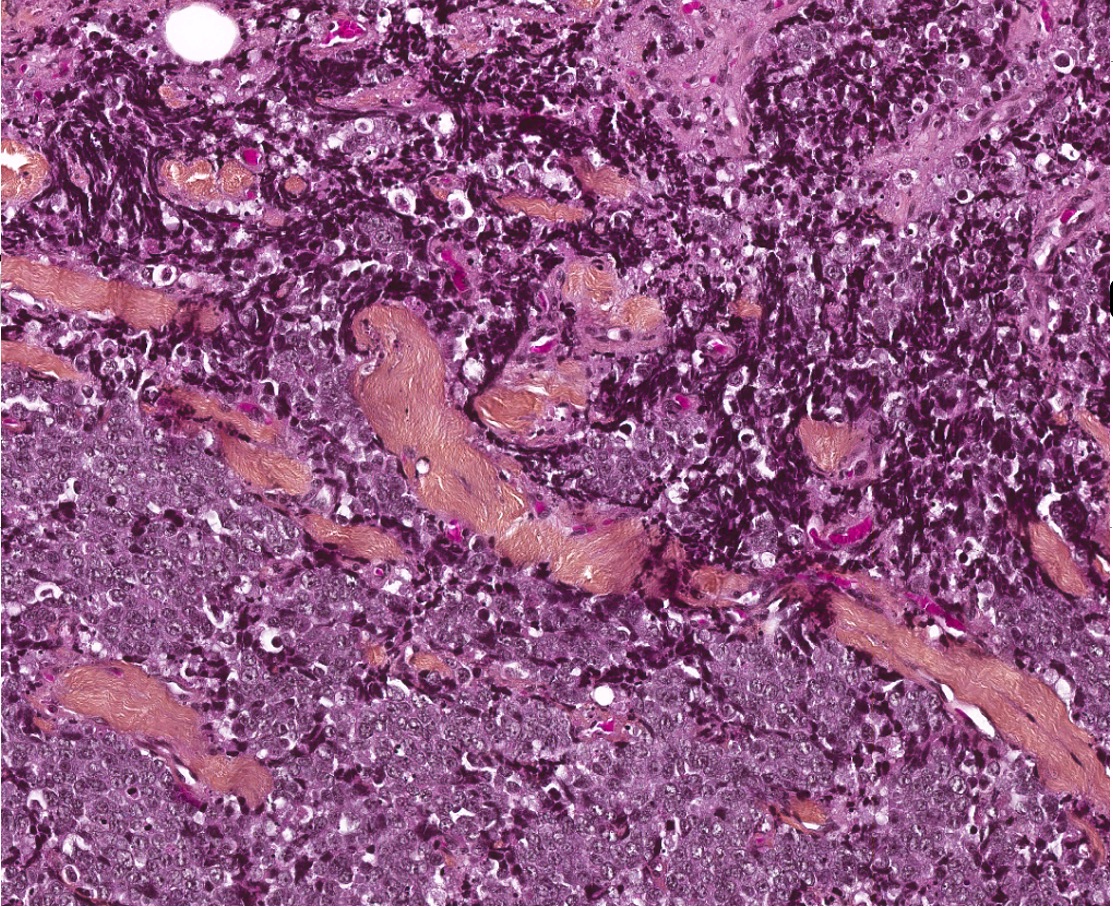
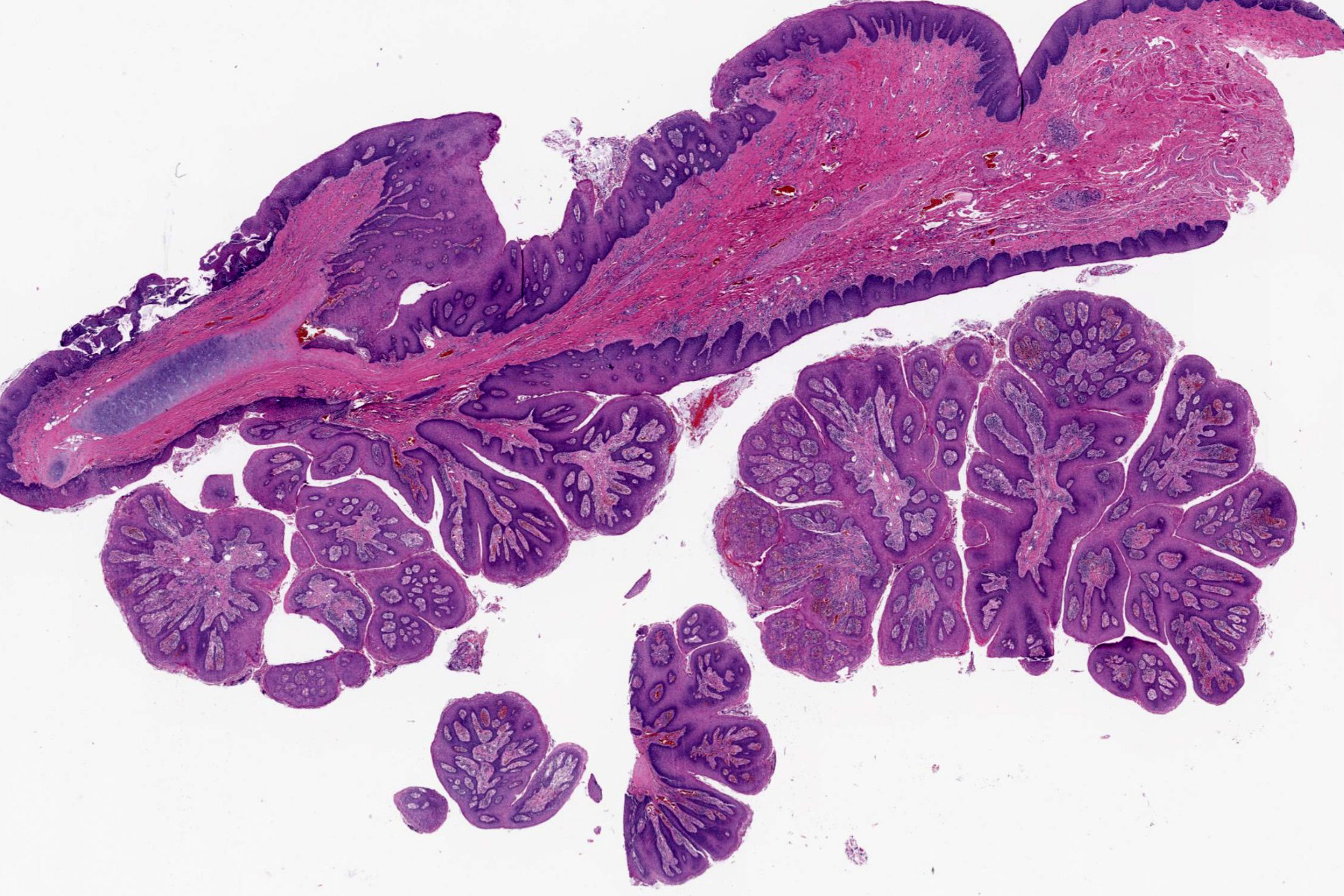
- Smooth intact surface without ulceration (Oncol Lett 2015;10:2303)
- Firm submucosal mass (Head Neck 2008;30:919)
- Frequent lateral submucosal spread, resulting in detection only at late stage (Am J Otolaryngol 2012;33:226)
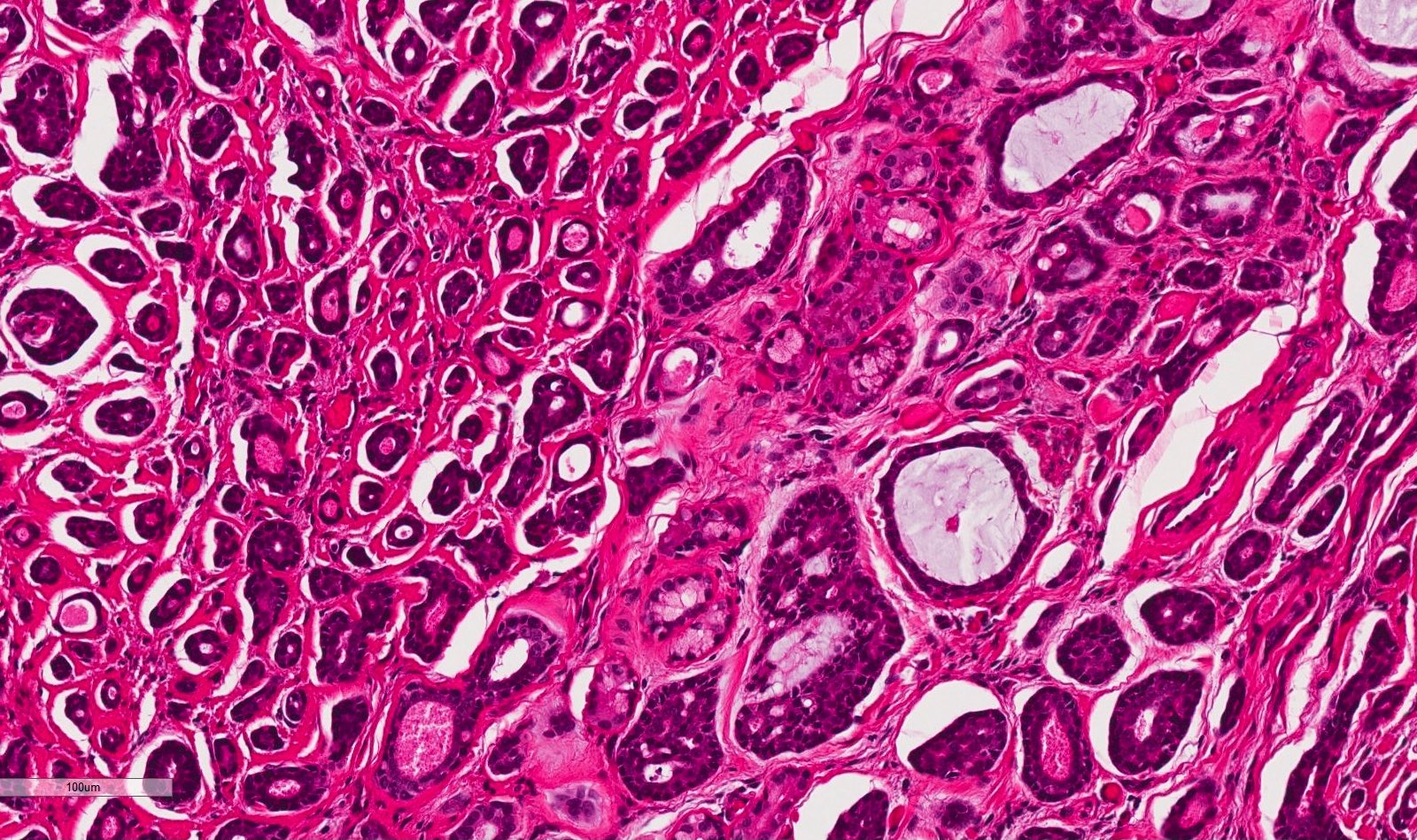
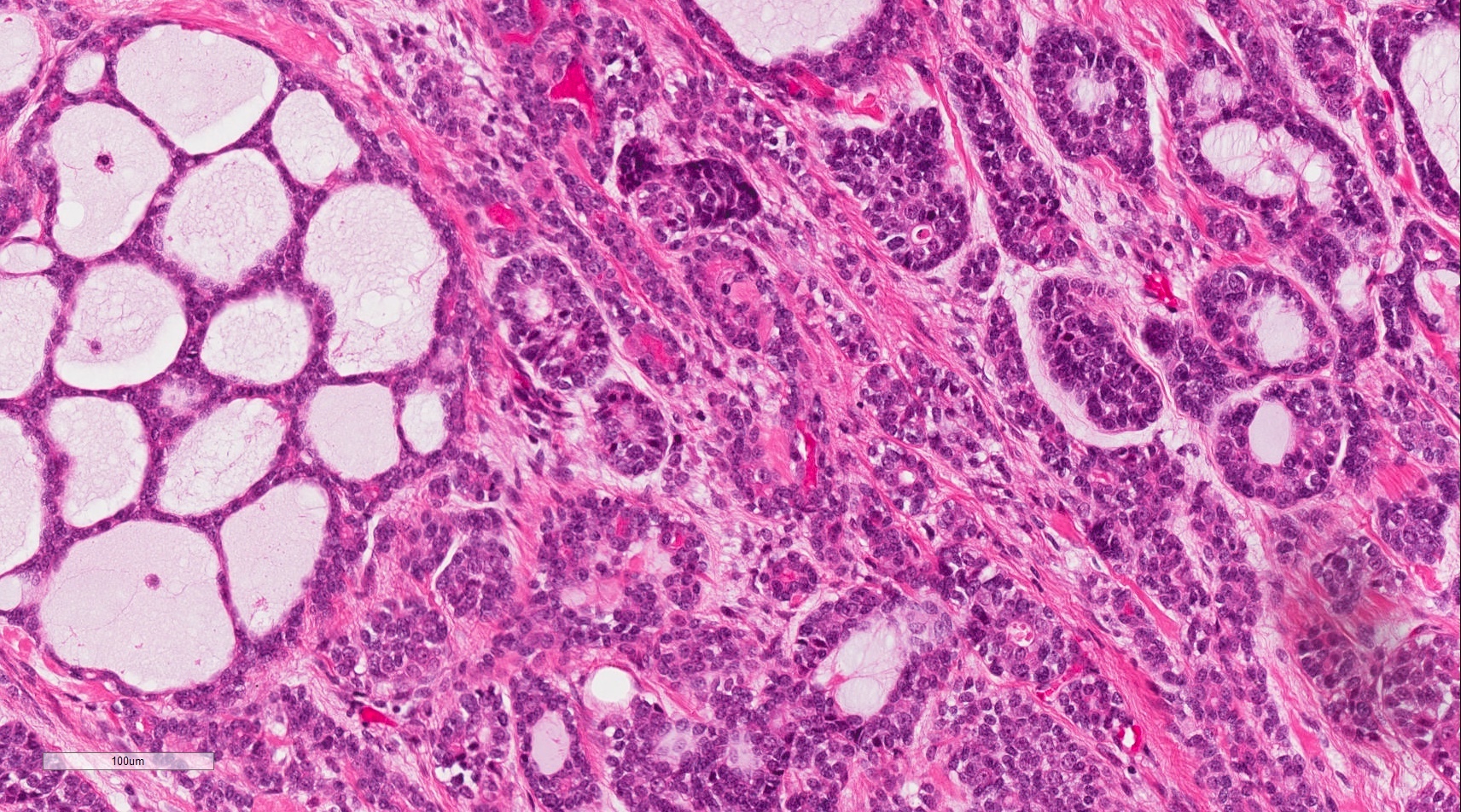
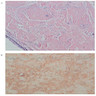
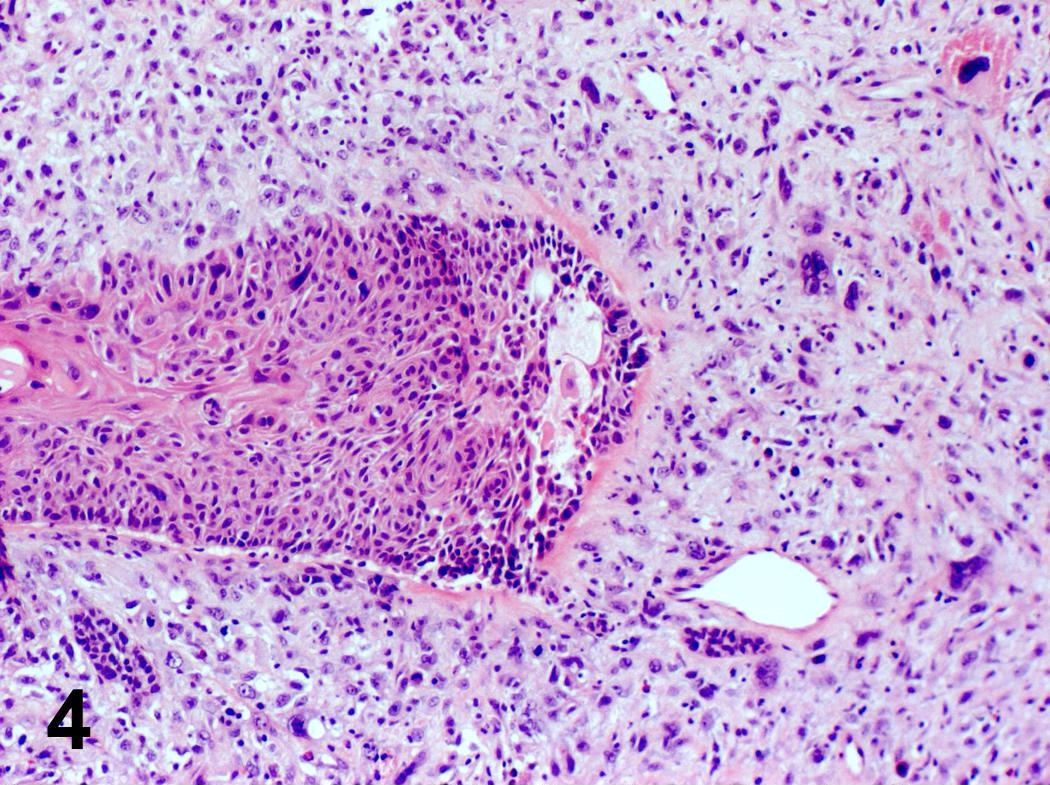
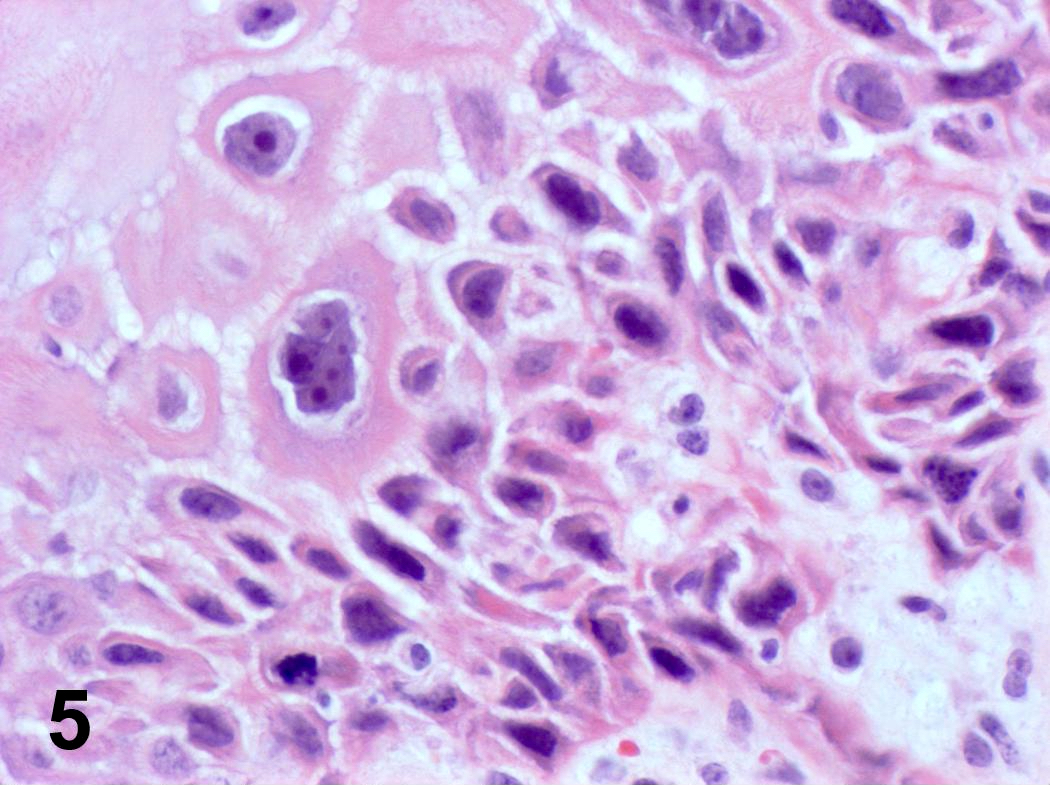
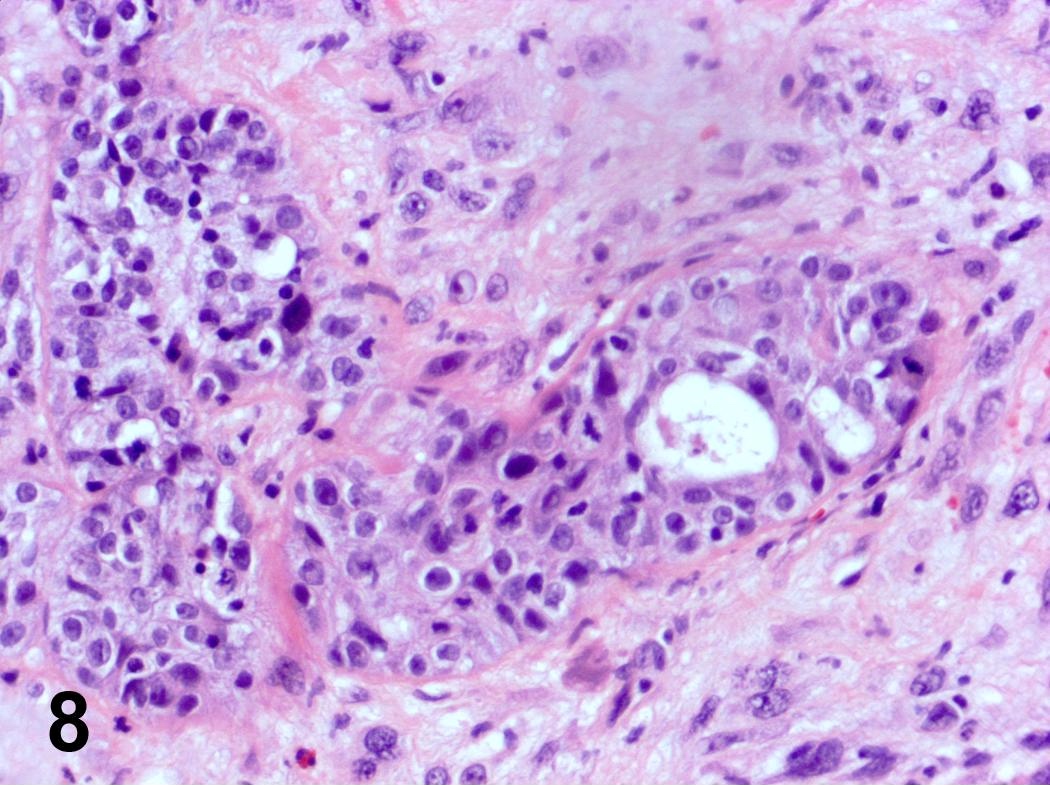
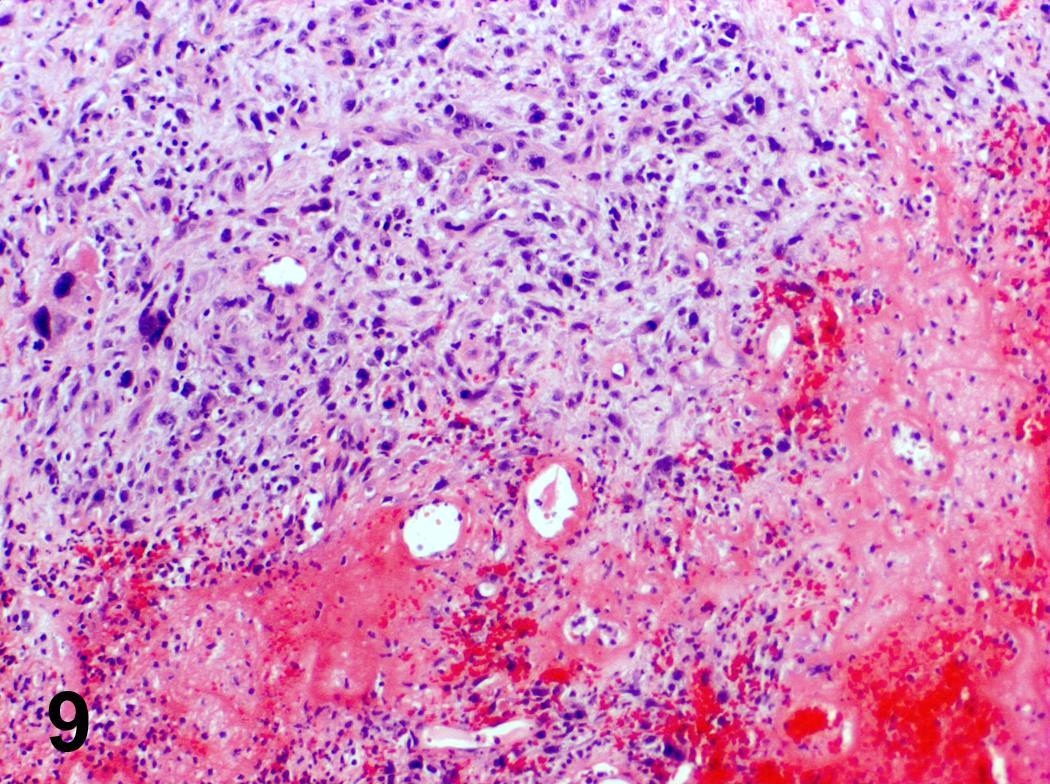
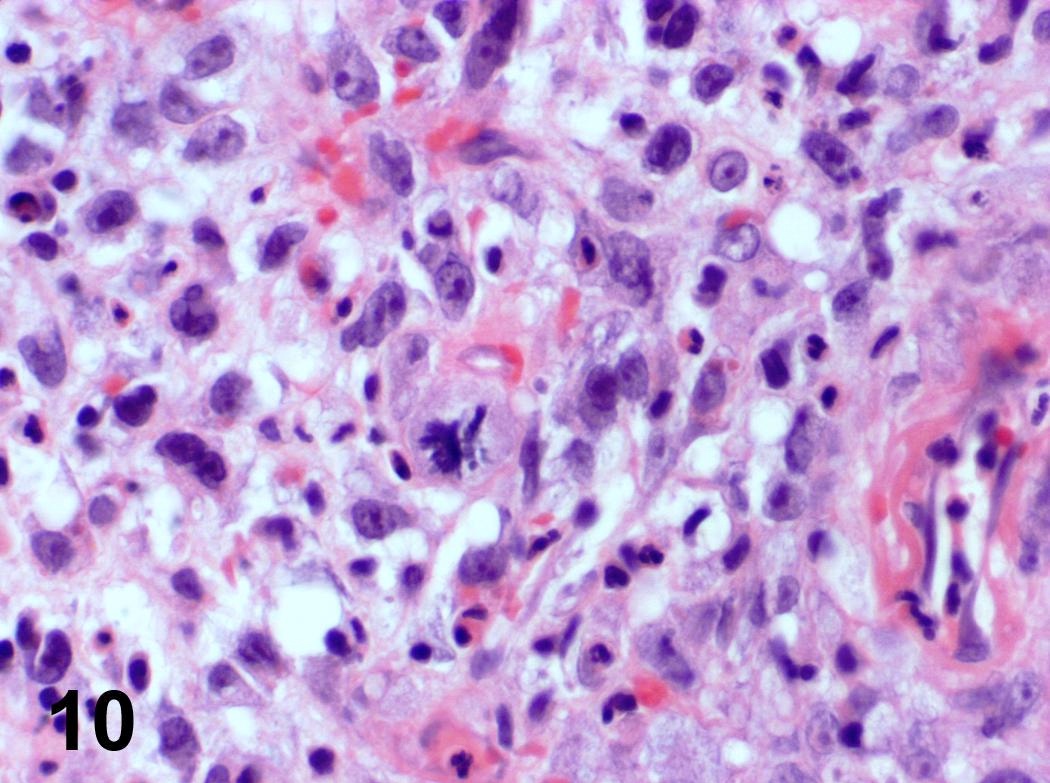
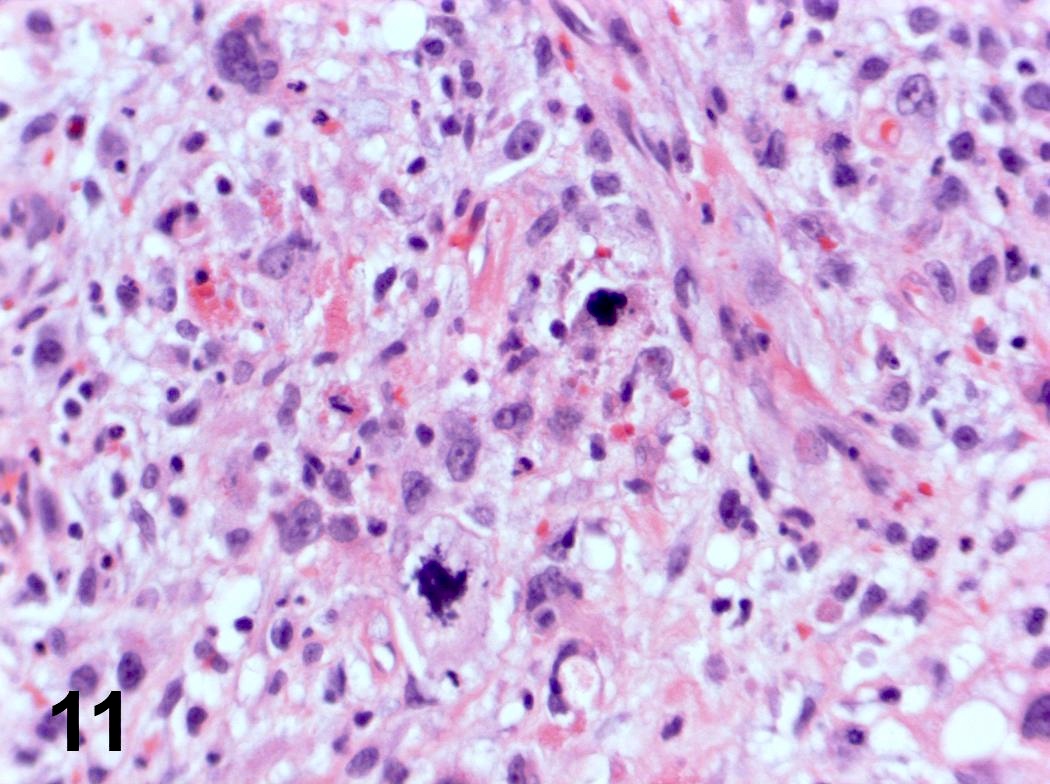
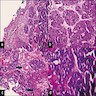
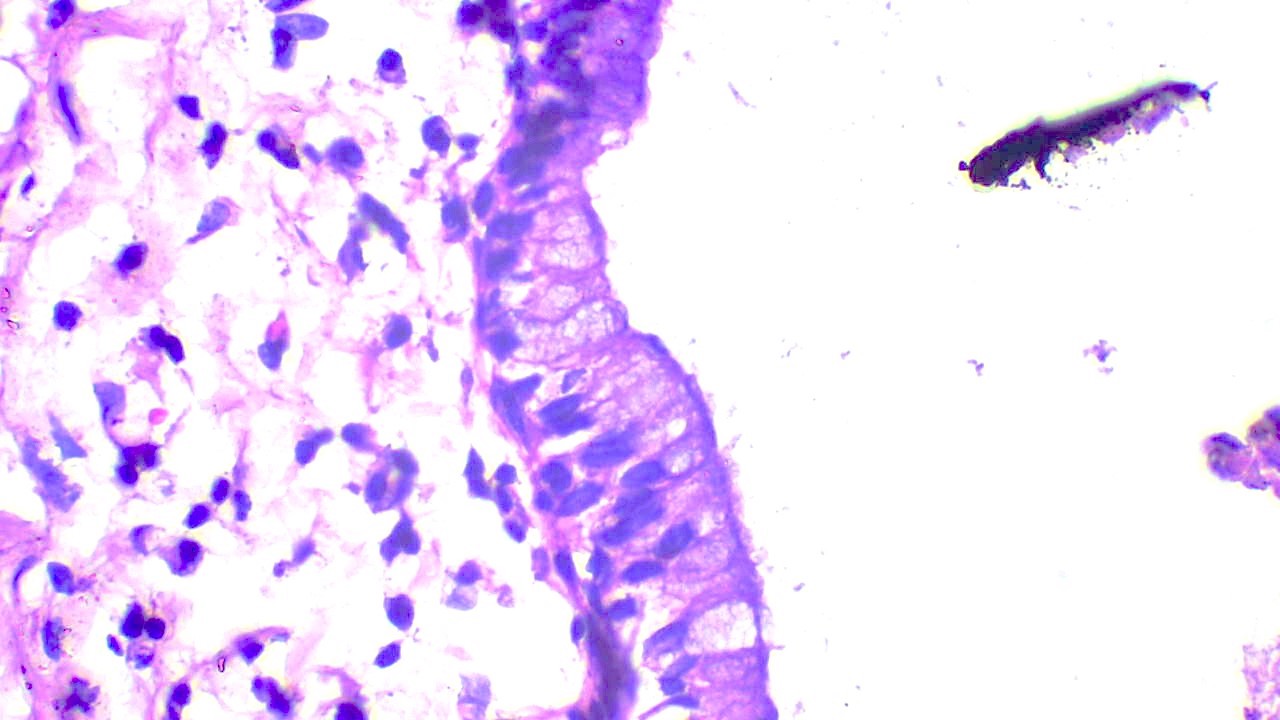
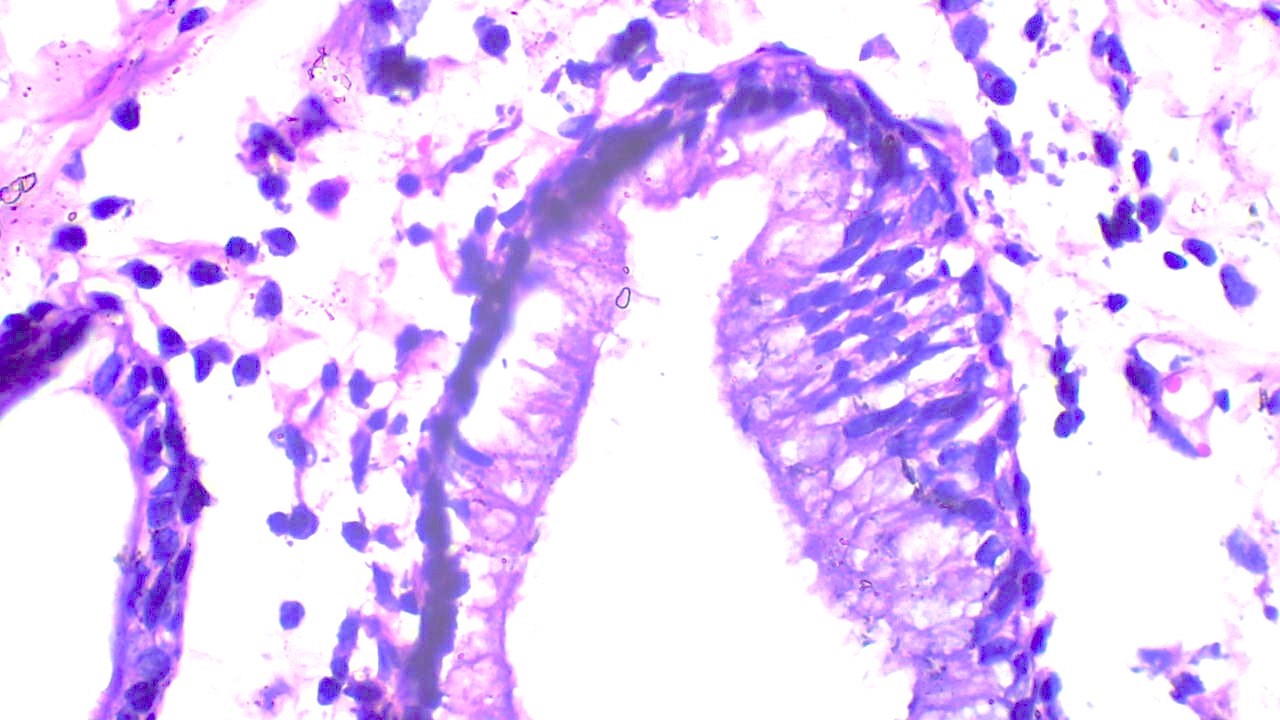
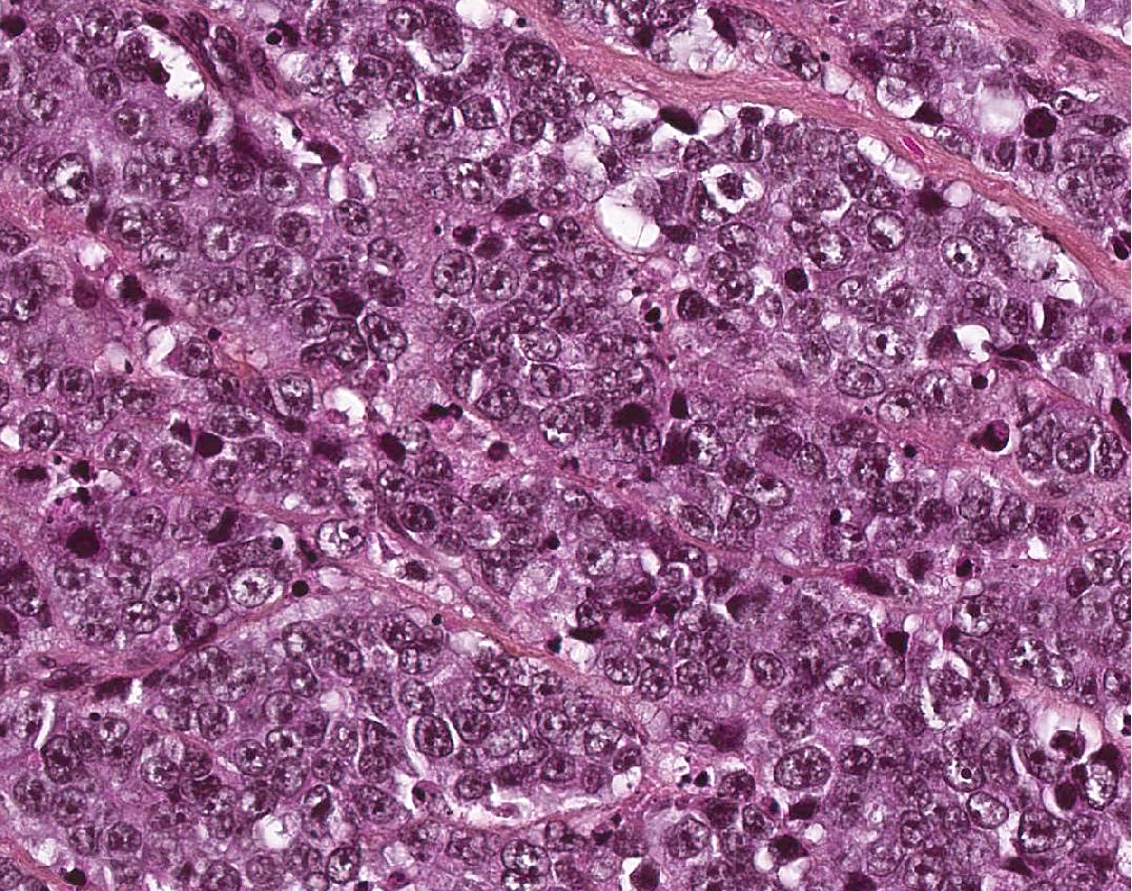
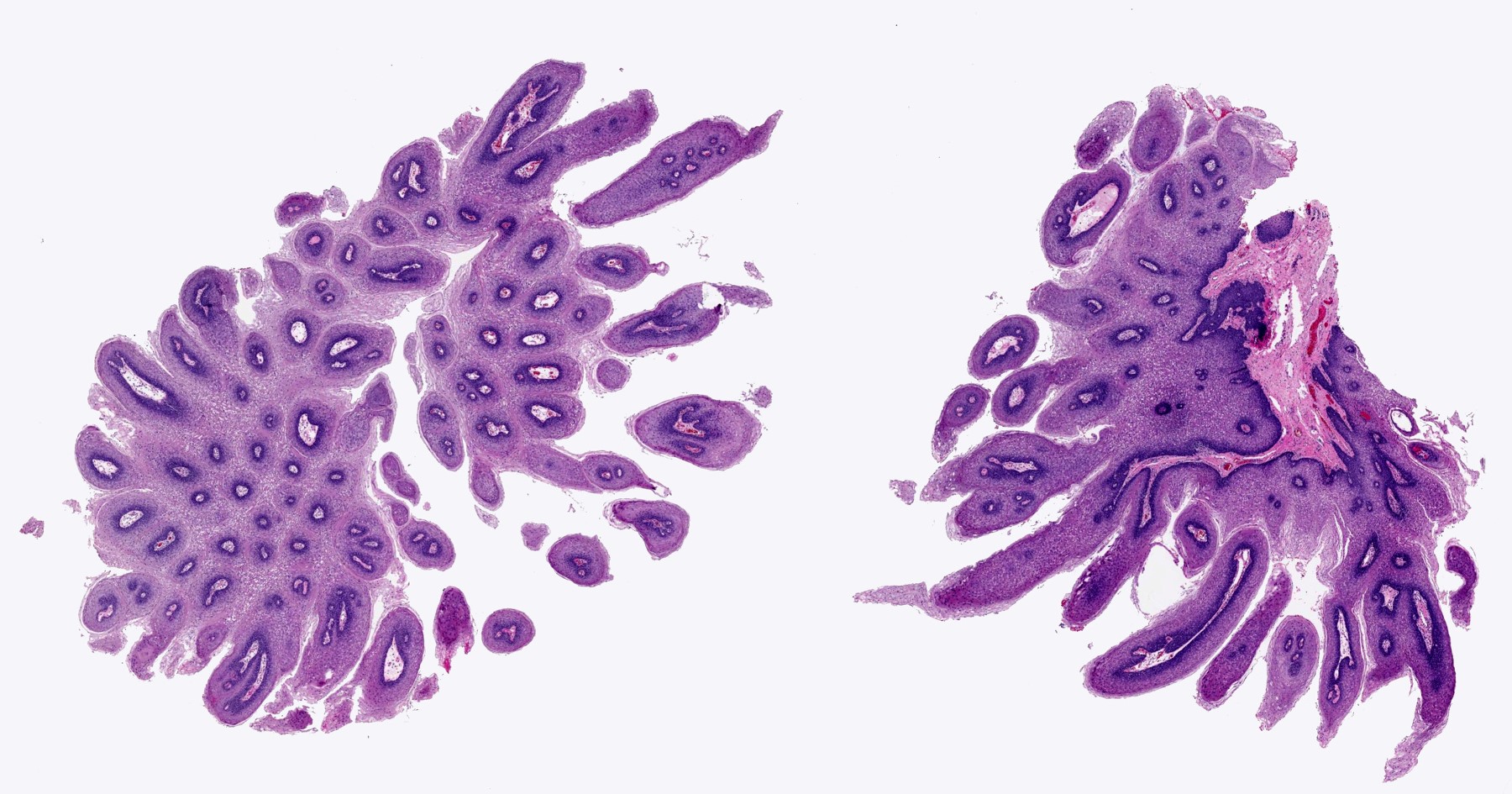
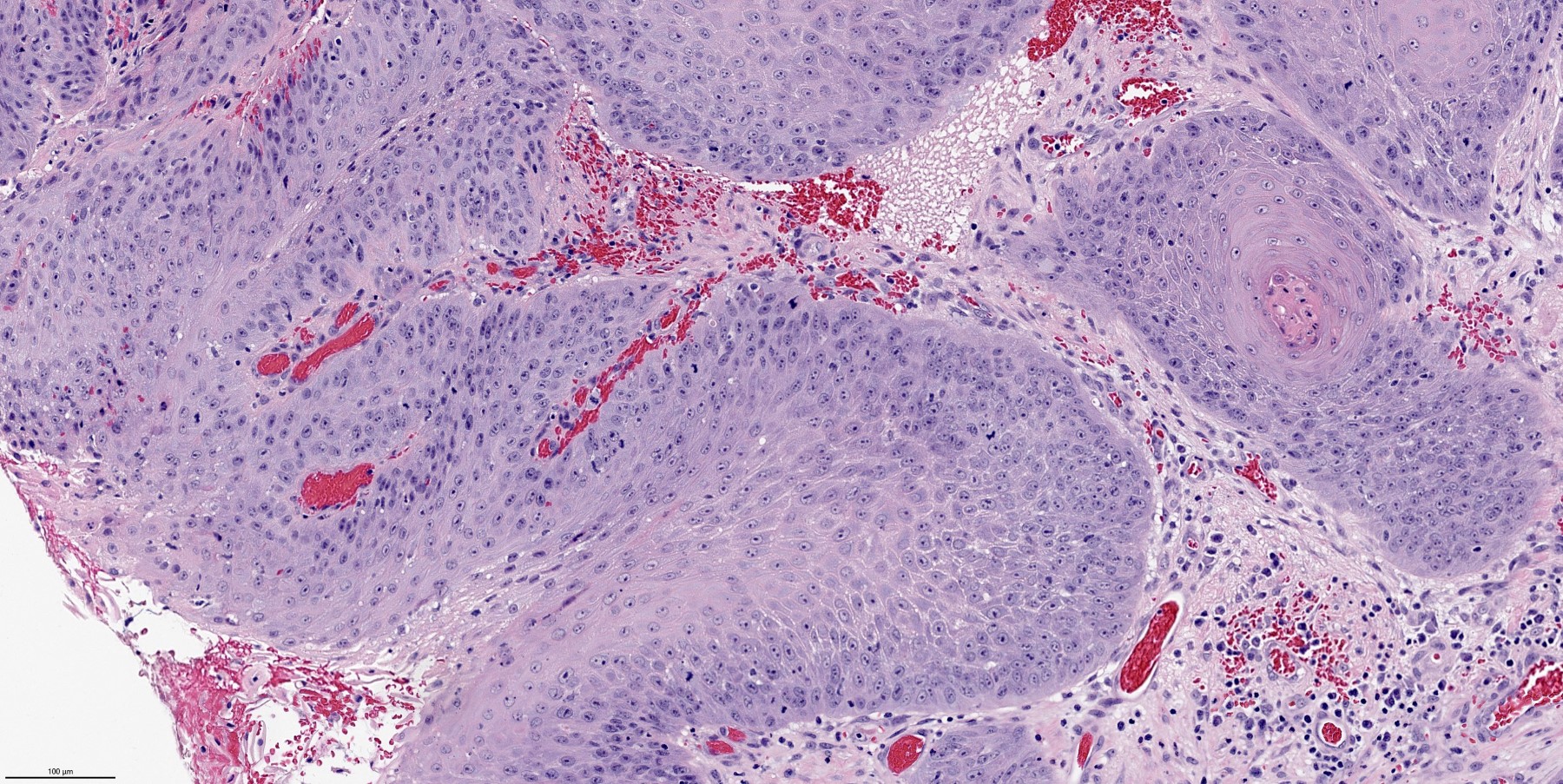
Microscopic (histologic) description
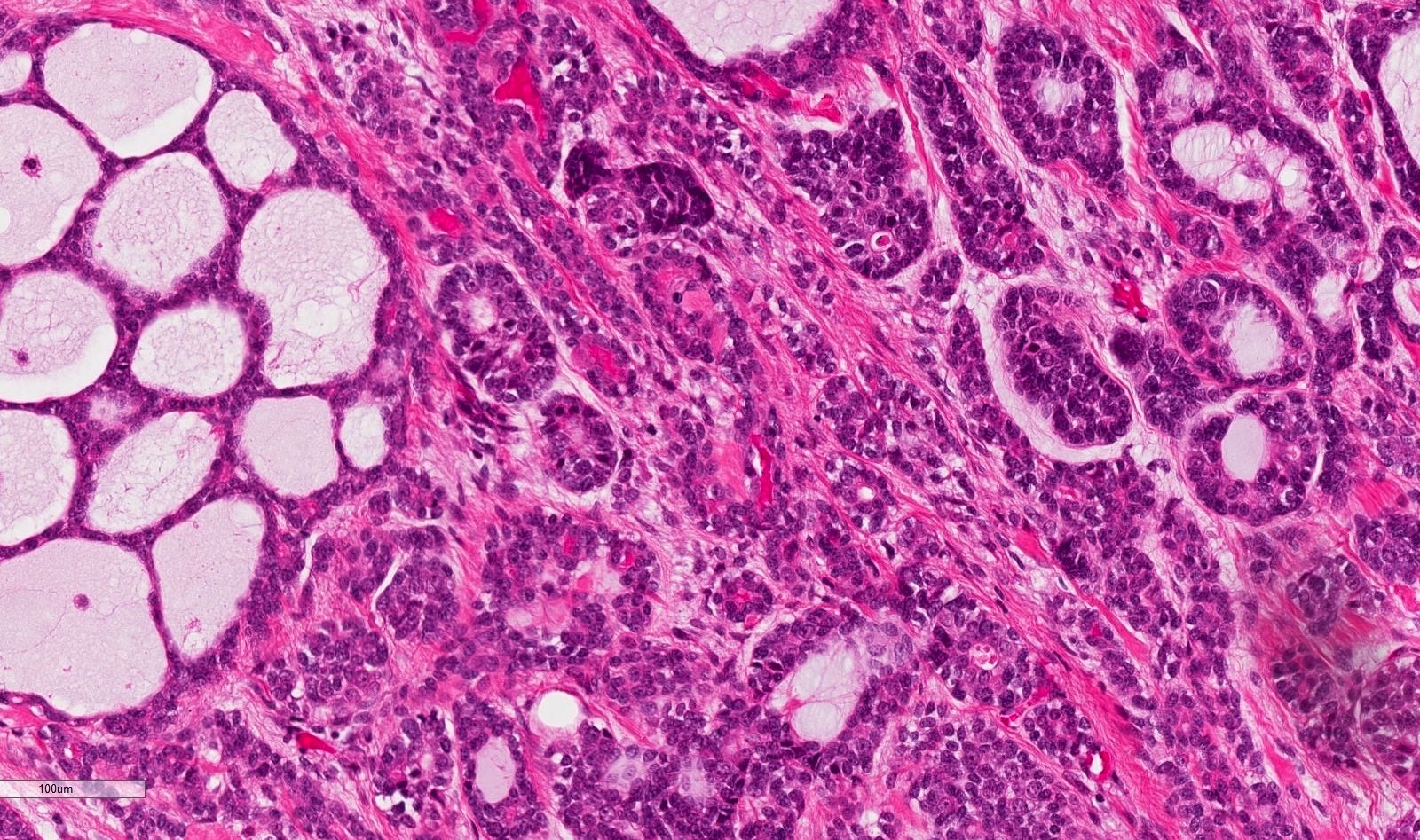
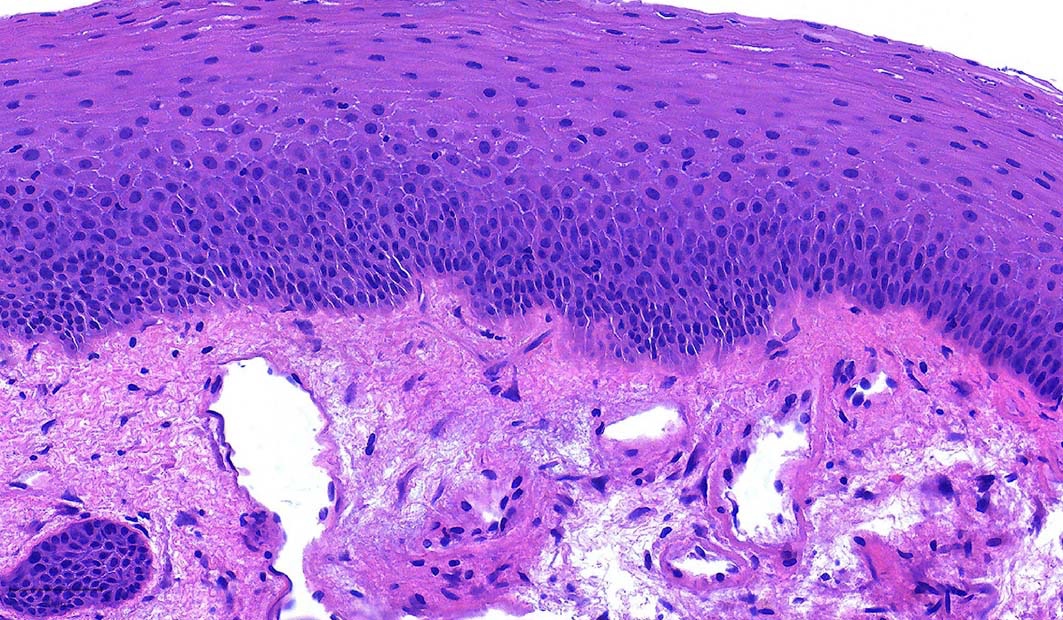
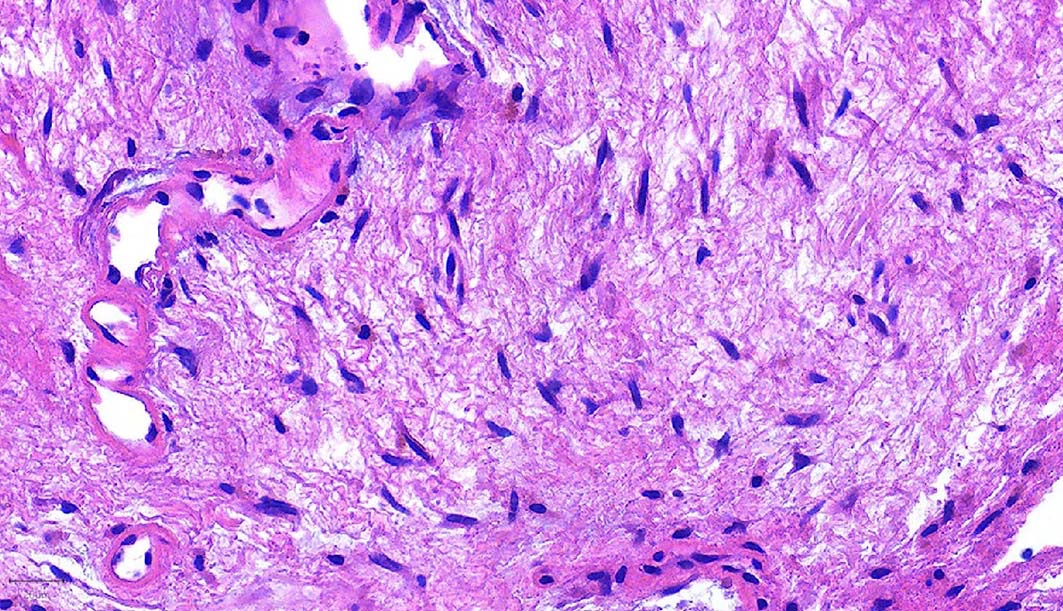
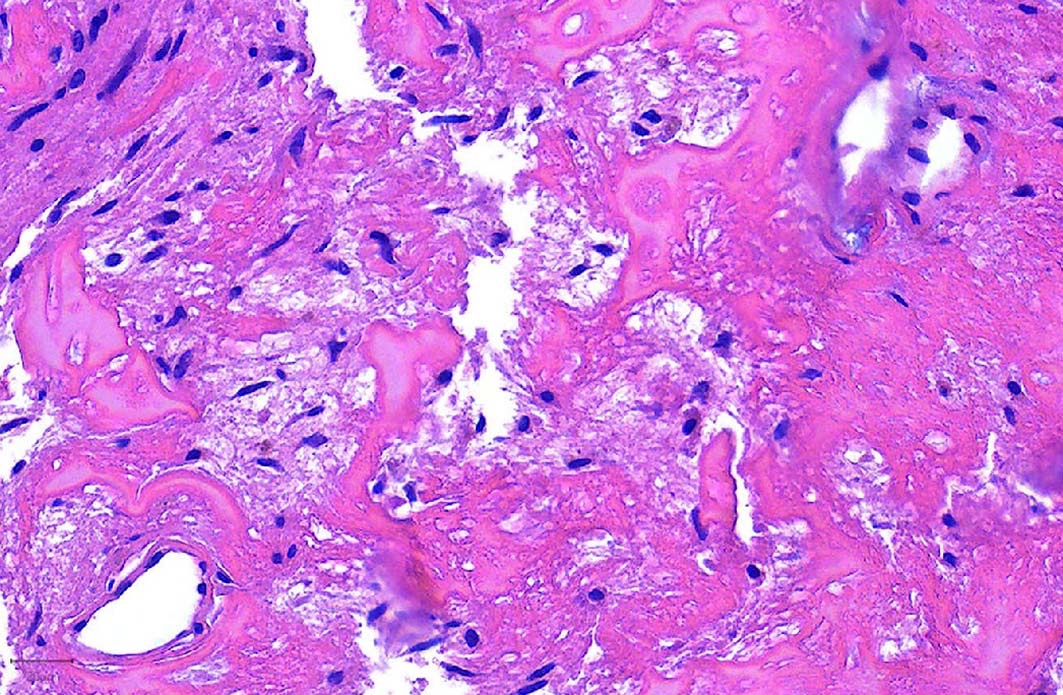
- Ductal cells: hyperchromatic angular nuclei, may have clear cytoplasm (Oral Oncol 2006;42:57)
- Myoepithelial cells: flattened nuclei (Oral Oncol 2006;42:57)
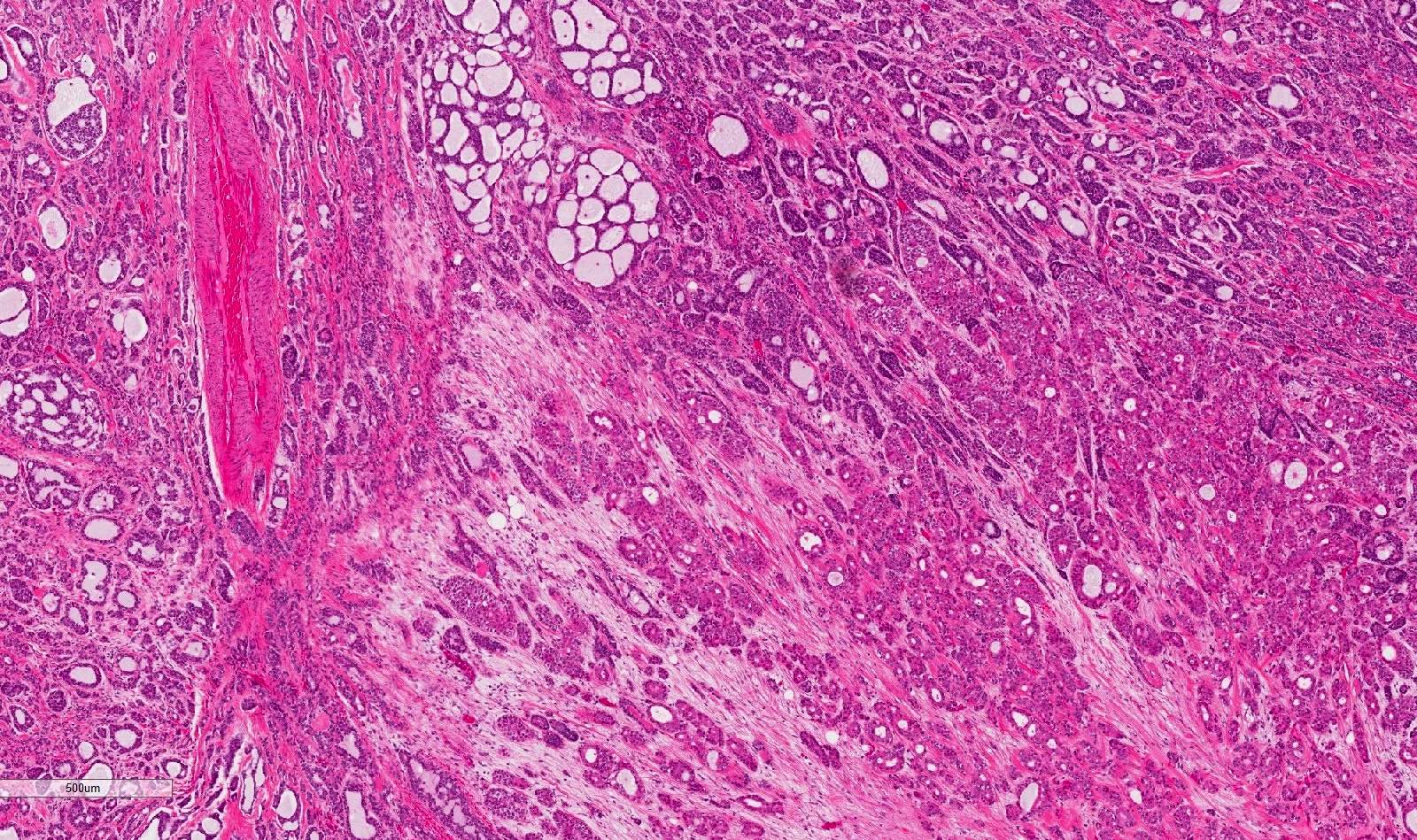
- Tubular pattern: multiple duct-to-tubule-like structures lined by multiple layers consisting of ductal or myoepithelial cells or both, uniform small cuboidal tumor cells, few mitotic figures (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:495)
- Cribriform pattern: islands of epithelioid basal cells, cystic spaces contain mucoid or hyalinized material, uniform small cuboidal tumor cells, few mitotic figures (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:495)
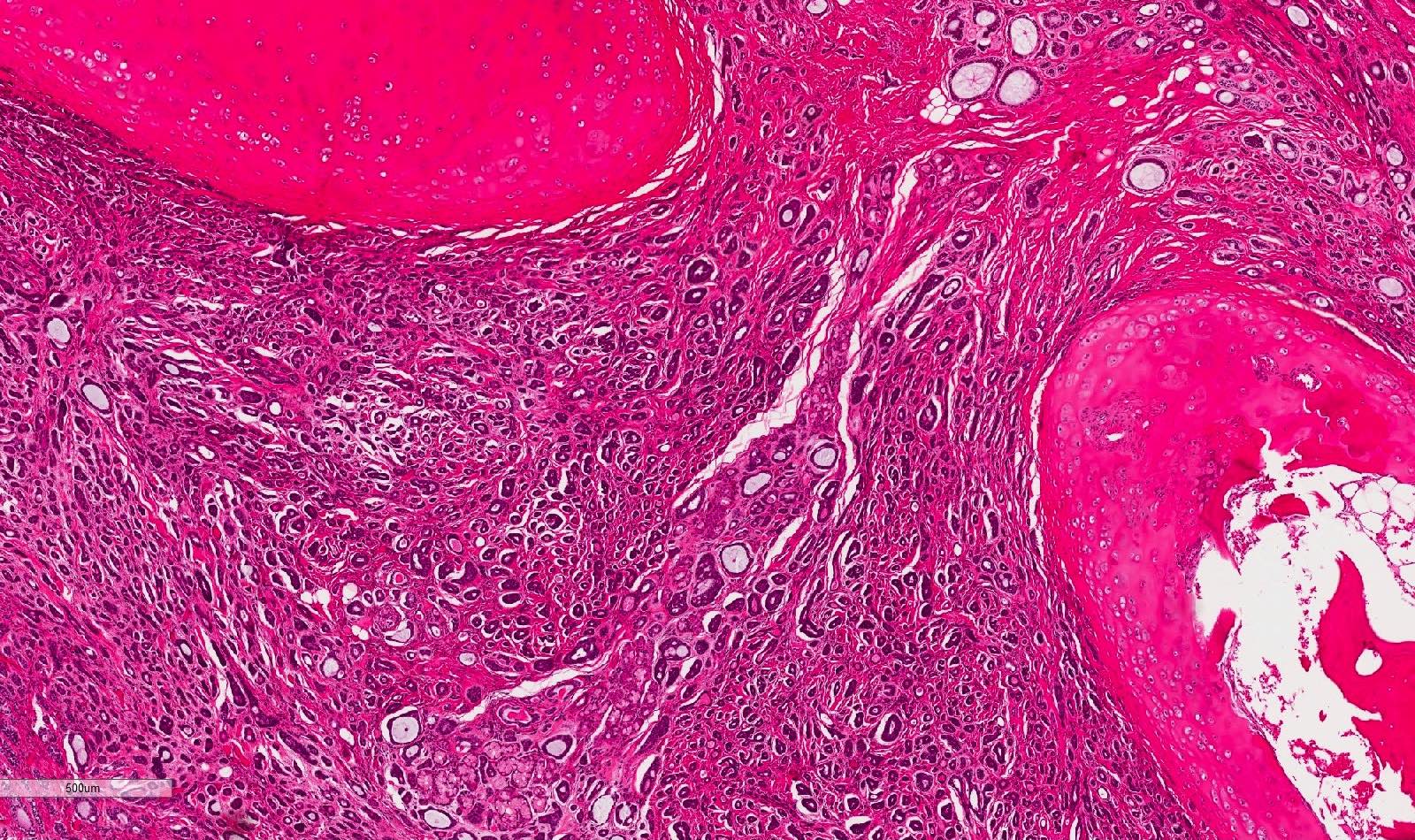
- Solid pattern: large solid sheets of tumor cells, may have focal central necrosis within tumor islands, pleomorphic tumor cells, many mitotic figures (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:495)
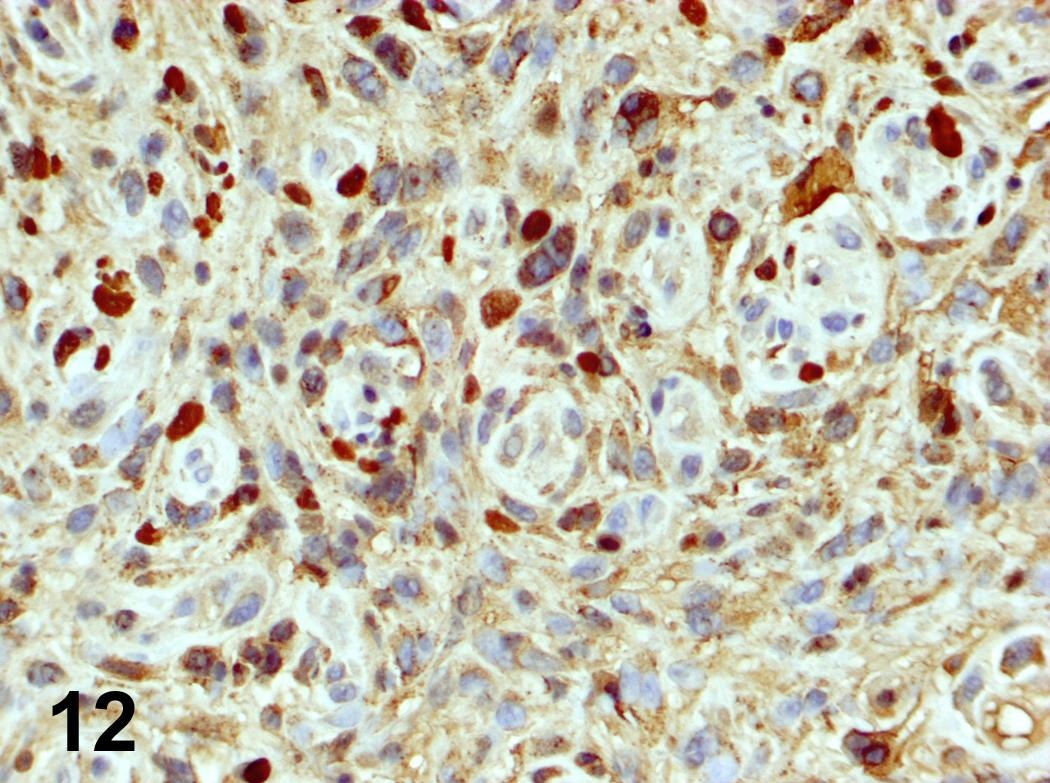
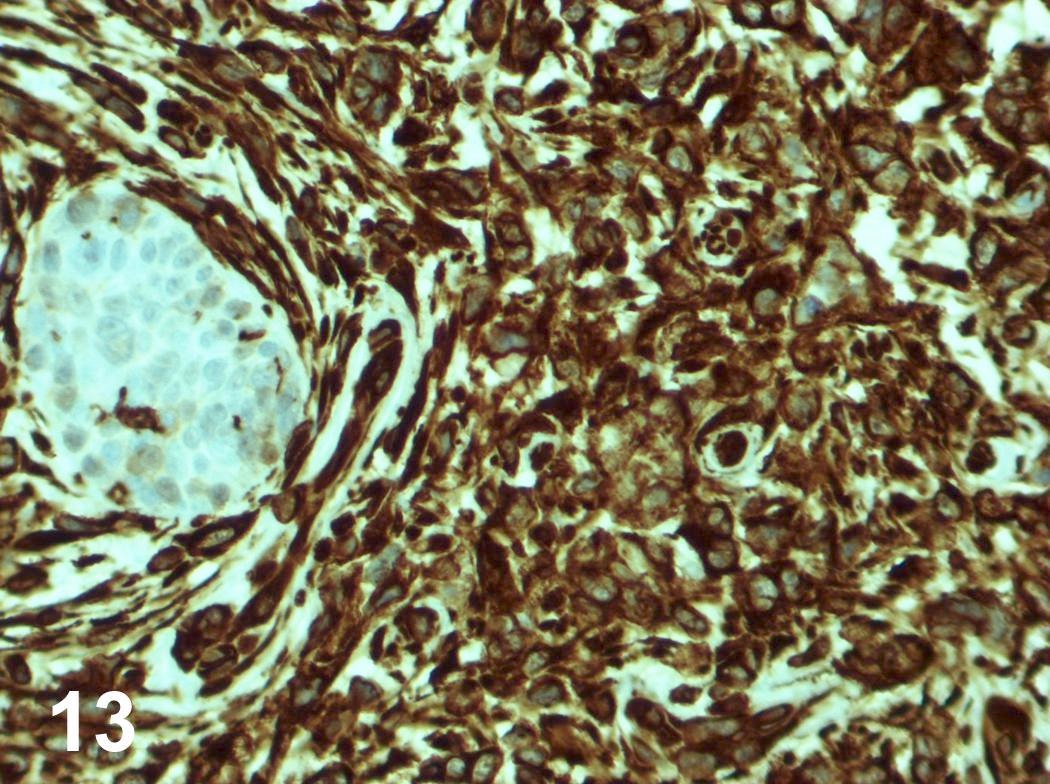
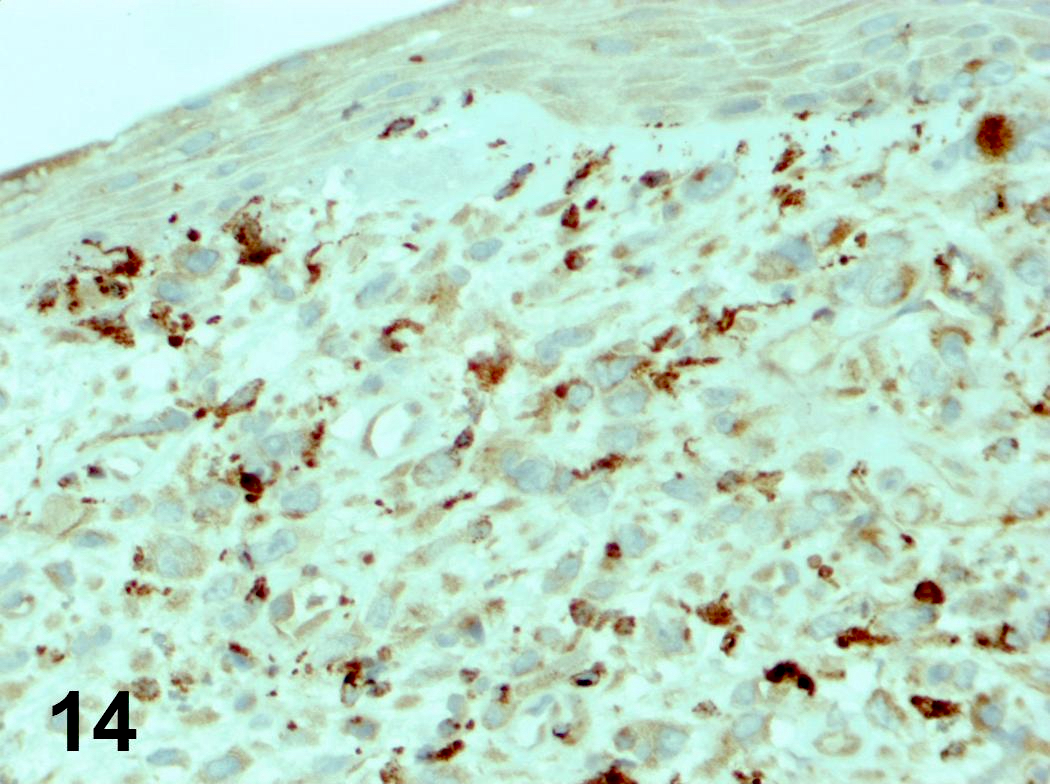
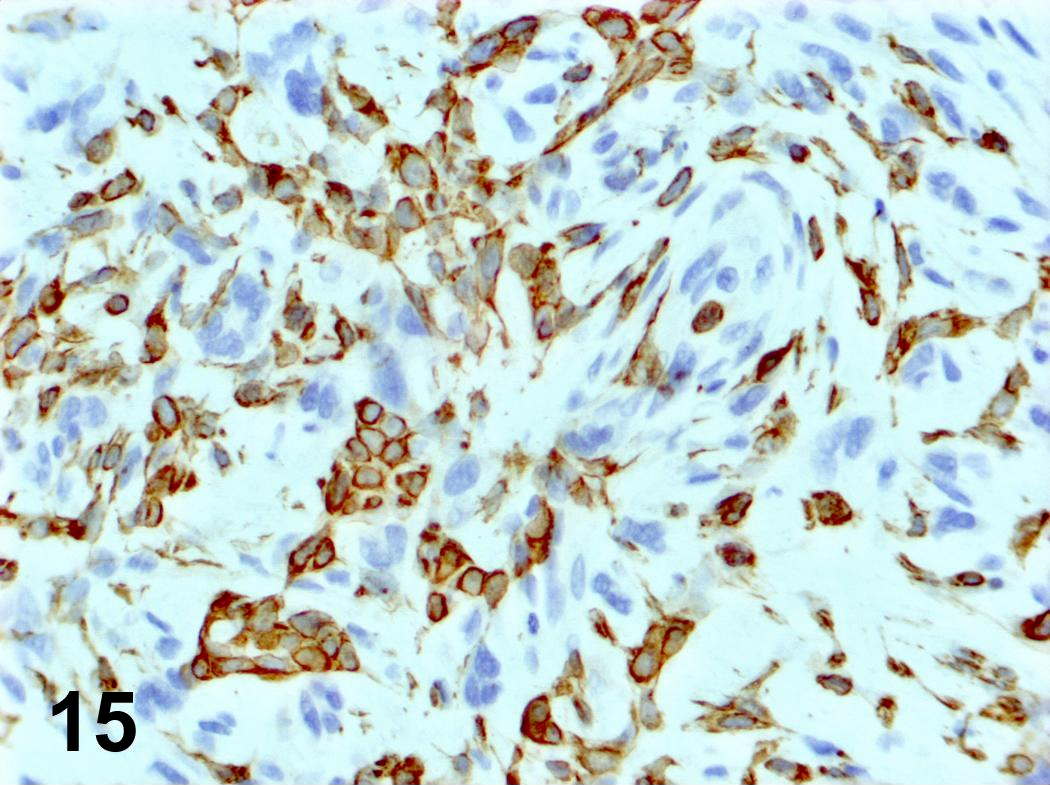
Microscopic (histologic) images
Positive stains
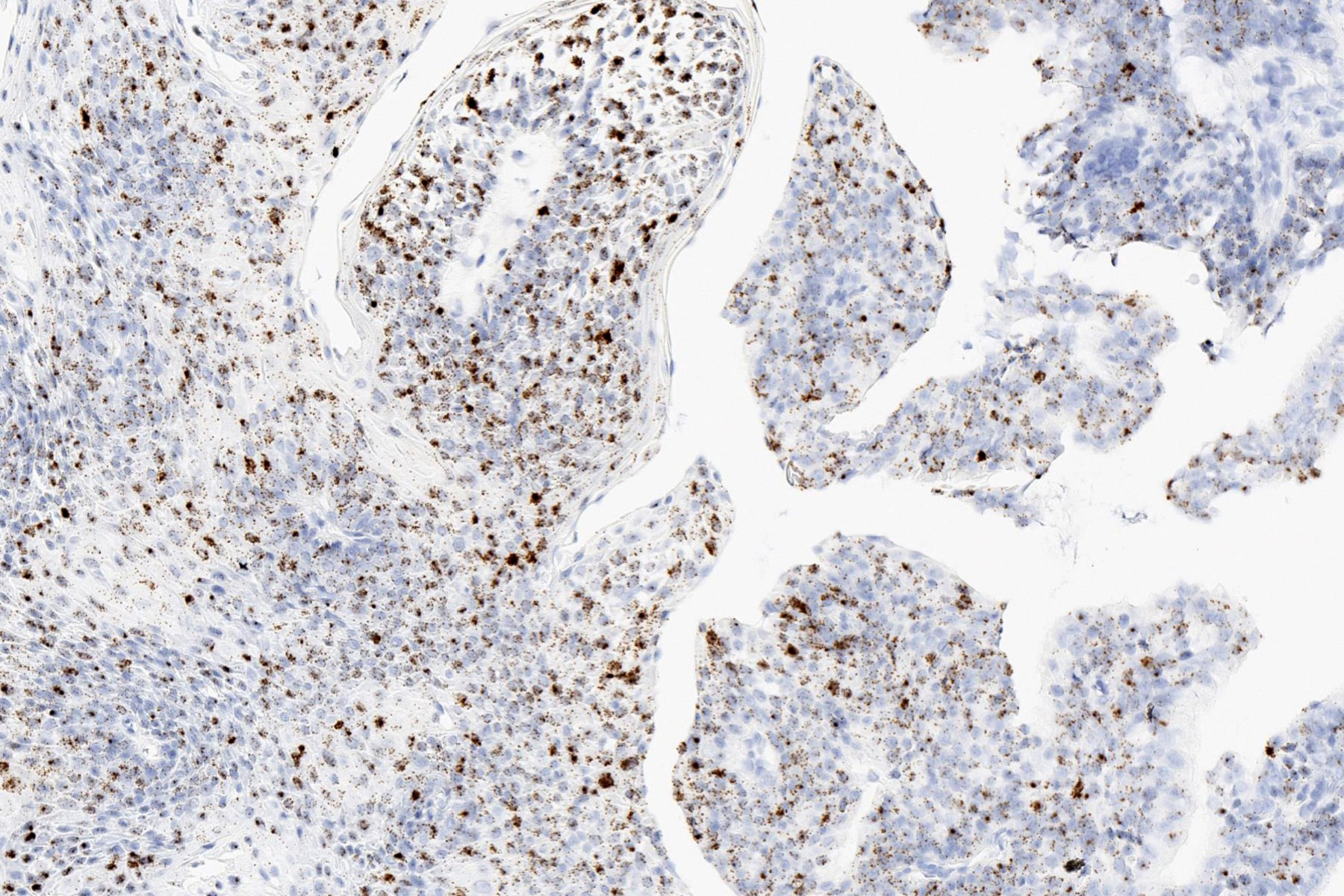
- Ductal: CK7, EMA, SOX10
- Myoepithelial: p63, SMA, calponin
- CD43 (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:495), CD117 / KIT (Oral Oncol 2006;42:57)
Molecular / cytogenetics description
- Associated with MYB-NFIB fusion gene (Mod Pathol 2011;24:1169)
- More recently MYBL1-NFIB fusion gene has also been identified (Oncotarget 2016;7:66239)
Sample pathology report
- Larynx, resection:
- Adenoid cystic carcinoma, differentiated tubular and cribriform growth patterns (see synoptic report)
- Maximum tumor size: at least 9 cm
- Tumor extensively involves lateral paratracheal and paralaryngeal margins
- Vocal cords and pyriform sinus negative for malignancy
- Perineural invasion present
Differential diagnosis
- Pleomorphic adenoma:
- Multiple patterns of growth and bland
- Polymorphous adenocarcinoma:
- Negative for CD117 / KIT staining
- Epithelial myoepithelial carcinoma:
- Consistent biphasic pattern of ductal cells with surrounding myoepithelial cells
- Basaloid squamous cell carcinoma:
- Peripheral palisading around nests with central comedonecrosis surrounded by hyalinized stroma
Board review style question #1
- Which of the following is true about this adenoid cystic carcinoma of the larynx?
- Immunohistochemical staining for CD117 will be negative in tumor cells
- Perineural invasion is extremely rare in this type of tumor
- The cystic spaces contain mucoid or hyalinized material
- The primary site of laryngeal adenoid cystic carcinoma is the vocal cords themselves
- This tumor is clinically aggressive, reflected by the high mitotic rate and basaloid cells
Board review style answer #1
C. The cystic spaces contain mucoid or hyalinized material. These spaces often contain a mildly basophilic mucoid material, a hyalinized eosinophilic product or a combined mucoid hyalinized appearance.
Comment Here
Reference: Adenoid cystic carcinoma
Comment Here
Reference: Adenoid cystic carcinoma
Board review style question #2
- What is the most common malignant minor salivary gland tumor of the larynx?
- Acinic cell carcinoma
- Adenoid cystic carcinoma
- Mucoepidermoid carcinoma
- Pleomorphic adenoma
- Polymorphous adenocarcinoma
Board review style answer #2
B. Adenoid cystic carcinoma. While minor salivary gland tumors of the larynx are rare overall, adenoid cystic carcinoma is the most common malignant entity in this location.
Comment Here
Reference: Adenoid cystic carcinoma
Comment Here
Reference: Adenoid cystic carcinoma
Adenosquamous carcinoma (pending)
[pending]
Amyloidosis-larynx
Table of Contents
Definition / general | Case reports | Laboratory | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Additional referencesDefinition / general
- 1% of laryngeal tumors; usually asymptomatic
- Mean age 38 years, more common in men
- Usually localized but may be associated with multifocal disease
- Does not develop into myeloma but may recur locally and occasionally cause death after a prolonged period
- May be due to immunocyte dyscrasias or MALT lymphoma (since lymphocytes are monoclonal)
Case reports
- 41 year old woman with apolipoprotein AI amyloidosis (Am J Pathol 2000;156:1911)
Laboratory
- Usually negative for monoclonal proteins by serum or urine electrophoresis
Treatment
- Surgical excision
Gross description
- Polypoid or granular lesions, mean 1.6 cm
Microscopic (histologic) description
- Acellular amorphous eosinophilic infiltrate in stroma, often accentuated around vessels and seromucous glands
- Sparse lymphoplasmacytic infiltrate
Microscopic (histologic) images
Positive stains
- Congo red; light chain restriction
Additional references
Amyloidosis-trachea
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- Presents as either diffuse narrowing (circumferential thickening of wall) of airway or solitary / multiple nodules (pseudotumoral)
- Associated with laryngeal or nasal involvement
Clinical features
- Symptoms of asthma, atelectasis, hemoptysis, obstruction
- May induce tracheomalacia in rheumatoid arthritis
- May be due to myeloma, lymphocytic interstitial pneumonia, lymphoplasmacytic lymphomas, plasma cell dyscrasias
- Does not usually evolve into systemic amyloidosis
- 15 - 40% die at mean 9 years after diagnosis from respiratory failure, pulmonary hemorrhage, pneumonia
Case reports
- 49 year old man with chronic cough and left lung hilar mass (Arch Pathol Lab Med 2003;127:e420)
- 50 year old man with nasal obstruction (Nihon Kyobu Shikkan Gakkai Zasshi 1997;35:1378)
- 53 year old man with tracheobronchopathia osteochondroplastica and AA amyloidosis (Yonsei Med J 2009;50:721)
- 60 year old woman with pseudotumoral tracheobronchial amyloidosis mimicking asthma (J Med Case Rep 2012;6:40)
- 64 year old woman with history of myasthenia gravis and type 1 diabetes mellitus (AJNR Am J Neuroradiol 2007;28:1557)
- 69 year old woman with seropositive erosive RA and bronchopneumonia (Clin Rheumatol 2008;27:807)
- 70 year old man with tracheobronchial circumferential wall thickening and mediastinal fat infiltration (Clin Nucl Med 2011;36:723)
- Cases with involvement of upper third of the trachea (Vestn Otorinolaringol 2008;(1):67)
- Atypical case necessitating tracheotomy (Eye Ear Nose Throat Mon 1952;31:193)
Treatment
- Laser therapy or bronchoscopic removal of deposits, radiation therapy, lung transplant
Gross description
- Focal to diffuse nodular thickening of trachea and proximal bronchial walls with patchy mural calcification
- Also extensive bronchial stenosis, postobstructive pneumonia, atelectasis
Microscopic (histologic) description
- Extensive thickening of submucosa due to irregular nodular masses or sheets of amyloid, reduced submucosal glands, calcification or osseous metaplasia of larger airways
- Variable multinucleated, osteoclast-like giant cells and plasma cells within amyloid
- Also amyloid deposition within submucosal vessel walls
Microscopic (histologic) images
Positive stains
- Congo red (apple green birefringence with polarized light)
Differential diagnosis
- Light chain deposition disease
- Pulmonary lymphoproliferative disorders
- Pulmonary scar tissue
- Systemic amyloidosis
- Tracheobronchopathia osteochondroplastica:
- Submucosal bony and cartilaginous tissue projects into tracheobronchial lumen, no amyloid
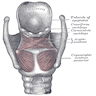
Anatomy & histology
Table of Contents
Definition / general | Staging anatomy | Structures | Diagrams / tables | Gross images | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
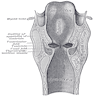
Larynx and hypopharynx
Trachea
- Tubular structure between pharynx / root of tongue and trachea at level of cervical vertebrae C4 - C6 in males, somewhat higher in females and during childhood
- Composed of cartilaginous tissue that undergoes ossification and may completely replace cartilage by age 20
- At puberty, increases in size in males due to enlargement of cartilages
- Cartilages are connected by ligaments and moved by numerous muscles
Trachea
- Composed of imperfect rings of hyaline cartilage, fibrous tissue, muscular fibers, mucous membranes and glands
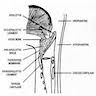
Staging anatomy
Larynx and hypopharynx
- Anterior limit is anterior or lingual surface of suprahyoid epiglottis, thyrohyoid membrane, anterior commissure and anterior wall of subglottic region (composed of thyroid cartilage, cricothyroid membrane and anterior arch of cricoid cartilage)
- Posterior and lateral limits include laryngeal aspect of aryepiglottic folds, arytenoids region, interarytenoid space and posterior surface of subglottic space (mucous membrane covering surface of cricoid cartilage)
- Superolateral limits are tip and lateral borders of epiglottis
- Inferior limit is plane passing through inferior edge of cricoid cartilage
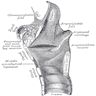
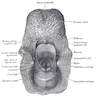
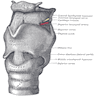
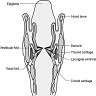
Structures
- Supraglottic portion:
- Epiglottis (lingual and laryngeal aspects), false vocal cords (ventricular bands), aryepiglottic folds (laryngeal aspect), arytenoid cartilages and ventricles
- Derived from third and fourth branchial pouches
- Inferior boundary is horizontal plane passing through lateral margin of ventricle at its junction with superior surface of vocal cord
- Glottic portion:
- True vocal cords (superior and inferior surfaces) and anterior and posterior commissures
- Derived from sixth branchial pouch
- Subglottic portion:
- Between lower border of true vocal cords and first tracheal cartilage (or lower margin of cricoid cartilage)
- Derived from sixth branchial pouch
- Anterior commissure:
- Convergence of thyroepiglottic, vestibular and vocal ligaments and conus elasticus
- Tendon provides anterior attachment for true vocal cords
- Tendon also separates glottic and supraglottic parts of larynx
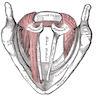
- Arytenoid cartilage:
- 2 hyaline cartilages at upper border of cricoid cartilage at back of larynx that support the vocal cords
- Each is pyramidal
- Apex is surmounted by small, conical, corniculate cartilage
- Conus elasticus:
- Extends from superior border of cricoid cartilage to free edge of vocal cord, then thickens to form vocal ligament, which runs length of true vocal cord close to mucosal surface, then continues along floor of ventricle as thyroglottic ligament
- Cricoid cartilage:
- Hyaline cartilage that is smaller but thicker and stronger than thyroid cartilage
- Upper edge is 1 cm below true vocal cords at mid larynx
- Forms the only complete trachiobronchial ring with posterior quadrate lamina (deep and broad, 2 - 3 cm high) and anterior arch that is narrow and convex
- Articulates with inferior horns of thyroid cartilage
- Cuneiform cartilages: 2 small, elongated pieces of cartilage on either side of aryepiglottic fold
- Epiglottis: thin, bicycle saddle-like and elastic fibrocartilage
- Apex is attached to inner thyroid cartilage just above anterior commissure by thyroepiglottic ligament
- Projects up behind tongue and body of hyoid bone, partly covers laryngeal entrance
- Sides are attached to arytenoid cartilages by aryepiglottic folds
- Upper and anterior surface is free, covered by mucous membrane reflected onto pharyngeal tongue and lateral wall of pharynx to form median and lateral glossoepiglottic folds
- Median glossoepiglottic fold divides area between base of tongue and epiglottis into 2 valleculae
- Not essential for respiration, phonation or deglutition
- Hypopharynx:
- Comprises posterolateral pharyngeal wall (from level of floor of valleculae to level of inferior border of cricoid cartilage), postcricoid esophagus (has anterior wall and extends from level of arytenoids cartilages superiorly to inferior border of cricoid cartilage) and pyriform sinuses (lie lateral to and below opening of larynx, each bounded laterally by medial aspect of thyroid lamina and medially by aryepiglottic fold)
- Trachea:
- Cartilaginous and membranous tube (also called windpipe), extending from lower larynx at C6 to upper border of T5 vertebrae, where it divides into right and left mainstem bronchi
- Flattened posteriorly
- 11 cm long, 2 - 2.5 cm in diameter, diameter greater in men than women, greater in adults than children
- Anteriorly: contacts thyroid isthmus, inferior thyroid veins, neck muscles, cervical fascia, anterior jugular veins, manubrium sterni, thymus, left innominate vein, aortic arch, innominate and left common carotid arteries, deep cardiac plexus
- Posteriorly: contacts esophagus
- Right bronchus appears to be a more direct continuation of trachea and so is the site of most foreign bodies
- Supplied by inferior thyroid arteries
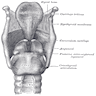
- Pre-epiglottic space: bounded posteriorly by epiglottic cartilage and thyroepiglottic ligament, anteriorly by thyroid cartilage and thyrohyoid membrane and superiorly by hypoepiglottic ligament; space communicates laterally with paraglottic space above ventricle
- Pyriform sinus: not strictly part of larynx; inverted 3 sided pyramid with apex inferiorly at level of cricopharyngeus muscle, bounded superiorly by glossoepiglottic folds, medially by aryepiglottic folds, laterally by pharyngeal wall
- Thyroid cartilage:
- Largest cartilage of larynx
- Shield shaped forming anterior surface of larynx and acute angle in midline of neck (laryngeal prominence, Adam's apple)
- Originally only hyaline cartilage but ossifies at age 25 at sites of muscle origin or insertion
- Lower edge is 1 cm below anterior commissure
- Lymphatics:
- Separate systems exist above and below ventricle
- Are also subdivided as superficial (mucosal) and deep
- Few lymphatics are present in true vocal cord
- Pyriform sinus drains laterally into deep cervical nodes, occasionally to paratracheal nodes
- Supraglottic lymphatics drain through thyrohyoid membrane into upper cervical and anterosuperior nodes
- Subglottic lymphatics drain into prelaryngeal (Delphian) node on cricothyroid membrane, then into pretracheal, paratracheal and supraclavicular nodes
Diagrams / tables
Microscopic (histologic) description
- Junction between epithelial types may be abrupt or separated by transitional area; patches of squamous epithelium in respiratory epithelium are common, particularly in smokers
- Dendritic melanocytes may be present in basal layer, particularly in blacks
- Epiglottis: stratified squamous epithelium similar to oral cavity with modified salivary glands that secrete thick mucous; laryngeal surface also has pits containing mucous glands
- Cartilage has full thickness fenestrae that communicate with pre-epiglottic space, which contains fat and areolar tissue
- False vocal cords and other supraglottic larynx: ciliated, columnar epithelium extending into ventricle of Morgagni with submucosal modified salivary gland epithelium
- Glottis: space between 2 vocal cords
- Hypopharynx: covered by nonkeratinizing stratified squamous epithelium; contains mucosal glands, scattered lymphoid aggregates and rich lymphatic plexus
- Reinke space: lamina propria of true vocal cord, between base of squamous epithelium and vocal ligament
- True vocal cords: stratified squamous epithelium with no / rare submucosal glands
- Subglottic larynx: epithelium resembles trachea / major bronchi - ciliated columnar epithelium with submucosal glands
- Trachea:
- Cartilage:
- 16 - 20 imperfect rings, with circular cartilaginous defect posterior and replaced by fibrous tissue and muscular fibers
- Each cartilage is 4 mm in depth, 1 mm in thickness
- Are elastic but may be calcified later in life
- Fibrous tissue:
- Thick layer covers outer surface of cartilaginous ring, thin layer covers inner surface
- Both layers merge at upper and lower margins of cartilaginous rings
- Muscular tissue:
- Longitudinal and transverse layers of smooth muscle
- Mucus membrane:
- Continuous with laryngeal and bronchial membranes
- Ciliated columnar epithelium overlying areolar and lymphoid tissue with elastic fibers, blood vessels, nerves, mucous glands
- Cartilage:
Basaloid squamous cell carcinoma
Table of Contents
Definition / general | Case reports | Treatment | Gross description | Microscopic (histologic) description | Positive stains | Negative stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Highly malignant, median survival 18 months (for all sites in head and neck)
- Heavy smokers or drinkers, often with advanced disease at diagnosis and other primary tumors in the area
- Usually men, ages 50+ years
- Usually base of tongue, hypopharynx or supraglottic larynx
Case reports
- Tumor with prominent spindle component (Arch Pathol Lab Med 1995;119:181)
Treatment
- Radical surgical excision, radical neck dissection and supplemental radiochemotherapy
Gross description
- Firm to hard, tan-white masses, may have central necrosis and up to 6 cm
Microscopic (histologic) description
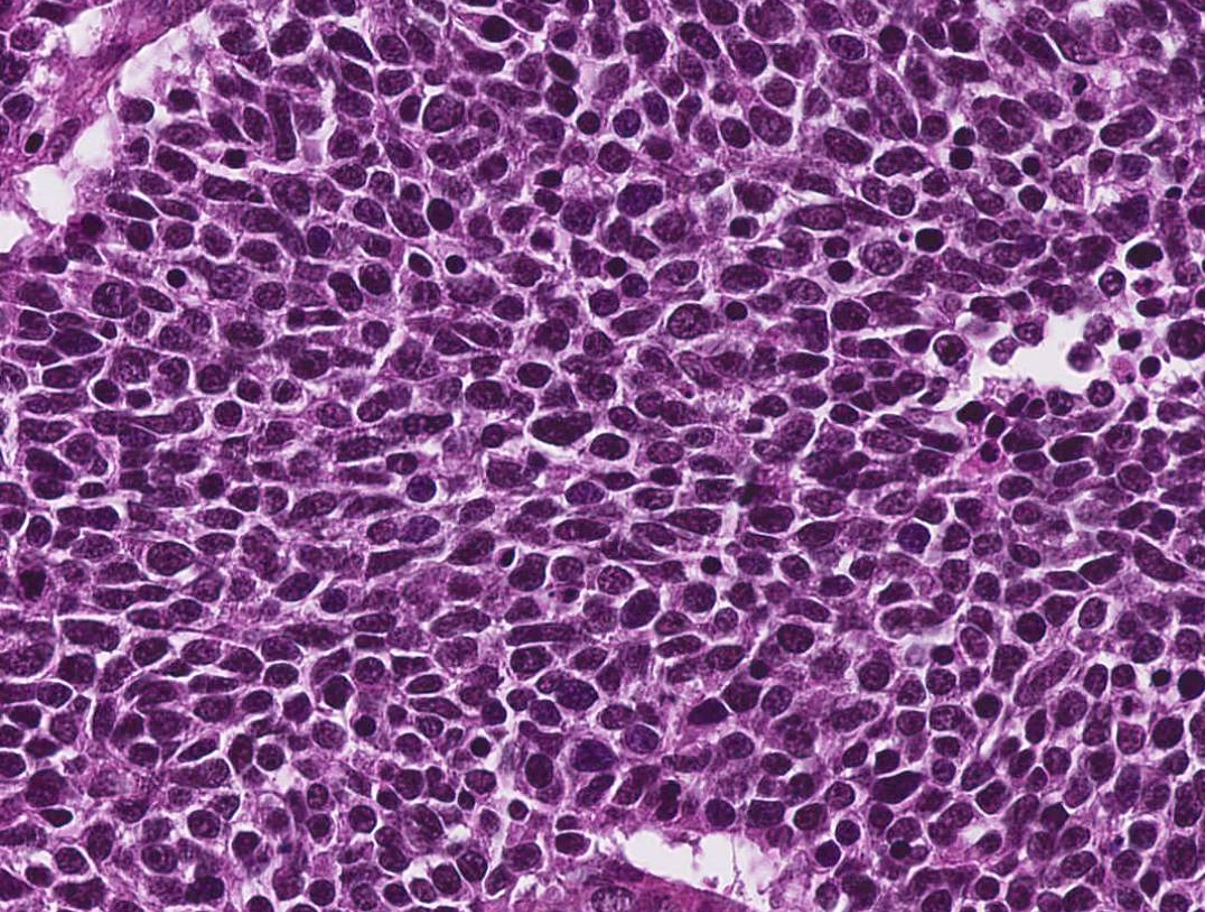
- Typical areas of squamous cell carcinoma (invasive and in situ) with nests or cords of small crowded cells with minimal cytoplasm, hyperchromatic nuclei, comedonecrosis, prominent hyalinization and peripheral palisading, small cystic spaces and mitotic activity
Positive stains
Negative stains
Electron microscopy description
- Rare tonofilaments, variable desmosomes
Differential diagnosis
- Adenoid cystic carcinoma: no significant pleomorphism, no mitotic activity and no squamous differentiation
- Small cell carcinoma: positive neuroendocrine markers
Additional references
Chondrosarcoma
Table of Contents
Definition / general | Case reports | Treatment | Gross description | Microscopic (histologic) description | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- 0.5% of primary laryngeal tumors
- Mean age 64 years, range 25 - 91 years; 80% male
- 69% arise in cricoid, 9% in thyroid cartilage and 14% in both
- 18 - 30% recur (more common with higher grade); death usually due to recurrence and not distant metastases
- 46% are grade I, 49% grade II, 5% grade III
- 37% have associated chondroma
- Myxoid and dedifferentiated variants may have worse prognosis; surprisingly, grade may not be associated with clinical outcome
- Clear cell variants are very rare
Case reports
- 57 year old man with clear cell tumor (Am J Surg Pathol 2002;26:386)
Treatment
- Complete excision with sparing of vocal cords
Gross description
- Usually 3 cm or more, invasion into bone of ossified laryngeal cartilage
Microscopic (histologic) description
- Diagnostic fields often small; have atypical, neoplastic chondrocytes with loss of normal architecture and distribution and invasion of bone; laryngeal cartilage usually has undergone endochondral ossification
- Low grade chondrosarcoma: slight increase in cellularity, mild atypia with binucleated chondrocytes within 1 lacuna; difficult to distinguish from chondroma; can report as "cartilaginous tumor without obvious evidence of malignancy, further classification pending removal of entire lesion"
- High grade chondrosarcoma: hypercellular with enlarged, binucleated and multinucleated atypical cells with increased nuclear to cytoplasmic ratio, irregular nuclear chromatin, prominent nucleoli, mitotic figures (may be atypical) and variable tumor necrosis
- Dedifferentiated chondrosarcoma: also called chondrosarcoma with additional malignant mesenchymal component (Am J Surg Pathol 1988;12:314)
Positive stains
- Clear cell variants: S100, collagen type II
Negative stains
- Clear cell variants: keratin
Molecular / cytogenetics description
- Clear cell variants: loss of 9p22 and 18q21
Differential diagnosis
- Chondroid metaplasia
- < https://www.pathologyoutlines.com/topic/softtissueeskchondroma.html">Chondroma
- Vocal process of arytenoid cartilage (normal finding)
Additional references
Contact ulcer
Table of Contents
Definition / general | Treatment | Microscopic (histologic) description | Additional referencesDefinition / general
- Also called granulomatous ulcer, posterior commissure ulcer
- At level of posterior commissure, near vocal process of arytenoid cartilage
- May recur after local excision but eventually subsides
Treatment
- Conservative (don't excise) because surgical trauma may cause recurrence
Microscopic (histologic) description
- Ulcerated or hyperplastic epithelium (occasionally pseudoepitheliomatous hyperplasia), overlying exuberant granulation tissue
Additional references
Conventional squamous cell carcinoma
Table of Contents
Definition / general | Clinical features | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosis | Additional referencesDefinition / general
- 9,000 new cases annually in US; 40% mortality
- Represents 90% of all laryngeal cancers
- 96% male; usually ages 40+ (but can occur in younger patients)
- Major risk factors are smoking, enhanced by heavy alcohol consumption
- HPV is not an early factor but positive in 20%, usually HPV 16 (Hum Pathol 1999;30:274)
- EBV a factor in 40% of hypopharyngeal carcinomas (Hum Pathol 1999;30:1071)
- Site influences histology and clinical behavior - either glottic, supraglottic or transglottic
- Spread is limited by tough membranes / ligaments
- Recurrence rate of 3% per year, second primary rate is 5% per year, usually in lung
- Metastases to regional lymph nodes and lungs; direct extension to thyroid gland and jugular vein
Clinical features
- Trachea:
- Exophytic, obstructive tumor usually in smokers but also children (J Am Coll Surg 2006;202:237)
- Most common primary malignancy of trachea (40 - 75%) (Cancer 1990;66:894)
- Usually arises in lower third of trachea (Eur Arch Otorhinolaryngol 1993;250:383, Acta Otolaryngol 1991;111:1162)
- May be extension of other head and neck primary
- May be associated with pneumoconiosis or posttracheotomy scar (Respiration 1993;60:250)
- Rapid clinical course, poor prognosis (Am J Clin Oncol 2011;34:32, Virchows Arch 2009;455:423, Jpn J Clin Oncol 1997;27:305)
Prognostic factors
- TNM; also tumor grade, tumor size, mitotic count, vascular invasion and margins
- 5 year survival by site:
- Glottic: I: 90%; II: 85%; III: 60%; IV: < 5%
- Supraglottic: I: 85%; II: 75%; III: 45%; IV: < 5%
- Transglottic: 50%
- Subglottic: 40%
Case reports
- 11 month old boy with severe dyspnea and stridor (Int J Pediatr Otorhinolaryngol 1998;43:163)
- 37 year old woman with hypohidrotic ectodermal dysplasia (J Laryngol Otol 2002;116:742)
- 50 year old man with unresectable basaloid squamous cell carcinoma (J Cancer Res Ther 2010;6:321)
- 52 year old woman with HPV and squamous cell carcinoma in a solitary tracheal papilloma (Ann Thorac Surg 2004;77:2201)
- 54 year old man with dyspnea, hemoptysis, cough and weight loss (Acta Otorhinolaryngol Ital 2010;30:209)
- 70 year old woman with double primary cancer of the lung and trachea (Nihon Kyobu Shikkan Gakkai Zasshi 1996;34:216)
- 78 year old man with spindle cell sarcomatoid carcinoma (Tuberk Toraks 2009;57:337)
- Nodal metastasis occurring postradiation therapy with mixture of squamous cell carcinoma and rhabdomyosarcoma (Am J Surg Pathol 1993;17:415)
Treatment
- Surgical excision with end to end anastomosis, radiation therapy (Med Princ Pract 2004;13:69)
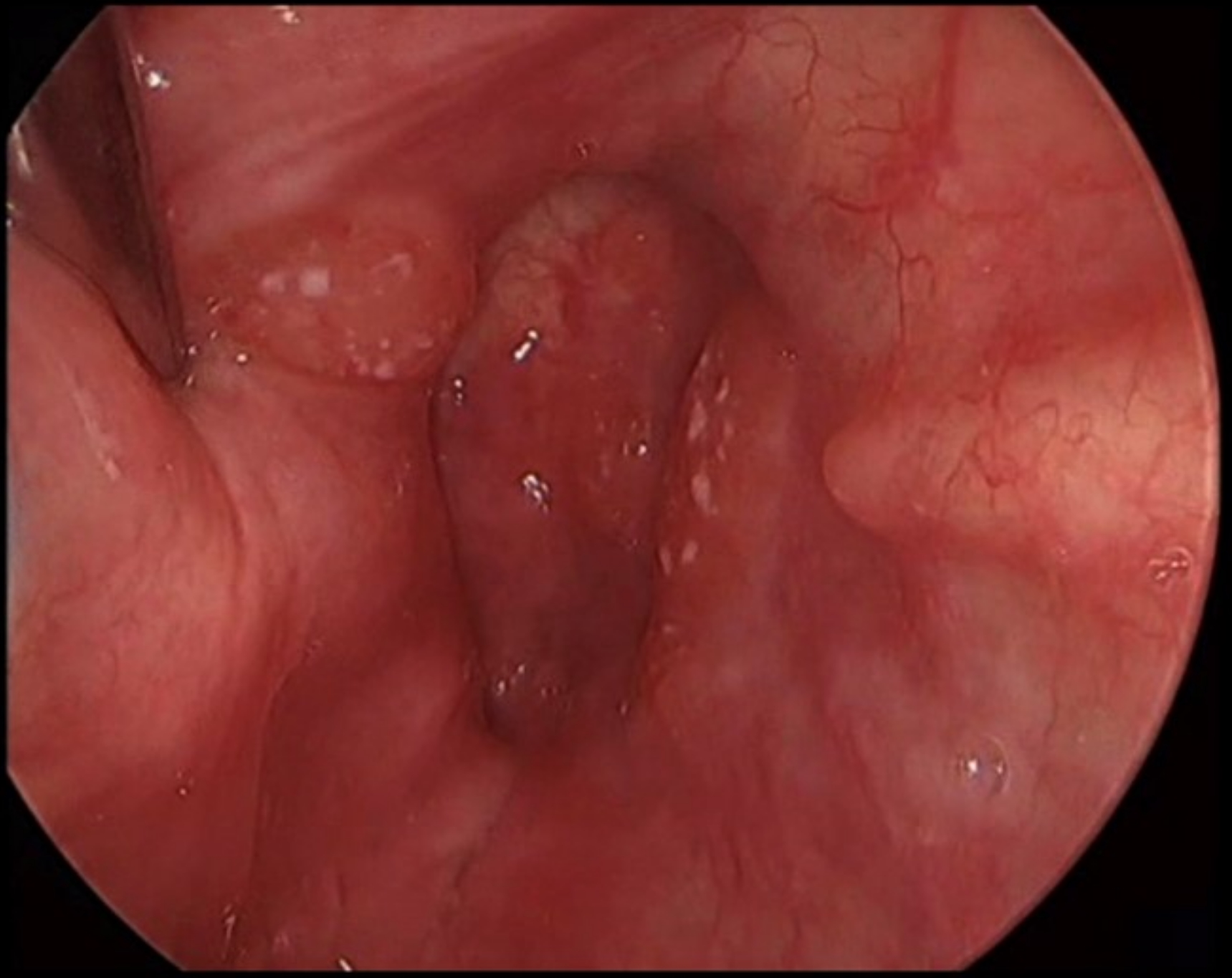
Clinical images
Gross description
- Pink to gray ulcerated mass; vocal cord lesions often keratotic
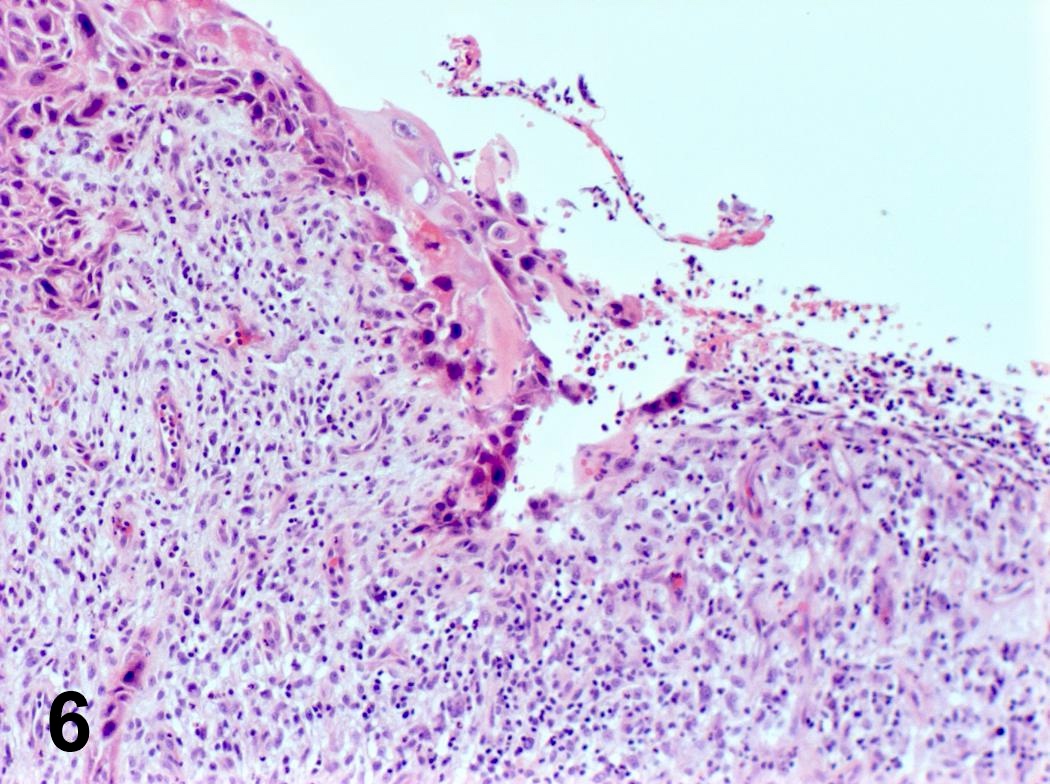
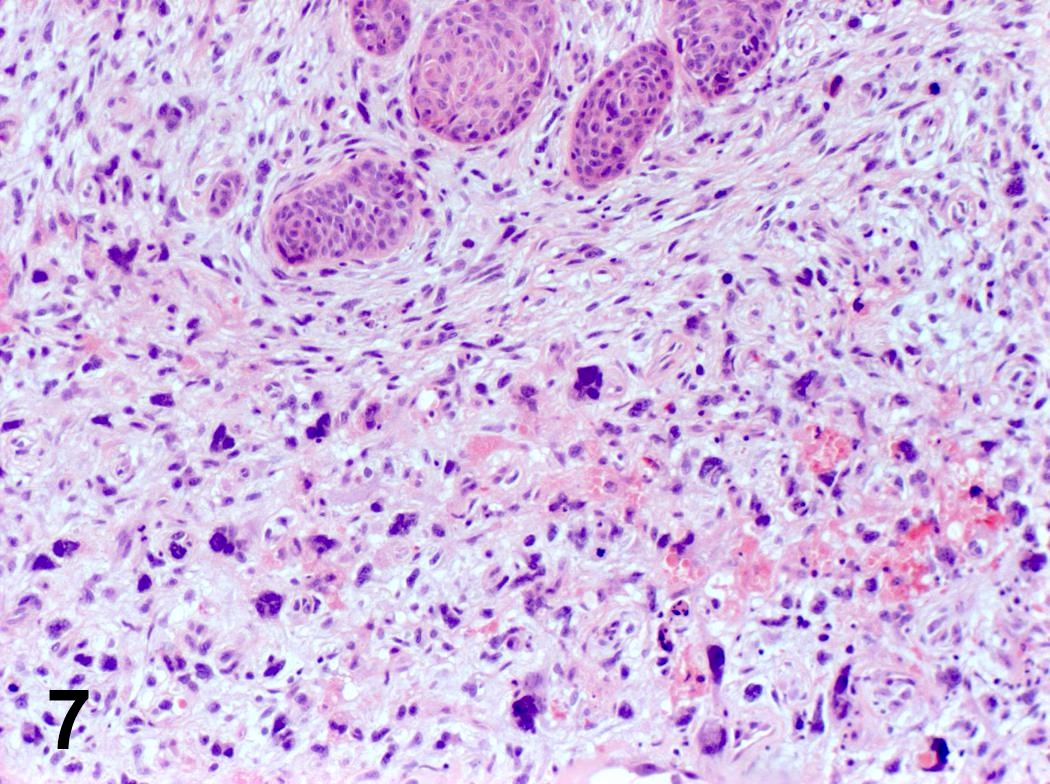
Microscopic (histologic) description
- Invasion indicated by desmoplasia around malignant squamous cells, often with keratinization at periphery
- Progression of columnar epithelium areas is similar to squamous cell carcinoma of cervical or lung
- Progression of vocal cord tumors is similar to squamous cell carcinoma of skin or esophagus
- Well, moderate or poorly differentiated, based on degree of keratinization, pearl formation, intercellular bridges and mitotic activity
- Smaller tumors are usually better differentiated
- Trachea:
- Variants include spindle cell, sarcomatoid
- May arise in papilloma
- May be combined with small cell and giant cell carcinoma
Microscopic (histologic) images
Contributed by Steven Catinchi-Jaime, M.D.
Images hosted on other servers:
Differential diagnosis
- Adenoid cystic carcinoma
- Extension of esophageal tumor (Arch Pathol Lab Med 1984;108:983)
- Metastasis from colon carcinoma (Can Assoc Radiol J 1989;40:198)
Additional references
Diphtheria
Definition / general
- Tracheal involvement in pharyngeal / laryngeal diphtheria is uncommon
- Late appearance of grey brown thick and firm false membranes of pharynx and larynx
- Death occurs rarely in nonimmunized children (Vestn Otorinolaringol 1995;(2):31)
Case reports
- Fatal case in a nonimmunized child (Pediatr Pathol 1994;14:391)
Gross images
Dysplasia
Table of Contents
Definition / general | Prognostic factors | Treatment | Gross description | Microscopic (histologic) description | Additional referencesDefinition / general
- For columnar epithelium, resembles cervical dysplasia
- Associated with HPV 16 and p53 expression
- Leukoplakia: clinical term describing any white lesion on a mucous membrane; usually associated with mucosal thickening and not dysplasia
- Erythroplakia: clinical term describing red lesion on a mucous membrane; usually associated with dysplasia or malignancy; in smokers, for squamous epithelium, features of nuclear pleomorphism, mitotic activity, abnormal mitotic figures and stromal inflammation are associated with progression to invasive carcinoma
- Keratosis: increase in surface keratin, often with prominent granular cell layer and orthokeratin (cells without nuclei) mixed with parakeratin (flat keratotic cells with pyknotic nuclei); not related to dysplasia
- Dyskeratosis: abnormal keratinization of epithelial cells
- Dysplasia: spectrum of abnormal epithelial maturation and cellular atypia that may or may not precede invasive carcinoma
- Carcinoma in situ: full thickness dysplasia of mucosa without violation of basement membrane; same as severe dysplasia
Prognostic factors
- Overall, low risk of development of invasive squamous cell carcinoma after dysplasia
- For mild dysplasia, 7% develop in situ or invasive carcinoma versus 24% with moderate dysplasia versus 25% with severe dysplasia
- High risk of progression to invasive carcinoma for severe keratinizing dysplasia versus nonkeratinizing dysplasia
Treatment
- Mild / moderate dysplasia may be reversible
- Severe dysplasia requires intervention (vocal cord stripping, surgery, radiation therapy, endoscopic laser resection) as well as surveillance of entire upper aerodigestive tract
Gross description
- Erythema of involved areas
Microscopic (histologic) description
- Mild dysplasia:
- Normal or mildly disordered basal layer with retained maturation and stratification of upper layers
- Mild nuclear atypia and possibly mitotic figures in basal third of epithelium
- No abnormal mitotic figures
- Variable keratosis and chronic inflammatory infiltrate
- Moderate dysplasia:
- Moderate nuclear atypia, usually with prominent nucleoli and mitotic figures, most pronounced in lower two - thirds of epithelium
- Cell maturation and stratification are present in upper layer
- No abnormal mitotic figures
- Variable keratosis
- Severe / high grade:
- Marked nuclear abnormalities and loss of maturation greater than two - thirds of epithelium
- Large atypical nuclei, some bizarre; nuclear pleomorphism is common
- May have prominent nucleoli
- Mitotic figures high in epithelium, often abnormal
- Keratinizing dysplasia:
- Defined as lesions in which epithelial alterations are so severe that there is a high probability of progression to invasive carcinoma
- Includes dyskeratotic cells and mitotic figures with variable atypical forms above basal zone, variable surface keratinization
- Carcinoma in situ:
- Full thickness nuclear abnormalities without stromal invasion
- Cells are usually keratinized but may be basal-like
- Often lumped together with severe dysplasia
- May represent peripheral portion of invasive carcinoma
- Papillary carcinoma in situ:
- Papillary fronds with a fibrovascular core covered by squamous epithelium with marked atypia
- Note: invasion may occur by dysplastic cells without full thickness epithelial involvement
Additional references
Grossing & features to report
Grossing
- Open in midline posteriorly, ink margins, take tissue for special studies
- Fix specimen
- Remove hyoid bone and inspect pre-epiglottic tissue
- Slice larynx (see below) and photograph
- For supraglottic and hypopharyngeal carcinomas, blocks should include relationship between tumor and anterior resection margin at base of tongue
- For partial laryngectomy specimens, inferior margin is usually most critical
- At least one section per 1 cm of tumor for large tumors, including tumor center and periphery and maximum depth of invasion
- Submit entire tumor if can do so in 5 sections or less
- Submit resection margins
- Nonneoplastic mucosa
- Bone or cartilage that is grossly involved by tumor
- Thyroid gland if present
- Lymph nodes
- Tracheostomy site
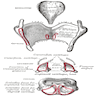
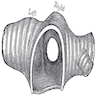
- Glottic tumors: show tumor relationship to ventricle, thyroid cartilage and cricoid cartilage by coronal section (plane dividing body into front and back, coronal section of normal larynx)
- Epiglottic tumors: determine extent of invasion of preepiglottic space by sagittal sections (plane dividing body into right and left, sagittal section of larynx)
- Pyriform sinus tumors: show invasion of supraglottic larynx and thyroid lamina by horizontal sections
Features to report
- Editor's note
- Tumor size and location
- Tumor histologic type and pattern
- Tumor histologic grade
- Depth of invasion of primary tumor
- Pattern of invasion
- Tumor extension to adjacent structures (indicate involvement or not)
- Status of resection margins (distance from invasive carcinoma to mucosal and deep margins)
- Vascular invasion
- Perineural invasion
- Presence of severe dysplasia / carcinoma in situ
- Lymph nodes: for each level, number obtained, number involved by tumor, size of nodal metastases and presence of extracapsular spread
- TNM staging
Additional references
Laryngeal cysts
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Laryngeal cysts are fluid filled, mucosa lined lesions that are unconnected to the laryngeal ventricle
Essential features
- Rare benign lesions in larynx; can affect both children and adults
- Mostly asymptomatic but can cause respiratory distress
- Computed tomography (CT): initial investigation of choice
- Histology is mandatory for definite diagnosis
- Oncocytic cysts are associated with recurrence particularly when multifocal, so long term follow up is recommended
- Numerous proposed classifications
Terminology
- Cystic laryngeal mass, saccule, laryngocele (not recommended)
ICD coding
- ICD-10: D14.1 - benign neoplasm of larynx
Epidemiology
- No gender predilection; oncocytic cysts more common in women (Eur Arch Otorhinolaryngol 2021;278:3381)
- In adults, more common after sixth decade
- Accounts for 5 - 10% of benign laryngeal lesions in adults
- 1.8 in 100,000 newborns
Sites
- Can occur in any part of the larynx but are more frequent in epiglottis and vallecula
Pathophysiology
- See Clinical features for classifications
- Ductal cyst
- Mucus retention cyst caused by obstruction of mucus gland ducts within mucus membrane; represents 75% of all laryngeal cysts
- Saccular cyst
- Saccule is a membranous sac in the larynx, located between the false vocal cords and the inner surface of the thyroid cartilage, which contains mucous secreting glands
- Obstruction of the mucous gland ducts of the laryngeal saccule with subsequent accumulation of secretions results in a submucosal cyst
- Represents 25% of all laryngeal cysts; further classified as anterior or lateral
- Lateral saccular cysts are predominantly congenital, formed during embryogenesis due to aberrant entrapment of developing saccule by third and fifth brachial arches; these remain quiescent until a trigger such as an upper respiratory tract infection causes expansion of the cyst
- References: Int J Phonosurg Laryngol 2016;6:53, Otolaryngol Head Neck Surg 2017;157:928
Etiology
- Oncocytic cysts more common in old age and in chronic smokers
Clinical features
- Nonspecific
- Hoarseness, foreign body sensation, dyspnea (Otolaryngol Head Neck Surg 2017;157:928)
- If located near the orifice of the ventricle or subglottic region, symptoms can range from stridor to significant respiratory obstruction
- Those in the vallecula can cause feeding problems
- Numerous proposed classifications
- DeSanto classification (Laryngoscope 1970;80:145)
- Ductal
- Saccular
- No importance to histology
- No separate class of congenital cysts
- Arens classification (Eur Arch Otorhinolaryngol 1997;254:430)
- Congenital
- Retention
- Inclusion cysts
- Forte classification of congenital cysts (Laryngoscope 2004;114:1123)
- Type I: confined to larynx, lined by endoderm
- Type II: extends beyond larynx
- Type IIa: composed of endoderm only
- Type IIb: composed of endoderm and mesoderm elements in cyst wall
- Newman classification (Am J Clin Pathol 1984;81:715)
- Epithelial cyst (includes ductal and saccular)
- Tonsillar cyst
- Oncocytic cyst
- DeSanto classification (Laryngoscope 1970;80:145)
Diagnosis
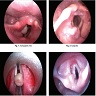
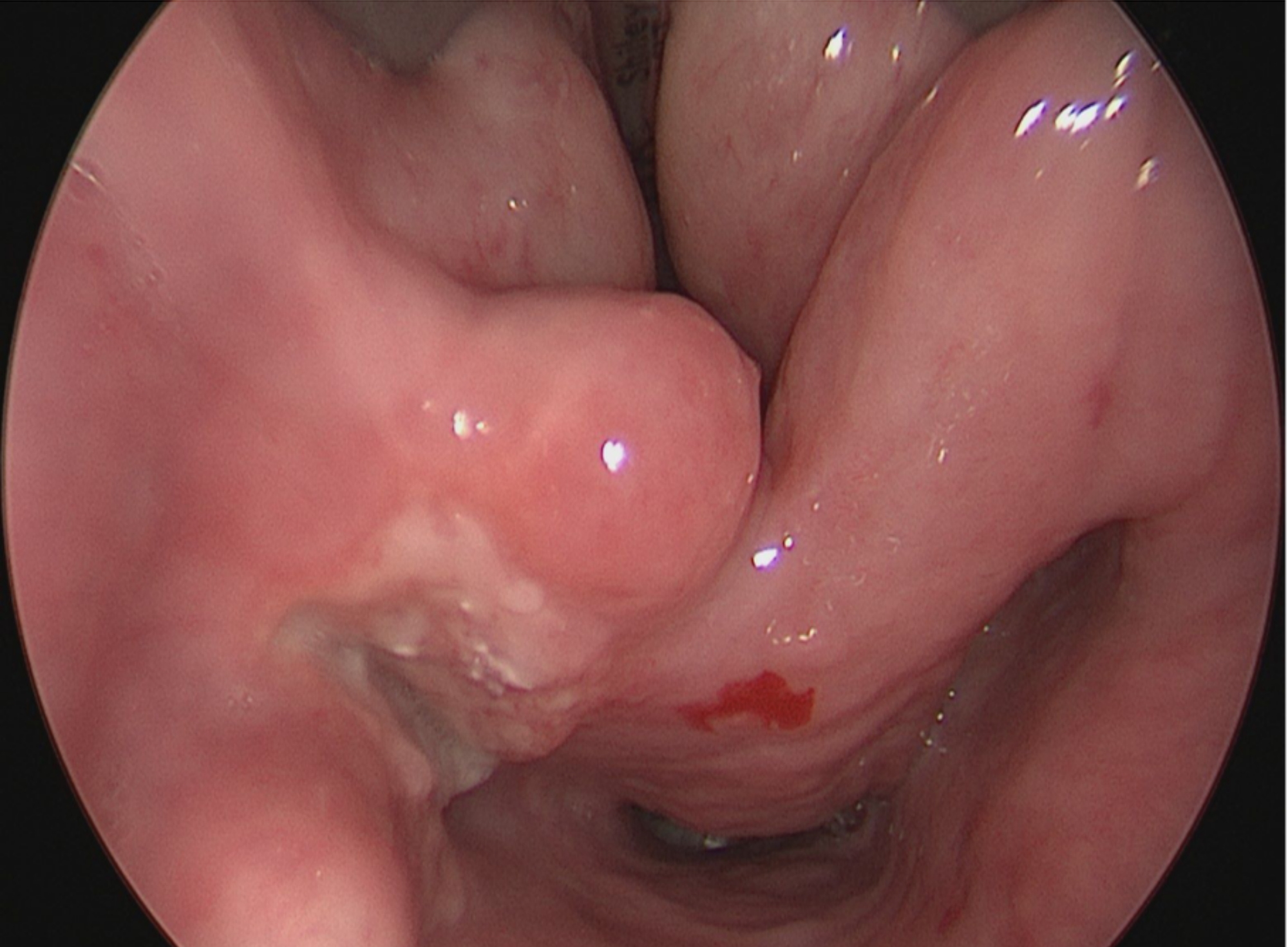
- Laryngoscopy
Radiology description
- Used to determine the location, size, extent and anatomical relationships
- Computed tomography (CT)
- Initial investigation of choice
- Well defined, fluid attenuation, nonenhancing rounded lesion
- Magnetic resonance imaging (MRI)
- Gold standard (Pediatr Pulmonol 2019;54:478)
- Well defined, fluid signal intensity, nonenhancing rounded lesion
- Endolaryngeal ultrasound (EUS) and optical coherence tomography (OCT)
- These are newer techniques that may improve preoperative evaluation of cysts
Prognostic factors
- Oncocytic cysts are associated with multiplicity and recurrence (Otolaryngol Head Neck Surg 2017;157:928)
Case reports
- 5 year old girl with dysphonia, moderate inspiratory dyspnea (J Med Life 2016;9:199)
- 34 year old man with a laryngeal cyst (Medicine (Baltimore) 2018;97:e11280)
- 64 year old man with laryngeal mass (Clin Pract 2016;6:822)
- 86 year old woman with oncocytic cyst (Forensic Sci Med Pathol 2022;18:554)
Treatment
- Complete excision by endoscopic excision, microlaryngeal surgery, laser excision or marsupialization
- Recurrence is associated with remnant of the cyst wall; therefore, total excision is recommended
Gross description
- Specimens are rarely received intact, so the following described features are from endoscopic findings
- Ductal cyst
- Usually measures < 1 cm in diameter
- Tends to be superficial (lies within mucus membrane)
- Saccular cyst
- Submucosal, ranges in size from 1 to > 7 cm in greatest dimension
- Reference: Medicine (Baltimore) 2018;97:e11280
Microscopic (histologic) description
- Histology is critical for definitive diagnosis
- Epithelial cyst
- Lined by respiratory or squamous epithelium or a flattened epithelium
- Epithelial lining may be papillary in architecture
- May contain keratin or mucin
- May have a lymphoid component in cyst wall
- Includes both ductal and saccular cysts; differentiation of ductal from saccular cysts is not possible histologically, so differentiation rests on clinical parameters
- Tonsillar cyst
- Resembles palatine tonsillar crypt
- Lined by stratified squamous epithelium
- Lymphoid follicles with germinal centers seen in cyst wall
- Oncocytic cyst
- Unilocular or multilocular cyst lined by uniform oncocytic epithelial cells
- May have prominent intraluminal papillary architecture or hyperplastic solid nests
- Unlike Warthin tumor, doesn't have bilayered oncocytes and lymphoid stroma
- Oncocytes originate from metaplasia of seromucinous gland ducts in response to aging and chronic smoking resulting in disturbance of mitochondrial enzyme organization
- Originates in ventricles or false vocal cords, presents as supraglottic mass
- Also called oncocytic papillary cystadenoma, oncocytic adenomatous hyperplasia, oncocytoma
- No atypia or invasion; mitosis: rare (Eur Arch Otorhinolaryngol 2021;278:3381)
Microscopic (histologic) images
Sample pathology report
- Larynx, epiglottic cyst, excision:
- Consistent with laryngeal epithelial cyst, lined by stratified squamous epithelium
Differential diagnosis
- Laryngocele:
- Dilatation of saccule
- Different from saccular cyst because these are distended with air and communicate with the laryngeal lumen
- Thyroglossal cyst:
- Difficult to distinguish from saccular cyst if deficient in thyroid tissue
- Differentiated on basis of exact anatomical location of cyst, as thyroglossal duct cysts are related with the hyoid bone and located in the midline of the neck
Additional references
Board review style question #1
Board review style answer #1
A. Laryngeal epithelial cyst. The section shows a distended cystic lesion lined by columnar epithelial cells with focal squamous metaplasia. The lesion is entirely confined within the larynx with no connection to lumen or dysplasia of overlying squamous epithelium. Answer B is incorrect because laryngocele is an air filled cavity communicating with the laryngeal lumen. Answer C is incorrect because squamous cell papilloma is an exophytic lesion lined by hyperplastic stratified squamous epithelium. Answer D is incorrect because vocal cord nodule / polyp is a surface lesion with no intralaryngeal extension.
Comment Here
Reference: Laryngeal cysts
Comment Here
Reference: Laryngeal cysts
Board review style question #2
Which type of laryngeal cyst needs follow up for a longer period of time after surgical excision?
- Ductal cyst
- Oncocytic cyst
- Saccular cyst
- Tonsillar cyst
Board review style answer #2
B. Oncocytic cyst. Oncocytic cysts need longer follow up because of their association with recurrence and multifocality. Anwers A, C and D are incorrect as surgical excision alone is sufficient and these cysts are not known to be associated with recurrence.
Comment Here
Reference: Laryngeal cysts
Comment Here
Reference: Laryngeal cysts
Laryngitis / laryngoepiglottitis
Table of Contents
Definition / general | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
Acute laryngoepiglottitis
Chronic (nonspecific) laryngitis
Histoplasmosis
- Also called acute supraglottitis
- Rare; due to Haemophilus influenza type B or Streptococcus pyogenes; reduced incidence due to childhood vaccine for H. influenza
- May produce sudden lethal swelling of epiglottis and vocal cords
- Croup: laryngotracheobronchitis in children; inflammatory narrowing produces inspiratory stridor
Chronic (nonspecific) laryngitis
- Commonly due to upper respiratory tract infection, overuse of voice, heavy exposure to tobacco smoke or alcohol
Histoplasmosis
- Common cause of fungal laryngitis in U.S. (also blastomycosis)
- Early lesions are in vocal cords and epiglottis
- Granulomatous lesion involving anterior larynx is likely to be histoplasmosis
Case reports
Chronic (nonspecific) laryngitis
Histoplasmosis
- CMV laryngitis and probable lymphoma in AIDS patient (Arch Pathol Lab Med 1992;116:539)
Histoplasmosis
- 78 year old man with epiglottic mass in nonendemic region (Arch Pathol Lab Med 2004;128:574)
Gross description
Acute laryngoepiglottitis
- Red and edematous epiglottis
Microscopic (histologic) description
Acute laryngoepiglottitis
Chronic (nonspecific) laryngitis
- Acute inflammatory infiltrate with edema, extending to adjacent soft tissues
Chronic (nonspecific) laryngitis
- Lymphocytic infiltrate with variable plasma cells and histiocytes
- Variable epithelial hyperplasia
Laryngocele (pending)
(pending)
Neuroendocrine neoplasm
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Neuroendocrine neoplasms are composed of neuroendocrine epithelial neoplasms that arise in the larynx and are either well differentiated or poorly differentiated (high grade)
Essential features
- Laryngeal neuroendocrine neoplasms are divided into 3 categories: well differentiated neuroendocrine neoplasms, poorly differentiated neuroendocrine carcinomas and mixed neuroendocrine - nonneuroendocrine neoplasm
- Well differentiated neuroendocrine neoplasm with immunohistochemical evidence of neuroendocrine differentiation and positivity for at least 1 keratin
- Ki67 proliferation index
- No abnormal p53 staining and no loss of Rb (in selected cases with Ki67 > 20%)
- Poorly differentiated neuroendocrine carcinomas
- Small cell neuroendocrine carcinoma
- High grade carcinoma
- Cell size smaller than diameter of 3 lymphocytes
- Prominent apoptotic bodies and necrosis
- Large cell neuroendocrine carcinoma
- High grade malignancy carcinoma
- Cell size larger than diameter of 3 lymphocytes
- Peripheral palisading, rosette formation or comedo pattern necrosis
- Small cell neuroendocrine carcinoma
- Well differentiated neuroendocrine neoplasm with immunohistochemical evidence of neuroendocrine differentiation and positivity for at least 1 keratin
Terminology
- Neuroendocrine neoplasms (NEN) are subclassified into 2 subtypes
- Well differentiated neuroendocrine neoplasms (NET)
- Well differentiated neuroendocrine tumor, grade 1
- Well differentiated neuroendocrine tumor, grade 2
- Well differentiated neuroendocrine tumor, grade 3
- Poorly differentiated neuroendocrine carcinomas (NEC)
- Small cell neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Well differentiated neuroendocrine neoplasms (NET)
- Mixed neuroendocrine - nonneuroendocrine neoplasm
ICD coding
Epidemiology
- Second most common group of neoplasms of the larynx after squamous cell carcinomas (Head Neck 2009;31:1634)
Sites
- Most frequent site: the supraglottic larynx (Head Neck Pathol 2022;16:375, Cancer 1988;62:2658)
- Most frequent type: grade 2 NET (Head Neck Pathol 2022;16:375, Cancer 1988;62:2658)
Pathophysiology
- Unknown
Etiology
- For NET: unclear
- Involvement of tobacco use (Adv Anat Pathol 2019;26:246)
- No well established association with human papillomavirus (HPV) (Eur Arch Otorhinolaryngol 2013;270:719)
- For NEC
- Tobacco use (Cancer Treat Rev 2019;78:42)
- No association with HPV (Am J Surg Pathol 2016;40:471)
Diagrams / tables
Table 1: WHO 2022 epithelial neuroendocrine neoplasms of the upper aerodigestive tract and salivary glands (Head Neck Pathol 2022;16:123)
| Neuroendocrine neoplasm | Tumor category | Diagnostic criteria |
| Well differentiated neuroendocrine neoplasm (neuroendocrine tumor, NET) | Well differentiated neuroendocrine tumor, grade 1 (NET, G1) | No necrosis and < 2 mitoses/2 mm²
Ki67 < 20% |
| Well differentiated neuroendocrine tumor, grade 2 (NET, G2) | Necrosis or 2 - 10 mitoses/2 mm²
Ki67 < 20% | |
| Well differentiated neuroendocrine tumor, grade 3 (NET, G3) | > 10 mitoses/2 mm²
Ki67 > 20% Absence of NEC cytomorphology | |
| Poorly differentiated neuroendocrine neoplasm (neuroendocrine carcinoma, NEC) | Small cell neuroendocrine carcinoma | > 10 mitoses/2 mm²
Ki67 > 20% (often > 70%) Small cell NEC cytomorphology |
| Large cell neuroendocrine carcinoma | > 10 mitoses/2 mm²
Ki67 > 20% (often > 50%) Large cell NEC cytomorphology |
Diagnosis
- Diagnosis is based on tissue examination findings of typical histologic and immunohistochemical features
Radiology description
- Structural imaging studies by CT and MRI: delineate extent and tumor location of NENs (Front Endocrinol (Lausanne) 2020;11:397)
Prognostic factors
- Adverse prognostic factors include
- Small cell neuroendocrine carcinoma (Cancer Treat Rev 2019;78:42)
- Stage IV disease (Head Neck 2015;37:707, Cancer Treat Rev 2019;78:42)
- Neck metastases (Head Neck 2015;37:707)
- 5 year survival rate
- Well differentiated neuroendocrine neoplasms: variable prognosis and based on the presence of distant metastasis and lymph node metastases (Head Neck 2015;37:707)
- Poorly differentiated neuroendocrine carcinomas: 5 - 20% (Ann Otol Rhinol Laryngol 2016;125:464, Laryngoscope 2017;127:1785)
- Regardless of site, lower stage tumors are associated with longer survival (Laryngoscope 2017;127:1785)
- Usually supraglottic
- Heavy smokers
- No association with HPV
Case reports
- 56 year old woman presented with painful subcutaneous skin lesions that were diagnosed as metastatic carcinoma at an outside facility; diagnosed as large cell neuroendocrine carcinoma (J Cutan Pathol 2018;45:229)
- 59 year old man presented to a community based hospital complaining of a left sided neck mass that has been present for ~1 month; diagnosed as small cell neuroendocrine carcinoma (Ear Nose Throat J 2022;101:NP96)
- 65 year old man, a smoker, and 63 year old man, a former smoker, presented with well differentiated neuroendocrine tumor (grade moderately differentiated neuroendocrine carcinoma) causing death 13 and 33 months after diagnosis (Arch Pathol Lab Med 1992;116:253)
Treatment
- G1 NET: local resection in the sense of either open or transoral endoscopic laser surgical partial laryngectomy is recommended (Laryngorhinootologie 2021;100:S1)
- G2 NET: radical surgical resection, elective neck dissection and radiotherapy (Laryngorhinootologie 2021;100:S1)
- G3 NET: not yet standardized because of lack of data
- NEC regardless of subtype: radiotherapy and chemotherapy (Head Neck 2015;37:707)
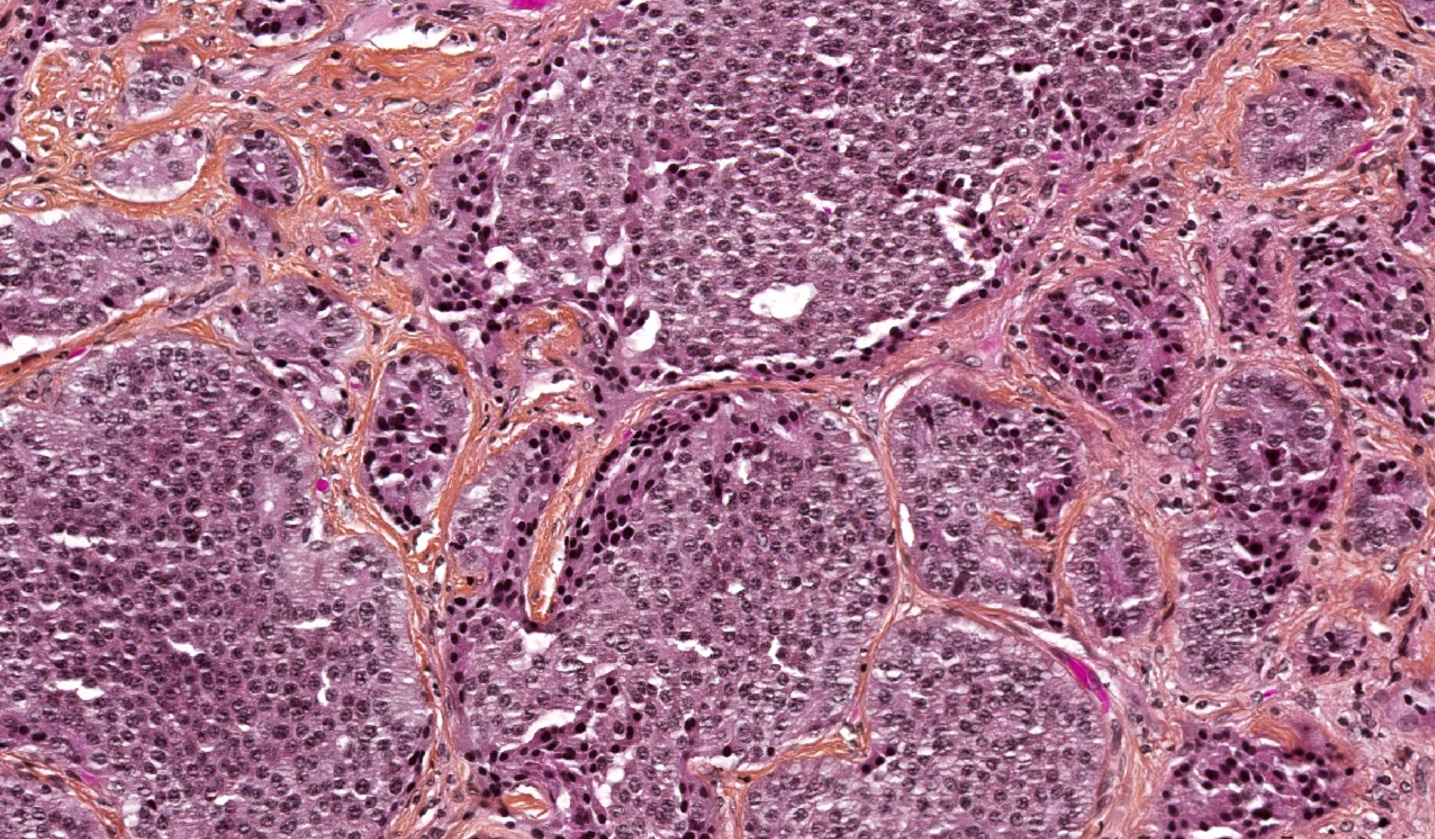
Microscopic (histologic) description
- Well differentiated neuroendocrine tumor (Head Neck Pathol 2022;16:123)
- Growth patterns: organoid (zellballen), trabecular, ribbon, solid, sheet-like (diffuse noncohesive) and individual cells
- Classical cytomorphology of tumor cells: uniform / monotonous round to oval nuclei, dispersed (salt and pepper) nuclear chromatin and ample granular pale eosinophilic cytoplasm often without distinct cell borders
- Uncommon cytomorphology of tumor cells: plasmacytoid, clear, oncocytic and rhabdoid (Head Neck Pathol 2022;16:375)
- Mild to focally moderate nuclear pleomorphism
- Necrosis, mitotic count and Ki67 proliferation index
- Grade 1: no necrosis and < 2 mitoses/2 mm² / Ki67 < 20%
- Grade 2: necrosis (often punctate [individual cell] and less commonly, coagulative [confluent foci]) or 2 - 10 mitoses/2 mm² / Ki67 < 20%
- Grade 3: > 10 mitoses/2 mm² / Ki67 > 20% without NEC cytomorphology
- Poorly differentiated neuroendocrine carcinomas (Head Neck Pathol 2022;16:123)
- Small cell neuroendocrine carcinoma
- Sheets, cords, nests, solid and diffuse (dyscohesive) but occasionally may be arranged in trabeculae
- May have peripheral nuclear palisading or rosettes
- Frequent necrosis in the form of apoptotic bodies or confluent (comedo type) foci
- Common: crush artifact with extravasated DNA coating blood vessels (Azzopardi phenomenon)
- Tumor cells are smaller than the diameter of 3 lymphocytes
- Hyperchromatic to finely granular or stippled appearing chromatin
- Inconspicuous nucleoli, scant cytoplasm and nuclear molding
- > 10 mitoses/2 mm²
- Ki67 > 20% (often > 70%)
- Large cell neuroendocrine carcinoma follows strict criteria
- Sheets, cords, nests, solid and diffuse (dyscohesive) but occasionally may be arranged in trabeculae
- May have peripheral nuclear palisading or rosettes
- Frequent necrosis in the form of apoptotic bodies or confluent (comedo type) foci
- Tumor cells are larger than the diameter of 3 lymphocytes with round to oval nuclei
- Vesicular chromatin
- Prominent nucleoli
- Abundant eosinophilic cytoplasm
- > 10 mitoses/2 mm²
- Ki67 > 20% (often > 50%)
- Small cell neuroendocrine carcinoma
- Mixed neuroendocrine - nonneuroendocrine neoplasm
- Rarely observed (J Laryngol Otol 2010;124:226)
- Typical cytomorphology of neuroendocrine neoplasm with the nonneuroendocrine components including squamous cell carcinoma or adenocarcinoma
Microscopic (histologic) images
Positive stains
- Not recommended staining for nonspecific biomarkers (CD56, CD57, PGP9.5 and neuron specific enolase)
- Recommended neuroendocrine staining
- > 80% staining of tumor cells of synaptophysin (the most sensitive), chromogranin A (the most specific) (Cancer Cytopathol 2016;124:871)
- Or diffuse INSM1 (useful in case of synaptophysin+ and chromogranin A-)
- Keratins including CK7 / 8 and CAM5.2
- Paranuclear dot-like cytokeratin reactivity
- Ki67 proliferation index > 20% and often > 70%
- p53 and pRb: useful for biopsy (no aberrant expression for NET versus aberrant expression of p53 and loss of pRb for NEC)
- p16: can be positive regardless of HPV status
- Calcitonin in G2 NETs (Head Neck Pathol 2022;16:375)
- Reference: Head Neck Pathol 2022;16:123
Negative stains
- TTF1 (focally positive in 13%)
- p63 / p40 (if present, tend to be very focal, limited to scattered nuclei), smooth muscle actin, CD117
- CEA
- Reference: Mod Pathol 2003;16:1041
Electron microscopy description
- Presence of membrane bound, electron dense neurosecretory granules (Ear Nose Throat J 2012;91:E20)
Sample pathology report
- Supraglottic larynx, biopsy:
- Well differentiated neuroendocrine tumor, WHO grade 2 (see comment)
- Comment: The presence of increased mitotic activity (4 mitoses/2 mm²) on this biopsy combined with the classic morphology of a well differentiated neuroendocrine tumor and positivity of immunohistochemical stains for cytokeratin AE1 / AE3, synaptophysin, chromogranin and 10% for Ki67 proliferative index support the diagnosis of well differentiated neuroendocrine tumor WHO grade 2 (out of 3).
Differential diagnosis
- Paraganglioma:
- Cytokeratin negative
- SOX10, S100 positive for sustentacular cells around tumor nests (Hum Pathol 2020;103:72)
- GATA3 positive (Hum Pathol 2020;103:72)
- Medullary thyroid carcinoma (MTC):
- Immunostaining in favor of MTC: strong positivity and diffuse for cytokeratin, calcitonin and TTF1 (Mod Pathol 2004;17:631)
- Association with elevated calcitonin levels (Ther Adv Endocrinol Metab 2022;13:20420188221099344)
- Adenoid cystic carcinoma:
- Positivity of myoepithelial cells markers
- Strongly positive for MYB / MYBL1 or fusion of MYB::MYBL1
- CD117 positivity is not specific and could be seen in neuroendocrine carcinoma and adenoid cystic carcinoma (Mod Pathol 2008;21:876, Mod Pathol 2003;16:1041)
- Basaloid squamous cell carcinoma:
- Has different morphology with nuclear pleomorphism, hyperchromatic and peripheral palisading, often with central comedo type necrosis, without expression neuroendocrine markers (focally may be seen)
- Diffusely positive for p63 and high molecular weight cytokeratins
Board review style question #1
Which of the following diagnoses regarding neuroendocrine neoplasms of the larynx with 15 mitoses/2 mm² and Ki67 30% without necrosis is correct?
- Poorly differentiated neuroendocrine carcinomas
- Well differentiated neuroendocrine tumor, grade 1
- Well differentiated neuroendocrine tumor, grade 2
- Well differentiated neuroendocrine tumor, grade 3
Board review style answer #1
D. Well differentiated neuroendocrine tumor, grade 3 because the diagnostic criteria applied with NET cytomorphology with > 10 mitoses/2 mm² and Ki67 > 20% and most importantly, without NEC cytomorphology.
Comment Here
Reference: Neuroendocrine neoplasm
Comment Here
Reference: Neuroendocrine neoplasm
Board review style question #2
Which of the following statements regarding differential diagnosis of neuroendocrine neoplasms of the larynx is true?
- Adenoid cystic carcinoma is diffusely positive for p16
- Basaloid squamous cell carcinoma has the identical morphology as the poorly differentiated neuroendocrine carcinoma
- Calcitonin and TTF1 may be expressed in neuroendocrine neoplasms
- Paraganglioma could express cytokeratin
- Treatment of neuroendocrine carcinomas is different based on the subtype
Board review style answer #2
C. Calcitonin and TTF1 may be expressed in neuroendocrine neoplasms.
Adenoid cystic carcinoma is diffusely positive for p16, as this biomarker can be seen in NEC or adenoid cystic carcinoma (A).
Basaloid squamous cell carcinoma is distinguished from poorly differentiated neuroendocrine carcinoma by its diffuse squamous marker expression (e.g., p40, CK5/6) and lack of neuroendocrine marker (synaptophysin, chromogranin, INSM1) and TTF1 staining (B).
Paraganglioma could not express cytokeratin (D).
Treatment of neuroendocrine carcinomas is not different based on the subtype of small and large cell neuroendocrine carcinoma (E).
Comment Here
Reference: Neuroendocrine neoplasm
Comment Here
Reference: Neuroendocrine neoplasm
Papillary squamous cell carcinoma
Table of Contents
Definition / general | Treatment | Gross description | Microscopic (histologic) description | Differential diagnosisDefinition / general
- Uncommon; exophytic growth pattern, features of carcinoma in situ plus foci of invasion
- Mean age in 60s
- Usually in larynx, oral cavity, oropharynx, hypopharynx and sinonasal tract
- Associated with HPV
- Relatively good prognosis; usually T2
- Difficult to determine invasion in biopsy specimens, although should consider invasive if clinically appreciable exophytic mass plus marked atypia, even without definitive stromal invasion
Treatment
- Surgery
Gross description
- Often solitary with exophytic or papillary growth; 2 mm to 4 cm
Microscopic (histologic) description
- Finger-like projections with fibrovascular cores or broad based bulbous growth with rounded projections and limited fibrovascular cores
- Overlying squamous epithelium is malignant; usually no / limited surface keratinization
Differential diagnosis
- Laryngeal papillomatosis:
- Bland epithelial proliferation with no / minimal atypia
- Verrucous carcinoma:
- Minimal atypia
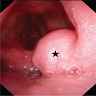
Papilloma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
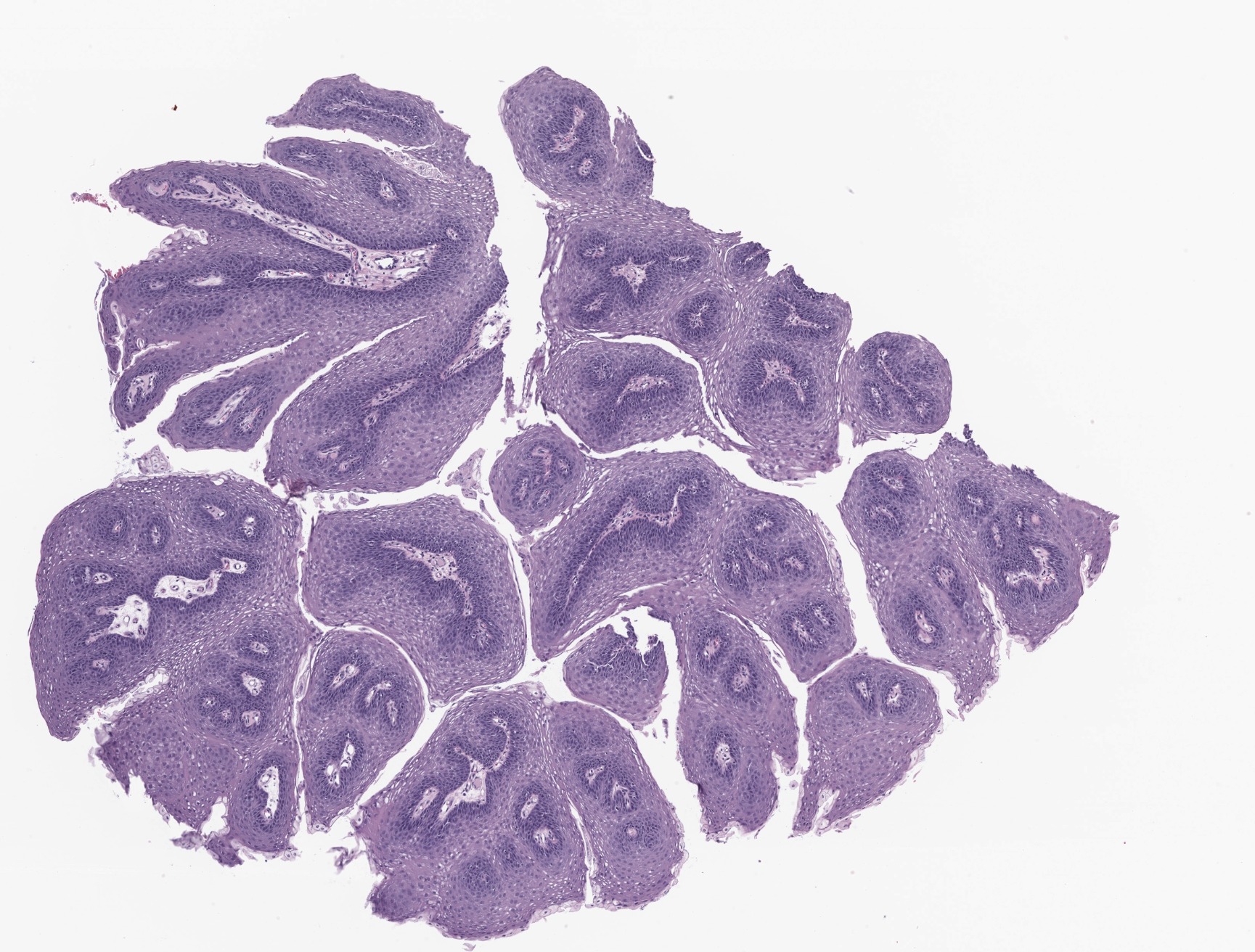
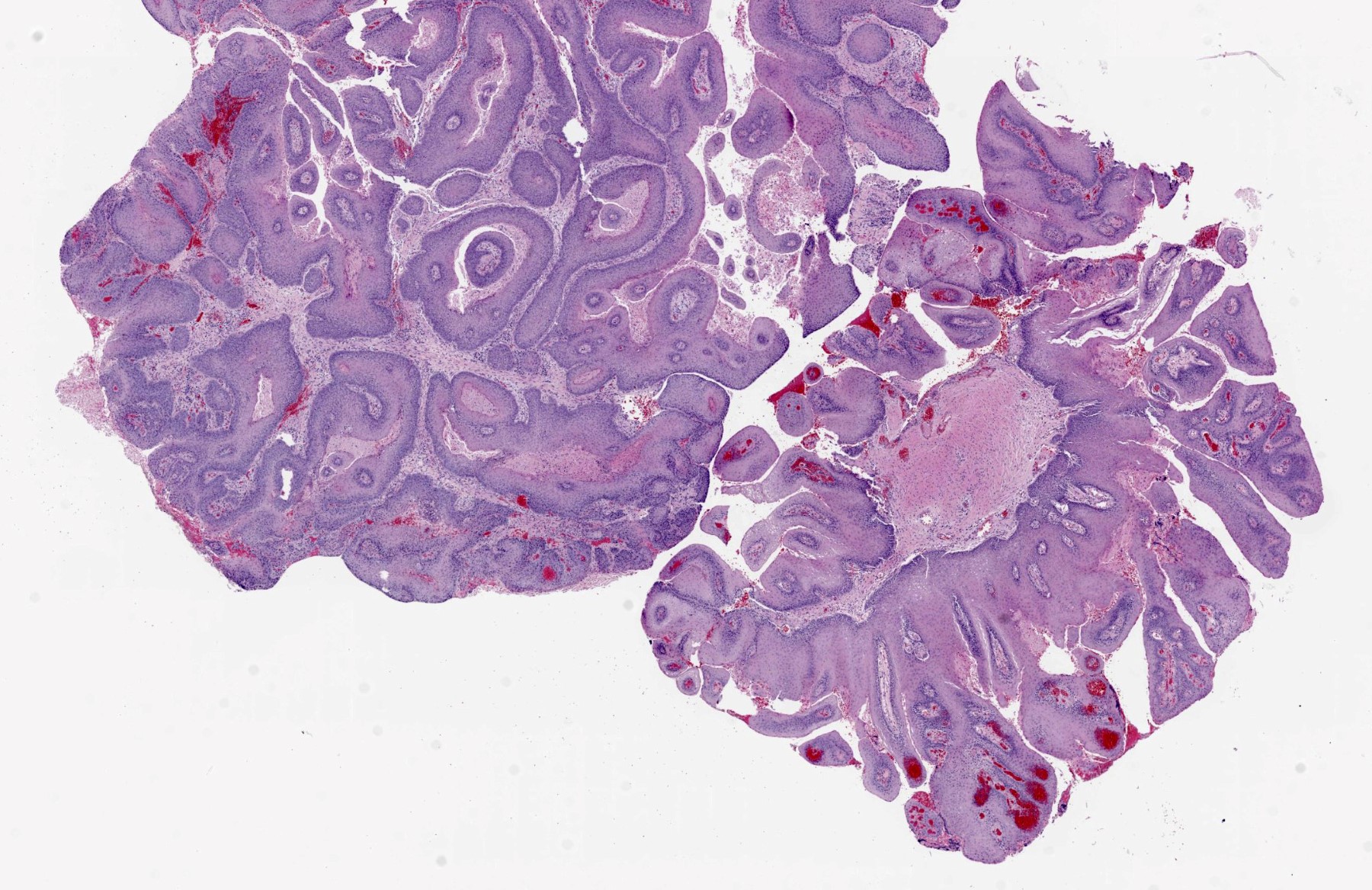
- Benign, exophytic squamous epithelial proliferation that is composed of branching papillary fronds with central fibrovascular cores lined by hyperplastic nonkeratinizing squamous epithelium, usually associated with low risk human papilloma virus (HPV) infection; in particular, genotypes 6 and 11
Essential features
- Benign exophytic / papillary squamous proliferation
- Branching papillae with central fibrovascular cores covered by hyperplastic squamous epithelium
- Associated with low risk HPV infection; in particular, genotypes 6 and 11
Terminology
- Squamous papilloma
- Squamous cell papilloma
- Squamous papillomatosis
- Squamous cell papillomatosis
- Recurrent respiratory papillomatosis
- Laryngeal papillomatosis
- Juvenile onset papillomatosis
- Adult onset papillomatosis
ICD coding
- ICD-O:
- ICD-10: D14.1 - benign neoplasm of larynx
- ICD-11: 2F00.1 & XA2RH5 & XH50N3 - recurrent respiratory papillomatosis & larynx & squamous papillomatosis
Epidemiology
- Most common benign epithelial tumor of the larynx
- Incidence: 43 per million in children, 18 - 23 per million in adults (Laryngoscope 2008;118:1236, Ther Clin Risk Manag 2015;11:731)
- Bimodal age distribution of disease onset:
- Children: often occurs in < 5 years, no sex predilection
- Adults: most frequently impacts patients in their 20s to 40s but may occur at any age; male predominant with M:F = ~3:2
- Decreased incidence in children since the introduction of quadrivalent HPV vaccination program (J Infect Dis 2018;217:208)
Sites
- Affects sites where ciliated respiratory type and squamous epithelium are juxtaposed
- Most frequently occurs in the larynx (involving the true vocal folds and ventricles), followed by the false vocal cords (Laryngoscope 2018;128:138)
- May rarely extend to extralaryngeal locations, such as epiglottis, subglottic area, hypopharynx and nasopharynx, tracheobronchial tree and pulmonary parenchyma (Respir Med 2017;126:116)
Pathophysiology
- HPV binds and replicates in the nuclei of the basal cell layer of metaplastic squamous mucosa (Laryngoscope Investig Otolaryngol 2019;4:89)
- The virus establishes itself through nonproductive replication as a low copy number episome by using the host DNA replication machinery
- Low viral load in the basal cells contributes to immune evasion and viral persistence (Laryngoscope Investig Otolaryngol 2019;4:89)
- HPV DNA replication can cause tumorogenesis and epithelial stratification
Etiology
- Virus induced lesion caused by low risk HPV
- Low risk HPV is detected in over 90% of patients
- Most common genotype is HPV6 (in 64%) followed by HPV11 (in 19%) (Viruses 2021;13:1624)
- Mode of infection differs according to patient's age
- Children: mostly vertical transmission at birth
- Firstborn child, vaginal delivery, maternal age < 20 years and active maternal genital HPV infection, are risk factors
- Cesarean section decreases the risk (Viruses 2021;13:1624)
- Adult: sexual contact and reactivation of latent infection from childhood
- Children: mostly vertical transmission at birth
- Unknown etiology in HPV negative papilloma / papillomatosis, which occurs mostly in adult setting and is associated with an increased risk of laryngeal carcinoma (PLoS One 2014;9:e99114)
Clinical features
- Can be divided into 4 subtypes: juvenile solitary, juvenile multiple, adult solitary and adult multiple
- Children: triad of progressive hoarseness, stridor and breathing difficulty (Respir Med 2017;126:116)
- Adults: most commonly hoarseness (Respir Med 2017;126:116)
- Symptoms tend to be more severe in children, due to the rapid growth of lesions and propensity for airway obstruction
- In severe cases, patients develop airway obstruction and respiratory distress
Diagnosis
- Diagnosis is typically rendered on biopsy, using laryngoscopy or bronchoscopy
- Direct laryngoscopy or fiberoptic bronchoscopy shows exophytic or papillary lesion(s)
Radiology description
- Helical computed tomography (CT) is an accurate method to identify the location of the disease and to characterize airway narrowing (Otolaryngol Clin North Am 2019;52:669, Respir Med 2017;126:116)
Prognostic factors
- Clinical course is unpredictable
- Adverse risk factors associated with aggressive behavior of the lesion (e.g., extralaryngeal spread and recurrence) include: HPV11 infection, younger age of onset, tracheostomy performed to avoid airway obstruction and previous invasive procedures (Viruses 2021;13:1624)
- Carcinomatous transformation reported in 3 - 6% of patients; reported to be associated with pulmonary disease, adult onset disease, previous irradiation and smoking (Viruses 2021;13:1624)
Case reports
- 34 year old man with concomitant tonsillar cyst and papilloma of the larynx (Medicine (Baltimore) 2018;97:e11280)
- 65 year old woman with dyspnea for the past 20 years (Respir Med Case Rep 2022;36:101607)
- 73 year old man with adult onset recurrent respiratory papillomatosis and sarcoma (Iran J Otorhinolaryngol 2022;34:59)
Treatment
- Surgical approach (including laser ablation) is the current standard of care (Laryngoscope Investig Otolaryngol 2018;3:22)
- Other treatments include antiviral therapy (such as cidofovir) and intralesional injections of anti-angiogenic drugs (such as bevacizumab) (Respir Med 2017;126:116)
- HPV vaccine may decrease recurrence after surgical debridement (Otolaryngol Clin North Am 2019;52:669)
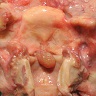
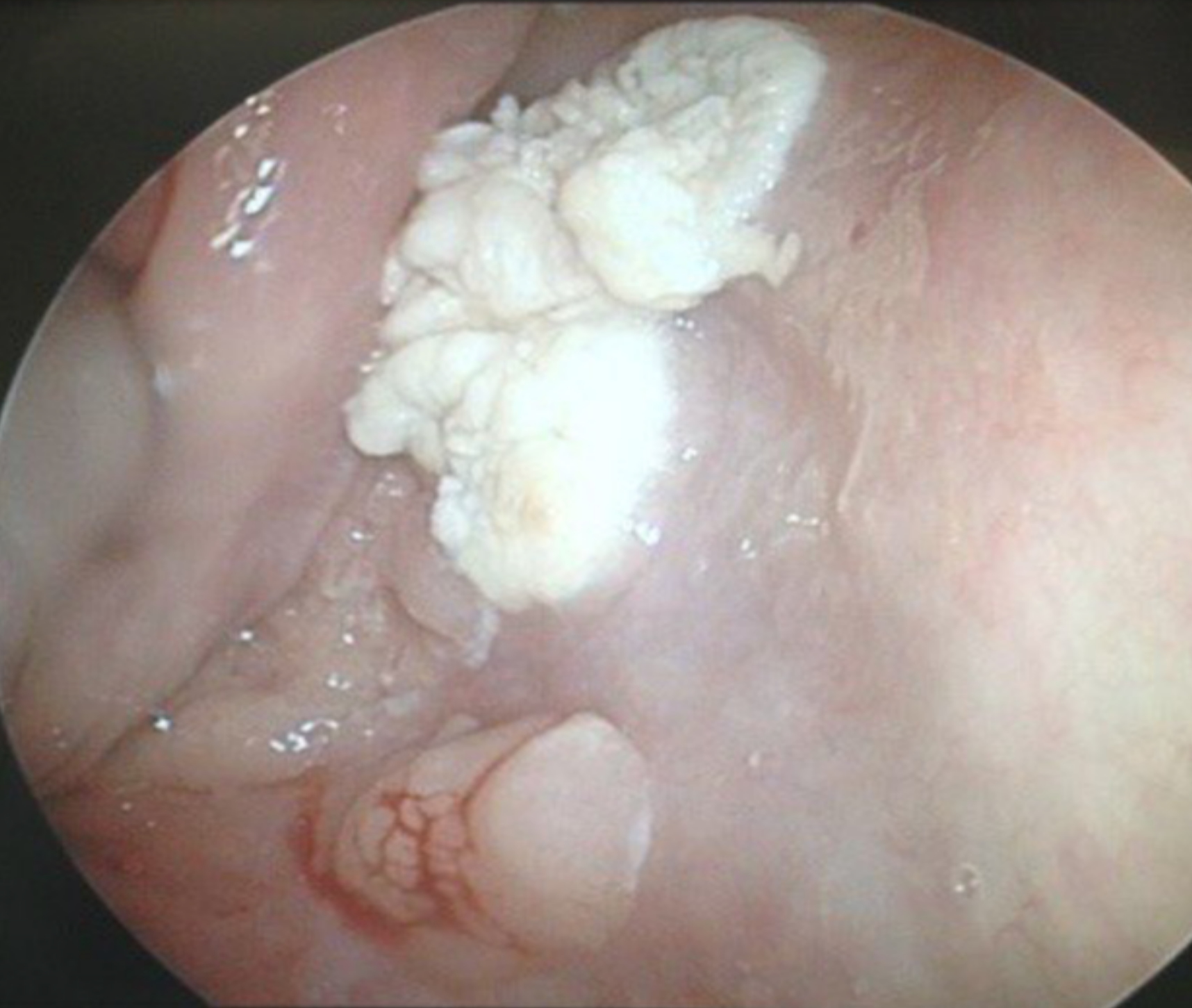
Gross description
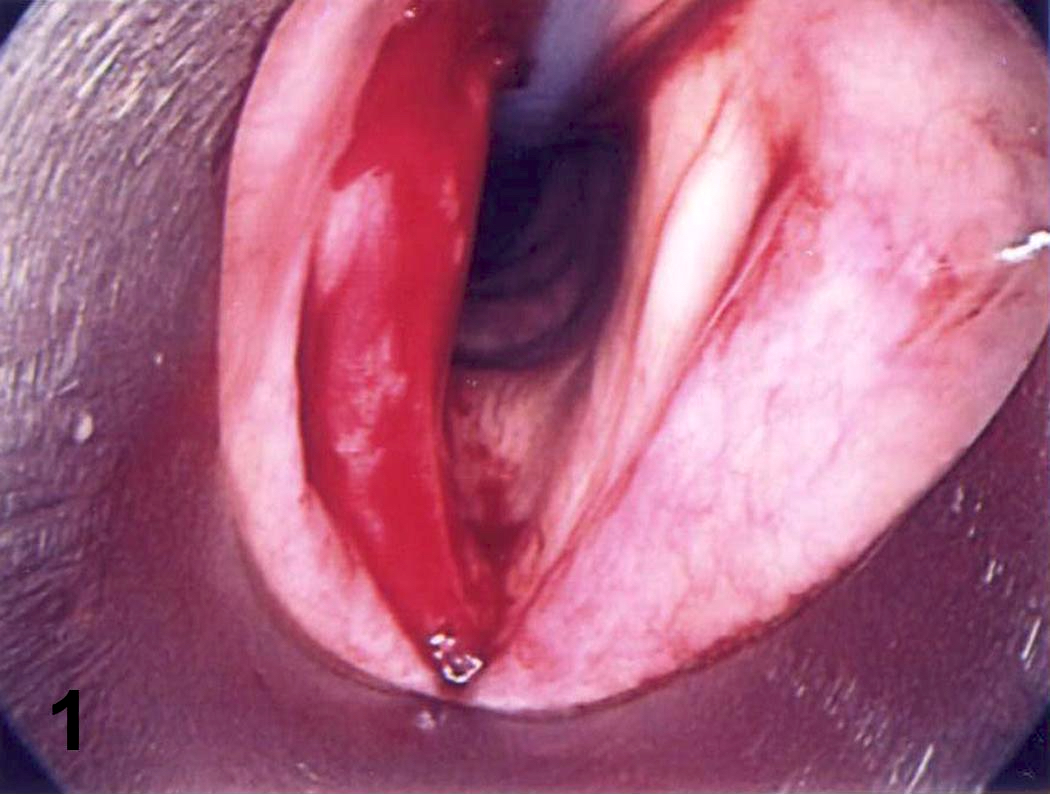
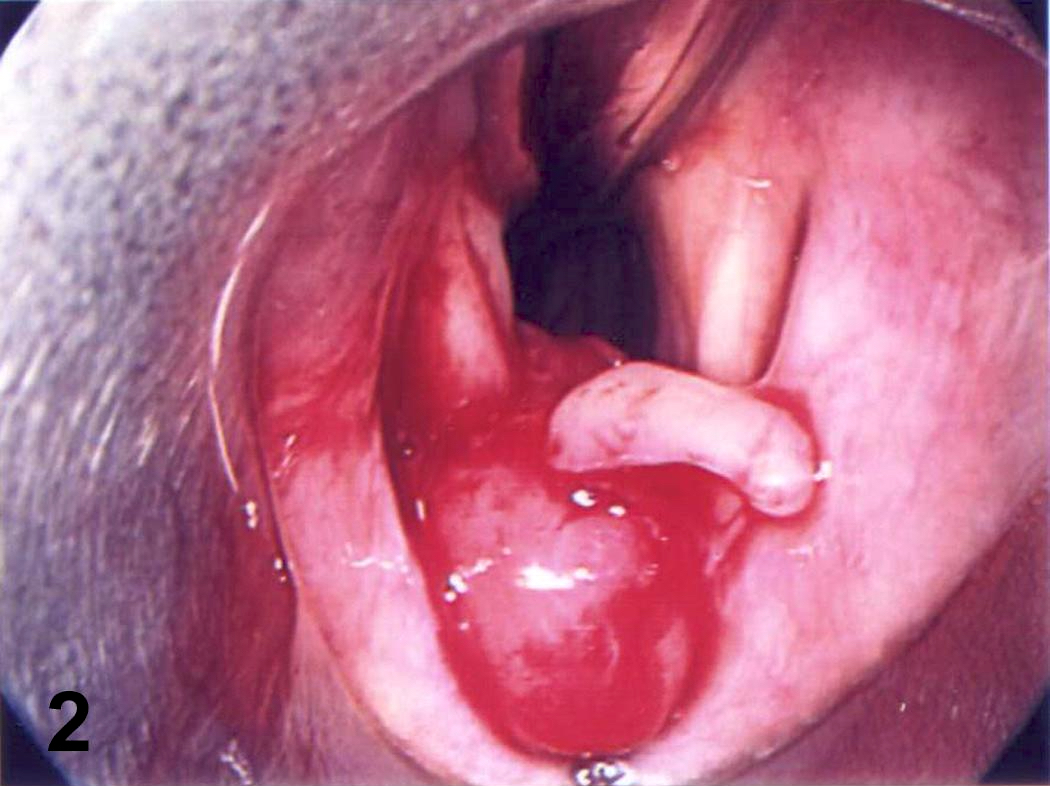
- Can be solid or multiple, variable size
- Exophytic cauliflower-like, sessile or pedunculated pinkish-whitish masses with bosselated surfaces
- Mucosa appears velvety in microscopic (small) papillomas
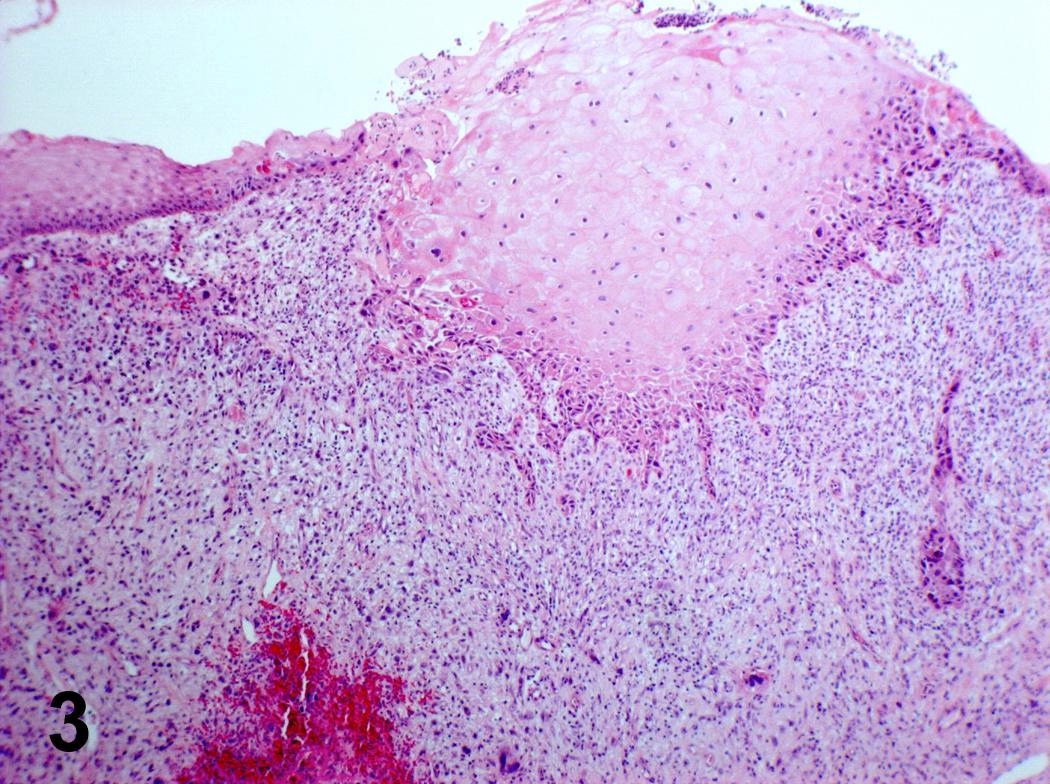
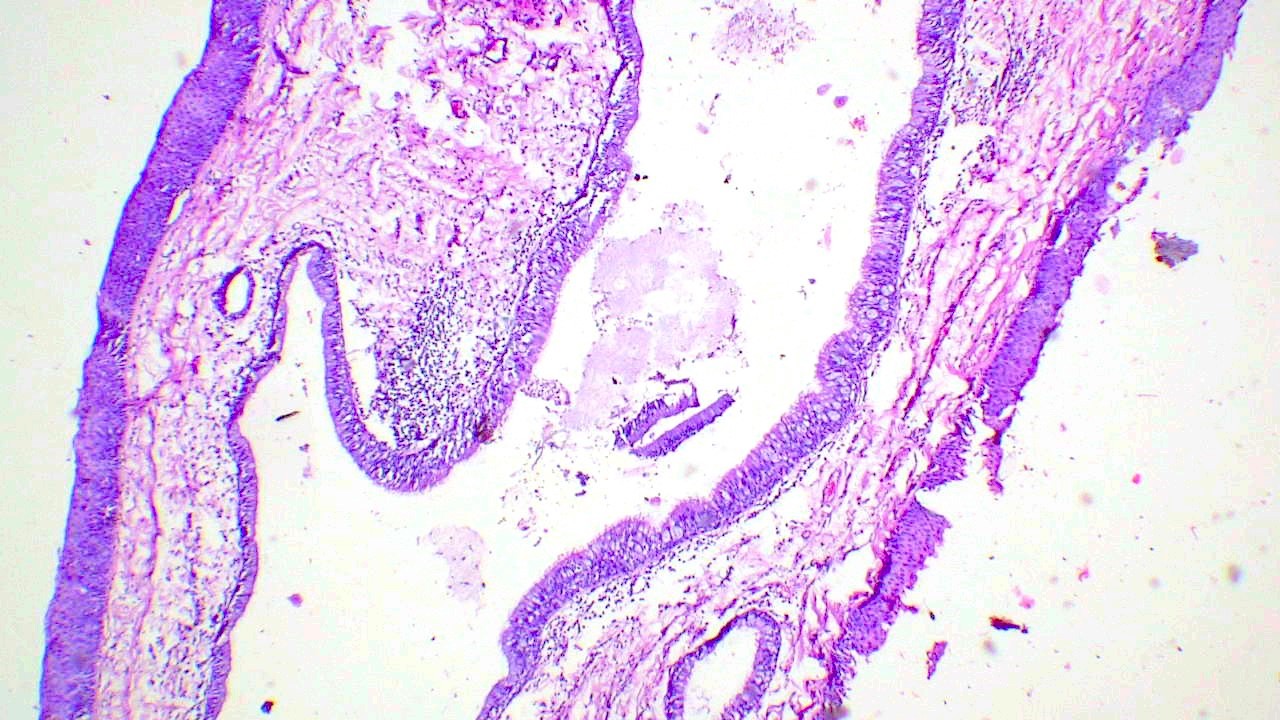
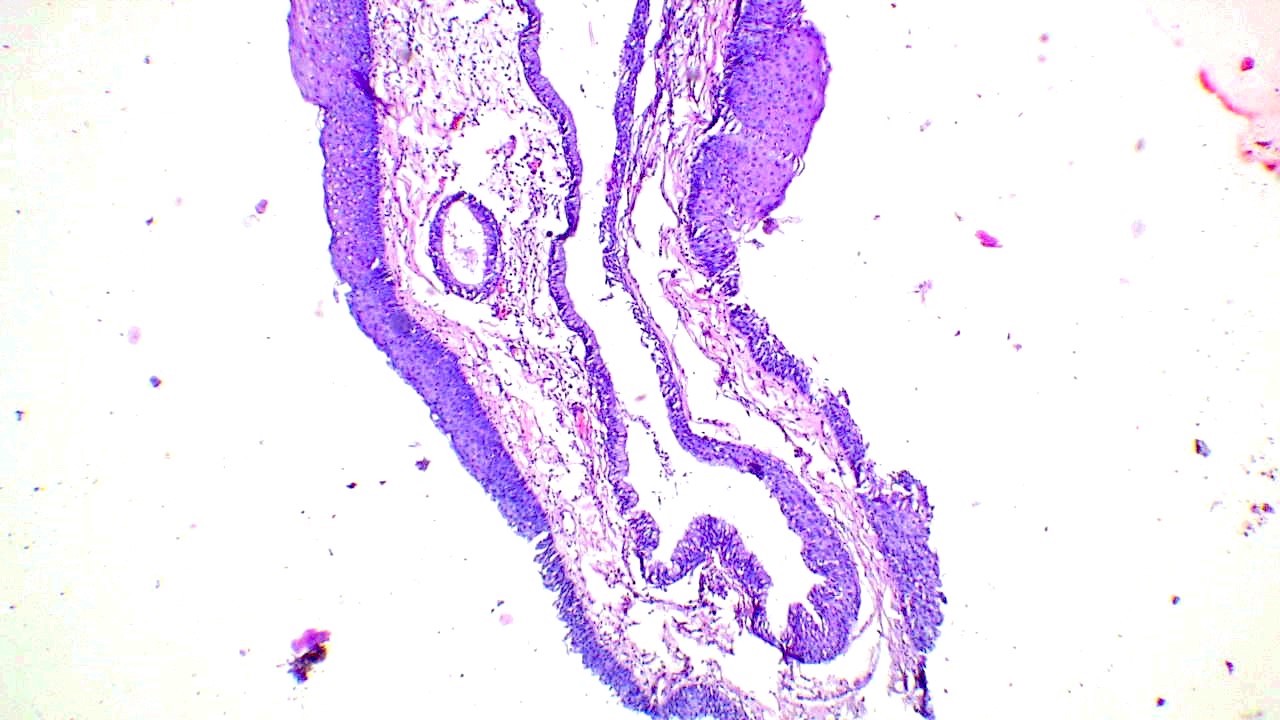
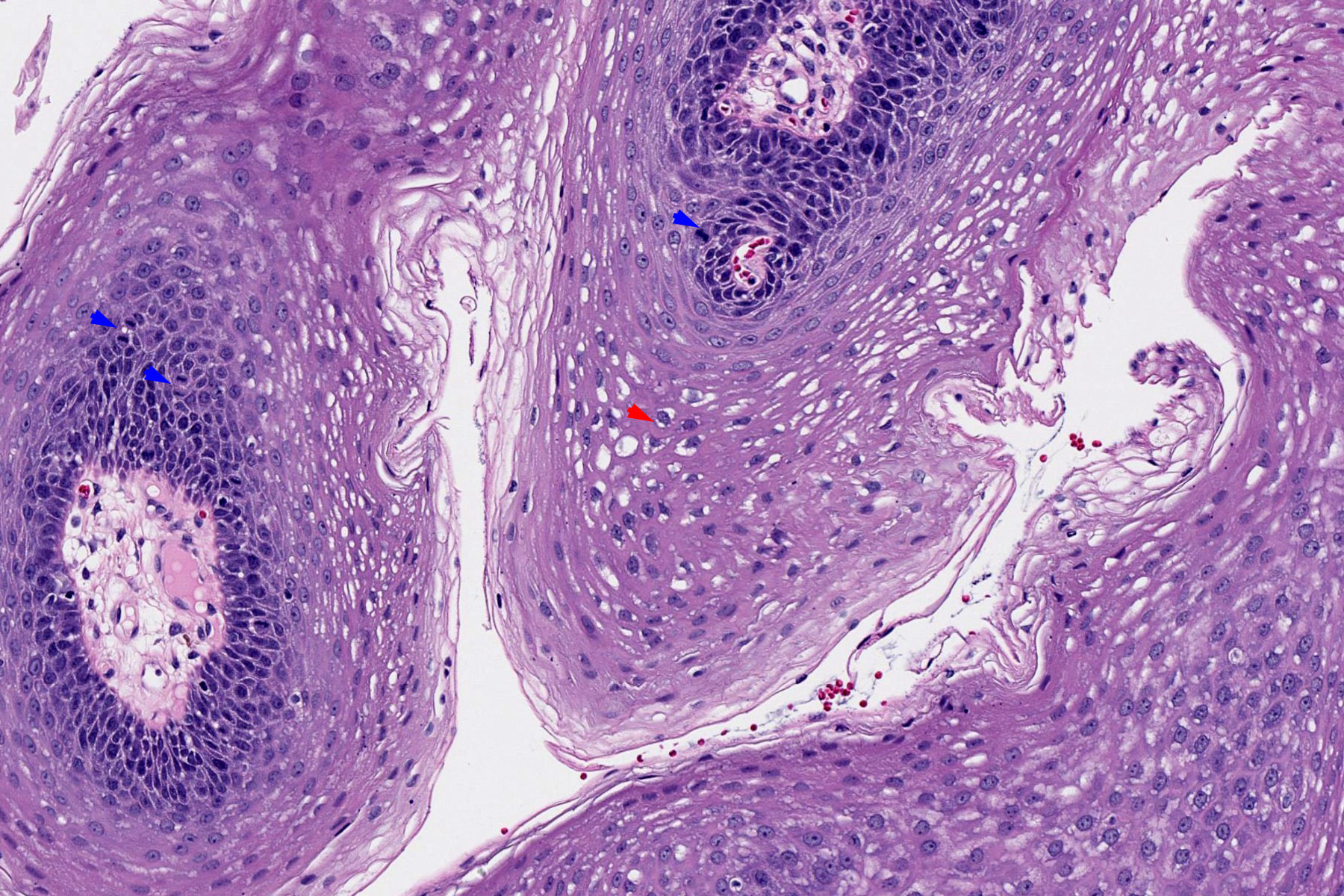
Microscopic (histologic) description
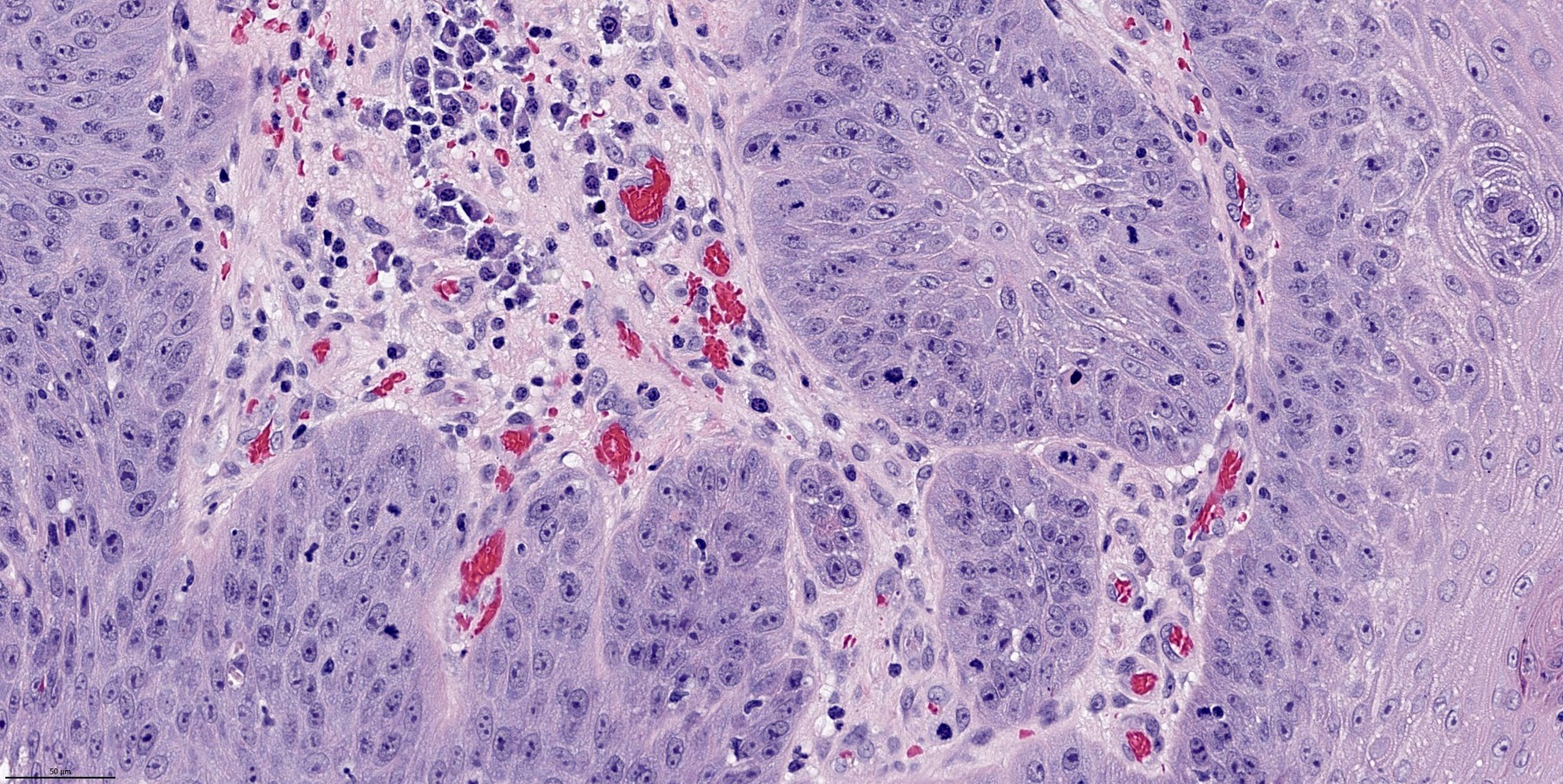
- Finger-like projections or multiple fronds with a central fibrovascular core, covered by benign hyperplastic stratified squamous epithelium
- Basal and parabasal hyperplasia
- Increased mitotic figures in the basal and parabasal layers
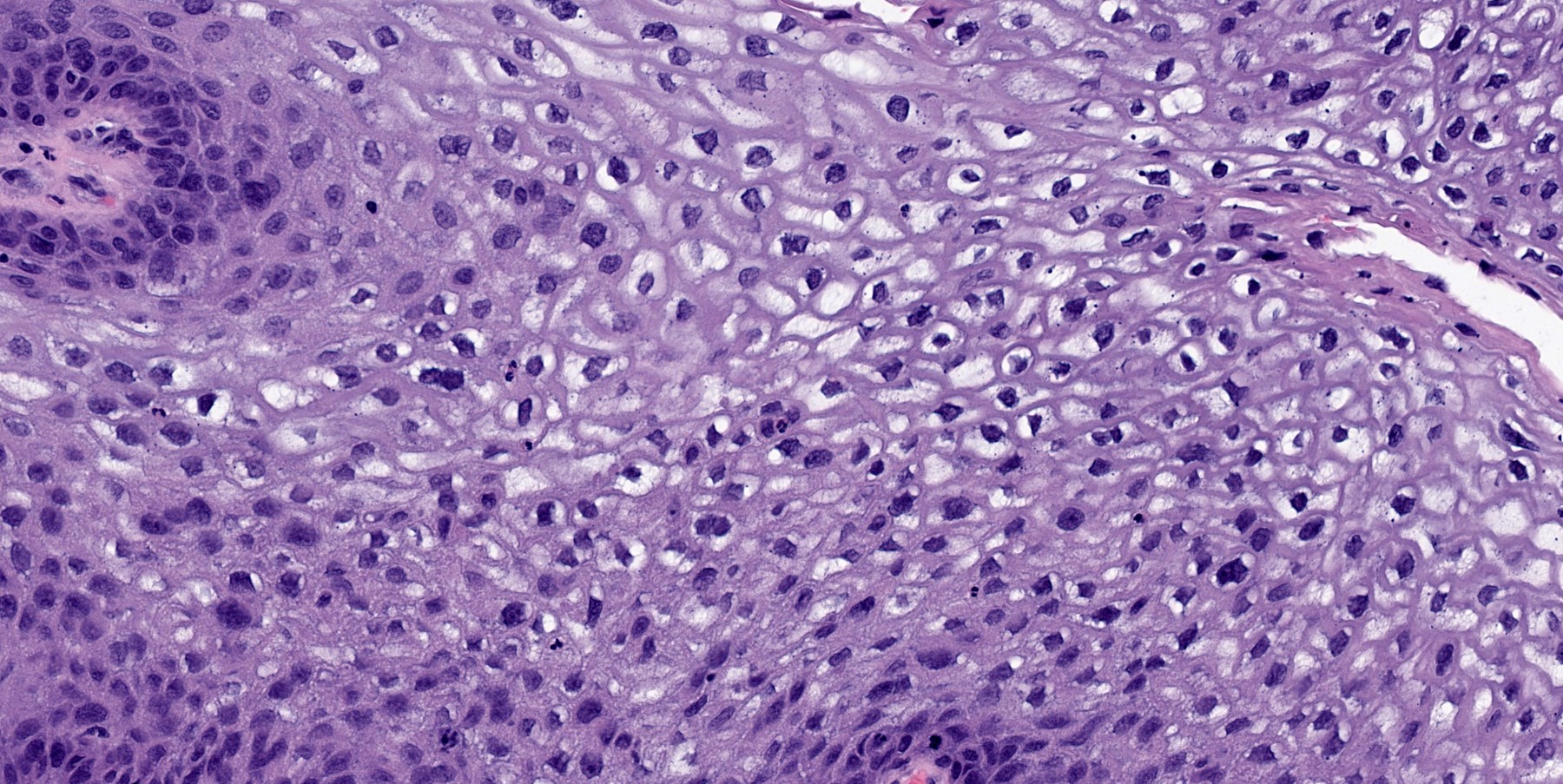
- Koilocytic changes in upper layer: may be pronounced or subtle
- Surface keratinization: absent to minimal
- Malignant transformation is characterized by aberrant (paradoxical) keratinization, marked cytological atypia, focal necrosis, increased mitoses and atypical mitoses not limited to the basal and parabasal layers, as well as invasive growth pattern (Respir Med 2017;126:116)
Microscopic (histologic) images
Positive stains
- RNA or DNA in situ hybridization for low risk HPV is typically positive
- Integrated pattern detected by DNA in situ hybridization is associated with increased risk of recurrence in pediatric patients (Head Neck Pathol 2012;6:3)
Sample pathology report
- Larynx, biopsy:
- Squamous papilloma
Differential diagnosis
- Squamous cell carcinoma:
- Verrucous carcinoma and papillary squamous cell carcinoma may show verruciform or papillary architecture
- Verrucous carcinoma shows keratinization and broad pushing invasion
- Papillary squamous cell carcinoma shows marked cellular pleomorphism, full thickness mitotic activity and atypical mitoses
- Squamous cell carcinoma of the larynx is typically negative for low risk HPV
- Verruca vulgaris:
- Marked hyperkeratosis and prominent keratohyalin granules
- Lack of branching fibrovascular cores
- Rare in larynx
Additional references
Board review style question #1
Board review style answer #1
B. Squamous papilloma. The H&E image shows a squamous papilloma, a benign squamous proliferation with papillary projections.
Comment Here
Reference: Papilloma
Comment Here
Reference: Papilloma
Board review style question #2
What is the most common etiology of laryngeal squamous papillomatosis?
- HPV 11 and HPV 6
- HPV 16 and HPV 6
- HPV 16 and HPV 11
- HPV 16 and HPV 18
Board review style answer #2
Board review style question #3
Which of the following statements is true regarding laryngeal papillomas?
- Although uncommon, they can extend into the trachea and bronchus
- Recurrence is more frequent in adult patients compared with pediatric patients
- Surface keratinization and hyperkeratosis are present in most cases
- They are related to high risk human papilloma virus (HPV)
Board review style answer #3
A. Although uncommon, they can extend into the trachea and bronchus
Comment Here
Reference: Papilloma
Comment Here
Reference: Papilloma
Reactive epithelial hyperplasia
Table of Contents
Definition / general | Gross description | Microscopic (histologic) description | Differential diagnosisDefinition / general
- Also called keratosis
- Usually involves true vocal cords and interarytenoid area
- Associated with smokers, singers and others who use voices excessively
Gross description
- White thickening of involved areas
- Verrucous keratosis if undulating warty configuration
- Pachyderma larynges if extensive keratinization
Microscopic (histologic) description
- When in respiratory (ciliated columnar) epithelium (false cord, ventricle, subglottic region), initially hyperplasia of reserve cells under epithelium, then replacement of epithelium by full thickness reserve cells, then complete squamous metaplasia; may have hyperkeratotic epithelium
- No nuclear abnormalities are present but underlying submucosal glands persist
- Pseudoepitheliomatous hyperplasia:
- Exuberant reactive overgrowth of squamous epithelium without atypia
- May resemble invasive squamous cell carcinoma
Differential diagnosis
- Dysplasia:
- Nuclear atypia
Spindle cell carcinoma
Table of Contents
Definition / general | Prognostic factors | Treatment | Gross description | Microscopic (histologic) description | Positive stains | Negative stains | Differential diagnosis | Additional referencesDefinition / general
- Also called sarcomatoid carcinoma, carcinosarcoma
- Uncommon in larynx; more common elsewhere in upper aerodigestive tract
- 90% male; mean age 66 years, range 35 - 92 years
- Associated with smoking (87%), alcohol (48%) and history of radiation therapy
- 71% are glottic; 59% are T1
- May recur locally (18%) or have distant metastases (14%); 18% die of disease
- Nodal metastases may have epithelial or stromal patterns or both
Prognostic factors
- Depth of invasion more important than differentiation of spindle component
Treatment
- Surgery with or without radiation; 45% recur
Gross description
- Polypoid (99%) tumors, mean size 2 cm
Microscopic (histologic) description
- Squamous cell carcinoma with spindle cell component (reactive, sarcomatous or variant of squamous cell carcinoma) that is often storiform or pleomorphic, may contain foci of benign or malignant cartilage or bone
- Frequent mitotic activity; often squamous cell carcinoma in situ
Positive stains
- Vimentin, 34betaE12 and AE1 / AE3; variable smooth muscle actin
Negative stains
Differential diagnosis
- Pure sarcoma
- Reactive conditions
Additional references
Staging-hypopharynx
Table of Contents
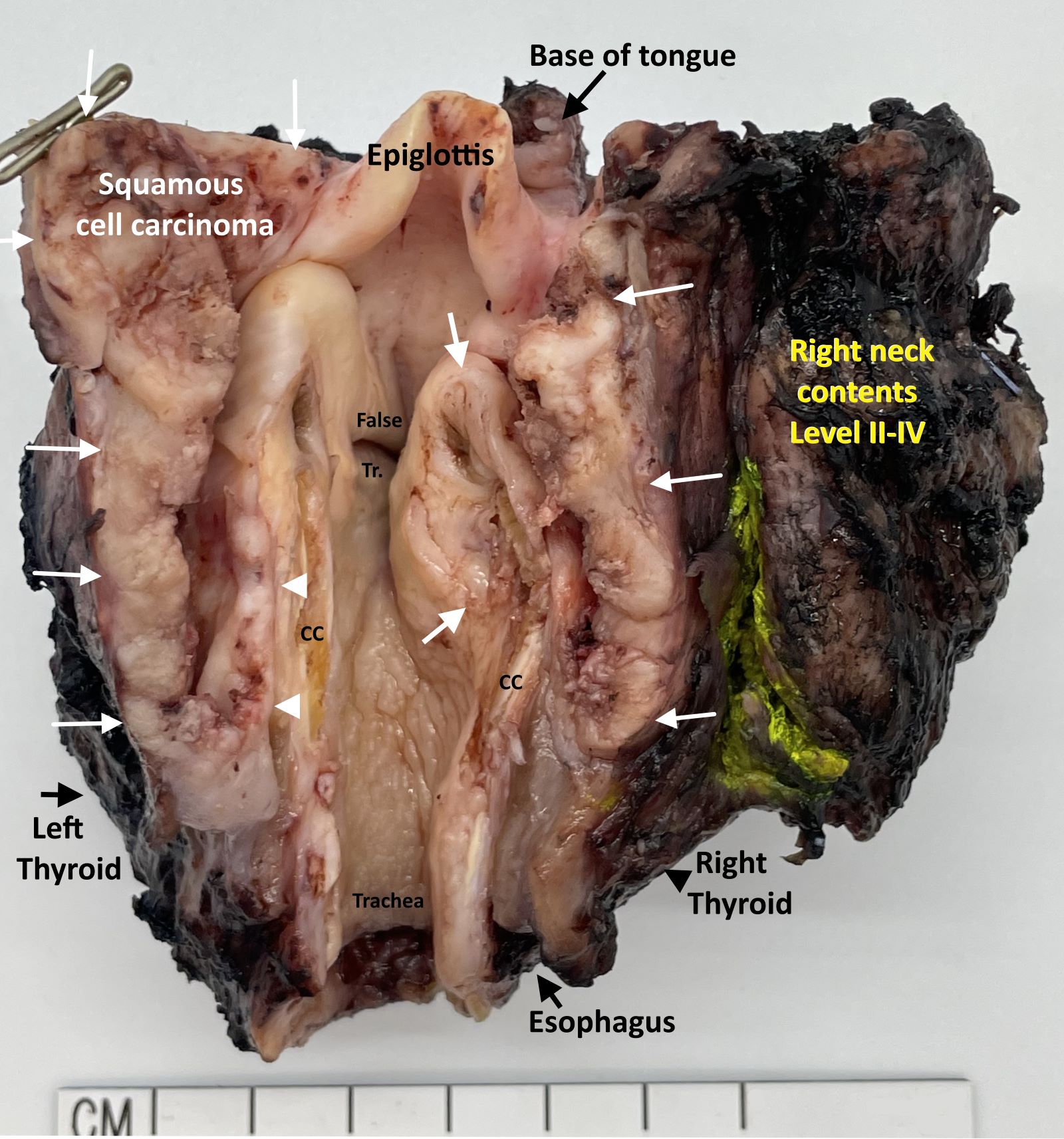
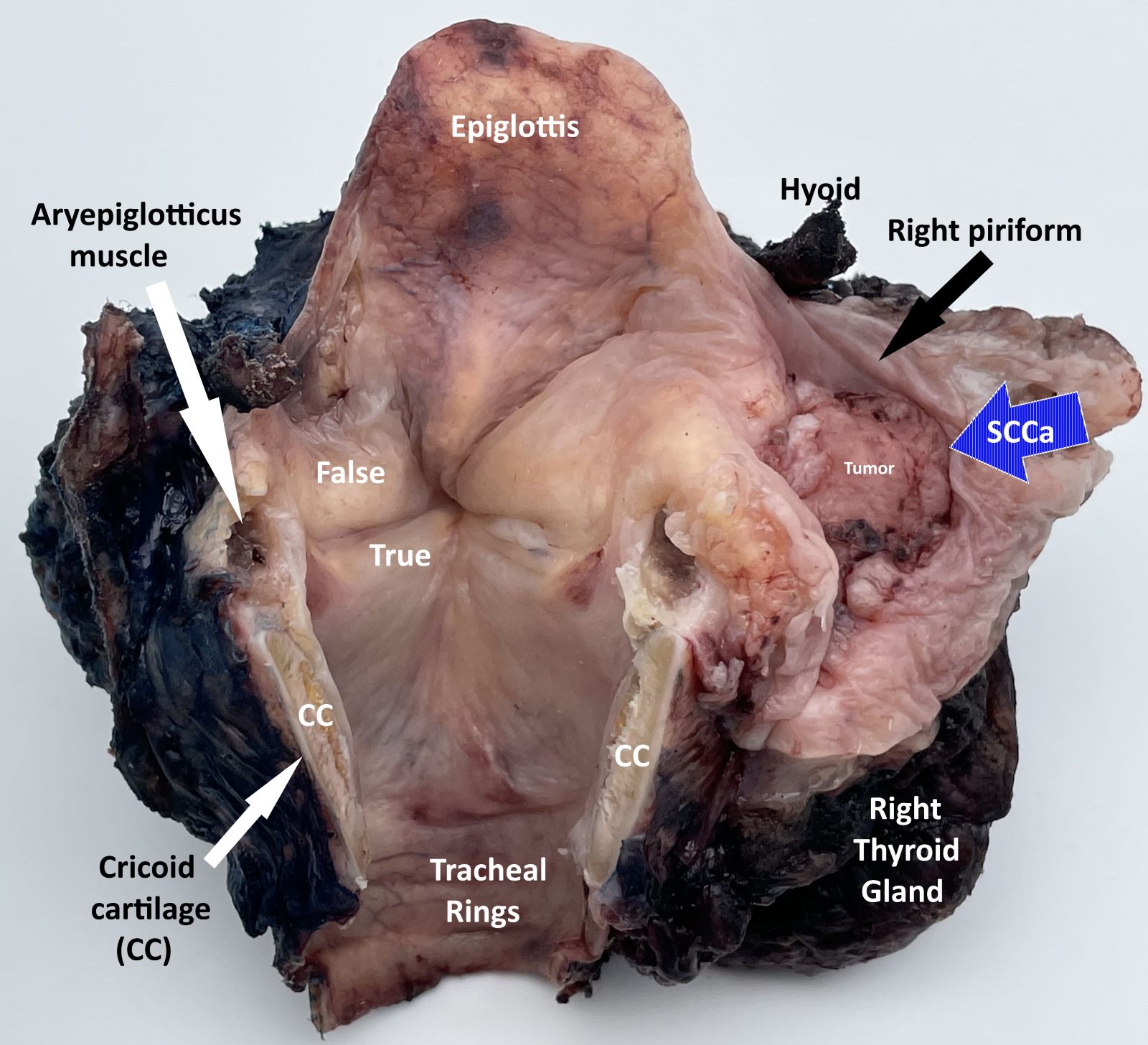
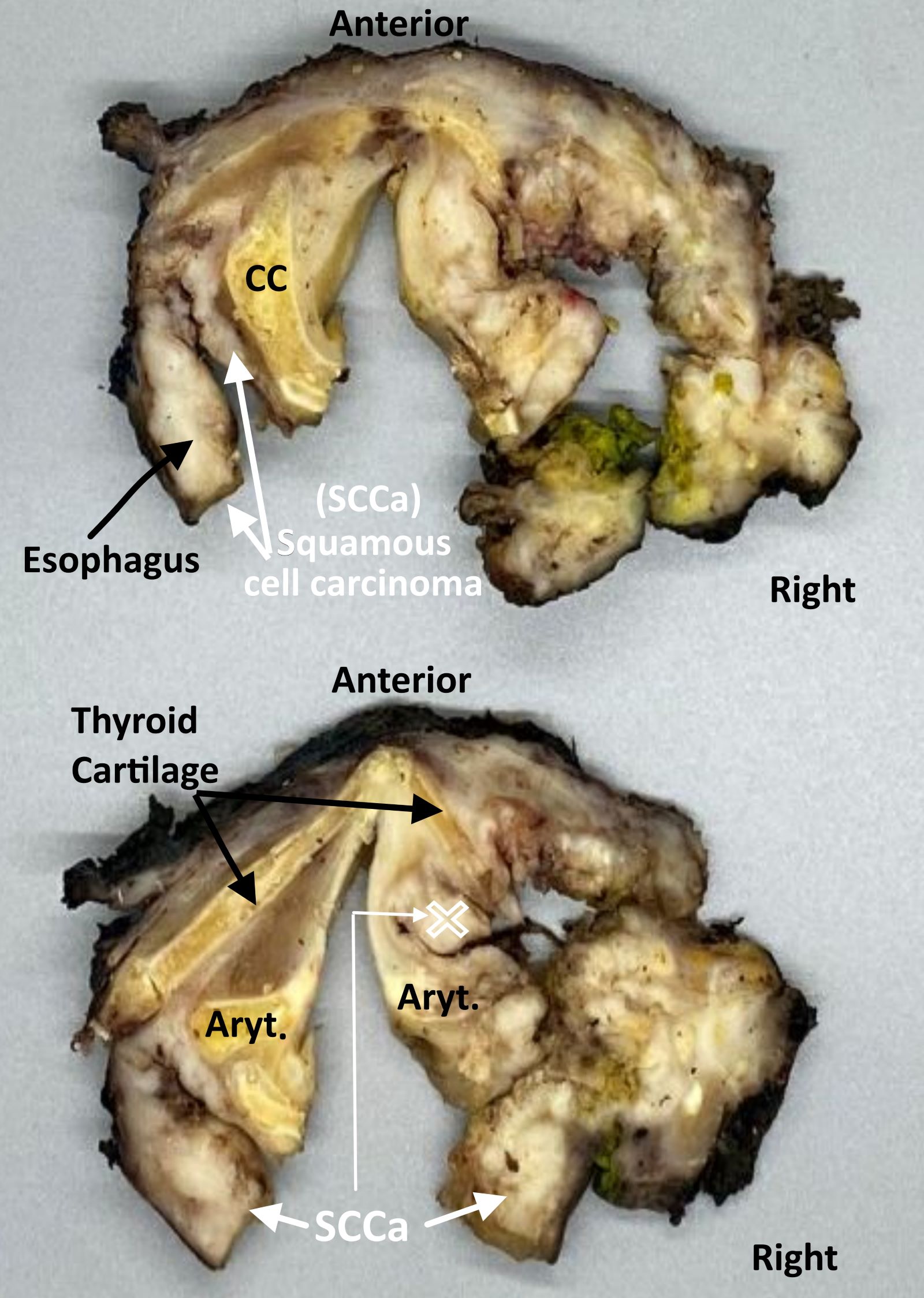
Definition / general | Essential features | Terminology | ICD coding | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | Prefixes | AJCC prognostic stage groups | Registry data collection variables | Histologic grade (G) | Histopathologic type | Clinical images | Gross images | Board review style question #1 | Board review style answer #1Definition / general
- All carcinomas of the hypopharynx are covered by this staging system
- The following topics are not covered: metastatic tumors to the hypopharynx, hematopoietic malignancies, sarcomas of the hypopharynx
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
Terminology
- Hypopharynx:
- Portion of the pharynx extending from the plane of the superior border of the hyoid bone (or floor of the vallecula) to the plane corresponding to the lower border of the cricoid cartilage
- Contents include:
- Pyriform sinus (right and left): represents part of the hypopharynx which expands bilaterally and forward around the sides of the larynx, and lies between the larynx and the thyroid cartilage
- Lateral and posterior hypopharyngeal walls
- Postcricoid region, extending from the level of the arytenoid cartilage and connecting folds to the inferior border of the cricoid cartilage; it connects the 2 piriform sinuses, thereby forming the anterior wall of the hypopharynx
ICD coding
- ICD-10:
- C10.0 - malignant neoplasm of vallecula
- C10.2 - malignant neoplasm of lateral wall of oropharynx
- C10.3 - malignant neoplasm of posterior wall of oropharynx
- C10.8 - malignant neoplasm of overlapping sites of oropharynx
- C10.9 - malignant neoplasm of oropharynx, unspecified
- C11.1 - malignant neoplasm of posterior wall of nasopharynx
- C12 - malignant neoplasm of pyriform sinus
- C13.0 - malignant neoplasm of postcricoid region
- C13.1 - malignant neoplasm of aryepiglottic fold, hypopharyngeal aspect
- C13.2 - malignant neoplasm of posterior wall of hypopharynx
- C13.8 - malignant neoplasm of overlapping sites of hypopharynx
- C13.9 - malignant neoplasm of hypopharynx, unspecified
Primary tumor (pT)
Regional lymph nodes (pN)
- pN: not assigned (no nodes submitted or found)
- pN: not assigned (cannot be determined based on available pathological information)
- pN0: no regional lymph node metastasis
- pN1: metastasis in a single ipsilateral lymph node, ≤ 3 cm and extranodal extension (ENE)-
- pN2: metastasis in a single ipsilateral lymph node, ≤ 3 cm in greatest dimension and ENE+; or > 3 cm but ≤ 6 cm in greatest dimension and ENE-; or metastases in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension and ENE-; or in bilateral or contralateral lymph node(s), none > 6 cm in greatest dimension and ENE-
- pN2a: metastasis in either:
- Single ipsilateral lymph node that is ≤ 3 cm and ENE+ or
- Single ipsilateral lymph node that is > 3 cm but ≤ 6 cm in greatest dimension and ENE-
- pN2b: metastasis in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension and ENE-
- pN2c: metastasis in bilateral or contralateral lymph node(s), none > 6 cm in greatest dimension and ENE-
- pN2a: metastasis in either:
- pN3: metastasis in a lymph node > 6 cm in greatest dimension and ENE-; or in a single ipsilateral node > 3 cm in greatest dimension and ENE+; or multiple ipsilateral, contralateral or bilateral nodes, any with ENE+; or a single contralateral node of any size and ENE+
- pN3a: metastasis in a lymph node that is > 6 cm in greatest dimension and ENE-
- pN3b: metastasis in either:
- Single ipsilateral lymph node, > 3 cm and ENE+ or
- Multiple ipsilateral, contralateral or bilateral lymph nodes, any with ENE+ or
- Single contralateral lymph node of any size and ENE+
Notes:
- Per AJCC 8th edition, for pN, a selective neck dissection will include 10+ lymph nodes and a comprehensive neck dissection (radical or modified radical neck dissection) will include 15+ lymph nodes
- Negative pathologic examination of a smaller number of nodes still mandates a pN0 designation
- Midline nodes are considered ipsilateral nodes
- Measurement of tumor metastasis:
- Cross sectional diameter of the largest lymph node metastatic deposit (not the lymph node itself) is measured in the gross specimen at the time of macroscopic examination or if necessary, using the histologic slide; may include matted or fused nodes
- Extranodal extension (ENE):
- AJCC 8th edition introduces the use of ENE in pN categorization
- Must be clearly defined as tumor present within the confines of the lymph node and as extending through the lymph node capsule into the surrounding connective tissue, with or without associated stromal reaction
Distant metastasis (pM)
- pM1: distant metastasis
Prefixes
- y: preoperative radiotherapy or chemotherapy
- r: recurrent tumor
AJCC prognostic stage groups
| Stage 0: | Tis | N0 | M0 | |||
| Stage I: | T1 | N0 | M0 | |||
| Stage II: | T2 | N0 | M0 | |||
| Stage III: | T3 | N0 | M0 | or | T1 - 3 | |
| Stage IVA: | T4a | N0 - 1 | M0 | or | T1 - 4a | |
| Stage IVB: | T4b | any N | M0 | or | any T | |
| Stage IVC: | any T | any N | M1 |
Registry data collection variables
- Extranodal extension clinical (present versus absent)
- Extranodal extension pathological (present versus absent)
- Extent of microscopic extranodal extension (distance of extension from the native lymph node capsule to the farthest point of invasion in extranodal tissue)
- Perineural invasion
- Lymphovascular invasion
- p16 / HPV status
- Performance status
- Tobacco use and pack years
- Alcohol use
- Depression diagnosis
Histologic grade (G)
- GX: cannot be assessed
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
Histopathologic type
- Carcinoma of the hypopharynx:
- Squamous cell carcinoma:
- HPV unrelated (negative) squamous cell carcinoma
- Variants of squamous cell carcinoma:
- Keratinizing
- Nonkeratinizing
- Acantholytic squamous cell carcinoma
- Adenosquamous carcinoma
- Basaloid squamous cell carcinoma
- Papillary squamous cell carcinoma
- Spindle cell squamous cell carcinoma
- Verrucous squamous cell carcinoma
- Lymphoepithelial carcinoma
- Carcinoma of minor salivary gland:
- Acinic cell carcinoma
- Adenoid cystic carcinoma
- Adenocarcinoma, NOS
- Basal cell adenocarcinoma
- Carcinoma ex pleomorphic adenoma
- Carcinoma type cannot be determined
- Carcinosarcoma
- Clear cell adenocarcinoma
- Cystadenocarcinoma
- Epithelial myoepithelial carcinoma
- Secretory carcinoma
- Mucoepidermoid carcinoma
- Mucinous carcinoma
- Myoepithelial carcinoma
- Oncocytic carcinoma
- Polymorphous adenocarcinoma
- Salivary duct carcinoma
- Neuroendocrine carcinoma:
- Small cell neuroendocrine carcinoma, HPV-
- Small cell neuroendocrine carcinoma, HPV+
- Large cell neuroendocrine carcinoma, HPV-
- Large cell neuroendocrine carcinoma, HPV+
- Squamous cell carcinoma:
Clinical images
Gross images
Board review style question #1
Which of the following is the pTNM stage (per the AJCC 8th edition) of a 3.1 cm right hypopharyngeal squamous cell carcinoma invading the right thyroid cartilage and true cord, with no lymph node metastases identified?
- pT1c N0, stage IC
- pT1c N0, stage IVB
- pT3b N0, stage IIIB
- pT3c N0, stage IVB
- pT4a N0, stage IVA
Board review style answer #1
E. pT4a N0, stage IVA. Invasion into the thyroid cartilage, irrespective of tumor size is pT4a.
Comment Here
Reference: Staging-hypopharynx
Comment Here
Reference: Staging-hypopharynx
Staging-larynx
Table of Contents
Definition / general | Essential features | Anatomy | ICD coding | Diagrams / tables | Primary tumor (pT) - supraglottis | Primary tumor (pT) - glottis | Primary tumor (pT) - subglottis | Pathological regional lymph nodes (pN) | Distant metastasis (pM) | AJCC prognostic stage grouping | Registry data collection variables | Histologic grade | Histopathologic type | Gross images | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Staging of definitive resections for carcinoma (squamous cell carcinoma, neuroendocrine carcinoma and minor salivary gland carcinoma) of the supraglottic, glottis and subglottic larynx should use this system
- Pyriform sinus represents part of the hypopharynx which expands bilaterally and forward around the sides of the larynx and lies between the larynx and the thyroid cartilage
- Cancers of the pyriform sinus are included in the protocol on pharynx cancers
- Staging protocols such as those of CAP document procedure, tumor site, transglottic extension, tumor laterality, tumor focality, tumor size, histologic type, histologic grade for squamous cell carcinoma, margin status, lymphovascular invasion, perineural invasion, regional lymph node findings and summarize these pathologic findings using TNM system
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- CAP protocol recommends the TNM staging system of the American Joint Committee on Cancer
- There are no significant alterations in the 8th edition to T stage of larynx
- However, extranodal extension (ENE) is now included in N stage
- In essence, pathologic ENE+ will increase the nodal category by 1
- Use of the CAP protocol is not required for recurrent tumors or for metastatic tumors that are resected at a different time than the primary tumor
- Use of the CAP protocol is not required for pathology reviews performed at a second institution (i.e., secondary consultation, second opinion or review of outside case at second institution)
- CAP larynx staging protocol (CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Larynx [Accessed 26 September 2018])
- Recently, the International Collaboration on Cancer Reporting (ICCR) guidelines have also become available for use (ICCR: Carcinomas of the Hypopharynx, Larynx and Trachea (TNM8) [Accessed 26 September 2018])
Anatomy
- Anatomic boundaries of the larynx (AJCC: Cancer Staging [Accessed 26 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Larynx [Accessed 26 September 2018]):
- Anterior limit of the larynx is composed of the anterior or lingual surface of the suprahyoid epiglottis, the thyrohyoid membrane, the anterior commissure and the anterior wall of the subglottic region, which is composed of the thyroid cartilage, the cricothyroid membrane and the anterior arch of the cricoid cartilage
- Posterior and lateral limits include the laryngeal aspect of the aryepiglottic fold, the arytenoid region, the interarytenoid space and the posterior surface of the subglottic space represented by the mucous membrane covering the surface of the cricoid cartilage
- Superolateral limits are composed of the tip and the lateral borders of the epiglottis
- Inferior limits are made up of the plane passing through the inferior edge of the cricoid cartilage
- Anatomic compartments, sites and subsites for the larynx (AJCC: Cancer Staging [Accessed 26 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Larynx [Accessed 26 September 2018], see figure 1 in Diagrams / tables):
- Supraglottis: extends from the tip of the epiglottis to a horizontal line passing through the apex of the ventricle
- Epilarynx, including marginal zone
- Suprahyoid epiglottis, including tip, lingual (anterior) and laryngeal surfaces
- Aryepiglottic fold, laryngeal aspect
- Arytenoid
- Supraglottis, excluding epilarynx
- Infrahyoid epiglottis
- Ventricular bands (false cords)
- Ventricle
- Epilarynx, including marginal zone
- Glottis: extends from the ventricle to approximately 0.5 - 1.0 cm below the free level of the true vocal cord
- Vocal cords
- Anterior commissure
- Posterior commissure
- Subglottis: extends approximately 1.0 cm below the level of the true vocal cord to the inferior rim of the cricoid cartilage
- Subglottis has no additional subsites
- Supraglottis: extends from the tip of the epiglottis to a horizontal line passing through the apex of the ventricle
- Additional anatomic compartments (AJCC: Cancer Staging [Accessed 26 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Larynx [Accessed 26 September 2018]):
- Paraglottic space is a potential space deep to the ventricles and saccules filled with adipose tissue and connective tissue (see figure 2 in Diagrams / tables)
- It is bounded by the conus elasticus inferiorly, the thyroid cartilage laterally, the quadrangular membrane medially and the piriform sinus posteriorly
- Pre-epiglottic space, like the paraglottic space, is filled with adipose tissue and connective tissue (see figure 3 in Diagrams / tables)
- It is triangular in shape and is bounded by the thyroid cartilage and thyrohyoid membrane anteriorly, the epiglottis and thyroepiglottic ligament posteriorly and the hyoepiglottic ligament at its base (see figures 1 and 2 Diagrams / tables)
- Paraglottic and preglottic spaces contain lymphatics and blood vessels but no lymph nodes
- Paraglottic space is a potential space deep to the ventricles and saccules filled with adipose tissue and connective tissue (see figure 2 in Diagrams / tables)
- Site specific carcinomas (AJCC: Cancer Staging [Accessed 26 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Larynx [Accessed 26 September 2018]):
- Supraglottic squamous cell carcinoma: a squamous cell carcinoma that involves the structures of the supraglottic larynx, including the epiglottis (laryngeal and lingual surfaces), aryepiglottic folds, arytenoids, false vocal cords and ventricles
- Glottic squamous cell carcinoma: a squamous cell carcinoma that involves the structures of the glottis, including the true vocal cords and the anterior and posterior commissures
- Subglottic squamous cell carcinoma: a squamous cell carcinoma that involves the subglottis, which begins 1 cm below the apex of the ventricle to its inferior border represented by the rim of the cricoid cartilage
- Transglottic carcinoma: a carcinoma that crosses the ventricles in a vertical direction arising in either the glottic or supraglottic larynx
ICD coding
Diagrams / tables
Primary tumor (pT) - supraglottis
- pTX: Primary tumor cannot be assessed
- pTis: Carcinoma in situ
- pT1: Tumor limited to 1 subsite of supraglottis with normal vocal cord mobility
- pT2: Tumor invades mucosa of > 1 adjacent subsite of supraglottis or glottis or region outside the supraglottis (e.g. mucosa of base of tongue, vallecula, medial wall of pyriform sinus) without fixation of the larynx
- pT3: Tumor limited to larynx with vocal cord fixation or invades any of the following: postcricoid area, pre-epiglottic space, paraglottic space or inner cortex of thyroid cartilage
- pT4a: Moderately advanced local disease: invades through the outer cortex thyroid cartilage or invades tissues beyond the larynx (e.g. trachea, soft tissues of neck including deep extrinsic muscle of tongue, strap muscles, thyroid or esophagus)
- pT4b: Very advanced local disease: invades prevertebral space, encases carotid artery or invades mediastinal structure
Primary tumor (pT) - glottis
- pTX: Primary tumor cannot be assessed
- pTis: Carcinoma in situ
- pT1: Tumor limited to the vocal cord(s) (may involve anterior or posterior commissure) with normal mobility
- pT1a: Limited to 1 vocal cord
- pT1b: Involves both vocal cords
- pT2: Tumor extends to supraglottis or subglottis or with impaired vocal cord mobility
- pT3: Tumor limited to the larynx with vocal cord fixation or invasion of paraglottic space or inner cortex of the thyroid cartilage
- pT4a: Moderately advanced local disease: invades through the outer cortex of the thyroid cartilage or invades tissues beyond the larynx (e.g. trachea, cricoid cartilage, soft tissues of neck including deep extrinsic muscle of the tongue, strap muscles, thyroid or esophagus)
- pT4b: Very advanced local disease: invades prevertebral space, encases carotid artery or invades mediastinal structures
Primary tumor (pT) - subglottis
- pTX: Primary tumor cannot be assessed
- pTis: Carcinoma in situ
- pT1: Tumor limited to subglottis
- pT2: Tumor extends to vocal cord(s) with normal or impaired mobility
- pT3: Tumor limited to larynx with vocal cord fixation or invasion of paraglottic space or inner cortex of the thyroid cartilage
- pT4a: Moderately advanced local disease: tumor invades cricoid or thyroid cartilage or invades tissues beyond the larynx (e.g. trachea, soft tissues of neck including deep extrinsic muscles of the tongue, strap muscles, thyroid or esophagus)
- pT4b: Very advanced local disease: tumor invades prevertebral space, encases carotid artery or invades mediastinal structures
Pathological regional lymph nodes (pN)
- pNX: Regional lymph nodes cannot be assessed
- pN0: No regional lymph node metastasis
- pN1: Metastasis in a single ipsilateral lymph node ≤ 3 cm in greatest dimension and extranodal extension (ENE)-
- pN2a: Single ipsilateral lymph node ≤ 3 cm and ENE+ or single ipsilateral lymph node > 3 cm but ≤ 6 cm in greatest dimension and ENE-
- pN2b: Metastases in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension and ENE-
- pN2c: Metastases in bilateral or contralateral lymph node(s), none > 6 cm in greatest dimension and ENE-
- pN3a: Metastasis in a lymph node that is > 6 cm in greatest dimension and ENE-
- pN3b: Metastasis in either
- Single ipsilateral lymph node, > 3 cm and ENE+ or
- Multiple ipsilateral, contralateral or bilateral lymph nodes, any with ENE+ or
- Single contralateral lymph node of any size and ENE+
Notes:
- Designation of "U" or "L" may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L)
- Per AJCC 8th edition, for pN, a selective neck dissection ordinarily will include > 10 lymph nodes and a radical or modified radical neck dissection ordinarily will include > 15 lymph nodes
- Negative pathologic examination of a smaller number of nodes still mandates a pN0 designation
- Midline nodes are considered ipsilateral nodes
- Extranodal extension (ENE)
- All macroscopically negative or equivocal lymph nodes should be submitted in toto
- Grossly positive nodes may be partially submitted for microscopic documentation of metastasis
- Reporting of lymph nodes containing metastasis should include whether there is presence or absence of extranodal extension (ENE), which is now part of N staging
- This finding consists of extension of metastatic tumor, present within the confines of the lymph node, through the lymph node capsule into the surrounding connective tissue, with or without associated stromal reaction
- Distance of extension from the native lymph node capsule is now suggested (but not yet required) with the proposed stratification of ENE into ENEma (> 2 mm) and ENEmi (≤ 2 mm)
- However, pitfalls in the measurement (i.e. in larger, matted lymph nodes, in nodes post fine needle aspiration and in nodes with near total replacement of lymph node architecture) and the disposition of soft tissue deposits are still not resolved
- In general, absence of ENE in a large (> 3 cm) lymph node, especially with traversing fibrous bands, should be viewed with skepticism
- Soft tissue deposits for lymph node metastases based on limited studies appear to be the equivalent of a positive lymph node with ENE and should be recorded as such
- Measurement of lymph node metastasis
- Cross sectional diameter of the largest lymph node metastatic deposit (not the lymph node itself) is measured in the gross specimen at the time of macroscopic examination or if necessary, on the histologic slide at the time of microscopic examination
Distant metastasis (pM)
- pM0: No distant metastasis
- pM1: Distant metastasis
AJCC prognostic stage grouping
| Stage 0: | Tis | N0 | M0 |
| Stage I: | T1 | N0 | M0 |
| Stage II: | T2 | N0 | M0 |
| Stage III: | T3 | N0 | M0 |
| T1 - 3 | N1 | M0 | |
| Stage IVA: | T4a | N0 - 1 | M0 |
| T1 - 4a | N2 | M0 | |
| Stage IVB: | Any T | N3 | M0 |
| T4b | Any N | M0 | |
| Stage IVC: | Any T | Any N | M1 |
Registry data collection variables
- ENE clinical (present versus absent)
- ENE pathological (present versus absent)
- Extent of microscopic ENE (distance of extension from the native lymph node capsule to the farthest point of invasion in extranodal tissue)
- Perineural invasion
- Lymphovascular invasion
- Performance status
- Tobacco use and pack year
- Alcohol use
- Depression diagnosis
Histologic grade
- GX: Cannot be assessed
- G1: Well differentiated
- G2: Moderately differentiated
- G3: Poorly differentiated
Notes:
- For histologic types of carcinomas that are amenable to grading, 3 histologic grades are suggested, as shown below (AJCC: Cancer Staging [Accessed 26 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Larynx [Accessed 26 September 2018])
- For conventional squamous cell carcinoma, histologic grading as a whole does not perform well as a prognosticator
- Nonetheless, it should be recorded when applicable as it is a basic tumor characteristic
- Selecting either the most prevalent grade or the highest grade for this synoptic protocol is acceptable
- Variants of squamous cell carcinoma (i.e. verrucous, basaloid, etc.) have an intrinsic biologic potential and currently do not appear to require grading
- Histologic (microscopic) grading of salivary gland carcinomas has been shown to be an independent predictor of behavior and plays a role in optimizing therapy
- However, most salivary gland carcinoma types have an intrinsic biologic behavior and attempted application of a universal grading scheme is merely a crude surrogate
- Thus, a generic grading scheme is no longer recommended for salivary gland carcinomas
- Carcinoma types for which grading systems exist and are relevant are incorporated into histologic type
- 3 major categories that are amenable to grading include adenoid cystic carcinoma, mucoepidermoid carcinoma and adenocarcinoma, not otherwise specified
- In some carcinomas, histologic grading may be based on growth pattern, such as in adenoid cystic carcinoma, for which a histologic high grade variant has been recognized based on the percentage of solid growth
- Those adenoid cystic carcinomas showing ≥ 30% of solid growth pattern are considered to be histologically high grade carcinomas
- Histologic grading of mucoepidermoid carcinoma includes a combination of growth pattern characteristics (e.g. cystic, solid, neurotropism) and cytomorphologic findings (e.g. anaplasia, mitoses, necrosis)
- Adenocarcinomas, not otherwise specified, do not have a formalized grading scheme and are graded intuitively based on cytomorphologic features
Histopathologic type
- Predominant cancer is squamous cell carcinoma
- Staging guidelines are applicable to all forms of epithelial carcinoma including those arising from minor salivary glands
- Other nonepithelial tumors such as lymphoid tissue, bone, cartilage (i.e. lymphoma and sarcoma) are not included
- Squamous cell carcinoma (conventional, variants)
- Carcinoma of minor salivary gland
- Acinic cell
- Adenoid cystic
- Adenocarcinoma, NOS
- Basal cell adenocarcinoma
- Carcinoma ex pleomorphic adenoma
- Carcinoma type cannot be determined
- Carcinosarcoma
- Clear cell adenocarcinoma
- Cystadenocarcinoma
- Epithelial myoepithelial carcinoma
- Mammary analogue secretory carcinoma
- Mucoepidermoid carcinoma
- Mucinous carcinoma
- Myoepithelial carcinoma
- Oncocytic carcinoma
- Polymorphous adenocarcinoma
- Salivary duct carcinoma
- Non salivary gland adenocarcinoma
- Neuroendocrine carcinoma
- Well differentiated neuroendocrine carcinoma (typical carcinoid tumor)
- Moderately differentiated neuroendocrine carcinoma (atypical carcinoid tumor)
- Poorly differentiated neuroendocrine carcinoma, small cell type
- Poorly differentiated neuroendocrine carcinoma, large cell type
- Combined (or composite) neuroendocrine carcinoma (specify types)
- Carcinoma type cannot be determined
Additional references
Board review style question #1
Regarding laryngeal squamous cell carcinoma, which of the pT category criteria in the 8th edition AJCC staging guide is likely to be satisfied by examination of this parasagittal section taken through the right side of a total laryngectomy specimen?
- pT2 due to infiltration of paraglottic space
- pT3 due to extension into pre-epiglottic space
- pT3 due to subglottic extension
- pT4a due to extralaryngeal extension
Board review style answer #1
D. pT4a due to extralaryngeal extension
Comment Here
Reference: Pathologic TNM staging of larynx (AJCC 8th edition)
Comment Here
Reference: Pathologic TNM staging of larynx (AJCC 8th edition)
Verrucous carcinoma
Table of Contents
Definition / general | Treatment | Gross description | Microscopic (histologic) description | Differential diagnosisDefinition / general
- 1 - 4% of laryngeal cancers
- Has cytologic and architectural features normally associated with a reactive process but with ability to invade normal tissue
- Locally destructive but almost never metastasizes; associated cervical adenopathy may be reactive and not metastatic disease
- Usually men in 50s to 60s; associated with tobacco smoking or chewing
- Occurs anywhere in upper aerodigestive tract
- 5 year survival 78% (better after surgery than radiation therapy)
- May coexist with conventional squamous cell carcinoma (if both present, must treat more aggressive component)
- HPV negative
- Difficult diagnosis to make, particularly from biopsies
Treatment
- Surgery; radiation not recommended in general since ineffective and may cause anaplastic transformation
Gross description
- Large, white-tan exophytic tumor fixed to normal structures
- Up to 10 cm; attached by broad base
Microscopic (histologic) description
- Invasive cancer with well differentiated squamous epithelium that lacks features of squamous cell carcinoma
- By definition has no dysplastic features above basal zone; uniform cells without atypia or mitotic figures
- Marked surface keratinization (church spire keratosis), broad rete pegs with pushing but not an infiltrative margin
- May have prominent lymphoplasmacytic and histiocytic infiltrate
Differential diagnosis
Verrucous hyperplasia
Table of Contents
Definition / generalDefinition / general
- Irreversible mucosal lesion of upper aerodigestive tract that tends to progress to verrucous carcinoma or conventional squamous cell carcinoma
Vocal cord polyp
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Common benign laryngeal lesion, also known as laryngeal nodule or singer's nodule
- Clinical terms: nodule (sessile) versus polyp (pedunculated) (J Voice 2004;18:125)
Essential features
- Benign exophytic, pedunculated (polyp) or sessile (nodule) lesion of the vocal cord, frequently related to voice overuse or irritation, lined by unremarkable squamous epithelium or with mild reactive atypia, overlying an edematous, myxoid or hyalinized fibroblastic stroma with telangiectatic vessels
Terminology
- Laryngeal nodule, singer's nodule, Reinke edema
Epidemiology
- Patients of all ages, even in pediatric population (Otolaryngol Clin North Am 2019;52:657)
- More common in heavy smokers, patients with history of chronic irritant inhalation or singers, due to inflammation, allergic or immunologic causes, possibly secondary to hemorrhage
- Can be secondary to irritation from gastroesophageal reflux disease (J Med Case Rep 2020;14:2)
Sites
- True vocal cords
Clinical features
- Hoarseness in patients with voice overuse or irritation (J Voice 2004;18:125)
Diagnosis
- Combination of clinical presentation, laryngoscopy findings and biopsy interpretation
Case reports
- 24 year old woman with laryngeal polyp secondary to gastroesophageal reflux disease (J Med Case Rep 2020;14:2)
- 35 year old man with a large laryngeal polyp secondary to voice overuse (Ear Nose Throat J 2011;90:E30)
- 47 year old man with longstanding hoarseness, increasing stridor and deteriorating shortness of breath (Ann Emerg Med 2016;68:421)
Treatment
- Nodule: voice therapy, phonomicrosurgery or intralesional corticosteroid injection
- Polyp: similar as for nodule, plus angiolytic laser treatment (Otolaryngol Clin North Am 2019;52:745)
Gross description
- Smooth, round, 1 - 3 mm growths on true vocal cords, often on anterior third
Microscopic (histologic) description
- Polyps and nodules are histologically indistinguishable, although polyps tend to be larger (J Voice 2004;18:125)
- Early stage lesions with myxoid edematous stromal changes and fibroblastic proliferation
- Later stage lesions with stromal hyalinization and telangiectatic blood vessels
- Overlying squamous mucosa is usually unremarkable or can show mild reactive atypia (J Med Case Rep 2020;14:2, Braz J Otorhinolaryngol 2013;79:434, J Voice 2004;18:125, J Laryngol Otol 1957;71:673)
Microscopic (histologic) images
Negative stains
Sample pathology report
- Larynx, left true vocal cord, biopsy:
- Vocal cord polyp
Differential diagnosis
- Lobular capillary hemangioma (pyogenic granuloma):
- Subepithelial lobular capillary proliferation, frequently secondary to mechanical trauma during intubation (Otolaryngol Clin North Am 2019;52:657)
- Spindle cell carcinoma:
- Frequently presents as vocal cord polyp, atypical subepithelial spindle cells (stains for keratins, p63, p40 can be helpful) with mitotic activity and associated dysplasia of the overlying squamous mucosa (Diagn Pathol 2007;2:1)
- Inflammatory myofibroblastic tumor:
- Myofibroblastic spindle cell tumor, frequently ALK positive, with associated inflammatory component, usually plasma cell rich (Diagn Pathol 2007;2:1)
- Localized amyloidosis:
- Very rare, subepithelial congophilic amorphous deposits (Open Med (Wars) 2020;15:327)
- Cavernous hemangioma:
- Usually supraglottic, vessel walls are thicker and doesn't respond to vocal rest (J Craniofac Surg 2013;24:687)
- Myxoma:
- Uncommon in the larynx, stellate spindle cells in abundant myxoid background (Head Neck Pathol 2014;8:204)
- Neurofibroma:
- Rare in the larynx, usually supraglottic localization in patients with neurofibromatosis type 1 (Arch Otolaryngol Head Neck Surg 2004;130:1400)
Board review style question #1
Which of the following is the most helpful diagnostic clue in the differential diagnosis of vocal cord polyp from spindle cell carcinoma?
- Dysplasia in the superficial squamous mucosa
- Immunoreactivity for keratins AE1 / AE3
- Presence of atypical stromal cells
- Telangiectatic blood vessels in the stroma
Board review style answer #1
A. Dysplasia in the superficial squamous mucosa of a polypoid laryngeal lesion should prompt the differential diagnosis of spindle cell carcinoma. The squamous mucosa of vocal cord polyps is usually unremarkable or can show mild atypia. Nonspecific immunostaining of keratins AE1 / AE3 in stomal fibroblasts or the presence of bizarre stromal cells can be diagnostic pitfalls, especially in the absence of overt dysplasia. Telangiectatic blood vessels can be seen in the stroma of both vocal cord polyps and spindle cell carcinomas.
Comment Here
Reference: Vocal cord polyp
Comment Here
Reference: Vocal cord polyp
Board review style question #2
Board review style answer #2
B. Congo red histochemical stain is a low cost, sensitive method of screening specimens for amyloid, although interpretation needs the use of special polarizing lenses. The remaining methods, although more specific, are much more expensive and their use should be reserved for the further workup of specimens with confirmed amyloidosis.
Comment Here
Reference: Vocal cord polyp
Comment Here
Reference: Vocal cord polyp
WHO classification of Laryngeal tumors (pending)
[Pending]
Recent Larynx, hypopharynx & trachea Pathology books
Find related Pathology books: head & neck/endocrine