Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Szeto W, Mannan R. Sinonasal carcinoma-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/nasalcarcinomageneral.html. Accessed August 25th, 2025.
Definition / general
- Rare and heterogenous group of malignancies that originate in the nasal cavity and paranasal sinuses and present with different histologic features and clinical behavior
Essential features
- Sinonasal carcinomas are a rare and heterogeneous group of malignancies that develop in the nasal cavity and the paranasal sinuses with diverse histology
- Most common are sinonasal squamous cell carcinoma and intestinal type adenocarcinomas
- Clinical presentation of sinonasal malignancies is usually nonspecific and diagnosed at advanced stages with poor prognosis
- Persistent exposure to wood and leather dust, textiles and organic solvents over a period of time is a strong risk factor for developing sinonasal carcinomas and is thought to be the result of chronic inflammation
ICD coding
- ICD-10: C30.0 - malignant neoplasm of the nasal cavity
Epidemiology
- Rare, < 1% of all human malignancies (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420, J Clin Med 2017;6:116)
- Sinonasal cancers comprise 3% of all head and neck cancers (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420, J Clin Med 2017;6:116)
- Affects 1 in 100,000 people per year worldwide (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Average age of presentation is between 50 - 70 years (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Epithelial tumors are the most common (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Originate from epithelial lining, accessory salivary glands, neuroendocrine tissue and olfactory epithelium
- Represent > 80% of all sinonasal tumors
- The 2022 5th edition of the WHO Classification of Tumours of the nasal cavity, paranasal sinuses and skull base include the following epithelial malignancies (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420, Cancers (Basel) 2021;13:2835, Head Neck Pathol 2022;16:1)
- Squamous cell carcinoma
- 50 - 80% of all sinonasal malignancies
- ~60% of these are keratinizing, ~40% are nonkeratinizing, smaller number of other variants
- NUT carcinoma
- Up to ~18% of upper aerodigestive tract poorly differentiated carcinomas
- SWI / SNF complex deficient sinonasal carcinoma
- Rare, with most tumors showing loss of SMARCB1 (INI1)
- Sinonasal lymphoepithelial carcinoma
- Strongly associated with Epstein-Barr virus infection, especially in endemic areas
- Sinonasal undifferentiated carcinoma
- ~3 - 5% of sinonasal carcinomas
- Teratocarcinosarcoma (Turk Neurosurg 2017;27:468, Brain Tumor Res Treat 2020;8:57)
- Extremely rare
- Mostly middle aged adults with a mean age of 54.5 years
- M:F = 7 - 8:1
- HPV related multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94)
- Strong association with HPV, particularly HPV 33
- Women affected more than men
- Mean age at presentation in sixth decade
- Adenocarcinomas
- Intestinal type adenocarcinoma represents ~20% of sinonasal malignancies
- Squamous cell carcinoma
- Sinonasal carcinomas occur more commonly in men (Nat Rev Clin Oncol 2014;11:460)
- M:F = 2:1 in squamous cell carcinoma
- M:F = 6:1 in intestinal type adenocarcinoma
- Male predominance is thought to be a result of occupational exposure (Nat Rev Clin Oncol 2014;11:460)
- Woodworkers have 500 - 900 times and 20 times the risk of developing intestinal type adenocarcinoma and squamous cell carcinoma, respectively (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Persistent exposure lasting longer than 20 years
- Association with woodworking not as strong outside of Europe, may reflect other susceptibility factors or exposure to different types of wood
- Leather dust, glues, formaldehyde, chrome, nickel, flour, textiles and organic solvents have also been associated with sinonasal carcinomas, mostly squamous cell carcinoma (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Smoking is also a risk factor, although not as strongly associated as other head and neck cancers (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Smoking can increase the risk of squamous cell carcinoma 2 to 3 fold
- HPV 16 and 18 are associated with squamous cell carcinoma (~30% of cases) (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Favorable prognosis
- Other lower risk factors include nasal polyposis, inverted sinonasal papilloma, chronic sinusitis and radiotherapy used in the treatment of retinoblastoma (Curr Oncol 2021;28:2420)
- Squamous cell carcinoma can arise from the 3 types of Schneiderian papillomas (exophytic, inverted and oncocytic) (Head Neck Pathol 2016;10:60)
- Very rare in exophytic type
- Most commonly arise in inverted papillomas (~10%, although reportedly as high as 27%)
- 4 - 17% in oncocytic type
- Squamous cell carcinoma can arise from the 3 types of Schneiderian papillomas (exophytic, inverted and oncocytic) (Head Neck Pathol 2016;10:60)
Sites
- Sinonasal tract comprises the nasal cavity, maxillary sinus, ethmoid sinus and sphenoid sinus (J Clin Med 2017;6:116)
- Includes the maxillary, ethmoid, nasal, frontal, palatine, sphenoid and lacrimal bones
- Lined with ciliated respiratory epithelium
- Sinonasal squamous cell carcinoma occurs predominantly in the nasal cavity and maxillary sinuses (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Virtually all intestinal type adenocarcinomas are located in the olfactory groove of the ethmoid sinuses (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Other histologic subtypes can occur anywhere within the sinonasal cavity (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
Pathophysiology
- Organic dust and occupational exposures associated with tumor development are not considered directly mutagenic (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- These substances may cause chronic inflammation due to continuous mucosal irritation
- Chronic inflammation is a recognized mechanism of malignancy in several cancer types
- Phagocytosis of inhaled organic dust, mineral fibers or fungal spores induce alveolar macrophages to secrete cytokines and chemokines involved in the inflammatory response, including (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Tumor necrosis factor (TNF) and IL1β, resulting in activation of transcription factor κβ (NFκβ)
- Expression of both NFκβ and cyclo-oxygenase 2 (COX2) are elevated in sinonasal carcinomas
- Stimulation by wood dust results in the release of reactive oxygen species and reactive nitrogen species by alveolar macrophages (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Peroxynitrites and nitrogen oxides can generate mutagenic DNA adducts
- Strong relationship between nitric oxide production through inducible nitric oxide synthase activity and G > A nucleotide transitions in TP53 gene
- TP53 G > T transitions have been observed virtually exclusively in nonsmoking woodworkers
- G > A transitions in KRAS was the most frequent mutation found in patients with intestinal type adenocarcinoma with a history of exposure to wood and leather dust
- Wnt / beta catenin pathway is also frequently affected (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Normally, beta catenin interacts with antigen presenting cell (APC) receptors, leading to ubiquitination of beta catenin
- When Wnt is overexpressed, beta catenin interacts with it and triggers translocation to the nucleus
- Acts as a transcription factor for genes encoding cyclin D1 and c-MYC, leading to cell cycle dysregulation
- Activating mutations of Wnt are detected in 30 - 50% of paranasal sinus tumors
- EGFR overexpression is found in 20 - 30% of sinonasal tumors (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Overexpression correlates with either amplification of EGFR gene or with hyperactivating point mutations
- EGFR overexpression is mutually exclusive with overexpression of p16, a surrogate marker of HPV infection
- Metaplasia of the normal respiratory or olfactory epithelium is thought to occur in early stages of development (Nat Rev Clin Oncol 2014;11:460)
- Histopathologic change that represents cellular and tissue response to chronic inflammation
- Squamous metaplasia followed by dysplasia are histologic changes that precede sinonasal squamous cell carcinoma
- Inverted papillomas are associated with a small proportion of sinonasal squamous cell carcinoma
- HPV 16 infection has been described in ~30% of malignant tumors, suggestive of inverted papilloma as a precursor lesion to this subset of squamous cell carcinoma
- Cuboidal and intestinal metaplasia are proposed to be a precursor to intestinal type adenocarcinoma
- These metaplastic tissues have been observed adjacent to these tumors and in people with exposure to environmental or occupational risk factors
- Metaplastic tissues show a switch from normal sinonasal epithelial immunophenotype (CK7+ / CK20- / CDX2- / villin-) to an abnormal intestinal epithelium immunophenotype (CK7- / CK20+ / CDX2+ / villin+)
Etiology
- Persistent environmental or occupational exposure to dust produced in the processing of wood, leather, flour, textiles, nickel and chrome (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Persistent exposure to glue, formaldehyde and organic solvents (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Smoking (Nat Rev Clin Oncol 2014;11:460)
- Human papillomavirus infection (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Radiotherapy for retinoblastoma (Curr Oncol 2021;28:2420)
Clinical features
- Tumors grow locally and may extend into adjacent structures (Curr Oncol 2021;28:2420)
- Clinical symptoms are often nonspecific (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Nasal obstruction, facial pain, persistent rhinorrhea or epistaxis
- Proptosis, diplopia or other neurologic symptoms may be seen in patients with advanced disease
- Due to nonspecific nature of symptoms in early stages of disease, sinonasal malignancies may have a prolonged period of time before diagnosis (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Lymph node metastasis at diagnosis in 10 - 20% of patients with sinonasal squamous cell carcinoma
- Lymphatic metastasis is rare in intestinal type adenocarcinoma, likely due to location and pattern of lymphatic drainage
- On follow up, 10% of patients develop distant metastasis; rarely occurs without local recurrence (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
Diagnosis
- Rigid nasal endoscopy
- Noninvasive imaging is essential in establishing the extent of the tumor (Nat Rev Clin Oncol 2014;11:460)
- Both CT and MRI should be performed to determine anatomic details on tumor localization and extension
- Critical in determining operability or planning radiotherapy
- MRI is standard imaging modality for postoperative surveillance (Nat Rev Clin Oncol 2014;11:460)
- Role of PET CT is important in staging of squamous cell carcinoma, allowing the identification of regional and distant metastases (Cancers (Basel) 2021;13:2835, Nat Rev Clin Oncol 2014;11:460)
- Biopsy
Radiology description
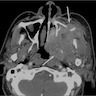
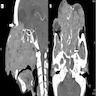
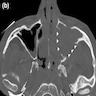
- Radiologic differentiation of sinonasal carcinomas is challenging due to overlap in imaging findings
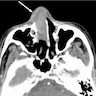
- Sinonasal squamous cell carcinomas show a soft tissue mass characterized by prominent bony destruction on CT (J Clin Med 2017;6:116, Head Neck Pathol 2016;10:1)
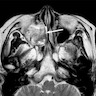
- MRI shows intermediate T1 signal intensity and hypointense T2 signal
- Variable enhancement on contrast enhanced T1 images that are less than half that of sinus mucosa
- Small lesions are homogenous in signal intensity
- Large tumors are more heterogenous with areas of necrosis and hemorrhage
- Many imaging manifestations of adenocarcinoma are often indistinguishable from squamous cell carcinoma (J Clin Med 2017;6:116, Head Neck Pathol 2016;10:1)
- May show areas of calcification corresponding with mucin content
- Mucin producing adenocarcinomas may show hyperintensity on T2, while those without mucin may show isointensity to hypointensity on T2
- Sinonasal undifferentiated carcinoma (J Clin Med 2017;6:116, Head Neck Pathol 2016;10:1)
- Noncalcified mass with variable contrast enhancement and areas of central necrosis on CT
- MRI shows isointensity on T1, isointensity to hyperintensity on T2 and heterogeneous enhancement on contrast enhanced T1
- Bony destruction and invasion into adjacent structures
- Difficult to distinguish from squamous cell carcinoma due to nonspecific findings
Radiology images
Prognostic factors
- Prognostic factors include age, performance status, tumor location and local extension, the histologic subtype and whether perineural invasion is present
- Carcinomas originating from the nasal cavity have a better prognosis than those from the paranasal sinuses
- This is attributed to nasal carcinomas producing symptoms that require earlier clinical attention
- Among carcinomas arising from the maxillary sinuses, those originating from the anterior inferior portion have a better prognosis than those from the superior posterior portion
- This is attributed to superior posterior tumors having accessible structures such as the orbit or skull base
- In T1 disease, 5 year survival is 80%; 30% in T4 disease
- Extensive local disease increases both surgical morbidity and local recurrence within 2 years; for example, maxillary sinus tumors have a 30 - 70% 5 year survival with resection but this drops to 10 - 20% in unresectable cases
- Intestinal type adenocarcinoma have a 50% 5 year survival after surgery and chemoradiation
- They usually grow locally, rarely involve regional lymph nodes and even more rarely give rise to distant metastases
- Papillary type and colonic type tumors have a better prognosis than solid or mucinous subtypes
- p53 and nuclear expression of beta catenin is associated with worse prognosis
- Histologic grade does not have prognostic value in sinonasal squamous cell carcinoma but molecular markers do
- EGFR overexpression and HPV negativity in squamous cell carcinoma is associated with worse prognosis
- p16 overexpression with EGFR negativity has a better prognosis
- References: Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420
Case reports
- 36 year old man with a history of nasal blockage and left sided polypoid mass (J Clin Diagn Res 2016;10:ED12)
- 43 year old woman with disfiguring facial swelling with exophytic lesions protruding from nostrils (Cureus 2017;9:e1386)
- 61 year old man with squamous cell carcinoma arising from sinonasal inverted papilloma (AJNR Am J Neuroradiol 2020;41:1156)
- 68 year old man with rapidly progressive visual loss (Case Rep Oncol 2019;12:277)
- 69 year old woman with progressively worsening right eye visual acuity (Cureus 2019;11:e5281)
Treatment
- Surgical resection has been the mainstay of sinonasal cancer management (Curr Oncol 2021;28:2420, Cancers (Basel) 2021;13:2835)
- Resection results in excellent control rates for T1 and T2 tumors
- For small, localized tumors, complete removal is possible with less invasive techniques (such as endoscopically)
- Radiotherapy is given postoperatively in most cases, even if margins are negative (Curr Oncol 2021;28:2420)
- Adjuvant chemotherapy is considered in cases of inadequate resection margin (Curr Oncol 2021;28:2420)
- Radical neck dissection or elective radiation therapy of the whole neck for patients with positive neck lymph nodes (Curr Oncol 2021;28:2420)
- Cisplatin or carboplatin with external beam radiation can be used for locally advanced and unresectable squamous cell carcinomas (Curr Oncol 2021;28:2420)
- Concurrent chemoradiation can be used for those with comorbidities contraindicating surgery (Curr Oncol 2021;28:2420)
- Intestinal type adenocarcinoma is typically less responsive to chemotherapy; use of radiotherapy is preferred (Curr Oncol 2021;28:2420)
- Radiotherapy in conjunction with chemotherapy, when possible, is beneficial in locally advanced or unresectable disease (Curr Oncol 2021;28:2420)
- Significant improvement in survival in patients treated with a combination of 2 or more multidisciplinary approaches (e.g., surgery, chemotherapy, radiotherapy) (Curr Oncol 2021;28:2420)
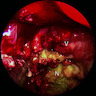
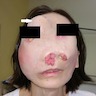
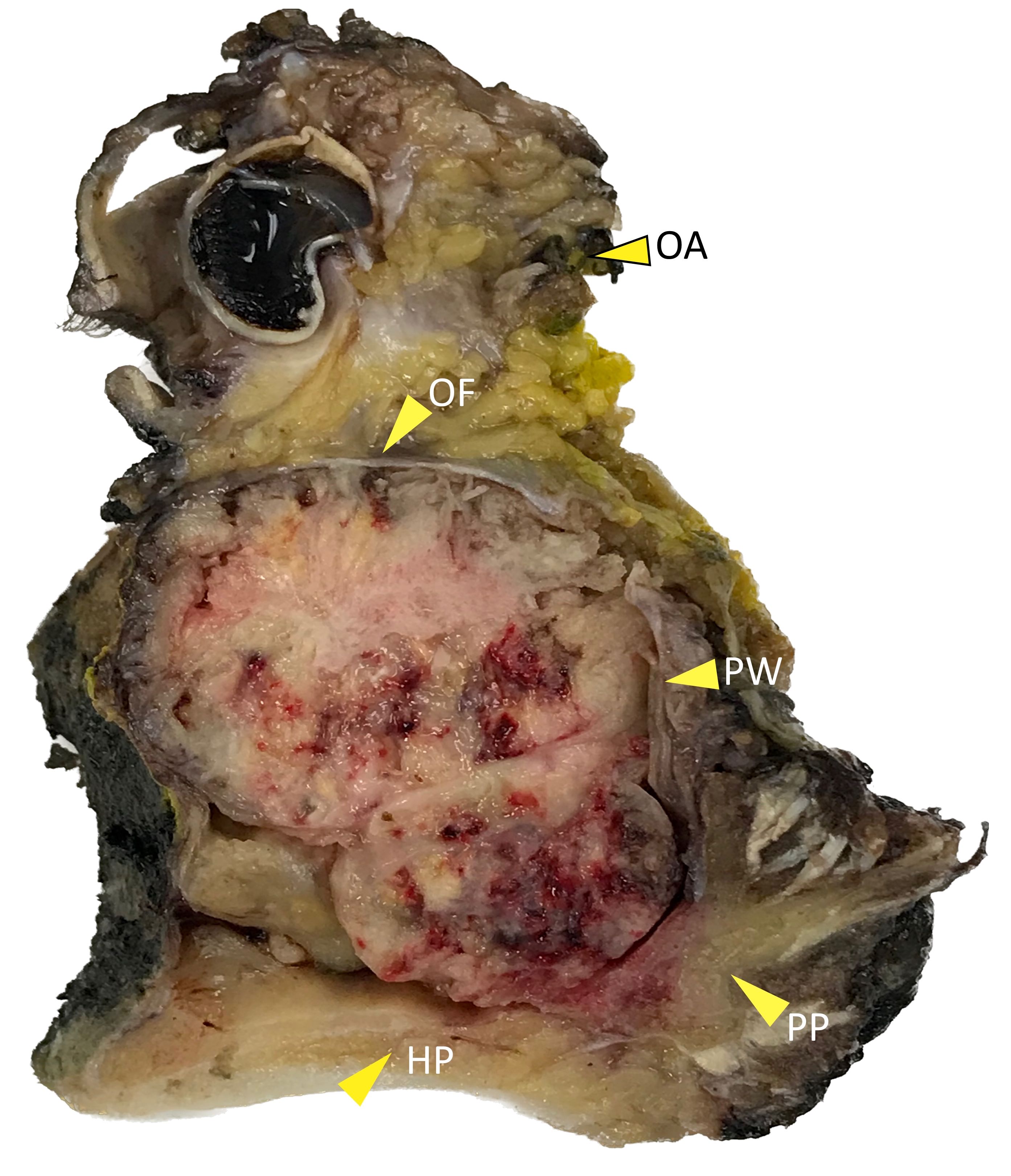
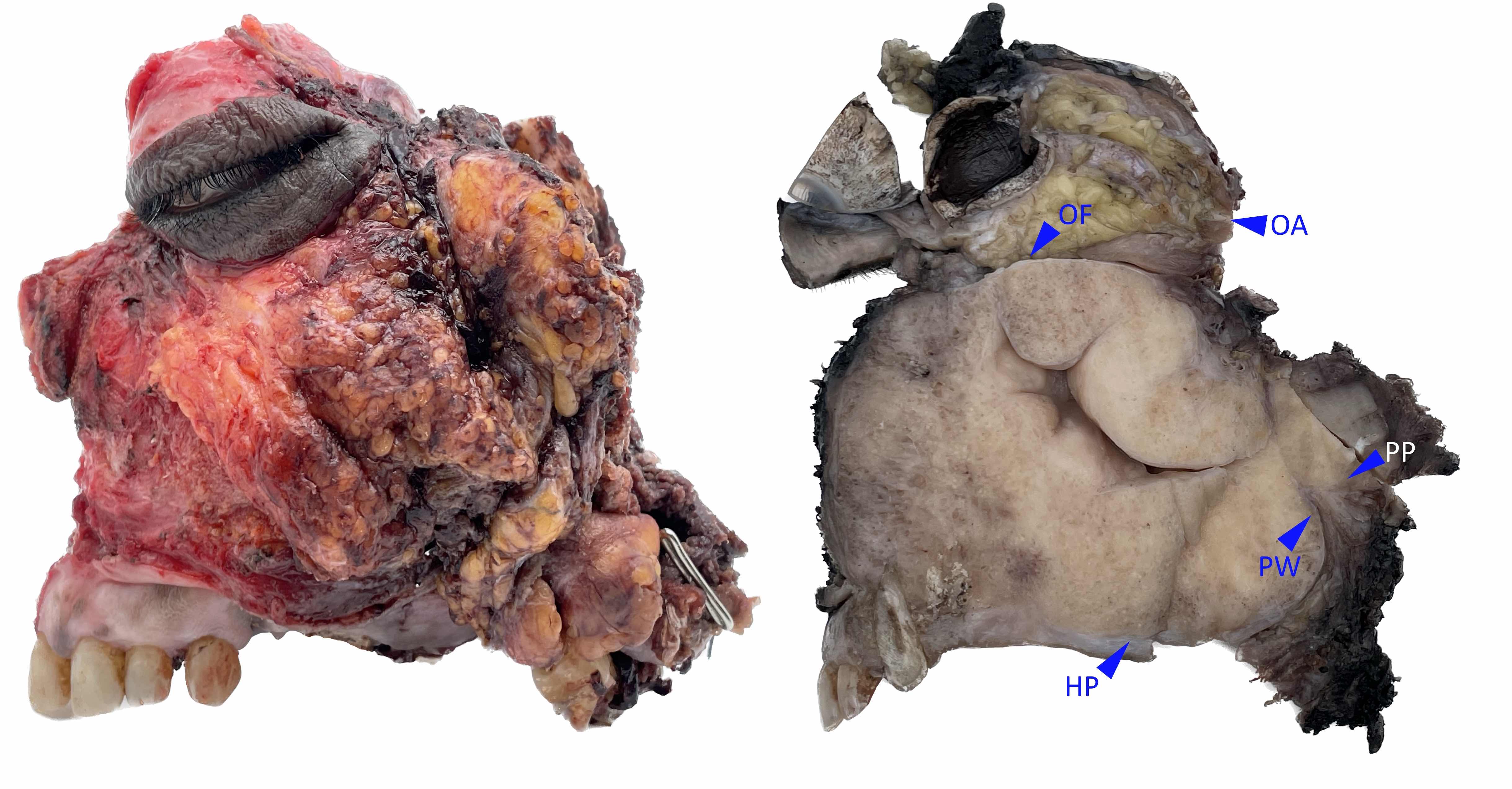
Clinical images
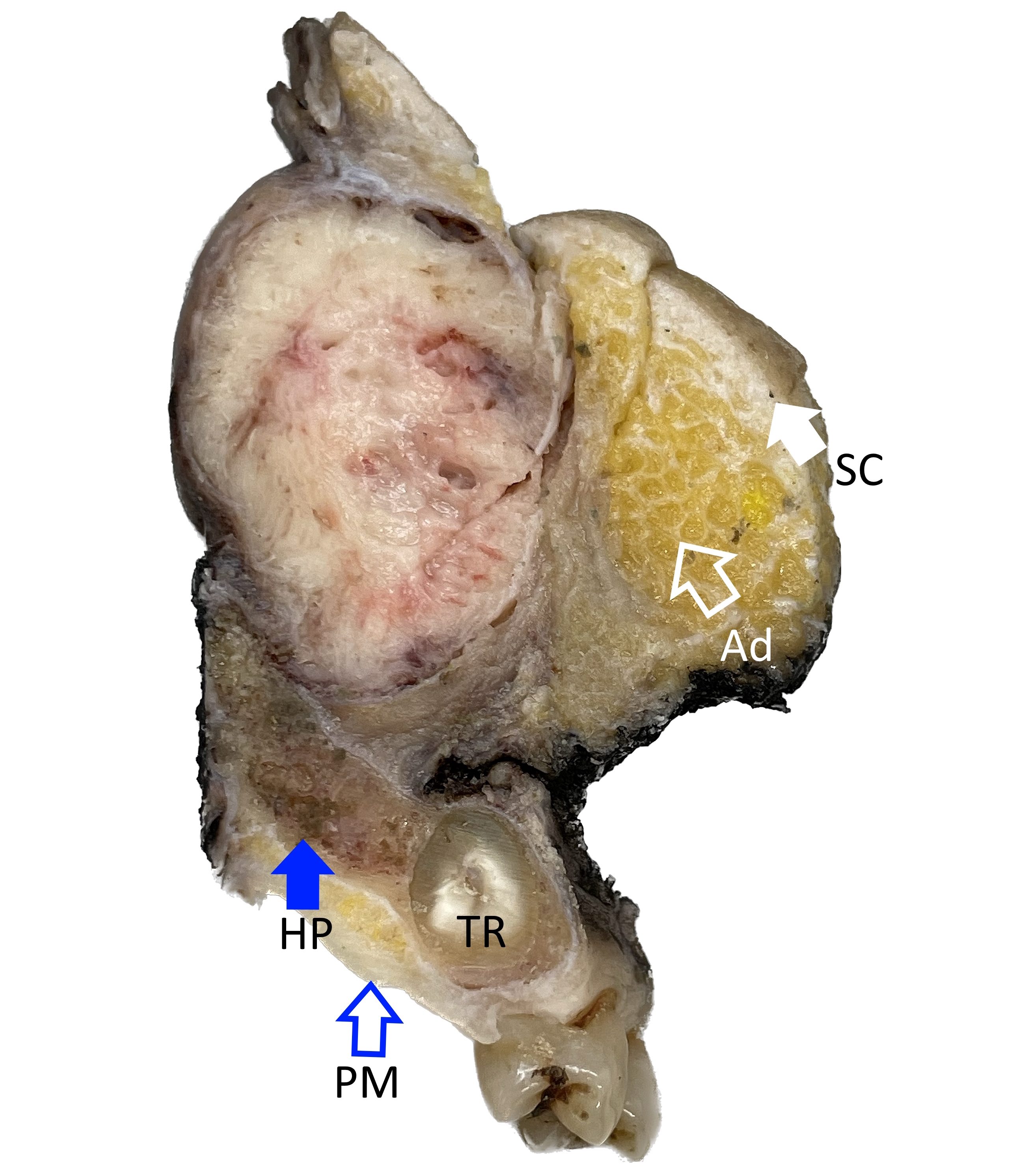
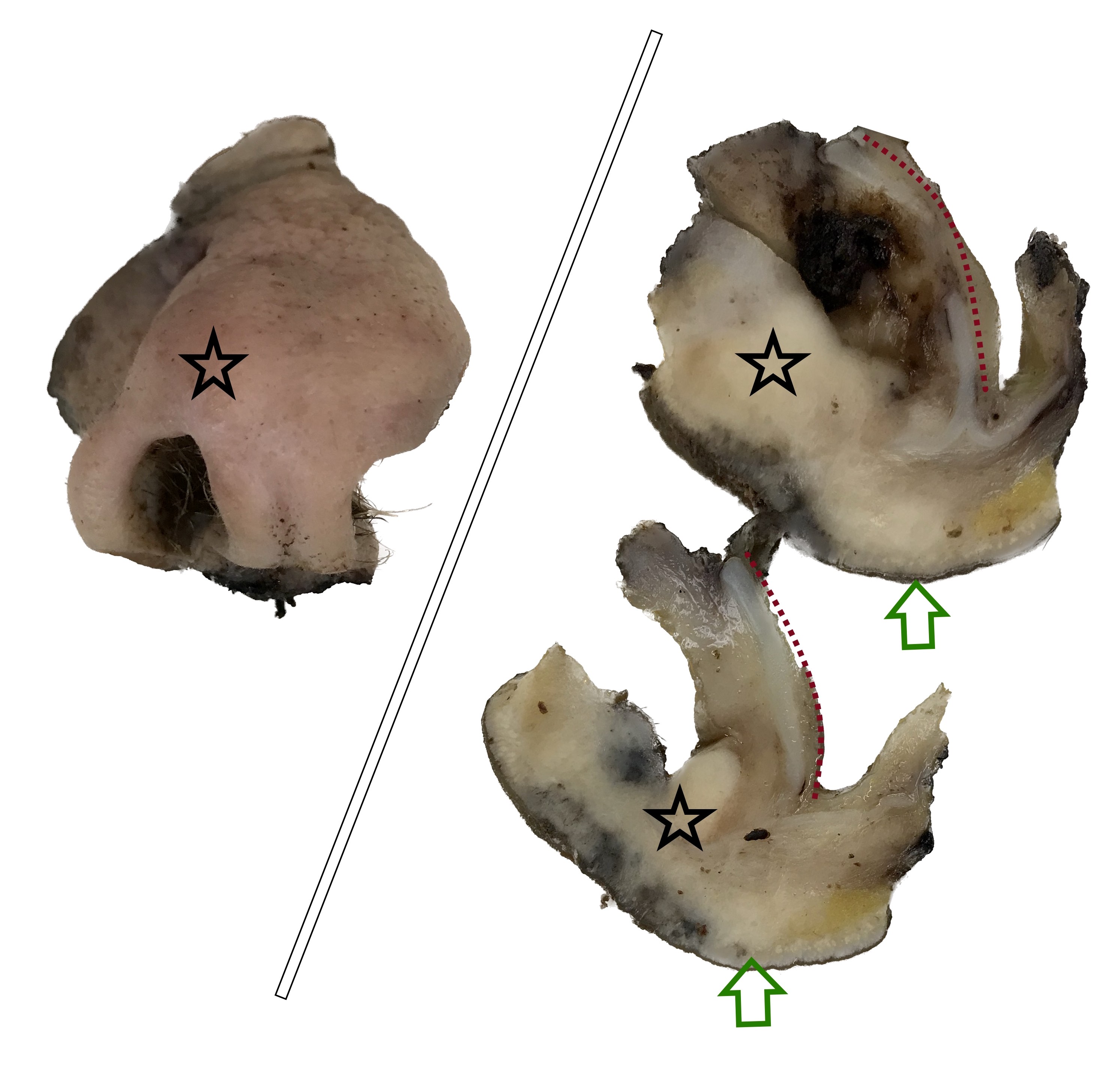
Gross description
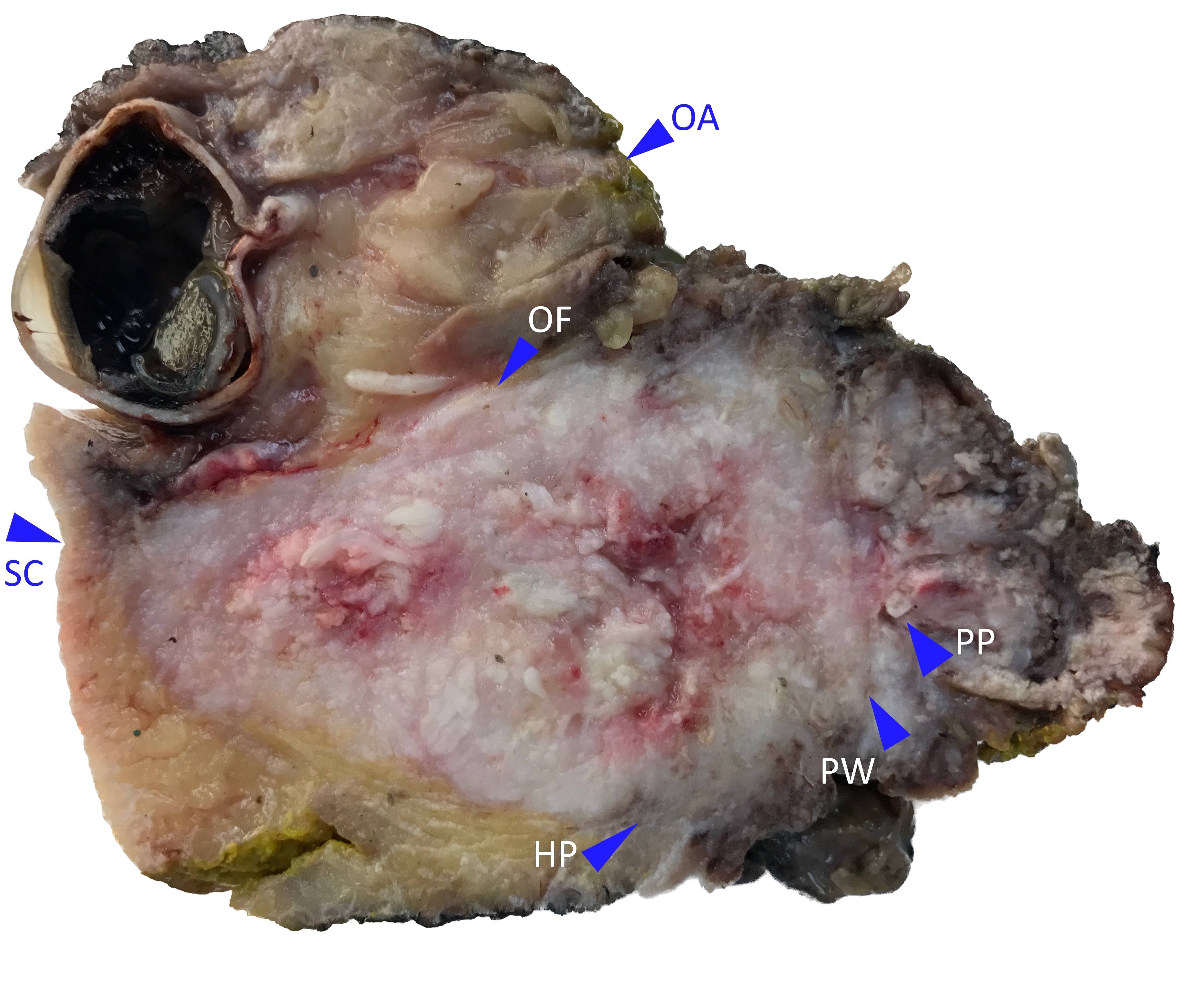
- Squamous cell carcinoma
- Soft, friable tan-white solid tumor
- Exophytic, papillary, polypoid, nodular or ulcerated mass
- May show hemorrhage and necrosis
- Adenocarcinoma
- Tan-white cut surface
- May be flat, polypoid, exophytic, papillary, ulcerated, mucinous
Gross images
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Images hosted on other servers:
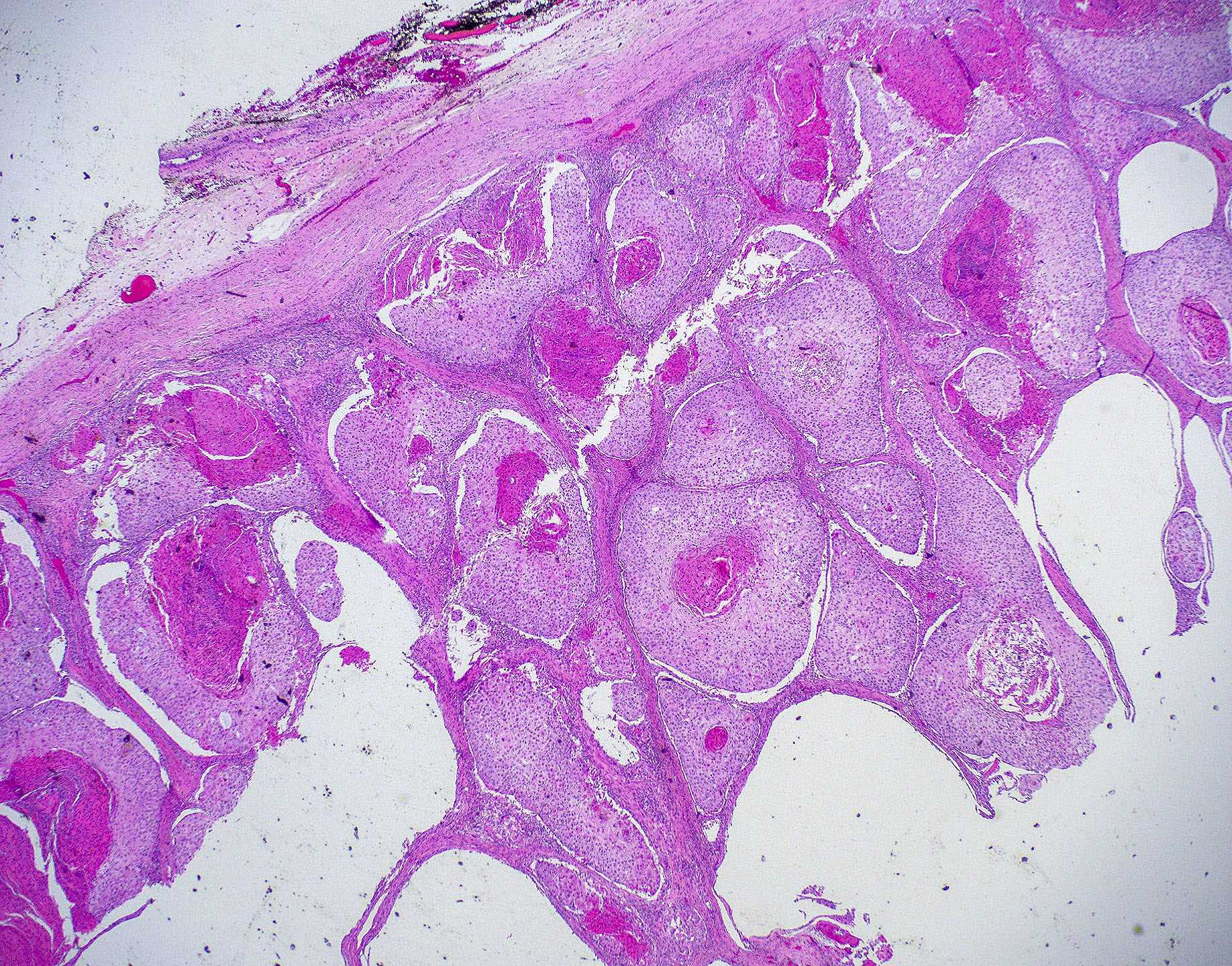
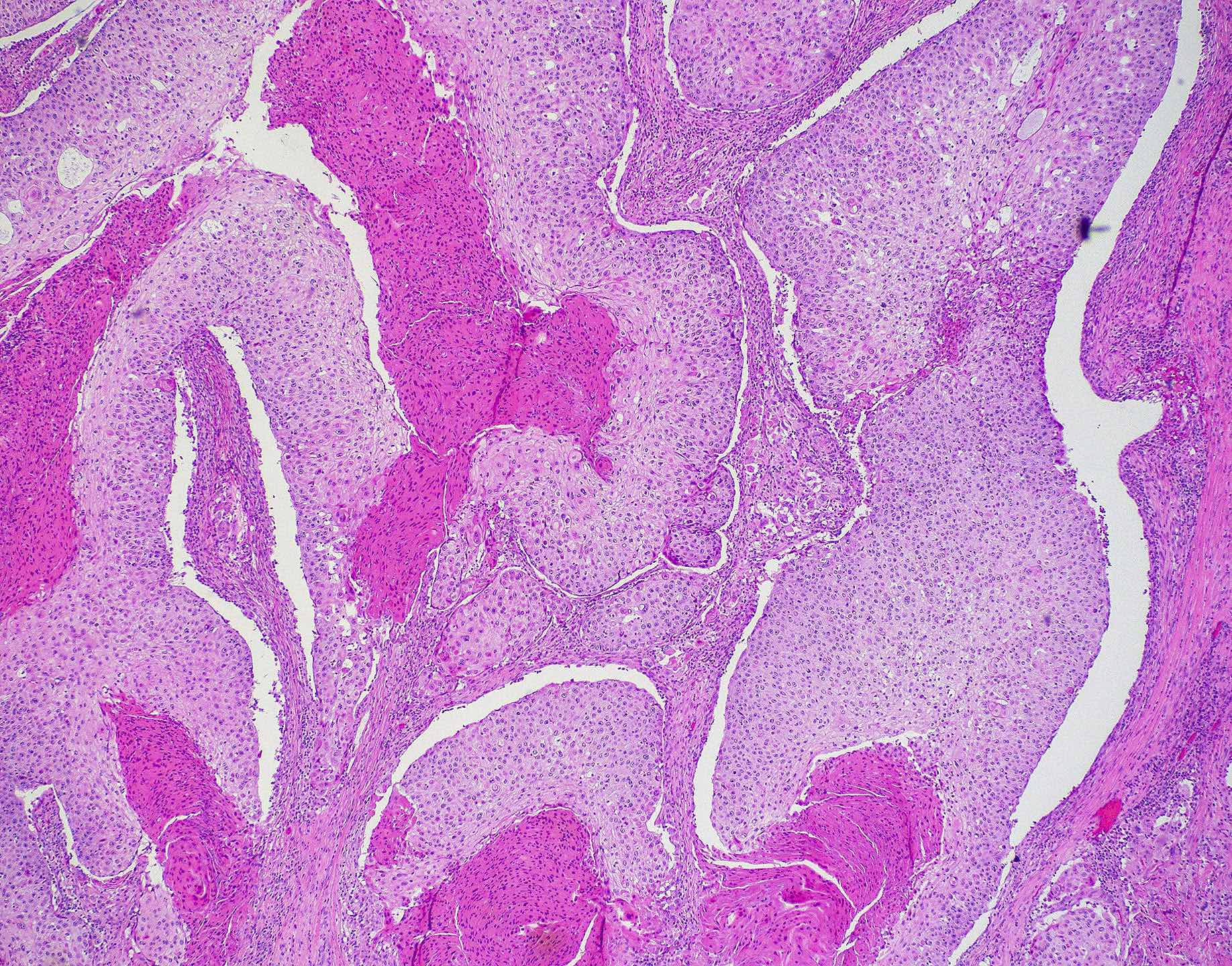
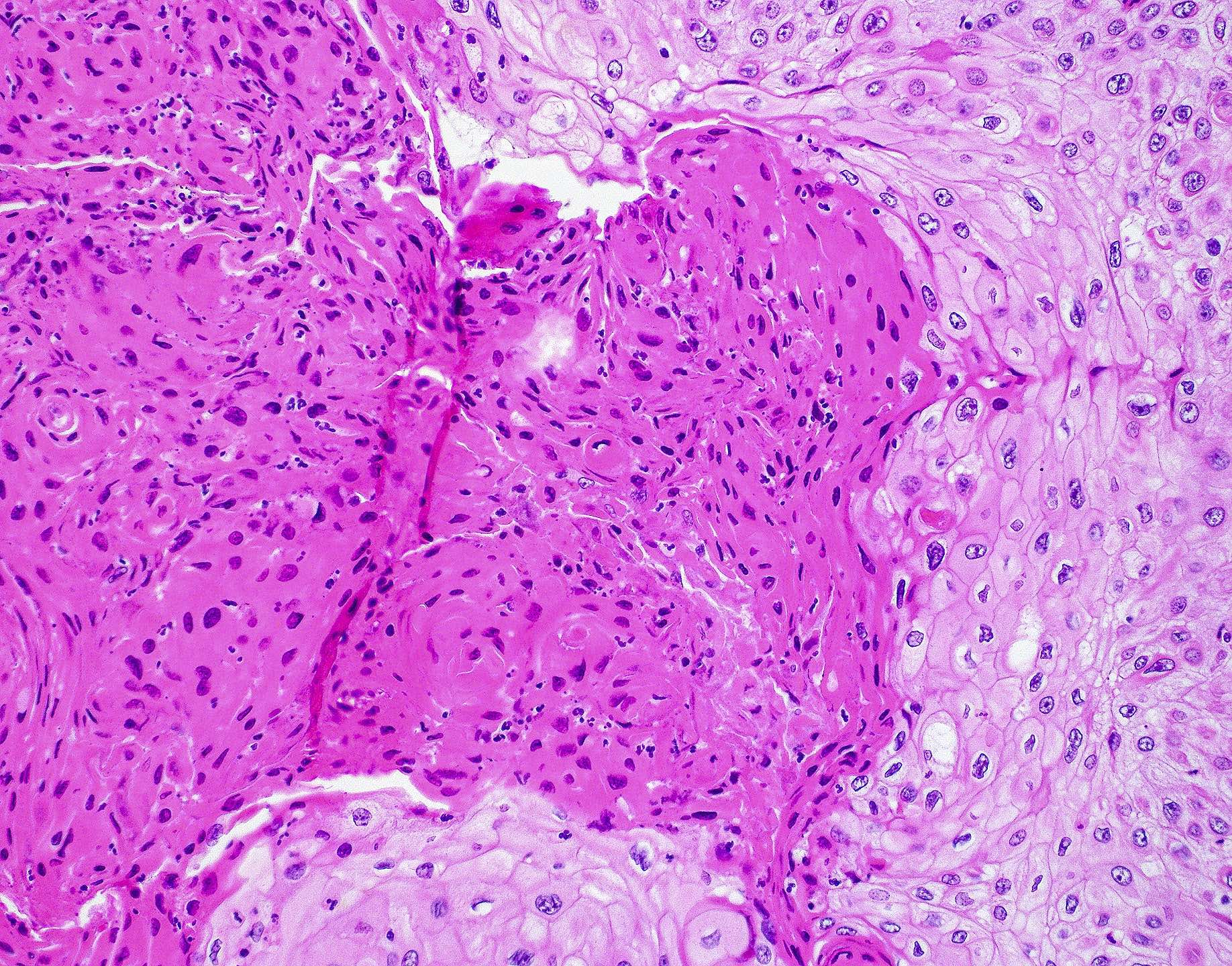
Microscopic (histologic) description
Squamous cell carcinoma (Head Neck Pathol 2016;10:60, Head Neck Pathol 2022;16:1)
NUT carcinoma (Head Neck Pathol 2022;16:1)
SWI / SNF complex deficient sinonasal carcinoma (Head Neck Pathol 2022;16:1, Am J Case Rep 2023;24:e939244)
Lymphoepithelial carcinoma (Curr Oncol 2021;28:2420, Ear Nose Throat J 2022;101:386)
Sinonasal undifferentiated carcinoma (Curr Oncol 2021;28:2420, Adv Anat Pathol 2020;27:51)
Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1)
HPV associated multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94, Head Neck Pathol 2022;16:1)
Adenocarcinoma (Curr Oncol 2021;28:2420, Head Neck Pathol 2016;10:68)
- Keratinizing squamous cell carcinoma
- Most common type, ~60%
- Morphologically identical to squamous cell carcinomas arising elsewhere
- Abundant, eosinophilic cytoplasm filled with keratin filaments
- Intercellular bridges prominent
- Stellate, irregular nests, cords or single tumor cells in desmoplastic stroma
- Keratinization and keratin pearl formation readily identifiable
- Graded as well, moderately or poorly differentiated
- Well differentiated shows squamous epithelium with abundant keratinization, prominent intercellular bridges, with minimal pleomorphism and low mitotic activity
- Moderately differentiated shows features between well and poorly differentiated, with focal keratinization
- Poorly differentiated carcinomas have more nuclear atypia, pleomorphism, increased mitotic activity and minimal keratinization
- Nonkeratinizing squamous cell carcinoma
- Squamous differentiation with minimal keratinization
- ~40% of all sinonasal squamous cell carcinomas
- Similar to the same tumor type arising in the oropharynx
- Blue cell tumor appearance with high N:C ratio, arranged in large, rounded nests or ribbons with smooth, usually well demarcated borders with little stromal desmoplasia
- Exophytic or inverted papillary architecture is common
- Minimal maturing squamous differentiation
- Prominent mitotic activity and apoptosis
- Central necrosis is common in nests
- Tumors tend to coat the mucosal surface with undulating, irregular contour
- In areas where tumor invades downward, has inverted appearance with rounded nests, mimicking Schneiderian papilloma with carcinoma in situ
- Combined with lack of stromal desmoplasia, gives a noninvasive appearance
- DEK::AFF2 subtype shows complex exophytic and endophytic growth
- Broad papillary fronds, anastomosing lobules, ribbons and nests
- Nuclear monotony and inflammatory infiltrate, especially neutrophils, typically present (Head Neck Pathol 2022;16:1)
- Requires confirmation with DEK::AFF2 FISH break apart probe
NUT carcinoma (Head Neck Pathol 2022;16:1)
- Composed of monotonous evenly spaced sheets of evenly sized nuclei that have vesicular chromatin and prominent nucleoli
- Inflammatory infiltrate commonly seen
- Abrupt keratinization may be seen in up to a third of cases
SWI / SNF complex deficient sinonasal carcinoma (Head Neck Pathol 2022;16:1, Am J Case Rep 2023;24:e939244)
- Most common subtype is SMARCB1 deficient
- Small to medium sized monomorphic basaloid cells, undifferentiated appearance
- Indistinct cytoplasmic borders and round nuclei with variably prominent nucleoli
- Up to a third of cases are more eosinophilic with plasmacytoid morphology, cytoplasmic vacuoles commonly seen
- SMARCB1 deficient sinonasal carcinoma may occasionally be gland forming or yolk sac-like
Lymphoepithelial carcinoma (Curr Oncol 2021;28:2420, Ear Nose Throat J 2022;101:386)
- Undifferentiated carcinoma with epithelial neoplastic cells accompanied by strong lymphocytic infiltrate
- Characterized by medium to large sized polygonal epithelioid cells with vesicular nuclei and prominent nucleoli
- Epithelioid cells form well circumscribed lobules or nests with dense mixed lymphoplasmacytic infiltrate
- Increased mitoses with atypical forms
- Dense lymphoid infiltrate may obscure epithelioid cells and mimic a lymphoproliferative disorder
- Strong association with EBV, positive by in situ hybridization
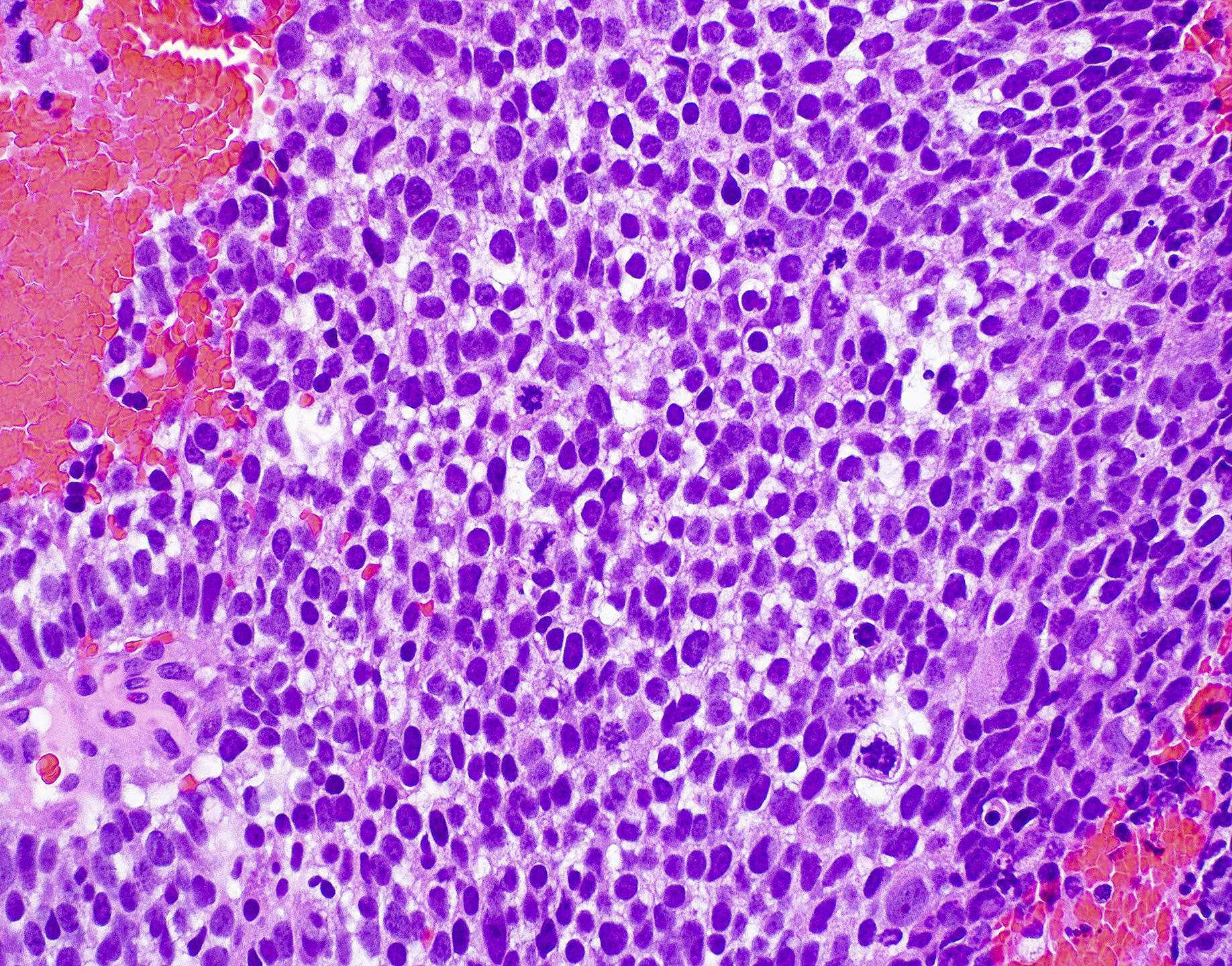
Sinonasal undifferentiated carcinoma (Curr Oncol 2021;28:2420, Adv Anat Pathol 2020;27:51)
- Undifferentiated polygonal medium to large cells arranged in nests, sheets, trabecular or solid patterns
- Round blue cell tumor
- Cells have round to oval hyperchromatic nuclei with variably prominent eosinophilic nucleoli and indistinct cell membranes
- High N:C ratio
- Strong mitotic activity with atypical forms
- Frequently with necrosis, lymphovascular invasion and perineural invasion
- Squamous, glandular or neuroectodermal differentiation should not be present
Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1)
- Has features of carcinosarcoma and teratoma with varying degrees of maturity
- Epithelial component typically consists of squamous epithelium and glandular structures
- May be benign or malignant
- Fetal appearing clear cell squamous epithelium is a characteristic finding
- Mesenchymal component may show myofibroblastic cell proliferation with a loose myxoid stroma
- Mature or immature mesenchymal elements seen (e.g., osteoid, cartilage, smooth or striated muscle)
- Primitive neuroepithelial cells in sheets and nests
- Cytologic atypia ranges from minimal to sarcomatous differentiation
HPV associated multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94, Head Neck Pathol 2022;16:1)
- Histologic and immunophenotypic features of both salivary gland type carcinoma and squamous cell carcinoma
- Biphasic ductal and myoepithelial differentiation, frequently in a cribriform arrangement reminiscent of adenoid cystic
- Frequent squamous cell carcinoma in situ, occasional squamous differentiation within invasive tumor
- Myoepithelial features show cytoplasmic clearing, plasmacytoid appearance and spindling
- May show sarcomatous transformation and cartilaginous differentiation
- May see surface squamous dysplasia
- High mitotic rate and areas of necrosis are frequently seen
Adenocarcinoma (Curr Oncol 2021;28:2420, Head Neck Pathol 2016;10:68)
- 20% of malignant neoplasms in the sinonasal tract
- Arises from either lining surface epithelium or seromucinous glands
- 3 main types: intestinal type, nonintestinal type and salivary type
- Intestinal type adenocarcinoma
- Originates from Schneiderian mucosa and is the most frequent type of adenocarcinoma (~6 - 13%)
- May recapitulate normal intestinal mucosa with villi and specialized cell types (e.g., goblet, Paneth)
- Similar features to adenomas and colorectal adenocarcinoma
- Cribriform glands with necrotic debris in lumen (dirty necrosis)
- Histologic types (modified Barnes classification): colonic, papillary, solid, mucinous, mixed
- Colonic type is most common (~40 - 50%)
- Glandular, tubular and trabecular architecture
- Rare papillae
- Glands lined by crowded columnar cells with anisonucleosis
- Intracellular and extracellular mucin and goblet cells may be present
- Papillary type
- Papillary architecture predominant with occasional tubular elements with minimal cytologic atypia
- May have columnar goblet cells mimicking intestinal adenomas
- Solid type
- Less differentiated with solid and trabecular architecture
- Minimal cytologic atypia with rare mitosis
- Mucinous
- Mimics mucinous variants of colorectal carcinoma
- Mucin filled glands or cell clusters that float in pools of extracellular mucin
- Colonic type is most common (~40 - 50%)
- Nonintestinal type adenocarcinoma
- Does not display features of either intestinal type or salivary type
- Morphologically heterogeneous
- Back to back small glands or acini
- Divided into high grade and low grade
- High grade
- More poorly differentiated with marked nuclear pleomorphism
- Glandular and papillary growth patterns
- Mitoses readily identifiable
- Tumor necrosis and apoptosis are frequently seen
- Low grade
- Varied with exophytic papillae and tubular or glandular patterns
- Papillae and glands lined by single layer of uniform columnar or cuboidal cells with minimal cytologic atypia and inconspicuous nucleoli
- Mild to moderate cellular pleomorphism and rare mitoses
- Tumor necrosis is not typically seen
- Sinonasal renal cell-like adenocarcinoma (Cureus 2021;13:e14285, Head Neck Pathol 2008;2:75, Head Neck Pathol 2022;16:1)
- Rare
- Morphologically similar to clear cell renal cell carcinoma with monotonous cuboidal to polyhedral cells with abundant clear cytoplasm
- Intranuclear holes may be seen
- Predominantly solid to nested, glandular growth pattern with fibrous septa and prominent vascularity
- Immunohistochemistry can distinguish from clear cell renal cell carcinoma
- Positive: CK7, CAIX, CD10; negative: RCC, PAX8 (Medicine (Baltimore) 2017;96:e7711)
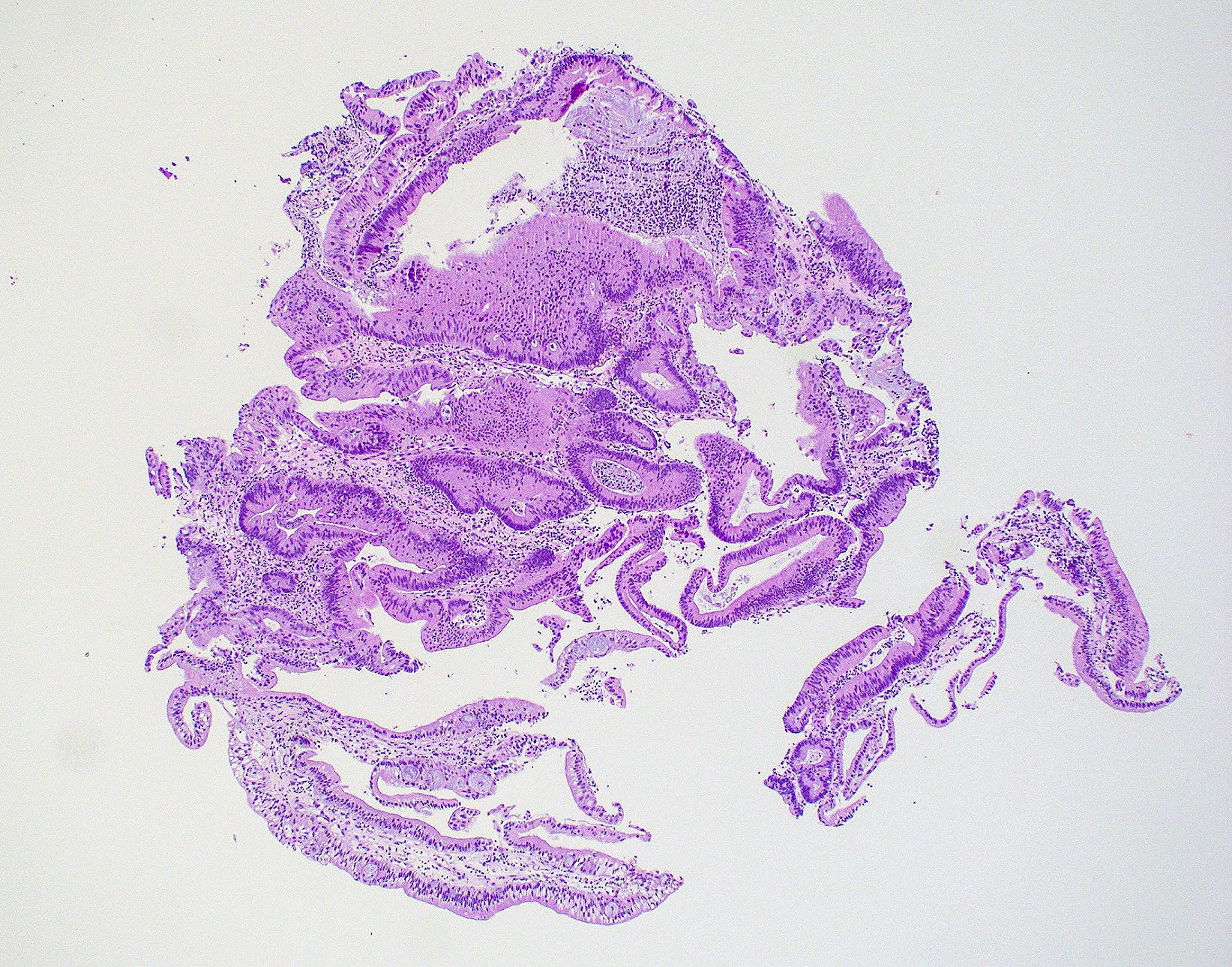
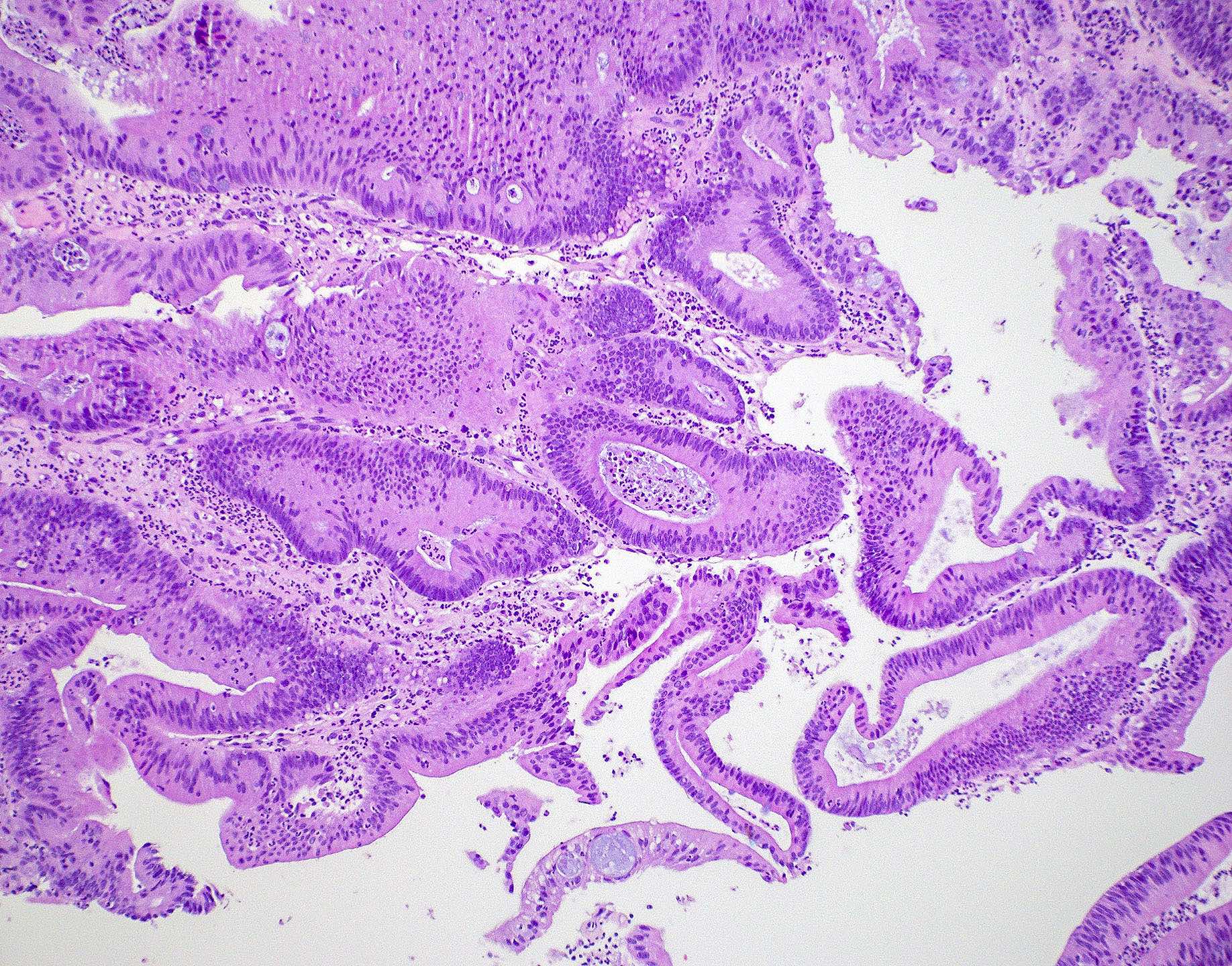
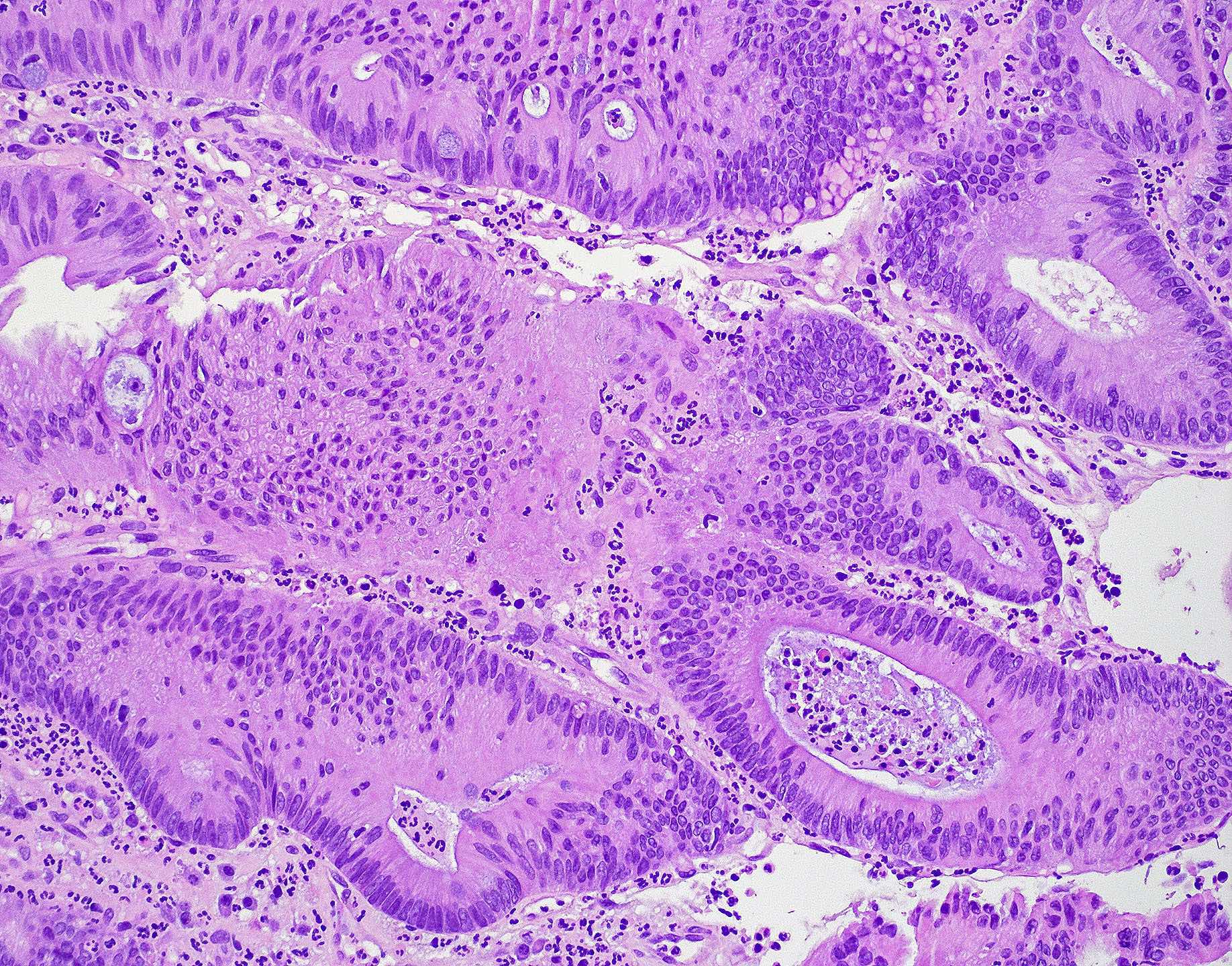
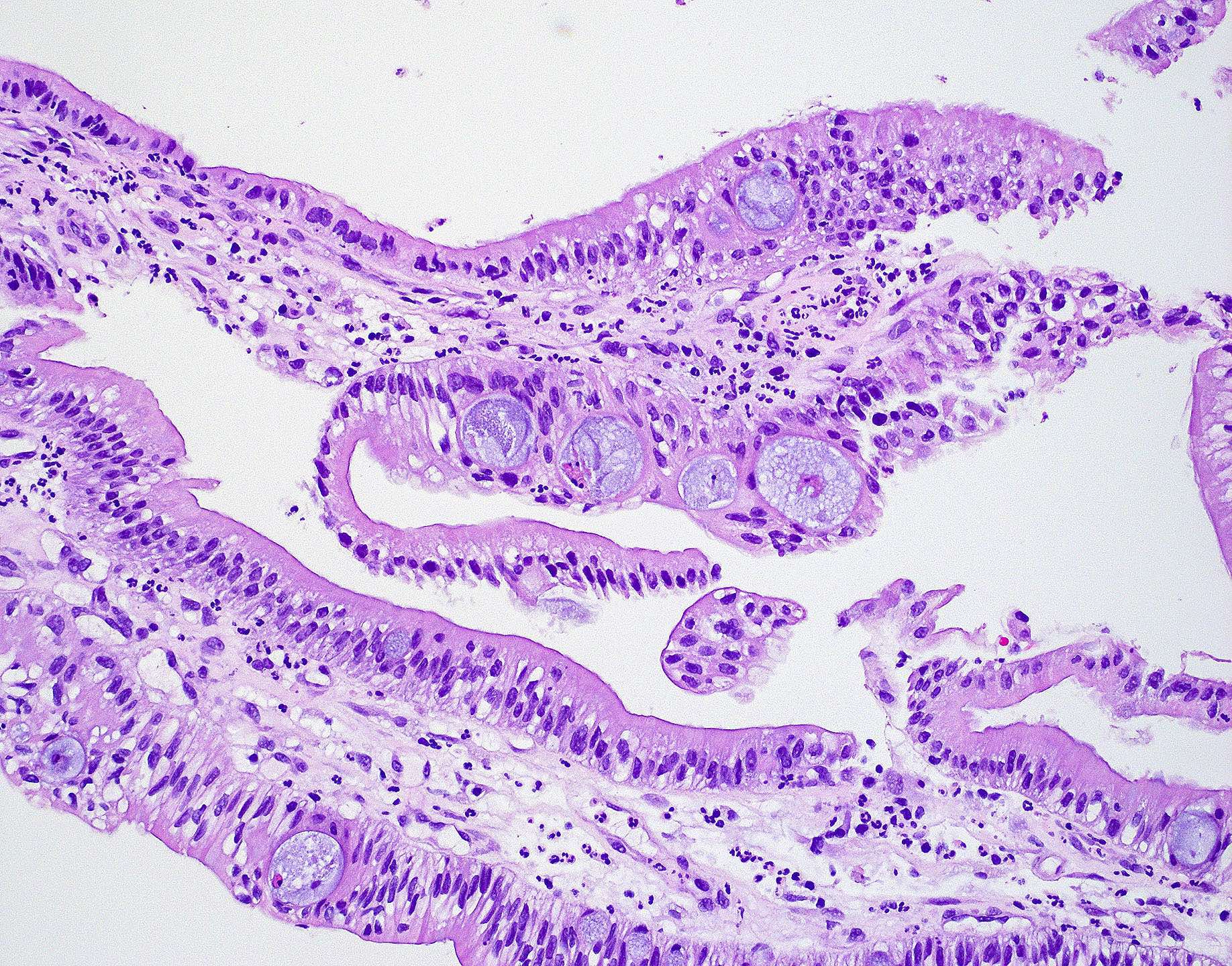
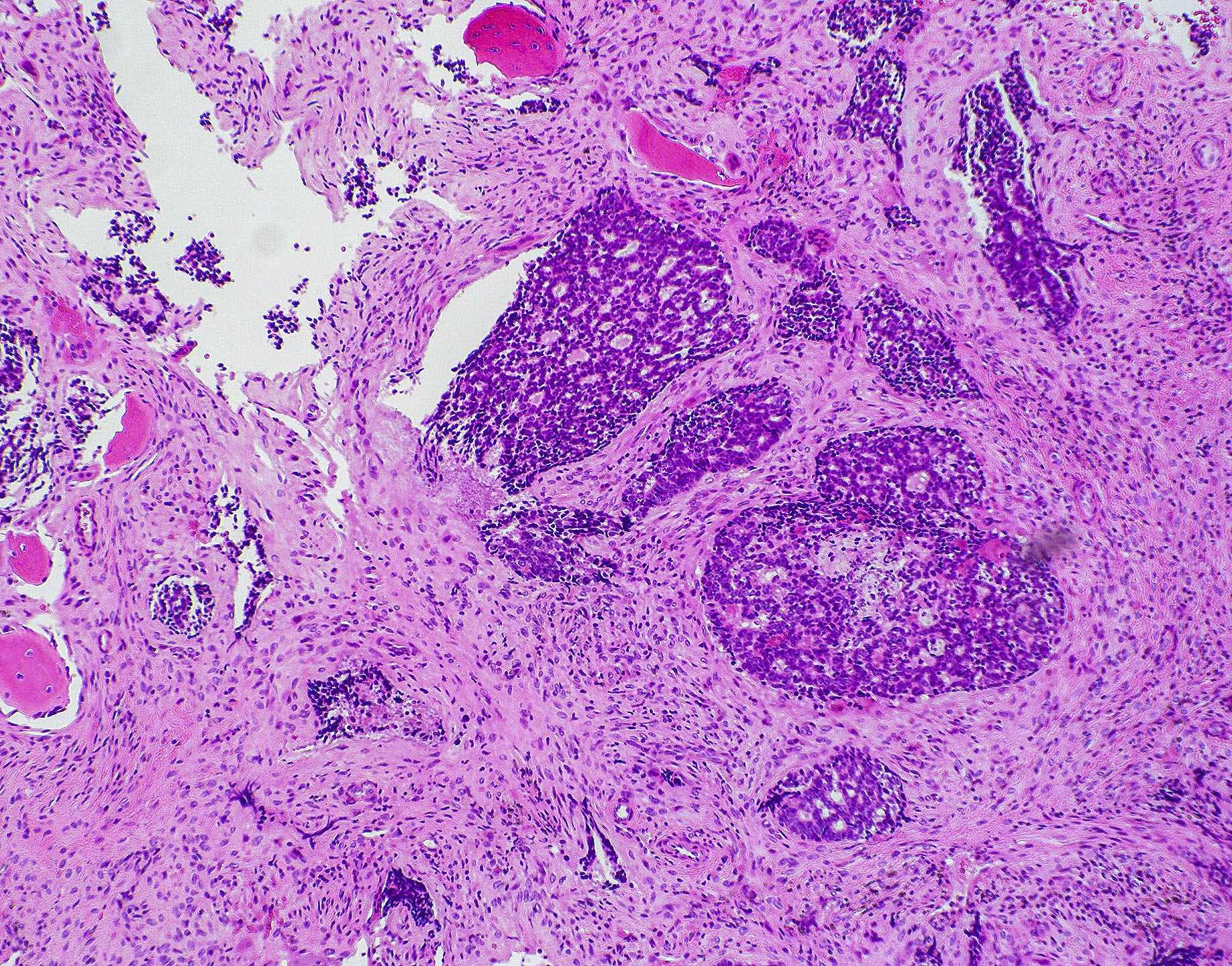
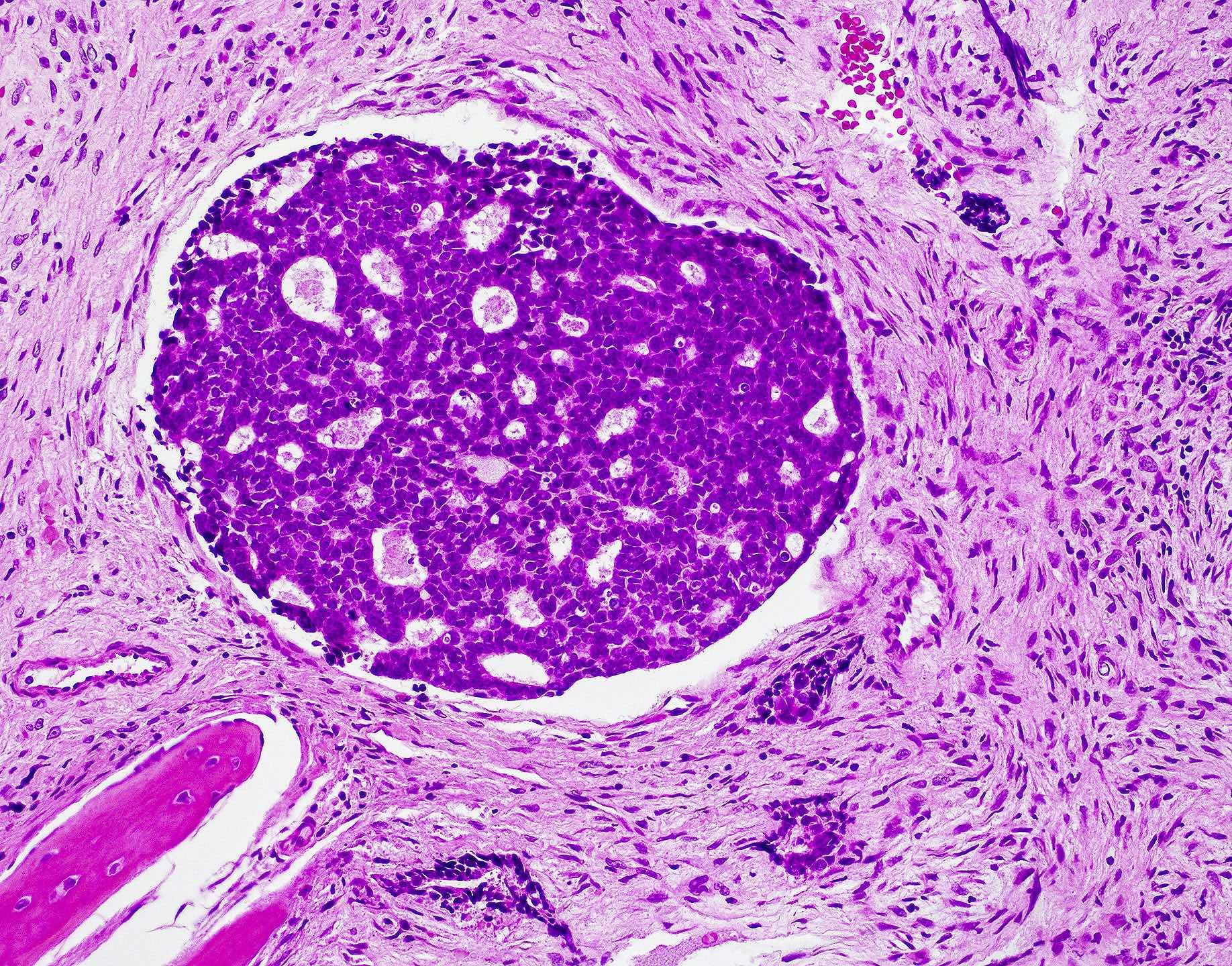
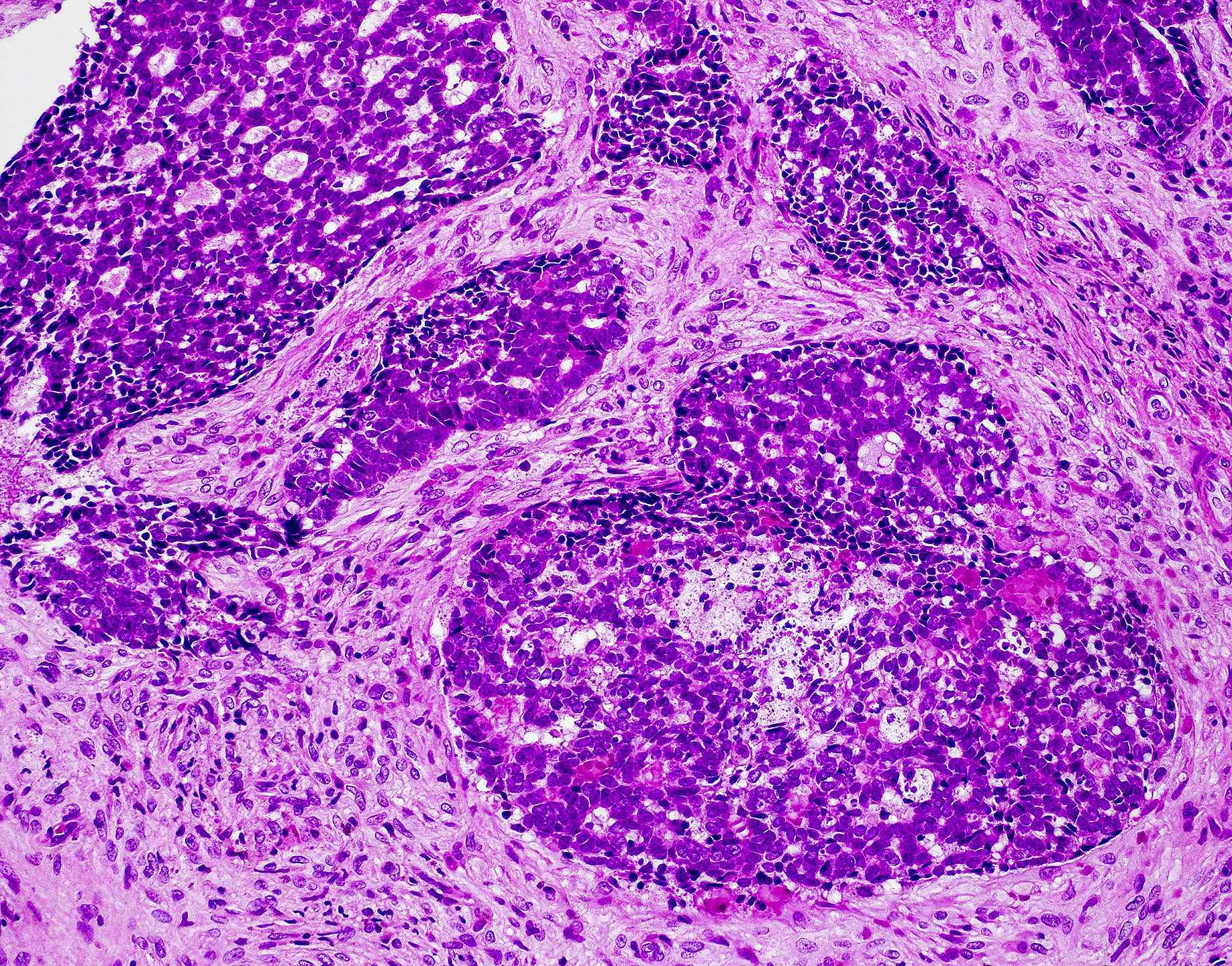
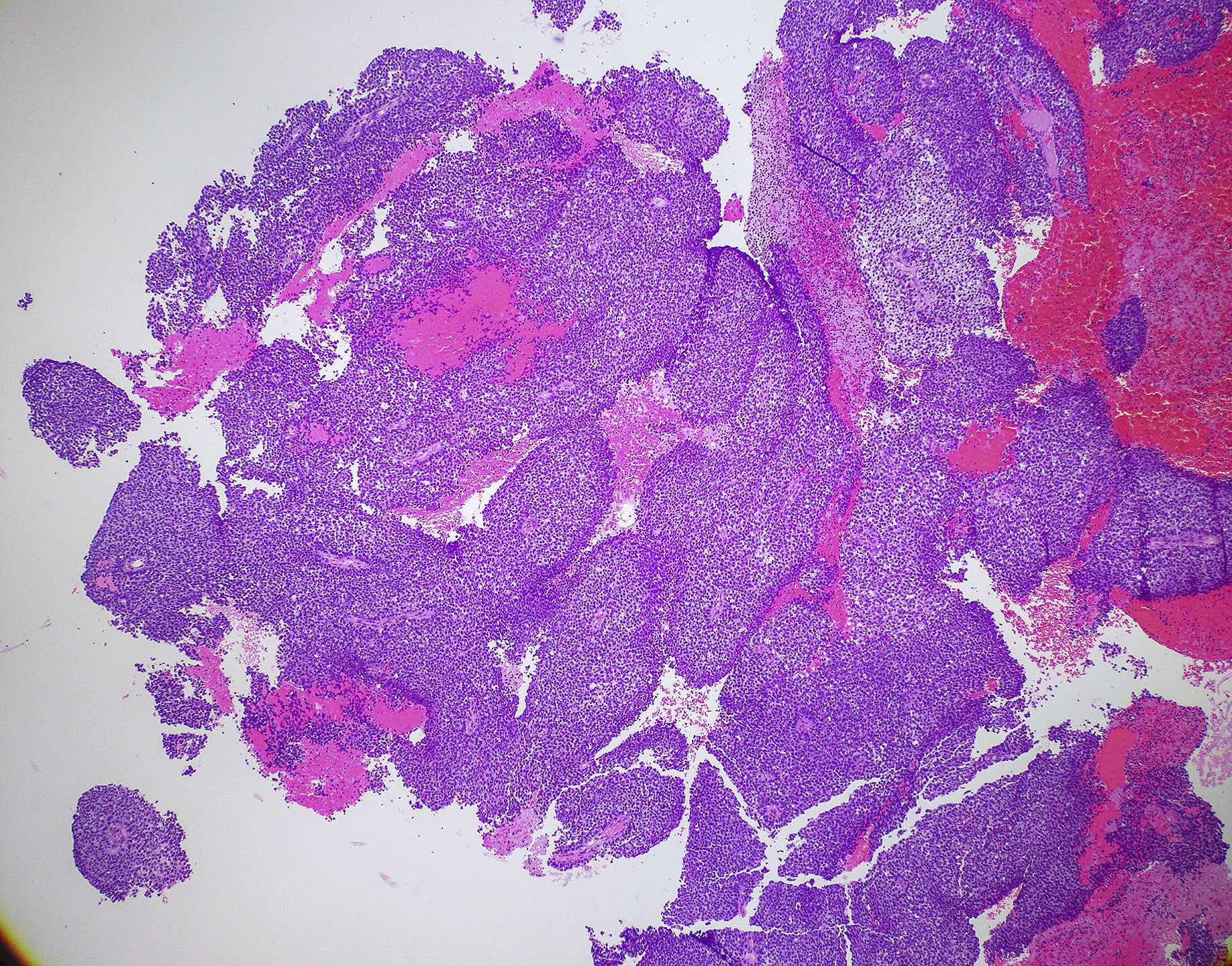
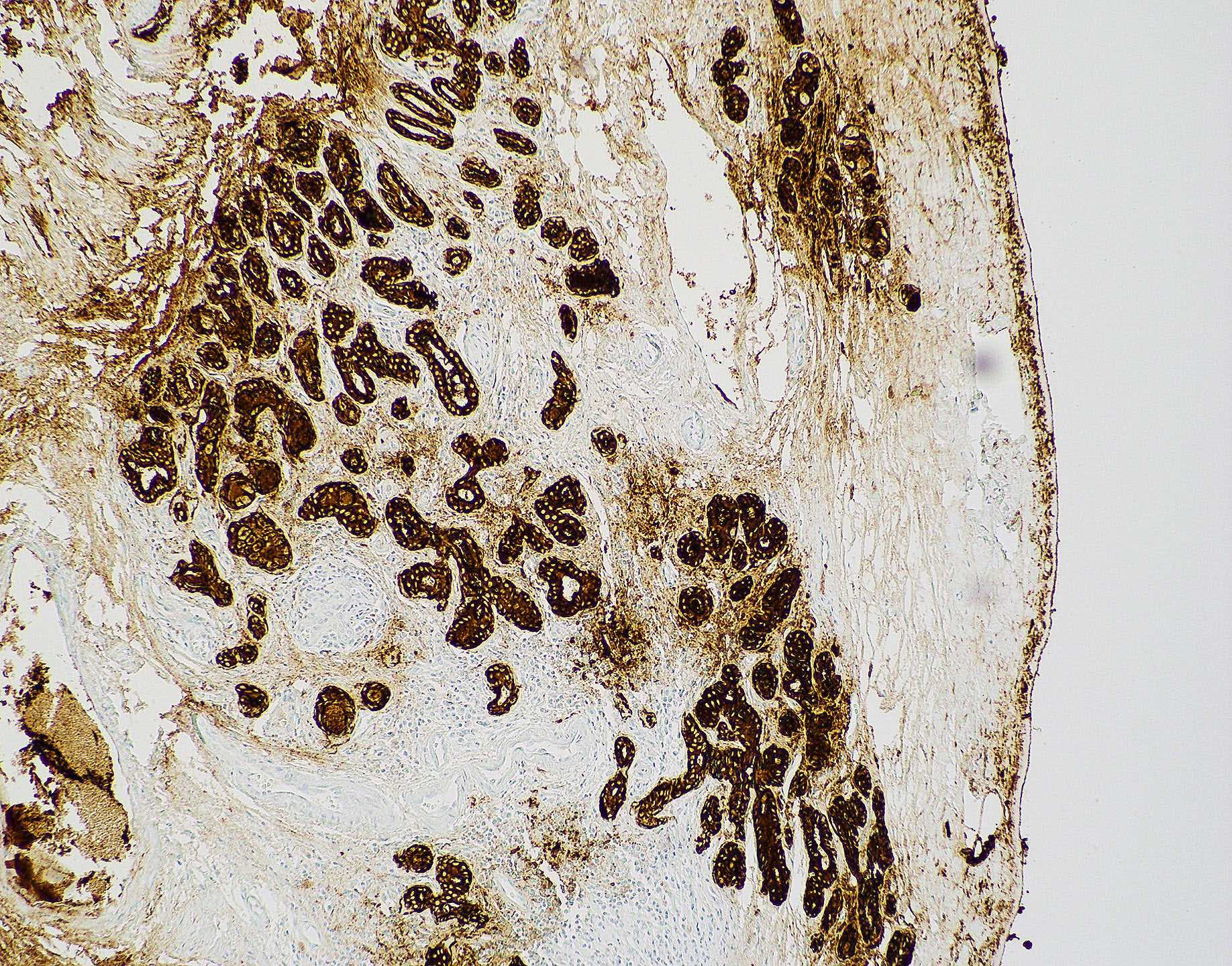
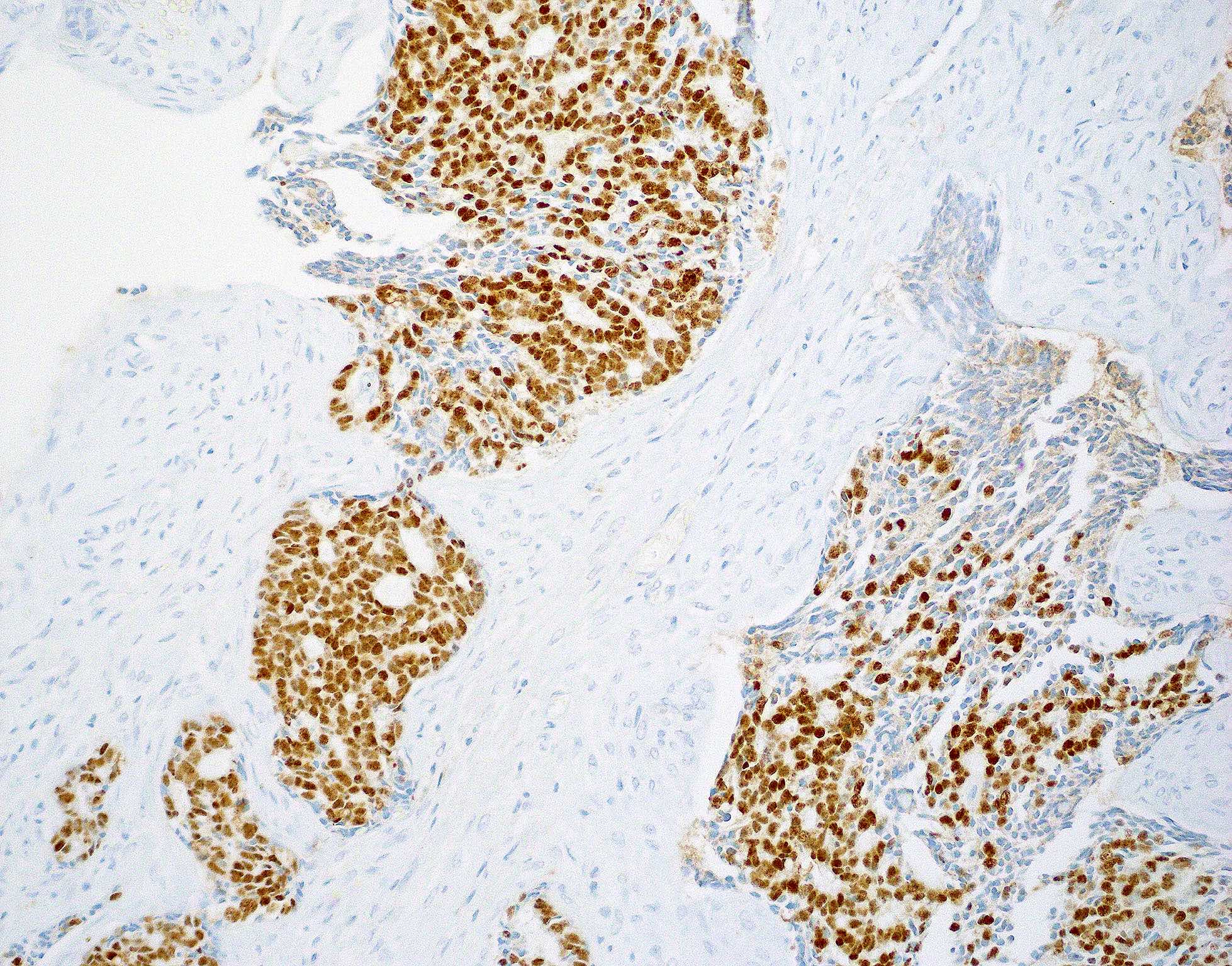
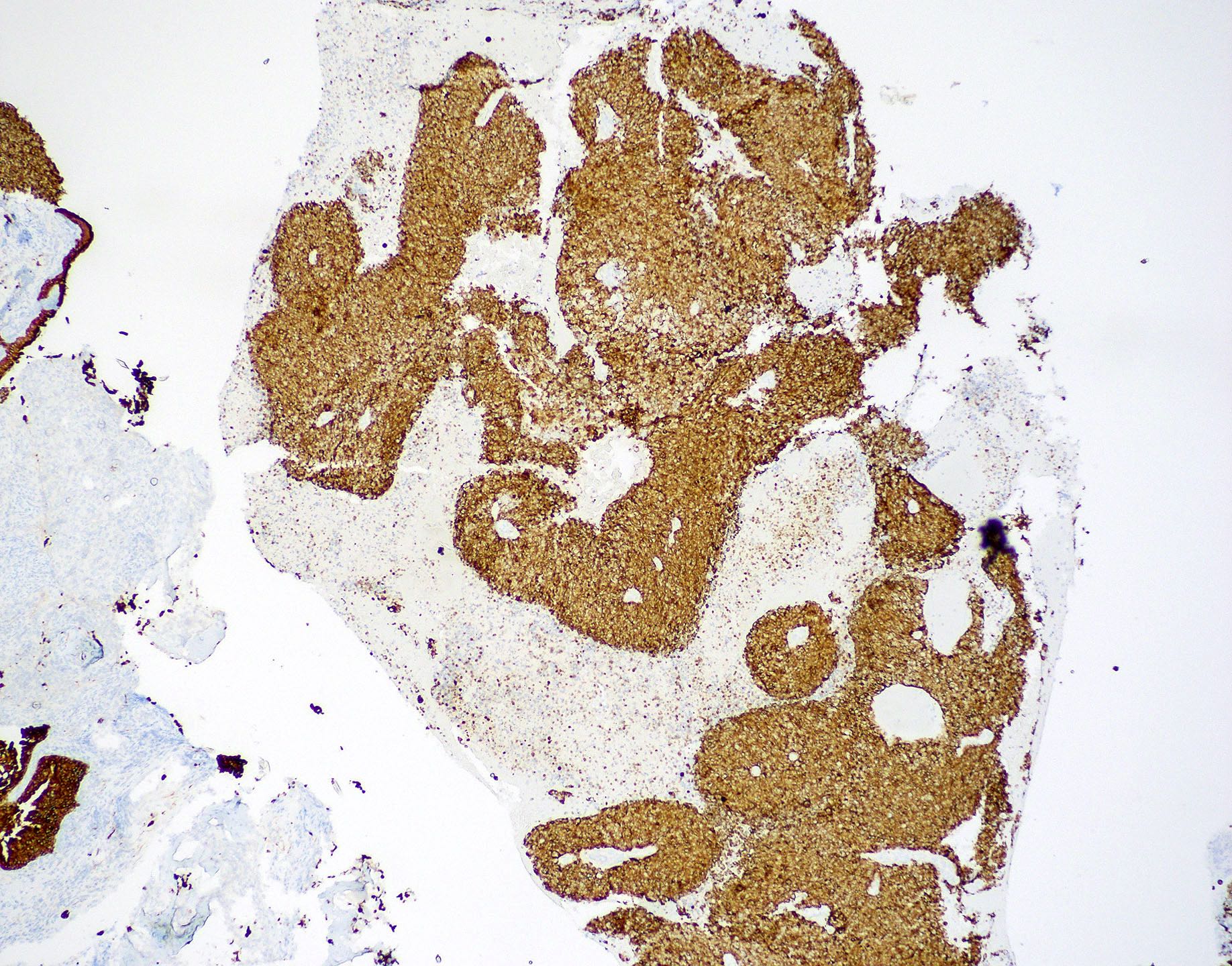
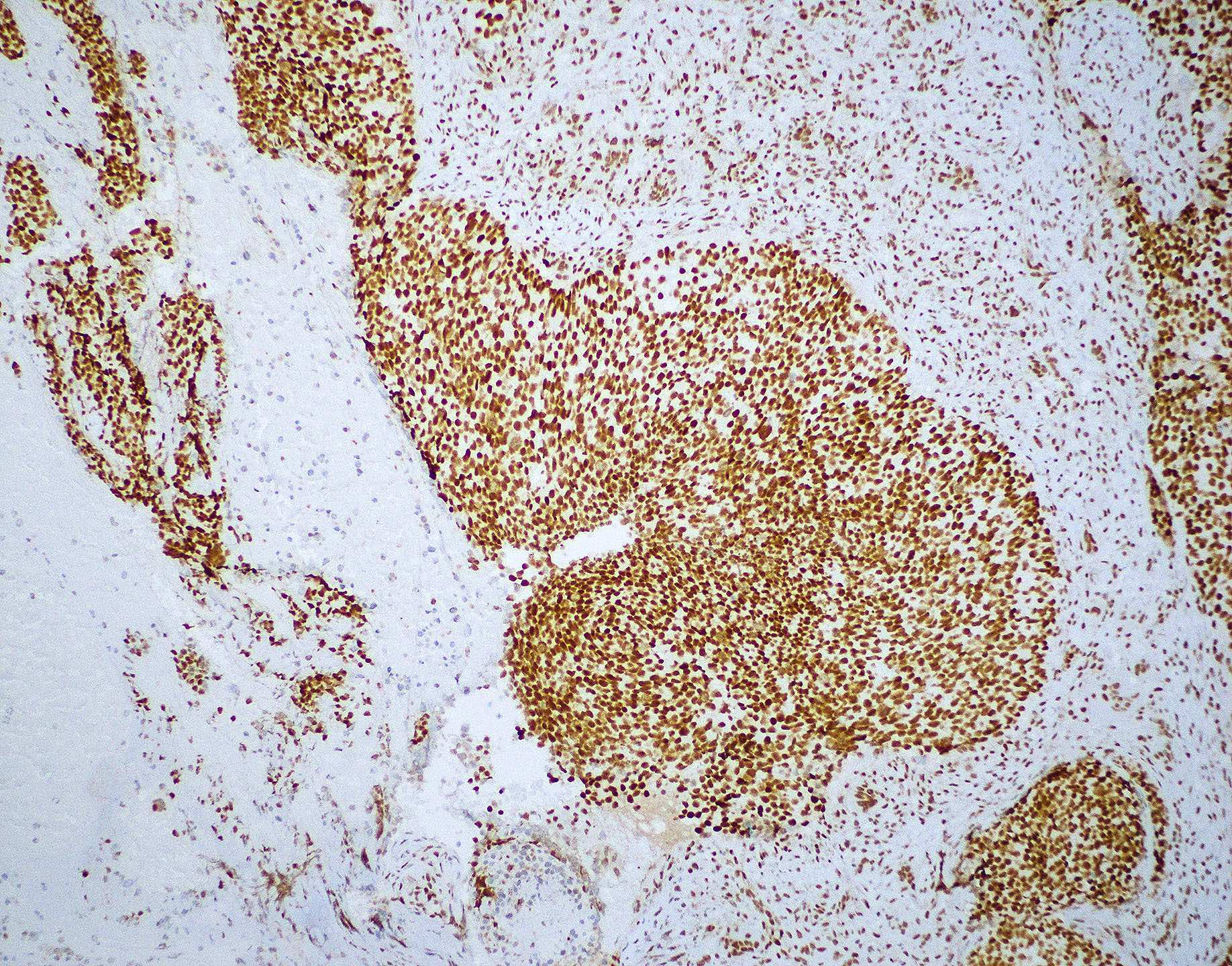
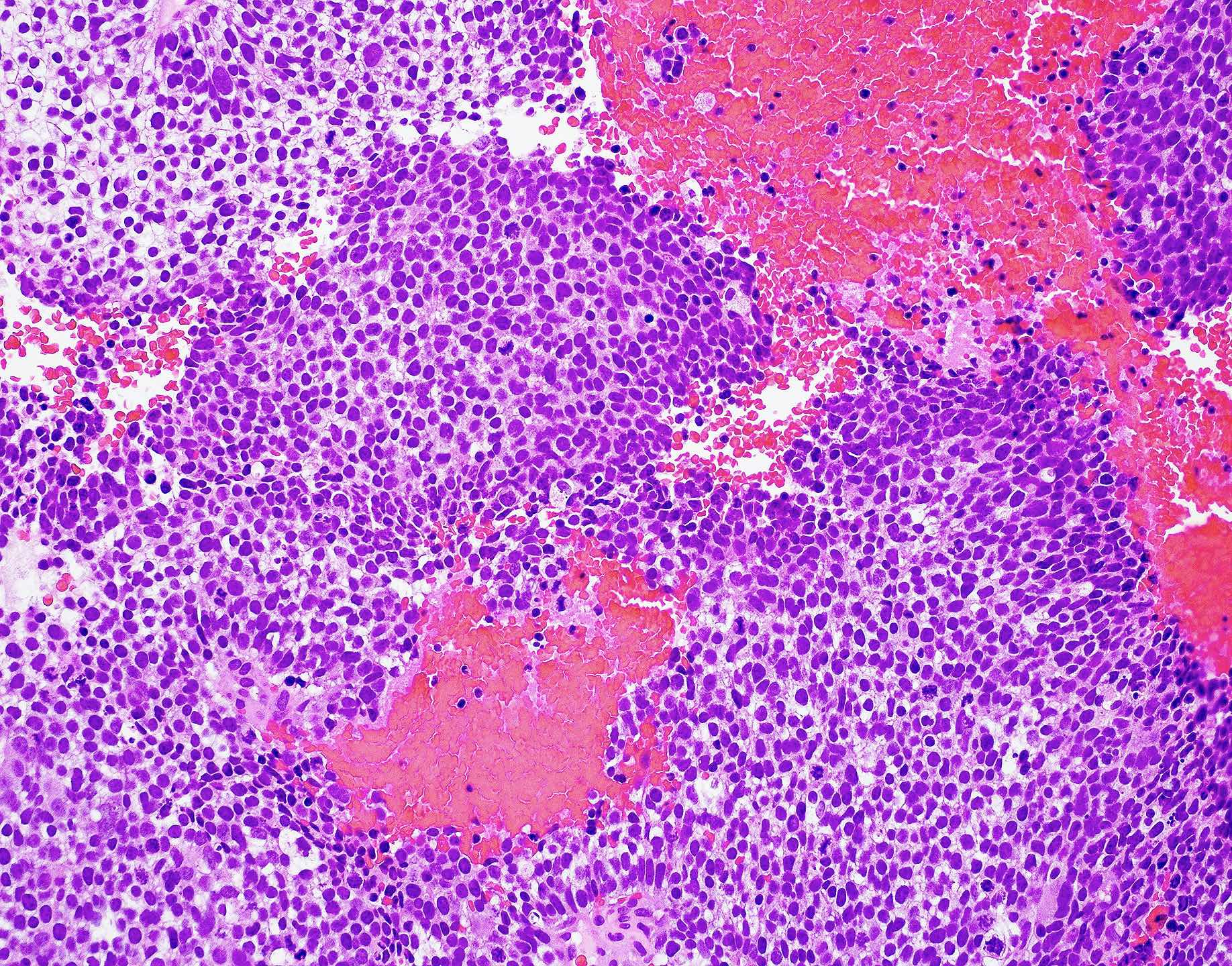
Microscopic (histologic) images
Contributed by Wai Szeto, M.D., M.S.
Positive stains
- Squamous cell carcinoma (Head Neck Pathol 2014;8:269, Am J Surg Pathol 2017;41:458)
- AE1 / AE3, CK5/6, CK7, CK903
- p40, p63
- EMA
- Nonkeratinizing squamous cell carcinoma
- p16 should be diffuse, block-like, with nuclear and cytoplasmic staining in > 70% of neoplastic cells
- SMARCB1 (INI1) and SMARCA4 (BRG1) retained
- NUT carcinoma (Am J Surg Pathol 2012;36:1216, Head Neck Pathol 2022;16:1)
- NUT1 in diffuse speckled pattern
- Pancytokeratin, p40 and p63 in majority of cases
- INI1 retained
- SWI / SNF complex deficient sinonasal carcinoma (Am J Surg Pathol 2017;41:458, Am J Case Rep 2023;24:e939244)
- Pancytokeratin
- p63, CK5 and CK7 in ~50% of cases
- Synaptophysin, chromogranin, CD56 and CD117 may occasionally show positivity
- p16 may show diffuse positivity but negativity for HPV by in situ hybridization
- Lymphoepithelial carcinoma (Semin Diagn Pathol 2015;32:74)
- Pancytokeratin
- EBER by in situ hybridization
- Sinonasal undifferentiated carcinoma (Mod Pathol 2017;30:S1, Mod Pathol 2019;32:205, Arch Pathol Lab Med 2015;139:55, Curr Oncol 2021;28:2420)
- Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1, Head Neck Pathol 2022;16:229)
- Complex immunoprofile due to many immunohistochemical stains highlighting different components
- Cytokeratins, AE1 / AE3, CAM5.2, CK5/6, EMA in epithelial components
- Desmin, vimentin, SMA in mesenchymal components
- S100, synaptophysin, chromogranin, CD56, CD99, INSM1, GFAP, NSE in immature neuroectodermal components
- HPV associated multiphenotypic sinonasal carcinoma (Head Neck Pathol 2019;13:331, Am J Surg Pathol 2017;41:1690, Head Neck Pathol 2019;13:220, Ear Nose Throat J 2020;99:94)
- Intestinal type adenocarcinoma (Head Neck Pathol 2017;11:295, Head Neck Pathol 2016;10:68)
- Pancytokeratin
- CK7, CEA and EMA are variably positive
- Intestinal markers: CK20, CDX2, villin, SATB2, MUC2
- Neuroendocrine markers may be focally positive (chromogranin, synaptophysin)
- Aberrant p53 expression
- Nonintestinal type adenocarcinoma (Head Neck Pathol 2016;10:68)
Negative stains
- Squamous cell carcinoma (Head Neck Pathol 2014;8:269, Am J Surg Pathol 2017;41:458)
- NUT carcinoma (Am J Surg Pathol 2012;36:1216, Head Neck Pathol 2022;16:1)
- Synaptophysin, chromogranin, S100 (may be focal)
- SWI / SNF complex deficient sinonasal carcinoma (Am J Surg Pathol 2017;41:458, Am J Case Rep 2023;24:e939244)
- Lymphoepithelial carcinoma (Semin Diagn Pathol 2015;32:74)
- p16 typically negative
- Sinonasal undifferentiated carcinoma (Mod Pathol 2017;30:S1, Mod Pathol 2019;32:205, Arch Pathol Lab Med 2015;139:55, Curr Oncol 2021;28:2420)
- Absent to minimal expression of lineage specific markers
- p40, p63, CK5/6, CEA, CD34, desmin, SMA, SMMHC, S100, calretinin, CD45
- May show weak or patchy expression of neuroendocrine markers (synaptophysin, chromogranin, CD56)
- Absent to minimal expression of lineage specific markers
- Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1, Head Neck Pathol 2022;16:229)
- SMARCA4 (BRG1) loss
- Β hCG, CD45
- HPV associated multiphenotypic sinonasal carcinoma (Head Neck Pathol 2019;13:331, Am J Surg Pathol 2017;41:1690, Head Neck Pathol 2019;13:220, Ear Nose Throat J 2020;99:94)
- Intestinal type adenocarcinoma (Head Neck Pathol 2017;11:295, Head Neck Pathol 2016;10:68)
- Annexin A1 loss
- Annexin A2 decreased
- Nonintestinal type adenocarcinoma (Head Neck Pathol 2016;10:68)
Molecular / cytogenetics description
- Nonkeratinizing squamous cell carcinoma
- p16 positive cases should be followed by high risk HPV ISH or PCR for confirmation (Cancers (Basel) 2022;14:1874)
- DEK::AFF2 fusion with RNA sequencing or DEK break apart FISH in DEK::AFF2 carcinoma (Am J Surg Pathol 2021;45:1682)
- Lymphoepithelial carcinoma (Head Neck Pathol 2022;16:1)
- EBER ISH positive
- Sinonasal undifferentiated carcinoma
- IDH2 R172 mutation is common (> 80%) (Mod Pathol 2019;32:205)
- NUT carcinoma (Head Neck Pathol 2013;7:11, Head Neck Pathol 2022;16:1)
- Rearrangement of NUT gene, most commonly BRD4::NUT [t(15;19)(q14, p13.1)]
- Remaining cases are BRD3::NUT [t(9;15)(q34.2;q14)] or other NUT variant fusions
- HPV associated multiphenotypic sinonasal carcinoma (Head Neck Pathol 2019;13:331, Am J Surg Pathol 2017;41:1690, Head Neck Pathol 2019;13:220, Ear Nose Throat J 2020;99:94)
- High risk HPV positive by DNA or RNA ISH and PCR
- HPV 33 is most commonly isolated
- Negative for MYB, MYBL1 or NFIB gene fusions that are seen in adenoid cystic carcinoma
Sample pathology report
- Nasal contents, biopsy:
- Undifferentiated carcinoma, most consistent with sinonasal undifferentiated carcinoma (see comment)
- Comment: Sections show a poorly differentiated epithelioid neoplasm growing in nests, sheets and trabeculae. Numerous mitotic figures are identified. Focal necrosis is present. The tumor cells have mild nuclear pleomorphism and prominent nucleoli. No overt squamous or glandular differentiation is identified. By immunohistochemical staining, the tumor is positive for pankeratin and CK7. The tumor is negative for synaptophysin, chromogranin, CK5/6, p40, p63, SMMS, S100, SOX10, myogenin and desmin. INI1 immunostain is retained. EBV EBER in situ hybridization is negative. Overall morphology and immunophenotypic profile are most consistent with sinonasal undifferentiated carcinoma.
Differential diagnosis
- Squamous cell carcinoma (Head Neck Pathol 2016;10:68):
- Schneiderian papillomas (inverted, exophytic, oncocytic):
- Lacks invasive growth
- Mild atypia
- Basaloid squamous cell carcinoma:
- Basaloid variant that grows in nests / nodules with peripheral palisading
- Schneiderian papillomas (inverted, exophytic, oncocytic):
- NUT carcinoma (Mod Pathol 2019;32:205, Head Neck Pathol 2022;16:1, Int J Surg Pathol 2022;30:273, Head Neck Pathol 2016;10:85, Curr Oncol 2021;28:2420):
- Basaloid squamous cell carcinoma:
- Sinonasal undifferentiated carcinoma:
- NUT1 negative
- IDH2 mutations frequently present
- Neuroendocrine carcinoma:
- Lacks keratinization
- Positivity for synaptophysin, chromogranin, CD56, NSE
- SWI / SNF complex deficient sinonasal carcinoma:
- SWI / SNF complex deficient sinonasal carcinoma (Am J Surg Pathol 2017;41:458, Am J Case Rep 2023;24:e939244, Head Neck Pathol 2022;16:1, Head Neck Pathol 2016;10:85, Curr Oncol 2021;28:2420):
- Sinonasal undifferentiated carcinoma:
- INI1 retained
- IDH2 mutations frequently present
- NUT carcinoma:
- NUT1 positive
- Neuroendocrine carcinoma:
- Positivity for synaptophysin, chromogranin, CD56, NSE
- HPV associated multiphenotypic sinonasal carcinoma:
- Sinonasal undifferentiated carcinoma:
- Lymphoepithelial carcinoma (Cureus 2022;14:e31103, Mod Pathol 2017;30:S1):
- Sinonasal undifferentiated carcinoma:
- Typically, does not have a prominent lymphoid infiltrate
- EBV negative
- Nonkeratinizing squamous cell carcinoma:
- Difficult to distinguish as the immunohistochemical profile will be virtually identical
- Melanoma:
- Lymphoma:
- Hematolymphoid markers showing B or T cell lineage
- Sinonasal undifferentiated carcinoma:
- Sinonasal undifferentiated carcinoma (CA Cancer J Clin 2023;73:72, Head Neck Pathol 2016;10:68):
- Neuroendocrine carcinoma:
- Shows nuclear molding, salt and pepper chromatin
- Strong positivity with neuroendocrine marker (synaptophysin, chromogranin, NSE)
- Nonkeratinizing squamous cell carcinoma:
- Keratinizing squamous cell carcinoma:
- p40, p63 and CK5/6 positive in keratinizing squamous cell carcinoma; negative in sinonasal undifferentiated carcinoma
- NUT carcinoma:
- Diffuse nuclear staining with NUT
- Lymphoma:
- Hematolymphoid markers showing B or T cell lineage
- Neuroendocrine carcinoma:
- Teratocarcinosarcoma (Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1):
- Olfactory neuroblastoma:
- Mesenchymal differentiation not present
- Carcinosarcoma:
- Neuroectodermal differentiation not present
- Olfactory neuroblastoma:
- HPV associated multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94, Head Neck Pathol 2022;16:1)
- Adenoid cystic carcinoma:
- MYB, MYBL1 or NFIB translocations present
- Squamous cell carcinoma:
- Lacks biphasic ductal and myoepithelial cell differentiation
- SWI / SNF complex deficient sinonasal carcinoma:
- Sinonasal undifferentiated carcinoma:
- IDH2 mutations frequently present
- HPV negative
- Diagnosis of exclusion
- Adenoid cystic carcinoma:
- Intestinal type adenocarcinoma (Head Neck Pathol 2016;10:68):
- Metastatic adenocarcinoma of colonic origin:
- Rare but must be considered
- Morphology and immunohistochemistry are virtually indistinguishable from a GI primary
- Exclusion primarily based on clinical and radiographic correlation
- Sinonasal nonintestinal type adenocarcinoma:
- Salivary gland type adenocarcinomas:
- Metastatic adenocarcinoma of colonic origin:
- Nonintestinal type adenocarcinoma (Head Neck Pathol 2016;10:68):
- Sinonasal intestinal type adenocarcinoma:
- Salivary gland type adenocarcinomas:
- Morphologically diverse
- Most common is adenoid cystic
- Follows immunophenotype of respective salivary gland type adenocarcinoma
Practice question #1
Practice answer #1
D. Sinonasal undifferentiated carcinoma. IDH1 or IDH2 mutations are common in sinonasal undifferentiated carcinoma and can be seen in more than 80% of cases. The most common is the IDH2 R172 mutation, which can be detected with immunohistochemistry. Answers A - C are incorrect because these mutations are not typically associated with those entities.
Comment Here
Reference: Sinonasal carcinoma-general
Comment Here
Reference: Sinonasal carcinoma-general
Practice question #2
A biopsy from a mass in the maxillary sinus showed histologic features of squamous cell carcinoma with a complex exophytic and endophytic growth pattern with broad papillary fronds. There is minimal keratinization. Which of the following gene rearrangements is frequently observed in this type of tumor?
- CASG::PRKD1 fusion
- CRTC1::MAML2 fusion
- DEK::AFF2 fusion
- MYB::NFIB fusion
Practice answer #2
C. DEK::AFF2 fusion. DEK::AFF2 is a novel fusion identified in a type of nonkeratinizing squamous cell carcinoma of the sinonasal tract called DEK::AFF2 fusion associated papillary squamous cell carcinoma that exhibits complex exophytic and endophytic growth with broad papillary fronds, anastomosing lobules, ribbons and nests. Detectable with DEK::AFF2 FISH break apart probe or RNA sequencing. Answer A is incorrect because CASG::PRKD1 is associated with polymorphous adenocarcinoma. Answer B is incorrect because CRTC::MAML2 is associated with mucoepidermoid carcinoma. Answer D is incorrect because MYB::NFIB is associated with adenocystic carcinoma.
Comment Here
Reference: Sinonasal carcinoma-general
Comment Here
Reference: Sinonasal carcinoma-general